Abstract
Palm Leaf Manuscripts are valuable cultural assets, and understanding the impact of environmental factors on their properties is essential for effective conservation. This study simulated aging to assess the effects of relative humidity (RH) on the manuscripts using various techniques, including SEM, DVS, TMA, FT-IR, and XRD. The results show that exposure to extreme dry or humid conditions negatively affects the manuscripts. In dry conditions, they experience bending, cracking, reduced mechanical strength, lower hygroscopicity, and chemical degradation. In humid conditions, fungal growth compromises the manuscripts’ structure. These changes affect color, gloss, hygroscopic behavior, cellulose crystallinity, and thermal stability, weakening mechanical properties. Manuscripts stored at 50% RH showed no significant damage, suggesting that this level is optimal for their preservation. This study provides insights into the degradation mechanisms of Palm Leaf Manuscripts under different humidity conditions and offers recommendations for their preservation.
Similar content being viewed by others
Introduction
Palm Leaf Manuscripts are a unique medium for writing, made from the leaves of palm species such as Talipot palm (Corypha umbraculifera L.) and palmyra palm (Borassus flabellifer L.). These leaves were processed for writing before the widespread use of paper and were widely used in South and Southeast Asia. Palm Leaf Manuscripts not only document valuable knowledge in fields such as history, literature, philosophy, art, and science but also play a crucial role in Buddhist culture and the spread of religion, carrying rich cultural significance1,2,3. Due to their immense historical and cultural value, Palm Leaf Manuscripts are recognized as precious cultural heritage, making their restoration and preventive conservation especially important.
Substantial progress has been made in the systematic analysis of the materials4,5,6,7 and production techniques8,9 of Palm Leaf Manuscripts. Researchers have classified common types of deterioration10,11 and conducted preliminary assessments of degradation levels12. Based on these insights, various preservation and restoration strategies have been explored13,14, providing essential data for understanding and conserving these artifacts scientifically, thus contributing significantly to their long-term protection.
However, while material analysis, production techniques, and conservation methods have been extensively studied, the effects and mechanisms of environmental factors on the aging of Palm Leaf Manuscripts remain insufficiently explored. Similar to paper and wood, Palm Leaf Manuscripts are cellulose-based organic materials primarily composed of cellulose, hemicellulose, and lignin. Changes in these components’ chemical structure influence key properties such as color, mechanical strength, and hygroscopicity4,15, thereby impacting the degradation process—these represent internal factors of aging.
External factors, particularly environmental conditions, also play a crucial role in the aging of Palm Leaf Manuscripts. Preliminary studies indicate that dry conditions reduce mechanical strength11,15, while excessive humidity increases hygroscopicity16. Extensive research on cellulose-based materials like paper and wood has shown that high temperatures accelerate thermo-oxidative degradation of cellulose and hemicellulose, leading to a decline in mechanical properties17,18,19. Similarly, prolonged UV radiation exposure damages cellulose molecular chains, promoting degradation and photochemical reactions in lignin that cause discoloration20,21,22. Acidic gases, such as sulfur compounds and nitrogen oxides, can trigger acid hydrolysis in the presence of moisture23, while dust deposition alters surface characteristics and color24.
Although these findings provide valuable insights into the preventive conservation of cellulose-based materials, Palm Leaf Manuscripts are often stored indoors in temples, libraries, or archival repositories. This largely protects them from temperature fluctuations, UV exposure, and atmospheric pollutants. However, humidity—subject to significant indoor fluctuations—emerges as the primary environmental factor affecting their preservation.
Palm Leaf Manuscripts, like other cellulose-based materials, are highly sensitive to humidity variations, which can induce significant physicochemical changes. Previous studies have found that frequent fluctuations in humidity, as well as excessively high or low humidity levels, can lead to a decline in the mechanical properties of Palm Leaf Manuscripts25. High humidity can also promote microbial growth and reproduction26, causing the degradation of the manuscripts’ main chemical components27. These studies indicate that inappropriate environmental humidity can alter the properties of Palm Leaf Manuscripts, accelerating their deterioration. However, most previous research has focused on the impact of relative humidity on a single property of Palm Leaf Manuscripts, such as mechanical performance or chemical structure, without comprehensively considering the combined changes in morphology, physical, and chemical properties under different relative humidity conditions. For example, a dry environment may not cause significant changes in the chemical structure of Palm Leaf Manuscripts, but it can result in dehydration and deformation, leading to a loss of mechanical properties. On the other hand, higher humidity may help the manuscripts maintain some degree of flexibility, but mold growth can significantly affect their morphology and degrade their main chemical components. Furthermore, these studies typically focus solely on comparing the performance of Palm Leaf Manuscripts before and after aging16,25,26, without examining the changes and trends in their performance at various time points throughout the aging process. Other studies have indicated that the raw palm leaves used in manuscripts not only exhibit anisotropic properties similar to other cellulose-based materials but also show differences between the upper and lower surfaces5,28. These characteristics suggest potential variations in performance based on direction and surface, warranting further investigation into whether such differences persist throughout the aging process. Therefore, comprehensively considering the changes in various properties of Palm Leaf Manuscripts under different relative humidity conditions and evaluating the optimal relative humidity for their preservation is of great significance for elucidating their aging mechanisms and developing effective restoration strategies and preventive conservation measures.
In this study, processed Palm Leaf Manuscripts were used as research samples. Simulated aging experiments were conducted to assess the long-term effects of varying relative humidity levels on their morphology, structure, color, gloss, surface contact angle, hygroscopicity, mechanical properties, chemical structure, and thermal stability. This study aims to identify the optimal relative humidity for palm leaf manuscript preservation and to enhance the understanding of environmental factors influencing their longevity. The results provide critical data to support research on degradation mechanisms and inform preventive conservation strategies for these invaluable cultural artifacts.
Methods
Materials
The experimental samples in this study consisted of initially processed Palm Leaf Manuscripts sourced from Yunnan Province, China. These samples were prepared following the traditional palm leaf manuscript production process, recognized as a National Intangible Cultural Heritage in China. Healthy talipot palm leaves (Corypha umbraculifera L.) underwent a series of treatments, including boiling, washing, air-drying, trimming, and flattening8, to obtain the research samples. To isolate the effects of relative humidity on the physical and chemical properties of the palm leaves themselves, no subsequent treatments such as writing or coloring were applied, thereby eliminating potential influences from pigments or adhesives on the experimental results.
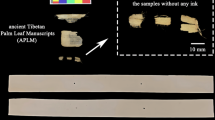
The overall appearance and localized morphological features of the upper and lower surfaces of the processed Palm Leaf Manuscripts are illustrated in Fig. 1.
The upper surface displays a prominent and well-defined longitudinal texture (Fig. 1b), whereas the lower surface exhibits clearer transverse patterns (Fig. 1c). These observations highlight the significant anisotropy in the physical and structural properties of Palm Leaf Manuscripts, a characteristic shared with other cellulose-based materials such as paper and wood29,30,31.
Simulated aging experiment
The samples were divided into five groups and subjected to different aging conditions in an environmental test chamber (GSH-64, Espec, Ōsaka, Japan). The temperature was consistently maintained at 25 °C, while the relative humidity (RH) levels were set at 10%, 30%, 50%, 70%, and 90%, respectively. Throughout the aging process, samples were periodically removed to monitor changes in their morphology and physicochemical properties. After 200 days of aging, the samples were analyzed to evaluate and compare their morphology and physicochemical properties before and after aging, as well as under the various RH conditions.
Morphology analysis
Morphological changes in the samples were examined using an ultra-depth optical microscope (VHX-6000, KEYENCE, Ōsaka, Japan) at magnifications ranging from 50x to 200x. The microscope’s integrated 3D scanning function was employed to scan and reconstruct the samples’ complete structure, enabling detailed analysis of overall morphology and structural alterations.
Scanning electron microscope (SEM) testing
To enhance conductivity, the samples were gold-coated using a sputter coater (Emitech K550X, Quorum Technologies, Laughton, UK) with high-purity gold (99.99% Au) as the target material. The sputtering process was conducted at a distance of 8 cm between the target and the samples, with a sputtering current of 20 mA for 60 s. Following gold coating, the microstructure of the samples was observed using a field emission scanning electron microscope (Regulus8100, HITACHI, Tokyo, Japan). The observation parameters were set to an accelerating voltage of 15 kV, a working distance of 15–20 mm, and magnifications ranging from 150× to 500×.
Color difference testing
A portable spectrophotometer (CM-26d, KONICA MINOLTA, Tokyo, Japan) was used to measure color changes in the samples before and after aging. The measurement parameters were as follows: light source: D65, measurement mode: SCI, measurement aperture: 8 mm, measurement angle: 8°, reflectance resolution: 0.001%, and spectral measurement range: 360–740 nm. Measurements were conducted every 10 days during the first 100 days of aging, and every 20 days thereafter. Both the upper and lower surfaces of each sample were tested, with each group consisting of five parallel samples. Color changes were characterized using the ΔE value:
Where \({L}^{* }\), \({a}^{* }\), and \({b}^{* }\) represent the measured color parameters after aging, and \({L}_{0}^{* }\), \({a}_{0}^{* }\), and \({b}_{0}^{* }\) are the corresponding parameters before aging.
Gloss testing
A gloss meter (SMN 268, Qi-li, Tianjin, China) was used to measure the gloss of the samples before and after aging at an incident light angle of 60°. The gloss reduction rate was determined by calculating the difference in gloss values before and after aging. Measurements were performed every 10 days during the first 100 days of aging and every 20 days thereafter. Both the upper and lower surfaces of each sample were tested, with each group comprising five parallel samples. The gloss reduction rate was calculated as follows:
Where \({G}_{0}\) is the initial gloss value, and \({G}_{t}\) is the gloss value after aging.
Surface contact angle testing
The surface contact angles of the samples with deionized water and diiodomethane (DIM) were measured using a contact angle goniometer (JC2000 DM, Zhong-chen, Shanghai, China). Measurements were conducted every 25 days on both the upper and lower surfaces of each sample, with each group comprising five parallel samples.
Surface energy calculation
The dispersive and polar components of the surface energy were then determined using the Owens–Wendt equation, and the total surface energy of the samples was calculated.
The \({\gamma }_{L}\) is the total surface tension of the reference liquid. For instance, the total surface tension value for water is 72.8 mN/m, while that for diiodomethane is 50.8 mN/m. \(\theta\) is the contact angle between the sample and the reference liquid. \({\gamma }_{S}^{d}\) and \({\gamma }_{S}^{p}\) are the dispersive and polar components of the sample (mN/m). \({\gamma }_{L}^{d}\) is the dispersive of the reference liquid. For instance, the dispersive value for water is 21.8 mN/m, while that for diiodomethane is 50.8 mN/m. \({\gamma }_{L}^{p}\) is the polar of the reference liquid. For instance, the polar value for water is 51.0 mN/m, while that for diiodomethane is 0.0 mN/m. \({\gamma }_{S}\) is the total surface energy of the sample (mN/m).
Dynamic vapor sorption (DVS) testing
A high-throughput dynamic vapor sorption analyzer (SPSx-1μ, ProUmid, Ulm, Germany) was used to measure the maximum moisture content of the samples under constant temperature and varying relative humidity (RH) conditions. The isothermal moisture adsorption and desorption curves were obtained within a humidity range of 0% to 95% RH. Humidity was controlled in 10% RH increments from 0% to 90% RH and 5% RH increments from 90% to 95% RH, with the desorption phase following the same gradient as the adsorption phase. The temperature was maintained at 25 °C. Equilibrium conditions were defined as a change in sample weight of no more than 0.1% within 10 min, or a maximum measurement duration of 360 min per gradient. Measurements were performed every 25 days, with each group consisting of three parallel samples.
Hardness testing
A handheld Type C Shore durometer (LXD-C, Wei-du, Wenzhou, China) was used to measure changes in the hardness of the samples before and after aging. Measurements were performed every 10 days during the first 100 days of aging and every 20 days thereafter. Both the upper and lower surfaces of each sample were tested, with each group consisting of ten parallel samples.
Flexural strength testing
The flexural strength of the samples was assessed using a thermomechanical analyzer (TMA 7100, Hitachi, Tokyo, Japan) equipped with quartz probes and components. The test was conducted with an initial load of 0.1 mN and a loading rate of 30 mN/min, continuing until the samples fractured to obtain the corresponding test curves. Measurements were taken every 25 days, with both the longitudinal and transverse directions of each sample tested, and each group comprising twenty samples.
The flexural strength (\(\sigma\)) and flexural modulus (\(E\)) of the samples were calculated using formulas (2) and (3), respectively:
Where\(\,\sigma \,\)(MPa) and\(\,E\,\)(MPa) are the flexural strength and flexural modulus of the sample, respectively; F (N) is the maximum load at the point of sample fracture; L (mm) is the span between the supports on the TMA flexural strength testing platform, fixed at 5 mm; b (mm) and d (mm) are the width and thickness of the sample, respectively, measured using a super-depth microscope (VHX-6000, KEYENCE, Ōsaka, Japan);\(\,\delta \,\)(mm) is the maximum displacement of the sample at the point of fracture.
Fourier-transform infrared spectroscopy (FT-IR) testing
The aged samples were finely ground and passed through a 200-mesh sieve. They were then mixed with spectrally pure KBr (Macklin, Shanghai, China) at a mass ratio of 1:100 and pressed into pellets. The chemical structure of the samples was analyzed using Fourier-transform infrared spectroscopy (FT-IR, Nicolet™ iS™5, Thermo Scientific, Waltham, MA, USA) to identify changes in characteristic functional groups before and after aging. Measurements were conducted every 25 days, with each group consisting of three parallel samples.
To further evaluate changes in the chemical structure, the infrared spectra were analyzed semi-quantitatively using the peak intensity method. Specific absorption peaks were selected to represent key components: 1730 cm−¹ (carbonyl group stretching vibration in hemicellulose), 1460 cm−¹ (methylene group stretching vibration in hemicellulose), 1505 cm−¹ (butyl propane backbone vibration in butyl lignin), 1370 cm−¹ (methyl group bending vibration in cellulose), and 1060 cm−¹ (glycosidic bond bending vibration in cellulose)32,33,34. The intensity values of these peaks were measured after baseline correction using OMNIC 9.2 software (Thermo Scientific, Waltham, MA, USA).
X-ray diffraction (XRD) testing
The crystal structure and crystallinity of the aged samples were analyzed using an X-ray diffractometer (D8 ADVANCE, Bruker, Munich, Germany). The operational parameters were set as follows: working voltage of 40 kV, current of 40 mA, and Cu-Kα radiation (λ = 1.5406 Å) as the radiation source. The scanning angle range was from 5° to 50° (2θ), with a scanning speed of 1°/min and a step size of 0.02°. Measurements were conducted every 50 days, with each group consisting of three parallel samples. Diffraction patterns were analyzed using MDI Jade 9 software (ICDD, Newtown Square, PA, USA), and the crystallinity index (CI) was calculated using the Segal method.
where CI is the crystallinity index of the sample; \({I}_{200}\) is the maximum intensity of the lattice diffraction angle of (200) near 2θ = 22.4°, which signifies both the crystalline and non-crystalline regions; and \({I}_{{am}}\) is the minimum intensity near the 2θ angle of 18°, indicating the non-crystalline region.
Thermogravimetric analysis (TGA)
The samples were analyzed for pyrolysis behavior using a thermogravimetric analyzer (Discovery TGA 550, TA Instruments, New Castle, DE, USA) under a nitrogen atmosphere. The gas flow rate was maintained at 20 mL/min, with the temperature ramped from 30 °C to 1000 °C at a heating rate of 20 °C/min. Thermogravimetric (TG) and derivative thermogravimetric (DTG) curves were obtained to track changes in sample mass as a function of temperature. Parameters such as weight ratios and pyrolysis temperatures at different decomposition stages were calculated using Origin 2024 software (OriginLab, Northampton, MA, USA).
Results
Performance of the samples before aging
The performance of the upper and lower surfaces, as well as the longitudinal and transverse directions of the samples before aging, was tested separately. The results are shown in Tables 1 and 2, respectively.
The results indicate that the upper and lower surfaces of the samples exhibit significant differences in color, gloss, hydrophilicity, and hardness (Table 1), which can be primarily attributed to structural differences between these surfaces. Studies have shown that, as plants predominantly growing in tropical regions, palm leaves develop structural adaptations to resist environmental factors such as ultraviolet radiation and high temperatures. The upper surface typically has a thicker lipid layer, while the lower surface contains more stomata to facilitate water exchange and airflow5. Consequently, the upper surface of the samples shows higher gloss, greater hydrophobicity, and increased hardness compared to the lower surface. Additionally, due to differences in fiber structure distribution28, the mechanical strength and flexibility of the samples are greater in the longitudinal direction than in the transverse direction (Table 2). This characteristic is similar to other cellulose-based materials, such as paper and wood.
The observed differences in performance between the upper and lower surfaces, as well as the longitudinal and transverse directions, may play a significant role during the aging process of Palm Leaf Manuscripts, potentially leading to variations in aging outcomes.
Aging results of the samples
Figure 2 shows the overall morphology and localized feature changes of the samples after 200 days of aging under different conditions (temperature at 25 °C, relative humidity at 10% RH, 30% RH, 50% RH, 70% RH, and 90% RH). It should be emphasized that similar phenomena were observed in all samples placed under the same relative humidity conditions, but only one representative sample is shown in the images.
The unaged samples exhibit a natural upward curvature of the upper surface (Fig. 2a), with a maximum height difference of 0.62 mm, which is likely caused by the natural growth process of the palm leaf and the processing techniques. After 200 days of aging at 10% RH (Fig. 2b) and 30% RH (Fig. 2c), cracks appeared on the sample surfaces. The cracks on the samples aged at 30% RH were smaller and barely visible to the naked eye, whereas those aged at 10% RH exhibited more severe cracking, with greater numbers and larger areas of cracks. These cracks result from significant deformation, as the natural upward curvature of the samples was further amplified in dry environments. The maximum height differences for samples aged at 10% RH and 30% RH increased to 4.08 mm and 2.4 mm, respectively. The anisotropy inherent to natural cellulose-based materials causes differing dimensional changes during moisture absorption and desorption. Typically, transverse dimensional changes are greater than longitudinal changes35. This leads to more pronounced transverse shrinkage during drying, generating compressive stress in the middle region. Coupled with the relatively weaker mechanical properties in the transverse direction (Table 2), the samples in dry environments experienced severe bending deformation and transverse cracks.
In contrast, the samples aged in humid conditions (70% RH and 90% RH) exhibited significant mold growth on their surfaces (Fig. 2e, f), with samples aged at 90% RH showing denser and more abundant mold. The fungal erosion and moisture-induced swelling prevented noticeable overall bending deformation but caused the surface to become uneven.
Only the samples aged at 50% RH (Fig. 2d) remained free of cracks or microbial damage after 200 days, with no significant changes in morphology compared to the unaged samples.
SEM images of the samples
The SEM images of the samples aged for 200 days under different conditions are shown in Fig. 3. It should be emphasized that similar phenomena were observed in all samples placed under the same relative humidity conditions, but only one representative sample is shown in the images.
Under the scanning electron microscope, the microscopic structures, such as leaf veins and stomata, on the sample surfaces are clearly visible. It is confirmed that the lower surface has a greater number and denser distribution of stomata compared to the upper surface (Fig. 3b, d).
After 200 days of aging at 10% RH (Fig. 3f) and 30% RH (Fig. 3j), significant cracks are observed on the upper surface of the samples. The cracks are more severe at 10% RH, with transverse cracks almost spanning the entire surface, reaching widths of approximately 20 μm. The stomatal structures on the upper surface are severely damaged, exhibiting collapse and closure. In contrast, the stomatal structures on the lower surface remain relatively intact (Fig. 3h, l). However, the originally distinct longitudinal leaf veins have disappeared, likely due to compressive stress caused by sample bending deformation.
To avoid contamination of the instrument, the SEM images of the samples aged at 70% RH (Fig. 3q–t) and 90% RH (Fig. 3u–x) were taken after most of the surface mold had been removed. The SEM images still reveal residual mold and its morphological features. The mold spores appear as spherical clusters, with a network of hyphae radiating outward from the spore clusters, some of which have already penetrated the sample surface (Fig. 3r). Samples aged at 90% RH show more severe fungal erosion, with numerous holes and cracks left by the mold on the surface (Fig. 3u). Additionally, hyphae have penetrated these structures, further invading the sample’s interior (Fig. 3v, x). The SEM images indicate that samples aged in humid environments (70% RH and 90% RH) are severely eroded by mold, which not only causes significant damage to the surface morphology but may also disrupt the internal structure of the samples. This damage is likely to have a significant impact on properties such as hygroscopicity and mechanical strength.
In contrast, samples aged at 50% RH for 200 days exhibit no cracks or microbial damage, with no significant morphological changes compared to the unaged samples (Fig. 3m–p).
Color difference and gloss reduction of the samples
The color difference (ΔE) and gloss reduction rates of the upper and lower surfaces of the samples during aging under different conditions and after 200 days of aging are shown in Fig. 4.
As a literary medium, color plays a critical role in determining the physical appearance of Palm Leaf Manuscripts and directly affects the readability of the inscriptions and illustrations on their surfaces. Therefore, color is one of the most important attributes of this type of cultural heritage36,37,38. The color difference values (∆E) of both the upper (Fig. 4a) and lower (Fig. 4b) surfaces of the samples increased progressively during aging. Samples aged in humid environments (70% RH and 90% RH) exhibited significant color changes after 30–40 days, whereas samples aged in relatively dry conditions (10% RH–50% RH) showed slower and less pronounced color changes. After 200 days of aging, all samples displayed varying degrees of color alteration (Fig. 4c). For samples aged at 70% RH and 90% RH, substantial color changes were observed compared to their pre-aging states, with ∆E values of 33.10 and 37.36 for the upper surfaces and 29.81 and 34.22 for the lower surfaces, respectively. These significant changes were primarily caused by extensive mold growth, which obscured the original appearance of the samples (Fig. 2e, f). Conversely, samples aged at 50% RH exhibited the least color change, with ∆E values of 2.02 and 1.96 for the upper and lower surfaces, respectively.
Overall, the lower surfaces of the samples exhibited less color change compared to the upper surfaces. This may be attributed to the lower surfaces’ originally more yellowish tone (Table 1), which is less susceptible to visible changes during oxidation-induced yellowing. Furthermore, the samples displayed an overall upward curvature, exposing the upper surfaces to a greater extent to oxygen and other environmental factors. This increased exposure may have accelerated the aging process and contributed to more significant color changes on the upper surfaces.
The gloss reduction rate of both the upper (Fig. 4d) and lower (Fig. 4e) surfaces of all samples increased progressively with aging. Samples aged in humid environments (70% RH and 90% RH) exhibited a more significant loss of gloss as aging progressed, with gloss reduction rates approaching nearly 100% after 200 days of aging (Fig. 4f). This indicates that extensive mold growth had caused the samples to almost completely lose their surface gloss. In contrast, samples aged in relatively dry conditions (10% RH–50% RH) exhibited gloss reduction rates ranging between 30% and 40%, with those aged at 50% RH showing the smallest change in gloss. Overall, no significant differences in gloss reduction rates were observed between the upper and lower surfaces of the samples.
Surface contact angle and surface energy of the samples
The surface contact angles of the upper and lower surfaces of the samples with two reference liquids during aging under different conditions and after 200 days of aging, as well as the surface energy of the upper and lower surfaces after 200 days, are shown in Fig. 5.
a, b Changes in contact angle of the upper surface. c Contact angle of the upper surface after 200 days. d Surface energy of the upper surface after 200 days. e,f Changes in contact angle of the lower surface. g Contact angle of the lower surface after 200 days. h Surface energy of the lower surface after 200 days.
In relatively dry environments (10% RH–50% RH), the contact angles of both the upper and lower surfaces of the samples with water increased significantly as aging progressed, indicating enhanced hydrophobicity (Fig. 5a, e). However, the contact angles with diiodomethane showed no significant changes compared to the pre-aging state (Fig. 5b, f). In contrast, for samples aged in humid environments (70% RH and 90% RH), the water contact angles on both surfaces decreased significantly with aging, suggesting increased hydrophilicity. Simultaneously, the contact angles with diiodomethane also decreased to some extent. After 200 days of aging in humid environments, the contact angles with both reference liquids were notably reduced (Fig. 5c, g). Specifically, for samples aged at 90% RH, the water and diiodomethane contact angles on the upper surface were 57.20° and 46.80°, respectively, while those on the lower surface were 58.62° and 47.04°, respectively. Conversely, samples aged in relatively dry environments exhibited a significant increase in water contact angles, whereas the contact angles with diiodomethane remained relatively unchanged. For samples aged at 10% RH, the water contact angles were 111.88° on the upper surface and 102.41° on the lower surface. Overall, the contact angles of the upper surfaces with both reference liquids were consistently larger than those of the lower surfaces. This discrepancy can be attributed to the thicker lipid layer on the upper surfaces5 and the collapse and closure of pores during the aging process (Fig. 3f, j).
After 200 days of aging, the dispersive component (\({\gamma }^{d}\)) of surface energy for samples aged in relatively dry environments (10% RH–50% RH) showed minimal change, while the polar component (\({\gamma }^{p}\)) decreased significantly. In particular, the polar component values for samples aged at 10% RH and 30% RH were nearly zero. In contrast, both the dispersive and polar components of surface energy increased notably for samples aged in humid environments (70% RH and 90% RH) (Fig. 5d, h). Regarding the total surface energy (\(\gamma\)), samples aged in relatively dry conditions exhibited a decrease compared to their pre-aging state, whereas those aged in humid environments showed a significant increase. Generally, materials with higher surface energy exhibit stronger hygroscopicity39. This suggests that the humid environment, coupled with mold-induced deterioration, may have caused a significant increase in the hygroscopicity of the samples.
Hygroscopic properties of the samples
The relationship between a material’s equilibrium moisture content and the relative humidity of its environment is described by the isothermal moisture adsorption curve40. The maximum equilibrium moisture content (measured at 95% RH in the isothermal adsorption curve) under various aging conditions, along with the moisture adsorption curve, desorption curve, and hysteresis curve after 200 days of aging (at 25 °C, 0% RH–95% RH), are shown in Fig. 6.
For samples aged in relatively dry environments (10% RH–50% RH), the maximum equilibrium moisture content decreased progressively with aging. In contrast, samples aged in humid environments (70% RH and 90% RH) exhibited an increase in maximum equilibrium moisture content over time (Fig. 6a). The isothermal adsorption curves followed the IUPAC Type II adsorption isotherm trend41, where the equilibrium moisture content increased with rising relative humidity, showing a gradual increase followed by a sharp rise. This behavior aligns with the typical hygroscopic characteristics of cellulose-based materials (Fig. 6b). At 95% RH, the equilibrium moisture content for unaged samples and those aged at 10% RH, 30% RH, 50% RH, 70% RH, and 90% RH were 25.01%, 21.46%, 22.95%, 23.60%, 28.85%, and 31.77%, respectively. These results indicate that relatively dry environments reduce the hygroscopicity of the samples, while humid environments and mold-induced degradation enhance it, consistent with the findings on surface energy.
The increased hygroscopicity of cellulose-based materials is typically attributed to the degradation of major components, such as cellulose, which exposes more adsorption sites15. This suggests that the chemical composition of the samples aged in humid environments, particularly those aged at 90% RH, underwent significant degradation due to mold activity, resulting in a greater degree of deterioration.
During the desorption phase, the equilibrium moisture content of the samples decreased with decreasing relative humidity but remained consistently higher than in the adsorption phase, demonstrating a certain degree of desorption hysteresis (Fig. 6c). Desorption hysteresis refers to the lag effect caused by differences in material behavior during adsorption and desorption, which is quantified as the hysteresis value (the difference in equilibrium moisture content between the desorption and adsorption phases). The hysteresis curves of the samples are shown in Fig. 6d.
During both adsorption and desorption, the hysteresis values increased initially with rising relative humidity and then decreased. After 100 days of aging under different conditions, the hysteresis values of the samples showed varying degrees of reduction. For samples aged in relatively dry environments (10% RH–50% RH), the overall changes in hysteresis were relatively minor compared to the pre-aging state, with the samples aged at 50% RH showing hysteresis values closest to those before aging. In contrast, samples aged in humid environments (70% RH and 90% RH) exhibited a significant reduction in hysteresis. Notably, for samples aged at 90% RH, the hysteresis values at the same humidity level decreased by up to 67.82% compared to their pre-aging state.
In general, greater desorption hysteresis indicates slower rates of moisture absorption or release during humidity changes, reflecting stronger stability in water retention42. These results suggest that humid environments and mold-induced deterioration not only significantly increased the hygroscopicity of the samples but also weakened their stability during humidity fluctuations. This loss of stability may lead to internal stresses caused by rapid moisture changes under variable humidity conditions, potentially resulting in cracking and other forms of degradation15.
Hardness of the samples
Figure 7 illustrates the hardness changes of the upper and lower surfaces of the samples during the aging process and after 200 days under different aging conditions.
The hardness of both the upper (Fig. 7a) and lower (Fig. 7c) surfaces of all samples decreased progressively with aging. Samples aged in humid environments (70% RH and 90% RH) exhibited a more pronounced reduction in surface hardness. After 200 days of aging, the hardness of the upper surfaces decreased by 62.07% and 68.97% for samples aged at 70% RH and 90% RH, respectively (Fig. 7b), while the lower surfaces showed reductions of 54.55% and 59.60%, respectively (Fig. 7d).
Samples aged in dry environments (10% RH and 30% RH) also exhibited significant reductions in surface hardness, particularly on the upper surfaces. This may be attributed to the formation of cracks caused by warping and deformation (Fig. 2b, c). The reduction in surface hardness typically weakens the fatigue resistance and overall structural strength of the material, making it more susceptible to mechanical damage.
Notably, samples aged at 50% RH for 100 days retained the highest surface hardness among all aged samples, with hardness values of 50.41 HC for the upper surface and 47.65 HC for the lower surface. This indicates that aging at 50% RH had the least impact on the mechanical strength of the samples.
Flexural strength and flexural modulus of the samples
The longitudinal and transverse flexural strength and flexural modulus of the samples during aging under different conditions and after 200 days of aging are shown in Fig. 8.
The longitudinal (Fig. 8a) and transverse (Fig. 8e) flexural strengths of all samples decreased progressively with aging. Samples aged in humid environments (70% RH and 90% RH) exhibited more significant reductions in flexural strength. After 200 days of aging, the longitudinal flexural strengths of samples aged at 70% RH and 90% RH were only 6.49 MPa and 4.41 MPa, respectively, representing reductions of 83.98% and 89.11% compared to their pre-aging states (Fig. 8c). The transverse flexural strengths also decreased by 62.00% and 75.43%, respectively (Fig. 8g). This degradation is likely caused by mold growth and proliferation, which promote the breakdown of cellulose, hemicellulose, and other components in the samples25,26.
Samples aged in dry environments (10% RH and 30% RH) also exhibited significant decreases in flexural strength. After 200 days, the longitudinal flexural strengths were 21.24 MPa and 25.20 MPa, reflecting reductions of 47.56% and 37.78%, respectively, compared to pre-aging values. Similarly, the transverse flexural strengths decreased by 30.28% and 23.71%, respectively. These reductions are likely due to surface cracking observed in the samples (Fig. 2b, c).
The trends in longitudinal and transverse flexural moduli mirrored those of flexural strength, both decreasing progressively with aging (Fig. 8b, f). Samples aged in both dry and humid environments exhibited substantial reductions in flexural modulus (Fig. 8d, h). Notably, samples aged at 50% RH for 200 days retained the highest flexural strength and modulus among all aged samples. Specifically, the longitudinal flexural strength and modulus were 32.91 MPa and 525.31 MPa, respectively, while the transverse flexural strength and modulus were 9.32 MPa and 149.12 MPa, respectively. This indicates that 50% RH had the least impact on the mechanical strength and stiffness of the samples, allowing them to maintain relatively high mechanical strength and good flexibility after 200 days of aging.
Additionally, the longitudinal flexural strength and modulus exhibited greater reductions compared to the transverse values during aging. This can be attributed to two factors: first, the initial longitudinal flexural strength and modulus values were higher, making the changes more pronounced; second, the formation of transverse surface cracks (Fig. 2b, c) likely caused breakage of longitudinal fibers, leading to significant declines in longitudinal flexural strength and modulus.
FT-IR of the samples
The changes in the relative intensities of cellulose and hemicellulose characteristic peaks during aging under different conditions and after 200 days of aging, as well as the infrared spectra after 200 days of aging, are shown in Fig. 9.
Changes in the relative intensities of cellulose and hemicellulose characteristic peaks during aging under different humidity conditions: a I1730/I1505. b I1460/I1505. c I1370/I1505. d I1060/I1505. e Infrared spectra after 200 days. Relative intensities of characteristic peaks after 200 days: f I1730/I1505. g I1460/I1505. h I1370/I1505. i I1060/I1505.
The results indicate that the relative intensities of the characteristic peaks for cellulose and hemicellulose decreased to varying degrees under different aging conditions. This suggests that cellulose and hemicellulose in the samples underwent oxidative and hydrolytic degradation during aging, with the degree of degradation increasing over time. During the aging process, the relative intensities of the characteristic peaks for hemicellulose (Fig. 9a, b) decreased more rapidly and significantly than those for cellulose (Fig. 9c, d). For example, in samples aged at 90% RH, the I1730/I1505 and I1460/I1505 peak intensity ratios showed noticeable changes by day 50, whereas significant changes in the I1370/I1505 and I1060/I1505 peak intensity ratios were observed only after day 75. By day 100, the I1730/I1505, I1460/I1505, I1370/I1505, and I1060/I1505 peak intensity ratios for samples aged at 90% RH had decreased by 50.13%, 37.02%, 21.87%, and 35.24%, respectively, compared to their pre-aging values. These results demonstrate that hemicellulose is less stable than cellulose and degrades more easily and rapidly27. This phenomenon was observed across all samples.
After 200 days of aging, the overall absorption intensity of the infrared spectra for all samples decreased to varying degrees (Fig. 9e), and the relative intensities of the characteristic peaks for cellulose and hemicellulose also declined (Fig. 9f–i). For samples aged at 70% RH, the I1730/I1505, I1460/I1505, I1370/I1505, and I1060/I1505 peak intensity ratios decreased by 54.76%, 52.92%, 45.89%, and 27.97%, respectively, compared to pre-aging values. In samples aged at 90% RH, these ratios decreased further, by 71.31%, 59.65%, 55.27%, and 52.88%, respectively. These results indicate that in high-humidity environments, combined with fungal activity, significant degradation of the main chemical components, such as cellulose and hemicellulose, occurred. The higher the humidity and the more severe the microbial damage, the greater the extent of degradation.
Previous studies have shown that the degradation of cellulose and hemicellulose leads to a reduction in the mechanical properties of materials, such as flexural strength and flexibility, which may result in physical deterioration43. These findings align with the observations from the mechanical performance tests of the samples. In contrast, samples aged in dry environments (10% RH and 30% RH) exhibited noticeable degradation, though to a lesser extent than those aged in humid conditions. Notably, the samples aged at 10% RH exhibited a higher degree of degradation than those aged at 30% RH. Research indicates that the rapid loss of moisture can cause internal stress concentration within the material, resulting in mechanical breakage of cellulose fibers15. On a macroscopic scale, this manifests as cracking, as confirmed by the SEM images of the samples (Fig. 3f, j). Large fractures and microcracks on the sample surface render cellulose and hemicellulose more susceptible to environmental factors, such as temperature and oxygen, accelerating the degradation process44,45 and further contributing to the decline in mechanical properties.
In contrast, the infrared spectra of samples aged at 50% RH for 200 days retained relatively strong absorption intensities, indicating minimal degradation of the main components. After 200 days, the I1730/I1505, I1460/I1505, I1370/I1505, and I1060/I1505 peak intensity ratios decreased by only 11.16%, 15.93%, 3.31%, and 4.85%, respectively, representing the least degree of degradation among all aging conditions.
XRD of the samples
The X-ray diffraction patterns of the samples after 200 days of aging, as well as the relative crystallinity of the samples during aging under different conditions and after 200 days of aging, are shown in Fig. 10.
The results indicate that all samples exhibited a prominent (200) peak near 22°, along with characteristic peaks corresponding to the (110) plane at approximately 15° and the (040) plane near 34°. This confirms that the cellulose crystal structure in all samples remained type I and did not undergo significant alterations under different aging conditions (Fig. 10a). Additionally, some non-cellulose diffraction peaks, such as those at 26° and 29°, likely originated from other crystalline substances, including Ca and Si, introduced during the preparation of Palm Leaf Manuscripts4. Overall, all samples displayed an increase in signal intensity and broadening of the amorphous regions (am) as aging progressed, suggesting that cellulose crystallinity may have been affected to varying extents during the aging process.
To further elucidate changes in the chemical structure and main components of the samples, the relative crystallinity of cellulose was calculated at different stages of aging and after 200 days under various aging conditions. The results show that for samples aged in relatively dry environments (10% RH, 30% RH, and 50% RH), the relative crystallinity of cellulose decreased over time (Fig. 9b). This reduction may be attributed to the natural degradation of cellulose and its transformation from crystalline to amorphous regions. Among these, samples aged at 50% RH exhibited the slowest rate of crystallinity loss.
In contrast, samples aged in humid environments (70% RH and 90% RH) exhibited a trend of an initial increase, followed by a rapid decrease in relative crystallinity. After approximately 100 days, the rate of decrease accelerated, surpassing the crystallinity loss observed in samples aged in dry conditions. This phenomenon suggests that, in the early stages of aging, high humidity and mold activity led to significant degradation of hemicellulose and the amorphous regions of cellulose, which degraded more rapidly and extensively27. As a result, the relative crystallinity of cellulose initially increased. However, as aging progressed and most of the hemicellulose degraded, cellulose became the primary target of mold-induced degradation, leading to a significant decrease in its relative crystallinity. This observation aligns with the findings from the infrared spectral analysis (Fig. 9).
After 200 days of aging under different conditions (Fig. 10c), the relative crystallinity of cellulose decreased by 8.01% (10% RH), 7.92% (30% RH), 6.61% (50% RH), 10.92% (70% RH), and 13.11% (90% RH) compared to the pre-aging state. Samples aged at 50% RH exhibited the smallest reduction in relative crystallinity, indicating greater structural stability under these conditions.
TGA of the samples
The TG and DTG curves, along with the related parameters for the samples after 200 days of aging under different conditions, are shown in Fig. 11. The results indicate that samples aged in humid environments (70% RH and 90% RH) began to decompose more rapidly in both the low-temperature decomposition stage (<200 °C) and the main pyrolysis stage (200–500 °C) (Fig. 11a). Additionally, these samples exhibited a significant increase in mass loss during the low-temperature decomposition stage compared to their pre-aging state, indicating a higher content of moisture and volatile small molecules. In the main pyrolysis stage, the mass loss percentage for all aged samples decreased to varying degrees compared to their pre-aging state, while the proportion of residual char increased (Fig. 11c). Among the primary chemical components of the samples, cellulose and hemicellulose typically decompose completely within the temperature range of 200–500 °C, resulting in low char yields. In contrast, lignin, due to its thermally stable aromatic ring structure, decomposes over a higher and broader temperature range and exhibits significant carbonization characteristics at elevated temperatures, leading to higher char yields46,47. These findings suggest that cellulose and hemicellulose underwent varying degrees of degradation during aging (as reflected by the reduced mass in the main pyrolysis stage), resulting in a relative increase in lignin content (as indicated by the increased residual char). Samples aged in humid environments experienced more severe degradation, likely due to mold activity, which is consistent with the infrared spectroscopy results.
Furthermore, the initial pyrolysis temperature and the maximum pyrolysis temperature of the aged samples were lower than those of the pre-aging samples (Fig. 11d), indicating an overall reduction in thermal stability. The decline in thermal stability was more pronounced for samples aged in humid environments, while samples aged at 50% RH retained relatively better thermal stability.
Discussion
The results of this study on the long-term effects of different relative humidity (RH) conditions on Palm Leaf Manuscripts demonstrate that both excessively dry and humid environments can significantly impact their overall performance, posing challenges for their preservation. In dry environments (10% RH and 30% RH), Palm Leaf Manuscripts undergo severe warping and surface cracking, which not only alter their morphology and structural integrity but also weaken their mechanical and hygroscopic properties. Furthermore, dry conditions accelerate the degradation of the primary chemical components. Conversely, humid environments (70% RH and 90% RH) lead to mold infestation, which severely damages the morphology and structure of the manuscripts. This results in noticeable changes in properties such as color, gloss, and hygroscopicity while also degrading the main chemical components, reducing cellulose crystallinity, and significantly impairing mechanical properties and thermal stability.
In contrast, samples aged at 50% RH exhibited no obvious signs of deterioration. The overall morphology, structure, color, gloss, hygroscopicity, mechanical properties, chemical composition, and thermal stability of these samples remained largely unchanged. This finding suggests that 50% RH is a relatively suitable humidity condition for the preservation of Palm Leaf Manuscripts.
Additionally, during the aging process of Palm Leaf Manuscripts, the differences in performance between the upper and lower surfaces, as well as along the longitudinal and transverse directions, play a significant role. For instance, in dry environments, the smaller dimensional changes and weaker mechanical properties in the transverse direction often lead to stress concentration in the central region of the samples, resulting in severe bending deformation and transverse cracking. The upward bending tendency of the sample increases the exposure of the upper surface to oxygen and other environmental factors, thereby accelerating the aging process and leading to more pronounced changes in color, gloss, and mechanical properties on the upper surface. Additionally, the longitudinal flexural strength and modulus exhibited greater reductions compared to the transverse values during aging. This can be attributed to two factors: first, the initial longitudinal flexural strength and modulus values were higher, making the changes more pronounced; second, the formation of transverse surface cracks (Fig. 2b, c) likely caused breakage of longitudinal fibers, leading to significant declines in longitudinal flexural strength and modulus.
This study investigates the physical and chemical changes in Palm Leaf Manuscripts under different relative humidity conditions, providing a more comprehensive understanding of their properties and aging mechanisms. The findings contribute to the development of more effective preventive conservation strategies. However, it is important to note that the preservation of Palm Leaf Manuscripts is typically influenced by a combination of environmental factors, such as temperature, humidity, and light exposure. The proposed condition of 50% relative humidity in this study is based solely on preliminary data and discussion. Moreover, the historical preservation environments experienced by simulated samples may not fully equate to those of actual artifacts. Therefore, the comprehensive impact of more complex environmental factors on the performance of Palm Leaf Manuscripts remains a crucial area for future research. In summary, this study provides valuable data to support the long-term preservation and preventive protection of these precious Palm Leaf Manuscripts, serving as a reference for further research.
Data availability
The author confirms that all data generated or analyzed during this study are included in this published article. The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request.
Code availability
Not applicable (this manuscript does not report data generation or analysis).
References
Kumar, D. U., Sreekumar, G. & Athvankar, U. Traditional writing system in southern India—palm leaf manuscripts. Des. Thoughts 7, 2–7 (2009).
Panigrahi, A. K. & Litt, D. Odia script in palm-leaf manuscripts. J. Humanit. Soc. Sci. 23, 13–19 (2018).
Meher, R. Tradition of palm leaf manuscripts in Orissa. Orissa Review, 43–46 (January 2009).
Chu, S., Lin, L. & Tian, X. Evaluation of the deterioration state of historical palm leaf manuscripts from Burma. Forests 14, 1775 (2023).
Sharma, D., Singh, M., Krist, G. & Velayudhan, N. M. Structural characterisation of 18th century Indian palm leaf manuscripts of India. Int. J. Conserv. Sci. 9, 257–264 (2018).
Sharma, D., Singh, M. R. & Dighe, B. Chromatographic study on traditional natural preservatives used for palm leaf manuscripts in India. Restaurator 39, 249–264 (2018).
Singh, M. R. & Sharma, D. Investigation of pigments on an Indian palm leaf manuscript (18th–19th century) by SEM-EDX and other techniques. Restaurator 41, 49–65 (2020).
Agrawal, O. P. Conservation of Manuscripts and Paintings of South-East Asia (Butterworth-Heinemann, 1984).
Sah, A. Palm leaf manuscripts of the world: material, technology and conservation. Stud. Conserv. 47, 15–24 (2002).
Wiland, J. et al. A literature review of palm leaf manuscript conservation—Part 1: A historic overview, leaf preparation, materials and media, palm leaf manuscripts at the British Library and the common types of damage. J. Inst. Conserv. 45, 236–259 (2022).
Zhang, M., Song, X., Wang, J. & Lyu, X. Preservation characteristics and restoration core technology of palm leaf manuscripts in Potala Palace. Arch. Sci. 22, 501–519 (2022).
Wiland, J. et al. A literature review of palm leaf manuscript conservation—Part 2: historic and current conservation treatments, boxing and storage, religious and ethical issues, recommendations for best practice. J. Inst. Conserv. 46, 64–91 (2023).
Joshi, Y. (ed.) Modern techniques of preservation and conservation of palm leaf manuscripts. In Proc. Conference on Palm Leaf and Other Manuscripts in Indian Languages (Institute of Asian Studies, Madras, 1995).
Suryawanshi, D. G., Nair, M. V. & Sinha, P. M. Improving the flexibility of palm leaf. Restaurator 13, 37–46 (1992).
Yu, D. et al. The effect of traditional processing craft on the hygroscopicity of palm leaf manuscripts. Herit. Sci. 12, 280 (2024).
Zhang, W., Wang, S. & Guo, H. Study on the effects of temperature and relative humidity on the hygroscopic properties of palm leaf manuscripts. Forests 15, 1816 (2024).
Liu, X. Y., Timar, M. C., Varodi, A. M. & Sawyer, G. An investigation of accelerated temperature-induced ageing of four wood species: colour and FTIR. Wood Sci. Technol. 51, 357–378 (2017).
Graminski, E. L., Parks, E. J. & Toth, E. E. The effects of temperature and moisture on the accelerated aging of paper. Restaurator 3, 175–199 (1979).
Méndez, C., Olmo, C., Renedo, C. J., Ortiz, A. & García, D. Kinetic assessment of Kraft and thermally upgraded Kraft papers aged in five alternative dielectric fluids. Polymers 16, 1196 (2024).
Botti, S., Di Lazzaro, P., Flora, F., Mezi, L. & Murra, D. Raman spectral mapping reveal molecular changes in cellulose aging induced by ultraviolet and extreme ultraviolet radiation. Cellulose 31, 749–758 (2024).
Mitsui, K., Takada, H., Sugiyama, M. & Hasegawa, R. Changes in the properties of light-irradiated wood with heat treatment: Part 1. Effect of treatment conditions on the change in color. Holzforschung 55, 601–605 (2001).
Niu, K. & Song, K. Hot waxing treatment improves the aging resistance of wood surface under UV radiation and water. Prog. Org. Coat. 151, 106468 (2021).
Havermans, J. Effects of air pollutants on the accelerated ageing of cellulose-based materials. Restaurator 16, 209–233 (1995).
Bartl, B. et al. The effect of dust particles on cellulose degradation. Stud. Conserv. 61, 203–208 (2016).
Zhang, W., Wang, S. & Guo, H. Influence of relative humidity on the mechanical properties of palm leaf manuscripts: short-term effects and long-term aging. Molecules 29, 5644 (2024).
Chu, S., Lin, L. & Tian, X. Analysis of Aspergillus niger isolated from ancient palm leaf manuscripts and its deterioration mechanisms. Herit. Sci. 12, 199 (2024).
Zhang, W., Wang, S. & Guo, H. Study on the aging effects of relative humidity on the primary chemical components of palm leaf manuscripts. Polymers 17, 83 (2025).
Yi, X. et al. Study on the material properties and deterioration mechanism of palm leaves. Restaurator 31, 749–758 (2024).
Brémaud, I., Gril, J. & Thibaut, B. Anisotropy of wood vibrational properties: dependence on grain angle and review of literature data. Wood Sci. Technol. 45, 735–754 (2011).
Katz, L. & Dvorak, G. J. On the anisotropic elastic properties of woods. J. Mater. Sci. 43, 2341–2353 (2008).
Lomunov, A., Smirnov, V. & Lomunov, K. Investigation of wood anisotropy under dynamic loading. Int. J. Impact Eng. 112, 37–45 (2018).
Schwanninger, M., Rodrigues, J. C., Pereira, H. & Hinterstoisser, B. Effects of short-time vibratory ball milling on the shape of FT-IR spectra of wood and cellulose. Vib. Spectrosc. 36, 23–40 (2004).
Marchessault, R. H. Application of infra-red spectroscopy to cellulose and wood polysaccharides. Pure Appl. Chem. 5, 107–130 (1962).
Pandey, K. K. & Pitman, A. J. FTIR studies of the changes in wood chemistry following decay by brown-rot and white-rot fungi. Int. Biodeterior. Biodegrad. 52, 151–160 (2003).
Wu, M. et al. A quasi-nondestructive evaluation method for physical-mechanical properties of fragile archaeological wood with TMA: a case study of an 800-year-old shipwreck. Forests 13, 38 (2022).
Ramírez Barat, B. et al. Design and validation of tailored colour reference charts for monitoring cultural heritage degradation. Herit. Sci. 9, 41 (2021).
Lorusso, S., Natali, A. & Matteucci, C. Colorimetry applied to the field of cultural heritage: examples of study cases. Conserv. Sci. Cult. Herit. 7, 13–27 (2007).
Ford, B. L. Monitoring colour change in textiles on display. Stud. Conserv. 37, 1–11 (1992).
Patera, A., Derluyn, H., Derome, D. & Carmeliet, J. Influence of sorption hysteresis on moisture transport in wood. Wood Sci. Technol. 50, 259–283 (2016).
Jamali, A. et al. Sorption isotherms of Chenopodium ambrosioides leaves at three temperatures. J. Food Eng. 72, 77–84 (2006).
Thommes, M. et al. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 87, 1051–1069 (2015).
Chen, Q. et al. The effect of graded fibrous structure of bamboo (Phyllostachys edulis) on its water vapor sorption isotherms. Ind. Crops Prod.151, 112467 (2020).
Bergander, A. & Salmén, L. Cell wall properties and their effects on the mechanical properties of fibers. J. Mater. Sci. 37, 151–156 (2002).
Lv, C. & Liu, J. Alkaline degradation of plant fiber reinforcements in geopolymer: a review. Molecules 28, 1868 (2023).
Ljungdahl, L. G. & Eriksson, K. E. Ecology of microbial cellulose degradation in Advances in Microbial Ecology, Vol. 8 (ed. Marshall, K. C.) (Springer, 1985).
Yu, J., Paterson, N., Blamey, J. & Millan, M. Cellulose, xylan and lignin interactions during pyrolysis of lignocellulosic biomass. Fuel 191, 140–149 (2017).
Apaydın Varol, E. & Mutlu, Ü. TGA-FTIR analysis of biomass samples based on the thermal decomposition behavior of hemicellulose, cellulose, and lignin. Energies 16, 3674 (2023).
Acknowledgements
The authors would like to thank Liusan Li and Li Li from the Chinese Academy of Cultural Heritage for their support. This research was funded by the National Key R&D Program of China, grant number 2022YFF0903905.
Author information
Authors and Affiliations
Contributions
Conceptualization: W.Z. and H.G.; Methodology: W.Z., S.W., L.H. and H.G.; Software: W.Z.; Investigation: W.Z.; Experiment: W.Z.; Formal analysis: W.Z.; Resources: H.G.; Writing—original draft preparation: W.Z.; Writing—review and editing: L.H. and H.G.; Visualization: L.H.; Project administration: S.W. and H.G.; Funding acquisition: H.G. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, W., Wang, S., Han, L. et al. Aging effects of relative humidity on palm leaf manuscripts and optimal humidity conditions for preservation. npj Herit. Sci. 13, 218 (2025). https://doi.org/10.1038/s40494-025-01794-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s40494-025-01794-9