Abstract
This study examines how natural aging affects the hygroscopic, kinetic, and thermodynamic properties of ancient Tibetan Palm Leaf Manuscripts. The results show aging increases equilibrium moisture content and hygroscopicity, with a noticeable hysteresis effect, suggesting enhanced moisture stability at low to moderate humidity. The GAB and H-H models indicate aging accelerates cellulose degradation, adds adsorption sites, and promotes physical adsorption. Kinetic studies reveal a faster moisture absorption and release rate with aging, especially under varying humidity. Thermodynamic analysis shows that as moisture content rises, the adsorption and desorption processes become more spontaneous. Infrared spectral analysis confirms significant degradation of cellulose and hemicellulose, increasing hydroxyl groups and hygroscopicity. The study suggests that these manuscripts are best preserved in a controlled environment with moderate humidity (50-60% RH) and cooler temperatures to maintain moisture stability and flexibility while preventing damage from excessive fluctuations.
Similar content being viewed by others
Introduction
Prior to the widespread use of paper, Palm Leaf Manuscripts were a dominant medium for literary works in South and Southeast Asia1. These manuscripts not only preserve invaluable knowledge in various fields such as history, literature, philosophy, art, and science, but also hold profound cultural significance, particularly within Buddhist traditions, playing a key role in the dissemination of the religion2. Tibet boasts the largest and most diverse collection of Palm Leaf Manuscripts in the world, with nearly 30,000 ancient manuscripts preserved in its temples, museums, and libraries. Research confirms that these manuscripts primarily originate from ancient India, with the majority dating back to the 11th to 14th centuries, while a few manuscripts can be traced to before the 11th century3.
Significant progress has been made in understanding the types of materials used in the production of ancient Palm Leaf Manuscripts, such as raw materials, pigments, and inks4,5,6, as well as the processes and principals involved in their preparation, including boiling and drying techniques3,7,8. Extensive research has also been conducted on the degradation of these manuscripts and their primary diseases9,10, as well as the underlying mechanisms contributing to their deterioration, such as inappropriate storage conditions and improper handling and transportation11,12,13. Additionally, researchers have implemented several conservation and restoration efforts for severely damaged manuscripts, including cleaning, reinforcement, and anti-mold and anti-insect treatments7,14, yielding encouraging results. Collectively, these studies offer valuable insights that advance scientific knowledge and enhance conservation strategies for ancient Palm Leaf Manuscripts, making crucial contributions to their long-term preservation and proper care.
Similar to other cellulose-based materials, such as paper and wood, Palm Leaf Manuscripts are highly sensitive to fluctuations in humidity, which can induce significant physical and chemical changes. Previous studies have shown that frequent humidity fluctuations, as well as exposure to excessively high (≥70%RH) or low (≤30%RH) humidity levels, can lead to a decline in the mechanical properties of healthy Palm Leaf Manuscripts15. High humidity (≥70%RH) also facilitates the growth of microorganisms, which accelerates the degradation of primary chemical components such as cellulose and hemicellulose in the healthy manuscript, with hemicellulose degrading more rapidly and to a greater extent12,13. Similar findings have been reported for other cellulose-based materials, confirming that humidity significantly affects the properties of paper and wood16,17. These observations highlight that improper environmental humidity can alter the properties of Palm Leaf Manuscripts, accelerating their deterioration and posing a threat to their long-term preservation. Consequently, investigating the hygroscopic properties of Palm Leaf Manuscripts is essential for understanding their inherent characteristics and degradation mechanisms, particularly in the context of developing effective conservation strategies for valuable cultural heritage, such as the ancient Tibetan Palm Leaf Manuscripts.
Dynamic Vapor Sorption (DVS) is a non-destructive technique employed to investigate the hygroscopic properties of materials and has proven to be highly effective in assessing the moisture absorption characteristics of cultural artifacts such as paper and wood18,19,20. Previous research utilizing this method has explored the impact of traditional palm leaf manuscript production techniques on the moisture absorption of the leaves, revealing that these techniques significantly reduce the hygroscopic properties of the manuscripts, thus enhancing their long-term preservation21. Other studies have integrated DVS with simulated aging experiments, showing that high-temperature environments reduce the moisture absorption of Palm Leaf Manuscripts, whereas humid conditions notably increase their hygroscopicity11. These studies have provided valuable insights into the degradation mechanisms of Palm Leaf Manuscripts and further corroborate the feasibility and effectiveness of using DVS to assess their hygroscopic properties. Classical adsorption models such as the GAB and H-H models can further explain DVS data to gain deeper insights into the hygroscopic properties of materials. Although some scholars argue that these models have certain limitations when studying wood and other lignocellulosic materials22, it is important to emphasize that the GAB and H-H models remain the most widely used and suitable adsorption models for studying the hygroscopicity of lignocellulosic materials at this stage. In particular, they provide valuable physical insights while explaining the hygroscopic behavior of materials. Moreover, DVS has been widely applied in other fields for evaluating moisture absorption23,24,25. Researchers have not only focused on the hygroscopic properties of materials but have also employed kinetic and thermodynamic approaches to assess the rate of water adsorption26,27, the spontaneity of adsorption, and the energy required for adsorption under various environmental conditions28,29. These studies contribute to a comprehensive understanding of a material’s moisture absorption characteristics and offer important insights for regulating environmental conditions during the production, use, and preservation of materials. However, previous studies have yet to systematically examine the differences in moisture absorption between ancient Tibetan Palm Leaf Manuscripts and modern, well-preserved manuscripts. Additionally, there has been no comprehensive use of kinetic and thermodynamic methods to assess the hygroscopic properties of these manuscripts.
This study focuses on ancient Tibetan Palm Leaf Manuscripts and modern, well-preserved manuscripts as its primary research subjects. Using Dynamic Vapor Sorption (DVS), the study aims to assess the differences in hygroscopic properties between the two types of manuscripts under varying temperature and relative humidity conditions. Additionally, it seeks to investigate the impact of long-term natural aging on the moisture absorption characteristics of these manuscripts. The study further incorporates kinetic and thermodynamic methods to comprehensively evaluate the kinetic and thermodynamic properties associated with the hygroscopic behavior of ancient Tibetan Palm Leaf Manuscripts. The primary objective of this research is to elucidate the hygroscopicity and its kinetic and thermodynamic properties in ancient Tibetan Palm Leaf Manuscripts, with the goal of providing insights into the degradation mechanisms of this invaluable cultural heritage and offering guidance for developing preventive conservation strategies.
Methods
Experimental materials
The ancient samples (APLM) in this study consist of fragments of Palm Leaf Manuscripts preserved in Tibet, China, dating back to approximately the 12th to 14th centuries. Due to the cold, dry climate and insufficient preservation conditions in Tibet, these manuscripts have undergone significant deterioration, with severe cases exhibiting breakage and fiber loss. To minimize the influence of ink and adhesives on the hygroscopicity results, portions of the manuscripts without ink were selected for testing.
The control samples (HPLM) in this study are unprocessed Palm Leaf Manuscripts from Yunnan Province, China. These samples were produced using traditional Palm Leaf Manuscript-making techniques, which have been recognized as a National Intangible Cultural Heritage of China. The leaves of the talipot palm (Corypha umbraculifera L.) underwent processes such as boiling, washing, drying, trimming, and flattening7, resulting in the healthy Palm Leaf Manuscripts used in this study. To avoid the influence of pigments and binding materials on the results, no additional treatments, such as writing or coloring, were applied to the control samples.
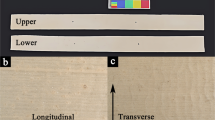
For each sample type, five non-continuous replicate samples (approximately 10 mm × 10 mm in size and weighing about 30 mg each) were selected for subsequent experiments, as shown in Fig. 1.
Simultaneous DVS
A high-throughput dynamic vapor sorption analyzer (SPSx-1μ, ProUmid, Germany) was utilized to measure the maximum moisture content of the samples under constant temperature and varying relative humidity conditions. This device was also used to obtain the isothermal moisture adsorption and desorption curves within the tested humidity range. The test range for relative humidity (RH) spanned from 0% to 90%, with humidity control gradients set at 10% RH increments from 0% to 90%. The desorption phase followed the same gradient as the adsorption phase. The temperature was maintained at 15 °C, 25 °C, and 35 °C, respectively. Equilibrium conditions were defined as a change in sample weight of no more than 0.1% within 10 minutes, or a maximum measurement duration of 720 minutes per gradient.
By measuring the equilibrium moisture content of the samples during both the adsorption and desorption phases, the desorption hysteresis curve of the sample can be further derived through data processing. Desorption hysteresis refers to the lag phenomenon that occurs when the adsorption and desorption processes of a substance on a material do not fully coincide. This phenomenon is quantified by the hysteresis value, which represents the difference in equilibrium moisture content between the adsorption and desorption processes at the same humidity level.
Adsorption models
The hygroscopicity test results were fitted using two classic models, and the model parameters were calculated using software (SPSS Statistics 22, IBM, USA) to further elucidate the hygroscopic characteristics of the samples.
The Guggenheim-Anderson-de Boer (GAB) model equation is as follows:
Where EMC (%) is the equilibrium moisture content; RH (%) is the RH; \({M}_{m\,}\) is the monolayer capacity; \({C}_{{\rm{GAB}}}\) (%) is an equilibrium constant related to monolayer sorption; and \({K}_{{\rm{GAB}}}\) (%) is an equilibrium constant related to multilayer sorption29.
The Hailwood-Horrobin (H-H) model equation is as follows:
Where \({M}_{h\,}\) is the monolayer moisture content (%); \({M}_{s\,}\) is the multilayer moisture content (%); \(w\) is the molecular weight of the wood at every adsorption site; and \({k}_{1}\) and \({k}_{2\,}\) are equilibrium constants in the sorption process20.
Dynamic model
The hygroscopic test results over time for the samples were fitted using the Page model, and the model parameters were calculated using software (SPSS Statistics 22, IBM, USA) to further elucidate the hygroscopic kinetics of the samples.
The Page model equation is as follows26:
Where MR represents is the moisture content variation at different time points; \(k\) is the adsorption or desorption rate constant used to describe the average adsorption or desorption rate of the sample under a given relative humidity condition; and \(n\) is the exponent parameter used to describe the nonlinear characteristics of the adsorption or desorption rate over time. The value of MR is calculated using the following equation:
Where \({M}_{t}\) (%)is the moisture content of the sample at time \(t\); \({M}_{0}\) (%) is the initial moisture content of the sample.
Thermodynamic characteristics
The net isosteric sorption heat (\({Q}_{{st}}\)) represents the minimum energy required to either absorb or remove moisture from a material during the adsorption or desorption process30. It is determined using the Clausius-Clapeyron equation by plotting \(\mathrm{ln}\left({a}_{w}\right)\) against (1/T), with the assumptions that the system maintains a constant equilibrium moisture content, and temperature does not affect the heat of pure water vaporization or excess sorption heat27,31. The net isosteric sorption heat is calculated using the following equation:
Where \({a}_{w}\) is the water activity; \(T\) (in Kelvin) is the temperature; \({Q}_{{st}}\) (kJ/mol) is the net isosteric sorption heat; and \(R\) is the ideal gas constant with 8.314×10−3kJ/(mol·K) value.
The sorption entropy (\({S}_{d}\)) reflects the interactions between water molecules and materials components and aids in understanding the changes in materials during the adsorption and desorption processes27,28. The sorption entropy is calculated using the following equation:
Where \({S}_{d}\) (kJ/(mol·K)) is the sorption entropy.
Furthermore, Gibbs free energy (\(\Delta G\)) offers insight into whether moisture adsorption or desorption occurs as a spontaneous process (indicated by \(-\Delta G\)) or requires energy input (indicated by \(+\Delta G\))32. The Gibbs free energy is calculated using the following equation:
Where \(\Delta G\) (kJ/mol) is the Gibbs free energy.
Infrared spectrum testing
The samples were finely ground and passed through a 200-mesh sieve. They were then mixed with KBr (spectrally pure, Macklin, Shanghai, China) at a mass ratio of 1:100 and pressed into pellets. The chemical structure of the samples was characterized using Fourier transform infrared spectroscopy (Nicolet™ iS™5, Thermo Scientific, Waltham, MA, USA), to compare the differences in the characteristic functional groups before and after aging.
In order to further characterize the changes to the chemical structure of the samples, the infrared spectra of the samples were analyzed semi-quantitatively, using the peak intensity method. The absorption peaks at 1730 cm−1 (stretching vibration by the carbonyl group on hemicellulose) and 1460 cm−1 (stretching vibration by the methylene group on hemicellulose) were selected as the characteristic peaks of hemicellulose, and the absorption peak at 1505 cm−1 (backbone vibration of butyl propane in butyl lignin) was chosen to represent lignin, and the absorption peaks at 1370 cm−1 (bending vibration by the methyl group on cellulose) and 1050 cm−1 (bending vibration by the glycosidic bond on cellulose) were chosen to represent cellulose33,34,35. Additionally, the absorption peak at 3300 cm⁻¹ (stretching vibration by the hydroxyl groups on cellulose and intermolecular hydrogen bonds) is used to represent the hydroxyl groups in the samples. The intensity values of the infrared spectral peaks were measured after baseline correction using software (OMNIC 9.2, Thermo Scientific, USA).
Results
Hygroscopicity of the samples
The relationship between the equilibrium moisture content (EMC) of the samples and the relative humidity (RH) of the environment is represented by the isothermal adsorption curves36. The isothermal moisture adsorption curves of the samples at different temperatures (15 °C, 25 °C, and 35 °C) are shown in Fig. 2a–c. The isothermal adsorption curves of the samples follow the Type II adsorption isotherm, as classified by IUPAC37, which is characteristic of the hygroscopic behavior of cellulose-based materials.
At lower relative humidity levels, the equilibrium moisture content of the two sample types is similar. However, as humidity increases, the equilibrium moisture content of the ancient Tibetan Palm Leaf Manuscripts (APLM) becomes significantly higher than that of the healthy Palm Leaf Manuscripts (HPLM). For example, at a temperature of 25 °C and 60% RH, the equilibrium moisture contents for APLM and HPLM are 9.42% and 8.91%, respectively, while at 90% RH, they are 23.76% and 18.85%, respectively (Fig. 2b). This trend is consistent at other temperatures, such as 15 °C, where the equilibrium moisture contents at 90% RH for APLM and HPLM are 25.20% and 20.14%, respectively (Fig. 2a), and at 35 °C, where they are 21.82% and 16.81%, respectively (Fig. 2c). These results indicate that the hygroscopicity of palm leaves significantly increases after prolonged natural aging.
Additionally, at the same relative humidity, the equilibrium moisture content of the samples decreases with increasing temperature and increases as the temperature decreases. For example, under 90% RH conditions, the equilibrium moisture contents for APLM are 25.20% at 15 °C, 23.76% at 25 °C, and 21.82% at 35 °C. For HPLM, the corresponding values are 20.14%, 18.85%, and 16.81%. This behavior occurs because higher temperatures increase molecular activity, which weakens the intermolecular forces that hold water molecules at the adsorption sites within the material, leading to a reduction in equilibrium moisture content38.
The hysteresis curves of the samples at different temperatures (15 °C, 25 °C, and 35 °C) are shown in Fig. 2d–f.
For all samples, the hysteresis values initially increase and then decrease with rising humidity, which is characteristic of cellulose-based materials. The key distinction lies in the peak hysteresis values: HPLM generally reaches its peak within the 70% to 80% RH range (Fig. 2d, e), while APLM peaks between 50% and 60% RH. Larger hysteresis values indicate slower absorption or release of moisture during humidity fluctuations, signifying more stable moisture retention39. This suggests that HPLM exhibits better hygroscopic stability in high-humidity environments (≥70% RH), whereas APLM demonstrates greater stability in medium humidity environments (50%RH ~ 60% RH). Notably, in medium to low humidity conditions, APLM’s hysteresis values are equal to or even exceed those of HPLM (Fig. 2d, e). This indicates that while long-term natural aging has increased the hygroscopicity of the palm leaves, it has also enhanced their moisture adsorption stability in medium to low humidity environments. Additionally, although HPLM exhibits better hysteresis values in high-humidity environments (≥70% RH), other studies have confirmed that such high humidity conditions can lead to mold growth, chemical composition degradation, and a decline in mechanical properties of the materials12,13,15. Therefore, storing the samples in medium to low humidity environments (≤60% RH) remains the better choice for their preservation.
As the temperature increases, particularly at 35 °C (Fig. 2f), the hysteresis values of the samples decrease significantly. For example, the peak hysteresis values for HPLM at 15 °C, 25 °C, and 35 °C are 3.03%, 3.07%, and 1.98%, respectively, while APLM’s peak values are 3.01%, 2.69%, and 1.81%. This phenomenon can be explained by the effect of temperature on enhancing molecular motion, which weakens the intermolecular forces that hold water molecules at adsorption sites within the material. As a result, moisture is more easily released, leading to a reduction in hysteresis values. Additionally, the increase in temperature causes APLM’s peak hysteresis value to shift towards lower humidity ranges, while the range for HPLM remains unaffected.
The hygroscopic and hysteresis results suggest that Tibetan Palm Leaf Manuscripts, after prolonged natural aging, are best preserved in an environment with 50% to 60% RH. This range helps maintain a stable moisture content and flexibility, while minimizing further damage from humidity fluctuations.
Adsorption models of the samples
To further investigate the adsorption behavior of palm leaves, the isothermal adsorption curves of the samples were fitted using the GAB model and the H-H model. Table 1 and Table 2 provide detailed listings of the model parameters calculated the samples, with high coefficients of determination (R² > 0.99) confirming the validity of these models.
GAB model of the samples
At 15 °C, 25 °C, and 35 °C, the monolayer capacity (\({M}_{m}\)) for HPLM is 4.638, 4.336, and 4.183, respectively, while APLM’s monolayer capacities are consistently higher at 5.154, 4.882, and 4.523 (Table 1). These data indicate that aged palm leaves possess more monolayer adsorption sites than healthier palm leaves. Studies on the hygroscopicity of cellulose-based materials have confirmed that a higher \({M}_{m}\) typically corresponds to lower cellulose crystallinity40. This suggests that prolonged natural aging has led to cellulose degradation in the samples, causing a transition from crystalline to amorphous regions41. This transition reduces the crystallinity of cellulose, thereby exposing more adsorption sites.
Furthermore, the \({M}_{m}\) values for both HPLM and APLM decrease as the temperature rises. This can be attributed to increased molecular activity at higher temperatures, which weakens the intermolecular forces holding water molecules at the adsorption sites, preventing water molecules from fully occupying these sites. Consequently, \({M}_{m}\) decreases at elevated temperatures. Previous research has indicated that high temperatures can promote the transition of cellulose from amorphous to crystalline regions42, thereby increasing cellulose crystallinity and reducing \({M}_{m}\). However, the maximum temperature applied in this study was only 35 °C for a short duration, which is insufficient to rapidly increase cellulose crystallinity. Therefore, the decline in \({M}_{m}\) is primarily due to heightened molecular activity and the escape of moisture induced by the increased temperature.
H-H model of the samples
In the H-H model, P represents the number of adsorption sites within the material. At 15 °C, 25 °C, and 35 °C, the P values for HPLM are 2.451, 2.409, and 2.317, respectively, whereas for APLM, they are 2.863, 2.826, and 2.679 (Table 2), consistently higher than those for HPLM. This suggests that prolonged natural aging has increased the number of adsorption sites in the samples. As the temperature increases, the number of effective adsorption sites—those actually occupied by water molecules—gradually decreases, which is consistent with the analysis from the GAB model.
According to the H-H model, the overall isothermal adsorption moisture content of a material can be divided into monolayer adsorption moisture content (\({M}_{h}\)) and multilayer adsorption moisture content (\({M}_{s}\))43. The \({M}_{h}\) and \({M}_{s}\) values obtained from the H-H model during the adsorption and desorption processes at different temperatures are shown in Fig. 3. It is generally accepted that in low humidity environments (0% to 40% RH), the adsorption of moisture by cellulose-based materials is primarily driven by monolayer adsorption21,44. As relative humidity increases and monolayer adsorption sites become occupied, the influence of monolayer adsorption gradually stabilizes, and multilayer adsorption begins to play a more significant role in the moisture adsorption process. This pattern is observable in all samples and also explains why the isothermal moisture adsorption curves of the samples follow a Type II isotherm, characterized by a ‘rapid-slow-rapid’ increase in equilibrium moisture content.
During the adsorption process, \({M}_{h}\) values for APLM consistently exceed those of HPLM. However, as temperature rises, \({M}_{h}\) for APLM gradually decreases, while \({M}_{h}\) for HPLM remains relatively stable (Fig. 3a–c). For example, at 15 °C, 25 °C, and 35 °C, the maximum \({M}_{h}\) values for APLM are 4.60%, 4.25%, and 4.21%, respectively, compared to 4.05%, 3.98%, and 4.00% for HPLM. This suggests that prolonged natural aging increases the number of monolayer adsorption sites in the palm leaves, thereby enhancing \({M}_{h}\). However, as temperature and molecular activity increase, these additional adsorption sites are not fully occupied, resulting in a decrease in \({M}_{h}\). \({M}_{s}\) typically reflects a material’s capacity for physical adsorption and capillary action in higher humidity environments, primarily associated with multilayer adsorption in microporous structures. During the adsorption process, \({M}_{s}\) for APLM also remains higher than for HPLM, and both values decrease as temperature rises (Fig. 3d–f). For APLM, the maximum \({M}_{s}\) values at 15 °C, 25 °C, and 35 °C are 20.63%, 19.95%, and 17.49%, respectively, while for HPLM, they are 15.75%, 15.13%, and 12.63%. This suggests that prolonged natural aging enhances the physical adsorption and capillary action of palm leaves, thereby increasing \({M}_{s}\). However, higher environmental temperatures weaken these properties, as reflected by the decrease in \({M}_{s}\) with rising temperature.
During desorption, both APLM and HPLM exhibit significantly higher \({M}_{h}\) values compared to the adsorption process. However, as temperature increases, APLM’s \({M}_{h}\) decreases more notably, while HPLM’s \({M}_{h}\) decreases less significantly. This is evident from APLM consistently having higher \({M}_{h}\) than HPLM at 15 °C and 25 °C (Fig. 3a, b), whereas at 35 °C and higher humidity, HPLM’s \({M}_{h}\) surpasses APLM’s (Fig. 3c). Specifically, during desorption, the maximum \({M}_{h}\) values for APLM at 15 °C, 25 °C, and 35 °C are 6.76%, 5.91%, and 5.37%, respectively, while for HPLM, they are 6.50%, 5.81%, and 5.71%. The change in \({M}_{s}\) during desorption, compared to adsorption, is less pronounced. In some cases, \({M}_{s}\) decreases at 15 °C and 35 °C (Fig. 3d, f). This suggests that the desorption hysteresis of palm leaves is primarily determined by the substantial increase in monolayer adsorption moisture content. This observation further explains why APLM exhibits stronger desorption hysteresis in medium to low humidity environments (≤60% RH), while HPLM shows greater desorption hysteresis in high-humidity environments (≥70% RH).
Kinetic characteristics of the samples
To investigate the kinetic characteristics of the samples’ hygroscopic properties, dynamic equilibrium curves for adsorption and desorption at different temperatures and relative humidity levels were plotted, as shown in Figs. 4 and 5. The initial slope of the curve indicates the sample’s initial adsorption or desorption rate in response to humidity changes, while the endpoint of the curve reflects the change in equilibrium moisture content and the time required to reach equilibrium within that gradient. For example, the adsorption curve at 90% RH represents the sample’s dynamic equilibrium moisture adsorption from 80% RH to 90% RH, whereas the desorption curve at 0% RH depicts the sample’s dynamic equilibrium moisture desorption from 10% RH to 0% RH.
During the adsorption process, the initial adsorption rates of HPLM at different relative humidities are generally consistent. However, the time required to reach equilibrium decreases initially and then increases as relative humidity rises (Fig. 4a–c). This trend is primarily determined by the increase in moisture content at the given relative humidity, with more moisture requiring a longer time to reach equilibrium. For instance, at 25 °C, the moisture content increases by 1.85%, 1.06%, and 5.14% at 10% RH, 30% RH, and 90% RH, respectively, with equilibrium times of 3.25 hours, 2.50 hours, and 6.25 hours. This behavior is explained by the H-H model, which suggests that as humidity increases, moisture adsorption transitions from being primarily driven by monolayer adsorption to multilayer adsorption (Fig. 3).
APLM also shows a similar trend of decreasing and then increasing equilibrium times with rising relative humidity. The difference lies in the significantly higher initial adsorption rate of APLM at both low and high humidities compared to moderate humidity (Fig. 4d–f). This is consistent with the findings on the sample’s hysteresis, which show that APLM absorbs or releases moisture more slowly and maintains moisture more stably at moderate humidity (Fig. 2d–f). Additionally, although APLM adsorbs more moisture than HPLM at the same relative humidity, it requires less time to reach equilibrium. For example, at 25 °C, the moisture content increases by 5.14% for HPLM and 7.24% for APLM at 90% RH, with equilibrium times of 6.25 hours and 4.25 hours, respectively. This suggests that, after prolonged natural aging, palm leaves not only have an improved capacity to adsorb moisture but also exhibit a significantly increased adsorption rate.
During the desorption process, the trend in equilibrium time is similar to that observed during the adsorption phase. The more moisture is removed at a given relative humidity, the longer it takes to reach equilibrium (Fig. 5). However, the time required to reach equilibrium during desorption is generally longer than during adsorption. This is primarily because desorbed water molecules must overcome intermolecular forces, such as hydrogen bonds and van der Waals forces, as well as the adsorption sites and capillary forces on the material’s surface38. These forces impede the desorption process, leading to a longer time to reach equilibrium, which is a significant manifestation of the sample’s desorption hysteresis.
Additionally, as temperature increases, the initial adsorption or desorption rates of the samples significantly rise, while the time required to reach equilibrium decreases markedly. For example, at 15 °C, 25 °C, and 35 °C, the equilibrium times for HPLM at 90% RH are 9.00 hours, 6.25 hours, and 3.25 hours, respectively (Fig. 5a–c), while for APLM, they are 5.75 hours, 4.25 hours, and 3.00 hours, respectively (Fig. 5d–f).
The underlying causes for this phenomenon are twofold: first, the increase in temperature promotes molecular motion, which enhances the rate at which the sample adsorbs or desorbs moisture; second, higher temperatures lower the sample’s equilibrium moisture content, reducing the change in moisture content during humidity fluctuations. This allows the sample to reach equilibrium more quickly.
To further investigate the kinetic characteristics of the samples’ hygroscopic properties, the dynamic equilibrium curves for adsorption and desorption were fitted using the Page model. Tables 3–5 provide detailed listings of the calculated model parameters for the samples. The high coefficients of determination (with most R² values exceeding 0.99 and a few exceeding 0.98) confirm the validity of these models.
Figure 6 shows the adsorption and desorption rate constants (\(k\)) for the samples at different temperatures and relative humidities, as determined by the Page model. To better illustrate the trends of \(k\) as a function of temperature and relative humidity, the values have been converted to logarithmic values with base 10.
During the adsorption phase, the trend of \(k\) for APLM and HPLM varies with relative humidity. As relative humidity increases, APLM’s \(k\) initially decreases and then increases, reaching a minimum at 60% RH, where its adsorption rate is the lowest (Fig. 6a–c). For example, at 25 °C, APLM’s \(k\) values at 10% RH, 60% RH, and 90% RH are 3.836, 1.752, and 2.384, respectively. This suggests that APLM adsorbs moisture faster in both high and low humidity environments but more slowly in moderate humidity, which aligns with the hysteresis findings. In contrast, HPLM’s \(k\) increases initially and then decreases with increasing relative humidity, exhibiting faster moisture adsorption at moderate humidity and slower adsorption at both high and low humidity. For instance, at 25 °C, HPLM’s \(k\) values at 10% RH, 60% RH, and 90% RH are 0.778, 1.354, and 0.854, respectively.
Moreover, under all temperature and relative humidity conditions, APLM’s \(k\) is consistently higher than HPLM’s. This further indicates that, after prolonged natural aging, palm leaves not only have an improved capacity to adsorb moisture but also exhibit a significantly increased adsorption rate. This trend supports the conclusion that aging enhances the hygroscopic properties of the palm leaves, enabling them to respond more quickly to changes in humidity.
During the desorption phase, the trend of \(k\) for APLM and HPLM with respect to relative humidity is generally similar. The \(k\) values are quite close in moderate to high humidity, but at low humidity, especially from 10% RH to 0% RH, the desorption rate is slower (Fig. 6d–f). This may be due to the fact that, at lower humidities, the material requires more energy to overcome hydrogen bonds, van der Waals forces, and other interactions to fully release moisture, resulting in a longer equilibrium time. Additionally, under all temperature and relative humidity conditions, APLM’s \(k\) is consistently higher than HPLM’s, which is consistent with the adsorption phase. This suggests that, after prolonged natural aging, palm leaves exhibit significantly increased rates of both moisture adsorption and desorption. The enhanced desorption rate of APLM further supports the conclusion that the aging process improves the palm leaves’ ability to quickly adjust to changes in humidity, allowing them to more effectively release moisture in both low and high humidity environments.
As the temperature increases, both APLM and HPLM exhibit a significant increase in \(k\) during both the adsorption and desorption phases. For example, at 15 °C, 25 °C, and 35 °C, APLM’s adsorption \(k\) at 90% RH are 1.913, 2.384, and 3.667, respectively, while HPLM’s adsorption \(k\) are 0.498, 0.845, and 1.556 (Fig. 6a–c). Similarly, at 15 °C, 25 °C, and 35 °C, APLM’s desorption \(k\) at 0% RH are 0.801, 1.332, and 1.731, respectively, while HPLM’s desorption \(k\) are 0.518, 0.631, and 1.229 (Fig. 6d–f). This increase is primarily due to the fact that higher temperatures intensify the molecular motion of water molecules, providing them with the necessary energy to more rapidly occupy or detach from the adsorption sites on the material’s surface. As a result, the rate of moisture adsorption or release is accelerated, leading to higher \(k\) values. The enhanced kinetic behavior at higher temperatures further illustrates the material’s increased responsiveness to humidity changes, improving both the adsorption and desorption processes.
The kinetic characteristics of the samples’ hygroscopic properties further indicate that Tibetan Palm Leaf Manuscripts, after prolonged natural aging, are best preserved in an environment with moderate humidity (50% to 60% RH) and relatively low temperatures. This environment allows the manuscripts to maintain a certain moisture content and flexibility while avoiding excessive internal moisture fluctuations. Such fluctuations, which could occur with more extreme humidity or temperature changes, have the potential to lead to further damage, compromising the integrity of the manuscripts. Therefore, controlling both humidity and temperature within this optimal range helps minimize the risks associated with environmental changes, ensuring better preservation of the manuscripts over time.
Thermodynamic characteristics of the samples
To investigate the thermodynamic characteristics of the samples’ hygroscopic properties, the net isosteric sorption heat (\({Q}_{{st}}\)) and sorption entropy (\({S}_{d}\)) at different temperatures and equilibrium moisture contents were calculated using the GAB model (with R² > 0.99) and the Clausius-Clapeyron equation. The results are shown in Fig. 7.
The \({Q}_{{st}}\) reflects the interaction strength between water molecules and the material’s adsorption sites. During the adsorption phase, the \({Q}_{{st}}\) values for APLM and HPLM range from 1.068 to 10.076 kJ/mol and 1.653 to 6.025 kJ/mol, respectively (Fig. 7a). In the desorption phase, \({Q}_{{st}}\) ranges from 1.642 to 14.791 kJ/mol for APLM and from 4.591 to 11.616 kJ/mol for HPLM (Fig. 7c). In both phases, \({Q}_{{st}}\) decreases progressively with increasing equilibrium moisture content. At low moisture content, the material primarily undergoes monolayer adsorption (Fig. 3), where the direct interaction between water molecules and the adsorption sites on the material surface results in stronger binding energy and, consequently, higher \({Q}_{{st}}\) values45. As the equilibrium moisture content increases, the adsorption mechanism transitions from monolayer to multilayer adsorption, with additional moisture primarily arising from intermolecular interactions. This weakens the interaction between the material surface and the water molecules, thereby reducing \({Q}_{{st}}\). Furthermore, at lower equilibrium moisture content (≤10%), \({Q}_{{st}}\) for APLM is higher than that for HPLM; however, as the equilibrium moisture content increases, \({Q}_{{st}}\) for APLM becomes lower than that for HPLM. This suggests that APLM exhibits stronger moisture adsorption at low-to-moderate humidity, but its adsorption capacity diminishes at higher humidity levels, making it more susceptible to moisture absorption or release compared to HPLM. These findings are consistent with the observed hysteresis (Fig. 2d–f). Moreover, \({Q}_{{st}}\) during the desorption phase is higher than during the adsorption phase at the same equilibrium moisture content, indicating that more energy is required for moisture release, which further reflects the desorption hysteresis of the samples.
The \({S}_{d}\) represents the degree of disorder in the adsorption or desorption process of water molecules on the material’s surface. Calculations show that the \({S}_{d}\) values for APLM and HPLM during the adsorption phase range from 2.843 to 12.022 J/(mol·K) and from 3.666 to 6.102 J/(mol·K), respectively (Fig. 7b). During the desorption phase, \({S}_{d}\) ranges from 4.735 to 36.274 J/(mol·K) for APLM and from 15.420 to 29.274 J/(mol·K) for HPLM (Fig. 7d). The variation in \({S}_{d}\) follows a trend similar to that of \({S}_{d}\), with both decreasing gradually as the equilibrium moisture content increases. At low moisture content, more adsorption sites on the material surface are available for water molecule binding, allowing the water molecules to be relatively free in their arrangement, which results in a higher degree of disorder. As the equilibrium moisture content increases and the adsorption sites become saturated, interactions such as hydrogen bonds and van der Waals forces between the water molecules and the material surface leaded to a more ordered and less mobile distribution of the water molecules, causing \({S}_{d}\) to decrease. Additionally, \({S}_{d}\) during the desorption phase is significantly higher than during the adsorption phase at the same equilibrium moisture content, indicating that water molecules exhibit greater mobility during desorption than during adsorption29,46.
Furthermore, Gibbs free energy (\(\Delta G\)) offers insight into whether moisture adsorption or desorption occurs as a spontaneous process (indicated by \(-\Delta G\)) or requires energy input (indicated by \(+\,\Delta G\))33. The Gibbs free energy (\(\Delta G\)) of the samples at different temperatures and equilibrium moisture contents is shown in Fig. 8.
The results indicate that the \(\Delta G\) values for all samples are greater than 0 and exhibit a decreasing trend as the equilibrium moisture content increases. For instance, at 25 °C, \(\Delta G\) for APLM during the adsorption process decreases from 6.494 kJ/mol to 0.221 kJ/mol with increasing equilibrium moisture content (Fig. 8b), while during the desorption process, \(\Delta G\) decreases from 3.981 kJ/mol to 0.230 kJ/mol (Fig. 8e). Similarly, for HPLM, \(\Delta G\) during the adsorption process decreases from 4.206 kJ/mol to 0.560 kJ/mol, and during the desorption process, it decreases from 2.892 kJ/mol to 0.014 kJ/mol. These findings suggest that both adsorption and desorption processes for APLM and HPLM are non-spontaneous and require external energy input. However, as the equilibrium moisture content increases, these processes gradually tend toward spontaneity. This trend aligns with similar observations in other materials28,29,45,46,47. Consequently, Palm Leaf Manuscripts should not be stored in excessively humid environments, as higher moisture content and the associated reduction in Gibbs free energy may facilitate increased moisture adsorption and desorption, contributing to further damage.
During the initial phase of adsorption, APLM exhibits a higher \(\Delta G\) than HPLM. However, as equilibrium moisture content increases, APLM’s \(\Delta G\) becomes lower than that of HPLM (Fig. 8a–c). This further suggests that APLM retains moisture more effectively in low-to-moderate humidity conditions. As environmental humidity rises, APLM’s \(\Delta G\) for moisture adsorption decreases, making the process more spontaneous and allowing APLM to absorb moisture more easily than HPLM. In contrast, during the desorption phase, APLM’s \(\Delta G\) consistently remains higher than HPLM’s (Fig. 8d–f), indicating that APLM requires more energy to release moisture at the same equilibrium moisture content. This finding is consistent with the hysteresis results (Fig. 2d–f).
Moreover, as temperature increases, the \(\Delta G\) for both adsorption and desorption processes decreases, indicating that the added thermal energy facilitates the adsorption and desorption of moisture, thus promoting these reactions48,49.
Infrared spectrum of the samples
The infrared spectra of the samples and the relative intensity ratios of the selected characteristic peaks are shown in Fig. 9. The functional groups and their assignments corresponding to the characteristic peaks and bands in the infrared spectra of the samples are detailed in Table 6.
Compared to HPLM, the infrared spectrum of APLM shows a general decrease in absorption intensity, especially at the characteristic absorption peaks of hemicellulose around 1730 cm−1 and 1460 cm−1, and cellulose around 1370 cm−1 and 1060 cm−1, where the absorption intensity is significantly weakened or even nearly completely disappeared (Fig. 9a). This indicates that, compared to healthy samples, the primary chemical components of the ancient Palm Leaf Manuscripts have undergone significant degradation due to prolonged natural aging. This degradation could result from the natural aging of cellulose and hemicellulose, or from external factors such as acidic environments and microorganisms’ growth.
To further elucidate the changes in chemical structure and primary components, a semi-quantitative analysis of the infrared spectra was conducted (Fig. 9b). The results indicate that the relative intensity of the characteristic peaks of cellulose and hemicellulose in APLM has decreased to varying extents compared to HPLM, with the relative intensity of the four characteristic peaks (I1730/I1505, I1460/I1505, I1370/I1505 and I1060/I1505) decreased by 77.38%, 58.33%, 28.89%, and 44.31%, respectively. This also indicates that, compared to healthy samples, the primary chemical components of the ancient Palm Leaf Manuscripts have undergone significant degradation due to prolonged natural aging, with hemicellulose showing more severe degradation than cellulose, which is consistent with previous research findings12,13. Additionally, the I3300/I1505 in APLM increased by 31.80% compared to HPLM, and indicates that the degradation of cellulose and hemicellulose has exposed more hydroxyl groups, which increases the moisture adsorption sites in APLM, thereby significantly enhancing its hygroscopicity.
Discussion
The results of the hygroscopicity study indicate that the equilibrium moisture content of ancient Tibetan Palm Leaf Manuscripts and healthy Palm Leaf Manuscripts is similar under low environmental relative humidity. However, as the humidity increases, the equilibrium moisture content of the ancient Tibetan Palm Leaf Manuscripts becomes significantly higher than that of the healthy Palm Leaf Manuscripts. This suggests that prolonged natural aging has significantly enhanced the hygroscopicity of the palm leaves. Additionally, both ancient Tibetan Palm Leaf Manuscripts and healthy Palm Leaf Manuscripts exhibit a hysteresis trend, which initially increases and then decreases with rising humidity. The key difference lies in the hysteresis values: ancient Tibetan Palm Leaf Manuscripts show higher hysteresis values in medium humidity environments (50%RH ~ 60% RH), whereas healthy Palm Leaf Manuscripts exhibit higher hysteresis values in high-humidity environments (≥70% RH). These findings imply that while prolonged natural aging has increased the hygroscopicity of the ancient manuscripts, it has also enhanced their stability in moisture absorption under low-to-moderate humidity conditions. Additionally, although HPLM exhibits better hysteresis values in high-humidity environments (≥70% RH), other studies have confirmed that such high humidity conditions can lead to mold growth, chemical composition degradation, and a decline in mechanical properties of the materials. Therefore, storing the samples in medium to low humidity environments (≤60% RH) remains the better choice for their preservation.
To further investigate the hygroscopic properties of the samples, the GAB and H-H models were applied to fit the hygroscopic data. The results from the GAB model reveal that ancient Tibetan Palm Leaf Manuscripts possess more adsorption sites and a higher monolayer capacity (\({M}_{m}\)) compared to healthy Palm Leaf Manuscripts. This suggests that prolonged natural aging has caused the degradation of cellulose and the transition from crystalline to amorphous regions, thereby reducing the crystallinity of the cellulose and exposing more adsorption sites. The results from the H-H model further support these findings, indicating that prolonged natural aging not only increases the number of monolayer adsorption sites but also significantly enhances the physical adsorption and capillary action of the palm leaves. Additionally, the H-H model confirms that the desorption hysteresis of the palm leaves is primarily driven by the substantial increase in the monolayer adsorption moisture content.
The hygroscopic properties of the samples also exhibit a significant correlation with environmental temperature. Under the same relative humidity conditions, the equilibrium moisture content of the samples decreases as the temperature increases. This is due to the increased activity of water molecules with rising temperature, which causes the water molecules to detach from the adsorption sites on the palm leaf. The intermolecular forces between the water molecules and the material weaken, leading to a reduction in equilibrium moisture content. The GAB and H-H models further support this observation, showing that higher temperatures prevent water molecules from fully occupying the adsorption sites on the palm leaf. This diminishes the material’s physical adsorption and capillary action, thereby reducing its hygroscopicity.
The kinetic characteristics of the samples’ hygroscopic properties reveal that ancient Tibetan Palm Leaf Manuscripts exhibit higher adsorption and desorption rates than healthy Palm Leaf Manuscripts under all temperature and relative humidity conditions. This suggests that, as a result of prolonged natural aging, not only has the moisture absorption capacity of the palm leaves increased, but the rate of moisture absorption has also significantly accelerated. Moreover, ancient Tibetan Palm Leaf Manuscripts absorb moisture more rapidly in both high and low humidity environments, while their moisture absorption rate is slower in moderate humidity conditions, consistent with the hysteresis findings. Additionally, the increase in temperature accelerates both the adsorption and desorption processes, leading to a decrease in equilibrium moisture content and allowing the samples to reach equilibrium more quickly during fluctuations in humidity.
The thermodynamic analysis of the samples’ hygroscopic properties reveals that \({Q}_{{st}}\), \({S}_{d}\), and \(\Delta G\) all decrease gradually with increasing equilibrium moisture content. This indicates that as moisture content increases, the interaction between the material surface and water molecules weakens, the mobility of water molecules decreases, and the processes of moisture adsorption and desorption gradually tend toward spontaneity. Furthermore, the \({Q}_{{st}}\), \({S}_{d}\), and \(\Delta G\) values for ancient Tibetan Palm Leaf Manuscripts are higher than those of healthy Palm Leaf Manuscripts at lower equilibrium moisture content (≤10%), but become lower as the equilibrium moisture content increases. This suggests that ancient Tibetan Palm Leaf Manuscripts exhibit stronger moisture adsorption under low-to-moderate humidity conditions, but their adsorption capacity weakens under higher humidity, making them more prone to absorbing or releasing moisture than healthy Palm Leaf Manuscripts—consistent with the observed hysteresis behavior.
Furthermore, the infrared spectral analysis also confirms that the primary chemical components such as cellulose and hemicellulose in the ancient Tibetan Palm Leaf Manuscripts have undergone significant degradation, exposing more hydroxyl groups and other adsorption sites, thereby significantly enhancing their hygroscopicity.
The study of the hygroscopic properties, including both the kinetic and thermodynamic characteristics, of ancient Tibetan Palm Leaf Manuscripts indicates that after prolonged natural aging, these manuscripts are best preserved in an environment with moderate humidity (50% to 60% RH) and relatively low temperatures. This preservation environment helps maintain a stable moisture content and flexibility, while avoiding excessive internal moisture fluctuations during environmental changes, thus preventing further damage.
This research explores the changes in the hygroscopic properties of ancient Tibetan Palm Leaf Manuscripts after prolonged natural aging, helping to provide a more comprehensive and in-depth understanding of the manuscript’s characteristics, which is crucial for developing more effective preventive conservation strategies. The results confirm that hygroscopicity can not only effectively reflect the degradation level of the manuscripts but also significantly influence their preservation. The study’s kinetic and thermodynamic analysis further clarifies the variation patterns of hygroscopicity under different temperature and relative humidity conditions, providing valuable guidance for the proper storage environment for these manuscripts. It is important to note that this study excluded the influence of ink, pigments, and other writing materials on the results. However, the impact of writing materials on the overall hygroscopicity of Palm Leaf Manuscripts and their changes under environmental factors remains an area for future research. Furthermore, the influence of more complex environmental factors on the hygroscopicity of the manuscripts is a direction that warrants further investigation. In conclusion, this study provides valuable data support for the long-term preservation and preventive conservation of these precious manuscripts, serving as a reference for future research.
Data availability
The author confirms that all data generated or analysed during this study are included in this published article. The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request.
Code availability
Not applicable (this manuscript does not report data generation or analysis).
References
Kumar, D. U., Sreekumar, G. & Athvankar, U. Traditional writing system in southern India—Palm leaf manuscripts. Des. Thoughts 7, 2–7 (2009).
Panigrahi, A. K. & Litt, D. Odia Script in Palm-Leaf Manuscripts. J. Humanit. Soc. Sci. 23, 13–19 (2018).
Yu, C. et al. Unraveling the ink composition of ancient palm leaf manuscript from Tibet: A multi-analytical study. npj Herit. Sci. 13, 73 (2025).
Sharma, D., Singh, M., Krist, G. & Velayudhan, N. M. Structural characterisation of 18th century Indian Palm leaf manuscripts of India. Int. J. Conserv. Sci. 9, 257–264 (2018).
Sharma, D., Singh, M. R. & Dighe, B. Chromatographic Study on Traditional Natural Preservatives Used for Palm Leaf Manuscripts in India. Restaurator 39, 249–264 (2018).
Singh, M. R. & Sharma, D. Investigation of Pigments on an Indian Palm Leaf Manuscript (18th–19th century) by SEM-EDX and other Techniques. Restaurator 41, 49–65 (2020).
Agrawal, O. P. Conservation of Manuscripts and Paintings of South-East Asia; Butterworth-Heinemann: London, UK, 1984.
Sah, A. Palm Leaf Manuscripts of the World: Material, Technology and Conservation. Stud. Conserv. 47, 15–24 (2002).
Chu, S., Lin, L. & Tian, X. Evaluation of the Deterioration State of Historical Palm Leaf Manuscripts from Burma. Forests 14, 1775 (2023).
Zhang, M., Song, X., Wang, J. & Lyu, X. Preservation characteristics and restoration core technology of palm leaf manuscripts in Potala Palace. Arch. Sci. 22, 501–519 (2022).
Zhang, W., Wang, S. & Guo, H. Study on the Effects of Temperature and Relative Humidity on the Hygroscopic Properties of Palm Leaf Manuscripts. Forests 15, 1816 (2024).
Chu, S., Lin, L. & Tian, X. Analysis of Aspergillus niger isolated from ancient palm leaf manuscripts and its deterioration mechanisms. Herit Sci 12, 199 (2024).
Zhang, W., Wang, S. & Guo, H. Study on the Aging Effects of Relative Humidity on the Primary Chemical Components of Palm Leaf Manuscripts. Polymers 17, 83 (2025).
Joshi Y., editor. Modern techniques of preservation and conservation of palm leaf manuscripts. Proceeding of Conference on Palm Leaf and Other Manuscripts in Indian Languages. Institute of Asian Studies, Madras, 1995.
Zhang, W., Wang, S. & Guo, H. Influence of Relative Humidity on the Mechanical Properties of Palm Leaf Manuscripts: Short-Term Effects and Long-Term Aging. Molecules 29, 5644 (2024).
Bylund Melin, C., Hagentoft, C.-E., Holl, K., Nik, V. M. & Kilian, R. Simulations of Moisture Gradients in Wood Subjected to Changes in Relative Humidity and Temperature Due to Climate Change. Geosciences 8, 378 (2018).
Kim, M.-J., Choi, Y.-S., Oh, J.-J. & Kim, G.-H. Experimental investigation of the humidity effect on wood discoloration by se-lected mold and stain fungi for a proper conservation of wooden cultural heritages. J. Wood Sci. 66, 31 (2020).
Ziegler, J. Testing Aquazol®. An Evaluation of its Suitability for the Conservation of Works on Paper. J. Pap. Conserv. 23, 48–58 (2022).
Liu, X. et al. Consolidation and Dehydration of Waterlogged Archaeological Wood from Site Huaguangjiao No.1. Forests 13, 1919 (2022).
Han, L. et al. Size Effect on Hygroscopicity of Waterlogged Archaeological Wood by Sim-ultaneous Dynamic Vapour Sorption. Forests 14, 519 (2023).
Yu, D. et al. The effect of traditional processing craft on the hygroscopicity of palm leaf manuscripts. Herit. Sci. 12, 280 (2024).
Thybring, E. E., Boardman, C. R., Zelinka, S. L. & Glass, S. V. Common sorption isotherm models are not physically valid for water in wood. Colloids Surf. A Physicochem. Eng. Asp. 627, 127214 (2021).
Liu, C., Li, X., Song, H. & Li, X. Moisture Sorption Isotherms of Polydextrose and Its Gelling Efficiency in Inhibiting the Retrogradation of Rice Starch. Gels 10, 529 (2024).
Duan, S., Geng, L., Li, G. & Ling, X. Water vapour adsorption isotherms of shales: Thermodynamic properties and microstructure. Fluid Phase Equilibria 563, 113583 (2023).
Song, X., Chen, C., Zhou, H., Shang, J. & Ren, T. Effect of high-temperature treatment on water vapour sorption of montmorillonite. Geoderma 436, 116563 (2023).
Efendi, M. Analysis of Courie-GAB-Peleg Models and Neural Networks in Jelly Candy by Corn Starch: Drying Kinetic and Moisture Sorption Isotherms. Food Biophysics 19, 1134–1146 (2024).
Lehmad, M. et al. Comprehensive Analysis of Adsorption–Desorption Isotherms, Drying Kinetics, and Nutritional Quality of Black Soldier Fly (Hermetia illucens) Larvae. Food Biophysics 19, 938–954 (2024).
Mutlu, C. Bee bread: sorption isotherms, thermodynamic characteristics of moisture adsorption and evaluation of adsorbed water. Heat Mass Transfer 60, 1745–1753 (2024).
Fikry, M. et al. Sorption Isotherms and Thermodynamic Characteristics of Gelatin Powder Extracted from Whitefish Skin: Mathematical Modeling Approach. Foods 13, 92 (2024).
Aksil, T., Abbas, M., Trari, M. & Benamara, S. Water adsorption on lyophilized Arbutus unedo L. fruit powder: Determination of thermodynamic parameters. Microchem. J. 145, 35–41 (2019).
Penteado Rosa, D., Rodrigues Evangelista, R., Borges Machado, A. L., Ribeiro Sanches, M. A. & Telis-Romero, J. Water sorption properties of papaya seeds (Carica papaya L.) formosa variety: An assessment under storage and drying conditions. LWT 138, 110458 (2021).
Bakhattar, I. et al. Thermodynamic analysis of batch adsorption isotherms of different types of olive pomace. Heat Mass Transfer 58, 613–630 (2022).
Schwanninger, M., Rodrigues, J. C., Pereira, H. & Hinterstoisser, B. Effects of short-time vibratory ball milling on the shape of FT-IR spectra of wood and cellulose. Vib. Spectrosc. 36, 23–40 (2004).
Marchessault, R. H. Application of infra-red spectroscopy to cellulose and wood polysaccharides. Pure Appl. Chem. 5, 107–130 (1962).
Pandey, K. K. & Pitman, A. J. FTIR studies of the changes in wood chemistry following decay by brown-rot and white-rot fungi. Int. Biodeterior. Biodegrad. 52, 151–160 (2003).
Jamali, A. et al. Sorption isotherms of Chenopodium ambrosioides leaves at three temperatures. J. Food Eng. 72, 77–84 (2006).
Thommes, M. et al. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 87, 1051–1069 (2015).
Moraes, M. A., Rosa, G. S. & Pinto, L. A. A. Moisture sorption isotherms and thermodynamic properties of apple Fuji and garlic. Free. Int. J. Food Sci. Technol. 43, 1824–1831 (2008).
Esteban, L. G. et al. Sorption and thermodynamic properties of juvenile Pinus sylvestris L. wood after 103 years of submersion. Holzforschung 62, 745–751 (2008).
Fernández, F. G. et al. Sorption and thermodynamic properties of Terminalia superba Engl. & Diels and Triplochiton scleroxylon K. Schum. through the 15, 35 and 50 °C sorption isotherms. Eur. J. Wood Wood Prod. 72, 99–106 (2014).
Han, L. et al. Even Samples from the Same Waterlogged Wood Are Hygroscopically and Chemically Different by Simultaneous DVS and 2D COS-IR Spectroscopy. Forests 14, 15 (2023).
Han, L. et al. Influence of Natural Aging on the Moisture Sorption Behaviour of Wooden Structural Components. Molecules 28, 1946 (2023).
Chen, Q. et al. The effect of graded fibrous structure of bamboo (Phyllostachys edulis) on its water vapor sorption isotherms. Ind. Crops Prod. 151, 112467 (2020).
Moussaoui, H., Bahammou, Y., Idlimam, A., Lamharrar, A. & Abdenouri, N. Investigation of hygroscopic equilibrium and modeling sorption isotherms of the argan products: A comparative study of leaves, pulps, and fruits. Food Bioprod. Process. 114, 12–22 (2019).
Ramadwa, T. E., Dzoyem, J. P., Adebayo, S. A. & Eloff, J. N. Ptaeroxylon obliquum leaf extracts, fractions and isolated compounds as potential inhibitors of 15-lipoxygenase and lipopolysaccharide-induced nitric oxide production in RAW 264.7 macrophage cells. South Afr. J. Bot. 147, 192–196 (2022).
Ouaabou, R. et al. Hygroscopic properties of sweet cherry powder: thermodynamic properties and microstructural changes. J. Food Qual. 3925572. https://doi.org/10.1155/2021/3925572 2021.
Arslan-Tontul, S. Moisture sorption isotherm and thermodynamic analysis of quinoa grains. Heat Mass Transf 57, 543–550 (2021).
Hassan, B., Mustapha, A. T., Al-Awaadh, A. M. & Ahmed, K. A. M. Physical and moisture sorption thermodynamic properties of Sukkari date (Phoenix dactylifera L.) powder. CyTA - J. Food 18, 264–273 (2020).
De Araújo, A. L. & da Silva Pena, R. Moisture desorption behavior and thermodynamic properties of pulp and seed of jambolan (Syzygium cumini). Heliyon 8, e09443 (2022).
Acknowledgements
The authors would like to thank Liusan Li and Li Li from the Chinese Academy of Cultural Heritage for the support. This research was funded by the National Key R&D Program of China, grant number 2022YFF0903905.
Author information
Authors and Affiliations
Contributions
Conceptualization: W.Z. and H.G.; Methodology: W.Z., S.W., L.H. and H.G.; Software: W.Z.; Investigation: W.Z.; Experiment: W.Z.; Formal analysis: W.Z.; Resources: H.G.; Writing—original draft preparation: W.Z.; Writing—review and editing: L.H. and H.G.; Visualization: L.H.; Project administration: S.W. and H.G.; Funding acquisition: H.G. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, W., Wang, S., Han, L. et al. Study on the hygroscopicity and kinetic and thermodynamic properties of ancient Tibetan Palm Leaf Manuscripts. npj Herit. Sci. 13, 412 (2025). https://doi.org/10.1038/s40494-025-01988-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s40494-025-01988-1