Abstract
Background
Impulsivity increases the risk for obesity and weight gain. However, the precise role of impulsivity in the aetiology of overeating behavior and obesity is currently unknown. Here we examined the relationships between personality-related measures of impulsivity, Uncontrolled Eating, body mass index (BMI), and longitudinal weight changes. In addition, we analyzed the associations between general impulsivity domains and cortical thickness to elucidate brain vulnerability factors related to weight gain.
Methods
Students (N = 2318) in their first year of university—a risky period for weight gain—completed questionnaire measures of impulsivity and eating behavior at the beginning of the school year. We also collected their weight at the end of the term (N = 1177). Impulsivity was divided into three factors: stress reactivity, reward sensitivity and lack of self-control. Using structural equation models, we tested a hierarchical relationship, in which impulsivity traits were associated with Uncontrolled Eating, which in turn predicted BMI and weight change. Seventy-one participants underwent T1-weighted MRI to investigate the correlation between impulsivity and cortical thickness.
Results
Impulsivity traits showed positive correlations with Uncontrolled Eating. Higher scores in Uncontrolled Eating were in turn associated with higher BMI. None of the impulsivity-related measurements nor Uncontrolled Eating were correlated with longitudinal weight gain. Higher stress sensitivity was associated with increased cortical thickness in the superior temporal gyrus. Lack of self-control was positively associated with increased thickness in the superior medial frontal gyrus. Finally, higher reward sensitivity was associated with lower thickness in the inferior frontal gyrus.
Conclusion
The present study provides a comprehensive characterization of the relationships between different facets of impulsivity and obesity. We show that differences in impulsivity domains might be associated with BMI via Uncontrolled Eating. Our results might inform future clinical strategies aimed at fostering self-control abilities to prevent and/or treat unhealthy weight gain.
Similar content being viewed by others
Introduction
The increase in the incidence of obesity in the past 50 years can be attributed to overeating in response to abundant and inexpensive calories [1]. Obesity is also heritable, and most of the implicated genes appear to be expressed in the central nervous system [2]. As such, obesity can be thought of as resulting from an interaction between a brain-based endophenotype and a disease-promoting environment [3]. Endophenotypes are intermediate phenotypes that link latent biological processes to observable outcomes [4, 5]. The neurobehavioral endophenotypes associated with obesity can be broadly categorized as domain-general and eating-specific [6].
One domain-general endophenotype is impulsivity [7], the tendency to act without full consideration of the consequences [8]. The literature is somewhat heterogeneous in its conceptualization of impulsivity. One of the behavioral models posits that impulsivity can be subdivided into three domains that align with personality factors: (1) low conscientiousness, reflecting self-control, or, in other terms, premeditation and perseverance; (2) neuroticism, a reflection of an individual’s sensitivity to stress and aversive events, also referred to as negative urgency, or the tendency to act impulsively when distressed, and (3) extraversion, reflecting sensitivity to rewards and sensation seeking [8,9,10]. Other models of impulsivity encompass dimensions such as reward drive and rash impulsiveness [11], reward discounting and rapid response [12], impaired cognitive control over emotional responses [13], or attentional impulsivity, motor impulsivity and non-planning impulsivity [14]. Here, however, we focused on the personality-related model of impulsivity as its three dimensions have been consistently associated with obesity [6].
Meta-analytic studies have shown that impulsivity shows weak positive correlations with BMI across independent studies [15]. The effects of impulsivity on body mass index (BMI), however, seem to be largely heterogeneous [15]. One reason for this heterogeneity may be the use of different impulsivity measures and domains across studies [6]. Another possibility is that the relation between personality measures of general impulsivity and BMI might be mediated by individually-varying eating-specific impulsivity characteristics [16]. The brain correlates of impulsivity seem to overlap with brain correlates of high BMI, which suggests that there is a relationship between obesity and impulsivity that might stem from the brain. For example, both high impulsivity and BMI are positively related to the volume of the striatum [17, 18], or negatively related to the volume of the prefrontal and orbitofrontal cortex [19, 20]. These brain regions are parts of the appetitive brain network and their activity reflects both the motivational value of food cues and the regulation of food intake, and can predict eating behavior [21]. In addition, high BMI has been associated with lower gray matter volume and cortical thickness in other areas such as the perception-related parietal lobe and temporal pole [20, 22,23,24,25]. However, it is possible that other brain structures involved in processes like impulsivity might be indirectly associated with weight gain. Identifying these regions might pave the way for new studies to investigate links between brain function and maladaptive eating patterns.
Eating-specific constructs associated with obesity include emotional eating [26], disinhibited eating [27], and power of food [28]. Scores on all these questionnaires consistently and strongly correlate with BMI [29] and with each other [30]. For this reason, in previous research we have proposed that different eating behavior questionnaires depict a common underlying latent factor, labeled Uncontrolled Eating [29, 31]. Preliminary results show that Uncontrolled Eating is associated with brain alterations from functional MRI studies, specifically higher brain activity in response to food cues or at rest in the cerebellum, and lower brain activity in the prefrontal cortex [32]. While both impulsivity traits and Uncontrolled Eating have been linked to increased BMI [16], the relationships between different impulsivity domains, Uncontrolled Eating and obesity remain to be tested. A hypothesis here is that some impulsivity traits might be associated with eating behaviors, which in turn will be linked to BMI [16]. However, comprehensive models have not been built for different impulsivity domains and eating questionnaires and have not been applied to the prediction of weight changes yet.
So far, most analyses have been conducted on cross-sectional datasets making it difficult to say if questionnaire-derived impulsivity is a risk factor or a consequence of obesity [32]. The freshman year of university is an ideal time period to test these hypotheses both cross-sectionally and longitudinally. During this time, students transition into a new environment with access to similar food and exercise options, allowing underlying vulnerability to express itself. Weight gain often happens in this short period of time and affects ~50–60% of students [33, 34]. Eating specific behaviors that revolve around the concept of impulsivity have been most commonly studied as risk factors for weight gain. The results, however, have been contradictory [35,36,37].
In the current study, we present a comprehensive examination of the relationships between impulsivity, Uncontrolled Eating, BMI and weight changes. We tested the hypothesis that the relations between general impulsivity, Uncontrolled Eating, BMI and longitudinal weight changes might be plausibly represented in a hierarchical structural equation model (SEM). In a sub-sample of participants, we additionally examined the relationship between domain-general impulsivity variables and brain structure. Here, we focused on cortical thickness as a measure genetically phenotypically independent of cortical volume and surface area that has been previously investigated in terms of its link with impulsivity, personality [38,39,40,41] or BMI [42, 43]. This post-hoc analysis was performed in order to extend our behavioral findings and identify new brain structures that might be relevant to weight status in an indirect manner.
Methods and materials
Participants
Participants were first year McGill university students, at least 18 years of age, recruited via an advertisement sent to the entire incoming class electronic mailing list. We enrolled 12% of first year students. Participants provided their consent online and data were collected using the online survey tool LimeSurvey (https://www.limesurvey.org) over three consecutive years (2013–2015). The study was approved by the Montreal Neurological Institute Research Ethics Board. Participants for the brain imaging experiment gave additional written consent before participating. There were no exclusion criteria for the behavioral part of the study and standard exclusion criteria for the MRI part of the study (e.g., metal parts in the body, claustrophobia, pregnancy).
Questionnaires
Participants filled out the following online questionnaires in the fall semester: “Big Five” personality dimensions (Openness/Imagination, Conscientiousness, Agreeableness, Extraversion, and Neuroticism) from the International Personality Item Pool (IPIP) [44], two subscales (lack of perseverance and sensation seeking) of the UPPS Impulsive Behavior Scale that best reflected two constructs of our impulsivity model – lack of self-control and reward sensitivity [6, 32, 45], Cohen’s Perceived Stress Scale (PSS) [46], and Rosenberg Self Esteem Scale [47], as well as eating specific questionnaires such as the disinhibition subscale of Three-Factor Eating Questionnaire (TFEQ) [27], emotional eating subscale of Dutch Eating Behavior Questionnaires (DEBQ) [26], and all subscales of Power of Food Scale (PFS) [28] (Table 1). Participants also reported their height and weight at two timepoints – together with online questionnaires during the initial assessment in the fall semester, and during a follow-up in the spring semester. Only a subset of participants reported their height and weight during the follow-up (N = 1145). Our questionnaires included two “catch” questions and three “catch-match” questions as a measure of the level of participants’ attention in completing the survey. Participants with total catch scores above three were excluded, resulting in a sample of 2318 participants (Males N = 750; Females N = 1538). After completion of the questionnaires during the initial assessment and after the follow-up, a subset of participants were asked to visit our laboratory to have their BMI measured. Height and weight were measured from 333 participants in the fall, and from 209 participants in the spring semester (N = 115 overlap with the fall group) using a medical scale and a stadiometer. Self-reported BMI was highly correlated with measured BMI in the fall (r = 0.91, p < 10−5) and in the spring (r = 0.92, p < 10−5). We replaced the reported BMI with measured BMI, when available, in the analyses for higher accuracy. BMI was further residualized for age, sex and a covariate to account for whether it was derived from self-report or measured. Brain imaging was conducted on a random subset of those participants who had expressed a willingness to undergo MRI in the fall (N = 71 participants, N = 69 of them were included in the cortical thickness analysis, see below).
Structural equation models
We performed a series of SEMs to analyze the relationships between Uncontrolled Eating, the three impulsivity traits defined above, and weight gain. First, we tested the hypothesis that Uncontrolled Eating, stress reactivity, lack of self-control and reward sensitivity can be considered separable latent constructs. We built a four-dimension model (Model 1, Fig. 1A), with Uncontrolled Eating, stress reactivity, lack of self-control and reward sensitivity modeled as latent constructs. Uncontrolled Eating (UE) was defined by the scores in Disinhibition, Power of Food and Emotional Eating, following previous work from our group [31, 32]. Stress reactivity was the latent variable that resulted from Perceived Stress Scale (PSS), Rosenberg self-esteem questionnaire and Neuroticism (IPIP). Lack of Self Control was a latent factor emerging from the observable variables Lack of Perseverance (UPPS) and Conscientiousness (IPIP). Finally, reward Sensitivity was formed by Extraversion (IPIP) and Sensation Seeking (UPPS). This model was compared to a simpler one-dimension model (Model 2, Fig. 1B), where all the observable factors were fit into a single latent variable.
A Model 1 representing 4 distinct latent variables; B Model 2 pooling all measures into 1 latent variable; C Model 3 representing a hierarchical model where stress reactivity, lack of self-control and reward sensitivity all influence Uncontrolled Eating; D Model 4 where all 4 latent variables influence BMI and BMI change. RSEQ Rosenberg self-esteem questionnaire, BMI body mass index.
We also built a hierarchical version of the four-dimension model (Model 3, Fig. 1C), where we examined the plausibility of a layered model structure, inspired by the watershed model of mental illness endophenotypes [5, 48]. In this model, stress reactivity, lack of self-control, and reward sensitivity were assumed to each contribute to the Uncontrolled Eating phenotype.
Finally, we examined the relationships between Uncontrolled Eating, the impulsivity traits, BMI and longitudinal changes in BMI in a hierarchical four-dimension model (Model 4, Fig. 1D). We tested whether BMI and longitudinal change in BMI (delta BMI) were predicted by Uncontrolled Eating, stress reactivity, lack of self-control and reward sensitivity.
We used the Lavaan package in R version 3.3. [49] to perform SEM. Model fit was assessed with the chi-square test (X2), the Comparative Fit index (CFI), Root Mean Square Error of Approximation (RMSEA) and the standardized root mean squared residuals (SRMR). The following guidelines were utilized for judging good fit: RMSEA (acceptable fit < 0.08, good fit < 0.05), CFI (acceptable fit 0.95–0.97, good fit > 0.97), SRMR (acceptable fit 0.05–0.10, good fit < 0.05) [50].
To test for the robustness of the preferred hierarchical SEM model (model 3, Fig. 1C), we repeated the analysis by randomly splitting the sample in half. Age and sex were regressed out of all the observable variables in the models. To account for the multiple models performed, we set a stringent p value threshold (p < 0.005).
Magnetic resonance imaging parameters and preprocessing
High-resolution T1-weighted anatomical images with voxel size = 1 x 1 x 1 mm were obtained (TR = 2.3 s; TE = 2.98 ms; FOV phase = 93.8°; FOV = 256 mm) with a Siemens Magnetom Trio 3 T MRI scanner at the Montreal Neurological Institute (MNI).
Pre-processing of T1-weighted MRIs included denoising using optimized non-local means filtering [51], correction for intensity inhomogeneity [52] and linear intensity scaling using histogram matching to the ICBM-MNI152 template. The images were linearly registered to the ICBM-MNI152 template (9 parameter registration) [53]. A mask of the brain was generated using BEaST, a nonlocal segmentation method applied to the linearly registered images in stereotaxic space [54].
Cortical thickness
All T1-weighted MRI images were processed using the CIVET pipeline (version 2.1; http://www.bic.mni.mcgill.ca/ServicesSoftware/CIVET). Native T1-weighted MRI scans were first corrected using the N3 algorithm, underwent brain masking, and registration to ICBM-MNI152 template. Images were then segmented into gray and white matter, cerebrospinal fluid and background with a neural net classifier. The white matter (inner) and gray matter (outer) cortical surfaces were generated using the Constrained Laplacian-based Automated Segmentation with Proximities algorithm. These surfaces were resampled to a stereotaxic surface template to allow vertex-based measurement of cortical thickness. All resulting images went through a stringent quality control by two inspectors in which 69 of 71 images were accepted for further analysis. Cortical thickness in MNI space was defined as the linked distance between the two surfaces across vertices.
Post-hoc brain-impulsivity analysis
We performed a post-hoc exploratory analysis to test which brain regions are associated with the three general-impulsivity domains. We extracted the latent individual scores for stress reactivity, lack of self-control and reward sensitivity of the participants with an available MRI. Next, using the SurfStat software package (http://www.math.mcgill.ca/keith/surfstat/), we computed three separate linear regression models to test for the associations between the three latent impulsivity scores and cortical thickness. The models were analyzed with random field theory with a threshold of p < 0.05 corrected for multiple comparisons over the entire surface [55]. Results are reported cluster- and vertex-wise corrected. Total surface area was included as a covariate. Age and sex were already regressed out of the observable variables forming the latent scores (see Structural Equation Models above). For this reason, these confounding variables were not included in the analyses. Moreover, since the latent scores were obtained from a SEM model that includes the 3 general impulsivity variables as latent variables, each latent score “accounts” for the other two.
Results
Participants’ characteristics are listed in Table 1. There were no sex differences in terms of weight gain in our sample (sex*time interaction p = 0.573).
Uncontrolled eating and general impulsivity traits can be considered independent latent variables (Models 1 and 2)
A four-dimension model, with Uncontrolled Eating, stress reactivity, lack of self-control and reward sensitivity as latent variables provided an acceptable fit (χ2(29) = 322.40; RMSEA = 0.068; CFI = 0.956; SRMR = 0.037). The first latent factor, Uncontrolled Eating, had positive loadings from Disinhibited Eating, Power of Food, and Emotional Eating. The latent factor stress reactivity had a negative loading from the Rosenberg self-esteem questionnaire and positive loadings from Neuroticism and the Perceived Stress Scale. Lack of self-control, the third latent factor had a positive loading from Lack of Perseverance and a negative loading from Conscientiousness. Reward sensitivity was characterized by positive loadings from the Extraversion and Sensation Seeking (UPPS) subscales. This model provided a better fit than an alternative single-dimension model, where all the observable variables were fit into a single latent score (difference in χ2(6) = 2770.5; p = 2.2e–16).
A four-dimensional hierarchical model, where general impulsivity traits predict uncontrolled eating, is plausible (Model 3)
We tested the plausibility of a four-dimension hierarchical model. In this model, the latent variables stress reactivity, lack of self-control and reward sensitivity were characterized as predictors of Uncontrolled Eating. All the paths were significant. Note that the fit parameters are the same as Model 2, since sample size and degrees of freedom did not change, and provided an acceptable fit (Fig. 2A).
A Model 3 (n = 2318), the relationships between Uncontrolled Eating (UE) and the general impulsivity-related variables stress reactivity, lack of self-control and reward sensitivity. B Model 4 (n = 1197), a model that additionally includes the associations between UE, general impulsivity-related variables, BMI and 6-month longitudinal change in BMI (delta BMI) are added. Numeric values are standardized beta weights (all of them p < 0.005). Dashed gray lines depict non-significant associations. PSS perceived stress scale, UE uncontrolled eating, BMI body mass index, Stress stress reactivity, Reward reward sensitivity, Disinh. disinhibition, Emot. Eating emotional eating. Lack Persev. lack of perseverance, Sensat. Seeking sensation seeking.
Relationship between uncontrolled eating, BMI and longitudinal weight change (Model 4)
Finally, we tested the relationship between Uncontrolled Eating, general impulsivity, BMI and longitudinal changes in BMI in the hierarchical four-dimension model. From these additional regression paths, the only one that was significant was the association between BMI and Uncontrolled Eating (B = 0.38; SE = 0.05; Beta = 0.09; p < 0.001). This model provided an acceptable fit (χ2(41) = 198.52; RMSEA = 0.055; CFI = 0.956; SRMR = 0.032) (Fig. 2B).
Validation of Model 4 in the half split dataset
All the previously observed relationships in Model 4 remained equivalent in the two subsamples. Fit parameters in the first half of the sample (N = 601 observation) were as follows: (χ2(41) = 102.97; RMSEA = 0.050; CFI = 0.965; SRMR = 0.032). The same model in the second half of the sample (N = 600 observations) had the following fit parameters (χ2(41) = 142.72; RMSEA = 0.064; CFI = 0.939; SRMR = 0.039).
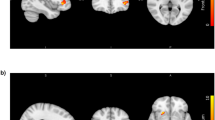
Post-hoc correlations between cortical thickness and general impulsivity scores
Higher stress sensitivity was associated with greater cortical thickness in the right temporoparietal junction extending to superior temporal gyrus. Lack of self-control was positively associated with greater thickness in the right superior frontal gyrus extending to midcingulate cortex. Finally, higher scores in reward sensitivity were associated with lower thickness in the bilateral inferior frontal gyrus (Fig. 3).
Discussion
The purpose of the current study was to provide a comprehensive examination of the relationship between domain-general (personality-related facets of impulsivity) and eating-specific obesity endophenotypes (Uncontrolled Eating), BMI, and weight changes. To do so, we tested a series of SEMs in a large sample size (N > 2300) of first-year students and accounted for the multidimensional nature of impulsivity. A final SEM model, which depicted a hierarchical relationship between general impulsivity, Uncontrolled Eating, BMI and weight gain, was developed in the first split-half of the sample and replicated in the second. In a follow-up analysis we also investigated neural correlates of different facets of impulsivity. We found that cortical thickness in parts of the frontal, cingulate and temporoparietal cortex were correlated with impulsivity and hence are potentially related to BMI via Uncontrolled Eating.
Following previous work, we first stratified impulsivity into three general latent variables: stress sensitivity, lack of self-control and reward sensitivity [8, 9]. A fourth latent variable, Uncontrolled Eating, was considered an eating-specific form of impulsivity. In line with previous publications [31], Uncontrolled Eating was defined using the total scores of relevant eating questionnaires. We showed that an SEM that separated these 4 latent factors was significantly better than a model in which all the observable variables were loaded into a single latent score. Moreover, a hierarchical relationship, in which the three general impulsivity domains were considered predictors of Uncontrolled Eating, was found to be plausible. Each general impulsivity domain had an independent and positive correlation with Uncontrolled Eating. We also found that higher scores in Uncontrolled Eating were positively associated with BMI, as demonstrated previously [31, 32]. However, none of the relations between general impulsivity domains and BMI were significant in the model that also included Uncontrolled Eating. The SEM model therefore suggests that the association between impulsivity and BMI occurs via eating-specific impulsivity, with impulsivity factors predicting Uncontrolled Eating, and Uncontrolled Eating predicting BMI.
Finally, we tested the hypothesis that Uncontrolled Eating underlies vulnerability for weight gain during the first year of university. We utilized the transition period into university because it is associated with a high risk of weight gain. In our sample, the average weight gain was small (0.52 kg over 10 months), but significant, and was similar to previous reports [33]. However, in this sample we did not find evidence that Uncontrolled Eating can longitudinally predict weight gain. This could be due to limited duration of the study. Moreover, we cannot exclude the possibility of seasonal weight gain [56], which would not necessarily be related to Uncontrolled Eating. The existence of bidirectional relationships between Uncontrolled Eating and BMI might also mask a relationship, since changes in body weight might lead to longitudinal variations in eating-specific impulsivity [57]. For instance, scores in different Uncontrolled Eating scales decrease after bariatric surgery [58] or voluntary weight loss [59].
Our results support a model in which BMI is associated with Uncontrolled Eating, which in turn is derived from general impulsivity. Impulsivity could compromise the maintenance of behaviors that promote healthy weight. High scores on conscientiousness have been consistently linked to a lower risk of having and developing obesity across independent samples [60, 61]. These results are generally derived from sample populations with a higher mean age than ours, suggesting that self-control deficits may be more associated with BMI as individuals age and start making their own food decision. Nonetheless, our findings point to reduced conscientiousness (lack of self-control) as a risk factor for overeating behaviors in adolescent and young adult populations.
In a subset of the sample, we performed additional post-hoc correlations between cortical thickness and impulsivity in all three tested domains. We found that the temporoparietal junction, superior frontal gyrus/midcingulate cortex and inferior frontal gyrus were associated with stress sensitivity, lack of self-control and reward sensitivity, respectively. These results are consistent with previous reports showing brain correlates of impulsivity [18, 19, 62] and partially overlap with obesity-related brain regions [20, 42]. Paralleled by our SEM results, these findings show that cortical thickness differences might indirectly influence BMI via Uncontrolled Eating. In the future we would like to update our SEM model to test the hypothesis that brain differences can act as independent predictors of impulsivity, which in turn are significant predictors of Uncontrolled Eating, and hence BMI. This hierarchy of relationships can be depicted as a watershed model [48], which will allow to test the hypothesis that the temporoparietal junction, midcingulate cortex and inferior frontal gyrus are indirectly involved in BMI, via their association with impulsivity. Unfortunately, due to low imaging sample size, this was not possible in this study.
The results of our study should be considered with regards to its limitations. Although we had a large sample size, whereby we were able to detect and replicate results from meta-analyses, most of our measures were self-reported. In the same vein, we did not collect any data on dieting among first year students, which can potentially be a confounding factor. There was also a large sex imbalance in our sample and while sex did not affect weight gain, it is still possible that this imbalance could have affected our results.
In addition, brain imaging was conducted in a small subset of the total sample. Because the brain analysis was a post hoc addition to this study, we did not conduct a formal power analysis – it is therefore possible that our sample size was too small to detect true effects of impulsivity on cortical thickness. Also, individuals who volunteered for brain imaging may not be representative of the entire sample. Finally, we only investigated a three-factor personality model of impulsivity. The results might not be generalizable to other models of impulsivity and hence further research is needed.
In sum, we showed that personality-related impulsivity is related to Uncontrolled Eating, which in turn is related to BMI, suggesting an indirect effect of impulsivity on BMI via Uncontrolled Eating. Future research could use larger sample sizes of neuroimaging data to replicate or refute our brain imaging results. Ideally, future designs should use longitudinal behavioral and neuroimaging data to investigate the directionality of relationships between impulsivity, Uncontrolled Eating, obesity and gray matter morphometry.
References
Hall KD. Did the food environment cause the obesity epidemic? Obesity. 2018;26:11–3. [Internet] Jan 1 [cited 2020 Aug 25] Available from: /pmc/articles/PMC5769871/?report=abstract.
Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, Day FR, et al. Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518:197–206. [Internet] Feb 12 [cited 2020 Aug 25] Available from: /pmc/articles/PMC4382211/?report=abstract.
O’Rahilly S, Farooqi IS. Human obesity: a heritable neurobehavioral disorder that is highly sensitive to environmental conditions. Diabetes. 2008;57:2905LP–2910.
Dagher A, Neseliler S, Han J-E. Chapter 32 - Appetite as motivated choice: hormonal and environmental influences. In: Dreher J-C, Tremblay LBT-DN, editors. Decision neuroscience: an integrative perspective. San Diego: Academic Press; 2017. p. 397–409.
Cannon TD, Keller MC. Endophenotypes in the genetic analyses of mental disorders. Annu Rev Clin Psychol. 2006;2:267–90.
Vainik U, Dagher A, Dubé L, Fellows LK. Neurobehavioural correlates of body mass index and eating behaviours in adults: a systematic review. Neurosci Biobehav Rev. 2013;37:279–99.
Michaud A, Vainik U, Garcia-Garcia I, Dagher A. Overlapping neural endophenotypes in addiction and obesity. Front Endocrinol (Lausanne) [Internet]. 2017 Jun 14 [cited 2019 Sep 3];8:127. Available from: http://journal.frontiersin.org/article/10.3389/fendo.2017.00127/full.
DeYoung CG. Impulsivity as a personality trait. In: Handbook of self-regulation: research, theory, and applications, 2nd ed. New York, NY, US: Guilford Press; 2011. p. 485–502.
Sharma L, Markon KE, Clark LA. Toward a theory of distinct types of “impulsive” behaviors: a meta-analysis of self-report and behavioral measures. Psychol Bull. 2014;140:374–408.
Settles RE, Fischer S, Cyders MA, Combs JL, Gunn RL, Smith GT. Negative urgency: a personality predictor of externalizing behavior characterized by neuroticism, low conscientiousness, and disagreeableness. J Abnorm Psychol. 2012;121:160–72. [Internet] Feb [cited 2020 Aug 25] Available from: /pmc/articles/PMC3299541/?report=abstract.
Stautz K, Dinc L, Cooper AJ. Combining trait models of impulsivity to improve explanation of substance use behaviour. Eur J Pers [Internet]. 2017 Jan 2 [cited 2021 Jun 4];31:118–32. Available from: http://journals.sagepub.com/doi/10.1002/per.2091.
Swann AC, Bjork JM, Moeller FG, Dougherty DM. Two models of impulsivity: relationship to personality traits and psychopathology. Biol Psychiatry. 2002;51:988–94.
Johnson SL, Carver CS, Joormann J. Impulsive responses to emotion as a transdiagnostic vulnerability to internalizing and externalizing symptoms. J Affect Disord. 2013;150:872–8.
Patton JH, Stanford MS, Barratt ES. Factor structure of the barratt impulsiveness scale. J Clin Psychol [Internet]. 1995 Nov 1 [cited 2021 Jun 4];51:768–74. Available from: https://onlinelibrary.wiley.com/doi/10.1002/1097-4679(199511)51:6%3C768::AID-JCLP2270510607%3E3.0.CO;2-1.
Emery RL, Levine MD. Questionnaire and behavioral task measures of impulsivity are differentially associated with body mass index: a comprehensive meta-analysis. Psychol Bull. 2017;143:868–902.
Meule A, Blechert J. Indirect effects of trait impulsivity on body mass. Eat Behav. 2017;26:66–9.
García‐García I, Morys F, Dagher A. Nucleus accumbens volume is related to obesity measures in an age‐dependent fashion. J Neuroendocrinol [Internet]. 2019 Nov 23 [cited 2019 Dec 2]; Available from: https://onlinelibrary.wiley.com/doi/abs/10.1111/jne.12812.
Tschernegg M, Pletzer B, Schwartenbeck P, Ludersdorfer P, Hoffmann U, Kronbichler M. Impulsivity relates to striatal gray matter volumes in humans: evidence from a delay discounting paradigm. Front Hum Neurosci [Internet]. 2015 Jul 2 [cited 2020 Jun 9];9:384. Available from: http://journal.frontiersin.org/Article/10.3389/fnhum.2015.00384/abstract.
Matsuo K, Nicoletti M, Nemoto K, Hatch JP, Peluso MAM, Nery FG. et al. A voxel-based morphometry study of frontal gray matter correlates of impulsivity. Hum Brain Mapp. 2009;30:1188–95.
Garc¡a-Garc¡a I, Michaud A, Dadar M, Zeighami Y, Neseliler S, Collins DL. et al. Neuroanatomical differences in obesity: meta-analytic findings and their validation in an independent dataset. Int J Obes. 2018;43:943–51.
Dagher A. Functional brain imaging of appetite. Trends Endocrinol Metab. 2012;23:250–60.
Beyer F, Kharabian Masouleh S, Kratzsch J, Schroeter ML, Röhr S, Riedel-Heller SG, et al. A metabolic obesity profile is associated with decreased gray matter volume in cognitively healthy older adults. Front Aging Neurosci. 2019;11:1–14.
Medic N, Ziauddeen H, Ersche KD, Farooqi IS, Bullmore ET, Nathan PJ, et al. Increased body mass index is associated with specific regional alterations in brain structure. Int J Obes.2016;40:1177–82.
Morys F, Dadar M, Dagher A. Association between mid-life obesity, its metabolic consequences, cerebrovascular disease and cognitive decline. J Clin Endocrinol Metab [Internet]. 2021 Mar 2 [cited 2021 Apr 13]; Available from: https://academic.oup.com/jcem/advance-article/doi/10.1210/clinem/dgab135/6156963.
Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–15.
van Strien T, Frijters JER, Bergers GPA, Defares PB. The Dutch Eating Behavior Questionnaire (DEBQ) for assessment of restrained, emotional, and external eating behavior. Int J Eat Disord. 1986;5:295–315.
Stunkard AJ, Messick S. The Three-Factor Eating Questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res. 1985;29:71–83.
Lowe MR, Butryn ML, Didie ER, Annunziato RA, Thomas JG, Crerand CE, et al. The Power of Food Scale. A new measure of the psychological influence of the food environment. Appetite. 2009;53:114–8.
Price M, Higgs S, Lee M. Self-reported eating traits: underlying components of food responsivity and dietary restriction are positively related to BMI. Appetite. 2015;95:203–10.
Mason AE, Vainik U, Acree M, Tomiyama AJ, Dagher A, Epel ES, et al. Improving assessment of the spectrum of reward-related eating: the RED-13. Front Psychol. 2017;8:1–14.
Vainik U, Neseliler S, Konstabel K, Fellows LK, Dagher A. Eating traits questionnaires as a continuum of a single concept. Uncontrolled eating. Appetite. 2015;90:229–39.
Vainik U, García-García I, Dagher A. Uncontrolled eating: a unifying heritable trait linked with obesity, overeating, personality and the brain. Eur J Neurosci. 2019;50:2430–45.
Vadeboncoeur C, Townsend N, Foster C. A meta-analysis of weight gain in first year university students: is freshman 15 a myth? BMC Obes. 2015;2:22.
Provencher V, Polivy J, Wintre MG, Pratt MW, Pancer SM, Birnie-Lefcovitch S, et al. Who gains or who loses weight? Psychosocial factors among first-year university students. Physiol Behav. 2009;96:135–41.
Finlayson G, Cecil J, Higgs S, Hill A, Hetherington M. Susceptibility to weight gain. Eating behaviour traits and physical activity as predictors of weight gain during the first year of university. Appetite. 2012;58:1091–8.
Lowe MR, Annunziato RA, Markowitz JT, Didie E, Bellace DL, Riddell L, et al. Multiple types of dieting prospectively predict weight gain during the freshman year of college. Appetite. 2006;47:83–90.
Meule A, Platte P. An examination of the “Freshman-15” in Germany: Predictors of weight change in female university students. Vol. 25, European Journal of Health Psychology. Meule, Adrian: Department of Psychology, University of Salzburg, Hellbrunner Strasse 34, Salzburg, Austria, 5020, adrian.meule@sbg.ac.at: Hogrefe Publishing; 2018. p. 2–8.
Riccelli R, Toschi N, Nigro S, Terracciano A, Passamonti L. Surface-based morphometry reveals the neuroanatomical basis of the five-factor model of personality. Soc Cogn Affect Neurosci. 2017;12:671–84. [Internet] Apr 1 [cited 2021 Aug 2] Available from: /pmc/articles/PMC5390726/.
Miglin R, Bounoua N, Goodling S, Sheehan A, Spielberg JM, Sadeh N. Cortical thickness links impulsive personality traits and risky behavior. Brain Sci [Internet]. 2019 Dec 1 [cited 2021 Jun 4];9. Available from: /pmc/articles/PMC6955970/.
Schilling C, Kühn S, Romanowski A, Schubert F, Kathmann N, Gallinat J. Cortical thickness correlates with impulsiveness in healthy adults. Neuroimage. 2012;59:824–30.
Winkler AM, Kochunov P, Blangero J, Almasy L, Zilles K, Fox PT, et al. Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. Neuroimage. 2010;53:1135–46. [Internet] Nov [cited 2021 Jun 4] Available from: /pmc/articles/PMC2891595/.
Vainik U, Baker TE, Dadar M, Zeighami Y, Michaud A, Zhang Y. et al. Neurobehavioral correlates of obesity are largely heritable. Proc Natl Acad Sci. 2018;115:9312–7.
Westwater ML, Vilar-López R, Ziauddeen H, Verdejo-García A, Fletcher PC. Combined effects of age and BMI are related to altered cortical thickness in adolescence and adulthood. Dev Cogn Neurosci [Internet]. 2019 Dec 1 [cited 2021 Aug 2];40. Available from: https://pubmed.ncbi.nlm.nih.gov/31751856/.
Donnellan MB, Oswald FL, Baird BM, Lucas RE. The Mini-IPIP Scales: Tiny-yet-effective measures of the Big Five Factors of Personality. Vol. 18, Psychological Assessment. Donnellan, M Brent: Department of Psychology, Michigan State University, East Lansing, MI, US, 48823, donnel59@msu.edu: American Psychological Association; 2006. p. 192–203.
Whiteside SP, Lynam DR, Miller JD, Reynolds SK. Validation of the UPPS impulsive behaviour scale: A four-factor model of impulsivity. Eur J Pers. 2005;19:559–74.
Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–96.
Rosenberg M Society and the adolescent self-image. 1965.
Fuhrmann D, Simpson-Kent IL, Bathelt J, Holmes J, Gathercole S, Astle D, et al. A hierarchical watershed model of fluid intelligence in childhood and adolescence. Cereb Cortex. 2019;30:1–14.
Rosseel Y. lavaan: An R Package for Structural Equation Modelinge human forearm during rythmic exercise. J Stat Softw. 2012;48:1–36.
Schermelleh-Engel K, Moosbrugger H, Müller H. Evaluating the fit of structural equation models: Tests of significance and descriptive goodness-of-fit measures. MPR-online. 2003;8:23–74.
Coupe P, Yger P, Prima S, Hellier P, Kervrann C, Barillot C. An optimized blockwise nonlocal means denoising filter for 3-D magnetic resonance images. IEEE Trans Med Imaging. 2008;27:425–41.
Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17:87–97.
Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersuject registration for MR volumetric data in standardized Talairach space. Journal of Computer Assisted Tomography. 1994;18:192–205.
Eskildsen SF, Coupé P, Fonov V, Manjón JV, Leung KK, Guizard N, et al. BEaST: Brain extraction based on nonlocal segmentation technique. Neuroimage. 2012;59:2362–73.
Worsley KJ, Taylor JE, Tomaiuolo F, Lerch J. Unified univariate and multivariate random field theory. In: NeuroImage. Academic Press; 2004. p. S189–95.
Yanovski JA, Yanovski SZ, Sovik KN, Nguyen TT, O’Neil PM, Sebring NG. A prospective study of holiday weight gain. N Engl J Med [Internet]. 2000 Mar 23 [cited 2020 Aug 24];342:861–7. Available from: /pmc/articles/PMC4336296/?report=abstract.
Arumäe K, Briley D, Colodro-Conde L, Mortensen EL, Jang K, Ando J, et al. Two genetic analyses to elucidate causality between body mass index and personality. Int J Obes [Internet]. 2021 Jul 10 [cited 2021 Aug 2]; Available from: http://www.nature.com/articles/s41366-021-00885-4.
Holsen LM, Davidson P, Cerit H, Hye T, Moondra P, Haimovici F, et al. Neural predictors of 12-month weight loss outcomes following bariatric surgery. Int J Obes. 2017;42:1–9.
Batra P, Das SK, Salinardi T, Robinson L, Saltzman E, Scott T, et al. Relationship of cravings with weight loss and hunger. Results from a 6month worksite weight loss intervention. Appetite. 2013;69:1–7.
Jokela M, Hintsanen M, Hakulinen C, Batty GD, Nabi H, Singh-Manoux A. et al. Association of personality with the development and persistence of obesity: a meta-analysis based on individual-participant data. Obes Rev. 2012;14:315–23.
Terracciano A, Sutin AR, McCrae RR, Deiana B, Ferrucci L, Schlessinger D. et al. Facets of personality linked to underweight and overweight. Psychosom Med. 2009;71:682–9.
Cho SS, Pellecchia G, Aminian K, Ray N, Segura B, Obeso I. et al. Morphometric correlation of impulsivity in medial prefrontal cortex. Brain Topogr. 2013;26:479–87.
Acknowledgements
This research was supported by a Canadian Institutes of Health Research Grant to AD. IGG was the recipient of a Postdoctoral Fellowship from the Canadian Institutes of Health Research. SN was supported by a Frederick Banting and Charles Best Canada Graduate Scholarship. UV was supported by Personal Post-doctoral Research Funding project PUTJD654 and by Fonds de recherche du Québec – Santé (FRQS) foreign post-doctoral training award.
Author information
Authors and Affiliations
Contributions
IGG, SN, SGS, UV, DLC, AD conceptualized the study, SN, SGS, NS collected the data, IGG, SN, FM, MD, YY, YZ, analyzed the data, all authors contributed to writing and editing of the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Garcia-Garcia, I., Neseliler, S., Morys, F. et al. Relationship between impulsivity, uncontrolled eating and body mass index: a hierarchical model. Int J Obes 46, 129–136 (2022). https://doi.org/10.1038/s41366-021-00966-4
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41366-021-00966-4
This article is cited by
-
Association between healthy neuroticism and eating behavior as revealed by the NKI Rockland Sample
Scientific Reports (2025)
-
Children’s eating behaviours and related constructs: conceptual and theoretical foundations and their implications
International Journal of Behavioral Nutrition and Physical Activity (2023)
-
Neuroanatomical correlates of genetic risk for obesity in children
Translational Psychiatry (2023)