Abstract
Background
Despite their large numbers and widespread use, very little is known about the extent to which per- and polyfluoroalkyl substances (PFAS) can cross the placenta and expose the developing fetus.
Objective
The aim of our study is to develop a computational approach that can be used to evaluate the of extend to which small molecules, and in particular PFAS, can cross to cross the placenta and partition to cord blood.
Methods
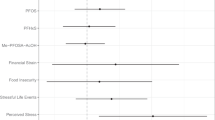
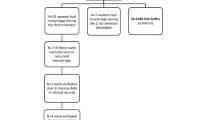
We collected experimental values of the concentration ratio between cord and maternal blood (RCM) for 260 chemical compounds and calculated their physicochemical descriptors using the cheminformatics package Mordred. We used the compiled database to, train and test an artificial neural network (ANN). And then applied the best performing model to predict RCM for a large dataset of PFAS chemicals (n = 7982). We, finally, examined the calculated physicochemical descriptors of the chemicals to identify which properties correlated significantly with RCM.
Results
We determined that 7855 compounds were within the applicability domain and 127 compounds are outside the applicability domain of our model. Our predictions of RCM for PFAS suggested that 3623 compounds had a log RCM > 0 indicating preferable partitioning to cord blood. Some examples of these compounds were bisphenol AF, 2,2-bis(4-aminophenyl)hexafluoropropane, and nonafluoro-tert-butyl 3-methylbutyrate.
Significance
These observations have important public health implications as many PFAS have been shown to interfere with fetal development. In addition, as these compounds are highly persistent and many of them can readily cross the placenta, they are expected to remain in the population for a long time as they are being passed from parent to offspring.
Impact
Understanding the behavior of chemicals in the human body during pregnancy is critical in preventing harmful exposures during critical periods of development. Many chemicals can cross the placenta and expose the fetus, however, the mechanism by which this transport occurs is not well understood. In our study, we developed a machine learning model that describes the transplacental transfer of chemicals as a function of their physicochemical properties. The model was then used to make predictions for a set of 7982 per- and polyfluorinated alkyl substances that are listed on EPA’s CompTox Chemicals Dashboard. The model can be applied to make predictions for other chemical categories of interest, such as plasticizers and pesticides. Accurate predictions of RCM can help scientists and regulators to prioritize chemicals that have the potential to cause harm by exposing the fetus.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
Data availability
The manuscript is accompanied by a supporting information document file and 3 supplementary spreadsheets with the all the underlying data (Supplementary Spreadsheet 1 – Database, Supplementary Spreadsheet 2 – Grid Search, Supplementary Spreadsheet 3 – Modeling Results). All code, Supplementary Documents and spreadsheets are available on GitHub (https://github.com/dimitriabrahamsson/pfas-maternal-cord).
References
Huppertz B. The anatomy of the normal placenta. J Clin Pathol. 2008;61:1296–302. https://doi.org/10.1136/jcp.2008.055277.
Needham LL, Grandjean P, Heinzow B, Jørgensen PJ, Nielsen F, Patterson DG, et al. Partition of environmental chemicals between maternal and fetal blood and tissues. Environ Sci Technol. 2011;45:1121–6. https://doi.org/10.1021/es1019614.
Eryasa B, Grandjean P, Nielsen F, Valvi D, Zmirou-Navier D, Sunderland E, et al. Physico-chemical properties and gestational diabetes predict transplacental transfer and partitioning of perfluoroalkyl substances. Environ Int. 2019;130:104874. https://doi.org/10.1016/j.envint.2019.05.068.
Li J, Cai D, Chu C, Li Q, Zhou Y, Hu L, et al. Transplacental transfer of per- and polyfluoroalkyl substances (PFASs): differences between preterm and full-term deliveries and associations with placental transporter MRNA expression. Environ Sci Technol. 2020;54:5062–70. https://doi.org/10.1021/acs.est.0c00829.
Chen A, Park J-S, Linderholm L, Rhee A, Petreas M, DeFranco EA, et al. Hydroxylated polybrominated diphenyl ethers in paired maternal and cord sera. Environ Sci Technol. 2013;47:3902–8. https://doi.org/10.1021/es3046839.
Li M, Zeng X-W, Qian ZM, Vaughn MG, Sauvé S, Paul G, et al. Isomers of Perfluorooctanesulfonate (PFOS) in cord serum and birth outcomes in China: Guangzhou Birth Cohort Study. Environ Int. 2017;102:1–8. https://doi.org/10.1016/j.envint.2017.03.006
Ferguson KK, Rosen EM, Rosario Z, Feric Z, Calafat AM, McElrath TF, et al. Environmental phthalate exposure and preterm birth in the PROTECT birth cohort. Environ Int. 2019;132:105099. https://doi.org/10.1016/j.envint.2019.105099.
Syme MR, Paxton JW, Keelan JA. Drug transfer and metabolism by the human placenta. Clin Pharmacokinet. 2004;43:487–514. https://doi.org/10.2165/00003088-200443080-00001.
Wang A, Padula A, Sirota M, Woodruff TJ. Environmental influences on reproductive health: the importance of chemical exposures. Fertil Steril. 2016;106:905–29. https://doi.org/10.1016/j.fertnstert.2016.07.1076.
Domingo JL, Nadal M. Human exposure to per- and polyfluoroalkyl substances (PFAS) through drinking water: a review of the recent scientific literature. Environ Res. 2019;177:108648. https://doi.org/10.1016/j.envres.2019.108648.
Sunderland EM, Hu XC, Dassuncao C, Tokranov AK, Wagner CC, Allen JG. A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects. J Exposure Sci Environ Epidemiol. 2019;29:131–47. https://doi.org/10.1038/s41370-018-0094-1.
U.S. Environmental Protection Agency. Chemistry Dashboard. https://comptox.epa.gov/dashboard/ (accessed 2021-03-09).
Williams AJ, Grulke CM, Edwards J, McEachran AD, Mansouri K, Baker NC, et al. The CompTox chemistry dashboard: a community data resource for environmental chemistry. J Cheminform. 2017;9:61. https://doi.org/10.1186/s13321-017-0247-6.
Highly fluorinated substances. https://www.kemi.se/en/chemical-substances-and-materials/highly-fluorinated-substances (accessed 2020-12-08).
Giesy JP, Kannan K. Global distribution of perfluorooctane sulfonate in wildlife. Environ Sci Technol. 2001;35:1339–42. https://doi.org/10.1021/es001834k.
Martin JW, Mabury SA, Solomon KR, Muir DCG. Bioconcentration and tissue distribution of perfluorinated acids in rainbow trout (Oncorhynchus Mykiss). Environ Toxicol Chem. 2003;22:196–204. https://doi.org/10.1002/etc.5620220126.
Gaballah S, Swank A, Sobus JR, Howey XM, Schmid J, Catron T, et al. Evaluation of developmental toxicity, developmental neurotoxicity, and tissue dose in Zebrafish exposed to GenX and other PFAS. Environ Health Perspect. 128, 047005. https://doi.org/10.1289/EHP5843.
Ahrens L, Bundschuh M. Fate and effects of poly- and perfluoroalkyl substances in the aquatic environment: a review. Environ Toxicol Chem. 2014;33:1921–9. https://doi.org/10.1002/etc.2663.
Fenton SE, Ducatman A, Boobis A, DeWitt JC, Lau C, Ng C, et al. Per- and polyfluoroalkyl substance toxicity and human health review: current state of knowledge and strategies for informing future research. Environ Toxicol Chem. 2021;40:606–30. https://doi.org/10.1002/etc.4890.
Grandjean P, Andersen EW, Budtz-Jørgensen E, Nielsen F, Mølbak K, Weihe P, et al. Serum vaccine antibody concentrations in children exposed to perfluorinated compounds. JAMA. 2012;307:391–7. https://doi.org/10.1001/jama.2011.2034.
Grandjean P, Heilmann C, Weihe P, Nielsen F, Mogensen UB, Budtz-Jørgensen E. Serum vaccine antibody concentrations in adolescents exposed to perfluorinated compounds. Environ Health Perspect. 2017;125:077018 https://doi.org/10.1289/EHP275
C8 Science Panel Website. http://www.c8sciencepanel.org/prob_link.html (accessed 2020-12-08).
Yamaguchi M, Arisawa K, Uemura H, Katsuura‐Kamano S, Takami H, Sawachika F, et al. Consumption of seafood, serum liver enzymes, and blood levels of PFOS and PFOA in the Japanese population. J Occup Health. 2013;55:184–94. https://doi.org/10.1539/joh.12-0264-OA.
Massoud O, Charlton M. Nonalcoholic fatty liver disease/nonalcoholic steatohepatitis and hepatocellular carcinoma. Clin Liver Dis. 2018;22:201–11. https://doi.org/10.1016/j.cld.2017.08.014.
Šabović I, Cosci I, De Toni L, Ferramosca A, Stornaiuolo M, Di Nisio A, et al. Perfluoro-octanoic acid impairs sperm motility through the alteration of plasma membrane. J Endocrinol Invest. 2020;43:641–52. https://doi.org/10.1007/s40618-019-01152-0.
Lam J, Koustas E, Sutton P, Johnson PI, Atchley DS, Sen S, et al. The navigation guide - evidence-based medicine meets environmental health: integration of animal and human evidence for PFOA effects on fetal growth. Environ Health Perspect. 2014;122:1040–51. https://doi.org/10.1289/ehp.1307923.
Chen F, Yin S, Kelly BC, Liu W. Isomer-specific transplacental transfer of perfluoroalkyl acids: results from a survey of paired maternal, cord sera, and placentas. Environ Sci Technol. 2017;51:5756–63. https://doi.org/10.1021/acs.est.7b00268.
Morello-Frosch R, Cushing LJ, Jesdale BM, Schwartz JM, Guo W, Guo T, et al. Environmental chemicals in an urban population of pregnant women and their newborns from San Francisco. Environ Sci Technol. 2016;50:12464–72. https://doi.org/10.1021/acs.est.6b03492.
Gao K, Zhuang T, Liu X, Fu J, Zhang J, Fu J, et al. Prenatal exposure to per- and polyfluoroalkyl substances (PFASs) and association between the placental transfer efficiencies and dissociation constant of serum proteins–PFAS complexes. Environ Sci Technol. 2019;53:6529–38. https://doi.org/10.1021/acs.est.9b00715.
Starling AP, Adgate JL, Hamman RF, Kechris K, Calafat AM, Ye X, et al. Perfluoroalkyl substances during pregnancy and offspring weight and adiposity at birth: examining mediation by maternal fasting glucose in the healthy start study. Environ Health Perspect. 125:067016. https://doi.org/10.1289/EHP641.
Spratlen MJ, Perera FP, Lederman SA, Robinson M, Kannan K, Herbstman J, et al. The Association Between Perfluoroalkyl Substances and Lipids in Cord Blood. J Clin Endocrinol Metab. 2020;105 https://doi.org/10.1210/clinem/dgz024.
Chen Q, Zhang X, Zhao Y, Lu W, Wu J, Zhao S, et al. Prenatal exposure to perfluorobutanesulfonic acid and childhood adiposity: a prospective birth cohort study in Shanghai, China. Chemosphere. 2019;226:17–23. https://doi.org/10.1016/j.chemosphere.2019.03.095.
Høyer BB, Ramlau-Hansen CH, Obel C, Pedersen HS, Hernik A, Ogniev V, et al. Pregnancy serum concentrations of perfluorinated alkyl substances and offspring behaviour and motor development at age 5–9 years – a prospective study. Environ Health. 2015;14:2. https://doi.org/10.1186/1476-069X-14-2.
Cahill TM, Cousins I, Mackay D. Development and application of a generalized physiologically based pharmacokinetic model for multiple environmental contaminants. Environ Toxicol Chem. 2003;22:26–34. https://doi.org/10.1002/etc.5620220104.
Verner M-A, Loccisano AE, Morken N-H, Yoon M, Wu H, McDougall R.et al. Associations of Perfluoroalkyl Substances (PFAS) with Lower Birth Weight: An Evaluation of Potential Confounding by Glomerular Filtration Rate Using a Physiologically Based Pharmacokinetic Model (PBPK. Environmental Health Perspectives. 2015;123:1317–24. https://doi.org/10.1289/ehp.1408837.
Forns J, Verner M-A, Iszatt N, Nowack N, Bach CC, Vrijheid M, et al. Early life exposure to perfluoroalkyl substances (PFAS) and ADHD: a meta-analysis of nine European population-based studies. Environ Health Perspect. 128 057002. https://doi.org/10.1289/EHP5444.
Verner M-A, Ngueta G, Jensen ET, Fromme H, Völkel W, Nygaard UC.et al. A Simple Pharmacokinetic Model of Prenatal and Postnatal Exposure to Perfluoroalkyl Substances (PFASs. Environ. Sci. Technol. 2016;50:978–86. https://doi.org/10.1021/acs.est.5b04399.
Mamsen LS, Björvang RD, Mucs D, Vinnars M-T, Papadogiannakis N, Lindh CH, et al. Concentrations of perfluoroalkyl substances (PFASs) in human embryonic and fetal organs from first, second, and third trimester pregnancies. Environ Int. 2019;124:482–92. https://doi.org/10.1016/j.envint.2019.01.010.
Mamsen LS, Jönsson BAG, Lindh CH, Olesen RH, Larsen A, Ernst E, et al. Concentration of perfluorinated compounds and cotinine in human foetal organs, placenta, and maternal plasma. Sci Total Environ. 2017;596–597:97–105. https://doi.org/10.1016/j.scitotenv.2017.04.058.
Pérez F, Nadal M, Navarro-Ortega A, Fàbrega F, Domingo JL, Barceló D, et al. Accumulation of perfluoroalkyl substances in human tissues. Environ Int. 2013;59:354–62. https://doi.org/10.1016/j.envint.2013.06.004.
Takaku T, Nagahori H, Sogame Y, Takagi T. Quantitative structure–activity relationship model for the fetal–maternal blood concentration ratio of chemicals in humans. Biol Pharm Bull. 2015;38:930–4. https://doi.org/10.1248/bpb.b14-00883.
Lancz K, Murínová Ľ, Patayová H, Drobná B, Wimmerová S, Šovčíková E, et al. Ratio of cord to maternal serum PCB concentrations in relation to their congener-specific physicochemical properties. Int J Hyg Environ Health. 2015;218:91–98. https://doi.org/10.1016/j.ijheh.2014.08.003.
Aylward LL, Hays SM, Kirman CR, Marchitti SA, Kenneke JF, English C, et al. Relationships of chemical concentrations in maternal and cord blood: a review of available data. J Toxicol Environ health Part B, Crit Rev. 2014;17:175–203. https://doi.org/10.1080/10937404.2014.884956.
Nakamura T, Nakai K, Matsumura T, Suzuki S, Saito Y, Satoh H. Determination of dioxins and polychlorinated biphenyls in breast milk, maternal blood and cord blood from residents of Tohoku, Japan. Sci Total Environ. 2008;394:39–51. https://doi.org/10.1016/j.scitotenv.2008.01.012.
Park J-S, Bergman Å, Linderholm L, Athanasiadou M, Kocan A, Petrik J, et al. Placental transfer of polychlorinated biphenyls, their hydroxylated metabolites and pentachlorophenol in pregnant women from Eastern Slovakia. Chemosphere. 2008;70:1676–84. https://doi.org/10.1016/j.chemosphere.2007.07.049.
Fourches D, Muratov E, Tropsha A. Trust, but verify: on the importance of chemical structure curation in cheminformatics and QSAR modeling research. J Chem Inf Model. 2010;50:1189–204. https://doi.org/10.1021/ci100176x.
Fourches D, Muratov E, Tropsha A. Trust, but Verify II: a practical guide to chemogenomics data curation. J Chem Inf Model. 2016;56:1243–52. https://doi.org/10.1021/acs.jcim.6b00129.
Moriwaki H, Tian Y-S, Kawashita N, Takagi T. Mordred: a molecular descriptor calculator. J Cheminformatics. 2018;10:4. https://doi.org/10.1186/s13321-018-0258-y.
Landrum G. RDKit: Using the New Fingerprint Bit Rendering Code. RDKit, 2018.
scikit-learn: machine learning in Python — scikit-learn 0.22.1 documentation. https://scikit-learn.org/stable/ (accessed 2020-02-20).
TensorFlow. TensorFlow. https://www.tensorflow.org/ (accessed 2020-02-17).
Welcome to Python.org. Python.org. https://www.python.org/ (accessed 2020-02-20).
Baskin II, Palyulin VA, Zefirov NS. Neural networks in building QSAR models. In Artificial Neural Networks: Methods and Applications; Livingstone DJ, Ed.; Methods in Molecular BiologyTM; Humana Press: Totowa, NJ, 2009; pp 133–54. https://doi.org/10.1007/978-1-60327-101-1_8.
Dearden JC, Rowe PH. Use of artificial neural networks in the QSAR prediction of physicochemical properties and toxicities for REACH Legislation. In Artificial Neural Networks; Cartwright H, Ed.; Methods in Molecular Biology; Springer: New York, NY, 2015; pp 65–88. https://doi.org/10.1007/978-1-4939-2239-0_5.
Polishchuk PG, Muratov EN, Artemenko AG, Kolumbin OG, Muratov NN, Kuz’min VE. Application of random forest approach to QSAR prediction of aquatic toxicity. J Chem Inf Model. 2009;49:2481–8. https://doi.org/10.1021/ci900203n.
Svetnik V, Liaw A, Tong C, Culberson JC, Sheridan RP, Feuston BP. Random forest: a classification and regression tool for compound classification and QSAR modeling. J Chem Inf Comput Sci. 2003;43:1947–58. https://doi.org/10.1021/ci034160g.
Darnag R, Mostapha Mazouz EL, Schmitzer A, Villemin D, Jarid A, Cherqaoui D. Support vector machines: development of QSAR models for predicting anti-HIV-1 activity of TIBO derivatives. Eur J Medicinal Chem. 2010;45:1590–7. https://doi.org/10.1016/j.ejmech.2010.01.002.
Maltarollo VG, Kronenberger T, Espinoza GZ, Oliveira PR, Honorio KM. Advances with support vector machines for novel drug discovery. Expert Opin Drug Discov. 2019;14:23–33. https://doi.org/10.1080/17460441.2019.1549033.
Rücker C, Rücker G, Meringer M. Y-randomization and its variants in QSPR/QSAR. J Chem Inf Model. 2007;47:2345–57. https://doi.org/10.1021/ci700157b.
Aniceto N, Freitas AA, Bender A, Ghafourian T. A novel applicability domain technique for mapping predictive reliability across the chemical space of a QSAR: reliability-density neighbourhood. J Cheminformatics. 2016;8:69. https://doi.org/10.1186/s13321-016-0182-y.
Netzeva TI, Worth AP, Aldenberg T, Benigni R, Cronin MTD, Gramatica P, et al. Current status of methods for defining the applicability domain of (Quantitative) structure-activity relationships: the report and recommendations of ECVAM Workshop 521,2. Alter Lab Anim. 2005;33:155–73. https://doi.org/10.1177/026119290503300209.
Gentile S. The safety of newer antidepressants in pregnancy and breastfeeding. Drug-Saf. 2005;28:137–52. https://doi.org/10.2165/00002018-200528020-00005.
Hallberg P, Sjöblom V. The use of selective serotonin reuptake inhibitors during pregnancy and breast-feeding: a review and clinical aspects. J Clin Psychopharmacol. 2005;25:59–73. https://doi.org/10.1097/01.jcp.0000150228.61501.e4.
Patil AS, Kuller JA, Rhee EHJ. Antidepressants in pregnancy: a review of commonly prescribed medications. Obstetrical Gynecol Surv. 2011;66:777–87. https://doi.org/10.1097/OGX.0b013e31823e0cbf.
Jeong Y, Lee S, Kim S, Park J, Kim H-J, Choi G, et al. Placental transfer of persistent organic pollutants and feasibility using the placenta as a non-invasive biomonitoring matrix. Sci Total Environ. 2018;612:1498–505. https://doi.org/10.1016/j.scitotenv.2017.07.054.
Li J, Sun X, Xu J, Tan H, Zeng EY, Chen D. Transplacental transfer of environmental chemicals: roles of molecular descriptors and placental transporters. Environ Sci Technol. 2021;55:519–28. https://doi.org/10.1021/acs.est.0c06778.
Acknowledgements
We want to thank Nisha Sipes (U.S. EPA) Ian Cousins (Stockholm University), and Matthew MacLeod (Stockholm University) for their thoughtful comments and immensely valuable suggestions.
Funding
This study was funded by the Office of Environmental Health Hazard Assessment (OEHHA) of the California Environmental Protection Agency (CalEPA) and by the National Institutes of Health/National Institute of Environmental Health Sciences (NIH/NIEHS) (grant numbers K99ES032892, P30-ES030284, P01ES022841, R01ES027051). The views expressed in this manuscript are those of the authors and do not necessarily represent the views and policies of OEHHA.
Author information
Authors and Affiliations
Contributions
DA collected the data, conducted the analyses and wrote the manuscript. A Si assisted in the data collection and analysis. JFR, JF, and TJW supervised, provided feedback and assisted with the writing of the manuscript. A So, SE, VC, EK, and LZ provided feedback on the analyses and assisted with the writing of the manuscript. CN, RA, WC constituted the advisory panel of the study, provided feedback and assisted with the analyses and the writing of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Abrahamsson, D., Siddharth, A., Robinson, J.F. et al. Modeling the transplacental transfer of small molecules using machine learning: a case study on per- and polyfluorinated substances (PFAS). J Expo Sci Environ Epidemiol 32, 808–819 (2022). https://doi.org/10.1038/s41370-022-00481-2
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41370-022-00481-2
Keywords
This article is cited by
-
Exposure forecasting – ExpoCast – for data-poor chemicals in commerce and the environment
Journal of Exposure Science & Environmental Epidemiology (2022)