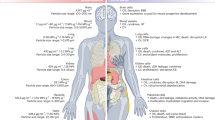
Abstract
Background
The human bioaccumulation of micro- and nano-plastics (MNPs) is increasingly being recognised in the aetiology and pathophysiology of human disease.
Objective
This systematic scoping review aims to provide a comprehensive investigation of studies examining the impacts of MNPs on the human cardiovascular system.
Methods
Five databases (PubMed, SCOPUS, CINAHL, Web of Science and EMBASE) were systematically searched.
Results
Forty-six articles were identified, 13 of which investigated the presence of MNPs within the human cardiovascular system, including atherosclerotic plaques, saphenous vein tissue, thrombi and venous blood. The effect of MNPs on cell lines suggest MNPs are cytotoxic, immunotoxic, and genotoxic.
Significance
The findings of this review, when evaluated together with additional studies utilising animal models, suggest MNPs may contribute to global cardiovascular morbidity and mortality. In particular, the ability of MNPs to induce endothelial damage, oxy-LDL formation, foam cell development and apoptosis, as well as to alter the clotting cascade, has potential implications for vascular diseases. In addition, MNPs may play a role in the aetiology and progression of congenital heart abnormalities, infective pathologies and cardiomyopathies. Despite an increasing awareness of the ability for MNPs to result in cardiovascular disease and dysfunction, a limited amount of research has been conducted to date characterising the presence of MNPs in the human cardiovascular system. Reseach is required to understand the extent of this rapidly emerging issue and to develop strategies that will support clinicians to appropriately manage and educate their patients in the future.

Similar content being viewed by others
Introduction
Cardiovascular disorders and environmental contamination from microplastics (MPs) are two major challenges within modern society [1, 2]. Currently, our understanding of the interrelationship between these two phenomena is limited [3]. Contemporary evidence points towards an increasing bioaccumulation of micro- and nano-plastics (MNPs) in humans, leading to increased disease and dysfunction within multiple organ systems, thereby presenting a threat to global public health [3, 4]. However, a lack of research and evidence synthesis to date currently leaves clinicians with insufficient data to guide the management of patients with MNP-associated disease and dysfunction.
In 2019, the World Health Organization (WHO) published a report entitled ‘Microplastics in Drinking-Water’, which minimised the significance of MNPs in drinking water in relation to their impact on human health [5]. The first report of MNPs in the human bloodstream was published in the same year. Since this time, the assertation that there is “no evidence to indicate a human health concern” [5], is increasingly being challenged by new studies. Once MNPs enter the human body, by means of inhalation, ingestion, or dermal absorption, they can cross biological barriers, leading to systemic exposure and bioaccumulation in vital organs and tissues [4, 6,7,8]. The ability of MNPs to influence inflammatory [6, 9], metabolic [6, 10], and endocrine pathways [11], in addition to their cytotoxic [12, 13], immunotoxic [6, 14], and genotoxic [12, 13] effects, suggests their implication in a number of disease processes. The discrepancy between the WHO report and current literature emphasises the need for urgent re-evaluation of the health impact of MNPs.
The definition of MNPs is a crucial starting point for this re-evaluation. A lack of consensus in literature elicits conflicts within public policy, legislation, research and medicine, compounding pre-existing challenges in monitoring and mitigating the impacts of MNPs. Moreover, the characteristics of these MNPs, such as their functionalisation, surface characteristics, shape, additives, pigmentation and polymer type, are essential in understanding their behaviour and impact on human health. However, these characteristics are yet to be considered in the literature, with most studies focusing only on particle size.
Cardiovascular disease remains a leading cause of morbidity and mortality globally, with data demonstrating that despite advances in recent decades, the mortality rate may be beginning to rise [1]. This concerning trend necessitates urgent research into the mechanisms surrounding the aetiology and progression of diseases relating to vascular pathologies, heart failure, and congenital and electrical abnormalities. This scoping review aims to systematically explore and summarise the literature surrounding MNPs in the human cardiovascular system and their pathological consequences, and explore the methodologies used in their detection and analysis, guided by the following research questions:
RQ 1. How are MNPs defined within the current cardiovascular literature?
RQ 2. What are the characteristics of plastics which have been found within human cardiovascular systems?
RQ 3. What methodology has been utilised to date to characterise plastics within human cardiovascular systems?
RQ 4. What are the pathophysiological considerations which have been explored regarding the presence of plastic in human cardiovascular systems?
For the purpose of this review, a broad definition of the term ‘cardiovascular system’ will be employed, inclusive of the heart, blood vessels, blood, and the components (e.g. immune cells) commonly found within human blood. While other reviews have previously provided broad insights into the potential health implications of MPs, this scoping review, through a rigorous and systematic interrogation of existing literature, attempts to solidify the field of knowledge surrounding MNPs and the cardiovascular system specifically, raise awareness of the scale of this emerging issue, and lay the foundation for further research which may assist in the development of health policies and clinical practice guidelines.
Methods
Protocol and registration
An a priori protocol was developed, informed by the recommendations of Arksey and O’Malley [15], the Joanna Briggs Institute (JBI) [16], and the PRISMA extension for scoping reviews reporting guidance (PRISMA-ScR). This protocol was published on the Open Science Framework (https://osf.io/w9hr5) on March 8th 2024. This review was conducted in accordance with the ethical principles set forth in the Declaration of Helsinki.
Eligibility criteria
A pre-determined eligibility criteria was developed, informed by the population (human), concept (microplastics or nanoplastics and their effects) and context (cardiovascular system). Any studies investigating the presence of MNPs within the human cardiovascular system, or their effects on human cardiovascular outcomes or on relevant human cells lines, were included. Pre-determined definitions were developed and outlined within the a priori protocol after careful interrogation of the existing literature. For clarity, plastics were defined as a synthetic or semi-synthetic material comprising organic polymers from plant extracts or fossil fuels. The term ‘cardiovascular system’ was defined simply as the heart, blood, and associated vessels. To ensure a broad and thorough scope of the literature was undertaken, all research methodologies were included except for abstracts, reviews, pre-prints, conference proceedings, poster presentations, and editorials. No date restrictions were applied to the search strategy.
Search strategy
A search strategy was developed utilising a three-step approach originally proposed by Arksey and O’Malley [15] and further outlined by the JBI. Firstly, a pilot search of PubMed and Google Scholar was undertaken on January 19th 2024. Secondly, results were reviewed to identify additional search terms, with the final search strategy being translated for additional search engines with the assistance of a validated search engine translation software (Systematic Review Accelerator [SRA] Polyglot) [17] (Appendix 1). The final search was executed on November 27th 2024. An additional search for grey literature was undertaken utilising Research Rabbit [18], TERA Farmer [19], and Perplexity [20].
Information sources
Five databases (PubMed, EMBASE, CINAHL, SCOPUS, and Web of Science) were searched on November 27th 2024. Results from database searches were exported into Endnote X9 [21].
Selection of sources of evidence
Duplicate results were removed utilising automation software (SRA Deduplicator) [22]. Articles were screened by two authors by title and abstract within SRA Screenatron [22]. Full text screening was undertaken within Covidence [23] by two authors with discrepancies resolved by a third author.
Charting of data items
A draft extraction table was developed within Microsoft Excel to align with the aims of the scoping review. This was piloted and refined prior to undertaking full data extraction. Where information was not relevant or not reported, this was recorded for clarity.
Synthesis of results
Data pertaining to definitions was extracted and, where possible, synthesised and visually represented. Similarly, data pertaining to the countries and years of publication was tabulated and visually represented. Studies identifying the presence of plastic in human specimens, and studies evaluating the effects of plastic on cellular viability, uptake, and function, have been tabulated separately.
Results
Selection of sources of evidence
Database searching led to the retrieval of 1188 articles, of which 743 articles were removed via automation within Systematic Review Accelerator and Covidence (Fig. 1). Title and abstract screening of the remaining 445 articles led to the exclusion of a further 375 articles. The full text of 69 out of the 70 identified articles was successfully retrieved and screened with substantial agreement between authors (Cohen’s Kappa = 0.760). This process led to the exclusion of a further 23 articles resulting in 46 articles being included within the review.
Synthesis of results
Of the 46 identified articles, 15 countries were represented, with China (n = 14), Spain (n = 5), the United States of America (n = 4), Italy (n = 4) and India (n = 4) representing over half (67%) of all identified publications (Fig. 2). Only one article was published prior to the WHO report on MPs in drinking water [5] being made publicly available. All articles defined MPs and nanoplastics (NPs) primarily based on the size of the particle (Table 1). Thirteen articles (Table 2) identified the presence of MNPs in venous blood samples, cardiac tissue, thrombi, saphenous veins and atherosclerotic plaques, with implications for all-cause mortality (Fig. 3). Sizes of identified particles varied greatly from 1 to 3000 μm (Fig. 4). The remaining 33 articles were in vitro investigations into the effect of MNPs on human cell lines relating to the cardiovascular system (Table 3). However, a discrepancy exists between the polymers used within in vitro studies and the types (Table 4) and characteristics of polymers that have actually been found in human vascular and cardiac tissue. The limited sensitivity of detection methodologies utilised to date has hindered the identification and characterisation of smaller NPs in human samples. These smaller NPs, as shown through in vitro studies, tend to have more pronounced adverse effects.
Definitions of microplastics and nanoplastics
Twenty-three (66%) articles provided a description of the term ‘microplastic’, 16 (41%) of the included articles defined the term ‘nanoplastic’, and nine utilised the descriptor MNP (25%). While earlier articles chose to define NPs as particles <100 nm in size [24,25,26,27,28,29], a shift occurred in 2022 whereby articles began to refer to NPs as particles less than 1000 nm in size [30,31,32,33] (Table 1). Increasingly, articles choose to refer to MNPs more generally as particles below 5 mm in size [34,35,36,37,38,39,40] while making specific reference to NPs as particles less than 1000 nm.
Presence of MNPs in atherosclerotic plaques and thrombi and their effects on clotting factors
Two articles were identified analysing the presence of MNPs in atherosclerotic plaques [34, 41] and thrombi [42] respectively (Table 2). Of the 257 patients who completed the 33-month follow up, Marfella et al. [41] identified plastic (polyethylene) in carotid artery atherosclerotic plaques of 150 (58.4%) patients. Additionally, 31 (12.1%) patients had PVC in atherosclerotic plaques. At the 33-month follow up, patients with detectable MNPs had an increased risk of composite outcomes, including myocardial infarction, stroke, or death from any cause, compared to those with MNP-free atherosclerotic plaques [41]. Yang et al. [38] more recently explored the presence of MNPs within the bloodstream of patients with acute coronary syndrome. This study found MNPs within 100% of patients [38]. Similar to Marfella et al. [41], these results found that higher rates of MNP contamination were associated with poorer patient prognosis, as evidenced by higher SYNTAX scores, representing more complex and severe coronary artery atherosclerosis [38].
Two articles were identified which investigated the presence of MNPs in thrombi [35, 42]. Wu et al. published the first study identifying MNPs in human thrombi in 2023, in which they observed a single low density polyethylene particle, alongside other foreign materials including pigments, iron compounds, and metallic oxide particles [42]. Since this time, a larger study has provided further evidence of the widespread presence of MNPs in human thrombi, identifying 384 MNPs in 80% (24/30) of thrombi [35]. Several factors including particle size and functionalisation (e.g. carboxylated or aminated surfaces) have been shown to influence clotting dynamics, with smaller, functional particles demonstrating a greater ability to derange clotting dynamics under low shear environments [30, 43]. Conversely, Arranz et al. found no statistically significant difference in coagulation or platelet function with the addition of 50–130 nm sized polystyrene particles to ex vivo human whole blood at a concentration of 100 μg/mL [44]. While this study provided constant agitation, these in vitro conditions do not account for biochemical and biomechanical factors, such as shear stress, which influence clotting dynamics.
Vascular tissue, endothelial cells and smooth muscle cells
A variety of polymer types have been reported within cardiac tissue obtained during open heart surgery and saphenous vein tissue, with significant variance in quantity per gram, shape and size [45, 46] (Table 2). When investigating the effect of MNPs on endothelial cells, identified articles commonly utilised human umbilical vein endothelial cells (HUVEC) [27, 28, 47,48,49] or vascular endothelial cells (EA.hy926) [50] (Table 3). Additionally, a single article by Lomonaco et al. [36] was identified, investigating the effects of polystyrene and polyethylene (both high and low density) on human coronary artery smooth muscle cells [36]. While Lu et al. [47] found little evidence of deleterious effects following exposure of HUVEC cells to 1 µm spheres, articles utilising smaller particle sizes found polystyrene MNPs to decrease cell viability and increase autophagy [48]. In particular, functionalised polystyrene particles were found to increase oxidative stress and lactate dehydrogenase (LDH), and induce mitochondrial damage, resulting in an 82% decrease in ATP production [27]. Similarly, aged MNPs significantly increased IL-6 and TNF, indicating increased inflammatory processes [36]. It is yet to be determined whether the increase in endothelial leakiness [28, 50] increases MNP interaction with vascular smooth muscle.
Genotoxic effects
The exposure of polystyrene to peripheral blood mononuclear cells was shown to induce micronucleation and damage [25, 51]. While Ballesteros et al. [25] reported no DNA damage associated with 0.04 to 0.1 µm polystyrene NP exposure, Sarma et al. [52], utilising a particle size of 50 nm, demonstrated DNA damage and genomic instability. Dailianis et al. [53] demonstrated that exposure of low-density polyethylene to ultraviolet rays was associated with higher cytotoxicity and genotoxicity. Finally, Li et al. [54] identified 523 differentially expressed genes in response to polystyrene exposure. These genes are involved in processes such as cell development, mitochondrial and lysosomal function, and the downregulation of key pluripotency markers associated with reduced stem cell renewal efficiency.
Discussion
Overview
This systematic scoping review demonstrates that research into the presence and effect of MNPs in the human cardiovascular system has rapidly increased since 2019. While inconsistencies exist in the definition of MNPs in the early literature base, a consistent approach of defining MPs as particles less than 5 mm and NPs as less than 1000 nm in size has emerged since August 2023 (Table 1). The majority of included studies utilised in vitro experimental designs with human samples and cell lines. The findings of the 13 identified articles which investigated MNPs in human tissue are alarming and warrant concern from public health authorities. Of particular note is a lack of research into the presence of MNPs in human samples from low socioeconomic countries, especially those in the Pacific, which are economically and culturally tied to an ocean facing increasing contamination by MNPs. Taken together, the findings of research to date demonstrating the genotoxic, cytotoxic, immunotoxic and neurotoxic effects of MNPs, in addition to their deleterious effects on cellular metabolism and inflammatory effects, raise significant concerns for their role in a range of cardiovascular pathologies including atherosclerosis, cardiomyopathies, electrical and congenital abnormalities, and infective pathologies.
The role of MNPs in atherosclerosis and coronary artery disease
In 2024, Marfella et al. [41] identified MNPs in 58.4% of atherosclerotic plaques, demonstrating that individuals with MNP-associated atherosclerosis had a higher rate of myocardial infarction, stroke, or death at 34-month follow up. Additionally, Yang et al. [38] identified a positive correlation between blood MP concentrations and coronary lesion complexity, as quantified by the SYNTAX (Synergy Between Percutaneous Coronary Intervention with Taxus and Cardiac Surgery) score. This study identified that acute coronary syndrome patients, particularly those with myocardial infarction, exhibited significantly higher microplastic burden, with associated elevations in inflammatory cytokines such as IL-6 and IL-12p70 [38]. Together, these studies highlight the concern that MNPs may not just play a role in the aetiology of atherosclerosis, but may actually be an important variable in understanding patient prognosis with implications for management decisions.
Investigations employing human and animal cell lines have revealed a multitude of biochemical mechanisms, providing evidence for MNPs in the aetiology and pathophysiology of atherosclerosis, as well as for their significant role in vascular pathologies (Fig. 5). For example, MNPs have been demonstrated to induce endothelial dysfunction, an early stage of atherosclerotic plaque development [55]. Studies utilising 1 µm PS spheres have demonstrated little effect in human umbilical vein endothelial cell lines to date. In contrast, articles utilising smaller and positively charged particles, similar in size to those found within the observational study by Marfella et al. [41], have demonstrated increased ROS and LDH production. Additionally, studies have described damage to mitochondrial membranes, leading to a >82% decrease in mitochondrial ATP production [27], decreased cell viability and impaired angiogenesis, thereby hindering endothelial healing [48, 49]. In addition to endothelial dysfunction, MNPs have deleterious impacts within smooth muscle [56] and lead to decreased levels of high density lipoproteins (HDLs) as well as increased low density lipoproteins (LDLs) [57] and systemic ROS, assisting in the formation of oxy-LDL [58]. Taken together, these results demonstrate the ability of MNPs to lay the foundation for atherosclerotic plaque development.
Following their rapid uptake into the cytoplasm of macrophages, NPs provoke lipid aggregation [59], promoting the differentiation of macrophages into foam cells and the development of atherosclerosis [60]. Their continued genotoxic and cytotoxic effect from increased endoplasmic reticulum stress, oxidative stress and disruption to mitochondrial membranes [61] results in apoptosis [62], potentially assisting in the development of a necrotic core, increasing plaque instability [63].
In cases where plaque rupture ensues, MNP contamination deranges the clotting cascade, impacting fibrin polymerisation rates and platelet aggregation. This modulates clot strength and the manner in which the clot adheres to the endothelial wall [64]. Of particular clinical concern is the ability of MNPs to impede the production of endothelium-derived nitric oxide [58, 65], impairing vasodilatory responses to clot formation [66]. Importantly, SGLT2 inhibitors within porcine endothelial models treated with NPs have been shown to upregulate endothelial nitric oxide synthase expression, decrease the formation of ROS, and ultimately inhibit NP-associated endothelial cell senescence [67]. Together, these two studies demonstrate that the production of nitric oxide is perturbed by MNPs, which may impact the delicate haemostatic balance between thrombosis and bleeding. The many pathways through which MNPs may cause cardiovascular disease provide potential pharmacological targets, requiring further exploration into their pervasive effects. Regardless, the involvement of MNPs in atherosclerotic disease provides significant cause for concern, not only in the context of coronary artery disease, but also in peripheral and cerebrovascular pathologies [41].
Valvular disorders, cardiomyopathies, and electrical abnormalities
In addition to vascular diseases, MNPs have been implicated in the dysfunction of cardiomyocytes [68] with potential implications for cardiomyopathies and electrical abnormalities [54, 56, 69] (Fig. 6). For example, the exposure of neonatal ventricular myocytes to NPs has been shown to significantly decrease intracellular Ca2+ levels, in addition to mitochondrial membrane potentials and cellular metabolism, resulting in a reduction in cardiomyocyte contraction forces [69]. Additionally, MNPs in rat models have been shown to induce cardiac fibrosis through activation of the Wnt/β-catenin pathway and cellular apoptosis [70]. Following polystyrene exposure, in vivo rat models have demonstrated increased troponin I and creatine kinase-MB (CK-MB) levels, as well as disruption of mitochondrial mtDNA and cGAS-STING signalling pathways, leading to cardiomyocyte apoptosis [68, 70, 71]. When exposed to MNPs at a concentration equivalent to human exposure, rats demonstrated a marked elevation in cardiac-specific markers and an increase in interventricular septal thickness [72]. This raises considerable concern and highlights a need for urgent research into MNP-associated cardiomyopathies [73].
Cardiac disorders of infective origins
The rough surface characteristics and size of MNPs found within the human cardiovascular system to date [41] provide an ideal environment to facilitate the adsorption of viruses or bacteria, the development of biofilms, and increased virus survival and infectivity [74,75,76]. MNPs have been shown to promote the infection of cells through the development of a protein corona facilitating a trojan horse mechanism, whereby NP particles shuttle viruses and bacteria into the cytoplasm [77, 78]. Additionally, the presence of MNPs has been shown to inhibit innate immune functions, in particular the actions of macrophages [77, 78]. Beijer et al. [79] demonstrated a dose-related immune response with the largest secretions of IL-1β, IL-8 and TNF-α elicited by polyethylene terephthalate, identified within both human blood and cardiac tissue [26, 46]. As a result, MNPs are likely to play an important role in pathologies such as infective endocarditis, rheumatic heart disease and pericarditis.
Congenital heart abnormalities
Of particular note, research highlighting the presence of MPs in human placentas (including on the fetal side), semen and the meconium of newborns raises important questions surrounding the potential role of MNPs in the aetiology of congenital cardiovascular abnormalities. Research investigating the potential abnormal development of the heart utilising pluripotent stem cells has demonstrated altered atrioventricular valve and cardiomyocyte formation following exposure to polystyrene NPs [80,81,82]. In animal models, NPs have been shown to alter umbilical and placental blood flow [83], with maternal polystyrene NP exposure leading to a 12% reduction in late gestational fetal weight [84]. With more specific reference to the cardiovascular system, maternal MP exposure in rats has also been observed to cause fetal aortic abnormalities [85]. Although current exposure levels are unlikely to cause significant cardiovascular anatomical or physiological abnormalities at birth, there is evidence that MNPs can affect cellular differentiation into cardiomyocytes, disrupt sarcomere organisation, impair contractility, and reduce calcium transients [54]. These findings raise concerns about potential subclinical alterations at birth that may contribute to clinical pathologies later in life [54].
Current gaps in the literature
Despite significant advances in the field of MNPs and cardiovascular health, research is urgently required to assist in the characterisation of MNPs contaminating the human cardiovascular system. Currently, a lack of research exists to appropriately inform animal and cell line research regarding the characteristics of human environmental exposure (Table 4). Without a comprehensive understanding of the types, sizes, characteristics (leachates, surface characteristics, electrical charge, shape, etc.) and concentrations of MNPs within the human cardiovascular system, it is unclear if cell line research currently provides a solid understanding of the effects of MNPs within the general population or in specific populations, such as those investigated by Marfella et al. [41] (carotid endarterectomy) or Yang et al. [38] (acute coronary syndrome). Results of in vitro studies should, therefore, be interpreted with caution until further research characterises the presence of MNPs in humans and explores the long-term effects of their bioaccumulation on disease outcomes through additional in vivo studies which include long-term follow up. To assist with this, researchers moving forward should consider consulting with scientists familiar with the challenges associated with MNP detection and characterisation to ensure sensitive laboratory-based methodologies are utilised, thereby limiting the potential for false positives and environmental contamination.
In addition, researchers and public health authorities alike are urged to begin investigating the presence of MNPs in low socioeconomic areas, especially those identified as high risk due to exposure to contaminated water, food and living environments. Furthermore, the contamination of various clinical populations requires attention to understand variances in exposure and physiological consequences. In conjunction with laboratory-based analysis, complementary methodologies utilising surveys to characterise behaviour, alongside longitudinal studies within both animals and humans, are required to understand how various behaviours and exposures influence MNP contamination and its long-term effects on chronic disease and mortality. Clinical trials using behavioural interventions modifying MNP exposure, for example through dietary modifications, are urgently required to inform public health advice and international industry policy development. Additionally, research should seek to elucidate the potential impacts of specific environments (e.g. cities) and/or occupational hazards, especially in industries such as construction where individuals may be exposed to higher rates of MNPs associated with cardiovascular disease, such as poly vinyl chloride and polyethylene, as suggested by in vivo studies to date. An interdisciplinary approach which seeks to understand the multiple organ system interactions should be considered in order to advance our understanding of individual organ systems.
Limitations
Due to the rapidly evolving nature of this research field, this scoping review will require updating within the next 2 years. At this time, further research that may allow for a systematic review and meta-analysis to be conducted on the presence of MNPs in various tissues is currently precluded by a lack of available data and consistency within methodologies and reporting. Additionally, a lack of research investigating the presence and effect of MNPs on the lymphatic system prohibits a robust discussion on how this complementary organ system affects cardiovascular function. Methodological limitations were noted within some articles which may have affected reported results. For example, reports of haemolytic activity may be overestimated considering Djapovic et al. [32] washed RBCs with hypertonic (0.99%) NaCl. Similarly, Gopinath et al. [86] isolated RBCs by centrifugation without a density gradient medium, which may have led to some leucocytes remaining with the RBC concentration, resulting in the release of haemolytic enzymes. Marfella et al. [41] highlighted the potential for laboratory contamination during MNP detection in atherosclerotic plaques, despite rigorous efforts to minimise this risk. They also noted that while pyrolysis-gas chromatography-mass spectrometry provides sensitive detection of MNPs, it does not differentiate between MPs and NPs, limiting precise characterisation of particle size and type.
Conclusion
This systematic scoping review highlights the notable increase in research interest in this field since 2019, with all currently published studies reporting adverse effects on the cardiovascular system. Throughout their lives, humans are exposed to a multitude of MNPs with varying functionality, surface characteristics, chemical compositions and sizes every day. To date, research has identified the presence of MNPs within venous blood samples, cardiac tissue, thrombi, saphenous veins and atherosclerotic plaques, with implications for the prognosis of patients with cardiovascular disease and all-cause mortality. These findings, in conjunction with in vitro experimental designs, raise significant concern for the potential contribution of MNPs to cardiovascular pathologies such as atherosclerosis, cardiomyopathies, electrical abnormalities, congenital cardiovascular defects and infective pathologies. Multiple health authorities, including the WHO and the American College of Physicians, continue to call for urgent research in this field to elucidate the presence and effect of MNP bioaccumulation in humans, as well as to explore potential solutions [5, 87, 88]. Without further research, policy makers will be unable to act appropriately, and clinicians will lack the necessary guidance on how to assess, manage and educate their patients and the general public.
References
McClellan M, Brown N, Califf RM, Warner JJ. Call to action: urgent challenges in cardiovascular disease: a Presidential Advisory from the American Heart Association. Circulation. 2019;139:e44–54.
Lamichhane G, Acharya A, Marahatha R, Modi B, Paudel R, Adhikari A, et al. Microplastics in environment: global concern, challenges, and controlling measures. Int J Environ Sci Technol. 2023;20:4673–94.
Molloy MA, Holt J, Charnetski M, Rossler K. Healthcare simulation standards of best practiceTM simulation glossary. Clin Simul Nurs. 2021;58:57–65.
Ghosh S, Sinha JK, Ghosh S, Vashisth K, Han S, Bhaskar R. Microplastics as an emerging threat to the global environment and human health. Sustainability. 2023;15:10821.
World Health Organization. Microplastics in drinking-water. 2019. Available from: https://www.who.int/publications/i/item/9789241516198. Accessed 22/02/2024.
Ali N, Katsouli J, Marczylo EL, Gant TW, Wright S, Bernardino de la Serna J. The potential impacts of micro-and-nano plastics on various organ systems in humans. EBioMedicine. 2024;99:104901.
Sun A, Wang W-X. Human exposure to microplastics and its associated health risks. Environ Health. 2023;1:139–49.
Yee MS, Hii LW, Looi CK, Lim WM, Wong SF, Kok YY, et al. Impact of microplastics and nanoplastics on human health. Nanomaterials. 2021;11:496.
Pulvirenti E, Ferrante M, Barbera N, Favara C, Aquilia E, Palella M, et al. Effects of nano and microplastics on the inflammatory process: in vitro and in vivo studies systematic review. Front Biosci Landmark. 2022;27:287.
Goodman KE, Hua T, Sang Q-XA. Effects of polystyrene microplastics on human kidney and liver cell morphology, cellular proliferation, and metabolism. ACS Omega. 2022;7:34136–53.
Kannan K, Vimalkumar K. A review of human exposure to microplastics and insights into microplastics as obesogens. Front Endocrinol. 2021;12:724989.
Shi X, Wang X, Huang R, Tang C, Hu C, Ning P, et al. Cytotoxicity and genotoxicity of polystyrene microplastics with different size and surface modification in A549 human lung cells. Int J Nanomedicine. 2021;17:4509–23.
Çobanoğlu H, Belivermiş M, Sıkdokur E, Kılıç Ö, Çayır A. Genotoxic and cytotoxic effects of polyethylene microplastics on human peripheral blood lymphocytes. Chemosphere. 2021;272:129805.
Hirt N, Body-Malapel M. Immunotoxicity and intestinal effects of nano- and microplastics: a review of the literature. Part Fibre Toxicol. 2020;17:57.
Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8:19–32.
Peters M, Godfrey C, McInerney P, Khalil H, Larsen P, Marnie C, et al. Best practice guidance and reporting items for the development of scoping review protocols. JBI Evid Synth. 2022;20:953–68.
Kung JY. Polyglot search translator. J Can Health Libr Assoc. 2022;43:35.
Research Rabbit. Available from: https://www.researchrabbit.ai/. Accessed 27/11/2024.
TERA Farmer. Available from: https://terafarmer.tera-tools.com/. Accessed 27/11/2024.
Perplexity.ai. 2024. Available from: https://www.perplexity.ai/. Accessed 27/11/2024.
EndNote X9. Available from: https://endnote.com/. Accessed 19/01/2024.
Systematic Review Accelerator. Available from: https://sr-accelerator.com/#/. Accessed 19/01/2024.
Covidence. Covidence.org. Available from: https://www.covidence.org/. Accessed 19/01/2024.
Bojic S, Falco MM, Stojkovic P, Ljujic B, Gazdic Jankovic M, Armstrong L, et al. Platform to study intracellular polystyrene nanoplastic pollution and clinical outcomes. Stem Cells. 2020;38:1321–5.
Ballesteros S, Domenech J, Barguilla I, Cortés C, Marcos R, Hernández A. Genotoxic and immunomodulatory effects in human white blood cells after: ex vivo exposure to polystyrene nanoplastics. Environ Sci Nano. 2020;7:3431–46.
Leslie HA, van Velzen M, Brandsma SH, Vethaak AD, Garcia-Vallejo JJ, Lamoree MH. Discovery and quantification of plastic particle pollution in human blood. Environ Int. 2022;163:107199.
Fu Y, Fan M, Xu L, Wang H, Hu Q, Jin Y. Amino-functionalized polystyrene nano-plastics induce mitochondria damage in human umbilical vein endothelial cells. Toxics. 2022;10:215.
Wei W, Li Y, Lee M, Andrikopoulos N, Lin S, Chen C, et al. Anionic nanoplastic exposure induces endothelial leakiness. Nat Commun. 2022;13:4757.
Malinowska K, Sicińska P, Bukowska B. P07-27 Polystyrene nanoparticles and their genotoxic properties in human peripheral blood mononuclear cells. Toxicol Lett. 2022;368:S130.
Tran DQ, Stelflug N, Hall A, Nallan Chakravarthula T, Alves NJ. Microplastic effects on thrombin-fibrinogen clotting dynamics measured via turbidity and thromboelastography. Biomolecules. 2022;12:1864.
Gettings SM, Timbury W, Dmochowska A, Sharma R, MacKenzie LE, Miquelard-Garnier G, et al. Polyethylene terephthalate (PET) micro- and nanoplastic particles affect the mitochondrial efficiency of human brain vascular pericytes without inducing oxidative stress. NanoImpact. 2023;34:100508.
Djapovic M, Apostolovic D, Postic V, Lujic T, Jovanovic V, Stanic-Vucinic D, et al. Characterization of nanoprecipitated PET nanoplastics by 1H NMR and impact of residual ionic surfactant on viability of human primary mononuclear cells and hemolysis of erythrocytes. Polymers. 2023;15:4703.
Wang Y, Li H, Lan J, Guan R, Bao Y, Du X, et al. The weakened physiological functions of human serum albumin in presence of polystyrene nanoplastics. Int J Biol Macromol. 2024;261:129609.
Liu S, Wang C, Yang Y, Du Z, Li L, Zhang M, et al. Microplastics in three types of human arteries detected by pyrolysis-gas chromatography/mass spectrometry (Py-GC/MS). J Hazard Mater. 2024;469:133855.
Wang T, Yi Z, Liu X, Cai Y, Huang X, Fang J, et al. Multimodal detection and analysis of microplastics in human thrombi from multiple anatomically distinct sites. EBioMedicine. 2024;103:105118.
Lomonaco T, Persiani E, Biagini D, Gisone I, Ceccherini E, Cecchettini A, et al. Type-specific inflammatory responses of vascular cells activated by interaction with virgin and aged microplastics. Ecotoxicol Environ Saf. 2024;282:116695.
Remigante A, Spinelli S, Gambardella L, Bozzuto G, Vona R, Caruso D, et al. Internalization of nano- and micro-plastics in human erythrocytes leads to oxidative stress and estrogen receptor-mediated cellular responses. Free Radic Biol Med. 2024;223:1–17.
Yang Y, Zhang F, Jiang Z, Du Z, Liu S, Zhang M, et al. Microplastics are associated with elevated atherosclerotic risk and increased vascular complexity in acute coronary syndrome patients. Part Fibre Toxicol. 2024;21:34.
Liu S, Yang Y, Du Z, Wang C, Li L, Zhang M, et al. Percutaneous coronary intervention leads to microplastics entering the blood: interventional devices are a major source. J Hazard Mater. 2024;476:135054.
Xu H, Dong C, Yu Z, Ozaki Y, Hu Z, Zhang B, et al. Detection and analysis of microplastics in tissues and blood of human cervical cancer patients. Environ Res. 2024;259:119498.
Marfella R, Prattichizzo F, Sardu C, Fulgenzi G, Graciotti L, Spadoni T, et al. Microplastics and nanoplastics in atheromas and cardiovascular events. N Engl J Med. 2024;390:900–10.
Wu D, Feng Y, Wang R, Jiang J, Guan Q, Yang X, et al. Pigment microparticles and microplastics found in human thrombi based on Raman spectral evidence. J Adv Res. 2023;49:141–50.
Christodoulides A, Hall A, Alves NJ. Exploring microplastic impact on whole blood clotting dynamics utilizing thromboelastography. Front Public Health. 2023;11:1215817.
Arribas Arranz J, Villacorta A, Rubio L, García-Rodríguez A, Sánchez G, Llorca M, et al. Kinetics and toxicity of nanoplastics in ex vivo exposed human whole blood as a model to understand their impact on human health. Sci Total Environ. 2024;948:174725.
Rotchell JM, Jenner LC, Chapman E, Bennett RT, Bolanle IO, Loubani M, et al. Detection of microplastics in human saphenous vein tissue using μFTIR: a pilot study. PLoS ONE. 2023;18:e0280594.
Yang Y, Xie E, Du Z, Peng Z, Han Z, Li L, et al. Detection of various microplastics in patients undergoing cardiac surgery. Environ Sci Technol. 2023;57:10911–8.
Lu YY, Cao M, Tian M, Huang Q. Internalization and cytotoxicity of polystyrene microplastics in human umbilical vein endothelial cells. J Appl Toxicol. 2023;43:262–71.
Lee HS, Amarakoon D, Wei CI, Choi KY, Smolensky D, Lee SH. Adverse effect of polystyrene microplastics (PS-MPs) on tube formation and viability of human umbilical vein endothelial cells. Food Chem Toxicol. 2021;154:112356.
Lu YY, Li H, Ren H, Zhang X, Huang F, Zhang D, et al. Size-dependent effects of polystyrene nanoplastics on autophagy response in human umbilical vein endothelial cells. J Hazard Mater. 2022;421:126770.
Chen YC, Chen KF, Andrew Lin KY, Su HP, Wu DN, Lin CH. Evaluation of toxicity of polystyrene microplastics under realistic exposure levels in human vascular endothelial EA.hy926 cells. Chemosphere. 2023;313:137582.
Babonaitė M, Čepulis M, Kazlauskaitė J, Lazutka JR. Evaluation of in vitro genotoxicity of polystyrene nanoparticles in human peripheral blood mononuclear cells. Toxics. 2023;11:627.
Sarma DK, Dubey R, Samarth RM, Shubham S, Chowdhury P, Kumawat M, et al. The biological effects of polystyrene nanoplastics on human peripheral blood lymphocytes. Nanomaterials. 2022;12:1632.
Dailianis S, Rouni M, Ainali NM, Vlastos D, Kyzas GZ, Lambropoulou DA, et al. New insights into the size-independent bioactive potential of pristine and UV-B aged polyethylene microplastics. Sci Total Environ. 2024;918:170616.
Li J, Weng H, Liu S, Li F, Xu K, Wen S, et al. Embryonic exposure of polystyrene nanoplastics affects cardiac development. Sci Total Environ. 2024;906:167406.
Gimbrone MA Jr, García-Cardeña G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ Res. 2016;118:620–36.
Persiani E, Cecchettini A, Ceccherini E, Gisone I, Morales MA, Vozzi F. Microplastics: a matter of the heart (and vascular system). Biomedicines. 2023;11:264.
Nnoruka UC, Okonkwo CJ, Ilechukwu I, Okonkwo CJ, Belonwu DC. Impact of polystyrene microplastic exposure on lipid profile and oxidative stress status of male and female Wistar rats. Environ Anal Health Toxicol. 2022;37:e2022024-0.
Batty M, Bennett MR, Yu E. The role of oxidative stress in atherosclerosis. Cells. 2022;11. https://doi.org/10.3390/cells11233843.
Florance I, Chandrasekaran N, Gopinath PM, Mukherjee A. Exposure to polystyrene nanoplastics impairs lipid metabolism in human and murine macrophages in vitro. Ecotoxicol Environ Saf. 2022;238:113612.
Florance I, Ramasubbu S, Mukherjee A, Chandrasekaran N. Polystyrene nanoplastics dysregulate lipid metabolism in murine macrophages in vitro. Toxicology. 2021;458:152850.
Koner S, Florance I, Mukherjee A, Chandrasekaran N. Cellular response of THP-1 macrophages to polystyrene microplastics exposure. Toxicology. 2023;483:153385.
Sukhorukov VN, Khotina VA, Bagheri Ekta M, Ivanova EA, Sobenin IA, Orekhov AN. Endoplasmic reticulum stress in macrophages: the vicious circle of lipid accumulation and pro-inflammatory response. Biomedicines. 2020;8:210.
Puylaert P, Zurek M, Rayner KJ, De Meyer G, Martinet W. Regulated necrosis in atherosclerosis. Arterioscler Thromb Vasc Biol. 2022;42:1283–306.
Rajendran D, Chandrasekaran N. Journey of micronanoplastics with blood components. RSC Adv. 2023;13:31435–59.
Khan A, Jia Z. Recent insights into uptake, toxicity, and molecular targets of microplastics and nanoplastics relevant to human health impacts. iScience. 2023;26:106061.
Chowdhury SR, Dey A, Mondal S, Gautam MK. Environmental microplastics and nanoplastics: effects on cardiovascular system. Toxicol Anal Clin. 2023;36:145–57.
Dhakal B, Shiwakoti S, Park EY, Kang KW, Schini-Kerth VB, Park SH, et al. SGLT2 inhibition ameliorates nano plastics-induced premature endothelial senescence and dysfunction. Sci Rep. 2023;13:6256.
Wei J, Wang X, Liu Q, Zhou N, Zhu S, Li Z, et al. The impact of polystyrene microplastics on cardiomyocytes pyroptosis through NLRP3/Caspase-1 signaling pathway and oxidative stress in Wistar rats. Environ Toxicol. 2021;36:935–44.
Roshanzadeh A, Oyunbaatar NE, Ganjbakhsh SE, Park S, Kim DS, Kanade PP, et al. Exposure to nanoplastics impairs collective contractility of neonatal cardiomyocytes under electrical synchronization. Biomaterials. 2021;278:121175.
Li Z, Zhu S, Liu Q, Wei J, Jin Y, Wang X, et al. Polystyrene microplastics cause cardiac fibrosis by activating Wnt/β-catenin signaling pathway and promoting cardiomyocyte apoptosis in rats. Environ Pollut. 2020;265:115025.
Wang K, Du Y, Li P, Guan C, Zhou M, Wu L, et al. Nanoplastics causes heart aging/myocardial cell senescence through the Ca2+/mtDNA/cGAS-STING signaling cascade. J Nanobiotechnol. 2024;22:96.
Leonard SVL, Liddle CR, Atherall CA, Chapman E, Watkins M, Calaminus SDJ, et al. Microplastics in human blood: polymer types, concentrations and characterisation using μFTIR. Environ Int. 2024;188:108751.
Zhou Y, Wu Q, Li Y, Feng Y, Wang Y, Cheng W. Low-dose of polystyrene microplastics induce cardiotoxicity in mice and human-originated cardiac organoids. Environ Int. 2023;179:108171.
Zhong H, Wu M, Sonne C, Lam SS, Kwong R, Jiang Y, et al. The hidden risk of microplastic-associated pathogens in aquatic environments. Eco Environ Health. 2023;2:142–51.
Lu J, Yu Z, Ngiam L, Guo J. Microplastics as potential carriers of viruses could prolong virus survival and infectivity. Water Res. 2022;225:119115.
Yang W, Li Y, Boraschi D. Association between microorganisms and microplastics: How does it change the host-pathogen interaction and subsequent immune response? Int J Mol Sci. 2023;24:4065.
Wolff CM, Singer D, Schmidt A, Bekeschus S. Immune and inflammatory responses of human macrophages, dendritic cells, and T-cells in presence of micro- and nanoplastic of different types and sizes. J Hazard Mater. 2023;459:132194.
Huang H, Hou J, Liao Y, Wei F, Xing B. Polyethylene microplastics impede the innate immune response by disrupting the extracellular matrix and signaling transduction. iScience. 2023;26:107390.
Beijer NRM, Dehaut A, Carlier MP, Wolter H, Versteegen RM, Pennings JLA, et al. Relationship between particle properties and immunotoxicological effects of environmentally-sourced microplastics. Front Water. 2022;4.
Braun T, Ehrlich L, Henrich W, Koeppel S, Lomako I, Schwabl P, et al. Detection of microplastic in human placenta and meconium in a clinical setting. Pharmaceutics. 2021;13:921.
Liu S, Guo J, Liu X, Yang R, Wang H, Sun Y, et al. Detection of various microplastics in placentas, meconium, infant feces, breastmilk and infant formula: a pilot prospective study. Sci Total Environ. 2023;854:158699.
Zhao Q, Zhu L, Weng J, Jin Z, Cao Y, Jiang H, et al. Detection and characterization of microplastics in the human testis and semen. Sci Total Environ. 2023;877:162713.
Hanrahan J, Steeves KL, Locke DP, O'Brien TM, Maekawa AS, Amiri R, et al. Maternal exposure to polyethylene micro- and nanoplastics impairs umbilical blood flow but not fetal growth in pregnant mice. Sci Rep. 2024;14:399.
Aghaei Z, Sled JG, Kingdom JC, Baschat AA, Helm PA, Jobst KJ, et al. Maternal exposure to polystyrene micro-and nanoplastics causes fetal growth restriction in mice. Environ Sci Technol Lett. 2022;9:426–30.
Cary CM, Fournier SB, Adams S, Wang X, Yurkow EJ, Stapleton PA. Single pulmonary nanopolystyrene exposure in late-stage pregnancy dysregulates maternal and fetal cardiovascular function. Toxicol Sci. 2024;199:149–59.
Gopinath PM, Saranya V, Vijayakumar S, Mythili Meera M, Ruprekha S, Kunal R, et al. Assessment on interactive prospectives of nanoplastics with plasma proteins and the toxicological impacts of virgin, coronated and environmentally released-nanoplastics. Sci Rep. 2019;9:8860.
World Health Organization. WHO calls for more research into microplastics and a crackdown on plastic pollution. 2024. Available from: https://www.who.int/news/item/22-08-2019-who-calls-for-more-research-into-microplastics-and-a-crackdown-on-plastic-pollution.
Crowley R, Mathew S, Hilden D. Environmental health: a position paper from the American College of Physicians. Ann Intern Med. 2022;175:1591–3.
Rubio L, Barguilla I, Domenech J, Marcos R, Hernández A. Biological effects, including oxidative stress and genotoxic damage, of polystyrene nanoparticles in different human hematopoietic cell lines. J Hazard Mater. 2020;398:122900.
Choi D, Bang J, Kim T, Oh Y, Hwang Y, Hong J. In vitro chemical and physical toxicities of polystyrene microfragments in human-derived cells. J Hazard Mater. 2020;400:123308.
Zhang M, Shi J, Huang Q, Xie Y, Wu R, Zhong J, et al. Multi-omics analysis reveals size-dependent toxicity and vascular endothelial cell injury induced by microplastic exposure: in vivo and in vitro. Environ Sci Nano. 2022;9.
Salvia R, Rico LG, Bradford JA, Ward MD, Olszowy MW, Martínez C, et al. Fast-screening flow cytometry method for detecting nanoplastics in human peripheral blood. MethodsX. 2023;10:102057.
Wang X, Zhao J, Ding S, Zhang H. Interaction of polystyrene nanoplastics with human fibrinogen. Int J Biol Macromol. 2023;238:124049.
Ghosal S, Bag S, Burman MD, Bhowmik S. Multispectroscopic investigations of the binding interaction between polyethylene microplastics and human hemoglobin. J Phys Chem Lett. 2023;14:10328–32.
Yu H, Li H, Cui C, Han Y, Xiao Y, Zhang B, et al. Association between blood microplastic levels and severity of extracranial artery stenosis. J Hazard Mater. 2024;480:136211.
Hwangbo S, Kim IY, Ko K, Park K, Hong J, Kang G, et al. Preparation of fragmented polyethylene nanoplastics using a focused ultrasonic system and assessment of their cytotoxic effects on human cells. Environ Pollut. 2024;362:125009.
Fleury JB, Baulin VA. Microplastics destabilize lipid membranes by mechanical stretching. Proc Natl Acad Sci USA. 2021;118.
Martín-Pérez J, Villacorta A, Banaei G, Morataya-Reyes M, Tavakolpournegari A, Marcos R, et al. Hazard assessment of nanoplastics is driven by their surface-functionalization. Effects in human-derived primary endothelial cells. Sci Total Environ. 2024;934:173236.
Płuciennik K, Sicińska P, Duchnowicz P, Bonarska-Kujawa D, Męczarska K, Solarska-Ściuk K, et al. The effects of non-functionalized polystyrene nanoparticles with different diameters on human erythrocyte membrane and morphology. Toxicol Vitr. 2023;91:105634.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Contributions
The authors confirm that all listed authors meet the requirements for authorship.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This manuscript is a review article and does not involve a research protocol requiring the approval by the relevant institutional review board or ethics committee.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix 1
Appendix 1
PubMed
(Microplastic*[tiab] OR Nanoplastic*[tiab])
AND
(cardiovascular[tiab] OR cardiac[tiab] OR blood[tiab] OR thrombi[tiab] OR artery[tiab] OR vein[tiab] OR microvascular[tiab] OR vascular[tiab] OR coronary[tiab] OR endothelial[tiab] OR clot*[tiab] OR thrombosis[tiab] OR pericard*l[tiab] OR endocard*[tiab] OR myocard*[tiab] OR adventitia[tiab] OR atherosclerosis[tiab] OR heart[tiab] OR cardiopulmonary[tiab] OR capillaries[tiab] OR bloodstream[tiab])
AND
(human*[tiab])
NOT
(Mice[tiab] OR Mouse[tiab] OR rodent[tiab] OR Rat[tiab] OR Rats[tiab] OR Murine[tiab] OR fish[tiab])
EMBASE
(Microplastic*:ti,ab OR Nanoplastic*:ti,ab)
AND
(cardiovascular:ti,ab OR cardiac:ti,ab OR blood:ti,ab OR thrombi:ti,ab OR artery:ti,ab OR vein:ti,ab OR microvascular:ti,ab OR vascular:ti,ab OR coronary:ti,ab OR endothelial:ti,ab OR clot*:ti,ab OR thrombosis:ti,ab OR pericard*l:ti,ab OR endocard*:ti,ab OR myocard*:ti,ab OR adventitia:ti,ab OR atherosclerosis:ti,ab OR heart:ti,ab OR cardiopulmonary:ti,ab OR capillaries:ti,ab OR bloodstream:ti,ab)
AND
(human*:ti,ab)
NOT
(Mice:ti,ab OR Mouse:ti,ab OR rodent:ti,ab OR Rat:ti,ab OR Rats:ti,ab OR Murine:ti,ab OR fish:ti,ab)
SCOPUS
(TITLE-ABS(Microplastic*) OR TITLE-ABS(Nanoplastic*))
AND
(TITLE-ABS(cardiovascular) OR TITLE-ABS(cardiac) OR TITLE-ABS(blood) OR TITLE-ABS(thrombi) OR TITLE-ABS(artery) OR TITLE-ABS(vein) OR TITLE-ABS(microvascular) OR TITLE-ABS(vascular) OR TITLE-ABS(coronary) OR TITLE-ABS(endothelial) OR TITLE-ABS(clot*) OR TITLE-ABS(thrombosis) OR TITLE-ABS(pericard*l) OR TITLE-ABS(endocard*) OR TITLE-ABS(myocard*) OR TITLE-ABS(adventitia) OR TITLE-ABS(atherosclerosis) OR TITLE-ABS(heart) OR TITLE-ABS(cardiopulmonary) OR TITLE-ABS(capillaries) OR TITLE-ABS(bloodstream))
AND
(TITLE-ABS(human*))
AND NOT
(TITLE-ABS(Mice) OR TITLE-ABS(Mouse) OR TITLE-ABS(rodent) OR TITLE-ABS(Rat) OR TITLE-ABS(Rats) OR TITLE-ABS(Murine) OR TITLE-ABS(fish))
Web of Science
((TI=Microplastic* OR AB=Microplastic*) OR (TI=Nanoplastic* OR AB=Nanoplastic*))
AND
((TI=cardiovascular OR AB=cardiovascular) OR (TI=cardiac OR AB=cardiac) OR (TI=blood OR AB=blood) OR (TI=thrombi OR AB=thrombi) OR (TI=artery OR AB=artery) OR (TI=vein OR AB=vein) OR (TI=microvascular OR AB=microvascular) OR (TI=vascular OR AB=vascular) OR (TI=coronary OR AB=coronary) OR (TI=endothelial OR AB=endothelial) OR (TI=clot* OR AB=clot*) OR (TI=thrombosis OR AB=thrombosis) OR (TI=pericard*l OR AB=pericard*l) OR (TI=endocard* OR AB=endocard*) OR (TI=myocard* OR AB=myocard*) OR (TI=adventitia OR AB=adventitia) OR (TI=atherosclerosis OR AB=atherosclerosis) OR (TI=heart OR AB=heart) OR (TI=cardiopulmonary OR AB=cardiopulmonary) OR (TI=capillaries OR AB=capillaries) OR (TI=bloodstream OR AB=bloodstream))
AND
((TI=human* OR AB=human*))
NOT
((TI=Mice OR AB=Mice) OR (TI=Mouse OR AB=Mouse) OR (TI=rodent OR AB=rodent) OR (TI=Rat OR AB=Rat) OR (TI=Rats OR AB=Rats) OR (TI=Murine OR AB=Murine) OR (TI=fish OR AB=fish))
CINAHL
(Microplastic*.tw. OR Nanoplastic*.tw.)
AND
(cardiovascular.tw. OR cardiac.tw. OR blood.tw. OR thrombi.tw. OR artery.tw. OR vein.tw. OR microvascular.tw. OR vascular.tw. OR coronary.tw. OR endothelial.tw. OR clot*.tw. OR thrombosis.tw. OR pericard*l.tw. OR endocard*.tw. OR myocard*.tw. OR adventitia.tw. OR atherosclerosis.tw. OR heart.tw. OR cardiopulmonary.tw. OR capillaries.tw. OR bloodstream.tw.)
AND
(human*.tw.)
NOT
(Mice.tw. OR Mouse.tw. OR rodent.tw. OR Rat.tw. OR Rats.tw. OR Murine.tw. OR fish.tw.)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Goldsworthy, A., O’Callaghan, L.A., Blum, C. et al. Micro-nanoplastic induced cardiovascular disease and dysfunction: a scoping review. J Expo Sci Environ Epidemiol 35, 746–769 (2025). https://doi.org/10.1038/s41370-025-00766-2
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41370-025-00766-2