Abstract
Objectives
To identify characteristics and outcomes of infants who received multiple doses of surfactant vs those who received one dose or none.
Study design
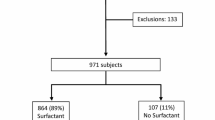
In this retrospective study, we included neonates of 22–28 weeks’ gestation admitted to NICUs in the Canadian Neonatal Network. Patients were divided into three groups: no surfactant, single dose, and multiple doses. The primary outcome was a composite of mortality or any of the major morbidities, including severe neurological injury, bronchopulmonary dysplasia, or ≥stage 3 retinopathy of prematurity.
Results
Of 8024 eligible neonates, 2461 (31%) did not receive surfactant, 3545 (44%) received one dose, and 2018 (25%) received >1 dose. Receiving one or more doses of surfactant was associated with significantly higher adjusted odds of mortality or major morbidities in a dose-dependent manner.
Conclusions
Receiving one or more doses of surfactant was associated with adverse neonatal outcomes. Receipt of more than one dose may reflect underlying severe lung immaturity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
The data sets generated and analyzed during the current study are with the corresponding author but current data transfer approval agreements do not allow for data to be made available to others.
References
Soll R, Ozek E. Multiple versus single doses of exogenous surfactant for the prevention or treatment of neonatal respiratory distress syndrome. Cochrane Database Syst Rev. 2009;21:CD000141.
Been JV, Rours IG, Kornelisse RF, Jonkers F, de Krijger RR, Zimmermann LJ. Chorioamnionitis alters the response to surfactant in preterm infants. J Pediatr. 2010;156:10–15.e1.
Horbar JD, Soll RF, Sutherland JM, Kotagal U, Philip AG, Kessler DL, et al. A multicenter randomized, placebo-controlled trial of surfactant therapy for respiratory distress syndrome. N Engl J Med. 1989;320:959–65.
Singh N, Halliday HL, Stevens TP, Suresh G, Soll R, Rojas-Reyes MX. Comparison of animal-derived surfactants for the prevention and treatment of respiratory distress syndrome in preterm infants. Cochrane Database Syst Rev. 2015;12:CD010249.
Rojas-Reyes MX, Morley CJ, Soll R. Prophylactic versus selective use of surfactant in preventing morbidity and mortality in preterm infants. Cochrane Database Syst Rev. 2012;3:CD000510.
Bahadue FL, Soll R. Early versus delayed selective surfactant treatment for neonatal respiratory distress syndrome. Cochrane Database Syst Rev. 2012;11:CD001456.
Subramaniam P, Ho JJ, Davis PG. Prophylactic nasal continuous positive airway pressure for preventing morbidity and mortality in very preterm infants. Cochrane Database Syst Rev. 2016;6:CD001243.
Stevens TP, Harrington EW, Blennow M, Soll RF. Early surfactant administration with brief ventilation vs. selective surfactant and continued mechanical ventilation for preterm infants with or at risk for respiratory distress syndrome. Cochrane Database Syst Rev. 2007;4:CD003063.
More K, Sakhuja P, Shah PS. Minimally invasive surfactant administration in preterm infants: a meta-narrative review. JAMA Pediatr. 2014;168:901–8.
Speer CP, Robertson B, Curstedt T, Halliday HL, Compagnone D, Gefeller O, et al. Randomized European multicenter trial of surfactant replacement therapy for severe neonatal respiratory distress syndrome: single versus multiple doses of Curosurf. Pediatrics. 1992;89:13–20.
Katz LA, Klein JM. Repeat surfactant therapy for postsurfactant slump. J Perinatol. 2006;26:414–22.
Herting E, Gefeller O, Land M, Sonderen LV, Harms K, Robertson B, et al. Surfactant treatment of neonates with group B streptococcal infection. Pediatrics. 2000;106:957–64.
Kattwinkel J, Bloom BT, Delmore P, Glick C, Brown D, Lopez S, et al. High-versus low-threshold surfactant retreatment for neonatal respiratory distress syndrome. Pediatrics. 2000;106:282–8.
Tsakaldis C, Giougki E, Karagianni P, Dokos C, Rallis D, Nikolaidis N. Is there a necessity for multiple doses of surfactant for respiratory distress syndrome of premature infants? Turk J Pediatr. 2015;54:368–75.
Hoekstra RE, Jackson JC, Myers TF, Frantz ID 3rd, Stern ME, Powers WF, et al. Improved neonatal survival following multiple doses of bovine surfactant in very premature neonates at risk for respiratory distress syndrome. Pediatrics. 1991;88:10–18.
Dunn MS, Shennan AT, Possmayer F. Single- versus multiple-dose surfactant replacement therapy in neonates of 30 to 36 weeks’ gestation with respiratory distress syndrome. Pediatrics. 1990;84:564–71.
Lee SK, Beltempo M, McMillan DD, Seshia M, Singhal N, Dow K, et al. Outcomes and care practices for preterm infants born at less than 33 weeks’ gestation: a quality-improvement study. CMAJ. 2020;192:E81–91.
The Canadian Neonatal Network. The Canadian Neonatal Network Abstractor’s Manual v.3.4.1. The Canadian Neonatal Network; 2019. http://www.canadianneonatalnetwork.org/portal/CNNHome/Publications.aspx.
Shah PS, Seidlitz W, Chan P, Yeh S, Musrap N, Lee SK, et al. Internal audit of the Canadian Neonatal Network data collection system. Am J Perinatol. 2017;34:1241–9.
Davis DJ, Barrington KJ. Recommendations for neonatal surfactant therapy. Pediatr Child Health. 2005;10:109–16.
Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500g. J Pediatr. 1978;92:529–34.
Lemyre B, Fusch C, Schmölzer GM, Bouali RN, Reddy D, Barrowman N, et al. Poractant alfa versus bovine lipid extract surfactant for infants 24+0 to 31+6 weeks gestational age: A randomized controlled trial. PLoS ONE. 2017;12:e0175922.
Beltempo M, Isayama T, Vento M, Lui K, Kusuda S, Lehtonen L, et al. Respiratory management of extremely preterm infants: an international survey. Neonatology. 2018;114:28–36.
Bel FV, Vaes J, Groenedaal F. Prevention, reduction and repair of brain injury of the preterm infant. Front Physiol. 2019;10:181.
Corbet A, Gerdes J, Long W, Avila E, Puri A, Rosenberg A, et al. Double-blind, randomized trial of one versus three prophylactic doses of synthetic surfactant in 826 neonates weighing 700 to 1100 grams: effects on mortality rate. American Exosurf Neonatal Study Groups I and IIa. J Pediatr. 1995;126:969–78.
Acknowledgements
The authors thank all site investigators and data abstractors of the CNN and the Canadian Preterm Birth Network (CPTBN). A full list of Network member investigators and their affiliations appears in Supplementary Information. We thank Heather McDonald Kinkaid, PhD, of the Maternal-infant Care Research Centre (MiCare) at Mount Sinai Hospital in Toronto, ON, Canada, for editorial support in preparing this manuscript; and other MiCare staff, for organizational support.
Funding
This study was supported by a grant from the Canadian Institutes of Health Research (CIHR) funding the Canadian Preterm Birth Network (PBN 150642). Organizational support for the CNN and the Canadian Preterm Birth Network was provided by the Maternal-infant Care Research Centre (MiCare) at Mount Sinai Hospital in Toronto, ON, Canada. MiCare is supported by a CIHR Team Grant (CTP 87518), the Ontario Ministry of Health and Long-Term Care, and the participating hospitals. PSS holds a CIHR Applied Research Chair in Reproductive and Child Health Services and Policy Research (APR-126340).
Author information
Authors and Affiliations
Contributions
HC and PSS conceptualized and designed the study, drafted the initial manuscript, and reviewed and revised the manuscript. AM, BL, EHN, RA, GE, and MB participated in study design and data interpretation and reviewed and revised the manuscript. EWY analyzed the data and reviewed and revised the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work in ensuring that questions related to its accuracy or integrity are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Coshal, H., Mukerji, A., Lemyre, B. et al. Characteristics and outcomes of preterm neonates according to number of doses of surfactant received. J Perinatol 41, 39–46 (2021). https://doi.org/10.1038/s41372-020-00779-9
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41372-020-00779-9
This article is cited by
-
Elective high frequency oscillatory ventilation versus conventional mechanical ventilation on the chronic lung disease or death in preterm infants administered surfactant: a systematic review and meta-analysis
Journal of Perinatology (2025)
-
Clinical predictors for surfactant retreatment in preterm infants with respiratory distress syndrome: the results of a pooled analysis
Italian Journal of Pediatrics (2025)
-
Risk factors for the time to development of retinopathy of prematurity in premature infants in Iran: a machine learning approach
BMC Ophthalmology (2024)
-
Complete blood count parameters as biomarkers of retinopathy of prematurity: a Portuguese multicenter study
Graefe's Archive for Clinical and Experimental Ophthalmology (2023)
-
Poractant alfa versus bovine lipid extract surfactant: prospective comparative effectiveness study
Journal of Perinatology (2022)