Abstract
The perinatal period is associated with high antibiotic exposure, which raises concerns about antimicrobial resistance (AMR) and future health impacts. The aim of this comprehensive systematic review, including publications from 2000 to 2022, is to describe the current evidence and state of antimicrobial stewardship (AMS) in the perinatal period and to identify gaps in knowledge for future research. The review included 36 studies from the Americas, Europe, Asia and Australia, involving a total of 64,798 pregnant women and 84,137 newborns. 33 out of 36 studies reported reduced antibiotic use, suggesting the potential to reduce antibiotic exposure. There is a lack of studies in the antepartum and intrapartum periods, of comprehensive AMS strategies across the entire perinatal period, and from low- and middle-income countries with a high burden of maternal and neonatal morbidity and mortality. Future research should include prospective, adequately powered studies including safety endpoints, clinical outcomes and AMR reports.
Similar content being viewed by others
Introduction
According to the World Health Organization, the perinatal period is defined as the period between the completed 22nd week of pregnancy and the first seven days after birth. Within the perinatal period, three distinct phases with specific health care priorities can be defined: antepartum, intrapartum and postpartum phase. An optimal start at the beginning of life has a significant impact on a person’s health and well-being [1, 2]. Use of antibiotics in the perinatal period is high with potential impact on antimicrobial resistance (AMR) and future health [3, 4]. AMR is one of the main challenges of medicine with currently more than 1.2 million deaths annually directly related [5, 6]. The perturbation of the development of the non-resilient microbiome in early life plays a key role for future health [7, 8]. Exposure to antibiotics in the perinatal period was reported to be associated with asthma, allergies, atopic dermatitis, obesity, celiac disease, diabetes and other immune disorders later in life [4, 8,9,10,11]. In addition, late onset sepsis and necrotizing enterocolitis (NEC) were reported as short-term adverse outcomes in preterm infants treated with prolonged exposure to antibiotics [10, 12,13,14]. Late onset sepsis and necrotizing enterocolitis are associated with impaired neurological long-term outcomes [15, 16].
Antibiotics are among the most frequently prescribed medications during pregnancy and it is estimated that, in approximately 40% of all pregnancies antibiotic treatments are used [4, 17,18,19,20]. The reasons for antibiotic treatment are variable and range from urogenital infections, suspected chorioamnionitis to the prophylactic therapy in cases of Group B streptococci (GBS) carriage [4]. Suspected chorioamnionitis, GBS prevention, prophylactic therapy in case of premature rupture of membranes (PROM) and prophylactic treatment in case of a caesarean section are the main reasons for intrapartum antibiotic administration affecting around two out of three pregnancies [21]. Within the first week of life, fear of early onset sepsis (EOS) is a key driver of antibiotic use [3]. But, the diagnosis of neonatal sepsis is challenging and there is still no accepted standardized definition [9, 21,22,23]. In case of true EOS, early start of antibiotic therapy is mandatory for optimal outcomes [9, 12, 13, 24,25,26,27,28]. The lack of predictive precision in current diagnostic tools and the need to start treatment early in cases of EOS lead to overtreatment: Up to 15% of all newborns and more than 75% of premature infants with a birth weight below 1500 g receive empirical antibiotics for suspected sepsis. In a recently published study comparing the burden of earl-onset sepsis versus the burden of antibiotic treatment, for one case of culture-proven sepsis, 58 newborns received antibiotics, and 273 antibiotic days were administered [29]. This, together with large variations between hospitals and countries, indicates that there is an enormous potential to safely reduce antibiotic exposure at the beginning of life and thereby reduce the threat of antimicrobial resistance and perturbations of the microbiome [3, 26, 29,30,31,32]. We hypothesize that there exists a knowledge gap regarding the efficiency of AMS interventions with lack of consideration of the perinatal period as a whole. We assume that most of the existing studies are of insufficient quality or inadequate size to prove safety of an approach. The aim of this review is to describe the current evidence and state of AMS during the perinatal period and to highlight knowledge gaps for future research.
Methods
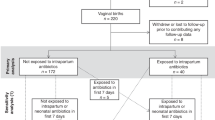
For this systematic review, we followed the requirements of the PRISMA checklist for data collection, analysis and reporting (amendment 1). The following four search strings were developed to search for suitable literature in the PubMed database between September 2022 and January 2023: “pregnancy AND antimicrobial stewardship”, “delivery AND antimicrobial stewardship”, “early-onset sepsis AND antimicrobial stewardship” and “perinatal AND antimicrobial stewardship”. The following filters were applied: Literature from 2000 to December 2022, English and German language, available fulltext or abstract. All article types not corresponding to a clinical study (for example reviews, perspectives, comments) were excluded. In a second step, articles which were retrieved from reference lists or were recommended by experts were added. Study selection was done by two authors independently (CW and MS). The flowchart in Fig. 1 provides an overview of the entire selection process.
For every selected study, the following seven characteristics were extracted, organized and summarized in a table (Table 1): Publication year and location, study design, bias assessment, number of participants, AMS intervention, outcome, and safety endpoints/adverse events. The publication year and location were chosen to observe a potential trend in AMS research activity within the last two decades and to describe the most active regions internationally. Within the study method we categorized all prospective randomized controlled trials or prospective quality improvement studies as high-quality, and all retrospective descriptive studies as low-quality study designs. The bias assessment was used to further describe the quality of the study and was done in the form of a separate risk of bias assessment table. Individual AMS interventions were described and, where possible, categorized according to the WHO definitions of AMS interventions (clinician education, patient and public education, institution-specific guidelines, cumulative antibiograms, prior authorization for restricted antimicrobials, de-labeling of spurious antibiotic allergies, prospective audit and feedback, antibiotic timeouts, antibiotic dose optimization, antibiotic duration), while AMS programs were summarized as multifaceted interventions. Interventions regarding diagnostics of suspected infections or empiric start of antibiotics were categorized into guidelines [33]. To analyze the results, we decided to represent the studies regarding their number of participants in two categories: Less than 1000 participants and more than 1000 participants. The threshold of 1000 was chosen pragmatically: Proven infections in the perinatal period are rare and therefore the studied population needs to be sufficient large to get potential generalizable results. The outcome of all studies was analyzed regarding the effect on antibiotic use. To assess safety, the studies were analyzed for the presence of an appropriate powered safety parameter and the incidence of adverse events, mortality or primarily missed sepsis cases with a delayed start of antibiotics. If any of the information were not available, we marked the variable as unknown.
All studies were sorted according to the time of their intervention in the perinatal period: Antepartum (prenatal), intrapartum (delivery), and postpartum (postnatal). Antepartum was defined after the completed 22nd week of gestation, intrapartum including all studies describing antibiotic use for delivery (including prophylactic antibiotics for GBS carriage, ROM and caesarean section), and postpartum including all studies within the first week of life. If a study included interventions in more than one phase, the study was categorized to the first phase.
Results
We identified 36 studies within the literature research according to the defined criteria (Fig. 1, Table 1). An overview of the most important results is available in Fig. 2. One study was published before 2010 and 34 out of the 36 studies (94%) were published between 2015 and 2022. 16 out of 36 studies originated from Europe, 15 from America and five from Asia and Australia. The study design was retrospective in 22 out of 36 studies (61%). The bias assessment showed in all 14 prospective studies at least one additional bias (Fig. 3). In total, 64’798 pregnant women and 84’137 neonates participated in all studies, whereas the exact number of participants were not clearly stated in three out of 36 studies. 17 of the studies included less than 1000 participants (47%) [34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50]. A total of 15 different interventions were presented across 5 AMS categories: guidelines (n = 20), antibiotic duration (n = 6), feedback and audit (n = 1), clinician education (n = 1) and multifaceted interventions (n = 8) aimed at reducing the use of antibiotics during pregnancy and the first week of life. The outcome showed a reduction in antibiotic use in 33 out of 36 studies (92%). Nine of the studies were powered for a safety outcome [37, 40, 50,51,52,53,54,55,56]. No increase in adverse events was observed in 29 out of 36 studies, while two studies did not report on adverse events or safety. 35 out of 36 studies included interventions in one phase, one in two phases and none in the complete perinatal period.
We identified three out of 36 studies (8%) in the antepartum phase [51, 56, 57]. The largest study in the antepartum phase was a randomized controlled trial investigating use of antibiotics in pregnancies with preterm prenatal rupture of membrane. The study showed a significant reduction (−16.5%) of a compound neonatal outcome (neonatal death, chronic lung disease, cerebral impairment) when erythromycin was given compared to placebo [51]. The use of co-amoxicillin (amoxicillin with clavulanic acid) was associated with an increased rate of neonatal NEC. The other prospective study focused on single dose surgical antimicrobial prophylaxis (SAP) in low-risk patients for elective surgeries during pregnancy and for caesarean section [56]. The result was a significant increase of single dose SAP-rate from 2% to over 60% within 6 months, maintained at 80–90% for more than two years with no increase in surgical site infection rate. The retrospective study reported about five rules regarding general prescriptions of antibiotics in pregnancy and showed a significant reduction in the use of antibiotics [57].
In the intrapartum phase, we identified five out of 36 studies (14%) [34,35,36, 52, 53]. Two studies focused on surgical antibiotic prophylaxis for cesarean section [52, 53]. One of them analyzed the difference between antibiotic use versus placebo [52], the other the time of application of antibiotic prophylaxis [53]. The study analyzing surgical antibiotic prophylaxis versus placebo showed a lower rate of infectious morbidity in the prophylaxis group [52], while an unchanged risk of surgical site infection was reported when antibiotic prophylaxis was given after cord clamping rather than before incision [53]. A third study monitored the effect of a new guideline restricting antibiotic prophylaxis in uncomplicated births and reported a 75% reduction in antibiotic use [34]. The last two studies looked at the impact of intrapartum polymerase chain reaction testing in GBS-positive mothers and reported a reduction in the need for intrapartum prophylaxis (IAP) by up to two-thirds [35, 36]. No adverse events were observed in four of the five studies, whereas 13 out of 913 infants in the study restricting antibiotic prophylaxis for uncomplicated birth developed sepsis within three days [34,35,36, 52, 53].
In the postpartum phase, we identified 28 out of 36 studies (78%). With eight out of 28 studies, the Kaiser Permanente Sepsis Calculator is the most analyzed single intervention [37,38,39,40,41,42, 58, 59]. Seven out of the eight studies were done in a retrospective design. The prospective study analyzing the Kaiser Permanente Sepsis Calculator was a before-after setting with a historical control group [39]. Multifaceted interventions, a combination of different interventions that were implemented at the same time or consecutively, were analyzed in eight studies [43,44,45,46, 60,61,62,63]. Most of these studies were quality improvement studies and five out of the eight studies had a retrospective design. An automatic stop-order was analyzed in three studies [47, 64, 65]. Two of the three studies were retrospective. The prospective study was an observational study with over 2’500 neonates included. In all three studies, antibiotic prescriptions were stopped automatically after 48 h of treatment. Serial physical examinations were analyzed in three studies [48, 66, 67]. Two of the three studies were retrospective. In all three studies, clinically healthy neonates with risk factors for EOS were observed for 48 h without antibiotic treatment. Additional two studies analyzed a biomarker-guided approach [54, 55]. Both studies had a prospective design with more than 1000 participants. One study analyzed the effect of c-reactive protein-guidance 18 h after start of antibiotic therapy, the other study used a procalcitonin-guided algorithm to shorten antibiotic treatment. The remaining four studies analyzed four different interventions based on placental analysis, leadership style for empowerment, infectious disease rounds and stratification of risk factors [13, 49, 50, 68]. All four studies were done in a retrospective design. In 27 out of the 28 studies in the postpartum phase, the outcome showed a reduction of antibiotic use, measured by various endpoints [13, 34,35,36,37,38,39,40,41, 43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68]. In one study analyzing the Kaiser Permanente Sepsis Calculator, the number of newborns identified for antibiotic therapy increased [42]. Regarding safety, five of the included studies were powered for a safety outcome showing different results [37, 40, 50, 54, 55]. No adverse events were observed in 22 out of the 28 studies, one study did not report about adverse events or safety [59]. In three studies, at least one EOS case was missed during the intervention phase [37, 42, 54]. An increased rate of delayed antibiotic treatment was observed in two studies [41, 45]. One study showed an increase in case fatalities, a re-analysis could not show any association of this result with a delayed or insufficient antibiotic therapy [44].
Discussion
Within the last decade, literature regarding AMS in the perinatal period increased remarkably. The increase of publication is mainly focused on the postpartum phase. Overall, a reduction of antibiotic use in the perinatal period is possible: In 33 out of the 36 studies analyzed, the introduction of an AMS intervention reduced exposure to antibiotics in the defined study population. On the other hand, this review shows a lack of studies in the ante- and intrapartum phase and a complete gap of AMS analysis including the whole perinatal period. Around two thirds of the studies were done in a retrospective and therefore low-quality design. In addition, most of the studies were not powered to assess the safety of the intervention.
Around half of the studies analyzed were published between 2014 and 2019 and their number doubled from 2020 to 2022, underlining a strong trend. First, the reason for this trend may be grounded in the call of international organizations as the World Health Organization to take global action on AMR to improve antibiotic treatment by increased surveillance and research [5]. Second, there is increasing evidence that unnecessary antibiotics in the perinatal period has an impact on the individual microbiome with potential impact for future health [3, 7]. And third, there is evidence that antibiotic exposure for only 48 h within the first week of life has major effects on the microbiome and AMR gene selection and that these changes are still relevant one year later [8]. The distribution of pathogens causing EOS and resistance patterns have changed over time and increased morbidity and mortality due to AMR is a major concern, particularly in middle- and low-income countries [5, 6]. Therefore, the lack of studies from Africa and Asia reported in this review is a worrying gap in the current knowledge and needs to be addressed in future AMS programs.
When analyzing the study’s design, it is noticeable that around two thirds of the 36 studies included were conducted in a retrospective design. This limits the significance of the results. In addition, around half of the studies had a sample size below 1000 participants. Whereas, a small sample size does not automatically mean low quality, an AMS study reducing antibiotic prescriptions must show a safety endpoint. The inclusion of safety parameters is missing in around a quarter of the studies analyzed, which represents an obstacle to safely introduce the interventions. A non-inferiority analysis reporting missed sepsis cases, delayed antibiotic initiation in culture-proven sepsis, antibiotic restarts due to recurrent infections, and morbidity and mortality are important safety parameters. Culture-proven bacterial infections in the perinatal period are overall relatively rare and power calculations for non-inferiority usually results in a high number of participants. As an example, the prospective, multicenter randomised controlled intervention trial NeoPInS analyzing a procalcitonin-guided algorithm to safely shorten antibiotic therapy in a cohort of more than 1’700 neonates with suspected EOS reported a highly significant result for superiority (reduction of antibiotics), but failed to prove non-inferiority [55]. Nevertheless, this trial together with some other prospective randomized trials published in high-stakes medical journals show the feasibility of large, prospective AMS studies.
Interestingly, 33 out of the 36 included studies reported a reduction of antibiotic prescriptions in the analyzed population. Whereas, we must consider a possible publication bias, it demonstrates that a reduction of antibiotic exposure in this vulnerable phase is feasible. An additional conclusion from this study is that there is overtreatment. Overall, 15 different interventions were used within the 36 analyzed studies. The Kaiser Permanente Sepsis Calculator and multifaceted AMS interventions as quality improvement programs were the most often studied strategies. Because of the large variety of interventions and study designs, it is not possible to conduct a meta-analysis comparing the effectiveness of different interventions.
This review shows important knowledge gaps regarding AMS within pregnancy and deliveries within the last two decades. Only three studies were included in the antepartum phase. One published in 2001 and two in 2022, hopefully indicating a start to fill this gap. This is urgently needed due to the estimation that around 40% of pregnancies are exposed to antibiotics [4]. Assessment tools such as the quick Sequential Organ Failure Assessment score (qSOFA) may help overcome some of the barriers to decision-making about antibiotic prescribing for pregnant women, but high-quality studies are lacking. Future studies are urgently needed to answer the main question about AMS in pregnancy: Which algorithm helps to diagnose bacterial infections in pregnancy with high accuracy and reduce unnecessary empirical antibiotic therapy? On the other hand, ethical concerns for clinical studies in pregnancies potentially increasing the risk to the pregnant women and the unborn child may be a reason for the low number of studies. Within the intrapartum phase, antibiotic prophylaxis for GBS was focused in clinical studies before 2000. The rate of neonatal EOS declined markedly within the last two decades [29, 69]. The strategy of prophylactic antibiotics for GBS positive pregnancies before delivery is probably responsible for a part of the decline, whereas the optimal strategy in the current area remains unknown: While there is probably no safe way to reduce overall prophylactic antibiotic exposure in GBS-positive pregnancies, the question remains, is there a way to safely reduce antibiotic prophylaxis in specific situations? What are the conditions necessary to safely administer surgical antibiotic prophylaxis for caesarean section after cord clamping rather than before incision? To answer these questions, we need to know exactly what effect a single dose of intrapartum antibiotics has on the developing neonatal microbiome and clinical outcomes. And does this effect depend on the type of antibiotic administered? In addition, the pathogen spectrum is changing over time and the resistance rates are increasing, reinforcing the need for new studies, particularly in low- and middle-income countries with a high burden of maternal and neonatal morbidity and mortality due to AMR. Last, it is striking that no study includes the perinatal period as a whole. Whereas the development and rise of perinatal centers internationally indicates an increased understanding of the importance of taking a holistic view of the perinatal period for clinical work, this needs to be further developed in clinical research. Antibiotic exposure during the whole perinatal period may have an impact on the neonate.
The main limitations restricting comparability and conclusions of this review are the low availability of high-quality studies and the large heterogeneity of study designs. Additionally, despite a thorough search of the PubMed database, possible relevant studies in other sources of biomedical and life science literature were not included. And third, because of the definition of the postpartum phase, antibiotic exposure and AMS opportunities in the neonatal intensive care unit beyond the first week of life are not covered. Nevertheless, various knowledge gaps and starting points for future research can be identified based on these findings (Table 2). First, the consideration of the perinatal period as a whole is key to support close communication of all involved clinical disciplines to plan and conduct clinical research improving AMS. Linked medical databases of the mother and the newborn facilitate to analyze the current state and to coordinate future research activities. Second, there is a need for prospective and adequately powered trials with clinical and safety endpoints in all three phases of the perinatal period. This need is most urgent in the antenatal and intrapartum periods. More studies are being published on AMS in the first week of life, but the heterogeneity of the interventions analyzed is high and safety or clinical outcomes are often not reported. In addition to AMR, clinical health outcomes in later life, such as asthma, allergies, atopic dermatitis, obesity, celiac disease, diabetes and other immune disorders, may demonstrate the burden of antibiotic therapy [4, 7,8,9,10,11]. Third, AMS interventions need to be tailored to the local context for implementation. There is most probably not one intervention fitting all context and different interventions need to be tested in various conditions. Therefore, future research must include more ethnically, racially and culturally diverse populations from low- and middle-income countries to reduce the high burden of maternal and neonatal morbidity and mortality. On the other hand, promising techniques for early detection of pathogens and AMR, such as nucleic acid amplification technologies (NAAT) and multiplex polymerase chain reaction (mPCR) need to be further tested in algorithms in high-income settings [35, 36]. For example, the incorporation of mPCR into algorithm-based approaches to electronical clinical records may help to support balanced decision-making on antibiotic therapy in the future [70, 71]. The development of a toolbox of various interventions for different situations may help for further dissemination and implementation of AMS. In the end, the implementation of AMS interventions is always a change process. Health care workers need to have a sense of urgency for AMS before adapting and changing their behavior. Therefore, the increase of the AMR challenge worldwide and the impact of antibiotic therapy on the child’s microbiome with potential impact of their future health are the cornerstones of every AMS program. Knowledge, communication, and education of all involved healthcare workers in the perinatal period are key to redirect the current increasing trends for AMR and chronic health conditions in the worldwide population.
Conclusion
In recent years, published studies regarding AMS in the perinatal period increased remarkably reporting the feasibility and possibility to reduce antibiotic therapy in this vulnerable phase. There is a lack of studies in the ante- and intrapartum phase and a complete gap of AMS analysis including the whole perinatal period. Many of the studies were done in a low-quality design or were not powered to assess the safety of the intervention.
Data availability
All the included studies are accessible via Pubmed.
References
World Health Organization. World Health Organization. 2024. Perinatal conditions. https://platform.who.int/mortality/themes/theme-details/topics/topic-details/MDB/perinatal-conditions.
World Health Organization. World Health Organization. 2024. Maternal and newborn health. https://www.who.int/europe/health-topics/maternal-health#tab=tab_1.
Stocker M, Klingenberg C, Navér L, Nordberg V, Berardi A, El Helou S, et al. Less is more: Antibiotics at the beginning of life. Nat Commun. 2023;14:2423.
Martinez de Tejada B. Antibiotic use and misuse during pregnancy and delivery: benefits and risks. Int J Environ Res Public Health. 2014;11:7993–8009.
World Health Assembly 69. Global action plan on antimicrobial resistance: options for establishing a global development and stewardship framework to support the development, control, distribution and appropriate use of new antimicrobial medicines, diagnostic tools, vaccines and other interventions: report by the Secretariat. Geneva: World Health Organization, 2016.
Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399:629–55.
Stiemsma LT, Michels KB. The role of the microbiome in the developmental origins of health and disease. Pediatrics. 2018;141:e20172437.
Reyman M, van Houten MA, Watson RL, Chu MLJN, Arp K, de Waal WJ, et al. Effects of early-life antibiotics on the developing infant gut microbiome and resistome: a randomized trial. Nat Commun. 2022;13:893.
Klingenberg C, Kornelisse RF, Buonocore G, Maier RF, Stocker M. Culture-negative early-onset neonatal sepsis - at the crossroad between efficient sepsis care and antimicrobial stewardship. Front Pediatr. 2018;6:285.
Achten NB, Klingenberg C, Benitz WE, Stocker M, Schlapbach LJ, Giannoni E, et al. Association of use of the neonatal early-onset sepsis calculator with reduction in antibiotic therapy and safety: a systematic review and meta-analysis. JAMA Pediatr. 2019;173:1032–40.
Fjalstad JW, Esaiassen E, Juvet LK, van den Anker JN, Klingenberg C. Antibiotic therapy in neonates and impact on gut microbiota and antibiotic resistance development: a systematic review. J Antimicrob Chemother. 2018;73:569–80.
Prusakov P, Goff DA, Wozniak PS, Cassim A, Scipion CEA, Urzúa S, et al. A global point prevalence survey of antimicrobial use in neonatal intensive care units: The no-more-antibiotics and resistance (NO-MAS-R) study. EClinicalMed. 2021;32:100727.
Steinmann KE, Lehnick D, Buettcher M, Schwendener-Scholl K, Daetwyler K, Fontana M, et al. Impact of empowering leadership on antimicrobial stewardship: a single center study in a neonatal and pediatric intensive care unit and a literature review. Front Pediatr. 2018;6:294.
Araujo da Silva AR, Marques A, Di Biase C, Faitanin M, Murni I, Dramowski A, et al. Effectiveness of antimicrobial stewardship programmes in neonatology: a systematic review. Arch Dis Child. 2020;105:563–8.
Adams-Chapman I. Necrotizing enterocolitis and neurodevelopmental outcome. Clin Perinatol. 2018;45:453–66.
Schlapbach LJ, Aebischer M, Adams M, Natalucci G, Bonhoeffer J, Latzin P, et al. Impact of sepsis on neurodevelopmental outcome in a swiss national cohort of extremely premature infants. Pediatrics. 2011;128:e348–57.
Broe A, Pottegård A, Lamont R, Jørgensen J, Damkier P. Increasing use of antibiotics in pregnancy during the period 2000–2010: prevalence, timing, category, and demographics. BJOG: Int J ournal Obstet Gynaecol. 2014;121:988–96.
Schilling AL, Rody A, Bossung V. Antibiotic use during pregnancy and childbirth: prospective observational study on prevalence, indications, and prescribing patterns in a german tertiary center. Geburtshilfe Frauenheilkd. 2023;83:192–200.
Ledger W, Blaser M. Are we using too many antibiotics during pregnancy? BJOG. 2013;120:1450–2.
Tran A, Zureik M, Sibiude J, Drouin J, Miranda S, Weill A, et al. Prevalence and associated factors of antibiotic exposure during pregnancy in a large French population-based study during the 2010–19 period. J Antimicrobial Chemother. 2023;78:2535–43.
Stoll BJ, Puopolo KM, Hansen NI, Sánchez PJ, Bell EF, Carlo WA, et al. Early-Onset Neonatal Sepsis 2015 to 2017, the Rise of Escherichia coli, and the Need for Novel Prevention Strategies. JAMA Pediatr. 2020;174(Jul):e200593.
Braye K, Foureur M, de Waal K, Jones M, Putt E, Ferguson J. Epidemiology of neonatal early-onset sepsis in a geographically diverse Australian health district 2006-2016. PLoS One. 2019;14:e0214298.
Martín V, Cárdenas N, Ocaña S, Marín M, Arroyo R, Beltrán D, et al. Rectal and Vaginal Eradication of Streptococcus agalactiae (GBS) in Pregnant Women by Using Lactobacillus salivarius CECT 9145, A Target-specific Probiotic Strain. Nutrients. 2019;11(Apr):810.
Zihlmann-Ji J, Braun C, Buettcher M, Hodel M, Lehnick D, Stocker M. Reduction of duration of antibiotic therapy for suspected early-onset sepsis in late-preterm and term newborns after implementation of a procalcitonin-guided algorithm: a population-based study in central Switzerland. Front Pediatr. 2021;9:702133.
Duvoisin G, Fischer C, Maucort-Boulch D, Giannoni E. Reduction in the use of diagnostic tests in infants with risk factors for early-onset neonatal sepsis does not delay antibiotic treatment. Swiss Med Wkly. 2014;144:w13981.
Mundal HS, Rønnestad A, Klingenberg C, Stensvold HJ, Størdal K. Norwegian neonatal network. antibiotic use in term and near-term newborns. Pediatrics. 2021;148:e2021051339.
Stocker M, Daunhawer I, van Herk W, El Helou S, Dutta S, Schuerman FABA, et al. Machine learning used to compare the diagnostic accuracy of risk factors, clinical signs and biomarkers and to develop a new prediction model for neonatal early-onset sepsis. Pediatr Infect Dis J. 2022;41:248–54.
van Herk W, el Helou S, Janota J, Hagmann C, Klingenberg C, Staub E, et al. Variation in current management of term and late-preterm neonates at risk for early-onset sepsis: an international survey and review of guidelines. Pediatr Infect Dis J. 2016;35:494–500.
Giannoni E, Dimopoulou V, Klingenberg C, Navér L, Nordberg V, Berardi A, et al. Analysis of antibiotic exposure and early-onset neonatal sepsis in Europe, North America, and Australia. JAMA Netw Open. 2022;5:e2243691.
Vatne A, Hapnes N, Stensvold HJ, Dalen I, Guthe HJ, Støen R, et al. Early empirical antibiotics and adverse clinical outcomes in infants born very preterm: a population-based cohort. J Pediatrics. 2023;253:107–114.e5.
Huncikova Z, Vatne A, Stensvold HJ, Lang AM, Støen R, Brigtsen AK, et al. Late-onset sepsis in very preterm infants in Norway in 2009–2018: a population-based study. Arch Dis Child Fetal Neonatal Ed. 2023;108:478–84.
Litz JE, Goedicke-Fritz S, Härtel C, Zemlin M, Simon A. Management of early- and late-onset sepsis: results from a survey in 80 German NICUs. Infection. 2019;47:557–64.
Antimicrobial stewardship interventions: a practical guide. 2024. https://www.who.int/europe/publications/i/item/9789289056267.
Sharma S, Kumari N, Sengupta R, Malhotra Y, Bhartia S. Rationalising antibiotic use after low-risk vaginal deliveries in a hospital setting in India. BMJ Open Qual. 2021;10:e001413.
Fullston EF, Doyle MJ, Higgins MF, Knowles SJ. Clinical impact of rapid polymerase chain reaction (PCR) test for group B Streptococcus (GBS) in term women with ruptured membranes. Ir J Med Sci. 2019;188:1269–74.
Hartvigsen CM, Nielsen SY, Møller JK, Khalil MR. Reduction of intrapartum antibiotic prophylaxis by combining risk factor assessment with a rapid bedside intrapartum polymerase chain reaction testing for group B streptococci. Eur J Obstet Gynecol Reprod Biol. 2022;272:173–6.
Money N, Newman J, Demissie S, Roth P, Blau J. Anti-microbial stewardship: antibiotic use in well-appearing term neonates born to mothers with chorioamnionitis. J Perinatol. 2017;37:1304–9.
Warren S, Garcia M, Hankins C. Impact of neonatal early-onset sepsis calculator on antibiotic use within two tertiary healthcare centers. J Perinatol. 2017;37:394–7.
Achten NB, Dorigo-Zetsma JW, van der Linden PD, van Brakel M, Plötz FB. Sepsis calculator implementation reduces empiric antibiotics for suspected early-onset sepsis. Eur J Pediatr. 2018;177:741–6.
Eason J, Ward H, Danko O, Richardson K, Vaitkute R, McKeon-Carter R. Early-onset sepsis: can we screen fewer babies safely? Arch Dis Child. 2021;106:86–8.
Morris R, Jones S, Banerjee S, Collinson A, Hagan H, Walsh H, et al. Comparison of the management recommendations of the Kaiser Permanente neonatal early-onset sepsis risk calculator (SRC) with NICE guideline CG149 in infants ≥34 weeks’ gestation who developed early-onset sepsis. Arch Dis Child Fetal Neonatal Ed. 2020;105:581–6.
Laccetta G, Ciantelli M, Tuoni C, Sigali E, Miccoli M, Cuttano A. Early-onset sepsis risk calculator: a review of its effectiveness and comparative study with our evidence-based local guidelines. Ital J Pediatr. 2021 ;47:73.
Arora V, Strunk D, Furqan SH, Schweig L, Lefaiver C, George J, et al. Optimizing antibiotic use for early onset sepsis: A tertiary NICU experience. J Neonatal Perinat Med. 2019;12:301–12.
Berardi A, Zinani I, Rossi C, Spaggiari E, D’Amico V, Toni G, et al. Antibiotic use in very low birth weight neonates after an antimicrobial stewardship program. Antibiotics (Basel). 2021;10:411.
Stritzke A, Tierney A, Keister F, Srivastava A, Dersch-Mills D, Hamilton C, et al. Antimicrobial stewardship at birth in preterm infants: not just about a decrease! Pediatr Infect Dis J. 2022;41:394–400.
Graus JM, Herbozo C, Hernandez R, Pantoja AF, Zegarra J. Managing antibiotics wisely in a neonatal intensive care unit in a low resource setting. J Perinatol. 2022;42:965–70.
Tolia VN, Desai S, Qin H, Rayburn PD, Poon G, Murthy K, et al. Implementation of an automatic stop order and initial antibiotic exposure in very low birth weight infants. Am J Perinatol. 2017;34:105–10.
Schmitt C, Novy M, Hascoët JM. Term newborns at risk for early-onset neonatal sepsis: Clinical surveillance versus systematic paraclinical test. Arch Pediatr. 2021;28:117–22.
Ykema JMA, D’Haens EJ, Havenith M, van Eyck J, van Lingen RA, Hemels MAC. Pilot study demonstrates that placental histology can provide an additional tool for diagnosing early-onset neonatal sepsis. Acta Paediatr. 2018;107:2086–91.
Capin I, Hinds A, Vomero B, Roth P, Blau J. Are early-onset sepsis evaluations and empiric antibiotics mandatory for all neonates admitted with respiratory distress? Am J Perinatol. 2022;39:444–8.
Kenyon SL, Taylor DJ, Tarnow-Mordi W Broad-spectrum antibiotics for preterm, prelabour rupture of fetal membranes: the ORACLE I randomised trial. THE LANCET. 2001;357.
Witt A. Antibiotic prophylaxis before surgery vs after cord clamping in elective cesarean delivery: a double-blind, prospective, randomized, placebo-controlled trial. Arch Surg. 2011;146:1404.
Swissnoso, Sommerstein R, Marschall J, Atkinson A, Surbek D, Dominguez-Bello MG, et al. Antimicrobial prophylaxis administration after umbilical cord clamping in cesarean section and the risk of surgical site infection: a cohort study with 55,901 patients. Antimicrob Resist Infect Control. 2020;9:201.
Lacaze-Masmonteil T, Rosychuk RJ, Robinson JL. Value of a single C-reactive protein measurement at 18 h of age. Arch Dis Child Fetal Neonatal Ed. 2014;99:F76–9.
Stocker M, van Herk W, El Helou S, Dutta S, Fontana MS, Schuerman FABA, et al. Procalcitonin-guided decision making for duration of antibiotic therapy in neonates with suspected early-onset sepsis: a multicentre, randomised controlled trial (NeoPIns). Lancet. 2017;390:871–81.
Puri M, Nain S, Gautam A, Chaudhary V, Jaiswal N, Gs T, et al. Rational use of antibiotics for major elective gynaecological and obstetrical surgical procedures: quality improvement journey from a tertiary care public facility. BMJ Open Quality. 2022;11. https://pubmed.ncbi.nlm.nih.gov/35545270/.
Yz Z, Tt L, W F Impact of antimicrobial stewardship programs on antibiotic use and drug resistance: analysis of data from maternal and child health care hospitals in Hubei Province, China. Current Med Sci. 2022;42. https://pubmed.ncbi.nlm.nih.gov/36184727/.
Mb D, S M, Km P Implementation of the sepsis risk calculator at an academic birth hospital. Hospital pediatrics [Internet]. 2018;8. https://pubmed.ncbi.nlm.nih.gov/29666161/.
Achten NB, Visser DH, Tromp E, Groot W, van Goudoever JB, Plötz FB. Early onset sepsis calculator implementation is associated with reduced healthcare utilization and financial costs in late preterm and term newborns. Eur J Pediatr. 2020;179:727–34.
Singh N, Gray JE. Antibiotic stewardship in NICU: De-implementing routine CRP to reduce antibiotic usage in neonates at risk for early-onset sepsis. J Perinatol. 2021;41:2488–94.
Malviya MN, Murthi S, Selim AA, Malik F, Jayraj D, Mendoza J, et al. A neonatologist-driven antimicrobial stewardship program in a neonatal tertiary care center in Oman. Am J Perinatol. 2024;41:e747–54.
Meyers JM, Tulloch J, Brown K, Caserta MT, D’Angio CT, GOLISANO CHILDREN’S HOSPITAL NICU ANTIBIOTIC STEWARDSHIP TEAM. A quality improvement initiative to optimize antibiotic use in a level 4 NICU. Pediatrics. 2020;146:e20193956.
Hamdy RF, Bhattarai S, Basu SK, Hahn A, Stone B, Sadler ED, et al. Reducing Vancomycin Use in a Level IV NICU. Pediatrics. 2020;146. https://pubmed.ncbi.nlm.nih.gov/32611807/.
Cantey JB, Wozniak PS, Pruszynski JE, Sánchez PJ. Reducing unnecessary antibiotic use in the neonatal intensive care unit (SCOUT): a prospective interrupted time-series study. Lancet Infect Dis. 2016;16(Oct):1178–84.
Astorga MC, Piscitello KJ, Menda N, Ebert AM, Ebert SC, Porte MA, et al. Antibiotic stewardship in the neonatal intensive care unit: effects of an automatic 48-hour antibiotic stop order on antibiotic use. J Pediatric Infect Dis Soc. 2019;8. https://pubmed.ncbi.nlm.nih.gov/29846666/.
Vatne A, Klingenberg C, Øymar K, Rønnestad AE, Manzoni P, Rettedal S. Reduced antibiotic exposure by serial physical examinations in term neonates at risk of early-onset sepsis. Pediatr Infect Dis J. 2020;39:438–43.
Frymoyer A, Joshi NS, Allan JM, Cohen RS, Aby JL, Kim JL, et al. Sustainability of a clinical examination-based approach for ascertainment of early-onset sepsis in late preterm and term neonates. J Pediatr. 2020;225:263–8.
Wang B, Li G, Jin F, Weng J, Peng Y, Dong S, et al. Effect of weekly antibiotic round on antibiotic use in the neonatal intensive care unit as antibiotic stewardship strategy. Front Pediatr. 2020;8:604244.
Benitz WE, Achten NB. Finding a role for the neonatal early-onset sepsis risk calculator. EClinicalMedicine. 2020;19:100255.
Ts R, Mp S, G B Syndromic Multiplex Polymerase Chain Reaction (mPCR) testing and antimicrobial stewardship: current practice and future directions. Current Infectious Dis Rep. 2021;23. https://pubmed.ncbi.nlm.nih.gov/33679252/.
Maurer FP, Christner M, Hentschke M, Rohde H. Advances in rapid identification and susceptibility testing of bacteria in the clinical microbiology laboratory: implications for patient care and antimicrobial stewardship programs. Infect Dis Rep. 2017;9:6839.
Funding
Open access funding provided by University of Luzern.
Author information
Authors and Affiliations
Contributions
CW and MS were responsible data analysis; CW wrote the first draft of the manuscript; MS, SA and MH reviewed the manuscript critically. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Winteler, C., Ardabili, S., Hodel, M. et al. A systematic review of Perinatal Antibiotic Stewardship – where we are, where to go?. J Perinatol 45, 1411–1422 (2025). https://doi.org/10.1038/s41372-025-02209-0
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41372-025-02209-0
This article is cited by
-
Do we need to reconsider our antibiotic regimen for preterm premature rupture of membranes? Pathogens and perinatal outcome in PPROM
BMC Pregnancy and Childbirth (2025)