Abstract
Point-of-care ultrasound (POCUS) is increasingly being utilized in the neonatal field; however, there is a lack of standardized guidance on the development of POCUS programs in neonatology. While subspecialties with decades of POCUS experience have established pathways for program development and training guidelines, such frameworks are absent in neonatal and perinatal medicine. Recent national and international publications have highlighted the need for structured program development and have established recommendations regarding standards for POCUS use, including minimum number of scans, competency-based assessments, and a strong quality improvement (QI) framework. This publication aims to provide steps toward program development for neonatal intensive care units eager to establish POCUS to augment the care of their patients.
Similar content being viewed by others
Introduction
The recognition in critical care medicine that Point-of-care ultrasound (POCUS) enhances triage, improves diagnostic accuracy, and benefits patient care has resulted in the rapid adoption of bedside ultrasound by clinicians [1]. POCUS has been utilized for decades in adult subspecialties such as anesthesia and emergency medicine for diagnostic and procedural applications. In 2001, emergency medicine guidelines for the use of POCUS were established to standardize programmatic development and include POCUS training in emergency medicine curricula [2].
Although the field of pediatrics has been a late adopter of POCUS, literature outlining the benefits of ultrasound in neonates, including assessment of endotracheal tube and central line tip positioning, was published over a decade ago [3, 4]. Recently, the use of POCUS has surged in pediatric subspecialties, with numerous publications addressing its indications and limitations [5,6,7,8]. However, guidelines standardizing programmatic development and integration into training programs are not yet available.
In 2022, the American Academy of Pediatrics, Section of Neonatal and Perinatal Medicine, recognized the value of POCUS as an adjunct to clinical care and published a clinical and technical report [5]. While the authors outlined indications as well as steps for programmatic development, they recognized the novelty of use in the neonatal intensive care unit (NICU) and barriers including lack of training guidelines, access to equipment, and insufficient expert facilitators. The Neonatal National POCUS Collaborative (NNPC) was established in 2023 to coalesce efforts towards standardizing POCUS implementation in neonatology and aid in providing a roadmap for program development. The mission of the Collaborative is ‘…to provide structure, standardize training programs and programmatic platforms, foster interdisciplinary collaboration with pediatric cardiology and pediatric radiology to ensure safe implementation of these programs, and promote innovation in the use of POCUS in the Neonatal Intensive Care units to enhance clinical care for our fragile population and improve patient outcomes’ (https://www.neopocuscollab.com/). This publication outlines the steps necessary to establish a neonatal POCUS program.
Programmatic needs
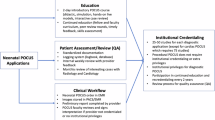
Successful development of a neonatal POCUS program involves the integration of 7 components: (1) defining the scope of practice, (2) Identifying stakeholders, (3) formalizing the educational curriculum, (4) acquiring equipment, (5) outlining clinical workflow, (6) establishing documentation protocols, (7) implementing a quality assurance and credentialing process. Each of these components may differ in development and maintenance based on institutional standards.
1) Scope of practice
POCUS is an adjunct to clinical care, providing time-sensitive information on a limited exam, refining differential diagnoses, and guiding clinical-decision making. Pediatric emergency medicine specialists have emphasized the role of POCUS as a valuable tool for rapid assessment, helping to streamline the triage process and accelerate patient management [9, 10].
Defining the scope of practice is a vital step in development of POCUS programs. Outlining basic and advanced POCUS applications in neonatology will provide a platform for further programmatic development including standardized training pathways. Neonatal POCUS scope of practice is dependent on local patient population, hospital structure, and clinical needs. This results in the identification of specific skills that will require structured training which will create a path to competence in practice.
2) Identification of stakeholders
POCUS program development requires the integration of key stakeholders to ensure successful implementation. Stakeholders include a NICU POCUS program director, providers from neonatology, cardiology, radiology, critical care and emergency medicine, divisional, and hospital leadership [5,6,7,8,9,10,11]. A need for a NICU program director was outlined in the AAP neonatal POCUS clinical report, who responsible for overseeing the development and maintenance of the program [5]. Ideally, the NICU program director should collaborate with the institutional credentialing body to incorporate POCUS into neonatal delineation of privileges, while also establishing a workflow to support billing, and the quality assurance (QA) process.
Non-NICU POCUS collaborators play a vital role in program development, as many pediatric specialties have already integrated POCUS into their standard of care. This is particularly evident in emergency medicine, where guidelines were published nearly a decade ago through collaborations with the AAP and emergency medicine societies [12]. Additionally, other POCUS-trained personnel within the hospital, such as anesthesia and pediatric intensive care specialists, can serve as valuable resources for educational support and contribute to shared infrastructure. Barriers identified in surveys for unsuccessful implementation of POCUS in the NICU included a perceived or actual lack of support from radiology and cardiology [13, 14]. However, cardiology’s recognition of the utility of POCUS has fostered greater opportunities for collaboration [6, 7]. Guidelines from the American Society of Echocardiography outline educational support for teaching cardiac POCUS [15]. This collaboration enhances educational support and quality improvement (QI) processes, with well-established metrics for assessment of image acquisition and interpretation [16].
3) Formalizing the educational curriculum
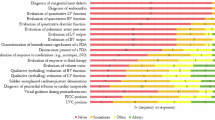
A key component in developing neonatal POCUS programs is structured training. While national guidelines for competency are unavailable, the literature supports a combination of didactic learning and hands-on experience for image acquisition and interpretation [17, 18]. A training pathway includes determining learning objectives, developing a curriculum, providing standardized scanning protocols, outlining expectations for image quality, interpretation, and competency standards. Basic and advanced applications of POCUS, as outlined by the NNPC in the accompanying publication, provide a framework for educational curricula. Integrating POCUS education within fellowship training and for faculty could be accomplished by ensuring participation in a 2-day POCUS “bootcamp”. This “bootcamp” encompasses didactic and hands-on sessions covering basic ultrasound principles and an overview of the scope of practice for bedside ultrasound [18,19,20].
Educational pathways include the development of competency specifications. Recognizing varied exposure to POCUS among learners, competency may be achieved at different rates. More fellows are exposed to ultrasound earlier in training, with medical schools and residency programs incorporating ultrasound education into their curricula [21, 22]. NICU POCUS training pathways may differ for a variety of learners, highlighting the need to develop a pathway toward competency assessment [23]. However, there is still uncertainty and lack of knowledge about the best pathway to develop competency in neonatal POCUS.
4) Equipment acquisition
Each institution should have a dedicated, fit-for-purpose ultrasound machine to allow users to acquire and archive high-quality images. It is critically important that the ultrasound machine meets the institutional POCUS requirements and supports the imaging needs of neonates. Therefore, ultrasound machines should be equipped with a variety of probes including phased array and high-frequency linear probes.
When new equipment is introduced, an agreement should be sought at local level on:
a) Maintenance of ultrasound machines and probes: Institutional support is needed to ensure safety of ultrasound machine and integration with an image repository and documentation. Ultrasound equipment needs to be serviced regularly, according to the manufacturer’s recommendation.
b) POCUS workflow: Ultrasound machines should be able to allow smooth patient workflow, electronic documentation and the ability to archive images. POCUS study findings should be documented in patient’s charts and all images should be archived for appropriate reference and QA purposes.
c) Local protocols: Each unit should develop standard protocols for POCUS image acquisition based on national and international recommendations, available literature and use appropriate strategies to incorporate POCUS workflow in patients’ clinical notes [24, 25].
d) Infection control and prevention: Ultrasound machines, cables and probes need to be cleaned and disinfected to minimize the risk of cross contamination before and after every use. Single-use sterile sachets are preferred over large gel bottles, which may become colonized with pathogens [26].
5) Outlining a clinical workflow
Image storage and documentation are crucial aspects of building a POCUS program [27]. Compliance with the Health Insurance Portability and Accountability act (HIPAA) regulations, safeguarding patient confidentiality, and ensuring data security are pivotal features of a well-designed image storage system. Images and videos obtained during clinical scans must be accurately tagged with appropriate patient identifiers and clinical demographics. An ideal image storage pathway would allow for the ability to link/accept the order placed for a POCUS scan in the electronic medical record on the ultrasound machine prior to performing the scan. Data collected during and after the scan for that specific order will then be automatically labeled with patient identifiers and stored in hospitals’ image storage system in the patient’s record (Fig. 1). A quality assurance process should be in place to regularly review and archive stored images, ideally by divisional POCUS champions. Ensuring secure storage of images acquired by learners during training presents a unique challenge, as most hospital image archive systems are typically limited to storing images obtained during clinical scans. HIPAA compliant, secure cloud-based image storage services specific for departmental POCUS needs could be considered, however these may pose a financial burden for smaller programs. As an alternative, external drives compliant with hospital regulations could be used to store deidentified images as an alternative practice (Table 1).
6) Documentation
Thorough documentation of observations and key findings from clinical scans is imperative for the long-term success of a POCUS program. Properly documented reports including patient demographics, type of scan, indications for the scan, descriptive summary of findings and impressions, with key images that inform clinical-decision making are essential components of the patient’s medical record. This practice not only ensures compliance with legal and regulatory standards but also promotes accountability and transparency. Appropriate documentation is required for quality assurance, effective billing, coding, and reimbursement. Creating templates within electronic medical records could promote consistent documentation among different providers. Furthermore, the contributions of these reports to future education and research could be invaluable.
Overall, a well-structured image storage and documentation pathway will enhance the credibility and quality of the POCUS program ultimately leading to improved patient care [28]. Utilizing an image repository, along with robust documenting and QA process is imperative for bedside ultrasound use to ensure accurate documentation [29].
The perceived liability, or risk of litigation, is theoretically generalized from litigations encountered by obstetricians and radiologists, where misinterpretation and misdiagnosis allegations represent a substantial portion of malpractice claims. However, there have been no reported cases of litigation related to the use of POCUS in neonatology. In contrast, there are two cases involving litigation due to the failure to use POCUS as a diagnostic tool [30].
7) Establishing QA and credentialing processes
In addition to hospital staff and divisional stakeholders, support from hospital leadership is crucial for program development. Their involvement is essential for resource allocation, including personnel support and machine acquisition, as well as other factors such as biomedical engineering and information technology (IT) support for image archiving and quality improvement mechanisms. Guidance from other departments within the hospital that have established QA and credentialing processes can provide an initial framework for program development, focusing on neonatal applications. Beyond structural support, shared educational expertise is invaluable, particularly when starting a program that may have limited NICU personnel available to provide education during its early stage.
Billing for neonatal POCUS
Billing for neonatal POCUS can be complex and challenging, particularly because many NICU services are included in the daily service bundle [31, 32]. While adult specialties such as emergency care medicine and anesthesiology have established billing frameworks [2, 33, 34], there remains a lack of consensus on neonatal POCUS billing. Efficient billing adheres to standards, including specific indications for POCUS use, image acquisition, documentation, interpretation, and payment requests. Optimal imaging acquisition, labeling, and storage are essential for developing an efficient and safe neonatal POCUS service. Documentation should include details such as the provider performing the procedure, date of service, type of exam, findings, and interpretations that guide medical decisions. Automating workflows offers a promising solution to enhance compliance, productivity, and efficient reimbursement, ultimately improving patient care [35, 36]. Such workflows have demonstrated significant increases in POCUS scans, user engagement, exam compliance, quality assurance, and potential revenue. Multidisciplinary collaboration among providers, coders, billing team, and institutional IT departments is essential for developing efficient workflows and regular optimization to meet evolving demands as the neonatal POCUS services grow.
Conclusion
The use of POCUS in neonatology is rapidly expanding, and a well-defined roadmap will help guide its development and streamline progress. The National Neonatal POCUS Collaborative was established to provide a forum for neonatal POCUS stakeholders to build consensus on programmatic development, including guideline creation, credentialing pathways, and training support. A multifaceted approach to NICU POCUS development through establishment of programmatic needs and maintenance will enable POCUS to become standard of care in the NICU, thereby enhance the care of this fragile patient population.
References
Moore CL, Copel JA. Point-of-care ultrasonography. N Engl J Med. 2011;364:749–57.
Ultrasound Guidelines. Emergency, point-of-care, and clinical ultrasound guidelines in medicine. Ann Emerg Med. 2023;82:e115–55.
Dennington D, Vali P, Finer NN, Kim JH. Ultrasound confirmation of endotracheal tube position in neonates. Neonatology. 2012;102:185–9.
Fleming SE, Kim JH. Ultrasound-guided umbilical catheter insertion in neonates. J Perinatol J Calif Perinat Assoc. 2011;31:344–9.
Stewart DL, Elsayed Y, Fraga MV, Coley BD, Annam A, Milla SS, et al. Use of point-of-care ultrasonography in the NICU for diagnostic and procedural purposes. Pediatrics. 2022;150:e2022060053.
McNamara PJ, Jain A, El-Khuffash A, Giesinger R, Weisz D, Freud L, et al. Guidelines and recommendations for targeted neonatal echocardiography and cardiac point-of-care ultrasound in the neonatal intensive care unit: an update from the American Society of Echocardiography. J Am Soc Echocardiogr Publ Am Soc Echocardiogr. 2024;37:171–215.
Lu JC, Riley A, Conlon T, Levine JC, Kwan C, Miller-Hance WC, et al. Recommendations for cardiac point-of-care ultrasound in children: a report from the American Society of Echocardiography. J Am Soc Echocardiogr Publ Am Soc Echocardiogr. 2023;36:265–77.
Singh Y, Tissot C, Fraga MV, Yousef N, Cortes RG, Lopez J, et al. International evidence-based guidelines on Point of Care Ultrasound (POCUS) for critically ill neonates and children issued by the POCUS Working Group of the European Society of Paediatric and Neonatal Intensive Care (ESPNIC). Crit Care Lond Engl. 2020b;24:65.
Plummer D, Brunette D, Asinger R, Ruiz E. Emergency department echocardiography improves outcome in penetrating cardiac injury. Ann Emerg Med. 1992;21:709–12.
Tayal VS, Kline JA. Emergency echocardiography to detect pericardial effusion in patients in PEA and near-PEA states. Resuscitation. 2003;59:315–8.
Tong JH, Manipon C, Vallandingham-Lee S, Dasani R, Davis AS, Bhombal S. The role of neonatal nurse practitioners in the implementation of a bedside point-of-care ultrasound program. Adv Neonatal Care J Natl Assoc Neonatal Nurses. 2024;24:536–43.
Marin JR, Lewiss RE. American Academy of Pediatrics, Committee on Pediatric Emergency Medicine, Society for Academic Emergency Medicine, Academy of Emergency Ultrasound, American College of Emergency Physicians, Pediatric Emergency Medicine Committee, World Interactive Network Focused on Critical Ultrasound. Point-of-care ultrasonography by pediatric emergency medicine physicians. Pediatrics 2015;135:e1113–22.
Nguyen J, Amirnovin R, Ramanathan R, Noori S. The state of point-of-care ultrasonography use and training in neonatal-perinatal medicine and pediatric critical care medicine fellowship programs. J Perinatol J Calif Perinat Assoc. 2016;36:972–6.
Mirza HS, Logsdon G, Pulickal A, Stephens M, Wadhawan R. A national survey of neonatologists: barriers and prerequisites to introduce point-of-care ultrasound in neonatal ICUs. Ultrasound Q. 2017;33:265–71.
Kirkpatrick JN, Grimm R, Johri AM, Kimura BJ, Kort S, Labovitz AJ, et al. Recommendations for echocardiography laboratories participating in cardiac point of care cardiac ultrasound (POCUS) and Critical Care Echocardiography Training: Report from the American Society of Echocardiography. J Am Soc Echocardiogr Publ Am Soc Echocardiogr. 2020;33:409–22.e4.
Lopez L, Saurers DL, Barker PCA, Cohen MS, Colan SD, Dwyer J, et al. Guidelines for performing a comprehensive pediatric transthoracic echocardiogram: recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr Publ Am Soc Echocardiogr. 2024;37:119–70.
Conlon TW, Himebauch AS, Fitzgerald JC, Chen AE, Dean AJ, Panebianco N, et al. Implementation of a pediatric critical care focused bedside ultrasound training program in a large academic PICU. Pediatr Crit Care Med J Soc Crit Care Med World Fed Pediatr Intensive Crit Care Soc. 2015;16:219–26.
Bhargava V, Haileselassie B, Rosenblatt S, Baker M, Kuo K, Su E. A point-of-care ultrasound education curriculum for pediatric critical care medicine. Ultrasound J. 2022;14:44.
Brant JA, Orsborn J, Good R, Greenwald E, Mickley M, Toney AG. Evaluating a longitudinal point-of-care-ultrasound (POCUS) curriculum for pediatric residents. BMC Med Educ. 2021;21:64.
Huang C, Morone C, Parente J, Taylor S, Springer C, Doyle P, et al. Advanced practice providers proficiency‐based model of ultrasound training and practice in the ED. J Am Coll Emerg Physicians Open. 2022;3:e12645.
Russell FM, Zakeri B, Herbert A, Ferre RM, Leiser A, Wallach PM. The state of point-of-care ultrasound training in undergraduate medical education: findings from a national survey. Acad Med J Assoc Am Med Coll. 2022;97:723–7.
Reaume M, Siuba M, Wagner M, Woodwyk A, Melgar TA. Prevalence and scope of point-of-care ultrasound education in internal medicine, pediatric, and medicine-pediatric residency programs in the United States. J Ultrasound Med J Am Inst Ultrasound Med. 2019;38:1433–9.
Restrepo D, Heyne TF, Schutzer C, Dversdal R. Teaching old dogs new tricks” – POCUS Education for Senior Faculty. POCUS J. 2023;8:9–12.
Liu J, Guo G, Kurepa D, Volpicelli G, Sorantin E, Lovrenski J, et al. Specification and guideline for technical aspects and scanning parameter settings of neonatal lung ultrasound examination. J Matern-Fetal Neonatal Med J Eur Assoc Perinat Med Fed Asia Ocean Perinat Soc Int Soc Perinat Obstet. 2022;35:1003–16.
Singh Y, Gupta S, Groves AM, Gandhi A, Thomson J, Qureshi S, et al. Expert consensus statement ’Neonatologist-performed Echocardiography (NoPE)’-training and accreditation in UK. Eur J Pediatr. 2016;175:281–7.
Nyhsen CM, Humphreys H, Koerner RJ, Grenier N, Brady A, Sidhu P, et al. Infection prevention and control in ultrasound - best practice recommendations from the European Society of Radiology Ultrasound Working Group. Insights Imaging. 2017;8:523–35.
Conlon TW, Yousef N, Mayordomo-Colunga J, Tissot C, Fraga MV, Bhombal S, et al. Establishing a risk assessment framework for point-of-care ultrasound. Eur J Pediatr. 2022;181:1449–57.
Mani N. Implementing a quality framework for storing emergency department point-of-care ultrasound examinations on a picture archiving and communication system. Ultrasound Leeds Engl. 2021;29:252–9.
Prager R, Wu D, Garber G, Finestone PJ, Zang C, Aslanova R, et al. Medico-legal risks of point-of-care ultrasound: a closed-case analysis of Canadian Medical Protective Association medico-legal cases. Ultrasound J. 2024;16:16.
Nguyen J, Cascione M, Noori S. Analysis of lawsuits related to point-of-care ultrasonography in neonatology and pediatric subspecialties. J Perinatol J Calif Perinat Assoc. 2016;36:784–6.
Lakshminrusimha S, Song C, Pearlman SA, Martin G, Duncan S. Controversies in CPT® coding in the neonatal intensive care unit: - critical vs. intensive care. J Perinatol J Calif Perinat Assoc. 2023;43:1535–40.
Chan B, Mnyavanu N, Bhombal S, Fraga MV, Groves AM, Marshall S, et al. Essentials of point-of-care ultrasound coding and billing at the neonatal intensive care unit setting in the United States. Am J Perinatol. 2024;41:2014–20.
Adhikari S, Amini R, Stolz L, O’Brien K, Gross A, Jones T, et al. Implementation of a novel point-of-care ultrasound billing and reimbursement program: fiscal impact. Am J Emerg Med. 2014;32:592–5.
Resnick J, Hoffenberg S, Tayal V, and Dickman E Ultrasound coding and reimbursement document, 2009. Emergency ultrasound section, American college of emergency physicians.
Lewiss RE, Cook J, Sauler A, Avitabile N, Kaban NL, Rabrich J, et al. A workflow task force affects emergency physician compliance for point-of-care ultrasound documentation and billing. Crit Ultrasound J. 2016;8:5.
Hughes D, Corrado MM, Mynatt I, Prats M, Royall NA, Boulger C, et al. Billing I-AIM: a novel framework for ultrasound billing. Ultrasound J. 2020;12:8.
Author information
Authors and Affiliations
Consortia
Contributions
SB, YS, SM, SM, and MVF conceptualized, designed and drafted the initial manuscript. AMG, BC, KGL, CBC, JZS, SHG, JS, CJ, MK, JLR, DVC, ASB reviewed and revised the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bhombal, S., Singh, Y., Marshall, S. et al. Neonatal point-of-care ultrasound—steps for program development. J Perinatol 46, 108–112 (2026). https://doi.org/10.1038/s41372-025-02359-1
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41372-025-02359-1
This article is cited by
-
A step towards the expansion of neonatal point-of-care ultrasound (POCUS)
Journal of Perinatology (2025)