Abstract
Point-of-care ultrasound (POCUS) has become essential for diagnosing and managing critically ill newborns. This technology offers rapid, non-invasive assessments and supports bedside clinical decision-making. Although POCUS applications in neonatology continue to expand, there remains a lack of standardized training, certification, and credentialing processes. This paper provides expert-based perspectives and guidelines for implementing neonatal POCUS, focusing on the core components of competency, credentialing, and quality assurance (QA). Recommendations include performing a minimum number of scans for various neonatal applications, integrating competency assessments into training programs, and ensuring a robust image repository and reporting pathway. Neonatal POCUS improves patient care, and establishing clear standards and frameworks will enhance provider performance, and ensure patient safety in neonatal intensive care units (NICUs).
Similar content being viewed by others
Introduction
Point-of-care ultrasound (POCUS) in neonatal care has expanded considerably in recent years and has become an essential clinical tool for managing critically ill newborns. Procedural POCUS is increasingly utilized as an adjunct technology to enhance performance by providing real-time visualization of anatomy [1, 2]. Diagnostic POCUS provides valuable information that complements physical examination findings and existing clinical data to support clinical decision-making [3, 4].
Growing evidence has prompted the American Academy of Pediatrics (AAP) to advocate for the use of POCUS in pediatric diagnostic and procedural practices within emergency medicine [5]. Additionally, the European Society for Pediatric and Neonatal Intensive Care (ESPNIC) issued international, evidence-based guidelines for the use of POCUS in pediatric and neonatal intensive care units (NICUs) [6]. More recently, the AAP endorsed a clinical and a technical report supporting the use of POCUS in the NICU for both diagnostic and procedural purposes [7].
While the use of POCUS continues to increase in neonatology, there are currently no standardized training curricula, formal accreditation, or national certification processes exist to guide program development. As the incorporation of POCUS into neonatal care expands, establishing a robust framework for its credentialing and quality assurance (QA) is essential to ensure safe and effective use. This manuscript provides practical guidelines based on expert opinions for implementing neonatal POCUS, focusing on the key components of training, credentialing, and ongoing competency assessment. Establishing clear standards and QA protocols will enhance the utility of this technology and improve patient care.
Methodology for consensus development and recommendations
The development of consensus and recommendations for the use of POCUS in neonatal care followed a structured and systematic approach to gathering expert opinions and evidence-based insights. Three lead authors (MVF, SB, YS) identified additional expert colleagues who significantly contributed to publications on neonatal POCUS and/or had developed POCUS training courses in the last 10 years. The expert panel selection (co-authors) was conducted prior to the literature review, ensuring representation from a broad range of POCUS fields and incorporation of perspectives that reflect regional variations and clinical practices relevant across the United States. All experts were members of the National Neonatal POCUS Collaborative (NNPC). This approach was intended to capture a comprehensive perspective on the use of POCUS in neonatal care, while also accounting for regional variations and clinical practices prevalent across the United States. A comprehensive review of literature was performed to gather the most up-to-date evidence, focusing on studies from the last ten years, clinical guidelines, and expert opinions regarding the use of POCUS in neonatology. The development of these practice guidelines and recommendations for neonatal POCUS practice involved multiple meetings and feedback exchanges with the expert panel and the NNPC Guidelines Subcommittee members. The guidelines were organized into five subsections, corresponding to key areas of application (heart, lungs, brain, abdomen, and procedural). Within each section, both basic and advanced POCUS applications were defined. Facilitated group discussions addressed specific issues such as training needs, accreditation standards, and competency assessments in neonatal POCUS. The grade of recommendation set forth by the Oxford Center for Evidence-Based Medicine (CEBM) was used for the level of evidence: A-level = Consistent level 1 studies, B-level = Consistent level 2 or 3 studies or extrapolations from level 1 studies, C-level = Level 4 studies or extrapolations from level 2 or 3 studies, and D-level = Level 5 evidence or troubling inconsistent or inconclusive studies at any level. The draft recommendations were shared with a broader group of neonatal POCUS colleagues for feedback through the NNPC. This ensured that the final recommendations were practical, feasible, and aligned with clinical practice. Feedback from these stakeholders was incorporated into the final document. The final set of recommendations was prepared in accordance with the international Appraisal of Guidelines, Research and Evaluation (AGREE) [8] and was reviewed and approved by the expert panel, ensuring that they reflected the committee’s consensus. The expert consensus was developed using majority vote among the panelists, following multiple online discussions. The resulting recommendations offer an evidence-based framework for implementing neonatal POCUS, with an emphasis on safe practice, QA, and ongoing professional development.
Scope of practice in neonatology: basic and advanced applications
With the expanding use of POCUS in the NICU, new clinical applications continue to emerge. As of 2025, position statements from the AAP [7], the American Society of Echocardiography (ASE) [9], and ESPNIC [6] offer valuable guidance and frameworks for POCUS use in the NICU.
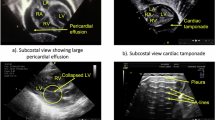
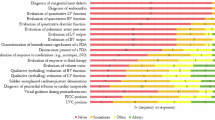
A comprehensive list of neonatal POCUS applications was developed following a literature review [10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47] with each application assigned a corresponding level of evidence. All diagnostic and procedural applications were categorized as either basic or advanced (Tables 1A and 1B). The list of basic POCUS applications is intended to serve as a foundational framework for developing a core neonatal POCUS curriculum. These basic indications are commonly used in the NICU and can typically be mastered by learners during the course of NICU fellowship training. In contrast, advanced indications require more specialized ultrasound training to achieve proficiency.
Competency, credentialing, and institutional privileges
Demonstrating competency, meeting credentialing requirements, and obtaining privileges in neonatal POCUS are essential to ensure that practitioners possess the necessary skills and knowledge to use ultrasound both effectively and safely. Competency in POCUS pertains to the individual provider and encompasses several key elements: understanding the clinical indications for its use, acquiring the technical skills to obtain high-quality images, accurately interpreting those images, and integrating the findings into clinical decision-making. Credentialing refers to institution specific criteria to define the scope of practice and its integration into clinical workflows. This process is critical for enabling practitioners to base medical decisions on POCUS findings, include images and interpretations in the medical record, and submit billing claims for POCUS using the appropriate Current Procedural Terminology (CPT) codes [48]. POCUS privileges are granted by the hospital and formally authorize a provider to perform POCUS within the institution.
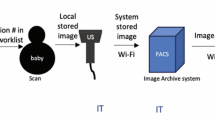
Achieving competency requires both theoretical knowledge and hands-on experience, including demonstrated proficiency in performing and interpreting ultrasound exams. Performing a set number of POCUS examinations is a component of reaching competency, which can be further assessed by POCUS experts. However, standardized training guidelines specific to the NICU clinical scope are currently lacking. The Emergency Medicine and the Pediatric Emergency Medicine Expert Guidelines recommend performing 25 to 50 POCUS examinations for each organ system to ensure proficiency [49, 50]. Extrapolating from these guidelines, the neonatology community recommends performing 25-50 studies per each diagnostic application with a mix of normal and abnormal exams, excluding cardiac POCUS. Currently, there is insufficient evidence to justify a specific minimum number of scans required to determine competency in neonatal cardiac POCUS training. However, due to the numerous cardiac views and multiple applications of neonatal cardiac imaging, it is recommended that trainees perform at least 75 scans, including a minimum of 25 exams focused on line placement and heart function evaluation and 50 exams focused on global systolic cardiac function, pericardial effusion and volume status assessment [9]. These numbers should be viewed as expert recommendations rather than strict requirements. Furthermore, imposing fixed minimum scan numbers may unnecessarily delay the implementation of proven POCUS techniques from reaching the bedside, particularly in simpler applications or when practitioners already have significant scanning experience in other organ systems. Therefore, a more pragmatic approach of competency-based rather than time-based assessment of skills should be considered. This competency-based approach may include synchronous evaluation methods such as observation at the patient bedside or via simulation and may also include asynchronous practices such as interactive image review for evaluation of acquisition quality and interpretation accuracy. These educational practices can also be incorporated into a QA program to ensure ongoing safe and effective practice. Figure 1 depicts a proposed framework for neonatal POCUS program development including training, a QA process and clinical workflow leading to institutional credentialing.
While the use of ultrasound for procedural guidance (e.g., central line placement, thoracentesis, paracentesis) is typically considered an adjunct technology that enhances provider performance and patient safety, it does not require formal credentialing or institutional privileging beyond baseline procedural credentialing. However, competency, training, and adherence to safety standards remain critical components of its use in clinical practice.
Collaborating with existing multidisciplinary POCUS teams within an institution can help leverage available resources and support the inclusion of neonatal POCUS within the hospital-wide credentialing framework.
Quality assurance process
QA in neonatal POCUS is essential to ensuring the safety of patients and healthcare institutions. This process involves systematic measures to verify the accuracy, reliability, and safety of ultrasound examinations. An inadequate QA process can compromise patient care and increase the risk of legal liability. Several organizations, including the ACEP (American College of Emergency Physicians) and ASE, have issued policy statements and guidelines related to POCUS QA [49,50,51,52]. To maintain patient safety, ongoing education of POCUS practitioners is vital. Practitioners should receive specialized training, demonstrate competency, and acquire appropriate credentialing and privileges. Regular competency assessments and continuing education help ensure that practitioners remain up-to-date with current guidelines and best practices. Additionally, practitioners should work within a clearly defined scope of POCUS practices to uphold safety standards. All POCUS images should be archived along with documentation of findings and interpretations to facilitate regular audits. The quality of these images should be reviewed to ensure they meet established standards. Similarly, findings and interpretations should undergo audits for accuracy, with interpretations validated against patient outcomes, results from other diagnostic tests, and/or surgical or pathology evaluations. It is essential that POCUS practitioners receive timely, constructive feedback in a nonjudgmental manner to support continuous improvement and ensure high-quality care. Institutions play a vital role in QA by establishing a review committee and supporting a dedicated POCUS program director. Advanced information technology may have electronic medical records linking POCUS order, POCUS machine image acquisition, image transmission to the patient chart, and documentation of image interpretation.
Initiating a POCUS program can present several challenges, particularly in establishing a robust QA process. Common obstacles include a shortage of POCUS experts within the subspecialty, the lack of standardized competency and educational frameworks, and resistance from administrators to provide necessary support [53]. During the early stages of program development, it is important to build relationships with multidisciplinary POCUS providers within the institution to support various aspects of programmatic development, including expertise in image acquisition and interpretation. Until a dedicated internal QA team is established, auditing all images and collaborating with radiologists, cardiologists, and local POCUS experts for quality control is imperative. In conclusion, the QA process for neonatal POCUS is a comprehensive, ongoing effort that encompasses education, equipment maintenance, institutional policy development and continuous evaluation. This multifaceted approach ensures that neonatal POCUS is performed to the highest standards, upholding both patient safety and the integrity of the healthcare system.
International relevance and adaptability of POCUS guidelines
International evidence-based ESPNIC POCUS guidelines provide comprehensive recommendations for the use of POCUS in the neonatal and pediatric intensive care units [5]. However, these are joint guidelines intended for use in both neonates and older children. In contrast, the currently proposed guidelines are neonatal specific and provide guidance on the basic and advanced applications. The authors believe these newly proposed guidelines can be adapted easily for international use in any neonatal settings and they are complimentary to the ESPNIC POCUS guidelines.
Conclusions
In summary, the use of POCUS in neonatology has significantly advanced, offering benefits in diagnostic and procedural guidance. However, to ensure its safe and effective use, it is essential to establish structured frameworks for competency, credentialing, and QA process. These frameworks should include training programs, ongoing competency assessments, and timely feedback for practitioners, as well as QA processes to monitor image quality and clinical accuracy. As neonatal POCUS evolves, institutions should focus on supporting these efforts through adequate resources and infrastructure.
References
de Souza TH, Brandão MB, Nadal JAH, Nogueira RJN. Ultrasound guidance for pediatric central venous catheterization: a meta-analysis. Pediatrics. 2018;142:e20181719.
Fraga MV, Stoller JZ, Glau CL, De Luca D, Rempell RG, Wenger JL, et al. Seeing is believing: ultrasound in pediatric procedural performance. Pediatrics. 2019;144:e20191401.
Conlon TW, Nishisaki A, Singh Y, Bhombal S, De Luca D, Kessler DO, et al. Moving beyond the stethoscope: diagnostic point-of-care ultrasound in pediatric practice. Pediatrics. 2019;144:e20191402.
Recker F, Kipfmueller F, Wittek A, Strizek B, Winter L. Applications of point-of-care ultrasound in neonatology: a systematic review of the literature. Life. 2024;14:658.
Marin JR, Lewiss RE. American Academy of Pediatrics, Committee on Pediatric Emergency Medicine; Society for Academic Emergency Medicine, Academy of Emergency Ultrasound; American College of Emergency Physicians, Pediatric Emergency Medicine Committee; World Interactive Network Focused on Critical Ultrasound. Point-of-care ultrasonography by pediatric emergency medicine physicians. Pediatrics. 2015;135:e1113–22.
Singh Y, Tissot C, Fraga MV, Yousef N, Cortes RG, Lopez J, et al. International evidence-based guidelines on Point of Care Ultrasound (POCUS) for critically ill neonates and children issued by the POCUS Working Group of the European Society of Paediatric and Neonatal Intensive Care (ESPNIC). Crit Care. 2020;24:65.
Stewart DL, Elsayed Y, Fraga MV, Coley BD, Annam A, Sarvis Milla S, et al. Use of point-of-care ultrasonography in the NICU for diagnostic and procedural purposes. Pediatrics. 2022;150:e2022060052.
Brouwers MC, Kerkvliet K, Spithoff K, AGREE Next Steps Consortium. The AGREE reporting checklist: a tool to improve reporting of clinical practice guidelines. BMJ. 2016;352:i1152.
McNamara PJ, Jain A, El-Khuffash A, Giesinger R, Weisz D, Freud L, et al. Guidelines and recommendations for targeted neonatal echocardiography and cardiac point-of-care ultrasound in the neonatal intensive care unit: an update from the American Society of Echocardiography. J Am Soc Echocardiogr. 2024;37:171–215.
Johnson KN, Thomas T, Grove J, Jarboe MD. Insertion of peripherally inserted central catheters in neonates less than 1.5 kg using ultrasound guidance. Pediatr Surg Int. 2016;32:1053–7.
de Souza TH, Brandão MB, Santos TM, Pereira RM, Nogueira RJN. Ultrasound guidance for internal jugular vein cannulation in PICU: a randomised controlled trial. Arch Dis Child. 2018;103:952–6.
Zanolla GR, Baldisserotto M, Piva J. How useful is ultrasound guidance for internal jugular venous access in children? J Pediatr Surg. 2018;53:789–93.
Verghese ST, McGill WA, Patel RI, Sell JE, Midgley FM, Ruttimann UE. Ultrasound-guided internal jugular venous cannulation in infants: a prospective comparison with the traditional palpation method. Anesthesiology. 1999;91:71–7.
Nardi N, Wodey E, Laviolle B, De La Brière F, Delahaye S, Engrand C, et al. Effectiveness and complications of ultrasound-guided subclavian vein cannulation in children and neonates. Anaesth Crit Care Pain Med. 2016;35:209–13.
Breschan C, Graf G, Jost R, Stettner H, Feigl G, Neuwersch S, et al. A retrospective analysis of the clinical effectiveness of supraclavicular, ultrasound-guided brachiocephalic vein cannulations in preterm infants. Anesthesiology. 2018;128:38–43.
Lausten-Thomsen U, Merchaoui Z, Dubois C, Eleni Dit Trolli S, Le Saché N, Mokhtari M, et al. Ultrasound-guided subclavian vein cannulation in low birth weight neonates. Pediatr Crit Care Med. 2017;18:172–5.
Aouad MT, Kanazi GE, Abdallah FW, Moukaddem FH, Turbay MJ, Obeid MY, et al. Femoral vein cannulation performed by residents: a comparison between ultrasound-guided and landmark technique in infants and children undergoing cardiac surgery. Anesth Analg. 2010;111:724–8.
McKinney A, Steanson K, Lebar K. A standardized training program in ultrasound-guided intravenous line placement: improving nurses’ confidence and success. Adv Neonatal Care. 2023;23:17–22.
Corder W, Stoller JZ, Fraga MV. A retrospective observational study of real-time ultrasound-guided peripheral arterial cannulation in infants. J Vasc Access. 2024;25:1643–8.
Oulego-Erroz I, González-Cortes R, García-Soler P, Balaguer-Gargallo M, Frías-Pérez M, Mayordomo-Colunga J, et al. Ultrasound-guided or landmark techniques for central venous catheter placement in critically ill children. Intensive Care Med. 2018;44:61–72.
Stoller JZ, Fraga MV. Real-time ultrasound-guided lumbar puncture in the neonatal intensive care unit. J Perinatol. 2021;41:2495–8.
Mohseny AB, van Velze V, Steggerda SJ, Smits-Wintjens VEHJ, Bekker V, Lopriore E. Late-onset sepsis due to urinary tract infection in very preterm neonates is not uncommon. Eur J Pediatr. 2018;177:33–38.
Tosif S, Baker A, Oakley E, Donath S, Babl FE. Contamination rates of different urine collection methods for the diagnosis of urinary tract infections in young children: an observational cohort study. J Paediatr Child Health. 2012;48:659–64.
Božičnik S, Díez Recinos A, Moreno Cantó MC, Pavlovič S, García-Muñoz Rodrigo F. Ultrasound-guided suprapubic bladder aspiration increases the success of the technique in infants less than 4 months-old. Pediatr (Barc). 2013;78:321–5.
Nazeer SR, Dewbre H, Miller AH. Ultrasound-assisted paracentesis performed by emergency physicians vs the traditional technique: a prospective, randomized study. Am J Emerg Med. 2005;23:363–7.
Dennington D, Vali P, Finer NN, Kim JH. Ultrasound confirmation of endotracheal tube position in neonates. Neonatology. 2012;102:185–9.
Katheria AC, Fleming SE, Kim JH. A randomized controlled trial of ultrasound-guided peripherally inserted central catheters compared with standard radiograph in neonates. J Perinatol. 2013;33:791–4.
Zaghloul N, Watkins L, Choi-Rosen J, Perveen S, Kurepa D. The superiority of point of care ultrasound in localizing central venous line tip position over time. Eur J Pediatr. 2019;178:173–9.
Doyle SC, Bergin NM, Young R, England A, McEntee MF. Diagnostic accuracy of ultrasound for localizing peripherally inserted central catheter tips in infants in the neonatal intensive care unit: a systematic review and meta-analysis. Pediatr Radio. 2022;52:2421–30.
Tauzin L, Sigur N, Joubert C, Parra J, Hassid S, Moulies ME. Echocardiography allows more accurate placement of peripherally inserted central catheters in low birthweight infants. Acta Paediatr. 2013;102:703–6.
Michel F, Brevaut-Malaty V, Pasquali R, Thomachot L, Vialet R, Hassid S, et al. Comparison of ultrasound and X-ray in determining the position of umbilical venous catheters. Resuscitation. 2012;83:705–9.
Fleming SE, Kim JH. Ultrasound-guided umbilical catheter insertion in neonates. J Perinatol. 2011;31:344–9.
Simanovsky N, Ofek-Shlomai N, Rozovsky K, Ergaz-Shaltiel Z, Hiller N, Bar-Oz B. Umbilical venous catheter position: evaluation by ultrasound. Eur Radio. 2011;21:1882–6.
Ades A, Sable C, Cummings S, Cross R, Markle B, Martin G. Echocardiographic evaluation of umbilical venous catheter placement. J Perinatol. 2003;23:24–8.
Kishigami M, Shimokaze T, Enomoto M, Shibasaki J, Toyoshima K. Ultrasound-Guided Umbilical Venous Catheter Insertion With Alignment of the Umbilical Vein and Ductus Venosus. J Ultrasound Med. 2020;39:379–83.
Kozyak BW, Fraga MV, Juliano CE, Bhombal S, Munson DA, Brandsma E, et al. Real-time ultrasound guidance for umbilical venous cannulation in neonates with congenital heart disease. Pediatr Crit Care Med. 20221;23:e257-e266.
Hébert A, Lavoie PM, Giesinger RE, Ting JY, Finan E, Singh Y, et al. Evolution of training guidelines for echocardiography performed by the neonatologist: toward hemodynamic consultation. J Am Soc Echocardiogr. 2019;32:785–90.
Singh Y, Bhombal S, Katheria A, Tissot C, Fraga MV. The evolution of cardiac point of care ultrasound for the neonatologist. Eur J Pediatr. 2021;180:3565–75.
Zaki HA, Albaroudi B, Shaban EE, Shaban A, Elgassim M, Almarri ND, et al. Advancement in pleural effusion diagnosis: a systematic review and meta-analysis of point-of-care ultrasound versus radiographic thoracic imaging. Ultrasound J. 2024;16:3.
Luo K, Wang H, Huang F, Tang J. Optimal timing and cutoff range of lung ultrasound in predicting surfactant administration in neonates: A meta-analysis and systematic review. PLoS One. 2023;18:e0287758.
De Luca D, Foti A, Alonso-Ojembarrena A, Condò V, Capasso L, Raschetti R, et al. UNION (Lung Ultrasound Features and Inflammation in NICU-Admitted Neonates) Study Group. Lung consolidation depth and gas exchange in different types of neonatal respiratory failure: the UNION multicenter study. Chest. 2024;165:1431–4.
Alonso-Ojembarrena A, De Luca D, Raimondi F. The use of lung ultrasound in neonatal units and the importance of critical revision of published data. J Matern Fetal Neonatal Med. 2024;37:2371541.
Surak A, Shaireen H, Elsayed Y. Applications of lung ultrasound as an emerging tool in neonates. J Neonatal Perinat Med. 2025;18:187–96.
Capasso L, Pacella D, Migliaro F, Salomè S, Grasso F, Corsini I, et al. Can lung ultrasound score accurately predict surfactant replacement? A systematic review and meta-analysis of diagnostic test studies. Pediatr Pulmonol. 2023;58:1427–37.
Pezza L, Alonso-Ojembarrena A, Elsayed Y, Yousef N, Vedovelli L, Raimondi F, et al. Meta-analysis of lung ultrasound scores for early prediction of bronchopulmonary dysplasia. Ann Am Thorac Soc. 2022;19:659–67.
Yousef N, Singh Y, De Luca D. Playing it SAFE in the NICU” SAFE-R: a targeted diagnostic ultrasound protocol for the suddenly decompensating infant in the NICU. Eur J Pediatr. 2022;181:393–8.
Elsayed Y, Wahab MGA, Mohamed A, Fadel NB, Bhombal S, Yousef N, et al. Point-of-care ultrasound (POCUS) protocol for systematic assessment of the crashing neonate-expert consensus statement of the international crashing neonate working group. Eur J Pediatr. 2023;182:53–66.
Chan B, Mnyavanu N, Bhombal S, Fraga MV, Groves AM, Marshall S, et al. Essentials of point-of-care ultrasound coding and billing at the neonatal intensive care unit setting in the United States. Am J Perinatol. 2024;41:2014–20.
Ultrasound guidelines: emergency, point-of-care, and clinical ultrasound guidelines in medicine. Ann Emerg Med. 2023;82:e115-e155.
Abo A, Alade K, Rempell R, Kessle D, Fischer J, Lewiss R, et al. Credentialing pediatric emergency medicine faculty in point-of-care ultrasound. Pediatr Emerg Care. 2021;37:e1687–e1694.
Ultrasound guidelines: emergency, point-of-care and clinical ultrasound guidelines in medicine. Ann Emerg Med. 2017;69:e27-e54.
Lu JC, Riley A, Conlon T, Levine JC, Kwan C, Miller-Hance WC, et al. Recommendations for cardiac point-of-care ultrasound in children: a report from the American Society of Echocardiography. J Am Soc Echocardiogr. 2023;36:265–77.
Conlon TW, Yousef N, Mayordomo-Colunga J, Tissot C, Fraga MV, Bhombal S, et al. Establishing a risk assessment framework for point-of-care ultrasound. Eur J Pediatr. 2022;181:1449–57.
Acknowledgements
We thank our colleagues from the NNPC Guidelines Subcommittee who provided insight and expertise in the development of these basic and advanced neonatal POCUS applications: Karena Lawrence, Cassandra Montoya, Dalibor Kurepa, Ekta Patel, Howard Chao, Indrani Bhattacharjee, John Wren, Krishna Dummula, Kristen Clark, Kshama Shah, Mark Weems, Michelle Elias Ruiz, Michelle Gontasz, Numra Aleem, Pablo Lohmann, Rachel Weinstein, Rupin Sharma, Sharada Gowda, Tom Nienaber and Vivek Saroha.
Author information
Authors and Affiliations
Consortia
Contributions
MVF, SB, CJ, MK, AMG, BC, and YS conceptualized, designed, and drafted the initial manuscript. SM, SM, KGL, CBC, JZS, SHG, JS, JLR, DVC, ASB, CP reviewed and revised the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fraga, M.V., Bhombal, S., Juliano, C. et al. Neonatal point-of-care ultrasound—guidelines for training, credentialing and quality assurance. J Perinatol 46, 113–118 (2026). https://doi.org/10.1038/s41372-025-02367-1
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41372-025-02367-1
This article is cited by
-
A step towards the expansion of neonatal point-of-care ultrasound (POCUS)
Journal of Perinatology (2026)
-
Utilization of integrated lung ultrasound and targeted neonatal echocardiography in preterm infant follow-up: is it feasible? Assessing value and practical challenges
European Journal of Pediatrics (2026)