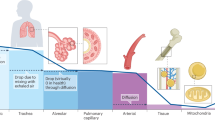
Abstract
The most abundant element on Earth, oxygen (O2) is essential for all complex, eukaryotic life. Because under- (hypoxia) or overexposure (hyperoxia) to O2 can be detrimental, achieving the ideal balance is crucial for human survival. In this perspective, we discuss the fundamental role of hemoglobin in O2 transport and tissue oxygenation. We also discuss the role that O2. can play in oxidative stress, sometimes initiating inflammatory cascades in vulnerable individuals, such as those with deficiencies in antioxidant defenses or with immune dysregulation. Preterm newborns may be especially prone to such oxidative injury.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Sanchez-Baracaldo P, Bianchini G, Wilson JD, Knoll AH. Cyanobacteria and biogeochemical cycles through Earth history. Trends Microbiol. 2022;30:143–57.
Winslow RM. Oxygen: the poison is in the dose. Transfusion. 2013;53:424–37.
Meng F, Kassa T, Jana S, Wood F, Zhang X, Jia Y, et al. Comprehensive biochemical and biophysical characterization of hemoglobin-based oxygen carrier therapeutics: All HBOCs are not created equally. Bioconjug Chem. 2018;29:1560–75.
Winslow RM. The role of hemoglobin oxygen affinity in oxygen transport at high altitude. Respir Physiol Neurobiol. 2007;158:121–7.
Pittman RN. Oxygen transport in the microcirculation and its regulation. Microcirculation. 2013;20:117–37.
Charlton M, Sims M, Coats T, Thompson JP. The microcirculation and its measurement in sepsis. J Intensive Care Soc. 2017;18:221–7.
Tsai AG, Johnson PC, Intaglietta M. Oxygen gradients in the microcirculation. Physiol Rev. 2003;83:933–63.
Wilson DF, Erecinska M. Effect of oxygen concentration on cellular metabolism. Chest. 1985;88:229S–32S.
Katona M, Gladwin MT, Straub AC. Flipping off and on the redox switch in the microcirculation. Annu Rev Physiol. 2023;85:165–89.
Benesch RE, Maeda N, Benesch R. 2,3-Diphosphoglycerate and the relative affinity of adult and fetal hemoglobin for oxygen and carbon monoxide. Biochim Biophys Acta. 1972;257:178–82.
Almeida AS, Figueiredo-Pereira C, Vieira HL. Carbon monoxide and mitochondria-modulation of cell metabolism, redox response and cell death. Front Physiol. 2015;6:33.
Di Cera E, Doyle ML, Morgan MS, De Cristofaro R, Landolfi R, Bizzi B, et al. Carbon monoxide and oxygen binding to human hemoglobin F0. Biochemistry. 1989;28:2631–8.
Leonard MB, Vreman HJ, Ferguson JE 2nd, Smith DW, Stevenson DK. Interpreting the carboxyhaemoglobin concentration in fetal cord blood. J Dev Physiol. 1989;11:73–6.
Friedman P, Guo XM, Stiller RJ, Laifer SA. Carbon monoxide exposure during pregnancy. Obstet Gynecol Surv. 2015;70:705–12.
Fossen Johnson S. Methemoglobinemia: infants at risk. Curr Probl Pediatr Adolesc Health Care. 2019;49:57–67.
Salguero KL, Cummings JJ. Inhaled nitric oxide and methemoglobin in full-term infants with persistent pulmonary hypertension of the newborn. Pulm Pharm Ther. 2002;15:1–5.
Srinivasan AJ, Morkane C, Martin DS, Welsby I. Should modulation of p50 be a therapeutic target in the critically ill? Expert Rev Hematol. 2017;10:449–58.
Seltzer JA, Bubic I, Winkler GA, Friedman NA, Bagby J, Tomaszewski CA, et al. Sulfhemoglobinemia and methemoglobinemia following acetaminophen overdose. Toxicol Rep. 2022;9:1725–7.
Narayan S, Petersen TL. Uncommon etiologies of shock. Crit Care Clin. 2022;38:429–41.
Brandenburg RO, Smith HL. Sulfhemoglobinemia; a study of 62 clinical cases. Am Heart J. 1951;42:582–8.
Esan AJ. Hematological differences in newborn and aging: a review study. Hematol Transfus Int J. 2016;3:178–90.
Hellstrom W, Martinsson T, Hellstrom A, Morsing E, Ley D. Fetal haemoglobin and bronchopulmonary dysplasia in neonates: an observational study. Arch Dis Child Fetal Neonatal Ed. 2021;106:88–92.
Prasad N, Dubey A, Kumar K, Shrivastava J. Role of fetal hemoglobin in the development and progression of retinopathy of prematurity in preterm infants. Indian J Ophthalmol. 2023;71:3478–83.
Fevereiro-Martins M, Aguiar L, Inacio A, Cardoso C, Santos AC, Marques-Neves C, et al. Fetal hemoglobin as a predictive biomarker for retinopathy of prematurity: a prospective multicenter cohort study in Portugal. Biomedicines. 2025;13:110.
Jiramongkolchai K, Repka MX, Tian J, Aucott SW, Shepard J, Collins M, et al. Lower foetal haemoglobin levels at 31- and 34-weeks post menstrual age is associated with the development of retinopathy of prematurity: PacIFiHER Report No. 1 PacIFiHER Study Group (Preterm Infants and Fetal Haemoglobin in ROP). Eye. 2021;35:659–64.
Orlando N, Pellegrino C, Valentini CG, Bianchi M, Barbagallo O, Sparnacci S, et al. Umbilical cord blood: current uses for transfusion and regenerative medicine. Transfus Apher Sci. 2020;59:102952.
Razak A, Malhotra A. Potential applications of umbilical cord blood-derived cells in neonatal diseases. Neoreviews. 2025;26:e297–e306.
Hassall OW, Thitiri J, Fegan G, Hamid F, Mwarumba S, Denje D, et al. Safety and efficacy of allogeneic umbilical cord red blood cell transfusion for children with severe anaemia in a Kenyan hospital: an open-label single-arm trial. Lancet Haematol. 2015;2:e101–7.
Bianchi M, Giannantonio C, Spartano S, Fioretti M, Landini A, Molisso A, et al. Allogeneic umbilical cord blood red cell concentrates: an innovative blood product for transfusion therapy of preterm infants. Neonatology. 2015;107:81–6.
Teofili L, Papacci P, Dani C, Cresi F, Remaschi G, Pellegrino C, et al. Cord blood transfusions in extremely low gestational age neonates to reduce severe retinopathy of prematurity: results of a prespecified interim analysis of the randomized BORN trial. Ital J Pediatr. 2024;50:142.
Ellsworth ML, Ellis CG, Popel AS, Pittman RN. Role of microvessels in oxygen supply to tissue. N Physiol Sci. 1994;9:119–23.
Klijn E, Den Uil CA, Bakker J, Ince C. The heterogeneity of the microcirculation in critical illness. Clin Chest Med. 2008;29:643–54, viii.
Rizzoni D, Mengozzi A, Masi S, Agabiti Rosei C, De Ciuceis C, Virdis A. New noninvasive methods to evaluate microvascular structure and function. Hypertension. 2022;79:874–86.
Perrone S, Bracciali C, Di Virgilio N, Buonocore G. Oxygen use in neonatal care: a two-edged sword. Front Pediatr. 2016;4:143.
Chan ED, Chan MM, Chan MM. Pulse oximetry: understanding its basic principles facilitates appreciation of its limitations. Respir Med. 2013;107:789–99.
Ross PA, Newth CJ, Khemani RG. Accuracy of pulse oximetry in children. Pediatrics. 2014;133:22–9.
Rosychuk RJ, Hudson-Mason A, Eklund D, Lacaze-Masmonteil T. Discrepancies between arterial oxygen saturation and functional oxygen saturation measured with pulse oximetry in very preterm infants. Neonatology. 2012;101:14–9.
Liao C, Zhang Q. Understanding the oxygen-sensing pathway and its therapeutic implications in diseases. Am J Pathol. 2020;190:1584–95.
Tenhunen R, Marver HS, Schmid R. Microsomal heme oxygenase. Characterization of the enzyme. J Biol Chem. 1969;244:6388–94.
Sedlak TW, Snyder SH. Bilirubin benefits: cellular protection by a biliverdin reductase antioxidant cycle. Pediatrics. 2004;113:1776–82.
Otterbein LE, Choi AM. Heme oxygenase: colors of defense against cellular stress. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1029–37.
Yet SF, Layne MD, Liu X, Chen YH, Ith B, Sibinga NE, et al. Absence of heme oxygenase-1 exacerbates atherosclerotic lesion formation and vascular remodeling. FASEB J. 2003;17:1759–61.
Poss KD, Tonegawa S. Heme oxygenase 1 is required for mammalian iron reutilization. Proc Natl Acad Sci USA. 1997;94:10919–24.
Konrad FM, Zwergel C, Ngamsri KC, Reutershan J. Anti-inflammatory effects of heme oxygenase-1 depend on adenosine A(2A)- and A(2B)-receptor signaling in acute pulmonary inflammation. Front Immunol. 2017;8:1874.
Alam J, Shibahara S, Smith A. Transcriptional activation of the heme oxygenase gene by heme and cadmium in mouse hepatoma cells. J Biol Chem. 1989;264:6371–5.
Alam J, Stewart D, Touchard C, Boinapally S, Choi AM, Cook JL. Nrf2, a Cap’n’Collar transcription factor, regulates induction of the heme oxygenase-1 gene. J Biol Chem. 1999;274:26071–8.
Igarashi K, Sun J. The heme-Bach1 pathway in the regulation of oxidative stress response and erythroid differentiation. Antioxid Redox Signal. 2006;8:107–18.
Leffler CW, Parfenova H, Jaggar JH, Wang R. Carbon monoxide and hydrogen sulfide: gaseous messengers in cerebrovascular circulation. J Appl Physiol. 2006;100:1065–76.
Zhao H, Wong RJ, Stevenson DK. The impact of hypoxia in early pregnancy on placental cells. Int J Mol Sci. 2021;22:9675.
Hartnett ME, Lane RH. Effects of oxygen on the development and severity of retinopathy of prematurity. J AAPOS. 2013;17:229–34.
Gaynon MW, Wong RJ, Stevenson DK, Sunshine P. Prethreshold retinopathy of prematurity: VEGF inhibition without VEGF inhibitors. J Perinatol. 2018;38:1295–300.
Zimmerman RA, Tsai AG, Intaglietta M, Tartakovsky DM. A mechanistic analysis of possible blood transfusion failure to increase circulatory oxygen delivery in anemic patients. Ann Biomed Eng. 2019;47:1094–105.
Tariket S, Hamzeh-Cognasse H, Laradi S, Arthaud CA, Eyraud MA, Bourlet T, et al. Evidence of CD40L/CD40 pathway involvement in experimental transfusion-related acute lung injury. Sci Rep. 2019;9:12536.
Funding
This work was supported by the Prematurity Research Fund; the March of Dimes Prematurity Research Center at Stanford University; the Charles B. and Ann L. Johnson Research Fund; the Christopher Hess Research Fund; the Providence Foundation Research Fund; the Roberts Foundation Research Fund; the Stanford Maternal and Child Health Research Institute; and the Stanford Cardiovascular Institute.
Author information
Authors and Affiliations
Contributions
DKS, RJW, JDR, IM, AMP and TLA drafted the initial manuscript, revised the manuscript, and approved the final version. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Stevenson, D.K., Wong, R.J., Reiss, J.D. et al. A clinician’s musings on oxygen: Too little or too much with life in the balance. J Perinatol 45, 1649–1652 (2025). https://doi.org/10.1038/s41372-025-02398-8
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41372-025-02398-8