Abstract
Objective
To evaluate the relationship between the incidence of surgical site infections (SSIs) and the duration of perioperative antibiotic prophylaxis (PAP) in neonatal surgery, and to identify risk factors for SSIs in neonates.
Methods
Eligible patients were neonates who underwent surgical procedures—primarily in the respiratory and gastrointestinal fields—between January 2014 and December 2023 at seven institutions. All data were retrospectively retrieved from electronic patient records. We estimated the risk difference using a modified least-squares regression model.
Results
Of the 983 patients included, 91 (9%) developed SSIs. A total of 735 patients (75%) received PAP for >24 h. There was no significant difference in risk when PAP duration was <24 h compared with ≥24 h. Independent risk factors for SSIs were an operative time exceeding 120 min, past surgical history, and open surgery.
Conclusion
In neonatal surgery, a short duration (<24 h) of PAP may not increase the risk of SSI.
Similar content being viewed by others
Perioperative antibiotic prophylaxis (PAP) is essential for the prevention of surgical site infections (SSIs). In adult surgery, sufficient evidence-based guidelines recommend short-term administration of PAP, i.e., discontinuing PAP within 24 h [1, 2]. In pediatric surgery, however, there was historically a lack of evidence, and guidelines for adults were often extrapolated or PAP was administered empirically. As a result, PAP tended to be prolonged. Evidence for pediatric patients has recently accumulated [3,4,5,6], and short-term administration is now also recommended and gradually becoming more widespread [7].
The situation in neonatal surgery is different. Some studies [8,9,10] suggest that short-term administration of PAP might be safe and effective for neonates as well as older children, and one of the few guidelines [11] recommends short-term administration of PAP for neonates. However, the latest studies reveal that PAP for neonates is still being administered for a long duration [12, 13].
Prolonged antibiotic administration is well known to be associated with many adverse effects, such as nephrotoxicity, Clostridium difficile infection [14], and multidrug-resistant organisms [15]. In recent years, antibiotic use in early childhood has also been implicated in the development of several diseases, such as asthma and inflammatory bowel disease, through alterations in the gut flora [16, 17]. This makes it an even more important issue for neonates, who have a long life ahead of them.
Unfortunately, this trend of long-term administration of PAP for neonates has been noted for 30 years [18], and the situation has not changed [12, 13]. The main reason for this is thought to be the weak level of evidence regarding PAP in neonates. There are no randomized controlled trials and only a few observational studies on PAP in this population. Conducting randomized controlled trials in neonatal surgery is challenging because of ethical concerns, the wide variety of comorbidities, and the small number of cases. Additionally, neonates often receive therapeutic antibiotics for hospital-acquired infections during the perioperative period because they are highly vulnerable to infection due to their immature immune systems [11]. These factors make it difficult to evaluate the efficacy of PAP in preventing SSIs in neonatal surgery.
The aims of this study were to evaluate the relationship between the incidence of SSIs and the duration of PAP in neonatal surgery and to identify risk factors for SSIs in neonates. To achieve these goals, we conducted a multi-institutional retrospective observational study involving several high-volume pediatric centers in Japan and collected detailed data on antibiotic usage from medical records.
Methods
Study design
This multi-institutional retrospective observational study was conducted at seven institutions: The University of Tokyo Hospital, National Center for Child Health and Development, Saitama Children’s Medical Center, Gunma Children’s Medical Center, Saitama Medical University Hospital, Kitasato University Hospital, and Japanese Red Cross Medical Center.
Patients
Eligible patients were children who underwent surgical procedures—mainly in the respiratory and gastrointestinal fields—during the neonatal period (within 28 days of birth) between 1 January 2014 and 31 December 2023 at the seven participating institutions. Patients were excluded if surgery was performed for peritonitis (due to intestinal perforation, necrotizing enterocolitis, or other causes), gastroschisis, omphalocele, biliary atresia, or myelomeningocele because these conditions inherently require prolonged perioperative antibiotic administration [19]. Patients who developed or were suspected of having a hospital-acquired infection other than SSI—such as urinary tract infection, pneumonia, or catheter-related bloodstream infection—after surgery and received therapeutic antibiotics were also excluded. Additionally, SSI was defined as occurring within 30 days postoperatively, and cases that could not be followed for the full 30 days were excluded from the analysis.
Data
All data were retrieved from electronic patient records. The variables assessed included sex, birth weight, gestational age at birth, birth place (in-hospital/out-of-hospital), mode of delivery, Apgar score (1 min/5 min), presence of underlying diseases such as chromosomal abnormalities or malformation syndromes, surgical diagnosis, surgical procedure, age and weight at surgery, surgical duration, American Society of Anesthesiologists (ASA) classification, wound class, past surgical history, SSI (occurrence, site, and day of occurrence since surgery), preoperative colonization of methicillin-resistant Staphylococcus aureus, and PAP (type and duration).
The duration of PAP was defined as the period from the start of the operation to the last administration of PAP. For patients with SSI, the duration of PAP was counted up to the day before the onset of SSI, even if antibiotics were continued for treatment of the infection.
The definitions of SSI and wound class followed the Centers for Disease Control and Prevention (CDC) manual [20]. In this classification, all small bowel operations—with or without opening the intestinal lumen (e.g., Ladd procedure and surgery for adhesive small bowel obstruction)—were classified as clean-contaminated. Operations with a dirty/infected wound class were excluded from the study because of the need for therapeutic antibiotic administration.
Statistical analysis
For descriptive statistics, continuous variables are presented as medians and interquartile ranges, while categorical variables are presented as frequencies and percentages.
To evaluate the association between the incidence of SSIs and the duration of PAP, we estimated the risk difference using a modified least-squares regression model and calculated 95% confidence intervals and P values [21]. The following variables—identified from previous studies and clinical judgment as potential risk factors—were included in the multivariable analysis: duration of PAP (<24 h, including no PAP, vs. ≥24 h), premature birth (<37 weeks vs. ≥37 weeks), Apgar score at 5 min (<4 vs. ≥4), age at operation (0 day vs. 1–2 days vs. ≥3 days), weight at surgery (<1500 g vs. ≥1500 g), operative time (<120 min vs. ≥120 min), ASA classification (<3 vs. ≥3), surgical approach (surface vs. open vs. thoracoscopy/laparoscopy), wound class (clean vs. clean-contaminated), and past surgical history (yes vs. no). Cases with missing variables were excluded from the multivariable analysis.
In addition, to adjust for two further confounders—institutions and surgical procedures—inverse probability weighting (IPW) using propensity scores was applied to prevent overfitting in the multivariable model, given the limited number of SSIs relative to the number of explanatory variables. The propensity scores were estimated using a logistic regression model that included key covariates from the primary analysis, along with institutions and surgical procedures. Surgical approach and wound class were excluded from the propensity score model because of their strong correlation with surgical procedures; this avoided multicollinearity and improved model stability. To assess the balance of confounders after weighting, a standardized mean difference plot was visually inspected, with a standardized mean difference of <0.2 considered indicative of good balance.
Statistical analyses were performed using JMP® Pro version 17.2.0 and SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Cohort description
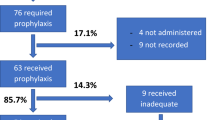
In total, 1643 neonates underwent surgical procedures during the study period at the 7 institutions. Of these, 660 patients were excluded based on the exclusion criteria, resulting in a final study population of 983 patients (Fig. 1). Among the excluded patients, 34 deaths occurred within 30 days, but none were attributed to SSI.
Incidence of SSIs and duration of PAP
The background characteristics of all 983 patients, as well as those in the No-SSI and SSI subgroups, are shown in Table 1. A total of 91 patients (9%) developed SSIs, with 79 cases (87%) classified as superficial SSIs. The median day of SSI occurrence was 10 days [6–20 days] postoperatively (Supplement 1).
Details of PAP administration are presented in Table 2 and Supplement 2. A total of 950 patients (97%) received PAP, with a median duration of 3 days [2–4 days]. Of these, 735 patients (75%) received PAP for more than 24 h. First- and second-generation cephalosporins were used in most cases, at rates of 35% and 41%, respectively.
Supplement 3 and Supplement 4 show trends over time in PAP duration and SSI rate, respectively. Although there was a gradual trend toward shorter PAP duration, no clear trend was observed in the SSI rate. Figure 2 illustrates the relationship between PAP duration and SSI rate across institutions. There was no clear association between the median duration of PAP and the SSI rate among the institutions.
The duration of perioperative antibiotic prophylaxis and the SSI rate at the seven participating hospitals are shown. Diamonds indicate the median duration of antibiotics, with vertical bars showing interquartile ranges. The width of each box represents the number of cases at that institution. SSI surgical site infection.
Multivariable regression analysis: relationship between incidence of SSIs and duration of PAP
Table 3 shows the results of the multivariable analysis using a modified least-squares regression model to estimate risk differences for factors associated with the incidence of SSIs. Seventy-five patients with missing data on risk factors (including eight patients with SSI) were excluded from the analysis.
There was no significant difference in risk when PAP was administered for ≤24 h vs. >24 h; the risk difference was −2.7% (95% confidence interval: −6.6% to 1.3%, P = 0.187). Similarly, no significant differences were observed when patients were grouped by PAP duration of ≤48 h vs. >48 h and ≤72 h vs. >72 h; the risk differences were 1.3% (95% confidence interval: −2.6% to 5.2%, P = 0.522) and 2.5% (95% confidence interval: −1.6% to 6.7%, P = 0.228), respectively.
Consistent results were obtained when additional confounders—institutions and surgical procedures—were included using IPW of the propensity scores. This analysis yielded a risk difference of −1.9% (for PAP duration ≤24 h vs. >24 h; 95% confidence interval: −7.2% to 3.4%, P = 0.482), with good covariate balance achieved (Supplement 5).
Other risk factors for SSI
In the multivariable analysis using modified least-squares regression models, the following factors were significantly associated with an increased risk of SSI: operative time exceeding 120 min (risk difference: 5.7%, 95% confidence interval: 1.3% to 10.2%, P = 0.012), past surgical history (risk difference: 13.3%, 95% confidence interval: 2.4% to 24.1%, P = 0.017), and open surgery (risk difference: 5.9%, 95% confidence interval: 2.0% to 9.8%, P = 0.003) (Table 3).
Discussion
This study retrospectively investigated PAP usage in 983 neonates who underwent surgery at multiple institutions and evaluated the relationship between the incidence of SSIs and the duration of PAP administration in neonatal surgery. A total of 950 patients (97%) received PAP, with approximately three quarters receiving it for ≥24 h. SSIs occurred in 91 of 983 patients (9%). In the multivariable analysis using a modified least-squares regression model and IPW of the propensity scores, no significant risk difference was observed when the PAP duration was ≤24 h compared with >24 h. However, a long operative time (≥120 min), past surgical history, and open surgery were significantly associated with an increased risk of SSI.
Previous studies of SSIs in neonatal surgery can be broadly divided into two main categories: those that investigated the incidence of SSIs and explored risk factors [22,23,24,25,26], and those that attempted to clarify the appropriate usage of PAP—both its type and duration [8, 9, 12, 13]. In this study, we addressed both themes by collecting a large volume of data related to perinatal factors, perioperative conditions, and antibiotic usage in neonates undergoing surgery. A unique feature of our study is the focus on “prophylactic” antibiotic use, achieved by excluding cases involving “therapeutic” antibiotic administration. While evidence on the incidence and risk factors of SSIs in neonatal surgery is gradually accumulating, data regarding the appropriate use of PAP remain limited. One major reason for this gap is that neonates often receive therapeutic antibiotics during the perioperative period [11]. For example, contaminated operations—such as those for intestinal perforation caused by immaturity of the intestinal tract—are more common in neonates. In such cases, antibiotics are used for treatment rather than prophylaxis, and prolonged administration is appropriate. Furthermore, because of their immunological immaturity, neonates are especially vulnerable to hospital-acquired infections such as urinary tract infections, pneumonia, and catheter-related bloodstream infections. As a result, antibiotic use often transitions from prophylactic to therapeutic. Previous studies [12, 13]—including those using large databases [6]—have not been able to clearly distinguish between prophylactic and therapeutic antibiotic use. By contrast, our study excluded cases involving therapeutic antibiotic administration for contaminated surgery or other infections. Therefore, we believe our analysis more accurately reflects the usage of prophylactic antibiotics in neonatal surgery.
In adult surgery, PAP administration typically does not exceed 24 h, except in special cases [1, 2]. In our study, even after excluding cases involving therapeutic antibiotic use, PAP was still administered for extended durations (>24 h) in many cases. This trend of prolonged administration is consistent with recent reports in neonatal surgery [12, 13].
Significant risk factors for SSIs identified in this study, such as a long operative time and past surgical history, have also been reported in previous studies on neonatal surgery [23, 26]. Other factors that are often considered clinically high risk for infection, such as low weight at surgery and short gestational age, did not show significant risk differences. Open surgery, another significant risk factor identified in our study, is also recognized as a risk factor in adult surgery [27]. However, there have been few laparoscopic or thoracoscopic procedures performed in neonates, and no prior studies have compared open surgery with laparoscopy as a risk factor for SSIs in this population. More cases involving laparoscopic surgery need to be accumulated to clarify whether laparoscopy or thoracoscopy is associated with the risk of neonatal SSI.
The incidence of SSIs in our study (9%) is comparable to that reported in previous studies of neonates, which range from 4.3% to 13.5% [22,23,24,25,26]. Although SSI rates vary widely depending on factors such as country, disease, and surgical procedure [2], the rate observed in our study appears higher than the recent overall rate in adults in Japan (5%) [28], but similar to the rate in the 2000s (approximately 10%). As SSI rates in adults have declined over time—partly due to improvements in perioperative management and the implementation of SSI surveillance—similar efforts, including the appropriate use of PAP, may contribute to a reduction in SSI rates in neonatal surgery as well.
Our study has important implications for the appropriate use of PAP. Regarding the duration of PAP, we categorized and compared durations of ≤24 h vs. >24 h, ≤48 h vs. >48 h, and ≤72 h vs. >72 h. In each comparison, shorter duration was not associated with an increased risk of SSI. Our findings are consistent with those of a previous study [8] on PAP in a mixed population of neonates and infants, which included 194 neonates and is often cited as evidence supporting short-term PAP in this population. Our study, which examined a larger cohort of 983 neonates, reached the same conclusion. We believe this is an important finding that supports and promotes the short and appropriate use of antibiotics in neonatal surgery.
This study has several limitations inherent to its design as a multi-institutional retrospective observational study without a unified perioperative protocol. First, we could not evaluate certain factors related to SSIs—such as preoperative skin preparation, timing of intraoperative antibiotic administration, and perioperative incision management—which may vary by institution or individual surgeon. Second, the participating institutions included a general hospital with a perinatal center, university hospitals, and children’s hospitals, introducing heterogeneity in patient backgrounds, although we adjusted for potential confounding factors identifiable from medical records. Third, both the type and duration of PAP were determined by the attending physician (pediatric surgeons or neonatologists) because there was no standardized protocol within each institution. Fourth, despite the large sample size, it was not possible to assess whether narrow-spectrum antibiotics were associated with SSI because broad-spectrum antibiotics were used in only a few cases. However, the incidence of SSI in this study was not higher than that reported in previous studies. Although broad-spectrum antibiotics are recommended for certain surgical procedures (e.g., sulbactam/ampicillin or tazobactam/piperacillin for esophageal anastomosis) [11, 29], narrow-spectrum antibiotics may be sufficient in neonates. Finally, the diagnosis of SSI relied on documentation in medical records, which may have led to overdiagnosis or underdiagnosis.
While antibiotic use clearly contributes to the prevention of SSIs, it is only one of many preventive measures, as noted in the CDC manual [2]. The lack of a consistent trend between the duration of PAP and the incidence of SSIs across facilities (Fig. 2) may also suggest that PAP administration alone is not sufficient and that comprehensive perioperative management is essential for SSI prevention. The next step in determining appropriate PAP use in neonates is to conduct a prospective observational study based on an antibiotic use protocol combined with unified, standardized perioperative management [30].
Conclusion
This observational study examined the relationship between the incidence of SSIs and the duration of PAP in neonatal surgery. The results suggest that a short duration (<24 h) of PAP may not increase the risk of SSI. However, PAP was administered for relatively long periods in many cases, indicating that further efforts are needed to define and promote appropriate durations of PAP administration.
Data availability
The datasets generated during/or analyzed during the current study and the code used to analyze and manage the data are available from the corresponding author on reasonable request.
References
Berríos-Torres SI, Umscheid CA, Bratzler DW, Leas B, Stone EC, Kelz RR, et al. Centers for disease control and prevention guideline for the prevention of surgical site infection, 2017. JAMA Surg. 2017;152:784–91.
Global Guidelines for the Prevention of Surgical Site Infection. Geneva: World Health Organization; 2018. https://www.who.int/publications/i/item/9789241550475 Accessed June 20, 2025.
Horwitz JR, Chwals WJ, Doski JJ, Suescun EA, Cheu HW, Lally KP. Pediatric wound infections: a prospective multicenter study. Ann Surg. 1998;227:553–8.
Khoshbin A, So JP, Aleem IS, Stephens D, Matlow AG, Wright JG. SickKids surgical site infection task force. antibiotic prophylaxis to prevent surgical site infections in children: a prospective cohort study. Ann Surg. 2015;262:397–402.
He K, Nayak RB, Allori AC, Brighton BK, Cina RA, Ellison JS, et al. Correlation between postoperative antimicrobial prophylaxis use and surgical site infection in children undergoing nonemergent surgery. JAMA Surg. 2022;157:1142–51.
He K, Iwaniuk M, Goretsky MJ, Cina RA, Saito JM, Hall B, et al. Incidence and relative burden of surgical site infections in children undergoing nonemergent surgery: implications for performance benchmarking and prioritization of prevention efforts. Ann Surg. 2023;278:280–7.
So JP, Aleem IS, Tsang DS, Matlow AG, Wright JG. SickKids surgical site infection task force. increasing compliance with an antibiotic prophylaxis guideline to prevent pediatric surgical site infection: before and after study. Ann Surg. 2015;262:403–8.
Vu LT, Vittinghoff E, Nobuhara KK, Farmer DL, Lee H. Surgical site infections in neonates and infants: is antibiotic prophylaxis needed for longer than 24 h? Pediatr Surg Int. 2014;30:587–92.
Walker S, Datta A, Massoumi RL, Gross ER, Uhing M, Arca MJ. Antibiotic stewardship in the newborn surgical patient: a quality improvement project in the neonatal intensive care unit. Surgery. 2017;162:1295–303.
Takayasu H, Tanaka K, Konishi K, Uematsu Y, Tomari T, Kumamoto Y. Postoperative continuation of antibiotic prophylaxis beyond 24 h is unnecessary for abdominal surgeries in children and neonates. J Pediatr Surg Open. 2024;8:100179.
Laituri C, Arnold MA. A standardized guideline for antibiotic prophylaxis in surgical neonates. Semin Pediatr Surg. 2019;28:53–56.
Wilhelm S, Tolkacz M, Kopel L, Stallion A, Novotny NM, Akay B, et al. Duration of perioperative antibiotic prophylaxis in neonatal surgery: Less is more. Am J Surg. 2024;236:115901.
Jadhav P, Choi PM, Ignacio R, Keller B, Gollin G. Antibiotic management after neonatal enteric operations in US Children’s Hospitals. J Pediatr Surg. 2025;60:162052.
Branch-Elliman W, O’Brien W, Strymish J, Itani K, Wyatt C, Gupta K. Association of duration and type of surgical prophylaxis with antimicrobial-associated adverse events. JAMA Surg. 2019;154:590–8.
Harbarth S, Samore MH, Lichtenberg D, Carmeli Y. Prolonged antibiotic prophylaxis after cardiovascular surgery and its effect on surgical site infections and antimicrobial resistance. Circulation. 2000;101:2916–21.
Hviid A, Svanström H, Frisch M. Antibiotic use and inflammatory bowel diseases in childhood. Gut. 2011;60:49–54.
Yamamoto-Hanada K, Yang L, Narita M, Saito H, Ohya Y. Influence of antibiotic use in early childhood on asthma and allergic diseases at age 5. Ann Allergy Asthma Immunol. 2017;119:54–58.
Fallat ME, Kristin AM. Random practice patterns of surgical antimicrobial prophylaxis in neonates. Pediatr Surg Int. 1994;9:479–82.
Wallace MW, Danko ME, Zamora IJ, Morris EA, Li J, Froehlich M, et al. Infectious Complications and Antibiotic Use in Gastroschisis. Surg Infect. 2023;24:405–13.
National Healthcare Safety Network. Surgical site infection (SSI) event; 2025. https://www.cdc.gov/nhsn/pdfs/pscmanual/9pscssicurrent.pdf Accessed June, 2025.
Hagiwara Y, Fukuda M, Matsuyama Y. The number of events per confounder for valid estimation of risk difference using modified least-squares regression. Am J Epidemiol. 2018;187:2481–90.
Segal I, Kang C, Albersheim SG, Skarsgard ED, Lavoie PM. Surgical site infections in infants admitted to the neonatal intensive care unit. J Pediatr Surg. 2014;49:381–4.
Clements KE, Fisher M, Quaye K, O’Donnell R, Whyte C, Horgan MJ. Surgical site infections in the NICU. J Pediatr Surg. 2016;51:1405–8.
Inoue M, Uchida K, Ichikawa T, Nagano Y, Matsushita K, Koike Y, et al. Contaminated or dirty wound operations and methicillin-resistant Staphylococcus aureus (MRSA) colonization during hospitalization may be risk factors for surgical site infection in neonatal surgical patients. Pediatr Surg Int. 2018;34:1209–14.
Woldemicael AY, Bradley S, Pardy C, Richards J, Trerotoli P, Giuliani S. Surgical site infection in a tertiary neonatal surgery centre. Eur J Pediatr Surg. 2019;29:260–5.
Yamamichi T, Yoshida M, Sakai T, Takayama K, Uga N, Umeda S, et al. Factors associated with neonatal surgical site infection after abdominal surgery. Pediatr Surg Int. 2022;38:317–23.
Kagawa Y, Yamada D, Yamasaki M, Miyamoto A, Mizushima T, Yamabe K, et al. The association between the increased performance of laparoscopic colon surgery and a reduced risk of surgical site infection. Surg Today. 2019;49:474–81.
Morikane K. Epidemiology and prevention of surgical site infection in Japan. J Hosp Infect. 2024;146:192–8.
Ichikawa S, Ishihara M, Okazaki T, Warabi K, Kato Y, Hori S, et al. Prospective study of antibiotic protocols for managing surgical site infections in children. J Pediatr Surg. 2007;42:1002–7.
Tobias J, Padilla BE, Lee J, Chen S, Wang KS, Kelley-Quon LI, et al. Standardized perioperative care reduces colorectal surgical site infection in children: a Western Pediatric Surgery Research Consortium multicenter analysis. J Pediatr Surg. 2023;58:45–51.
Funding
Open Access funding provided by The University of Tokyo.
Author information
Authors and Affiliations
Contributions
TS conceptualized and designed the study, collected data, carried out the initial analyses, and drafted the initial manuscript. JF coordinated and supervised data collection, and critically reviewed and revised the manuscript. KO and YM carried out analyses, and critically reviewed and revised the manuscript. TI, CO, HK, AN, KM, KT, HT, YT, KO, RY, AI critically reviewed and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics
The study was conducted with the approval of the Ethics Committee of the University of Tokyo Graduate School of Medicine and Faculty of Medicine (approval number: 2024212NI) and with permission obtained from each participating institution. Given the retrospective nature of the study, the Ethics Committee did not require informed consent and all methods were performed in accordance with the relevant guidelines and regulations. The study was performed in accordance with the Declaration of Helsinki.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sunouchi, T., Fujishiro, J., Oba, K. et al. Impact of prophylactic antibiotic duration on surgical site infection rate in neonatal surgery: a multicenter retrospective observational study. J Perinatol 45, 1443–1449 (2025). https://doi.org/10.1038/s41372-025-02400-3
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41372-025-02400-3