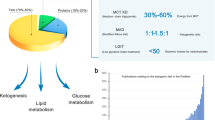
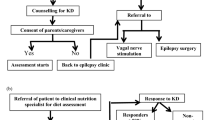
Abstract
The application of ketogenic diet (KD) in newborns is rare and mainly reserved for specific metabolic and seizure disorders. Furthermore, only case reports describe the effects of KD in premature infants. Here, we summarize the recent advances, indications, mechanisms of action and practical issues related to KD. We also provide a paradigm of a preterm male infant born at 34 weeks with pyruvate dehydrogenase deficiency, highlighting the therapeutic challenges and outcomes with the KD. This report underscores the complexities of managing metabolic disorders in neonates and the effects of KD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
No datasets are available since this is a review/perspective article.
References
Nordli DR Jr., Kuroda MM, Carroll J, Koenigsberger DY, Hirsch LJ, Bruner HJ, et al. Experience with the ketogenic diet in infants. Pediatrics. 2001;108:129–33.
Youngson NA, Morris MJ, Ballard JWO. The mechanisms mediating the antiepileptic effects of the ketogenic diet, and potential opportunities for improvement with metabolism-altering drugs. Seizure. 2017;52:15–9.
Wilder R. The effects of ketonemia on the course of epilepsy. Mayo Clin Bull. 1920;2:307–8.
Martin K, Jackson CF, Levy RG, Cooper PN. Ketogenic diet and other dietary treatments for epilepsy. Cochrane Database Syst Rev. 2016;2:CD001903.
Martin-McGill KJ, Bresnahan R, Levy RG, Cooper PN. Ketogenic diets for drug-resistant epilepsy. Cochrane Database Syst Rev. 2020;6:CD001903.
Phitsanuwong C, Kim JA, Schimpf S, Nordli DR Jr. Experience with the ketogenic diet in premature neonates. Epilepsia Open. 2023;8:200–4.
van der Louw E, van den Hurk D, Neal E, Leiendecker B, Fitzsimmon G, Dority L, et al. Ketogenic diet guidelines for infants with refractory epilepsy. Eur J Paediatr Neurol. 2016;20:798–809.
Wells J, Swaminathan A, Paseka J, Hanson C. Efficacy and Safety of a Ketogenic Diet in Children and Adolescents with Refractory Epilepsy-A Review. Nutrients. 2020;12:1809.
Thompson L, Fecske E, Salim M, Hall A. Use of the ketogenic diet in the neonatal intensive care unit-Safety and tolerability. Epilepsia. 2017;58:e36–e9.
Dressler A, Trimmel-Schwahofer P, Reithofer E, Groppel G, Muhlebner A, Samueli S, et al. The ketogenic diet in infants-advantages of early use. Epilepsy Res. 2015;116:53–8.
Kossoff EH, Zupec-Kania BA, Auvin S, Ballaban-Gil KR, Christina Bergqvist AG, Blackford R, et al. Optimal clinical management of children receiving dietary therapies for epilepsy: updated recommendations of the International Ketogenic Diet Study Group. Epilepsia Open. 2018;3:175–92.
Hawkes CP, Roy SM, Dekelbab B, Frazier B, Grover M, Haidet J, et al. HypercAlcemia In Children Using The Ketogenic Diet: A Multicenter Study. J Clin Endocrinol Metab. 2021;106:e485–e95.
Armeno ML, Kossoff EH. Let food be thy medicine. The interaction between ketogenic diet therapy and anti-seizure medications: a systematic review. Epileptic Disord. 2023;25:18–27.
Dahlin MG, Beck OM, Amark PE. Plasma levels of antiepileptic drugs in children on the ketogenic diet. Pediatr Neurol. 2006;35:6–10.
Coppola G, Verrotti A, D’Aniello A, Arcieri S, Operto FF, Della Corte R, et al. Valproic acid and phenobarbital blood levels during the first month of treatment with the ketogenic diet. Acta Neurol Scand. 2010;122:303–7.
Welzel T, Ziesenitz VC, Weber P, Datta AN, van den Anker JN, Gotta V. Drug-drug and drug-food interactions in an infant with early-onset SCN2A epilepsy treated with carbamazepine, phenytoin and a ketogenic diet. Br J Clin Pharm. 2021;87:1568–73.
He F, Ye L, Wang L, Zhou J, Shao X, Miao P, et al. Ketogenic diet therapy leads to antiseizure medication reduction in children and adults with drug-resistant epilepsy. CNS Neurosci Ther. 2024;30:e14854.
Inui T, Wada Y, Shibuya M, Arai-Ichinoi N, Okubo Y, Endo W, et al. Intravenous ketogenic diet therapy for neonatal-onset pyruvate dehydrogenase complex deficiency. Brain Dev. 2022;44:244–8.
Rho JM. How does the ketogenic diet induce anti-seizure effects?. Neurosci Lett. 2017;637:4–10.
Bough KJ, Rho JM. Anticonvulsant mechanisms of the ketogenic diet. Epilepsia. 2007;48:43–58.
Danial NN, Hartman AL, Stafstrom CE, Thio LL. How does the ketogenic diet work? Four potential mechanisms. J Child Neurol. 2013;28:1027–33.
Likhodii SS, Serbanescu I, Cortez MA, Murphy P, Snead OC 3rd, et al. Anticonvulsant properties of acetone, a brain ketone elevated by the ketogenic diet. Ann Neurol. 2003;54:219–26.
Sullivan PG, Dube C, Dorenbos K, Steward O, Baram TZ. Mitochondrial uncoupling protein-2 protects the immature brain from excitotoxic neuronal death. Ann Neurol. 2003;53:711–7.
Thio LL, Wong M, Yamada KA. Ketone bodies do not directly alter excitatory or inhibitory hippocampal synaptic transmission. Neurology. 2000;54:325–31.
Fraser DD, Whiting S, Andrew RD, Macdonald EA, Musa-Veloso K, Cunnane SC. Elevated polyunsaturated fatty acids in blood serum obtained from children on the ketogenic diet. Neurology. 2003;60:1026–9.
Dahlin M, Elfving A, Ungerstedt U, Amark P. The ketogenic diet influences the levels of excitatory and inhibitory amino acids in the CSF in children with refractory epilepsy. Epilepsy Res. 2005;64:115–25.
DeVivo DC, Leckie MP, Ferrendelli JS, McDougal DB Jr. Chronic ketosis and cerebral metabolism. Ann Neurol. 1978;3:331–37.
Shimazu T, Hirschey MD, Newman J, He W, Shirakawa K, Le Moan N, et al. Suppression of oxidative stress by beta-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science. 2013;339:211–4.
Rowley S, Patel M. Mitochondrial involvement and oxidative stress in temporal lobe epilepsy. Free Radic Biol Med. 2013;62:121–31.
Bough KJ, Wetherington J, Hassel B, Pare JF, Gawryluk JW, Greene JG, et al. Mitochondrial biogenesis in the anticonvulsant mechanism of the ketogenic diet. Ann Neurol. 2006;60:223–35.
Masino SA, Li T, Theofilas P, Sandau US, Ruskin DN, Fredholm BB, et al. A ketogenic diet suppresses seizures in mice through adenosine A(1) receptors. J Clin Invest. 2011;121:2679–83.
Taha AY, Ryan MA, Cunnane SC. Despite transient ketosis, the classic high-fat ketogenic diet induces marked changes in fatty acid metabolism in rats. Metabolism. 2005;54:1127–32.
Cunnane SC, Musa K, Ryan MA, Whiting S, Fraser DD. Potential role of polyunsaturates in seizure protection achieved with the ketogenic diet. Prostaglandins Leukot Ess Fat Acids. 2002;67:131–5.
Yehuda S, Carasso RL, Mostofsky DI. Essential fatty acid preparation (SR-3) raises the seizure threshold in rats. Eur J Pharm. 1994;254:193–8.
Voskuyl RA, Vreugdenhil M, Kang JX, Leaf A. Anticonvulsant effect of polyunsaturated fatty acids in rats, using the cortical stimulation model. Eur J Pharm. 1998;341:145–52.
Yuen AW, Sander JW, Fluegel D, Patsalos PN, Bell GS, Johnson T, et al. Omega-3 fatty acid supplementation in patients with chronic epilepsy: a randomized trial. Epilepsy Behav. 2005;7:253–8.
Bromfield E, Dworetzky B, Hurwitz S, Eluri Z, Lane L, Replansky S, et al. A randomized trial of polyunsaturated fatty acids for refractory epilepsy. Epilepsy Behav. 2008;12:187–90.
Patel KP, O’Brien TW, Subramony SH, Shuster J, Stacpoole PW. The spectrum of pyruvate dehydrogenase complex deficiency: clinical, biochemical and genetic features in 371 patients. Mol Genet Metab. 2012;106:385–94.
Stanley CA, Anday EK, Baker L, Delivoria-Papadopolous M. Metabolic fuel and hormone responses to fasting in newborn infants. Pediatrics. 1979;64:613–9.
Prins ML. Cerebral ketone metabolism during development and injury. Epilepsy Res. 2012;100:218–23.
Sofou K, Dahlin M, Hallbook T, Lindefeldt M, Viggedal G, Darin N. Ketogenic diet in pyruvate dehydrogenase complex deficiency: short- and long-term outcomes. J Inherit Metab Dis. 2017;40:237–45.
Persson B, Settergren G, Dahlquist G. Cerebral arterio-venous difference of acetoacetate and D-hydroxybutyrate in children. Acta Paediatr Scand. 1972;61:273–8.
Angelis D, Jaleel MA, Brion LP. Hyperglycemia and prematurity: a narrative review. Pediatr Res. 2023;94:892–903.
Herrero-Mendez A, Almeida A, Fernandez E, Maestre C, Moncada S, Bolanos JP. The bioenergetic and antioxidant status of neurons is controlled by continuous degradation of a key glycolytic enzyme by APC/C-Cdh1. Nat Cell Biol. 2009;11:747–52.
Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci USA. 1994;91:10625–9.
Izumi Y, Benz AM, Zorumski CF, Olney JW. Effects of lactate and pyruvate on glucose deprivation in rat hippocampal slices. Neuroreport. 1994;5:617–20.
Angelis D, Brion LP, Chalak L. L-lactate provision in preterm newborns: friend or foe? Pediatr Res. 2025. https://doi.org/10.1038/s41390-025-04104-y. Epub ahead of print.
Omar Ibrahim I, Perrot C, Roumes H, Beauvieux MC, Brissaud O, Cramaregeas S, et al. Positive impact of sodium L-lactate supplementation on blood acid-base status in preterm newborns. Pediatr Res. 2025. https://doi.org/10.1038/s41390-025-03963-9. Epub ahead of print.
Acknowledgements
We would like to acknowledge the Crystal Charity Ball and the NeuroNICU program at the University of Texas Southwestern Medical Center for supporting this project. Figure 1 was created with BioRender.com and appropriate permission has been obtained (#v25k736).
Funding
No financial support was received for the development of this review.
Author information
Authors and Affiliations
Contributions
SK wrote the initial draft and critically reviewed the manuscript and approved it as submitted. DA contributed to the concept of the paper, wrote part of the initial and revised drafts of this manuscript, and approved the final manuscript as submitted. LAU, RC, JT, and MM contributed equally to the conceptualization of the paper, reviewed, and revised the manuscript, and approved the final manuscript as submitted.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study was approved by the University of Texas Southwestern Institutional Review Board, and informed parental consent was waived per IRB guidelines. All methods were performed in accordance with the relevant guidelines and regulations.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Katsoudas, S., Umana, L.A., Clarke, R. et al. Ketogenic diet in neonates: effects, pitfalls, and a paradigm from a preterm newborn affected by pyruvate dehydrogenase deficiency. J Perinatol (2025). https://doi.org/10.1038/s41372-025-02427-6
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41372-025-02427-6