Abstract
When two lymphomas occur concurrently or sequentially in a patient, it is a major question whether they derive from the same lymphocyte or hematopoietic precursor cell or developed independently. We studied four composite classic Hodgkin lymphomas (HL) and other mature B-cell lymphomas, and two composite mature B- and T-cell neoplasias by whole exome sequencing (WES). Analysis of their IGV genes revealed that three composite B-cell lymphomas originated from common germinal center-experienced B cells. WES identified shared somatic mutations in the lymphomas of these clonally related composite lymphomas, indicating their derivation from a common, pre-malignant precursor. Most mutations were restricted to one or the other of these lymphomas, likely explaining how distinct lymphomas developed from a common ancestral B cell. In the two B-cell/T-cell lymphoma cases, and a composite clonally unrelated HL/chronic lymphocytic leukemia, the lymphoma partners did not share any somatic mutations. In three cases, we identified potentially oncogenic variants also in cells serving as constitutional controls. These variants may have contributed to development of a composite lymphoma/leukemia. We provide additional evidence of frequent clonal relation in composite lymphomas, highlight the multistep transformation process of related lymphomas with a likely pre-malignant intermediate common precursor, and support the importance of constitutional variants in lymphomagenesis.
Similar content being viewed by others
Introduction
In rare instances, two histopathologically distinct lymphomas occur concurrently in a patient, which is termed a composite lymphoma [1, 2]. Many composite lymphomas consist of a Hodgkin lymphoma (HL) and another mature B-cell lymphoma. Sequentially occurring histopathologically distinct lymphomas are also considered composite lymphomas in this study, because they have a similar pathogenesis as concurrent composite lymphomas [1]. It is a major question whether synchronous and metachronous composite lymphomas are a chance occurrence of two hematological malignancies in a patient, or whether they have a common origin. In more than half of the composite lymphomas consisting of a HL and another B-cell lymphoma investigated, the cases were clonally related and derived from the same mature B cells, as evidenced by common immunoglobulin (IG) V gene rearrangements [1, 3, 4]. If two distinct lymphomas derive from a common precursor, it is likely that the latter carried shared genetic lesions and hence represented a pre-malignant cell. Moreover, the lymphomas likely also carry distinct genetic lesions explaining the distinct histopathology of the lymphomas. This issue was so far addressed by a candidate gene approach for a few genes in a small number of cases [2, 5,6,7], revealing, for example, that the prototypical BCL2::IGH translocation was present in both partners in composite HL and follicular lymphoma (FL) [1, 2, 5, 8]. Recently, deep sequencing approaches indicated that composite lymphomas share some genetic lesions, but also carry distinct lesions [9,10,11,12]. However, these studies were restricted to selected gene panels, and with one exception performed on whole tissue sections or macrodissected areas, so that an unequivocal assignment of mutations to one and/or the other lymphoma remained uncertain.
In some types of lymphomas and leukemias representing transformed mature B or T cells [e.g., chronic lymphocytic leukemia (CLL), hairy cell leukemia], the first somatic mutation(s) can already occur in hematopoietic stem or precursor cells (HSC/HPC) [13,14,15]. Additionally, mutations in particular genes, such as TET2, can be found in descendants of clonally expanded HSC/HPC in elderly individuals [16]. Hence, combinations of a B- and a T-cell malignancy in a patient may also originate from a common HSC/HPC with somatic mutations. Furthermore, constitutional genetic variants might also contribute to the development of two lymphomas in one patient [17, 18].
In the present study, we performed whole exome sequencing (WES) of isolated lymphoma and non-tumor cells of four composite mature B-cell neoplasias, each with a classic HL as one of the partners, as well as two instances of composite mature B- and T-cell lymphoma to further clarify the complex somatic and constitutional genomic features underlying their pathogenesis, presenting these six cases as a starting point for further studies.
Materials and methods
Materials and Methods are provided as supplementary material.
Results
Description of six cases of composite lymphomas
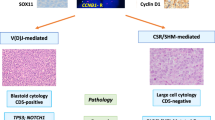
We studied four composite B-cell lymphomas and two combinations of B-cell and T-cell lymphoma (Table 1). Case 1 is a composite HL and CLL. Case 2 represents a sequential occurrence of a splenic marginal zone lymphoma (SMZL) and an HL appearing in a lymph node three years later. Case 3 is a composite HL and mantle cell lymphoma (MCL). In case 4, a FL was diagnosed, and three years later a HL was identified. In case 1, the Hodgkin and Reed-Sternberg (HRS) tumor cells (but not the CLL cells) were infected by Epstein-Barr virus (EBV) (not shown). In the three other HL, HRS cells were EBV-negative. Immunohistochemical stainings supported the diagnoses (Table 1 and Supplementary Figs. 1–3). In case 5, a CLL was initially diagnosed. Four months later, a T-prolymphocytic leukemia (T-PLL) appeared in the peripheral blood in addition to the still present CLL (Suppl. Fig. S4A). In case 6, a plasma cell leukemia (PCL) was first diagnosed, and two years later, an anaplastic large cell lymphoma (ALCL) and the PCL occurred concurrently in the peripheral blood (Supplementary Fig. 4B).
IGV gene analysis of composite B-cell lymphomas
In the four composite B-cell lymphomas, we first performed an analysis of the rearranged IGV genes of microdissected lymphoma cells to clarify whether the two lymphomas in the same patient were clonally related. The IGV genes were also analyzed for somatic mutations, as their presence indicates germinal center (GC) experience of the lymphoma precursor cell (Supplementary Fig. 5) [19].
In case 1, a HL and CLL, both lymphomas carried different IG heavy and light chain rearrangements, revealing that these lymphomas were derived from two clonally unrelated B cells (Table S4). The distinct lymphomas in cases 2, 3, and 4 each share a heavy chain rearrangement, and the lymphomas of cases 2 and 3 also have identical light chain rearrangements (Table S4). In case 4, no light chain was identified in the FL. This may, for example, be due to mutations in the primer binding site(s) which could impair binding and consequently amplification. All three lymphomas had mutated heavy and light chain rearrangements. The somatic mutations of the clonally related IGHV genes of cases 2, 3, and 4 and, for case 2, the IGKV genes, were identical, indicating that the somatic hypermutation process was largely silenced at the stage when the common lymphoma precursors started to develop into the distinct related lymphomas (Table S4).
Taken together, the IGV gene analysis of the four composite B-cell lymphomas revealed GC experience of the HRS cells in all instances, and a clonal relationship of the HRS cells to the other lymphoma in cases 2, 3, and 4. Conversely, the lymphomas of case 1 derive from two clonally unrelated B cells.
WES of composite lymphomas
WES was performed on the six composite lymphomas/leukemias to identify shared and distinct somatic mutations. For cases 1, 2, 3, and 4, the lymphoma cells as well as non-tumor cells (NTCs) used as constitutional control were microdissected from frozen tissue sections, whereas the leukemic cells and NTCs in cases 5 and 6 were isolated by flow-cytometric cell sorting. We focussed on non-synonymous mutations, as these are more likely to be functionally relevant than silent mutations. The samples were sequenced at a mean depth of 63.3x (range: 30.6–154.6x). We considered as shared somatic mutations variants which were detectable in both tumor samples with a VAF of at least 10% and covered but absent (defined as VAF below 3%) in the NTC sample.
Shared somatic mutations
The HRS and CLL cells of case 1, which did not share rearranged IGV genes, also did not share any somatic mutations. These lymphomas therefore were clonally unrelated and developed separately from each other.
Shared mutations were found in the three composite B-cell lymphomas, which also shared IGV gene rearrangements, further proving that these lymphomas derive from a single mature B cell (Tables 2, 3, Fig. 1, and Supplementary Table S5). Case 2, the composite HL/SMZL, shared five mutations, all representing missense single nucleotide variants (SNVs). The gene phenylalanyl-tRNA synthetase alpha chain (FARSA) showed a clonal heterozygous mutation (VAF 40% in HL; 46% in FL). FARSA is involved in the pathobiology of MCL, where it is generally downregulated and also shows a tumor suppressor-like profile in diffuse large B-cell lymphoma [20]. MKRN1, also harboring a shared mutation in case 2, is a transcriptional co-regulator and E3 ubiquitin ligase [21]. It ubiquitinates proteins such as p53 and p21 and can be both pro- and anti-apoptotic [22]. It was not previously reported as recurrently mutated in hematological malignancies.
VAF of the mutation in the tumor partners is shown on the respective axes. Distinct mutations cluster on the X or Y axes, while shared mutations lie in between. The size of the dots corresponds with the mean coverage of the mutation site. Mutations in selected lymphoma-associated genes are labeled with the gene name. A–C display cases 2, 3, and 4, which each encompass clonally related lymphomas, as evidenced by both shared IGV gene rearrangements and shared somatic mutations, displayed in gray. D–F Display cases 1, 5, and 6, none of which share somatic mutations between their partner lymphomas. Distinct mutations in orange (HL), green (other mature B-cell lymphoma), or blue (T-cell lymphoma). Gene symbols marked according to mutation type: red: truncating mutation, blue: frameshift mutation, violet: in-frame insertion/deletion, black: missense mutation, green: splice site mutated. Mutations in IGV genes were excluded.
In case 3, the composite MCL and HL share eight somatic mutations. These include a probably originally heterozygous mutation in the tumor suppressor gene TP53, where the other allele may have been deleted in the MCL, resulting in a hemizygous mutation (55% VAF in HL, 100% VAF in MCL). This TP53 hotspot mutation (rs28934576, p.R273L) [23] is associated with pathogenicity in various cancers, including B-cell lymphomas [24]. TP53 mutations have been identified in HRS cells before [25, 26], and are frequent in MCL [27]. DUSP22, a known tumor suppressor gene in lymphomas, is mutated in both lymphomas as well.
The composite HL and FL in case 4 shared six mutations, one of them being a nonsense mutation in S1PR2 (25% VAF in FL, 19% VAF in HL), a tumor suppressor gene commonly silenced in aggressive B-cell lymphomas [28,29,30]. A further mutated gene, midnolin (MIDN), facilitates the ubiquitin-independent proteasomal degradation of a number of transcription factors that are known as oncogenic, such as FOSB [31] and EGR1, indicating a possible role in lymphomagenesis.
In cases 5 and 6, the two composite B- and T-cell lymphomas, no shared mutations were identified, showing that these lymphomas were not clonally related.
Distinct somatic mutations in composite lymphomas
In addition to the shared somatic mutations, all lymphomas in this study also carried distinct mutations, i.e., mutations present in only one of the tumors and absent also in the NTC samples (Tables 2, 4, and Supplementary Table 6). These mutations occurred after the lineages of the lymphomas diverged. Each lymphoma harbored between 9 and 367 non-synonymous distinct mutations (Table 2). Case 4, with 367 (HL) and 258 (FL) distinct somatic mutations, likely represents hypermutating lymphomas. In all cases, mutations in lymphoma-associated genes were identified.
In case 4, the HL prominently carried somatic mutations in HL-related genes, such as TNFAIP3, CFLAR/c-FLIP, and KLHDC8B [32,33,34,35]. SOCS1, one of the most frequently mutated genes in HL [36], was mutated in three different positions in the HRS cells of case 1. Other affected genes in the HRS cells with implications in lymphomagenesis include FAF1 [37] (case 1), TNIP1/ABIN-1 [38] (case 2), and PRDM1/BLIMP1 [39, 40] (case 4). The non-Hodgkin lymphoma partners of cases 1–4 all also carried mutations in lymphoma-related genes (Table 4).
For the B-cell/T-cell lymphoma combinations, typical mutations were also identified, such as a mutation in DIS3 in the PCL [41], or a mutation of MSC (p.E116K) in ALCL of case 6 [42].
Taken together, we identified numerous mutated genes with roles in lymphomagenesis which likely contributed to the development of the lymphomas.
Somatic copy number variations and a chromosomal translocation
We also performed an analysis of copy number variations (CNVs) (Fig. 2). The analysis of CNVs in WES data, as performed here, is mostly restricted to large CNVs. Accordingly, we focused our analysis on large events and selected hotspots for CNVs. In cases 2 and 3, no reliable CNV data were obtained, likely because of issues with data quality due to whole gene amplification used in these cases.
We identified several CNVs that are known recurrent events in HL, including an amplification of JAK2 and the neighboring PD-L1 and PD-L2 genes (9p23-p24) in the HL of case 1, and a REL amplification (2p13) in the HL of case 4 (Fig. 2, and Supplementary Fig. 6).
The T-PLL possesses a large deletion in 11q (including ATM), a gain of 8q (including the oncogene MYC), and an amplification of TCL1A and TCL1B (14q32), which are all hallmarks of T-PLL. The CLL partner lost a region of 13q14, an event shared with more than 50% of CLL cases [43]. In case 6, the ALCL lost a large part of 17p, including TP53. The PCL had gains in 1q, as is typical for plasma cell malignancies and lost 13q, which encompassed the tumor suppressor genes BRCA2 and RB1.
Since WES data cover only parts of the genome, they are not suitable for in silico breakpoint analyzes. We therefore performed FISH analyzes to identify translocations. Indeed, the clonally related HL/MCL carried an identical IGH::CCND1 translocation as confirmed by CD30-FICTION (not shown). Conversely, both partners of the HL/FL composite lymphoma were negative for the IGH::BCL2 translocation typical of classic FL. The FL did not carry a mutation in STAT6, which has been reported to be present in 55–80% of IGH::BCL2-negative FL [44, 45]. However, we detected a 16p13 deletion in this FL (Fig. 2), and this event is enriched in IGH::BCL2-negative FL (seen in 39% of IGH::BCL2-negative, but only 9% of translocation-positive FL) [44].
Candidate constitutional variants or rare germline polymorphisms
The analysis primarily focused on somatic mutations present in one or both of the lymphomas, but missing in the NTCs. However, constitutional polymorphisms or variants may also contribute to the pathogenesis of composite lymphomas [46, 47]. To study the potential contribution of constitutional variants or rare polymorphisms in the development of composite lymphomas, we performed an analysis of genetic variants present in the lymphoma cells as well as the NTCs.
We define constitutional events as non-synonymous genetic variants that are shared between both tumors and the NTCs at high VAFs (≥30% in both tumors and NTCs). These include constitutional polymorphisms that were not excluded by the panel of normal filtering, such as novel mutations, extremely rare alleles, or mutations in hematological cells.
As expected, non-tumor-shared events were identified in all cases analyzed (Supplemantary Table S7), with 400–653 events per case, case 2 being an outlier with only 67 events. While most of these represent unrelated constitutional polymorphisms or somatic mutations in non-cancer genes, a small number of events in each case were found in lymphoma-related genes. Some of these events are specifically related to lymphomas, such as in case 5, which includes nine single-nucleotide events associated with lymphomas, e.g., the p.P925T missense variant in EP300 (COSV54337074) [48, 49], or events in MCL1 and PTPN13 (Table 5).
Case 1 harbors an activating missense mutation in JAK3 (p.V722I, COSV71685519) that is related to various types of lymphomas and leukemias, and found recurrently in other cancers as well [50, 51]. This constitutively phosphorylated mutant protein induces cytokine-independent cell growth in the murine cell line Ba/F3, indicating a transforming effect [52, 53]. In all six cases, SNVs were detected in well-known tumor suppressors and oncogenes or B cell- and lymphoma-associated genes (Table 5). Some of these variants were detected as somatically mutated in cancers according to the COSMIC database, often in a neoplasm of the hematopoietic lineage.
Additionally, variants were processed with an artificial intelligence-based variant prioritization platform to interpret whether these events represent “true” constitutional events linked to lymphomagenesis, cancer predisposition, and/or immune dysfunction. This analysis identified a pathogenic BRCA2 mutation (c.9672dup, p.Tyr3225IlefsTer30) in case 4 as a likely contributor to a cancer predisposition syndrome, possibly intensified by a variant of unknown significance in ATM (c.8187A > T, p.Gln2729His) (Table 5). These DNA damage repair deficiencies might explain why both lymphomas in case 4 show unusually high mutational frequencies.
Discussion
Composite lymphomas consisting of a classic HL and another B-cell lymphoma are frequently clonally related and derive from GC-experienced B cells
In the present study, we analyzed six cases of composite lymphomas regarding their shared or distinct origin. We confirmed previous results that concurrent or sequential combinations of an HL and another B-cell lymphomas are frequently clonally related [1,2,3], as evidenced by shared IGV gene rearrangements in three of the four composite lymphomas analyzed here. These shared rearrangements were all somatically mutated, indicating that the separation into two different lymphomas happened after the progenitor B cells gained GC experience. Case 1, a composite HL and CLL, was the only B-cell composite lymphoma in our study that was clonally unrelated. The CLL carried unmutated IGV genes suggesting a pre-GC B-cell derivation, whereas the HRS cells carried somatically mutated IGV genes, in line with the known GC derivation of HL [54].
Shared somatic mutations drive tumorigenesis
In all three cases with shared IGV gene rearrangements (cases 2, 3, and 4), we also identified shared somatic mutations in the WES analysis, further corroborating that the lymphoma partners derive from one cell of origin. Many of these mutations were found in lymphoma-related genes such as TP53, DUSP22, and S1PR2. These may represent driver mutations, such as a known destructive mutation in TP53 in case 3, or less deleterious events that only “nudge” the cells towards becoming cancerous.
The numbers of shared non-synonymous somatic mutations in the clonally related composite lymphomas were relatively low, with only 5, 8, and 6 of these mutations in cases 2, 3, and 4, respectively (Table 2). The number of distinct mutations was higher in each case. This indicates that the common precursor of both lymphomas was a pre-malignant cell with relatively few mutations and that most mutations happened after the two separate lymphoma precursors had developed. Multiple distinct mutations may be needed to generate an HL versus another B-cell lymphoma from a GC B cell that was the origin of both lymphomas. However, we likely underestimate the number of shared mutations, as WES from pools of microdissected cells does not cover the exome completely at sufficient sequencing depth, and mutations outside the exome were not covered.
Distinct somatic mutations determine the lymphoma type in composite lymphomas
Distinct mutations that are only present in one lymphoma partner contribute to the differentiation between both lymphomas, as evidenced by finding entity-typical mutations in the lymphomas. In addition to these mutations, we also analyzed large CNVs in cases 1, 4, 5, and 6. The majority of distinct CNVs we observed were hallmarks of the respective lymphoma subtype, e.g., 8q and 11q deletions in the T-PLL, a 13q14 deletion in the case 5 CLL, and the gains of JAK2/PD-L1/PD-L2 and REL in the HL of cases 1 and 4, respectively. All of these events likely contributed to the differentiation into a specific lymphoma type.
Lymphoma-related constitutional SNVs might contribute to multi-step lymphomagenesis
In all cases analyzed, we identified sequence variants that were shared between both lymphomas and the NTCs of a given case, which may represent constitutional variants. We classified constitutional variants according to the ACMG criteria and with few exceptions, most of them were called (likely) benign. Among the exceptions was a likely pathogenic mutation in the DNA damage repair gene BRCA2 in case 4. We also screened the literature for variants that were called (likely) benign, but had some measure of functional data that indicated a role in lymphomagenesis, and identified, e.g., a variant in JAK3 that is scored as benign by all algorithms we employed, but is nonetheless functionally relevant in lymphomagenesis due to it being a phosphomimetic mutation that constitutionally activates JAK3. This discrepancy underscores the fundamental differences of in silico approaches to variant classification in the constitutional (germline) versus the tumor (somatic) setting and a need to functionally validate candidate variants in the respective context. It is also intriguing that many of the (likely) benign variants affect commonly altered pathways or complexes in lymphoma. Thus, one could speculate that they might contribute to lymphomagenesis via a kind of polygenic or low-penetrance predisposition.
We also identified variants in multiple genes that are often mutated in clonal hematopoiesis [55]. However, since these events all had VAFs higher than 35% in both lymphomas and NTCs, it is unlikely that these events represent clonal hematopoiesis, as in that case, one would expect much lower VAFs in the NTCs.
Some possibly oncogenic constitutional events that are shared between lymphomas and NTCs might contribute to cancer development later on, analogous to tumor predisposition syndromes. Genetic predisposition to lymphomas is well-known [56] and has been described in the context of familial increased risk of lymphoma/leukemia as well [47]. As far as we know, none of the patients included in this study was clinically diagnosed with a tumor predisposition syndrome, but the development of multiple distinct lymphomas that are not clonally related might hint at a common constitutional predisposition.
Our data add to the growing evidence that constitutional or early somatic events can contribute to tumorigenesis [46] and have implications for the genesis of both singular and composite lymphomas: the first hit(s) of a composite lymphoma can happen early during hematopoiesis or might even be a constitutional variant, as is the case for singular lymphomas as well.
Two models of multi-step lymphomagenesis in composite lymphomas
Based on our data, we propose two models of multi-step lymphomagenesis in composite lymphomas (Fig. 3): Model A, representing GC-derived lymphomas, is based on cases 2, 3, and 4, all of which were composite B-cell lymphomas sharing mutated IGV gene rearrangements and somatic mutations. These cases likely represent a “typical” B-cell lymphomagenesis originating in the GC reaction due to factors such as rapid cell division and DNA damage during somatic hypermutation. These processes can generate pre-malignant cells, which may later split into two different lymphoma entities. A common GC B-cell derivation is indeed seen in nearly all cases of clonally related composite B-cell lymphomas [1].
Model A depicts tumorigenesis in lymphomas with a GC-experienced B cell as a shared origin, with case 4, a composite HL/FL, as an example. B shows a model of tumor predisposition that includes shared constitutional/HSC variants, with case 5, a composite CLL/T-PLL, as an example. Other composite lymphomas may have no relation and be a chance occurrence. Figure created with biorender.com.
The as-of-yet speculative model B is based on cases 1, 5, and 6. These cases do not share an IGV gene rearrangement or somatic mutations but have some early candidate transforming events in common with NTCs. We regard these cases as possible examples of a “predisposition” model, in which oncogenic lesions would occur early during hematopoietic differentiation or are present as constitutional variants, predisposing the patient to develop multiple lymphomas.
These different mechanisms of pathogenesis may be combined, such as in case 4, which had both shared somatic mutations and a constitutional variant of BRCA2, which predisposes to cancer. In addition to the two models, there are likely also cases with completely unrelated composite lymphomas, where the patient is not predisposed to lymphomagenesis either.
As our analysis was restricted to exonic SNVs and large CNVs, analyzing additional dimensions, such as environmental factors, immunodeficiencies, epigenetic changes, mutations in areas not covered by WES, or therapy effects in sequential lymphomas, may also shed more light on the development of composite lymphomas.
Study limitations
Our approach is limited primarily by the low sample availability since composite lymphomas are rare and we needed to obtain samples from both lymphoma partners as frozen material to perform laser microdissection. Furthermore, all six cases consist of different lymphoma entities, further limiting cross-case comparisons. There is also a lack of “true” constitutional sequencing information from a non-hematopoietic tissue. The comparatively shallow mean sequencing depth of 63.3 limits the analysis of subclonal mutations, so we focused on clonal mutations. Working with WES data also limits our approach to exonic variants, which means that, e.g., shared mutations in regulatory regions would not have been identified. We did not only identify variants that are already linked to lymphomagenesis, but a large number of candidate variants as well. Functional studies will be needed to clarify the relevance of these variants.
References
Küppers R, Dührsen U, Hansmann M-L. Pathogenesis, diagnosis, and treatment of composite lymphomas. Lancet Oncol. 2014;15:e435–46.
Trecourt A, Donzel M, Fontaine J, Ghesquières H, Jallade L, Antherieu G, et al. Plasticity in classical Hodgkin composite lymphomas: a systematic review. Cancers. 2022;14:5695.
Bräuninger A, Hansmann ML, Strickler JG, Dummer R, Burg G, Rajewsky K, et al. Identification of common germinal-center B-cell precursors in two patients with both Hodgkin’s disease and non-Hodgkin’s lymphoma. N Engl J Med. 1999;340:1239–47.
Marafioti T, Hummel M, Anagnostopoulos I, Foss HD, Huhn D, Stein H. Classical Hodgkin’s disease and follicular lymphoma originating from the same germinal center B cell. J Clin Oncol. 1999;17:3804–9.
Schmitz R, Renné C, Rosenquist R, Tinguely M, Distler V, Menestrina F, et al. Insights into the multistep transformation process of lymphomas: IgH-associated translocations and tumor suppressor gene mutations in clonally related composite Hodgkin’s and non-Hodgkin’s lymphomas. Leukemia. 2005;19:1452–8.
Schneider S, Crescenzi B, Schneider M, Ascani S, Hartmann S, Hansmann M-L, et al. Subclonal evolution of a classical Hodgkin lymphoma from a germinal center B-cell-derived mantle cell lymphoma. Int J Cancer. 2014;134:832–43.
Murray C, Quinn F, Illyes G, Walker J, Castriciano G, O’Sullivan P, et al. Composite blastoid variant of mantle cell lymphoma and classical Hodgkin lymphoma. Int J Surg Pathol. 2017;25:281–6.
Nakamura N, Ohshima K, Abe M, Osamura Y. Demonstration of chimeric DNA of bcl-2 and immunoglobulin heavy chain in follicular lymphoma and subsequent Hodgkin lymphoma from the same patient. J Clin Exp Hematopathol. 2007;47:9–13.
Tashkandi H, Petrova-Drus K, Batlevi CL, Arcila ME, Roshal M, Sen F, et al. Divergent clonal evolution of a common precursor to mantle cell lymphoma and classic Hodgkin lymphoma. Cold Spring Harb Mol Case Stud. 2019;5:a004259.
Singh K, Lezama LS, Kurzer J, Oak J, Schultz LM, Walkush A, et al. Targeted mutational profiling reveals clonal relationships in metachronous occurrence of classic Hodgkin and mediastinal large B-cell lymphomas. Am J Surg Pathol. 2023;47:81–90.
Trecourt A, Mauduit C, Szablewski V, Fontaine J, Balme B, Donzel M, et al. Plasticity of mature B cells between follicular and classic Hodgkin lymphomas: A series of 22 cases expanding the spectrum of transdifferentiation. Am J Surg Pathol. 2022;46:58–70.
Wang H, Yang L, Li Q, Song H, Ji H. Case report: composite mantle cell lymphoma and classical Hodgkin lymphoma. Pathol Oncol Res. 2023;29:1611051.
Chung SS, Kim E, Park JH, Chung YR, Lito P, Teruya-Feldstein J, et al. Hematopoietic stem cell origin of BRAFV600E mutations in hairy cell leukemia. Sci Transl Med. 2014;6:238ra71.
Damm F, Mylonas E, Cosson A, Yoshida K, Della Valle V, Mouly E, et al. Acquired initiating mutations in early hematopoietic cells of CLL patients. Cancer Discov. 2014;4:1088–101.
Quivoron C, Couronné L, Della Valle V, Lopez CK, Plo I, Wagner-Ballon O, et al. TET2 inactivation results in pleiotropic hematopoietic abnormalities in mouse and is a recurrent event during human lymphomagenesis. Cancer Cell. 2011;20:25–38.
Jaiswal S, Ebert BL. Clonal hematopoiesis in human aging and disease. Science. 2019;366:eaan4673.
Berndt SI, Vijai J, Benavente Y, Camp NJ, Nieters A, Wang Z, et al. Distinct germline genetic susceptibility profiles identified for common non-Hodgkin lymphoma subtypes. Leukemia. 2022;36:2835–44.
Wang SS, Slager SL, Brennan P, Holly EA, de Sanjose S, Bernstein L, et al. Family history of hematopoietic malignancies and risk of non-Hodgkin lymphoma (NHL): a pooled analysis of 10 211 cases and 11 905 controls from the International Lymphoma Epidemiology Consortium (InterLymph). Blood. 2007;109:3479–88.
de Silva NS, Klein U. Dynamics of B cells in germinal centres. Nat Rev Immunol. 2015;15:137–48.
Feng M, Yang K, Wang J, Li G, Zhang H. First report of FARSA in the regulation of cell cycle and survival in mantle cell lymphoma cells via PI3K-AKT and FOXO1-RAG1 axes. Int J Mol Sci. 2023;24:1608.
Hildebrandt A, Brüggemann M, Rücklé C, Boerner S, Heidelberger JB, Busch A, et al. The RNA-binding ubiquitin ligase MKRN1 functions in ribosome-associated quality control of poly(A) translation. Genome Biol. 2019;20:216.
Lee E-W, Lee M-S, Camus S, Ghim J, Yang M-R, Oh W, et al. Differential regulation of p53 and p21 by MKRN1 E3 ligase controls cell cycle arrest and apoptosis. EMBO J. 2009;28:2100–13.
Freed-Pastor WA, Prives C. Mutant p53: one name, many proteins. Genes Dev. 2012;26:1268–86.
Janssens V, van Hoof C, de Baere I, Merlevede W, Goris J. The phosphotyrosyl phosphatase activator gene is a novel p53 target gene. J Biol Chem. 2000;275:20488–95.
Chen WG, Chen YY, Kamel OW, Koo CH, Weiss LM. p53 mutations in Hodgkin’s disease. Lab Invest. 1996;75:519–27.
Maggio EM, Stekelenburg E, van den Berg A, Poppema S. TP53 gene mutations in Hodgkin lymphoma are infrequent and not associated with absence of Epstein-Barr virus. Int J Cancer. 2001;94:60–66.
Jares P, Campo E. Advances in the understanding of mantle cell lymphoma. Br J Haematol. 2008;142:149–65.
Stelling A, Hashwah H, Bertram K, Manz MG, Tzankov A, Müller A. The tumor suppressive TGF-β/SMAD1/S1PR2 signaling axis is recurrently inactivated in diffuse large B-cell lymphoma. Blood. 2018;131:2235–46.
Meyer SN, Koul S, Pasqualucci L. Mouse models of germinal center derived B-cell lymphomas. Front Immunol. 2021:12:710711.
Al-Kawaaz M, Sanchez T, Kluk MJ. Evaluation of S1PR1, pSTAT3, S1PR2, FOXP1 expression in aggressive, mature B cell lymphomas. J Hematopathol. 2019;12:57–65.
Qi M, Sun L, Zheng L, Zhang J, Han Y, Wu F, et al. Expression and potential role of FOSB in glioma. Front Mol Neurosci. 2022:15:972615.
Schmitz R, Hansmann M-L, Bohle V, Martin-Subero JI, Hartmann S, Mechtersheimer G, et al. TNFAIP3 (A20) is a tumor suppressor gene in Hodgkin lymphoma and primary mediastinal B cell lymphoma. J Exp Med. 2009;206:981–9.
Mathas S, Lietz A, Anagnostopoulos I, Hummel F, Wiesner B, Janz M, et al. c-FLIP mediates resistance of Hodgkin/Reed-Sternberg cells to death receptor-induced apoptosis. J Exp Med. 2004;199:1041–52.
Krem MM, Salipante SJ, Horwitz MS. Mutations in a gene encoding a midbody protein in binucleated Reed-Sternberg cells of Hodgkin lymphoma. Cell Cycle. 2010;9:670–5.
Kato M, Sanada M, Kato I, Sato Y, Takita J, Takeuchi K, et al. Frequent inactivation of A20 in B-cell lymphomas. Nature. 2009;459:712–6.
Weniger MA, Melzner I, Menz CK, Wegener S, Bucur AJ, Dorsch K, et al. Mutations of the tumor suppressor gene SOCS-1 in classical Hodgkin lymphoma are frequent and associated with nuclear phospho-STAT5 accumulation. Oncogene. 2006;25:2679–84.
Menges CW, Altomare DA, Testa JR. FAS-associated factor 1 (FAF1): diverse functions and implications for oncogenesis. Cell Cycle. 2009;8:2528–34.
Dong G, Chanudet E, Zeng N, Appert A, Chen Y-W, Au W-Y, et al. A20, ABIN-1/2, and CARD11 mutations and their prognostic value in gastrointestinal diffuse large B-cell lymphoma. Clin Cancer Res. 2011;17:1440–51.
Mandelbaum J, Bhagat G, Tang H, Mo T, Brahmachary M, Shen Q, et al. BLIMP1 is a tumor suppressor gene frequently disrupted in activated B cell-like diffuse large B cell lymphoma. Cancer Cell. 2010;18:568–79.
Pasqualucci L, Compagno M, Houldsworth J, Monti S, Grunn A, Nandula SV, et al. Inactivation of the PRDM1/BLIMP1 gene in diffuse large B cell lymphoma. J Exp Med. 2006;203:311–7.
Lionetti M, Barbieri M, Todoerti K, Agnelli L, Fabris S, Tonon G, et al. A compendium of DIS3 mutations and associated transcriptional signatures in plasma cell dyscrasias. Oncotarget. 2015;6:26129–41.
Luchtel RA, Zimmermann MT, Hu G, Dasari S, Jiang M, Oishi N, et al. Recurrent MSCE116K mutations in ALK-negative anaplastic large cell lymphoma. Blood. 2019;133:2776–89.
Calin GA, Dumitru CD, Shimizu M, Bichi R, Zupo S, Noch E, et al. Frequent deletions and down-regulation of micro- RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci USA. 2002;99:15524–9.
Xian RR, Xie Y, Haley LM, Yonescu R, Pallavajjala A, Pittaluga S, et al. CREBBP and STAT6 co-mutation and 16p13 and 1p36 loss define the t(14;18)-negative diffuse variant of follicular lymphoma. Blood Cancer J. 2020;10:69.
Nann D, Ramis-Zaldivar JE, Müller I, Gonzalez-Farre B, Schmidt J, Egan C, et al. Follicular lymphoma t(14;18)-negative is genetically a heterogeneous disease. Blood Adv. 2020;4:5652–65.
Mosquera Orgueira A, Cid López M, Peleteiro Raíndo A, Díaz Arias JÁ, Antelo Rodríguez B, Bao Pérez L, et al. Detection of rare germline variants in the genomes of patients with B-cell neoplasms. Cancers. 2021;13:1340.
Wang X, Deng L, Ping L, Shi Y, Wang H, Feng F, et al. Germline variants of DNA repair and immune genes in lymphoma from lymphoma-cancer families. Int J Cancer. 2024;155:93–103.
Pasqualucci L, Dominguez-Sola D, Chiarenza A, Fabbri G, Grunn A, Trifonov V, et al. Inactivating mutations of acetyltransferase genes in B-cell lymphoma. Nature. 2011;471:189–95.
Margolskee E, Jobanputra V, Jain P, Chen J, Ganapathi K, Nahum O, et al. Genetic landscape of T- and NK-cell post-transplant lymphoproliferative disorders. Oncotarget. 2016;7:37636–48.
Tabone T, Abuhusain HJ, Nowak AK, Erber WN, McDonald KL. Multigene profiling to identify alternative treatment options for glioblastoma: a pilot study. J Clin Pathol. 2014;67:550–5.
Oliveira DM, Laudanna C, Migliozzi S, Zoppoli P, Santamaria G, Grillone K, et al. Identification of different mutational profiles in cancers arising in specific colon segments by next generation sequencing. Oncotarget. 2018;9:23960–74.
Yin C, Sandoval C, Baeg G-H. Identification of mutant alleles of JAK3 in pediatric patients with acute lymphoblastic leukemia. Leuk Lymphoma. 2015;56:1502–6.
Malinge S, Ragu C, Della-Valle V, Pisani D, Constantinescu SN, Perez C, et al. Activating mutations in human acute megakaryoblastic leukemia. Blood. 2008;112:4220–6.
Weniger MA, Küppers R. Molecular biology of Hodgkin lymphoma. Leukemia. 2021;35:968–81.
Bernstein N, Spencer Chapman M, Nyamondo K, Chen Z, Williams N, Mitchell E, et al. Analysis of somatic mutations in whole blood from 200,618 individuals identifies pervasive positive selection and novel drivers of clonal hematopoiesis. Nat Genet. 2024;56:1147–55.
Szmyd B, Mlynarski W, Pastorczak A. Genetic predisposition to lymphomas: Overview of rare syndromes and inherited familial variants. Mutat Res. 2021;788:108386.
Acknowledgements
This work was supported by the Deutsche Krebshilfe (70112112), the Deutsche Forschungsgemeinschaft (SFB1530, C01), the Deutsche José Carreras Leukämie-Stiftung (DJCLS 02R/2020), Associazione Italiana Ricerca sul Cancro (AIRC IG 2019-23732 to E.T.) and Fondazione Beretta, Brescia for E. Albertini. We thank the IMCES facility and Klaus Lennartz for support with cell sorting. We thank Gwen Hoffmann, Julia Bein, Yvonne Michels, and Kerstin Heise for technical assistance. We thank the NGS Core Facility, German Cancer Research Center, for providing sequencing services.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
Contribution: AL, M-LH, and RK designed the study; AL, MS, and MAW performed the experiments; PJ, LL, EA, FF, SA, ET, SH, M-LH, and WK provided clinical data, pathological evaluation and essential material; SB and RS performed and evaluated FISH studies, AF, AM, and RS evaluated constitutional variants; BB, VB, AL, and RK analyzed the data; VB and RK wrote the manuscript. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study was conducted in accordance with the ethical standards of the institutional and/or national research committees and with the ethical standards as laid down in the Declaration of Helsinki, and all methods were performed in accordance with the relevant guidelines and regulations. The study was approved by the local ethics committees of the University Hospitals of Frankfurt/Main (8/15) and Essen (15-6185-BO). The biopsy samples were obtained with informed consent by the patients, or they were derived from deceased patients, with allowance by the ethics committee to use them in anonymized form.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Berg, V., Lollies, A., Schneider, M. et al. Common origin and somatic mutation patterns of composite lymphomas and leukemias. Leukemia 39, 1960–1971 (2025). https://doi.org/10.1038/s41375-025-02549-y
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41375-025-02549-y