Abstract
The European LeukemiaNet recently proposed specific Clinical Signs and Symptoms (CSSs) that may trigger cytoreduction in patients with polycythemia vera (PV) at low thrombotic risk (LR). To evaluate the impact of CSSs on the thrombotic risk of patients at LR, high risk by age only (HR-AGE) or by previous thrombosis (HR-THRO), we conducted a multicenter cooperative study (NCT06134102) involving 739 PV patients treated with first-line hydroxyurea. At hydroxyurea start, 443 patients had at least one CSS. In patients with and without CSSs, the incidence rate ratio of thrombosis was 2.2 and 0.7 per 100 patient-years, respectively (p < 0.001), and the thrombosis-free survival (TFS) adjusted for delayed entry at 5 years was 88.7% and 96.1% (p < 0.001). The best 5-years TFS was observed in LR and HR-AGE with no CSSs (LR, 100%; HR-AGE: 98.1%). LR, HR-AGE patients with CSSs and HR-THRO patients without CSSs had comparable TFS (89.2%, 92.1% and 88.8%, respectively). TFS of HR-THRO patients was 80.2%. In multivariate analysis including each CSS, inadequate hematocrit control (HR: 2.32, p < 0.001), relevant CVRFs (HR: 2.87, p = 0.006), progressive splenomegaly (HR: 4.02, p = 0.03) and previous thrombosis (HR: 3.76, p < 0.001) remained significantly associated with thrombotic risk. CSSs identify an increased thrombotic risk phenotype across all risk categories.

Similar content being viewed by others
Introduction
Polycythemia vera (PV) is a rare hematologic cancer belonging to the family of chronic myeloproliferative neoplasms (MPN) and driven by mutations in the Janus kinase 2 (JAK2) gene, most frequently the JAK2V617F. The presence of an activating JAK2 mutation drives the aberrant proliferation of hematopoietic cells [1, 2] leading to erythrocytosis, thrombocytosis, leukocytosis and increased cytokine production resulting in systemic symptoms typical of PV [3, 4].
Patients with PV face a substantial risk of thrombotic complications, with a 2.7- to 13.1-fold higher likelihood of developing arterial and venous thrombosis, respectively, compared to age- and sex-matched controls [5]. Therefore, the clinical management of PV is focused on reducing the risk of such events, which remain the leading cause of morbidity and mortality.
Advanced age and a history of previous thrombotic events are two well-established clinical risk factors for predicting future thromboembolic episodes [6,7,8,9,10]. Based on these two risk factors, patients with PV are currently classified as low-risk (LR) (absence of both risk factors) and high-risk (HR) (history of thrombosis and/or age ≥60 years) [4, 11]. However, a growing body of literature suggests that advanced age and prior thrombosis history may rather reflect a limited view of the overall risk profile and are sometimes perceived as suboptimal for proper management of PV. The fact that various biomarkers are currently investigated for risk assessment of PV (including but not limited to allelic ratio [12, 13], red blood cell distribution width (RDW)/neutrophil-lymphocyte ratio [14, 15], and others) emphasizes the importance of improved risk stratification.
In light of this evidence, the European LeukemiaNet (ELN) has recently indicated the use of cytoreductive therapy also in LR patients presenting specific clinical signs and symptoms (CSSs), namely, intolerance to phlebotomy, uncontrolled hematocrit, symptomatic progressive splenomegaly, persistent and/or progressive leukocytosis, extreme thrombocytosis, relevant cardiovascular risk factors [16]. However, the prognostic role of these factors remains so far elusive.
Hydroxyurea (HU) is currently the most widely used first-line cytoreductive therapy for PV patients [17,18,19], offering effective control of the disease and playing a key role in thrombosis prevention with generally acceptable tolerance [20, 21].
In a large multicenter cooperative study, we assessed the impact of ELN clinical signs and symptoms on the thrombotic risk of PV patients homogeneously treated with first-line HU.
Materials and methods
Patients’ collection and study design
This is an observational, retrospective cohort study (PV-ARC, NCT06134102) that was promoted by the IRCCS Azienda Ospedaliero-Universitaria S. Orsola-Malpighi, Bologna, Italy.
The study now involves 1162 patients with PV diagnosed between January 1985 and December 2023. Among them, 739 patients were treated with first-line HU and had data on CSSs available at the start of HU.
Following approval of each IRB, participating centers collectively submitted diagnostic and follow-up information. The totality of medical files from each center was reported via data input into an electronic database that was developed to label all study data with an alphanumeric code after the de-identification of patients to protect personal privacy.
The data collected included patient demographics, medications, clinical/laboratory tests at diagnosis and during follow-up, type of PV therapy and thromboses. All information about concomitant diseases, body mass index (BMI), cardiovascular risk factors (CVRFs), thrombosis history, and drug usage was recorded in each case history and, thereafter, used for retrospective evaluation. Any treatment decision, including the use of phlebotomies, HU and antiplatelet/anticoagulant drugs, was at the physician’s discretion and independent from participation in this study.
After the first data entry, follow-up information was validated with revision of the clinical data, and specific queries were addressed to the participating centers in cases of inconsistent data. All patients were followed until death or the data cut-off date (March 2024).
Statistical analysis
Statistical analysis was carried out at the biostatistics laboratory of the MPN Unit, IRCCS Azienda Ospedaliero-Universitaria S. Orsola-Malpighi, Bologna.
Continuous variables are expressed as mean ± standard deviation (SD), whereas categorical variables are presented as frequencies and percentages. Comparisons of quantitative variables between two groups of patients were carried out using the Wilcoxon–Mann–Whitney rank-sum test and among three groups using the Kruskal–Wallis equality-of-populations rank test. Associations between categorical variables were tested using the χ2 test.
The mean thrombotic risk scores associated with each CVRF were analyzed by a one-way analysis of variance (ANOVA).
A Poisson regression model was applied to calculate the incidence rate ratios (IRR) of thrombotic events. The IRR was described as the number of events per 100 patient-years (%p-y), considering the time between HU start and the first thrombotic event or last contact.
Variables associated with thrombotic events were identified using univariate and multivariate (MVA) Cox proportional hazards model. Hazard ratio (HR) has been reported with 95% confidence intervals (CI).
Thrombosis-free survival (TFS) was assessed from HU start to the first thrombosis or last contact, using Kaplan–Meier analysis, adjusted for delayed entry, considering the time between PV diagnosis and the start of HU. Differences were evaluated with the log-rank test.
Tests were two-sided and p values < 0.05 were considered significant. Analyses were performed using STATA/SE software version 18.0 (StataCorp).
Definitions
PV was diagnosed according to the WHO 2022 classification [22]. In patients diagnosed before 2022, bone marrow biopsies were internally reviewed to adhere the data to current criteria [22]. In patients without a bone marrow histology (n. 49, 6.6%), PV diagnosis was based on the presence of elevated hemoglobin levels (i.e., >18.5 and 16.5 g/dL in males and females, respectively), the JAK2 Exon 14 or Exon 12 mutations, and low serum erythropoietin. Conventional criteria were used for the diagnosis of post Polycythemia Vera Myelofibrosis (PPV-MF) [23]; leukemic transformation was defined by blast cells being at least 20% in peripheral blood and/or bone marrow [22].
According to conventional criteria, LR patients were defined by age <60 years and no previous thrombotic history. HR patients were defined by age ≥60 years and/or a history of thromboembolism [24]. In this analysis, HR patients were further stratified in two groups: HR-AGE (patients at high risk only due to age ≥60 years) and HR-THRO (patients at high risk due to previous thrombosis, regardless of age).
Thromboses were defined according to the International Classification of Diseases (9th revision) [25] and graded according to the Common Terminology Criteria for Adverse Events (CTCAE) v5.0 [26]. Only grade ≥2 thromboses occurring during HU therapy were recorded and analyzed in the present study.
At HU start, presence and type of CSSs were initially searched as per the ELN definition [16]. However, three criteria were adapted based on real-life experience. In particular: a platelet (PLT) count >1500 × 109/L was never observed in our cohort, so the threshold has been lowered to a PLT count >1000 × 109/L; for the same reason, the need for at least 6 phlebotomies (PHLs) for at least 2 years has been modified to at least 6 PHLs and/or a persistently higher hematocrit (HCT) of 50% for at least 1 year before HU start. This threshold was selected according to the results of the Cyto-PV trial, where patients randomized to maintain HCT 45–50% had poorer outcomes [27].
In absence of a unified definition of “relevant” CVRF [16, 28], the ANOVA test was used to identify the combination of CVRFs most strongly correlated with the risk of thrombosis. Five predefined CVRFs, namely active smoking, hypertension, overweight (BMI ≥ 25), diabetes, and dyslipidemia, were included in the analysis [29].
In summary, CSSs were defined as follows: (1) persistent/progressive leukocytosis: 100% increase if white blood cells (WBC) < 10 × 109/L or 50% increase if WBC ≥ 10, or WBC > 15 × 109/L before HU start; (2) extreme thrombocytosis: PLT > 1000 × 109/L at HU start; (3) progressive splenomegaly: increase by more than 5 cm below costal margin (BCM) in the year before HU start; (4) inadequate HCT control: ≥6 PHLs/year or HCT ≥ 50% at two evaluations within 1 year before HU start or PHL intolerance; (5) “relevant” CVRFs: co-occurrence of active smoking, hypertension and overweight (with or without the presence of diabetes and/or dyslipidemia); (6) severe itching.
In patients at low thrombotic risk who did not receive cytoreduction during follow-up, the presence of CSSs was assessed at least 2 years after diagnosis to ensure the most accurate assessment of the criteria for inadequate hematocrit control.
The presence and severity of itching at the start of HU were assessed during clinical visits. In recent years, the 10-item Myeloproliferative Neoplasm Symptom Assessment Form Total Symptom Score (MPN10-TSS) [30] has been used and “severe” itching was considered with a score ≥5 according to ELN definition. Alternatively, the itching score was considered ≥5 if refractory to specific medication (e.g., antihistamines).
Additional reasons to start hydroxyurea were defined as follows: spleen palpable at 2–5 cm below costal margin, mild leukocytosis or thrombocytosis (WBC > 11 × 109/L or PLT > 450 × 109/L but not meeting the definition of persistent/progressive or extreme), presence of microvascular disturbances despite appropriate phlebotomies and antiplatelet drug therapy (including vascular headaches, dizziness, visual disturbances, burning pain sensation in the palms of the hands and soles of the feet, distal paresthesia and acrocyanosis) [31], ≥3 PHLs in the 12 months before HU start.
Results
Patient cohort
Overall, 739 patients received treatment with first-line HU and were included in this analysis. At therapy start, 137 (18.5%) patients were categorized as LR and 602 (81.5%) as HR. Among them, 424 (70.4%) were HR-AGE and 178 (29.6%) were HR-THRO, with 46 (25.8%) patients only due to previous thrombosis and 132 (74.2%) due to both older age and previous thrombosis (Supplementary Fig. 1).
At least one CVRF was present in 577 (78.1%) patients; specifically, 276 (37.3%), 181 (24.5%), 82 (11.1%) and 38 (5.1%) patients had one, two, three or ≥4 CVRFs. The frequency of individual CVRFs and their inter-relationships are shown in Table 1 and in Fig. 1a.
Co-occurring CVRFs (a) and CSSs (b) are indicated in the clockwise direction. In the Circos representation, the length of the arc corresponds to the frequency of the CVRF/CSS (color coded) and the width of the ribbon corresponds to the frequency of patients who also had a second CVRF/CSS. The frequency of each individual CVRF/CSS in this cohort is shown on the right side. Specifically, b shows the prevalence proportion of individual CSS.
The ANOVA scores for correlation with thrombosis were: 1.21, active smoking (IRR: 2.26%p-y); 1.15, hypertension (IRR: 1.83%p-y); 0.58, overweight (IRR: 1.93%p-y); 0.14, diabetes (IRR: 1.78%p-y) and 0.03, dyslipidemia (IRR: 1.79%p-y). In accordance with the findings of this test, patients with concomitant active smoking, hypertension and overweight were included in the “relevant CVRFs” category (ANOVA score: 4.73; p = 0.03). Overall, “relevant” CVRFs were present in 41 (5.5%) patients; in 15 of these patients, diabetes and/or dyslipidemia were also present.
At HU start, CSSs were present in 443 (60.0%) patients, more frequently in LR (n. 95, 69.3%) compared to HR patients (n. 348, 57.8%, p = 0.02), mainly due to higher presence of persistent/progressive leukocytosis (p = 0.006), progressive splenomegaly (p = 0.004) and severe itching (p = 0.002) (Table 1). Patients not presenting CSSs (296, 40.0%) were more frequently female (44.2% vs. 36.6%, p = 0.04), whereas age at HU start was comparable to CSSs cohort (66.7 ± 11.1 vs. 65.6 ± 11.6 years, p = 0.1).
The frequency of CSSs was comparable between HR-AGE (n. 239, 56.4%) and HR-THRO (n. 109, 61.2%) patients (p = 0.27). This was also observed in HR-THRO patients due to both older age and previous thrombosis (62.1%) and HR-THRO patients due to previous thrombosis only (58.7%, p = 0.69).
Overall, more than one CSS was present in 155 (out of 443, 35.0%) patients, mainly in low risk (43.2% vs. 32.8% HR, p = 0.05). The frequency of individual CSS and their inter-relationships are shown in Fig. 1b.
At HU start, median leukocytes and platelet counts, and mean spleen length BCM were significantly higher in LR compared to HR patients, with no significant differences between HR-AGE and HR-THRO patients.
The mean time from PV diagnosis to HU initiation in the CSS low-risk cohort was 3 years, with 22.1% of patients initiating HU more than 5 years after diagnosis. Conversely, all HR patients started HU soon after entering the high-risk category (mean: 2.3 months).
Median HU starting dose was comparable across the three risk categories, while LR patients more frequently received a maximum dose ≥1 g (Table 1). Anticoagulants were used more frequently in HR patients (12.3% vs. 4.4% LR, p = 0.007). Conversely antiplatelets, excluding patients receiving anticoagulants, were used comparably across risk categories (LR: 95.4% vs. HR: 98.8%, p = 0.21).
Additional reasons for starting HU were present in 92.9% of patients without CSSs. Distribution of additional reasons according to conventional risk categories is shown in Supplementary Table 1.
Thrombosis during hydroxyurea therapy
The median time on HU was 6.2 years (SD ± 4.3). During HU therapy, 72 patients had a thrombosis, for a total of 92 thrombotic events. Thromboses were equally distributed between arterial (n. 45, 48.9%) and venous (n. 47, 51.1%). Supplementary Table 2 details type and site of thrombotic events.
Globally, the IRR of thrombosis during HU was 2.02%p-y and significantly higher (3.0%p-y) in HR-THRO patients (Table 2). Among HR-THRO patients, patients older than 60 years had the highest IRR of thrombosis (3.5%p-y).
Thrombotic events in low-risk patients with CSSs and not receiving HU
To explore whether providing HU therapy in LR patients with CSSs may reduce the thrombotic risk, we evaluated an additional cohort of 44 LR patients who did not receive cytoreduction despite the presence of CSSs. Characteristics and thrombotic outcomes of these patients are reported in Supplementary Table 3.
Overall, in these 44 patients, 6 thrombotic events occurred, resulting in an IRR of thrombosis of 2.1%p-y. This figure was numerically higher than 1.6%p-y observed in low-risk patients with CSSs who received HU (Table 2).
Impact of clinical signs and symptoms on the incidence of thrombosis during hydroxyurea therapy
The IRR of thrombosis was estimated according to the presence of CSSs, without considering additional reasons for starting HU.
In the total cohort, CSSs were associated with a significantly increased IRR of thrombosis (2.2 vs. 0.7%p-y in patients without CSSs, p < 0.001). This was confirmed across all the risk categories (Table 2).
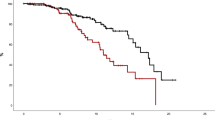
TFS at 5 years was 88.6% in patients with CSSs compared to 96.2% in those without CSSs (p < 0.001) (Fig. 2a). Examining the three risk categories separately, the TFS curve of patients with CSSs was always significantly worse than that of patients without CSSs (Fig. 2b–d). Across all risk categories, the best TFS at 5 years was observed in LR and HR-AGE (LR with no CSSs, 100%; HR-AGE with no CSSs: 98.1%). LR and HR-AGE patients with CSSs had comparable TFS than HR-THRO patients without CSSs (89.2%, 92.1% and 88.8%, respectively). HR-THRO patients with CSSs had the most dismal TFS (80.2%) (Supplementary Fig. 2).
Prognostic impact of each individual clinical sign and symptom on the thrombotic risk
In univariate Cox analysis, history of thrombosis (p < 0.001), age ≥60 years (p = 0.04), extreme thrombocytosis (p = 0.01), progressive splenomegaly (p = 0.05), inadequate hematocrit control (p = 0.01), relevant CVRFs (p = 0.01) were significantly associated with a higher thrombotic risk.
In MVA, four factors maintained significant association with the thrombotic risk: inadequate hematocrit control (HR: 2.32, 95% CI: 1.45–3.72, p < 0.001); relevant CVRFs (HR: 2.87, 95% CI: 1.36–6.06, p = 0.006); progressive splenomegaly (HR: 4.02, 95% CI: 1.18–13.65, p = 0.03) and history of thrombosis (HR: 3.76, 95% CI: 2.32–6.10, p < 0.001) (Fig. 3).
CVRFs cardiovascular risk factors, HU hydroxyurea, HR hazard ratio, CI confidence interval. Red dots identify variable statistically significantly associated with a higher probability of thrombosis during hydroxyurea in both multivariate and univariate analysis; Yellow dots identify variable statistically significantly associated with a higher probability of thrombosis during hydroxyurea only in univariate analysis.
Discussion
HU is the primary cytoreductive agent used in the treatment of PV and plays a crucial role in the overall anti-thrombotic strategy. Its use significantly reduces the incidence of thrombotic events; however, a notable number of events still occur, with an incidence rate of 3.3% p-y, which increases with age [32]. The effectiveness of HU is greater in preventing initial or recurrent arterial events, but it has a higher failure rate in preventing venous events [31, 33, 34]. Therefore, it is essential to identify patient groups undergoing cytoreductive therapy who remain at an increased risk for thrombosis.
In our cohort, the overall incidence of thrombosis during HU treatment is lower than what was estimated in the aforementioned meta-analysis [32], likely due to the younger median age of our patients, which includes LR patients. However, the incidence of recurrent thrombosis in the HR-THRO group (3% p-y) is comparable to the rate reported in the meta-analysis (4.5% p-y), indicating that the patients we studied reflect real-world scenarios.
To ensure homogeneous therapeutic exposure and reduce variability in thrombotic risk factors, only patients treated with HU as first-line therapy were included. Indeed, interferon therapy is mainly used in younger patients and has been made available over the years in different formulations, each potentially providing different degrees of thrombotic protection [35,36,37,38]. As for ruxolitinib, in Italy this agent is only used as second-line therapy. Therefore, ruxolitinib-treated patients represent a different cohort, characterized by more advanced disease, in whom HU had been initiated earlier and had already failed [39].
It has been more than 2 years since the publication of the ELN recommendations on the relevance of specific clinical signs and symptoms to consider for earlier therapy in conventionally defined low-risk PV patients [16]. However, the strength of these recommendations remains weak due to a lack of specific data on the prognostic impact of such CSSs on the thrombotic risk.
Our results show that CSSs are common and significantly influence thrombotic risk, isolating a cohort of patients at further increased risk within each risk category. Additionally, three CSSs were more significantly associated with thrombosis: inadequate hematocrit control, relevant cardiovascular risk factors and progressive splenomegaly.
Elevated hematocrit is one of the major risk factors for thrombosis in patients with PV [3, 11]. In the CYTO-PV study, patients with hematocrit below 45% had a lower incidence of deaths from cardiovascular causes or major thrombotic events (1.1%p-y) than those with a target hematocrit of 45–50% (4.4%p-y) [40]. A higher risk of thrombosis has also been observed in the subset of HU-treated patients requiring ≥3 phlebotomies per year [41].
There is a significant association between major thromboembolic events and hypertension, diabetes, hyperlipidemia, obesity and smoking [6, 8,9,10, 13, 42,43,44,45]. Nevertheless, a consensus on which cardiovascular risk factors and/or combinations thereof should be designated as “relevant” in patients with PV remains elusive. As observed in this study, patients frequently exhibit multiple concomitant cardiovascular risk factors. These CVRFs often co-occur with other clinical signs and symptoms that correlate with the thrombotic risk, such as inadequate hematocrit control (Fig. 1b). Furthermore, the impact of individual CVRFs can sometimes be masked by the thrombotic risk associated with PV, complicating the identification of a single CVRF that is associated with a heightened thrombotic risk. We ultimately adopted a composite definition, requiring the concurrent presence of at least three CVRFs, namely smoke, hypertension, and overweight. This definition was derived from statistical associations with thrombotic risk and relative incidence ratio rates of thrombosis associated with each factor. From a biological perspective, this composite definition is more likely to reflect the synergistic effects of CVRFs. The potential for cumulative risk actions associated with the concurrent presence of two risk factors cannot be disregarded. However, this definition aligns with previous guidelines and may serve as a practical clinical guidance tool [16, 46]. Overall, these findings recommend that CVRFs in all PV patients should be adequately controlled and reinforce the necessity of a multidisciplinary approach [47].
We also observed a correlation between progressive splenomegaly and an increased risk of thrombosis. This correlation has been reported by previous groups [9] but not confirmed in other cohorts [48]. The assessment of spleen size by palpation may be inaccurate and/or not adequately recorded in medical charts. However, the ELN criteria focus exclusively on patients with persistent and progressive splenomegaly, whose clinical burden is unlikely to be overlooked, and recommend physical examination of the spleen, to enable its application in daily clinical practice. However, some patients with progressive but minimally symptomatic splenomegaly may have been excluded from this analysis. Overall, further investigation into the dynamic evaluation of the spleen may provide valuable insights for the management of PV.
Here, we could not confirm the association between thrombocytosis/leukocytosis and thrombosis. The association between the number of platelets and PV outcomes is controversial, with some studies [6, 49], but not others [31], reporting an association between higher platelet count and thrombosis. While in the ECLAP and in the CYTO-PV studies an association between leukocytosis and vascular events was noted [6, 49], hyperleukocytosis at diagnosis or during follow-up was not found to correlate with thrombotic risk in other studies [50, 51]. Neutrophil-to-lymphocyte ratio (NLR) values ≥ 5 was independently associated with an increased risk of venous thrombosis, which suggests that NLR, rather than leukocyte count alone, may be a valuable prognostic biomarker for thrombosis in PV and should be further investigated [15, 52].
The JAK2V617F variant allele frequency is also a significant prognostic factor in PV. Nonetheless, the 2024 and 2025 National Comprehensive Cancer Network (NCCN) guidelines, as well as the ELN recommendations, do not yet include molecular evaluation as a criterion for initiating cytoreductive therapy [16, 53, 54]. In this context, the results of this study remain valid and valuable. However, the collection of molecular data would provide valuable additional insights and should be included in future research agendas of cooperative groups.
Our study confirmed the pivotal role of thrombotic history [6, 8,9,10, 42, 55]. The loss of statistical significance of older age in multivariable analysis should not imply that age is not an independent risk factor for thrombosis. Indeed, the IRRs of thrombosis demonstrated a linear correlation between thrombotic risk and age. It is notable that the IRR for low-risk patients (aged under 60 with no previous history of thrombosis) is 1.1%p-y, while the IRR for high-risk patients aged 60 or over (but with no previous thrombosis) is higher at 1.3%p-y. This incidence is comparable to that of patients at high risk for a previous thrombotic history (but aged under 60 years), who have an IRR of 1.9%p-y. The simultaneous presence of both risk factors identifies the highest risk category, with an IRR of 3.5%p-y. The results are comparable to those of the ECLAP study, which found that the IRRs of thrombosis were similar in older age groups without thrombosis (4.9%p-y) and in younger age groups with thrombosis (5.0%p-y) [55].
Finally, the IRR of thrombosis in low-risk patients with CSSs but who did not receive HU showed that lack of cytoreduction was associated with a numerically higher rate of thrombosis. This finding may further support the relevance of CSSs in clinical decision making but should be prospectively confirmed. Indeed, in the present study the decision to start HU was at the discretion of the treating hematologist and occurred at different follow-up times. As a result, the exposure to CSSs was not uniform across patients’ cohorts, potentially confounding their thrombotic risk.
Intrinsic limitations of the present study are due to its retrospective nature and include uncontrolled patient adherence to HU therapy, consistency in the assessment of CSSs, and reporting of thrombotic events, which may vary and affect the generalizability of the results.
In conclusion, CSSs may identify an increased risk phenotype across all thrombotic risk categories and may be actionable markers that can inform more aggressive and personalized management strategies.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request to the corresponding author (francesca.palandri@unibo.it). https://doi.org/10.5281/zenodo.14017557.
References
Perner F, Perner C, Ernst T, Heidel FH. Roles of JAK2 in aging, inflammation, hematopoiesis and malignant transformation. Cells. 2019:8:854.
Perner F, Pahl HL, Zeiser R, Heidel FH. Malignant JAK-signaling: at the interface of inflammation and malignant transformation. Leukemia. 2025;39:1011–30.
Tefferi A, Barbui T. Polycythemia vera: 2024 update on diagnosis, risk-stratification, and management. Am J Hematol. 2023;98:1465–87.
Masarova L, Chifotides HT. SOHO state of the art update and next questions: novel therapies for polycythemia vera. Clin Lymphoma Myeloma Leuk. 2024;24:141–8.
Hultcrantz M, Björkholm M, Dickman PW, Landgren O, Derolf ÅR, Kristinsson SY, et al. Risk for arterial and venous thrombosis in patients with myeloproliferative neoplasms: a population-based cohort study. Ann Intern Med. 2018;168:317–25.
Landolfi R, Di Gennaro L, Barbui T, De Stefano V, Finazzi G, Marfisi R, et al. Leukocytosis as a major thrombotic risk factor in patients with polycythemia vera. Blood. 2007;109:2446–52.
Finazzi G. A prospective analysis of thrombotic events in the European collaboration study on low-dose aspirin in polycythemia (ECLAP). Pathol Biol. 2004;52:285–8.
Enblom-Larsson A, Renlund H, Andréasson B, Holmberg H, Liljeholm M, Själander A. Thromboembolic events, major bleeding and mortality in essential thrombocythaemia and polycythaemia vera—a matched nationwide population-based study. Br J Haematol. 2024;204:1740–51.
Cerquozzi S, Barraco D, Lasho T, Finke C, Hanson CA, Ketterling RP, et al. Risk factors for arterial versus venous thrombosis in polycythemia vera: a single center experience in 587 patients. Blood Cancer J. 2017;7:1–7.
Barbui T, Carobbio A, Rumi E, Finazzi G, Gisslinger H, Rodeghiero F, et al. In contemporary patients with polycythemia vera, rates of thrombosis and risk factors delineate a new clinical epidemiology. Blood. 2014;124:3021–3.
Barbui T, Tefferi A, Vannucchi AM, Passamonti F, Silver RT, Hoffman R, et al. Philadelphia chromosome-negative classical myeloproliferative neoplasms: revised management recommendations from European LeukemiaNet. Leukemia. 2018;32:1057–69.
Guglielmelli P, Mora B, Gesullo F, Mannelli F, Loscocco GG, Signori L, et al. Clinical impact of mutated JAK2 allele burden reduction in polycythemia vera and essential thrombocythemia. Am J Hematol. 2024;99:1550–9.
Guglielmelli P, Loscocco GG, Mannarelli C, Rossi E, Mannelli F, Ramundo F, et al. JAK2V617F variant allele frequency >50% identifies patients with polycythemia vera at high risk for venous thrombosis. Blood Cancer J. 2021;11:199.
Barbui T, Carobbio A, Guglielmelli P, Ghirardi A, Fenili F, Loscocco GG, et al. Neutrophil/lymphocyte ratio identifies low-risk polycythaemia vera patients for early Ropeginterferon alfa-2b therapy. Br J Haematol. 2024;205:2287–94.
Verstovsek S, Krečak I, Heidel FH, De Stefano V, Bryan K, Zuurman MW, et al. Identifying patients with polycythemia vera at risk of thrombosis after hydroxyurea initiation: the Polycythemia Vera-Advanced Integrated Models (PV-AIM) Project. Biomedicines. 2023;11:1925.
Marchetti M, Vannucchi AM, Griesshammer M, Harrison C, Koschmieder S, Gisslinger H, et al. Appropriate management of polycythaemia vera with cytoreductive drug therapy: European LeukemiaNet 2021 recommendations. Lancet Haematol. 2022;9:e301–11.
Crodel CC, Jentsch-Ullrich K, Reiser M, Jacobasch L, Sauer A, Tesch H, et al. Cytoreductive treatment in real life: a chart review analysis on 1440 patients with polycythemia vera. J Cancer Res Clin Oncol. 2022;148:2693–705.
Jentsch-Ullrich K, Eberhardt J, Zeremski V, Koehler M, Wolleschak D, Heidel FH. Characteristics and treatment of polycythemia vera patients in clinical practice: a multicenter chart review on 1476 individuals in Germany. J Cancer Res Clin Oncol. 2016;142:2041–9.
Palandri F, Rossi E, Auteri G, Breccia M, Paglia S, Benevolo G, et al. Predictors of response to hydroxyurea and switch to ruxolitinib in HU-resistant polycythaemia VERA patients: a real-world PV-NET study. Cancers. 2023;15:3706.
Podoltsev NA, Zhu M, Zeidan AM, Wang R, Wang X, Davidoff AJ, et al. The impact of phlebotomy and hydroxyurea on survival and risk of thrombosis among older patients with polycythemia vera. Blood Adv. 2018;2:2681.
Gilbert HS. Current management in polycythemia vera. Semin Hematol. 2001;38:25–8.
Arber DA, Orazi A, Hasserjian RP, Borowitz MJ, Calvo KR, Kvasnicka HM, et al. International consensus classification of myeloid neoplasms and acute leukemias: integrating morphologic, clinical, and genomic data. Blood. 2022;140:1200–28.
Barosi G, Mesa RA, Thiele J, Cervantes F, Campbell PJ, Verstovsek S, et al. Proposed criteria for the diagnosis of post-polycythemia vera and post-essential thrombocythemia myelofibrosis: a consensus statement from the International Working Group for Myelofibrosis Research and Treatment. Leukemia. 2008;22:437–8.
Mora B, Passamonti F. SOHO state of the art updates and next questions | polycythemia vera: is it time to rethink treatment?. Clin Lymphoma Myeloma Leuk. 2023;23:79–85.
Slee VN. The International Classification of Diseases: ninth revision (ICD-9). Ann Intern Med. 1978;88:424–6.
Cancer Institute N. Common Terminology Criteria for Adverse Events (CTCAE) Common Terminology Criteria for Adverse Events (CTCAE) v5.0. 2017.
Marchioli R, Finazzi G, Specchia G, Masciulli A, Mennitto MR, Barbui T. The CYTO-PV: a large-scale trial testing the intensity of CYTOreductive therapy to prevent cardiovascular events in patients with polycythemia vera. Thrombosis. 2011;2011:1–9.
Greenlund KJ, Zheng ZJ, Keenan NL, Giles WH, Casper ML, Mensah GA, et al. Trends in self-reported multiple cardiovascular disease risk factors among adults in the United States, 1991-1999. Arch Intern Med. 2004;164:181–8.
Tsao CW, Vasan RS. Cohort profile: the Framingham Heart Study (FHS): overview of milestones in cardiovascular epidemiology. Int J Epidemiol. 2015;44:1800–13.
Emanuel RM, Dueck AC, Geyer HL, Kiladjian JJ, Slot S, Zweegman S, et al. Myeloproliferative neoplasm (MPN) symptom assessment form total symptom score: prospective international assessment of an abbreviated symptom burden scoring system among patients with MPNs. J Clin Oncol. 2012;30:4098–103.
De Stefano V, Za T, Rossi E, Vannucchi AM, Ruggeri M, Elli E, et al. Recurrent thrombosis in patients with polycythemia vera and essential thrombocythemia: incidence, risk factors, and effect of treatments. Haematologica. 2008;93:372–80.
Ferrari A, Carobbio A, Masciulli A, Ghirardi A, Finazzi G, de Stefano V, et al. Clinical outcomes under hydroxyurea treatment in polycythemia vera: a systematic review and meta-analysis. Haematologica. 2019;104:2391–9.
De Stefano V, Rossi E, Carobbio A, Ghirardi A, Betti S, Finazzi G, et al. Hydroxyurea prevents arterial and late venous thrombotic recurrences in patients with myeloproliferative neoplasms but fails in the splanchnic venous district. Pooled analysis of 1500 cases. Blood Cancer J. 2018;8:1–7.
Barbui T, De Stefano V, Ghirardi A, Masciulli A, Finazzi G, Vannucchi AM. Different effect of hydroxyurea and phlebotomy on prevention of arterial and venous thrombosis in Polycythemia Vera. Blood Cancer J. 2018;8:124.
Mascarenhas J, Kosiorek HE, Prchal JT, Rambaldi A, Berenzon D, Yacoub A, et al. A randomized phase 3 trial of interferon-α vs hydroxyurea in polycythemia vera and essential thrombocythemia. Blood. 2022;139:2931–41.
Silver RT. Long-term effects of the treatment of polycythemia vera with recombinant interferon-α. Cancer. 2006;107:451–8.
Them NCC, Bagienski K, Berg T, Gisslinger B, Schalling M, Chen D, et al. Molecular responses and chromosomal aberrations in patients with polycythemia vera treated with peg-proline-interferon alpha-2b. Am J Hematol. 2015;90:288–94.
Kiladjian JJ, Cassinat B, Chevret S, Turlure P, Cambier N, Roussel M, et al. Pegylated interferon-alfa-2a induces complete hematologic and molecular responses with low toxicity in polycythemia vera. Blood. 2008;112:3065–72.
AIFA (Agenzia Italia del Farmaco). Determina n. 381/2024. 2024. https://www.aifa.gov.it/documents/20142/961234/Determina_381-2024_Jakavi.pdf.
Marchioli R, Finazzi G, Specchia G, Cacciola R, Cavazzina R, Cilloni D, et al. Cardiovascular events and intensity of treatment in polycythemia vera. N Engl J Med. 2013;368:22–33.
Alvarez-Larrán A, Pérez-Encinas M, Ferrer-Marín F, Hernández-Boluda JC, José Ramírez M, Martínez-López J, et al. Risk of thrombosis according to need of phlebotomies in patients with polycythemia vera treated with hydroxyurea. Haematologica. 2017;102:103–9.
Benevolo G, Elli EM, Bartoletti D, Latagliata R, Tiribelli M, Heidel FH, et al. Impact of comorbidities and body mass index on the outcome of polycythemia vera patients. Hematol Oncol. 2021;39:409–18.
Barbui T, Vannucchi AM, Carobbio A, Rumi E, Finazzi G, Gisslinger H, et al. The effect of arterial hypertension on thrombosis in low-risk polycythemia vera. Am J Hematol. 2017;92:E5–6.
Horvat I, Boban A, Zadro R, Antolic MR, Serventi-Seiwerth R, Roncevic P, et al. Influence of blood count, cardiovascular risks, inherited thrombophilia, and JAK2 V617F burden allele on type of thrombosis in patients with Philadelphia chromosome Negative Myeloproliferative Neoplasms. Clin Lymphoma Myeloma Leuk. 2019;19:53–63.
Komatsu N, Jun GJ, Yonezu T, Ohashi Y. Real-world, retrospective study evaluating thromboembolic events, associated risk factors, and health-care resource utilization in Japanese patients with polycythemia vera. Int J Hematol. 2020;112:176–84.
Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41:255–323.
Palandri F, Branzanti F, Venturi M, Dedola A, Fontana G, Loffredo M, et al. Real-life use of ropeg-interferon α2b in polycythemia vera: patient selection and clinical outcomes. Ann Hematol. 2024;103:2347–54.
Lee MW, Yeon SH, Ryu H, Song IC, Lee HJ, Yun HJ, et al. Volumetric Splenomegaly In Patients With Polycythemia Vera. J Korean Med Sci. 2022;37:e87.
Barbui T, Masciulli A, Marfisi MR, Tognoni G, Finazzi G, Rambaldi A, et al. White blood cell counts and thrombosis in polycythemia vera: a subanalysis of the CYTO-PV study. Blood. 2015;126:560–1.
De Stefano V, Za T, Rossi E, Vannucchi AM, Ruggeri M, Elli E, et al. Leukocytosis is a risk factor for recurrent arterial thrombosis in young patients with polycythemia vera and essential thrombocythemia. Am J Hematol. 2010;85:97–100.
Ronner L, Podoltsev N, Gotlib J, Heaney ML, Kuykendall AT, O’Connell C, et al. Persistent leukocytosis in polycythemia vera is associated with disease evolution but not thrombosis. Blood. 2020;135:1696–703.
Carobbio A, Vannucchi AM, De Stefano V, Masciulli A, Guglielmelli P, Loscocco GG, et al. Neutrophil-to-lymphocyte ratio is a novel predictor of venous thrombosis in polycythemia vera. Blood Cancer J. 2022;12:28.
Ali H, Castells M, σ Þ, Dunbar A, George TI, Green S, et al. NCCN guidelines version 2.2024 myeloproliferative neoplasms patient advocate. 2024.
Ali H, Castells M, σ Þ, Fein Revell R, Green S, Gundabolu K, et al. NCCN guidelines version 1.2025 myeloproliferative neoplasms patient advocate. 2025.
Marchioli R, Finazzi G, Landolfi R, Kutti J, Gisslinger H, Patrono C, et al. Vascular and neoplastic risk in a large cohort of patients with polycythemia vera. J Clin Oncol. 2005;23:2224–32.
Acknowledgements
This work was supported by Ministero della Salute Ricerca corrente and by BolognAIL.
Funding
The work reported in this publication was funded by Italian Ministry of Health, RC-2024-2790083 project. FHH was supported in part by grants of the German Research Council (DFG): HE6233/15-1 and 16-1, project number 517204983.
Author information
Authors and Affiliations
Contributions
FrP: conceptualization; investigation; funding acquisition; writing—original draft; writing—review & editing; data curation; resources; and visualization. FB: conceptualization, writing—original draft; writing—review & editing; data curation; formal analysis; and visualization. EM, ADe, GBe, MT, GAP, MBr, EME, RL, ER, VDS: conceptualization; investigation; visualization; writing—review & editing; Resources. FrC, AT, MFa, AD, FaC: investigation; writing—review & editing; and resources. FHH: visualization; writing—review & editing.
Corresponding authors
Ethics declarations
Competing interests
FP participated in the speaker’s bureau and advisory board of Novartis, BMS, AOP, Sierra Oncology, Incyte, Telios, Abbvie, Constellation-Morphosys, Sobi and GSK. MB reports honoraria from Novartis, BMS, Pfizer, Incyte. GB reports honoraria from Novartis, Janssen, Amgen, Takeda, and BMS. MT reports honoraria from and has served on speakers’ bureaus for Novartis, BMS, Pfizer, and Incyte. FHH served as an advisor for Novartis, CTI, Celgene/BMS, Janssen, Abbvie, GSK, Merck and AOP and received research funding from Novartis, Celgene/BMS and CTI. GAP reports consultancy and honoraria from Abbvie, AOP, AstraZeneca, BMS, Incyte, GSK, Morphosys, and Novartis. VDS participated in the speaker’s bureau and advisory board of Abbvie, AOP Health, Bristol Myers Squibb, Glaxo Smith Kline, Grifols, Novartis, Novo Nordisk, Sanofi, SOBI, Takeda.
Ethical approval
The PV-ARC study was performed in accordance with the guidelines of the IRBs of the participating centers and the standards of the Helsinki Declaration. For patients currently under follow-up with the experimental center, informed consent was obtained as part of one of the visits in their normal care pathway. For deceased patients, Italian regulations authorized the processing of personal data carried out for scientific research purposes (Gazzetta Ufficiale no. 72 dated 26 March 2012). Therefore, the processing of personal data is considered authorized upon approval of the study by the Ethics Committee. The promoter of this study was the IRCCS Azienda Ospedaliero-Universitaria S. Orsola-Malpighi, Bologna, which obtained approval from the Area Vasta Emilia Centro (AVEC) Ethics Committee (approval file number: 483/2018/Oss/AOUBo). This study was also approved by the local ethics committees of participating centers (protocol code: PV-ARC) and had no commercial support.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Palandri, F., Breccia, M., Elli, E.M. et al. Impact of ELN clinical signs and symptoms on the thrombotic risk in polycythemia vera patients treated with front-line hydroxyurea. Leukemia 39, 1928–1936 (2025). https://doi.org/10.1038/s41375-025-02646-y
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41375-025-02646-y
This article is cited by
-
Response to the commentary by Xu et al. on the PV-AIM study
Leukemia (2026)
-
Patient-reported outcomes regarding the use of complementary and alternative medicine (CAM) in BCR::ABL1-negative myeloproliferative neoplasias
Journal of Cancer Research and Clinical Oncology (2026)
-
Long-term real-world thrombotic and clinical outcomes in polycythemia vera – a hospital-based i2b2 cohort study
Annals of Hematology (2026)
-
Challenges in predicting hydroxyurea resistance and reducing thrombotic risk in polycythemia vera patients: unmasking the limits of its machine learning study
Leukemia (2025)