Abstract
Resistance to the Bcl-2-specific inhibitor, Venetoclax (VEN), poses a therapeutic challenge in the management of chronic lymphocytic leukemia and acute myeloid leukemia. Although VEN resistance has been linked to Mcl-1 upregulation, thereby switching survival dependence from Bcl-2 to Mcl-1, the mechanism underlying increased Mcl-1 expression remains elusive. Given that changes in cellular redox state affect cancer cell fate, we investigated the crosstalk between intracellular redox milieu and Mcl-1 upregulation in VEN-resistant cells. Results show that increased Mcl-1 protein levels in VEN-resistant hematologic malignant cells are associated with elevated intracellular superoxide (O2.−) levels. Validating that, augmenting intracellular O2.− in VEN-sensitive cells increases Mcl-1 phosphorylation at threonine-163 (T163pMcl-1) and protein stability via reduced Mcl-1 ubiquitination and degradation. Furthermore, redox-activated AKT/PKB is implicated in O2.−-induced T163pMcl-1, as reducing intracellular O2.− or inhibiting AKT significantly decreases T163pMcl-1 and Mcl-1 accumulation, which amplifies mitochondrial apoptotic priming and restores VEN sensitivity. Importantly, combination therapy with AKT inhibitor, capivasertib, and VEN reduced VEN-resistant cells systemically and prolonged survival in a murine model. Collectively, a novel redox-dependent mechanism of Mcl-1 stability is demonstrated for the acquisition of VEN resistance, which has therapeutic implications for employing redox modulating strategies and AKT inhibitors against VEN-resistant hematologic malignancies.

Similar content being viewed by others
Introduction
Apoptosis evasion is a cancer hallmark, mediated by aberrant expression and survival dependence on anti-apoptotic members of the Bcl-2 family (Bcl-2, Bcl-xL, and Mcl-1), which function to sequester pro-apoptotic proteins (Bak and Bax). As such, the development of selective anti-apoptotic Bcl-2 inhibitors was explored to reinstate apoptotic functions [1, 2].
Venetoclax/ABT199 (VEN) is a Bcl-2-specific inhibitor, clinically approved for the treatment of chronic lymphocytic leukemia (CLL) and acute myeloid leukemia (AML) [1, 3, 4]. While VEN triggers apoptosis in Bcl-2-dependent hematological cancers, other anti-apoptotic proteins such as Mcl-1 or Bcl-xL appear to play an important role(s) in VEN responsiveness [5, 6]. Prolonged VEN therapy has been shown to reduce survival dependence of hematological cancers on Bcl-2, thereby promoting the selective survival of VEN-refractory cells [7, 8]. While reports have linked BCL2 mutation(s), such as the G101V to inefficient VEN binding to the BH3 domain of Bcl-2 [9,10,11], this is believed to be a rare occurrence with low allelic fraction [9, 10]. A probable mechanism underlying VEN resistance is the upregulation of the anti-apoptotic protein Mcl-1 [7, 12,13,14,15]. Cells with higher Mcl-1 levels are resistant to VEN treatment [7, 16], while inhibiting Mcl-1 restores sensitivity of VEN-R cells to VEN, thus indicating a switch of dependence from Bcl-2 to Mcl-1 [13, 17, 18]. Nonetheless, the underlying mechanism of Mcl-1 upregulation in VEN-R cells has not been well-established to date.
Mcl-1 was first identified in human myeloblastic leukemia and shown to have anti-apoptotic and other physiological functions. Mcl-1 is a short-lived protein, with its expression regulated at multiple cellular levels. Structurally, besides the Bcl-2 homology (BH)1-BH4 domains, Mcl-1 also contains a PEST domain in the N-terminus that functions as signal for protein turnover [19]. Mcl-1 stability is regulated by several ubiquitination and phosphorylation sites [20]. Threonine-163 (T163) phosphorylation stabilizes Mcl-1, while multisite phosphorylation (T92, S121, S159) promotes Mcl-1 degradation [21,22,23,24,25,26]. Notably, the complex nature of its regulation, coupled with non-apoptotic functions, such as in cardiomyocyte physiology [27], impact the systemic use of Mcl-1 inhibitors. Therefore, a better understanding of the mechanism(s) and/or signaling networks involved in regulating Mcl-1 stability could have potential therapeutic implications, particularly in the setting of VEN resistance with a switch to Mcl-1 dependence.
While the canonical anti-apoptotic activity of the Bcl-2 proteins involves mediating mitochondria outer membrane permeabilization (MOMP), our work has linked altered cellular redox status as the non-canonical pro-survival mechanism [28], such as increased mitochondrial oxygen consumption and intracellular superoxide (O2.−) levels upon overexpression of Bcl-2 [29,30,31]. Reduction of intracellular O2.− restored sensitivity of Bcl-2-expressing cells to drug-induced apoptosis. Mechanistically, elevated intracellular O2.− resulted in sustained phosphorylation of Bcl-2 at serine-70 (S70pBcl-2) via redox-dependent inactivation of the protein phosphatase-2A (PP2A) [32, 33].
Against the backdrop of the crosstalk between cellular redox state and the anti-apoptotic activity of Bcl-2, we questioned the role of cellular redox milieu in the acquisition of VEN-R and the associated increase in Mcl-1 levels. Herein, we provide evidence linking an increase in intracellular O2.− to Mcl-1 abundance in VEN-resistant (VEN-R) hematologic malignant cells. Furthermore, O2.−-induced Mcl-1 stability is a function of AKT-mediated T163pMcl-1, decreased overall mitochondrial priming and increased cell survival.
Results
Venetoclax resistance is associated with increased intracellular O2 .−
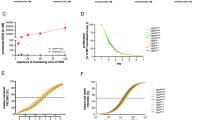
To study the interplay between intracellular redox state and Mcl-1 upregulation upon the acquisition of VEN resistance, we generated the VEN-R AML cells (VEN-R MOLM14 and VEN-R OCI-AML2) from VEN-sensitive or parental (VEN-S) MOLM14 and OCI-AML2 cells (Figs. 1A, S1A, Supplementary Method). Consistent with published reports [5, 15], we verified Mcl-1 abundance in the acquired VEN-R cells (R) compared to the VEN-S (S) cells of AML as well as in our previously established lymphoid VEN-R diffused large B-Cell lymphoma (DLBCL) cells (Fig. 1B) [7, 13]. Similarly, our previously characterized inherent VEN-R DLBCL cells, TMD8, in comparison to the VEN-S OCI-Ly1 cells [7], showed increased Mcl-1 levels (Fig. 1B). As previously described in VEN-R lymphoid malignancies [7], we also observed an increase in S70pBcl-2 in these VEN-R AML cells (Fig. 1B), indicative of an altered cellular redox milieu [32]. As Mcl-1 regulates apoptosis and mitochondrial bioenergetics [34], we further examined the oxidative phosphorylation (OXPHOS) status of VEN-R cells. Both VEN-R cell lines exhibited higher basal oxygen consumption rate (OCR) and maximal respiration rate compared to VEN-S cells (Fig. 1C, D). Given that enhanced OXPHOS has been linked to the generation of a more pro-oxidant and drug-resistant milieu [13, 35], we observed a significant increase in intracellular O2.− in acquired VEN-R MOLM14, OCI-AML2, and OCI-Ly1 cells (Fig. 1E). Corroborating the latter, inherently VEN-R OCI-AML3 (relative to VEN-S OCI-AML2, Fig. 1F) and TMD8 cells also exhibited higher intracellular O2.− compared to VEN-S OCI-AML2 and OCI-Ly1 cells, respectively (Fig. 1G, H). Importantly, treatment with VEN for 48 h could also increase reactive oxygen species (ROS) in VEN-S MOLM14 cells (Fig. S2A). Collectively, these data suggest an interplay between intracellular redox state and Mcl-1 accumulation upon VEN resistance acquisition.
A Cell viability of venetoclax-sensitive (VEN-S) MOLM14, OCI-AML2, and acquired venetoclax-resistant (VEN-R) MOLM14 and OCI-AML2 cells following treatment with increasing concentration of venetoclax (VEN) for 48 h. Cell viability was measured using MTT assay. N = 3. B Western blot analyses showing Mcl-1, S70pBcl-2, Bcl-2, and Tubulin/β-Actin levels in MOLM14, OCI-AML2, OCI-Ly1 of VEN-S/parental or VEN-R cells, and TMD8 (inherently resistant) cells. N = 3. C Representative graph showing the kinetics of oxygen consumption rate (OCR) when MOLM14 and OCI-AML2 of VEN-S and VEN-R cells were subjected to oligomycin (1 µM), FCCP (1 µM), and rotenone/antimycin A (Rot/AA) (0.5 µM/0.5 µM) injection at the indicated timepoints of the assay. D Graph showing fold change in oxygen consumption rate (OCR) of MOLM14 and OCI-AML2 of VEN-S and VEN-R cells. OCR was measured via Seahorse assay (Agilent, USA). N = 3. Unpaired t-test was used. E Graph showing fold change in intracellular O2.− levels of MOLM14 (N = 4), OCI-AML2 (N = 3), OCI-Ly1 (N = 3) of VEN-S and VEN-R cells. Unpaired t-test was used. F Cell viability of VEN-S OCI-AML2 and inherently VEN-R OCI-AML3 cells following treatment with increasing concentrations of VEN (µM) for 48 h. Cell viability was measured via MTT assay. N = 3. Sidak’s multiple comparisons test was used. G-H Graph showing fold change in intracellular O2.− levels of inherently VEN-R OCI-AML3 or TMD8 cells relative to VEN-S OCI-AML2 or OCI-Ly1 cells, respectively. N = 4. Unpaired t-test was used. *MOLM14 and VEN-S MOLM14 or OCI-Ly1 and VEN-S OCI-Ly1 are used interchangeably.
Intracellular O2 .− increases Mcl-1 and reduces mitochondrial apoptotic priming
Next, we questioned if the intracellular increase in O2.− was upstream of Mcl-1 accumulation. Intracellular O2.− was manipulated via pharmacological inhibition (diethyldithiocarbamate; DDC) of superoxide dismutase-1 (SOD1), an enzyme responsible for the conversion of O2.− to hydrogen peroxide (H2O2), or its genetic knockdown (siSOD1). DDC treatment resulted in a dose-dependent increase in intracellular O2.− in VEN-S MOLM14 and OCI-AML2 AML cells and three other hematopoietic cell lines, RPMI8226 myeloma, Jurkat T-ALL, and OCI-Ly3 DLBCL (Figs. 2A and S3A). Importantly, increased intracellular O2.− resulted in Mcl-1 accumulation in all 5 cell lines, indicating that the effect of O2.− on Mcl-1 was not limited to AML cell lines (Figs. 2B and S3A). Similarly, siSOD1 resulted in an increase in intracellular O2.− and Mcl-1 protein levels (Figs. 2C, D and S3B). To further validate these in vitro data, we analyzed the expression of Mcl-1 and SOD1 in a panel of patient-derived lymphoma biopsies. Indeed, analysis of lysates from 13 lymphoma patients revealed a significant inverse correlation between Mcl-1 and SOD1 protein levels in vivo, validated by Pearson correlation analysis (Fig. 2E).
A Line graph showing raw O2.− detection following treatment with diethyldithiocarbamate (DDC) (50–200 µM) in MOLM14 or Jurkat cells for 4 h. Bar graphs directly below show fold change of averaged intracellular O2.− levels upon treatment with indicated doses of DDC in MOLM14 (N = 4) or Jurkat (N = 3) cells for 4 h. Intracellular O2.− level was measured via lucigenin chemiluminescence assay. Holm–Sidak’s comparisons test was used. B Western blot analyses showing increase in Mcl-1 levels upon treatment with indicated doses of diethyldithiocarbamate (DDC) in MOLM14 or Jurkat cells for 4 h. N = 3. C Graph showing fold change in averaged intracellular O2.− levels upon treatment with siRNA-mediated knockdown of superoxide dismutase 1 (SOD1) (100 nM) in Jurkat (left panel) or MOLM14 (right panel) cells for 48 h. Intracellular O2.− level was measured via lucigenin chemiluminescence assay. N = 3. Holm–Sidak’s comparisons test was used. D Western blot analyses showing Mcl-1, SOD1, and tubulin levels after siRNA-mediated knockdown of SOD1 (100 nM) in Jurkat or MOLM14 cells for 48 h. N = 3. E Western blot showing Mcl-1 and SOD1 levels in 13 lymphoma patient samples. The top panel (s.e) represents short exposure of the blot whereas the panel below (l.e) represents a long exposure of the blot. Densitometric analysis and Pearson correlation analysis of Mcl-1 (l.e.) vs SOD1 protein levels were performed from 13 lymphoma patient samples. Raw densitometric values obtained from ImageJ were used for plotting the graph. N = 13. F Cell viability of MOLM14 cells treated with DDC (25 µM) for 2 h before treatment with VEN (0.02 μM) for 48 h, measured via MTT assay. N = 4. Holm–Sidak’s comparisons test was used. G Western blot showing Mcl-1 levels of MOLM14 cells following treatment with DDC (25 µM) for 2 h before treatment with VEN (0.02 μM) for 48 h. N = 3. H Co-immunoprecipitation of BIM isoforms and immunoblots of BIM isoforms and Mcl-1 in MOLM14 cells treated with DDC (25 µM) for 2 h before treatment with VEN (0.02 μM) for subsequent 24 h. Input showing levels of Mcl-1, BIM, β-Actin. N = 2. I Co-immunoprecipitation of BIM isoforms and immunoblots of BIM isoforms and Mcl-1 in VEN-S and VEN-R MOLM14 cells. Input showing levels of Mcl-1, BIM, β-Actin. N = 2. J Cell viability of RPMI8226 cells treated with DDC (100 µM) for 2 h before treatment with S63845 (0.075 μM) for 24 h, measured via MTT assay. N = 4. Holm–Sidak’s comparisons test was used. K Co-immunoprecipitation of Mcl-1 and immunoblots of Bak, and Mcl-1 in RPMI8226 cells treated with DDC (100 µM) for 2 h before treatment with S63845 (0.075 μM) for subsequent 48 h. Input showing levels of Mcl-1, pro and cleaved caspase-3, Bak and GAPDH. N = 3. L Heatmap of BH3-profiling showing percentage of cytochrome c loss from CLL patient samples after ex vivo treatment with DDC (50 µM) for 4 h and subsequent exposure to different BH3 peptide or mimetic concentrations. N = 6. BH3 Peptides—BIM or PUMA targets all pro-apoptotic proteins, MS1 targets Mcl-1, BAD targets Bcl-2 and Bcl-xL, HRK targets Bcl-xL, ABT199 targets Bcl-2, and A1331852 targets Bcl-xL. M Western blot showing Mcl-1 levels of CLL patient samples after ex vivo treatment with DDC (50 µM) for 4 h. N = 6. Densitometric analyses of Mcl-1/β-Actin levels in CLL patient samples treated with DDC (50 µM) for 4 h, normalized to non-treated control. N = 6. Paired t-test was used.
Intrigued by the association between O2.− and Mcl-1 in VEN-R cells, we next evaluated if increase in O2.− could affect sensitivity to VEN. Pre-treatment of VEN-S MOLM14 cells with DDC significantly reduced cell sensitivity to VEN (Fig. 2F), in conjunction with increased Mcl-1 expression (Fig. 2G). In support of the latter finding as well as our recent publication demonstrating that reduced binding of the pro-apoptotic executioner BIM to BCL-2, is concurrently sequestered by the increased Mcl-1 protein in VEN-R DLBCL cells [7], our immunoprecipitation assay revealed that increased Mcl-1 could further scavenge BIM in VEN-S MOLM14 cells (Fig. 2H), thus suggesting that the increased Mcl-1 protein is phenotypically functional through the reduction of apoptosis. Importantly, the protein-protein interactive effect between stabilized Mcl-1 and BIM was also recapitulated in the VEN-R MOLM14 cells (Fig. 2I). These data provide testimony that O2.−-mediated decrease in VEN sensitivity is a function of active Mcl-1 protein. To further confirm that, we assessed the effect of increased O2.− on cell death upon exposure to Mcl-1 specific inhibitor, S63845. Similar to results observed with VEN, incubation of S63845-sensitive RPMI8226 cells with DDC resulted in a significant reduction in cell death (Fig. 2J). Furthermore, immunoprecipitation analysis revealed that S63845-mediated dissociation of Mcl-1 from Bak was reversed upon DDC-induced increase in O2.−, corresponding to an increased Mcl-1 level and decreased cleaved caspase 3 (Fig. 2K). These data imply that increased Bak and/or BIM sequestration by Mcl-1 could reduce apoptotic sensitivity by interfering with MOMP in cancer cells harboring elevated O2.− levels. Indeed, annexin V assay confirms that DDC could rescue VEN- and S63845-induced apoptotic cell death in VEN-S MOLM14 cells (Fig. S4A). To further corroborate our findings, we employed BH3-profiling to evaluate whether elevated intracellular O2.− affects mitochondria priming in CLL patient samples. Patient-derived CLL cells were treated ex vivo with DDC and cytochrome c loss was measured upon incubation with various BH3-peptides/mimetics. Results showed decreased overall apoptotic priming (reduced cytochrome c loss for all peptides) in DDC-treated patient-derived CLL cells (Fig. 2L). Importantly, reduced priming was also associated with a significant increase in Mcl-1 levels in the 6 patient-derived CLL cells treated ex vivo with DDC (Fig. 2M). Together, these findings establish the role of aberrant O2.− signaling on Mcl-1 upregulation, which regulates MOMP by sequestering pro-apoptotic proteins to render cells resistant to VEN-induced apoptosis.
Reduction in intracellular O2 .− levels decreases Mcl-1 and restores venetoclax sensitivity
To validate that increased Mcl-1 is a function of increased O2.−, we employed two different strategies to reduce intracellular O2.− – scavenging O2.− with Tiron or inhibiting NADPH oxidase(NOX)-dependent O2.− production with diphenyleneiodonium (DPI) (Fig. 3A). Tiron pre-treatment neutralized DDC-induced increases in intracellular O2.− and Mcl-1 expression in VEN-S MOLM14 cells (Fig. 3B, C). Similarly, DPI treatment reduced intracellular O2.− levels as well as baseline and DDC-induced Mcl-1 expression in VEN-S MOLM14 cells (Fig. 3D, E), thus confirming that increased Mcl-1 protein is attributed to increased intracellular O2.−.
A Diagram illustrating the conversion of superoxide (O2.−) to hydrogen peroxide (H2O2) is mediated by superoxide dismutase 1 (SOD1), which could be inhibited by diethyldithiocarbamate (DDC). Tiron and DPI are scavengers and inhibitors of O2.− production, respectively. The diagram was created in BioRender. Pervaiz, S. (2022) BioRender.com/v93n781. B Graph showing fold change in intracellular O2.− levels in MOLM14 cells upon treatment with Tiron (5 mM) for 1 h followed by DDC (50 µM) for 4 h. N = 4. Holm–Sidak’s comparisons test was used. C Western blot showing Mcl-1 levels in MOLM14 cells treated with O2.− scavenger, Tiron (5 mM) for 1 h followed by DDC (50 µM) for 4 h. N = 3. D Graph showing fold change of intracellular O2.− levels in MOLM14 cells upon treatment with DPI for 20 h. Intracellular O2.− levels was measured via lucigenin chemiluminescence assay. N = 3. Dunnett’s multiple comparisons test was used. E Western blot showing Mcl-1 levels in MOLM14 cells upon treatment with increasing diphenyleneiodonium (DPI) doses for 20 h (left panel) or pre-treatment with DPI (1 μM) for 20 h and in combination with DDC (50 μM) for subsequent 4 h (right panel). N = 3. F, G Western blot showing Mcl-1, pro and cleaved caspase-3, GAPDH and/or β-Actin levels of VEN-R MOLM14 or VEN-R OCI-Ly1 cells following treatment with O2.− scavenger, Tiron (1 mM/VEN-R MOLM14, 2.5 mM/VEN-R OCI-Ly1) for 2 h followed by VEN (0.05 µM/VEN-R MOLM14, 1 µM/VEN-R OCI-Ly1) for 48 h. N = 3. H, I Cell viability of VEN-R MOLM14 (N = 4) or VEN-R OCI-Ly1 (N = 4) cells following treatment with O2.− scavenger, Tiron, for 2 h followed by increasing VEN doses for 48 h, measured via CTG assay. Tukey’s multiple comparisons test was used. J Western blot showing Mcl-1, pro and cleaved caspase-3, GAPDH and/or β-Actin levels of inherently VEN-R Su-DHL4 cells following treatment with O2.− scavenger, Tiron (5 mM) for 2 h followed by VEN (0.5 µM) for 48 h. N = 3. K Cell viability of inherently VEN-R Su-DHL4 cells following treatment with O2.− scavenger, Tiron (5 mM) for 2 h followed by VEN (0.5 µM) for 48 h, measured via CTG assay. N = 3. Sidak’s multiple comparisons test was used. L Cell viability of acquired VEN-R OCI-AML2 cells following treatment with DPI (0.1 µM) for 2 h followed by VEN (0.2 µM) for 24 h. Cell viability was measured via MTT assay. N = 2. Sidak’s multiple comparisons test was used. M Western blot showing Mcl-1, pro and cleaved caspase-3 and GAPDH levels of VEN-R OCI-AML2 cells treated with DPI (0.1 µM) for 2 h followed by VEN (0.2 µM) for 24 h. N = 3.
To further evaluate the effects of O2.− on Mcl-1 specifically in VEN-R cells, we proceeded to treat VEN-R MOLM14 cells with VEN at increasing concentrations. We observed a concomitant increase in O2.− and Mcl-1, suggesting a similar regulation of O2.− on Mcl-1 in VEN-R cells (Fig. S5A, B). To confirm that O2.− is upstream of Mcl-1 stability, we treated VEN-R cells with the O2.− scavenger, Tiron, and successfully reduced not only baseline Mcl-1 but also VEN-induced Mcl-1 in VEN-R MOLM14 and VEN-R OCI-Ly1 cells, thus confirming that O2.− is indeed an upstream regulator of Mcl-1 stability (Fig. 3F, G). Importantly, reduced intracellular O2.− by Tiron was further translated to an increased caspase-3 cleavage and decrease in cell viability upon co-treatment with VEN in VEN-R MOLM14 and VEN-R OCI-Ly1 cells, as measured by Western blot analysis, CellTiter-Glo (CTG), and Annexin V cell viability assays (Figs. 3F–I and S6A). Consistent effects of scavenging O2.− were also observed in inherently VEN-R Su-DHL4 [7] and OCI-AML3 cells (Figs. 3J, K and S6B, C). To further corroborate these findings, we assessed the effect of inhibiting NOX-dependent O2.− generation (DPI) on VEN sensitivity in VEN-R OCI-AML2 cells. DPI treatment similarly reduced cell viability, Mcl-1 levels and increase caspase-3 cleavage in VEN-R OCI-AML2 cells upon co-treatment with VEN (Fig. 3L, M). Finally, it is worth pointing out that Tiron or DPI alone significantly reduced Mcl-1 levels, thus strongly supporting the involvement of intracellular O2.− on Mcl-1 protein levels. Together, these data indicate that modulating intracellular O2.− reduces Mcl-1 levels and restores sensitivity of VEN-R cells to VEN.
Increased O2 .− induces Mcl-1 abundance by reducing its protein turnover
We next investigated the underlying mechanism of O2.−-mediated Mcl-1 accumulation. As Mcl-1 could be regulated through gene transcription or protein stability, we first investigated whether increased O2.− could affect gene transcription. Real-time PCR analysis of MCL1 mRNA following DDC treatment revealed no significant difference in MCL1 mRNA levels in VEN-S MOLM14 or RPMI8226 cells (Fig. S7A, B), thus indicating that the effect of increased intracellular O2.− on Mcl-1 could be downstream of gene transcription. We next performed a cycloheximide (CHX) time-chase experiment to determine if the effect was mediated at the post-translational level. Results showed that pre-treatment with DDC for 2 h followed by CHX for the indicated timepoints clearly increased the half-life of Mcl-1 protein in RPMI8226 or VEN-S MOLM14 cells (Fig. 4A, B). Notably, similar results of longer Mcl-1 half-life were observed in VEN-R MOLM14 and VEN-R OCI-AML2 cells, thus corroborating our findings in the context of VEN resistance (Fig. 4C, D). These data indicate that Mcl-1 is post-translationally stabilized in VEN-R cells in a milieu of heightened intracellular O2.− levels.
Western blot showing Mcl-1 and tubulin levels in A RPMI8226, B MOLM14 cells following pre-treatment with DDC (100 µM) for 120 min followed by cycloheximide (CHX) 25 µg/ml, for the indicated timepoints (in minutes). Right panels: Graph plotting the densitometric analysis of RPMI8226 or MOLM14 cells treated with DDC and CHX or CHX alone. Fold change in densitometry values (Mcl-1/Tubulin) and were normalized to untreated control at 0 min of the experiment. Densitometric values were obtained using ImageJ. N = 3. C, D Western blot showing Mcl-1 and GAPDH levels in MOLM14, VEN-R MOLM14, OCI-AML2, VEN-R OCI-AML2 following treatment of cycloheximide (CHX) (25 µg/ml) for the indicated time points. Bottom panels: Graph plotting the densitometric analysis of MOLM14, VEN-R MOLM14, OCI-AML2, and VEN-R OCI-AML2 cells. Densitometric values were obtained using ImageJ. N = 3. E, F Co-Immunoprecipitation of Mcl-1 and immunoblots of Lys48 (K48) and Mcl-1 in RPMI8226 or MOLM14 cells pre-treated with MG132 (5 µM) for 1 h, followed by DDC (100 µM) for 4 h. Input showing levels of Mcl-1 and β-Actin. N = 2. G Western blot showing T163pMcl-1, S159pMcl-1, Mcl-1, Tubulin levels in MOLM14 cells after treatment with indicated doses of DDC (µM) for 4 h. N = 3. H Western blot showing T163pMcl-1, S159pMcl-1, Mcl-1, SOD1, Tubulin levels in MOLM14 cells after siRNA-mediated knockdown of SOD1 (100 nM) for 48 h. N = 3. I Western blot showing T163pMcl-1, Mcl-1, β-Actin levels in VEN-R MOLM14 or VEN-R OCI-Ly1 following treatment with O2.− scavenger, Tiron (1 mM/VEN-R MOLM14, 2.5 mM/VEN-R OCI-Ly1) for 2 h followed by VEN (0.05μM/VEN-R MOLM14, 1μM/VEN-R OCI-Ly1) for 48 h. N = 3. J Western blot showing T163pMcl-1, Mcl-1, β-Actin levels of CLL patient samples after ex vivo treatment with DDC (50 µM) for 4 h. Densitometric analyses of T163pMcl-1/β-Actin levels in CLL patient samples treated with DDC (50 µM) for 4 h, normalized to non-treated control. N = 6. Mcl-1 and β-Actin are from the same set in Fig. 2M. Paired t-test was used. K Western blot showing T163pMcl-1, Mcl-1, and SOD1 levels in 13 lymphoma patient samples. Densitometric analysis and Pearson correlation analysis of T163pMcl-1 vs SOD1 protein levels was performed from 13 lymphoma patient samples. Raw densitometric values obtained from ImageJ were used for plotting the graph. Mcl-1 and SOD1 are from the same set in Fig. 2E. N = 13.
Since O2.−-mediated Mcl-1 stability occurs post-translationally, we investigated if this was due to a reduction in protein turnover. As Mcl-1 protein stability is regulated by the proteasomal degradation pathway [20], we inhibited proteasomal degradation pathway with the proteasomal inhibitor, MG132, to accumulate Mcl-1 and subsequently treated cells with DDC. We hypothesized that MG132-accumulated Mcl-1 that are stabilized by DDC-induced O2.− would display lower Lys48 (K48) polyubiquitin chain modification, a key signal for protein degradation via the proteasome [20, 36], as compared to MG132-accumulated Mcl-1 without DDC. Indeed, we observed vividly lower levels of Lys48 polyubiquitinated-Mcl-1 in cells that were treated with DDC (Fig. 4E). Importantly, lower Lys48 polyubiquitination corresponded accurately with higher levels of Mcl-1 in our input (Fig. 4E). These results were consistently observed in two different cell lines of RPMI8226 and VEN-S MOLM14 cells (Fig. 4E, F), thereby indicating that increase in intracellular O2.− promotes Mcl-1 protein stabilization and accumulation by inhibiting its proteasomal degradation.
O2 .−-mediated Mcl-1 stability is a function of its Thr163 phosphorylation
Mcl-1 stability is regulated by its phosphorylation at Thr163 (T163pMcl-1) and/or S159 (S159pMcl-1), whereby T163pMcl-1 promotes its stability while S159pMcl-1 promotes its proteasomal degradation [22, 25]. Since we observed a prolonged half-life of Mcl-1 and reduced ubiquitination-mediated degradation of Mcl-1, we postulated that Mcl-1 phosphorylation is affected by the altered O2.− levels. Indeed, significant increases in the stabilizing T163pMcl-1 and Mcl-1, but not the degrading S159pMcl-1, were detected upon DDC-induced O2.− elevation in VEN-S MOLM14 cells (Fig. 4G). Similar results were obtained upon knocking down SOD1 with siSOD1 (Fig. 4H). Reciprocally, scavenging O2.− with Tiron in VEN-R MOLM14, OCI-Ly1 or Su-DHL4 cells resulted in reduced T163pMcl-1 levels (Figs. 4I and S8A). We further recapitulated these in vitro findings in ex vivo DDC-treated CLL primary cells by observing that DDC could elevate T163pMcl-1 (Fig. 4J). Finally, in an in vivo setting, we observed a significant inverse correlation between T163pMcl-1 and SOD1 protein expression levels in a range of 13 lymphoma patient samples (Fig. 4K). These data provide evidence that increased O2.− stabilizes Mcl-1 by promoting T163 phosphorylation.
To validate the role of T163pMcl-1 in promoting Mcl-1 stability upon increased intracellular O2.−, we transfected cells with empty vector (EV), wild-type MCL1 (MCL1wt), or phospho-deficient mutant-T163A MCL1 (MCL1T163A), followed by DDC treatment. Notably, the DDC-induced increase in Mcl-1 was observed in cells overexpressing MCL1wt, but not upon overexpression of MCL1T163A (Fig. 5A, B). Subsequently, we generated a stable CRISPR/Cas9 Mcl-1 knockout (KO) clone of Jurkat cells. Note that VEN-R MOLM14 cells were not used due to their survival dependence on Mcl-1 (Fig. S9A). These cells were not suitable for the generation of a stable CRISPR/Cas9 Mcl-1 KO clone due to the potential significant cell death or adaptive mechanisms that may hinder subsequent investigation pertaining to Mcl-1 and its dependence. The Jurkat Mcl-1 KO cells were subsequently transiently transfected with EV, MCL1wt, or MCL1T163A and treated with DDC. Notably, while DDC-induced O2.− increase resulted in Mcl-1 upregulation in MCL1wt-expressing cells, there was no observable increase upon transfection with MCL1T163A (Fig. 5C). Together, these data provide evidence to link O2.--mediated Mcl-1 stability to T163pMcl-1.
A Western blot showing T163pMcl-1, Mcl-1, Tubulin levels in RPMI8226 cells after transfection with 2 µg of empty vector (EV), MCL1wt, or non-phosphorylatable MCL1T163A for 48 h followed by treatment with DDC (100 µM) for 4 h. N = 2. B Densitometric analyses of Mcl-1 levels in RPMI8226 cells after transfection with 2 µg of empty vector (EV), MCL1wt, or non-phosphorylatable MCL1T163A for 48 h followed by treatment with DDC (100 µM) for 4 h. Densitometric values were normalized to tubulin (Mcl-1/Tubulin) and results are relative to EV. N = 2. Sidak’s multiple comparisons test was used. C Western blot showing T163pMcl-1, Mcl-1 levels in Jurkat Mcl-1 KO cells after transfection with 2 µg of empty vector (EV), MCL1wt, or non-phosphorylatable MCL1T163A for 48 h followed by treatment with DDC (100 µM) for 4 h. N = 3. D Graph showing cell viability of VEN-R MOLM14 cells transfected with 2 µg of empty vector (EV), MCL1wt, or non-phosphorylatable MCL1T163A for 24 h followed by treatment with VEN (0.05 μM) for 48 h. Cell viability was measured using MTT assay. N = 5. Tukey’s multiple comparisons test was used. E Western blot showing T163pMcl-1, Mcl-1, and GAPDH of VEN-R MOLM14 cells transfected with 2 µg of empty vector (EV), MCL1wt, or non-phosphorylatable MCL1T163A for 24 h. N = 3. F Western blot showing T163pMcl-1, Mcl-1, and GAPDH of VEN-R MOLM14 cells transfected with 2 µg of empty vector (EV), MCL1wt, or non-phosphorylatable MCL1T163A for 24 h followed by treatment with VEN (0.05 μM) for 24 h. N = 4. G Densitometric analyses of Mcl-1 levels in VEN-R MOLM14 cells transfected with 2 µg of empty vector (EV), MCL1wt, or non-phosphorylatable MCL1T163A for 24 h followed by treatment with VEN (0.05 μM) for 24 h. Densitometric values were normalized to GAPDH (Mcl-1/GAPDH), and results are relative to EV. N = 4. Sidak’s multiple comparisons test was used.
Next, we verified if T163pMcl-1 was involved in regulating Mcl-1 stability in the context of VEN resistance. VEN-R MOLM14 cells were transfected with EV, MCL1wt or MCL1T163A for 24 h followed by treatment with VEN for 48 h (Fig. 5D, E). Notably, MCL1T163A-expressing cells were significantly more sensitive to VEN compared to MCL1wt-expressing cells (Fig. 5D). Furthermore, unlike MCL1wt-expressing cells, VEN treatment failed to upregulate Mcl-1 in MCL1T163A-expressing cells (Fig. 5F, G). Collectively, these data indicate that enhanced Mcl-1 stability induced by increased O2.− is dependent on T163pMcl-1, which appears to be critical in the context of acquired VEN resistance.
AKT activation underlies O2 .−-induced T163pMcl-1 and Mcl-1 stability
T163pMcl-1 has been linked to ERK activation [24] and we observed that ERK inhibition via PD98059 blocked DDC-induced Mcl-1 upregulation, thus indicating the involvement of ERK in promoting O2.−-mediated Mcl-1 stability (Fig. 6A). Interestingly, earlier reports provide evidence that AKT could physically interact with Mcl-1 [37] as well as its enzymatic activity is activated by O2.− [38]. Given these observations, we questioned the role of AKT in mediating redox-dependent T163pMcl-1 and Mcl-1 stability. First, DDC treatment resulted in AKT activation, evidenced by increased S473 phosphorylation (S473pAKT) and downstream GSK3β S9 phosphorylation (S9pGSK3β), as well as increased T163pMcl-1 and Mcl-1 (Fig. 6B). Second, pre-treatment with PI3K inhibitor, LY294002, prevented DDC-induced AKT activation and blocked T163pMcl-1 and Mcl-1 accumulation (Fig. 6B). Third, AKT-specific inhibitor-VIII (AKTI-VIII) prevented DDC-induced increase in S473pAKT, and importantly, inhibited the increase in T163pMcl-1 and Mcl-1 (Fig. 6C). Intriguingly, although blocking PI3K or AKT reduced inhibitory S9pGSK3β, there was no significant increase in GSK3β-dependent S159pMcl-1, which promotes Mcl-1 degradation (Fig. 6B, C). Fourth, we used capivasertib, an AKT inhibitor currently approved for breast cancer treatment [39], and observed that capivasertib could significantly reduce T163pMcl-1 and Mcl-1 levels without affecting S159pMcl-1 (Fig. 6D). As capivasertib does not prevent S473pAKT but competitively binds to its ATP-binding pocket to inhibit downstream phosphorylation of its target [40, 41], we therefore used S9pGSK3β as a positive control (Fig. 6D). Furthermore, pre-treatment with capivasertib for 1 h prevented O2.−-mediated T163pMcl-1 and Mcl-1 accumulation by DDC (Fig. 6E). Similarly, siAKT1 could prevent DDC-induced increases in T163pMcl-1 and Mcl-1 levels in MOLM14 and OCI-AML3 cells (Figs. 6F and S10A). It is worth pointing out that the link between AKT activation and O2.−-mediated Mcl-1 accumulation is not exclusive to hematopoietic cancers; stable knockout of AKT1/2 in HCT116 colorectal carcinoma cells (AKT-DKO) also exhibited reduced T163pMcl-1 and Mcl-1 levels, which was not due to reduction in MCL1 mRNA levels (Fig. S10B, C). Importantly, ex vivo DDC-treated primary CLL patient samples showed a significant increase in S473pAKT (Fig. 6G), in conjunction with downstream increase in T163pMcl-1 and Mcl-1 (Fig. 4J). Further validating the involvement of O2.− in regulating S473pAKT and T163pMcl-1 in the context of VEN resistance, results show that scavenging O2.− with Tiron reduced S473pAKT, T163pMcl-1, and Mcl-1 in VEN-R MOLM14 cells (Fig. 6H). Together, these data highlight the importance of AKT in redox-dependent stability of Mcl-1 via T163pMcl-1, particularly in VEN-R cells (Fig. 6I).
A Western blot showing T163pMcl-1, Mcl-1, T202/Y204pERK, ERK1/2, Tubulin levels of RPMI8226 cells pre-treated with PD98059 (10 µM) followed by DDC (100 µM) for 4 h. N = 3. B Western blot showing T163pMcl-1, S159pMcl-1, Mcl-1, S473pAKT, AKT1, S9pGSK3β, GSK3β, GAPDH levels of MOLM14 cells pre-treated with LY294002 (25/50 µM) for 2 h followed by DDC (100 µM) for 4 h. N = 3. C Western blot showing T163pMcl-1, S159pMcl-1, Mcl-1, S473pAKT, AKT1, S9pGSK3β, GSK3β, GAPDH levels of MOLM14 cells pretreated with AKT inhibitor VIII (25/50 µM) for 2 h followed by DDC (100 µM) for 4 h. N = 3. D Western blot showing T163pMcl-1, S159pMcl-1, Mcl-1, S9pGSK3β, GSK3β, GAPDH levels of MOLM14 cells treated with indicated doses of capivasertib (µM) for 5 h. N = 3. E Western blot showing T163pMcl-1, S159pMcl-1, Mcl-1, S9pGSK3β, GSK3β, GAPDH levels of MOLM14 cells pre-treated with capivasertib (1 µM) for 1 h and followed by DDC (100 µM) for 4 h. N = 3. F Western blot showing T163pMcl-1, S159pMcl-1, Mcl-1, AKT1, GAPDH levels of MOLM14 cells transfected with Neg or siAKT1 for 48 h before treatment with DDC (50–100 µM) for 4 h N = 3. G Western blot showing S473pAKT, AKT levels of CLL patient samples after ex vivo treatment with DDC (50 µM) for 4 h. Densitometric analyses of S473pAKT/AKT levels in CLL patient samples treated with DDC (50 µM) for 4 h, normalized to non-treated control. N = 6. Paired t-test was used. H Western blot showing S473pAKT, AKT, T163pMcl-1, Mcl-1, β-Actin levels in VEN-R MOLM14 following treatment with O2.− scavenger, Tiron (1 mM) for 2 h before treatment with VEN (0.05 μM) for 48 h. N = 3. I Diagram illustrating that O2.−-induced T163pMcl-1, but not S159pMcl-1, is dependent on AKT activation. The diagram was Created in BioRender. Pervaiz, S. (2022) BioRender.com/p32o555.
Targeting hyperactivated AKT in venetoclax-resistant cells restores sensitivity to venetoclax
Given that VEN-R cells exhibit heightened O2.−, and AKT is activated by O2.−, we next questioned if AKT is hyperactivated in VEN-R cells. If so, would treatment with AKT inhibitors re-sensitize VEN-R cells to VEN? Indeed, S473pAKT levels (together with T163pMcl-1 and Mcl-1) were significantly higher in VEN-R MOLM14 and VEN-R OCI-AML2 (Fig. 7A). Simultaneously, T308pAKT was higher in VEN-R MOLM14 cells (Fig. S11A). Interestingly, VEN-R OCI-Ly1 and, inherently VEN-R Su-DHL4 also displayed higher S473pAKT levels (Fig. 7A). Since S473pAKT is upregulated in all VEN-R cells, we subsequently evaluated the effects of AKT inhibition on Mcl-1 levels and VEN sensitivity. Indeed, capivasertib not only reduced T163pMcl-1 and Mcl-1, but also enhanced caspase-3 cleavage upon VEN treatment in VEN-R MOLM14 and VEN-R OCI-AML2 cells (Fig. 7B). Additionally, BH3-profiling revealed that cells became more dependent on Bcl-2, thus more sensitive to Bcl-2 inhibition upon treatment with capivasertib (increased cytochrome c loss by BAD peptide or ABT199), which contrasted with MS1 peptide or S63845 where no further cytochrome c loss was induced (Fig. 7C). The increase in Bcl-2 dependence by capivasertib resulted in a synergistic reduction in cell viability of VEN-R MOLM14 cells when co-treated with VEN (Fig. 7D, highest single agent (HSA) synergy model, Figs. 7E [42, 43] and S12A). A 7-day cell proliferation chase assay via Trypan Blue exclusion further accentuated the cytotoxic effect of this treatment combination in significantly suppressing proliferation of VEN-R MOLM14 cells as compared to all control groups (Fig. 7F). The effects of this treatment combination were also recapitulated in other VEN-R cell lines of OCI-AML2, OCI-Ly1, and Su-DHL4 (Figs. 7B, G and S12A, B) and primary CLL patient samples (Figs. 7H, I and S13A). Interestingly, capivasertib did not drastically change the levels of S70pBcl-2 as compared to T163pMcl-1 and Mcl-1 in VEN-R MOLM14 and VEN-R OCI-AML2 cells (Figs. S14A and 7B), thus suggesting that the reduction in Mcl-1 alone is sufficient to re-sensitize VEN-R cells to VEN treatment. This indicates that Mcl-1 may play a more prominent role in VEN resistance, at least in myeloid malignancy.
A Western blot showing S473pAKT, AKT, T163pMcl-1, Mcl-1, Tubulin and/or β-Actin levels of VEN-S and VEN-R MOLM14, OCI-AML2, and OCI-Ly1 cells, as well as inherently VEN-R Su-DHL4 cells. B Western blot showing T163pMcl-1, Mcl-1, pro and cleaved caspase-3, β-Actin levels in VEN-R MOLM14 or VEN-R OCI-AML2 cells treated with capivasertib (1 µM/VEN-R MOLM14, 5 µM/VEN-R OCI-AML2) and/or VEN (0.1 µM/VEN-R MOLM14, 10 µM/VEN-R OCI-AML2) for 24 h. N = 3. C Heatmap of BH3-profiling showing percentage of cytochrome c loss from VEN-R MOLM14 cells following treatment with capivasertib (2 µM) or DMSO for 4 h, and subsequent exposure to different BH3 peptide or mimetic concentrations to indicate specific increase in Bcl-2 dependence. N = 3. D Graph showing cell viability assay of VEN-R MOLM14 cells treated with capivasertib (1–5 µM) and/or VEN (0.02–0.075 μM) for 48 h. Cell viability was assessed using CTG assay and data were normalized to untreated control cells. N = 3. Sidak’s multiple comparisons test was used. E Heatmap below showing synergy score based on Highest Single Agent (HSA) synergy model, calculated using SynergyFinder. F Graph showing a 7-day chase assay of VEN-R MOLM14 live cell count in fold change following treatment with DMSO, capivasertib (2 μM), VEN (0.1 μM), or combination. Day 3—DMSO vs combo P < 0.0001, Capi vs combo P < 0.02, VEN vs combo P < 0.001; Day 5—DMSO vs combo P < 0.0001, Capi vs combo P < 0.001, VEN vs combo P < 0.002; Day 7—DMSO vs combo P < 0.0001, Capi vs combo P < 0.0001, VEN vs combo P < 0.001. Tukey’s multiple comparisons test was used. G Cell viability assay of VEN-R OCI-AML2 (N = 3), VEN-R OCI-Ly1 (N = 3), Su-DHL4 (N = 4) cells treated with capivasertib and/or VEN for 48 h. Cell viability was assessed using the CTG assay and data were normalized to untreated control cells. Concentrations used for capivasertib (5 µM/VEN-R OCI-AML2, 0.5 µM/OCI-Ly1 VEN-R, 2 µM/Su-DHL4) and/or VEN (10 µM/VEN-R OCI-AML2, 1 µM/OCI-Ly1 VEN-R, 0.5 µM/Su-DHL4). Sidak’s multiple comparisons test was used. H Cell viability of CLL patient cells following ex vivo treatment with capivasertib (2 µM) and/or VEN (0.005 μM) for 24 h, measured via DAPI+/Annexin V−. N = 10. Sidak’s multiple comparisons test was used. I Western blot showing Mcl-1, S9pGSK3β, GSK3β, GAPDH levels in CLL patient cells following ex vivo treatment with capivasertib (2 µM) for 6 h. Representative blots shown for 2 CLL patient samples. Densitometric analyses of Mcl-1/β-Actin levels in CLL patient samples treated with capivasertib (2 µM) for 6 h, normalized to DMSO control. N = 5. Paired t-test was used. J Tumor burden of mice displayed by %hCD45 + hCD33 + VEN-R MOLM14 cells in blood sample harvested from mice that has been treated in vivo with vehicle, capivasertib, VEN or combo. N = 4 per study arm. Day 15 is the treatment initiation day. K Probability of survival following treatment with vehicle, capivasertib, VEN or combo in mice harboring VEN-R MOLM14 cells. N = 4 per study arm.
Lastly, to verify our in vitro and ex vivo findings, we implanted VEN-R MOLM14 cells in NRG-SGM3 mice via tail vein and treated these mice according to our treatment schedule (Fig. S15A). Indeed, we observed that the treatment combination (capivasertib + VEN) not only reduced VEN-R MOLM14 cells systemically but also prolonged the survival of mice compared to those of the control groups (Fig. 7J, K). Importantly, all mice from the control groups reached the endpoint, but not those from the treatment combination group (Fig. 7K). Collectively, our finding demonstrates the interplay between AKT and Mcl-1 in driving VEN resistance and strongly suggest that AKT inhibition could be a potential therapeutic strategy to manage VEN-R cells and restore VEN sensitivity.
Discussion
The use of VEN for clinical management of CLL and AML is a key milestone since the discovery of BH3-mimetic Bcl-2 inhibitor(s); however, relapse occurs due to the upregulation of a different anti-apoptotic protein Mcl-1 and switch in survival dependence. The upstream mechanism(s) underlying these changes upon VEN resistance acquisition still remain elusive. We present evidence linking intracellular O2.− to the prolonged half-life of Mcl-1 via phosphorylation-mediated protein stability. These data corroborate our earlier findings linking O2.−-driven signaling to apoptotic inhibition [44], such as the constitutive Rac1 activation, phosphorylation-mediated activation of c-Myc, Bcl-2, Stat-3, and NF-κB [29, 32, 33, 35, 45,46,47,48,49].
The role of redox adaptation in mediating resistance to targeted and/or chemotherapy is well-documented [33, 47, 50,51,52]. While an overwhelming oxidative stress, such as chemotherapy-induced, triggers cancer cell execution [53, 54], a mild pro-oxidant milieu endows cells with a survival advantage [33, 47, 55, 56]. Corroborating the latter, a mild increase in ROS is essential for maintaining a transformed phenotype of cancer cells [55, 57]. This is vividly demonstrated by our current study, that a mild increase in intracellular O2.− promotes Mcl-1 stability as a mechanism of VEN resistance acquisition. Interestingly, recent studies have identified that increased OXPHOS potential plays a critical role in determining sensitivity to Bcl-2 inhibition. These studies further demonstrated that the deregulated expressions of AMPK subunits, anti-apoptotic protein, and/or mitochondrial complex VI subunit as critical phenomena to metabolic reprogramming and the increase in OXPHOS potential in VEN-R cells [13, 52]. Given that our earlier work has suggested that increased OXPHOS could increase the probability of mitochondrial electron leakage onto oxygen to generate mitochondrial O2.− [30, 35], these collective evidence provide a mechanistic hypothesis that deregulated expression of these proteins could be upstream players to the enhanced OXPHOS, mitochondrial O2.− production, AKT activation, Mcl-1 phosphorylation and stability, which ultimately drives VEN resistance. Notably, VEN-induced pro-oxidant milieu was demonstrated by another study in which Nrf2 inhibition and mitochondrial ROS production were implicated [58]. It is, therefore, plausible that the sustained pro-oxidant milieu in VEN-R cells is an adaptation to oxidative stress upon prolonged VEN exposure. Alternatively, intracellular O2.− generation in VEN-R cells could be due in part to outgrowth of Ras-mutant clones, as reported in AML patients treated with VEN-containing regimens [15]. In this regard, O2.− has been implicated in oncogenic signaling upon Ras activation [59, 60].
While Mcl-1 expression is regulated at multiple cellular levels, redox-dependent increase in its stability in VEN-R cells is a function of post-translational modification and supports earlier findings that T163pMCL-1 promotes Mcl-1 stability [24, 25]. We highlight the importance of O2.−-induced T163pMcl-1 in maintaining the viability of VEN-R cells. Interestingly, the unstructured N-terminus of Mcl-1 promotes its proteasomal degradation, and several E3-ligases promote Mcl-1 degradation [61,62,63]. Ubiquitination and proteasomal degradation are facilitated by interaction with NOXA, whereas BIM binding stabilizes Mcl-1 [12, 64]. Although the E3-ligase regulation in VEN-R cells remains elusive, we demonstrated that increased O2.− stabilizes Mcl-1 by reducing its ubiquitination, and by enhancing its binding to BIM.
Interestingly, O2.−-dependent AKT activation is linked to increased T163pMcl-1. AKT plays an important role in mediating survival and proliferation in AML cells [65, 66]. While AKT activation has been described in VEN-R cells [67], its association with altered intracellular redox environment, including O2.−-induced S473pAKT, T163pMcl-1, and stabilized Mcl-1, is novel. As to how O2.− triggers AKT activation, a previous report demonstrated PTEN inactivation via S-nitrosylation as an underlying mechanism of AKT activation [38]. Whether this is involved in VEN-R cells remains to be explored. Notably, interaction between Mcl-1 PEST domain and pleckstrin homology (PH) domain of AKT has been reported [37]. The interaction between AKT and Mcl-1 is further supported by the fact that T163 resides in the PEST domain. Whether this physical interaction is involved or further enhanced in the context of VEN resistance, in conjunction with the potential involvement of redox-inactivation of PTEN and interaction between PTEN, AKT, and Mcl-1, remains an interesting topic to be addressed. AKT activation also explains the absence of S159pMcl-1, as GSK3β is inactivated upon AKT phosphorylation. Furthermore, as the PI3K/AKT pathway is frequently activated together with mutated FMS-related receptor tyrosine kinase (FLT3) in AML [65], it is not surprising that S159pMcl-1 is undetected in VEN-R cells. Finally, our work demonstrates that capivasertib prevented T163pMcl-1 and Mcl-1 stability and restored VEN sensitivity. Given that capivasertib has recently been approved as part of a treatment combination for breast cancer [39], and is being tested pre-clinically [68] and in relapsed and refractory (R/R) non-Hodgkin lymphoma patients (NCT05008055), increase the likelihood of utilizing this regimen due to its existing and potentially manageable patient safety profile as well as provide a strong rationale for its clinical utility in patients who have relapsed on or are refractory to VEN therapy.
In summary, while Mcl-1 upregulation has been a key feature in relapse and refractory patients treated with the FDA-approved VEN as well as in various experimental models of VEN resistance, there has yet to be a comprehensive study delineating the mechanism of this upregulation. Our finding addresses this gap by providing a clear mechanism of Mcl-1 stabilization and accumulation, which involves an increase in intracellular O2.− level and subsequent AKT activation to promote T163 phosphorylation and stability of Mcl-1. As we showed that this novel resistance mechanism is conserved across different major hematologic malignancies, our study highlights actionable nodes for therapeutic intervention to restore sensitivity to VEN via modulating intracellular O2.− and/or inhibiting AKT. Given the pressing issue of relapse and refractory cases in VEN-treated CLL and AML patients, our findings may ultimately impact treatment strategies and patient outcomes.
Methods
Western blot analyses
Western blot analysis was used to detect phosphorylation or total protein levels, as previously described [32, 33]. Precision Plus ProteinTM KaleidoscopeTM prestained protein standards (Cat.#1610375, Bio-Rad, California, USA) were used. ImageJ was used for densitometric quantification of western blot bands.
Plasmids, siRNAs, and cell transfection
pcDNA3.1-MCL1wt, pcDNA3.1-MCL1T163A plasmids were gifts from Roger Davis (Addgene, #25375, #25376). pcDNA3-HA-Ubiquitin was a gift from Edward Yeh (Addgene, #18712). onTARGETplus human AKT1 siRNA smartpool (Cat.#L-003000-00), and onTARGETplus SOD1 siRNA sequences (Cat.#J-008364–09,J-008364–10) were purchased from Dharmacon Technologies, Cambridge, UK. Cells were transfected with 2 µg pcDNA3.1, MCL1wt, MCL1T163A plasmids, or 100 nM siRNA using the Neon Electroporation system (Invitrogen, California, USA).
Detection of ROS and O2 .−
Detections of ROS and O2.− were performed using the O2.−-sensitive lucigenin chemiluminescence assay, 2’,7’-dichlorofluorescein diacetate (DCFDA) assay, and MitoSOXTM Red O2.− assay, as described previously [46,47,48]. A detailed protocol is included as Supplementary Information.
BH3-profiling technique
BH3-profiling was used to measure the priming of cells towards apoptosis and to determine the cellular dependency on specific anti-apoptotic proteins for survival [69]. A detailed protocol is included as supplementary information.
Statistical analyses
Results are represented in graphs with mean ± SD. Two-tailed T test, one-way or two-way ANOVA, Sidak’s, Tukey’s, Dunnett’s, or Holm–Sidak’s multiple comparison test was used depending on the comparisons made. Pearson's correlation coefficient, r, was used to test for correlations between two variables. All experiments were repeated at least two times unless otherwise stated. Statistical significance was set at P < 0.05.
Other materials and methods could be found in the supplementary materials file.
Data availability
All materials and data are available from the corresponding authors upon reasonable request.
References
Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J, et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med. 2013;19:202 01/06/online.
Diepstraten ST, Anderson MA, Czabotar PE, Lessene G, Strasser A, Kelly GL. The manipulation of apoptosis for cancer therapy using BH3-mimetic drugs. Nat Rev Cancer. 2022;22:45–64. 2022/01/01.
DiNardo CD, Pratz K, Pullarkat V, Jonas BA, Arellano M, Becker PS, et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood. 2019;133:7–17.
Roberts AW, Davids MS, Pagel JM, Kahl BS, Puvvada SD, Gerecitano JF, et al. Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. N Engl J Med. 2016;374:311–22.
Lin KH, Winter PS, Xie A, Roth C, Martz CA, Stein EM, et al. Targeting MCL-1/BCL-XL forestalls the acquisition of resistance to ABT-199 in acute myeloid leukemia. Sci Rep. 2016;6:27696.
Ramsey HE, Fischer MA, Lee T, Gorska AE, Arrate MP, Fuller L, et al. A novel MCL1 inhibitor combined with venetoclax rescues venetoclax-resistant acute myelogenous leukemia. Cancer Discov. 2018;8:1566–81.
Chong SJF, Zhu F, Dashevsky O, Mizuno R, Lai JX, Hackett L, et al. Hyperphosphorylation of BCL-2 family proteins underlies functional resistance to venetoclax in lymphoid malignancies. J Clin Investig. 2023;133:e170169.
DiNardo CD, Tiong IS, Quaglieri A, MacRaild S, Loghavi S, Brown FC, et al. Molecular patterns of response and treatment failure after frontline venetoclax combinations in older patients with AML. Blood. 2020;135:791–803.
Blombery P, Thompson ER, Nguyen T, Birkinshaw RW, Gong J-n, Chen X, et al. Multiple BCL2 mutations cooccurring with Gly101Val emerge in chronic lymphocytic leukemia progression on venetoclax. Blood. 2020;135:773–7.
Blombery P, Anderson MA, Gong JN, Thijssen R, Birkinshaw RW, Thompson ER, et al. Acquisition of the recurrent Gly101Val mutation in BCL2 confers resistance to venetoclax in patients with progressive chronic lymphocytic leukemia. Cancer Discov. 2019;9:342–53.
Chong SJF, Davids MS. Breaking through BCL-2 inhibition in CLL. Blood. 2020;135:709–11.
Niu X, Zhao J, Ma J, Xie C, Edwards H, Wang G, et al. Binding of released bim to Mcl-1 is a mechanism of intrinsic resistance to ABT-199 which can be overcome by combination with daunorubicin or cytarabine in AML cells. Clin Cancer Res. 2016;22:4440–51.
Guieze R, Liu VM, Rosebrock D, Jourdain AA, Hernandez-Sanchez M, Martinez Zurita A, et al. Mitochondrial reprogramming underlies resistance to BCL-2 inhibition in lymphoid malignancies. Cancer Cell. 2019;36:369–384.e313.
Pei S, Pollyea DA, Gustafson A, Stevens BM, Minhajuddin M, Fu R, et al. Monocytic subclones confer resistance to venetoclax-based therapy in patients with acute myeloid leukemia. Cancer Discov. 2020;10:536–51.
Zhang Q, Riley-Gillis B, Han L, Jia Y, Lodi A, Zhang H, et al. Activation of RAS/MAPK pathway confers MCL-1 mediated acquired resistance to BCL-2 inhibitor venetoclax in acute myeloid leukemia. Signal Transduct Target Ther. 2022;7:51.
Gong J-N, Khong T, Segal D, Yao Y, Riffkin CD, Garnier J-M, et al. Hierarchy for targeting prosurvival BCL2 family proteins in multiple myeloma: pivotal role of MCL1. Blood. 2016;128:1834.
Kotschy A, Szlavik Z, Murray J, Davidson J, Maragno AL, Le Toumelin-Braizat G, et al. The MCL1 inhibitor S63845 is tolerable and effective in diverse cancer models. Nature. 2016;538:477.
Li Z, He S, Look AT. The MCL1-specific inhibitor S63845 acts synergistically with venetoclax/ABT-199 to induce apoptosis in T-cell acute lymphoblastic leukemia cells. Leukemia. 2019;33:262–6.
Sancho M, Leiva D, Lucendo E, Orzáez M. Understanding MCL1: from cellular function and regulation to pharmacological inhibition. FEBS J. 2022;289:6209–34.
Wu X, Luo Q, Liu Z. Ubiquitination and deubiquitination of MCL1 in cancer: deciphering chemoresistance mechanisms and providing potential therapeutic options. Cell Death Dis. 2020;11:556.
Inoshita S, Takeda K, Hatai T, Terada Y, Sano M, Hata J, et al. Phosphorylation and inactivation of myeloid cell leukemia 1 by JNK in response to oxidative stress. J Biol Chem. 2002;277:43730–4.
Maurer U, Charvet C, Wagman AS, Dejardin E, Green DR. Glycogen synthase kinase-3 regulates mitochondrial outer membrane permeabilization and apoptosis by destabilization of MCL-1. Mol Cell. 2006;21:749–60.
Nifoussi SK, Ratcliffe NR, Ornstein DL, Kasof G, Strack S, Craig RW. Inhibition of protein phosphatase 2A (PP2A) prevents Mcl-1 protein dephosphorylation at the Thr-163/Ser-159 phosphodegron, dramatically reducing expression in Mcl-1-amplified lymphoma cells. J Biol Chem. 2014;289:21950–9.
Domina AM, Vrana JA, Gregory MA, Hann SR, Craig RW. MCL1 is phosphorylated in the PEST region and stabilized upon ERK activation in viable cells, and at additional sites with cytotoxic okadaic acid or taxol. Oncogene. 2004;23:5301–15.
Nifoussi SK, Vrana JA, Domina AM, De Biasio A, Gui J, Gregory MA, et al. Thr 163 phosphorylation causes Mcl-1 stabilization when degradation is independent of the adjacent GSK3-targeted phosphodegron, promoting drug resistance in cancer. PloS ONE. 2012;7:e47060.
Harley ME, Allan LA, Sanderson HS, Clarke PR. Phosphorylation of Mcl-1 by CDK1-cyclin B1 initiates its Cdc20-dependent destruction during mitotic arrest. EMBO J. 2010;29:2407–20.
Wang X, Bathina M, Lynch J, Koss B, Calabrese C, Frase S, et al. Deletion of MCL-1 causes lethal cardiac failure and mitochondrial dysfunction. Genes Dev. 2013;27:1351–64.
Chong SJF, Marchi S, Petroni G, Kroemer G, Galluzzi L, Pervaiz S. Noncanonical cell fate regulation by Bcl-2 proteins. Trends Cell Biol. 2020;30:537–55.
Clement MV, Hirpara JL, Pervaiz S. Decrease in intracellular superoxide sensitizes Bcl-2-overexpressing tumor cells to receptor and drug-induced apoptosis independent of the mitochondria. Cell Death Differ. 2003;10:1273–85.
Chen ZX, Pervaiz S. Bcl-2 induces pro-oxidant state by engaging mitochondrial respiration in tumor cells. Cell Death Differ. 2007;14:1617–27.
Chong SJF, Lai JXH, Eu JQ, Bellot GL, Pervaiz S. Reactive oxygen species and oncoprotein signaling-A dangerous liaison. Antioxid Redox Signal. 2018;29:1553–88.
Low ICC, Loh T, Huang Y, Virshup DM, Pervaiz S. Ser70 phosphorylation of Bcl-2 by selective tyrosine nitration of PP2A-B56δ stabilizes its antiapoptotic activity. Blood. 2014;124:2223–34.
Chong SJF, Lai JXH, Qu J, Hirpara J, Kang J, Swaminathan K, et al. A feedforward relationship between active Rac1 and phosphorylated Bcl-2 is critical for sustaining Bcl-2 phosphorylation and promoting cancer progression. Cancer Lett. 2019;457:151–67.
Perciavalle RM, Stewart DP, Koss B, Lynch J, Milasta S, Bathina M, et al. Anti-apoptotic MCL-1 localizes to the mitochondrial matrix and couples mitochondrial fusion to respiration. Nat Cell Biol. 2012;14:575–83.
Chen ZX, Pervaiz S. Involvement of cytochrome c oxidase subunits Va and Vb in the regulation of cancer cell metabolism by Bcl-2. Cell Death Differ. 2010;17:408–20.
Wang B, Xie M, Li R, Owonikoko TK, Ramalingam SS, Khuri FR, et al. Role of Ku70 in deubiquitination of Mcl-1 and suppression of apoptosis. Cell Death Differ. 2014;21:1160–9.
Chen G, Park D, Magis AT, Behera M, Ramalingam SS, Owonikoko TK, et al. Mcl-1 interacts with akt to promote lung cancer progression. Cancer Res. 2019;79:6126–38.
Lim S, Clément MV. Phosphorylation of the survival kinase Akt by superoxide is dependent on an ascorbate-reversible oxidation of PTEN. Free Radic Biol Med. 2007;42:1178–92.
Mullard A. FDA approves first-in-class AKT inhibitor. Nat Rev Drug Discov. 2024;23:9.
Andrikopoulou A, Chatzinikolaou S, Panourgias E, Kaparelou M, Liontos M, Dimopoulos MA, et al. “The emerging role of capivasertib in breast cancer”. Breast. 2022;63:157–67.
Lang L, Shay C, Zhao X, Xiong Y, Wang X, Teng Y. Simultaneously inactivating Src and AKT by saracatinib/capivasertib co-delivery nanoparticles to improve the efficacy of anti-Src therapy in head and neck squamous cell carcinoma. J Hematol Oncol. 2019;12:132.
Zheng S, Wang W, Aldahdooh J, Malyutina A, Shadbahr T, Tanoli Z, et al. SynergyFinder plus: toward better interpretation and annotation of drug combination screening datasets. Genom Proteom Bioinform. 2022;20:587–96.
Ianevski A, Giri AK, Aittokallio T. SynergyFinder 3.0: an interactive analysis and consensus interpretation of multi-drug synergies across multiple samples. Nucleic Acids Res. 2022;50:W739–w743.
Pervaiz S, Clement MV. Superoxide anion: oncogenic reactive oxygen species? Int J Biochem Cell Biol. 2007;39:1297–304.
Pervaiz S, Cao J, Chao OS, Chin YY, Clement MV. Activation of the RacGTPase inhibits apoptosis in human tumor cells. Oncogene. 2001;20:6263–8.
Yee YH, Chong SJF, Kong LR, Goh BC, Pervaiz S. Sustained IKKβ phosphorylation and NF-κB activation by superoxide-induced peroxynitrite-mediated nitrotyrosine modification of B56γ3 and PP2A inactivation. Redox Biol. 2021;41:101834.
Chong SJF, Iskandar K, Lai JXH, Qu J, Raman D, Valentin R, et al. Serine-70 phosphorylated Bcl-2 prevents oxidative stress-induced DNA damage by modulating the mitochondrial redox metabolism. Nucleic Acids Res. 2020;48:12727–45.
Raman D, Chong SJF, Iskandar K, Hirpara JL, Pervaiz S. Peroxynitrite promotes serine-62 phosphorylation-dependent stabilization of the oncoprotein c-Myc. Redox Biol. 2020;34:101587.
Kang J, Chong SJ, Ooi VZ, Vali S, Kumar A, Kapoor S, et al. Overexpression of Bcl-2 induces STAT-3 activation via an increase in mitochondrial superoxide. Oncotarget. 2015;6:34191–205.
Landriscina M, Maddalena F, Laudiero G, Esposito F. Adaptation to oxidative stress, chemoresistance, and cell survival. Antioxid Redox Signal. 2009;11:2701–16.
Pennington JD, Wang TJ, Nguyen P, Sun L, Bisht K, Smart D, et al. Redox-sensitive signaling factors as a novel molecular targets for cancer therapy. Drug Resist Update. 2005;8:322–30.
Chong SJF, Lu J, Valentin R, Lehmberg TZ, Eu JQ, Wang J, et al. BCL-2 dependence is a favorable predictive marker of response to therapy for chronic lymphocytic leukemia. Mol Cancer. 2025;24:62.
Mizutani H, Tada-Oikawa S, Hiraku Y, Kojima M, Kawanishi S. Mechanism of apoptosis induced by doxorubicin through the generation of hydrogen peroxide. Life Sci. 2005;76:1439–53.
Tsang WP, Chau SP, Kong SK, Fung KP, Kwok TT. Reactive oxygen species mediate doxorubicin induced p53-independent apoptosis. Life Sci. 2003;73:2047–58.
Irani K, Xia Y, Zweier JL, Sollott SJ, Der CJ, Fearon ER, et al. Mitogenic signaling mediated by oxidants in Ras-transformed fibroblasts. Science. 1997;275:1649–52.
Szatrowski TP, Nathan CF. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991;51:794–8.
Hole PS, Zabkiewicz J, Munje C, Newton Z, Pearn L, White P, et al. Overproduction of NOX-derived ROS in AML promotes proliferation and is associated with defective oxidative stress signaling. Blood. 2013;122:3322–30.
Nguyen LXT, Troadec E, Kalvala A, Kumar B, Hoang DH, Viola D, et al. The Bcl-2 inhibitor venetoclax inhibits Nrf2 antioxidant pathway activation induced by hypomethylating agents in AML. J Cell Physiol. 2019;234:14040–9.
Wang FS, Wang CJ, Chen YJ, Chang PR, Huang YT, Sun YC, et al. Ras induction of superoxide activates ERK-dependent angiogenic transcription factor HIF-1alpha and VEGF-A expression in shock wave-stimulated osteoblasts. J Biol Chem. 2004;279:10331–7.
Mitsushita J, Lambeth JD, Kamata T. The superoxide-generating oxidase Nox1 is functionally required for ras oncogene transformation. Cancer Res. 2004;64:3580–5.
Zhong Q, Gao W, Du F, Wang X. Mule/ARF-BP1, a BH3-Only E3 ubiquitin ligase, catalyzes the polyubiquitination of Mcl-1 and regulates apoptosis. Cell. 2005;121:1085–95.
Inuzuka H, Shaik S, Onoyama I, Gao D, Tseng A, Maser RS, et al. SCF(FBW7) regulates cellular apoptosis by targeting MCL1 for ubiquitylation and destruction. Nature. 2011;471:104–9.
Ding Q, He X, Hsu JM, Xia W, Chen CT, Li LY, et al. Degradation of Mcl-1 by beta-TrCP mediates glycogen synthase kinase 3-induced tumor suppression and chemosensitization. Mol Cell Biol. 2007;27:4006–17.
Gomez-Bougie P, Menoret E, Juin P, Dousset C, Pellat-Deceunynck C, Amiot M. Noxa controls mule-dependent Mcl-1 ubiquitination through the regulation of the Mcl-1/USP9X interaction. Biochem Biophys Res Commun. 2011;413:460–4.
Brandts CH, Sargin BL, Rode M, Biermann C, Lindtner B, Schwäble J, et al. Constitutive activation of Akt by Flt3 internal tandem duplications is necessary for increased survival, proliferation, and myeloid transformation. Cancer Res. 2005;65:9643–50.
Martelli AM, Nyåkern M, Tabellini G, Bortul R, Tazzari PL, Evangelisti C, et al. Phosphoinositide 3-kinase/Akt signaling pathway and its therapeutical implications for human acute myeloid leukemia. Leukemia. 2006;20:911–28.
Alkhatabi HA, Zohny SF, Shait Mohammed MR, Choudhry H, Rehan M, Ahmad A, et al. Venetoclax-resistant MV4-11 leukemic cells activate PI3K/AKT pathway for metabolic reprogramming and redox adaptation for survival. Antioxidants. 2022;11:461.
Willis BS, Mongeon K, Dry H, Neveras IL, Bryan N, Pandya M, et al. Potent combination benefit of the AKT inhibitor capivasertib and the BCL-2 inhibitor venetoclax in diffuse large B cell lymphoma. Leukemia. 2024;38:2663–74.
Ryan J, Montero J, Rocco J, Letai A. iBH3: simple, fixable BH3 profiling to determine apoptotic priming in primary tissue by flow cytometry. Biol Chem. 2016;397:671–8.
Acknowledgements
The authors would like to thank Dr Thomas Loh, National University Hospital, Singapore for kindly providing us with lymphoma patient-derived samples. We also acknowledge Jennifer Smith, Ph.D., and Richard Siu for the generation of the BH3-profiling plates. SJFC is a scholar of the Lee Kuan Yew Postdoctoral Fellowship by the National University of Singapore (NUS), and the Simeon J. Fortin Charitable Foundation Postdoctoral Fellowship by the Simeon J. Fortin Charitable Foundation, Bank of America Private Bank, Co-Trustees—a Charles A. King trust postdoctoral fellowship program. MSD received funding from the National Institute of Health grant R01CA266298. CJW received funding from the National Cancer Institute grant NCI-P01CA206978. SJFC received funding from the Ministry of Education, Singapore, Academic Research Fund Tier 1, FY2024, and the National Medical Research Council (NMRC), Singapore, Open Fund—Young Individual Research Grant NMRC/OFYIRG24JUL-0013. AMSC received funding from the NMRC Open Fund—Individual Research Grant NMRC/OFIRG21NOV-0025. CC received funding from the NMRC Centre Grant Programme—Targeted Therapy for Blood Cancer II (TETRAD II) grant NMRC/CG21APR2002. SP received funding from the NMRC Open Fund— Individual Research Grant NMRC/OFIRG/0041/2017 and Yong Loo Lin School of Medicine, NUS, grant.
Author information
Authors and Affiliations
Contributions
Conceptualization: SJF Chong, JXH Lai, S Pervaiz. Methodology: SJF Chong, JXH Lai, AMS Cheung, C Chuah, MS Davids, S Pervaiz. Data acquisition: SJF Chong, JXH Lai, K Iskandar, BJ Leong, R Guièze, C Wang, Y Wang, D Raman, AMS Cheung. Data analysis and interpretation: SJF Chong, JXH Lai, K Iskandar, C Wang, Y Wang, WJ Chng, MS Davids, S Pervaiz. Writing, review, and/or revision of the manuscript: All authors. Project administrative or material support: SJF Chong, RHF Lim, CJ Wu, AMS Cheung, WJ Chng, C Chuah, MS Davids, S Pervaiz. Study supervision, funding and final version: SJF Chong, S Pervaiz.
Corresponding authors
Ethics declarations
Competing interests
MSD has received institutional research funding from Ascentage Pharma, MEI Pharma, Novartis, Surface Oncology, TG Therapeutics, and personal consulting income from AbbVie, Adaptive Biosciences, Ascentage Pharma, AstraZeneca, BeiGene, BMS, Eli Lilly, Genentech, Genmab, Janssen, Merck, Mingsight Pharmaceuticals, Secura Bio, TG Therapeutics, and Takeda. CJ Wu holds equity in BioNTech, and receives research funding from Pharmacyclics. R. Guièze has received travel and research funding, and honoraria for consulting from Johnson and Johnson, AbbVie, BeOne Medicine, AstraZeneca, Eli Lilly, Amgen, and Roche. All other authors have no disclosures of conflict of interest.
Ethics approval and consent to participate
All experimental methods in this study were performed in accordance with the relevant guidelines and regulations. Patient samples were obtained following informed consent and approval by relevant institutional review board (IRB). Lymphoma patient samples were obtained from National University Hospital (NUH), Singapore (IRB-#2012/00579) and CLL patient samples were obtained from DFCI, Boston, USA (IRB-#17-561). All experiments pertaining to animal study were approved by the SingHealth experimental medicine centre (SEMC) institutional animal care and use committee (IACUC-#1681).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chong, S.J.F., Lai, J.X.H., Iskandar, K. et al. Superoxide-mediated phosphorylation and stabilization of Mcl-1 by AKT underlie venetoclax resistance in hematologic malignancies. Leukemia 39, 2477–2491 (2025). https://doi.org/10.1038/s41375-025-02694-4
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41375-025-02694-4