Abstract
The addition of gemtuzumab ozogamicin (GO) to intensive chemotherapy (IC) has become a mainstay in treating patients with core binding factor acute myeloid leukemia (CBF-AML). However, evidence for the efficacy of GO in this particular subgroup is primarily based on meta-analytic data from different trials conducted more than a decade ago. In this registry-based study, we evaluated the impact of adding GO to IC in 265 CBF-AML patients from the SAL, AMLCG, and CELL cooperative study groups. Patients receiving GO had a 2-year overall survival of 90% compared with 80% in those without GO (hazard ratio [HR] 0.45, 95% confidence interval [CI] 0.21–0.95, P = 0.036) and a 2-year event-free survival of 51% versus 36% (HR 0.69, 95% CI 0.48–0.99, P = 0.046). While complete remission rates in GO vs. non-GO patients were comparable (89% vs. 90%, P = 0.81), more GO patients achieved measurable residual disease-negative remission (77% vs. 49%, P < 0.001), resulting in numerically reduced cumulative incidence of relapse (HR 0.67, 95% CI 0.43–1.02, P = 0.06). Despite delayed platelet recovery, high-grade toxicities were not increased in GO-treated patients. These findings support the integration of GO into treatment protocols for IC-eligible patients with CBF-AML.
Similar content being viewed by others
Introduction
Gemtuzumab ozogamicin (GO) is an antibody-drug conjugate that targets CD33, a marker expressed on most acute myeloid leukemia (AML) cells. It combines a CD33 monoclonal antibody with the cytotoxic agent calicheamicin and was first approved in 2000. However, concerns about increased induction mortality led to its market withdrawal in 2010 [1, 2]. In the AML15 trial, which enrolled 137 core-binding factor AML (CBF-AML) patients, 72 of whom received GO, Burnett et al. were the first to demonstrate an improvement in overall survival (OS), particularly in favorable-risk AML, when GO was added to induction [3]. Following the introduction of dose fractionation, the ALFA-0701 trial showed significantly prolonged event-free survival (EFS) and relapse-free survival (RFS), as well as a numerically longer OS, in 271 treatment-naïve AML patients receiving GO in addition to intensive chemotherapy (IC) [4]. A meta-analysis by Hills et al. pooled data from five randomized trials with a total of 3325 AML patients, revealing an OS benefit for the addition of GO [5]. Consistent with the AML15 trial results, the meta-analysis highlighted a pronounced efficacy in patients with cytogenetically favorable AML. It reported a 22.5% absolute OS benefit at 5 years in the subgroup with CBF-AML compared to IC alone. As a result, GO was re-approved by the FDA in 2017 and the EMA in 2018 for the first-line treatment of CD33-expressing AML [2], leading to its incorporation into standard induction regimens, particularly for patients with CBF-AML harboring the t(8;21)(q22;q22.1)/RUNX1::RUNX1T1 or inv(16)(p13.1q22)/t(16;16)(p13.1;q22)/CBFB::MYH11 fusion genes [6,7,8].
Although many centers worldwide have adopted the practice of adding GO to induction chemotherapy in patients with CBF-AML, the relatively modest OS in non-GO patients with favorable cytogenetics in the meta-analysis (55% at five years), along with the variability in IC regimens and inconsistent use of high-dose cytarabine consolidation, remain subjects of debate. Here, to further assess the potential benefit of GO in both young and elderly adult CBF-AML, we retrospectively analyzed data from 265 CBF-AML patients who received first-line IC with or without GO, registered in the databases of the German Study Alliance Leukemia (SAL), the German AML Cooperative Group (AMLCG), and the Czech Leukemia Study Group for Life (CELL).
Materials and methods
A database search of the registries of the German Study Alliance Leukemia (SAL), the AML Cooperative Study Group (AMLCG), the Czech Leukemia Study Group (CELL), and the University Hospital of Hamburg-Eppendorf identified all adult patients ≥18 years with first diagnosis of CBF-AML registered between January 2014 and December 2023. Patients provided written consent at their respective AML centers, and the registry studies were approved by the ethics committees of the participating study groups.
Endpoints
Primary endpoints were OS, EFS, and the cumulative incidence of relapse (CIR) in patients achieving composite complete remission (CR). Composite CR included CR and CRi (CR with incomplete hematological recovery). CRi was defined as CR with residual neutropenia <1.0/nl or thrombocytopenia <100/nl [6]. Induction failure was defined as failure to achieve composite CR after 1–2 cycles of induction therapy.
Following current European LeukemiaNet (ELN) guidelines, OS was defined as the time from diagnosis to death [6]. EFS was calculated from the date of diagnosis to the date of induction failure, morphological relapse, molecular failure, or death. CIR was calculated from the date of composite CR to either morphological relapse or molecular failure, and death without relapse or molecular failure was considered a competing risk. Molecular failure was defined as failure to achieve a <3 log10 reduction of the transcript by qPCR, conversion from undetectable to detectable, or a ≥1 log10 increase according to ELN definitions [9]. Secondary endpoints included achievement of measurable residual disease (MRD)-negative remission, time to MRD negativity, safety, and toxicity. Time to MRD negativity was defined as the time from the start of induction therapy to MRD negativity by PCR. MRD testing was performed in accredited, non-centralized laboratories using standardized quantitative PCR assays (sensitivity 10−⁴–10−⁵), in accordance with established protocols [10,11,12].
Safety and toxicity analyses included the time to platelet (≥50/nl) and ANC (≥0.5/nl) recovery after first induction, as well as the occurrence of sinusoidal obstruction syndrome (SOS) of any grade, hemorrhage Common Terminology Criteria of Adverse Events (CTCAE) grade ≥3, and infections CTCAE grade ≥3. Bleeding and infections were analyzed during the first induction only.
Statistical analysis
Time-to-event outcomes were estimated using the Kaplan–Meier method, and differences between groups were assessed with the log-rank test. Cumulative incidence functions for competing risks were compared using Gray’s test. Patient characteristics were analyzed with the Mann–Whitney U test for continuous variables and either Fisher’s exact test or the chi-square test for categorical variables, as appropriate. Multivariable analysis was performed using a Cox proportional hazards regression model. For competing risks endpoints, a regression model according to Fine and Gray was applied [13]. All tests were two-tailed, and a P-value < 0.05 was considered statistically significant. All analyses were performed using RStudio (version 2023.12.1).
Results
Patient characteristics
A total of 265 CBF-AML patients diagnosed in the participating sites were identified and included in the analysis. Amongst those, 80 patients received GO at a median (range) cumulative dose of 9 (3-9) mg/m2 as part of induction chemotherapy, and 185 patients receiving induction therapy without GO served as the non-GO control group. Patient characteristics of the GO and non-GO cohorts are shown in Table 1. The majority of patients were diagnosed with de novo CBF-AML (90% vs. 91%). There were no significant differences between the two groups with respect to median age (GO vs. non-GO patients, 47 vs. 50 years), female sex (43% vs. 50%), or ECOG performance status (ECOG ≤1, 92% vs. 89%) at the time of diagnosis. In the GO and non-GO groups, 41% and 45% of patients, respectively, harbored the RUNX::RUNX1T1 fusion gene. Both cohorts were also similar in terms of other AML characteristics at the time of diagnosis, including white blood cell (WBC) count, peripheral blood and bone marrow blasts, lactate dehydrogenase (LDH) levels, additional cytogenetic aberrations otherwise considered adverse according to ELN criteria, and selected co-mutations, including KIT, FLT3-ITD and NRAS mutations. The frequency of FLT3-TKD as well as of other co-mutations was higher in GO versus non-GO patients (FLT3-TKD, 18% vs. 7%, P < 0.001; other co-mutations, 34% vs. 16%, P < 0.001), which however, may relate to the fact that GO patients were treated at a later time point when a more comprehensive molecular panel became standard of care based on updated guidelines (2018 or later).
Patients received a median number of one induction cycle in both groups. In the GO group, all patients received standard 7 + 3 cytarabine/daunorubicin as chemotherapy backbone, and 8 patients (10%) additionally received the kinase inhibitor midostaurin. The median (range) GO dose was 9 (3-9) mg/m2, with 19 patients (24%) receiving <3 doses of GO in induction. In the non-GO group, 159 patients (86%) received 7 + 3, with 7 patients (4%) also receiving midostaurin. Twenty-six (14%) non-GO patients received other induction regimens, including 23 patients who received high-dose cytarabine and mitoxantrone (HAM).
A median number of 3 consolidation cycles was administered in both groups. Twenty-five (36%) patients in the GO cohort received GO also in consolidation. In the GO cohort, 28 (37%) and 46 (61%) of patients received intermediate-dose (IDAC) and high-dose cytarabine (HiDAC), respectively, and 15 (20%) patients received anthracycline-containing consolidation, following a consolidation strategy that was used in the ALFA-0701 trial [14]. In the non-GO group, 106 (67%) received HiDAC and 42 (26%) received IDAC. Twenty-one (13%) non-GO patients received an anthracycline during consolidation, including 9 patients receiving TAD and 2 patients receiving lower doses of cytarabine in combination with daunorubicin. A total of 4 (5%) and 8 (5%) patients received midostaurin during consolidation in the GO and non-GO cohort, respectively.
Composite CR rates after induction therapy were similar in the GO (89%) and non-GO (90%) groups (P = 0.81). In the GO group, 28 (35%) patients underwent allogeneic hematopoietic cell transplantation (HCT) vs. 90 (49%) in the non-GO group (P = 0.040). The proportion of patients transplanted either in CR1, ≥CR2, or with active disease was similar in GO and non-GO patients (Table 1).
Primary endpoints
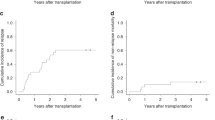
Median (range) follow-up was 2.2 (0.4–4.6) and 5.5 (0.3–9.5) years in the GO and non-GO cohorts, respectively. OS was significantly longer in the GO cohort compared to the non-GO cohort (HR 0.45; 95% CI 0.21–0.95; P = 0.036; Fig. 1A) with 2-year OS estimates of 90% (95% CI, 84–98%) vs. 80% (95% CI, 74–86%). Similarly, estimated EFS was significantly improved in GO vs. non-GO-treated patients (HR 0.69, 95% CI, 0.48–0.99, P = 0.046, Fig. 1B), with 2-year EFS estimates of 51% (95% CI, 40–64%) vs. 36% (95% CI, 29–43%). CIR showed a numerical benefit in favor of the GO group (2-year estimate 42% (95% CI, 29–54%) vs. 55% (95% CI, 48–63%) (HR 0.67, 95% CI 0.43–1.02, P = 0.06; Fig. 1C)). Two-year non-relapse mortality was 1% (95% CI, 0–4%) vs. 5% (95% CI, 2–8%) in GO vs. non-GO patients (HR 4.05, 95% CI, 0.53–30.9, P = 0.18). Thus, EFS differences in GO vs. non-GO patients were primarily driven by an increased incidence of relapse.
The addition of GO was associated with significantly improved OS (A) and EFS (B). CIR was numerically lower in GO-treated patients compared to those who did not receive GO (C), although the difference did not reach statistical significance. GO gemtuzumab ozogamicin, HR hazard ratio, CI confidence interval.
In multivariable analyses adjusting for age, sex, AML ontogeny, CBF fusion gene, and additional cytogenetic or molecular alterations, treatment with GO remained associated with a favorable OS (HRadjusted 0.42, 95% CI 0.19–0.92, P = 0.031) and EFS (HRadjusted 0.65, 95% CI 0.44–0.96, P = 0.031; Table 2), while CIR was non-significantly reduced in GO vs. non-GO patients (HRadjusted 0.66, 95% CI 0.42–1.04, P = 0.07). There was no significant heterogeneity of GO treatment effects on OS among patients with different baseline characteristics, including age (≤50 and >50 years), sex, ECOG performance status, WBC at diagnosis (<20 and ≥20/nl), CBF fusion gene, or the presence of additional cytogenetic or molecular alterations (Fig. 2). Survival curves stratified by CBF fusion genes are shown in Supplementary Fig. 1. Notably, survival outcomes were similar regardless of whether GO patients received 1 or 2 versus 3 doses of GO during induction (HR 0.48, 95% CI, 0.06–3.90, P = 0.49), suggesting consistent efficacy across dosing regimens. However, this finding should be interpreted with caution, given the small sample size and the potential bias introduced by dose reductions due to early toxicity.
No heterogeneity in treatment effect was detected across subgroups, as assessed by tests for interaction. Missing values for ECOG, WBC, karyotype aberrations, and co-mutations were not imputed. CBF core-binding factor, ECOG Eastern Cooperative Oncology Group performance score, GO gemtuzumab ozogamicin, HR hazard ratio, CI confidence interval, WBC white blood count.
To address potential selection bias, specifically the possibility that GO was predominantly administered to fitter patients, we compared the outcomes of non-GO patients diagnosed before (n = 114) and after (n = 71) the re-approval of GO in April 2018. Although a higher proportion of non-GO CBF-AML patients after April 2018 received IDAC vs. HiDAC, there were no significant differences between these two groups with respect to established or potential risk factors such as age, comorbidities, presence of other cytogenetic aberrations or co-mutations (Supplementary Table 1), nor with respect to OS (HR 0.95, 95% CI 0.56–1.61, P = 0.84; Supplementary Fig. 2). Of note, the number of CBF-AML patients not treated with GO diminished after the end of 2019. In a further sensitivity analysis, excluding the 9 (GO) and 10 (non-GO) patients receiving concomitant therapy with a KIT inhibitor either during induction or consolidation, GO patients continued to have a favorable OS compared to non-GO patients (HR 0.50, 95% CI, 0.20–1.01, P = 0.05).
Time to MRD negativity
Seventy (88%) patients in the GO cohort and 158 (85%) patients in the non-GO cohort achieved a composite CR and had evaluable MRD data. A total of 77% (54/70) of evaluable GO patients achieved MRD-negative remission at any time point after induction therapy compared with 49% (77/158) of evaluable non-GO patients (P < 0.001). The median time to MRD negativity was significantly shorter in GO vs. non-GO patients at 5.6 (1-23) vs. 8.4 (0-85) months (P < 0.001). No difference in overall MRD negativity rates was observed in patients who received GO only during induction or also during consolidation therapy (74 vs. 75%, P = 0.92), while the time to MRD-negative remission was 5.7 vs. 4.4 months (P = 0.23).
Safety and toxicity
Analyses included the time to platelet and ANC recovery after first induction, as well as the occurrence of selected adverse events. Data on platelet and ANC recovery were available for 199 (75%) and 198 (75%) patients with CR/CRi after induction therapy, respectively. GO treatment was associated with a significantly longer time to platelet recovery of 2 days (GO vs. non-GO, median days 24 vs. 22, P < 0.001), whereas the median time to ANC recovery was identical in both groups (26 vs. 26 days, P = 0.48; Supplementary Table 2). SOS was observed in 4 (5%) patients treated with GO compared to 2 (1%) in the non-GO group. All patients with reported SOS had received more than one dose of GO. No cases of SOS-related deaths were reported. Higher-grade bleeding events CTCAE grade ≥3 occurred in 9 (11%) and 15 (8%), respectively, and infections grade ≥3 after first induction occurred in 41 (51%) and 87 (49%) of the GO and non-GO patients, respectively (Supplementary Table 3).
Discussion
For decades, the combination of cytarabine and daunorubicin has been the cornerstone of AML induction therapy. The use of the 7 + 3 protocol followed by high-dose cytarabine consolidation in CBF-AML has demonstrated remission rates of approximately 90% and 3- and 5-year OS rates of 65–70% and 58–65%, respectively [15,16,17,18,19]. In the meta-analysis by Hills et al., the addition of GO to IC provided the greatest benefit in AML patients with favorable (i.e., CBF) cytogenetics, with a 5-year OS probability of 78% versus 55% [5]. While the difference in OS between GO and non-GO-treated patients was encouraging, some limitations of the meta-analysis should be considered. Notably, only 7% of the included patients had a CBF fusion gene, and the overall results were largely driven by the AML15 trial [3]. Moreover, survival in non-GO patients was comparatively low relative to other contemporary studies of IC-eligible CBF-AML patients [16,17,18, 20], possibly reflecting heterogeneity or suboptimal chemotherapy backbones in some of the included trials. Despite these limitations, however, the addition of GO to induction therapy has gained broad acceptance in the treatment of CBF-AML.
Against this background, our multicenter retrospective analysis of 265 CBF-AML patients confirmed a significantly longer OS of 90% vs. 80% at 2 years with the addition of GO to IC induction, adding an OS benefit of approximately 10%. Our data align with previous reports showing improved outcomes following the addition of GO in CBF-AML patients [1, 3, 5, 21]. Notably, survival outcomes of non-GO patients in our cohort are consistent with previously reported data on CBF-AML [16,17,18], supporting the validity of our analysis. While remission rates were comparable between GO-treated and non-treated patients, GO treatment resulted in a higher proportion of MRD-negative remissions and a numerically reduced incidence of AML relapse. These findings are consistent with two recent studies that have highlighted the benefits of GO in terms of enhanced MRD clearance and reduced relapse risk [22, 23]. In contrast, a recent multicenter retrospective analysis in a cohort of 200 CBF-AML patients reported comparable OS for treatment regimens with and without GO [24]. Differences in treatment protocols, particularly chemotherapy backbones and GO dosage, may account for these divergent findings.
Both SOS and prolonged myelotoxicity have been associated with GO [1, 4, 14, 25]. Indeed, our study confirmed a significant delay in platelet recovery by two days, which did not translate into an increased incidence of clinically significant bleeding. Consistent with previous studies, we found a numerically increased risk of SOS among patients treated with GO, which did not adversely affect survival outcomes.
Our results provide compelling evidence supporting the feasibility of incorporating GO into induction therapy for CBF-AML, although some limitations should be acknowledged. First, the retrospective nature of our study introduces a potential risk of selection bias. While patient characteristics, particularly age and performance status, did not differ significantly among patients who were or were not treated with GO after its re-approval in April 2018, and OS among non-GO patients did not differ significantly when comparing those treated before versus after the reintroduction of GO, we cannot fully exclude the possibility of unmeasured confounding. Temporal changes in clinical practice, such as the sporadic use of small-molecule inhibitors or advances in supportive care, may have influenced outcomes. Notably, our sensitivity analysis, which excluded patients treated with KIT inhibitors at any point during therapy, still demonstrated a benefit associated with GO, suggesting that the observed effect was not significantly confounded by KIT inhibitor use. Second, although MRD assessments were conducted using similar techniques and sensitivity thresholds, the analyses were not centralized, and monitoring schedules may have varied across centers. Third, the heterogeneity of consolidation regimens, particularly among non-GO patients, may have impacted outcomes, despite the fact that survival in the non-GO group was consistent with other contemporary CBF-AML cohorts.
To our knowledge, this study represents the largest analysis to date comparing outcomes in intensively treated CBF-AML patients with and without GO. Although follow-up in the GO cohort was relatively short, the observed 2-year survival benefit appears to be primarily attributable to a reduced risk of relapse rather than higher remission rates, consistent with the findings reported by Hills et al. in the favorable-risk cytogenetic subgroup. In a contemporary cohort, our data show an absolute survival advantage of 10% at 2 years with GO-containing induction therapy and acceptable toxicity, thereby supporting the integration of GO into treatment algorithms for patients with CBF-AML.
Data availability
The datasets analyzed in the current study are available from the corresponding author upon reasonable request.
References
Petersdorf SH, Kopecky KJ, Slovak M, Willman C, Nevill T, Brandwein J, et al. A phase 3 study of gemtuzumab ozogamicin during induction and postconsolidation therapy in younger patients with acute myeloid leukemia. Blood. 2013;121:4854–60.
Swaminathan M, Cortes JE. Update on the role of gemtuzumab-ozogamicin in the treatment of acute myeloid leukemia. Ther Adv Hematol. 2023;14:20406207231154708.
Burnett AK, Hills RK, Milligan D, Kjeldsen L, Kell J, Russell NH, et al. Identification of patients with acute myeloblastic leukemia who benefit from the addition of gemtuzumab ozogamicin: results of the MRC AML15 trial. J Clin Oncol J Am Soc Clin Oncol. 2011;29:369–77.
Lambert J, Pautas C, Terré C, Raffoux E, Turlure P, Caillot D, et al. Gemtuzumab ozogamicin for de novo acute myeloid leukemia: final efficacy and safety updates from the open-label, phase III ALFA-0701 trial. Haematologica. 2019;104:113–9.
Hills RK, Castaigne S, Appelbaum FR, Delaunay J, Petersdorf S, Othus M, et al. Addition of gemtuzumab ozogamicin to induction chemotherapy in adult patients with acute myeloid leukaemia: a meta-analysis of individual patient data from randomised controlled trials. Lancet Oncol. 2014;15:986–96.
Döhner H, Wei AH, Appelbaum FR, Craddock C, DiNardo CD, Dombret H, et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood. 2022;140:1345–77.
Khoury JD, Solary E, Abla O, Akkari Y, Alaggio R, Apperley JF, et al. The 5th edition of the World Health Organization classification of haematolymphoid tumours: myeloid and histiocytic/dendritic neoplasms. Leukemia. 2022;36:1703–19.
Arber DA, Orazi A, Hasserjian RP, Borowitz MJ, Calvo KR, Kvasnicka H-M, et al. International consensus classification of myeloid neoplasms and acute leukemias: integrating morphologic, clinical, and genomic data. Blood. 2022;140:1200–28.
Heuser M, Freeman SD, Ossenkoppele GJ, Buccisano F, Hourigan CS, Ngai LL, et al. 2021 Update on MRD in acute myeloid leukemia: a consensus document from the European LeukemiaNet MRD Working Party. Blood. 2021;138:2753–67.
Schuurhuis GJ, Heuser M, Freeman S, Béné M-C, Buccisano F, Cloos J, et al. Minimal/measurable residual disease in AML: a consensus document from the European LeukemiaNet MRD Working Party. Blood. 2018;131:1275–91.
Perea G, Lasa A, Aventín A, Domingo A, Villamor N, Queipo de Llano MP, et al. Prognostic value of minimal residual disease (MRD) in acute myeloid leukemia (AML) with favorable cytogenetics [t(8;21) and inv(16)]. Leukemia. 2006;20:87–94.
Viehmann S, Teigler-Schlegel A, Bruch J, Langebrake C, Reinhardt D, Harbott J. Monitoring of minimal residual disease (MRD) by real-time quantitative reverse transcription PCR (RQ-RT-PCR) in childhood acute myeloid leukemia with AML1/ETO rearrangement. Leukemia. 2003;17:1130–6.
Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509.
Castaigne S, Pautas C, Terré C, Raffoux E, Bordessoule D, Bastie J-N, et al. Effect of gemtuzumab ozogamicin on survival of adult patients with de-novo acute myeloid leukaemia (ALFA-0701): a randomised, open-label, phase 3 study. Lancet. 2012;379:1508–16.
Begna KH, Xu X, Gangat N, Alkhateeb H, Patnaik MM, Al-Kali A, et al. Core-binding factor acute myeloid leukemia: long-term outcome of 70 patients uniformly treated with “7+3”. Blood Cancer J. 2022;12:55.
Löwenberg B, Pabst T, Vellenga E, van Putten W, Schouten HC, Graux C, et al. Cytarabine dose for acute myeloid leukemia. N Engl J Med. 2011;364:1027–36.
Schlenk RF, Benner A, Krauter J, Büchner T, Sauerland C, Ehninger G, et al. Individual patient data-based meta-analysis of patients aged 16 to 60 years with core binding factor acute myeloid leukemia: a survey of the German Acute Myeloid Leukemia Intergroup. J Clin Oncol J Am Soc Clin Oncol. 2004;22:3741–50.
Miyawaki S, Ohtake S, Fujisawa S, Kiyoi H, Shinagawa K, Usui N, et al. A randomized comparison of 4 courses of standard-dose multiagent chemotherapy versus 3 courses of high-dose cytarabine alone in postremission therapy for acute myeloid leukemia in adults: the JALSG AML201 Study. Blood. 2011;117:2366–72.
Marcucci G, Geyer S, Laumann K, Zhao W, Bucci D, Uy GL, et al. Combination of dasatinib with chemotherapy in previously untreated core binding factor acute myeloid leukemia: CALGB 10801. Blood Adv. 2020;4:696–705.
Paschka P, Döhner K. Core-binding factor acute myeloid leukemia: can we improve on HiDAC consolidation?. Hematology. 2013;2013:209–19.
Burnett AK, Russell NH, Hills RK, Kell J, Freeman S, Kjeldsen L, et al. Addition of gemtuzumab ozogamicin to induction chemotherapy improves survival in older patients with acute myeloid leukemia. J Clin Oncol. 2012;30:3924–31.
Freeman SD, Thomas A, Thomas I, Hills RK, Vyas P, Gilkes A, et al. Fractionated vs single-dose gemtuzumab ozogamicin with determinants of benefit in older patients with AML: the UK NCRI AML18 trial. Blood. 2023;142:1697–707.
Döhner H, Weber D, Krzykalla J, Fiedler W, Kühn MWM, Schroeder T, et al. Intensive chemotherapy with or without gemtuzumab ozogamicin in patients with NPM1-mutated acute myeloid leukaemia (AMLSG 09-09): a randomised, open-label, multicentre, phase 3 trial. Lancet Haematol. 2023;10:e495–509.
Rojek AE, McCormick BJ, Cwykiel J, Odetola O, Abaza Y, Nai N, et al. Real-world outcomes of intensive induction approaches in core binding factor acute myeloid leukemia. EJHaem. 2024;5:728–37.
Giles FJ, Kantarjian HM, Kornblau SM, Thomas DA, Garcia-Manero G, Waddelow TA, et al. Mylotarg (gemtuzumab ozogamicin) therapy is associated with hepatic venoocclusive disease in patients who have not received stem cell transplantation. Cancer. 2001;92:406–13.
Acknowledgements
The authors are grateful to the patients who consented to participate in the aforementioned registries and to the families who supported them.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
JR, PM, JHM, BW, JS, JV, JN, PZ, TS, ZK, CK, JMU, TSa, LR, SK, JSc, LPM, SKa, DN, LF, PPH, MC, EJ, JM, JG, RR, MJ, LH, SKl, FM, NM, KF, WB, MR, MRi, JL, BS, SH, MK, SWK, RK, KS, HS, UP, CDB, CMT, GL, HCR, JMa, MB, CR, CS, and MH were responsible for clinical data collection, data curation, and patient management. JR, PM, CS, and MH were responsible for the design of the study, collection of the data, interpretation of the results, and writing the report. JR and PM analyzed the data. SZ helped with statistical analyses. CS and MH supervised the study. All authors reviewed and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
TS received honorarium, financial support, and congress participation from Pfizer and served as a consultant for Pfizer. FM received support for meeting attendance from Servier, AbbVie, Incyte, Gilead, Jazz Pharmaceuticals, Novartis, Teva, Pfizer, and Amgen; received support for medical writing from Servier, and Springer Verlag; received research grants from Apis Technologies, and Daiichi Sankyo; received speaker honoraria from Servier, Jazz Pharmaceuticals, Daiichi Sankyo, and AbbVie; participated in advisory boards for Otsuka Pharma, Servier, Pfizer, and Daiichi Sankyo. SK received honorarium as a speaker from Eickeler and travel support from AbbVie, Alexion, and Jazz. KS received speaker honoraria and/or travel grants from and/or has been a consultant to: AbbVie, Alexa, Bristol Myers Squibb, Jazz Pharmaceuticals, Leo, Pfizer, Sanofi, Servier, Sobi, Stemline, Takeda, MSD, Gilead. GL received research grants not related to this manuscript from AGIOS, AQUINOX, AstraZeneca, Bayer, Celgene, Gilead, Janssen, MorphoSys, Novartis, F. Hoffmann-La Roche Ltd, and Verastem. GL received honoraria not related to this study from ADC Therapeutics, AbbVie, Amgen, AstraZeneca, Bayer, BeiGene, BMS, Celgene, Constellation, Genase, Genmab, Gilead, Hexal/Sandoz, Immagene, Incyte, Janssen, Karyopharm, Lilly, Miltenyi, MorphoSys, MSD, NanoString, Novartis, PentixaPharm, Pierre Fabre, F. Hoffmann-La Roche Ltd, and Sobi. HCR received consulting and lecture fees from AbbVie, Roche, KinSea, Vitis, Cerus, Lilly, Novartis, Takeda, AstraZeneca, Vertex, and Merck. HCR received research funding from AstraZeneca and Gilead Pharmaceuticals. HCR is a co-founder of CDL Therapeutics GmbH. MBo has no conflict of interest regarding this work. Outside this study, he has received lecture fees and served on advisory boards for Jazz, MSD, and ActiTrexx. CR has received honoraria from AbbVie, Astellas, Bristol-Meyer-Squibb, Daiichi Sankyo, Jazz, Janssen, Novartis, Otsuka, Pfizer, Servier, and institutional research funding from AbbVie, Astellas, Novartis, Pfizer. CS received honoraria/has served on Advisory Boards for AbbVie, Astellas, AstraZeneca, BMS, Daiichi Sankyo, Laboratories Delbert, Jazz Pharmaceuticals, Novartis, Otsuka, Pfizer, Roche. CS received institutional research support from Jazz Pharmaceuticals and congress/travel grants from AbbVie, BMS, Jazz Pharmaceuticals, and Pfizer.
Ethics approval and consent to participate
All procedures were performed in accordance with the relevant guidelines and regulations. The bio-registry of the SAL (NCT03188874), which encompasses 59 centers specialized in the treatment of hematologic neoplasms across Germany and the Czech Republic, is approved by the ethics committee of the Technical University Dresden (EK 98032010). The registry at the University Hospital Hamburg-Eppendorf is approved by the ethics committee of the Medical Association Hamburg (PV3469). Written informed consent was obtained from all participants.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ronnacker, J., Muller, P.J., Mikesch, JH. et al. Gemtuzumab ozogamicin in first-line treatment of CBF-AML: insights from a retrospective multi-center analysis. Leukemia 39, 2174–2180 (2025). https://doi.org/10.1038/s41375-025-02700-9
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41375-025-02700-9
This article is cited by
-
Emerging strategies and novel therapeutic targets in acute myeloid leukemia: current advances and future directions
Biomarker Research (2025)
-
Anti-CD33 Antibody-Drug Conjugate (Gemtuzumab ozogamicin) in Adult Acute Myeloid Leukemia: Real Life Data from Iraq
Indian Journal of Hematology and Blood Transfusion (2025)