Abstract
Lanthanide (Ln)-based metal halides with excellent luminescence properties, large Stokes shifts, and low toxicity have aroused wide attention as scintillators for X-ray imaging. However, the lack of fast and mild synthesis methods of Ln-based metal halides, as one of the technical challenges, limits their applications. Here, benefiting from the innovative selection of methanol and ethanol as the solvent and anti-solvent, respectively, a series of Cs3LnCl6 (Ln = Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu) microcrystals (MCs) were prepared via the recrystallization method at room temperature for the first time. This recrystallization method could also realize large-scale production at one time and recyclable recrystallization of single-element MCs and the preparation of high-entropy five-element Cs3{TbDyHoErTm}1Cl6 crystals. Among these Cs3LnCl6 MCs, Cs3TbCl6 MCs with 4f → 5d absorption transition possess the highest photoluminescence quantum yield of 90.8%. Besides, under X-ray irradiation, Cs3TbCl6 MCs show a high light yield of ~51,800 photons MeV−1 and the as-fabricated thin films possess promising X-ray imaging ability and excellent spatial resolutions (12 lp mm−1). This work provides a new method for ultrafast preparing Ln-based metal halides under mild synthetic conditions and highlights their excellent potential as scintillators for X-ray imaging.
Similar content being viewed by others
Introduction
X-ray scintillators can convert X-ray photons to visible photons with lower energy and have been widely employed in the fields of industrial flaw detection, medical diagnosis, safety inspection, petroleum logging, environmental monitoring, etc. Recently, lead-based metal halides with large X-ray absorption coefficients, excellent photoelectric properties and solution processability have displayed remarkable scintillation performance and great promise for X-ray detection and imaging1,2,3,4,5,6,7. However, the toxicity of Pb2+ and self-absorption caused by small Stokes shifts inhibit their large-scale applications as scintillators. To overcome these issues, replacing Pb2+ with other elements to obtain lead-free metal halides with low toxicity and large Stokes shifts has captured great interest8,9,10,11,12.
Trivalent lanthanide (Ln3+) ions with low toxicity, large Stokes shifts, and distinct energy level transitions usually exhibit abundant and unique emissions with sharp lines in the range from ultraviolet (UV) to near-infrared (NIR) region13,14,15. Besides, quantum yield improvement, quantum cutting effect, defects passivation, multimode luminescence, etc., caused by the introduction of Ln3+ ions dopants, can bring lead-free metal halides excellent potentials in the applications of X-ray imaging, solid-state lighting, night vision, information storage, optical thermometry, etc16,17,18,19,20,21,22,23. To date, there are many reports on Ln-doped metal halides24, but a few on Ln-based metal halides due to the difficulty in synthesis, which limits the development of Ln-based metal halides as scintillators in the field of X-ray imaging. According to the previous reports, it is difficult to prepare Ln-based metal halides with high crystallinity by the traditional solvothermal method because Ln elements have strong oxygen affinity and hydrophilicity, and the solubility of Ln halides is quite different from other metal halides in mixed solutions25. Although there have been some reports of Ln-based metal halides synthesized by high-temperature solid-state synthesis or hot-injection method, such high-temperature conditions extremely limit their development26,27,28,29. Hence, it is necessary to develop a simple, fast, and mild synthesis method to prepare Ln-based metal halides and further explore their potential as scintillators in the field of X-ray imaging.
Herein, a series of Cs3LnCl6 (Ln = Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu) metal halide microcrystals (MCs) were synthesized via a recrystallization method at room temperature. Based on this method, the feasibility of large-scale production at one time and recyclable recrystallization of single-element MCs, and the preparation of related high-entropy Ln-based metal halide crystals were investigated. Density functional theory (DFT) calculations were adopted to explore the 4f → 5d transitions or Cl → Ln charge transfer transitions in parts of Cs3LnCl6 MCs, which could overcome 4 f → 4 f parity-forbidden transitions and bring them better absorption ability in the near UV region and great PL performance. Subsequently, Cs3TbCl6, with the highest photoluminescence quantum yield (PLQY) of 90.8% among these Cs3LnCl6, was selected to investigate the potential in the application of X-ray imaging. Under X-ray irradiation, Cs3TbCl6 MCs powder with excellent X-ray scintillation performance was combined with polydimethylsiloxane (PDMS) to fabricate related thin films, which displayed great X-ray imaging ability.
Results
Considering the radioactivity of Pm3+, Ln3+ ions involved in this work do not include Pm3+. All the samples were prepared through a simple synthetic route at room temperature. As illustrated in Fig. 1a, CsCl and LnCl3·xH2O were dissolved in methanol (MeOH) under ultrasound, and then Cs3LnCl6 (Ln = Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu) metal halides MCs can be obtained quickly within 2 min by introducing moderate ethanol (EtOH) into the mixed MeOH solution to precipitate them as powder. To prepare Cs3LaCl6, it requires the addition of cyclohexane as an anti-solvent after adding EtOH. However, the obtained metal halide is Cs3LaCl6·3H2O rather than Cs3LaCl6. The structural and morphological characterizations of as-prepared Cs3LaCl6·3H2O are shown in Supplementary Fig. S1. Therefore, in subsequent discussions, we will focus on the remaining thirteen Cs3LnCl6 MCs (Ln = Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu).
The X-ray diffraction (XRD) patterns of the prepared Cs3LnCl6 MCs are displayed in Fig. 1b, Supplementary Fig. S2–9 and S10–14. Cs3LnCl6 MCs exhibit similar diffraction patterns, suggesting these MCs share the same crystal structure and space group. Specifically, the diffraction patterns of Cs3CeCl6, Cs3PrCl6, Cs3NdCl6, Cs3SmCl6, Cs3EuCl6, Cs3GdCl6, Cs3TbCl6, and Cs3ErCl6 match well with the standard patterns (PDF#04-007-9649, 04-007-9650, 04-007-9651, 04-007-9652, 04-007-9653, 04-007-9654, 04-006-9440, 04-010-7422), conforming to monoclinic crystal structure (C2/c space group) without any secondary phases (Supplementary Figs. S2–9). Nevertheless, the XRD patterns of Cs3DyCl6, Cs3HoCl6, Cs3TmCl6, Cs3YbCl6, and Cs3LuCl6 are not filed in the PDF or ICSD database. Hence, the Rietveld refinements of their diffraction patterns were performed. As shown in Supplementary Fig. S10–14 and Table S1, the Rietveld refinement X-ray diffraction plots and structural parameters of Cs3DyCl6, Cs3HoCl6, Cs3TmCl6, Cs3YbCl6, and Cs3LuCl6 were provided, and the results demonstrate their monoclinic crystal structure (C2/c space group) without any secondary phases. Therefore, the crystal structure of all Cs3LnCl6 MCs adopts a monoclinic C2/c space group (#15) (Fig. 1a), consisting of a 0D framework of spatially independent octahedra [LnCl6]3-, which are completely separated by surrounding Cs+ ions27. Meantime, from Cs3CeCl6 to Cs3LuCl6, the XRD peak (\(\bar{1}\)13) slightly shifts to a larger angle. This progressive shrinking of the lattice can be attributed to the gradual reduction of ionic radius from Ce3+ to Lu3+. Compared with the XRD patterns of other Cs3LnCl6, the relative intensity between parts of the XRD peaks changes in Cs3CeCl6 for the crystals could grow selectively along with different crystal planes. As displayed in the scanning electron microscopy (SEM) images (Fig. 1c), from Cs3CeCl6 to Cs3LuCl6, their morphologies transform from thin plate shape to flower shape, which implies the different crystal growth processes, possibly caused by the increasing rate of crystallization. Subsequently, the crystallization rates of these Cs3LnCl6 MCs are reflected by the productivities after adding EtOH for 60 seconds. As presented in Supplementary Fig. S15, the crystallization rates become faster and faster from Cs3CeCl6 to Cs3EuCl6, and then almost identical from Cs3EuCl6 to Cs3LuCl6, which matches well with morphological change in SEM images30.
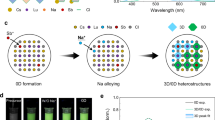
In addition, this recrystallization method could also realize large-scale production at one time and excellent recyclability of single-element Cs3LnCl6 MCs and the preparation of high-entropy five-element Ln-based metal halide crystals. As shown in Fig. 2a, large quantities of Cs3TbCl6 MCs (~11.0 g) can be easily obtained at one time by enlarging metal salts in equal proportions by a factor of 100 and dissolving them in MeOH, followed by adding anti-solvent EtOH. Subsequently, the recyclability of as-prepared MCs was explored to avoid the waste of resources after completing a specific application mission. An appropriate amount of MeOH was employed to recover as-prepared Cs3TbCl6 MCs. Then, Cs3TbCl6 MCs could be precipitated again with sufficient EtOH as an anti-solvent. As shown in Fig. 2b and Supplementary Fig. S16, Cs3TbCl6 MCs could be utilized and recycled repeatedly. In addition, based on this recrystallization method, high-entropy five-element Ln-based metal halide crystals could be prepared successfully. High-entropy materials, as excellent functional materials, have attracted increasing attention, however, high temperature (~1000 °C) is generally necessary in synthetic procedures that limits their development31,32. Here, instead of being indirectly added, EtOH as anti-solvent was diffused slowly into the mixed MeOH solution, including metal (Cs+, Tb3+, Dy3+, Ho3+, Er3+, Tm3+) salts. After standing for 12 hours, five-element Cs3{TbDyHoErTm}1Cl6 metal halide crystals were successfully acquired at room temperature (Fig. 2c). High-resolution transmission electron microscopy (HRTEM) exhibits distinct lattice fringes with a lattice spacing of 0.29 nm that is indexed as crystal plane (\(\bar{6}\)22) of the Cs3{TbDyHoErTm}1Cl6 crystals phase (Fig. 2d). SEM elemental mappings reveal homogeneous distribution of all five incorporated Ln3+ ions within five-element Cs3{TbDyHoErTm}1Cl6 crystals (Fig. 2e). In Supplementary Table S2, inductively coupled plasma optical emission spectrometry (ICP-OES) provided the actual molar ratio of the five Ln3+ ions (at 17-25%). The XRD pattern of as-prepared five-element Cs3{TbDyHoErTm}1Cl6 crystals displays a similar monoclinic structure with the physical mixture of five corresponding single-element crystals (Fig. 2f). Moreover, after fine scanning the primary characteristic diffraction peak, no peak splitting happens and the full width at half maximum (FWHM) displays a smaller value compared with that of the physical mixture with the multi-phase structure, implying the single-phase structure of Cs3{TbDyHoErTm}1Cl6 crystals. As a result, as-prepared Cs3{TbDyHoErTm}1Cl6 crystals could be confirmed as single-phase high-entropy crystals, indicating that high-entropy Ln-based metal halide crystals could be successfully prepared at room temperature based on this recrystallization method.
Structure characterizations of as-prepared high entropy Cs3{TbDyHoErTm}1Cl6. a Representative photograph of large-scale synthetic crystals of Cs3TbCl6 under 365 nm UV light irradiation. b Schematic diagram of the reversible synthesis of Cs3TbCl6 crystals. c The schematic illustration of high-entropy Cs3{TbDyHoErTm}1Cl6 crystals. The (d) HRTEM image and (e) SEM elemental mappings of high-entropy Cs3{TbDyHoErTm}1Cl6 crystals. f The general XRD and fine scans over the (\(\bar{1}\)13) reflection of high entropy Cs3{TbDyHoErTm}1Cl6 crystals and the related physical mixture of Cs3TbCl6, Cs3DyCl6, Cs3HoCl6, Cs3ErCl6, and Cs3TmCl6
To investigate the optical properties of as-prepared Cs3LnCl6 MCs, the absorption spectra are carried out (Supplementary Fig. S17). The absorption bands located at 340 nm for Cs3CeCl6 and 285 nm for Cs3TbCl6 could be ascribed to 4f → 5d transitions of Ce3+ and Tb3+ ions, respectively. In the absorption spectrum of Cs3PrCl6, there are a weak broadband (300–420 nm) and sharp peaks (420–600 nm), which could be attributed to 4f → 5d and 4f → 4f transitions of Pr3+ ions, respectively. For Cs3EuCl6 and Cs3YbCl6, the broad absorption bands are charge transfer bands (CTB) in [EuCl6]3− and [YbCl6]3− octahedra. While for other Cs3LnCl6, there are mainly sharp peaks from 4f → 4f transitions of Ln3+ ions or no obvious absorption peaks. The parity forbidden 4f → 4f transitions of Cs3LnCl6 MCs may limit their photoluminescence (PL) performance and optoelectronic applications. Then, PL spectra of these Cs3LnCl6 MCs under the excitations of specific wavelengths are shown in Fig. 3a, Supplementary Figs. S18 and S19. Among these MCs, Cs3CeCl6, Cs3PrCl6, Cs3TbCl6, and Cs3EuCl6 possess the strongest visible emissions and emit bright blue (around 400 nm; Cs3CeCl6 and Cs3PrCl6), green (547 nm; Cs3TbCl6), and red (592 nm and 612 nm; Cs3EuCl6) emissions, respectively. In the NIR range, Cs3YbCl6 MCs display the strongest emission at ~1000 nm. While for other Cs3LnCl6, only weak Ln3+ characteristic emissions or host emissions could be observed. The PLE spectra of Cs3LnCl6 MCs monitored at the position of Ln3+ characteristic emissions are shown in Fig. 3b and Supplementary Fig. S20, the excitation bands display similar patterns with their absorption spectra. Then, under the excitation of specific wavelength, absolute PLQYs of these Cs3LnCl6 MCs could be obtained (Fig. 3c and Supplementary Table S3). Among them, Cs3TbCl6 possesses the highest PLQY of 90.8%, exceeding most lead-free metal halides (Supplementary Table S4)33,34,35,36,37,38,39. From the values of PLQYs, among all Cs3LnCl6 MCs, those Cs3LnCl6 (Ln = Ce, Pr, Eu, Tb, Yb) with great absorption ability usually possess great PL performance as well. In Fig. 3d–h and Supplementary Fig. S21, the PL decay curves of Ln3+ characteristic emissions or host emissions in Cs3LnCl6 could provide their lifetimes that match the characteristics of the lifetimes of 5d/4f → 4f transitions of Ln3+ ions.
To further investigate the great absorption abilities in parts of Cs3LnCl6. The electronic structures of Cs3LnCl6 were investigated by theory calculation. The partial charge density maps and partial density of states (PDOS) of Cs3CeCl6 and Cs3EuCl6 were carried out for Ce3+ and Eu3+ act as typical Ln3+ ions to display 4f → 5d transition and Cl → Ln charge transfer transition, respectively (Fig. 4a–d). As disclosed in the partial charge density maps and PDOS of Cs3CeCl6, the valence band (VB) was composed of Cl-3p and Ce-4f-occupied orbitals. While the conduction band (CB) was composed of Ce-5d and Ce-4f empty orbitals. For comparison, the PDOS of Cs3LnCl6 (Ln = Pr, Nd, Sm, and Gd) are also calculated (Supplementary Fig. S22). It is found that the 4f occupied orbitals are much closer to 5d empty orbitals in Cs3CeCl6, which could be responsible for their greater possibility for 4f → 5d transitions. For Cs3EuCl6, the VB and CB are contributed by Cl-3p occupied orbitals and Eu-4f empty orbitals, respectively. Similarly, the Eu-4f empty orbitals are much closer to Cl-3p occupied orbitals in Cs3EuCl6, implying a higher possibility for Cl → Eu charge transfer transitions.
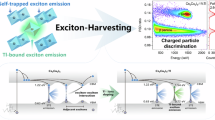
Tb3+ and Pr3+ ions could display 4f → 5d transitions, and Yb3+ ions possess Cl → Yb charge transfer transition as well, which may be attributed to the similar energy level conditions with that of Cs3CeCl6 and Cs3EuCl6, respectively. In Fig. 4e, the possible PL mechanisms of these Cs3LnCl6 (Ln = Ce, Pr, Tb, Eu, and Yb) with 4f → 5d transitions or charge transfer transitions are proposed. For Cs3CeCl6 and Cs3PrCl6, the electrons in the 4f ground states are excited to the 5 d excited states and then relaxed to the 4f ground states, giving out blue emissions due to 5d → 4f radiative recombination. For Cs3TbCl6, after being excited from 4f ground state to the 5d excited state, the electrons are nonradiatively relaxed to the Tb3+ 4f excited states and further relaxed to 4f ground states along with Tb3+ characteristic emissions from 4f → 4f transitions. While for Cs3EuCl6 and Cs3YbCl6, after being excited from Ln3+ 4f ground states to charge transfer states, the electrons are relaxed to Ln3+ 4f excited states and then relaxed to 4f ground states, resulting in corresponding Ln3+ emissions for radiative recombination of their 4f → 4f transitions.
For other Cs3LnCl6 without 4f → 5d transitions or charge transfer transitions, their 4f occupied orbitals may be far away from 5 d empty orbitals, and their 4f empty orbitals may be far from Cl-3p occupied orbitals, resulting in higher energy required for 4f → 5d transitions and Cl → Ln charge transfer transitions, thus decreasing the probability for these transitions under the excitation of near UV light. Therefore, it is difficult for these Cs3LnCl6 to exhibit great absorption ability based on 4f → 5d transitions or Cl → Ln charge transfer transitions. Hence, among all Cs3LnCl6, small energy level intervals from Ln-4f occupied orbitals to Ln-5d empty orbitals or from Cl-3p occupied orbitals to Ln-4f empty orbitals could result in great possibilities for 4f → 5d transitions or Cl → Ln charge transfer transitions that replace the parity forbidden 4f → 4f transitions and could be responsible for their great PL performance.
The thermogravimetric (TG) curves (Supplementary Fig. S23) show no significant weight loss (remaining > 94%) until 600 °C for these Cs3LnCl6 MCs, indicating their excellent structural stability. In Supplementary Figs. S24 and S25, the PL intensities of Cs3LnCl6 MCs remain above 88% of their initial values, and XRD patterns remain essentially unchanged after the MCs were left in a sealed environment without light exposure for 300 days, indicating the great air stability of these Cs3LnCl6 MCs.
The light yield (LY), as one of the important indicators for evaluating the scintillator performance, is proportional to the PLQYs of scintillators. Among these Cs3LnCl6, Cs3TbCl6 MCs with the highest PLQY and large Stokes shift could be very promising to exhibit great scintillator performance and have great potential for X-ray imaging40. Therefore, the X-ray scintillation performance of Cs3TbCl6 MCs was explored. The band gap of Cs3TbCl6 was calculated as 4.3 eV in Supplementary Fig. S26. Compared with commercially available scintillators, Cs3TbCl6 MCs exhibit a higher absorption coefficient than that of NaI:Tl and the equivalent absorption coefficient with that of Lu3Al5O12:Ce (LuAG:Ce) at 8–10 keV (Fig. 5a). For quantifying the X-ray LY of Cs3TbCl6 MCs, commercially available scintillator LuAG:Ce (with the thickness of 1 mm; the LY of 25,000 photons MeV−1) was selected as the reference40,41. Meantime, as shown in Supplementary Fig. S27a, Cs3TbCl6 sample with the thickness of 0.5 mm was prepared to unify the absorbed X-ray energy with LuAG:Ce sample. Radioluminescence (RL) spectra of Cs3TbCl6 MCs and LuAG:Ce are presented in Fig. 5b, similar Tb3+ characteristic emissions of Cs3TbCl6 imply the same radiative recombination channel with that under the excitation of UV light. Besides, as the X-ray dose rate increases, the RL integral intensity of Cs3TbCl6 MCs displays a linear increasing response curve (Supplementary Fig. S27b). As exhibited in Fig. 5c, the response of Cs3TbCl6 MCs is 2.07 times higher than that of LuAG:Ce and the outstanding LY of Cs3TbCl6 MCs is ~51,800 photons MeV−1. Compared with other Ln-based metal halide scintillators obtained via solid-state synthesis, hydrothermal synthesis or hot-injection methods in previous reports, as-prepared Cs3TbCl6 MCs could not only be synthesized quickly under mild synthetic conditions but also possess excellent LY of X-ray scintillator (Fig. 5d and Supplementary Table S5)22,28,33,42. Meantime, as shown in Supplementary Fig. S27c, the LY of Cs3TbCl6 MCs exceeds that of parts of commercial scintillation crystals43,44,45. Moreover, compared with commercial scintillation crystals Gd2O2S:Ce,Pr,F (LY = 35000 photons MeV−1), Cs3TbCl6 MCs with higher LY have great potential to replace Gd2O2S:Ce,Pr,F and be used as the next generation sensitization screen in the field of X-ray computed tomography imaging. In Supplementary Fig. S27d, when the signal-to-noise ratio (SNR) is 3, the detection limit of Cs3TbCl6 MCs is 63 nGy s−1, which is 87.3 times lower than those required for medical X-ray diagnosis standards (5.5 μGy s−1)46. Furthermore, under the X-ray irradiation with a cumulative dose of 1.38 Gy, the RL intensity remains unchanged, demonstrating the robust radiation hardness of Cs3TbCl6 MCs as X-ray scintillators (Fig. 5e). Subsequently, for X-ray imaging, flexible scintillation thin film (50 mm × 50 mm × 1 mm) based on Cs3TbCl6 MCs powder was prepared via blending the sample with polydimethylsiloxane (PDMS) and put it in X-ray imaging system (Fig. 5f and Supplementary Fig. S27e). As shown in Fig. 5g–i, a smartphone, a headset, and a wireless network interface controller were utilized as target objects to research the X-ray imaging ability of Cs3TbCl6@PDMS film. Under X-ray illumination, the distinct inside structure can be distinguished, indicating the realization of non-destructive testing for internal electronic components in these target objects. As presented in line-pair card imaging in Fig. 5j, the spatial resolution is derived as 12 lp mm−1, exceeding that of most scintillator thin films based on Ln-based metal halides in the previous reports (Supplementary Table S6)22,27,28,42. It is suggested that Cs3TbCl6 MCs synthesized by the recrystallization method possess excellent scintillator performance and have great potential for X-ray imaging.
Application of Cs3TbCl6 in X-ray imaging. a The absorption coefficients of Cs3TbCl6 MCs, NaI:Tl and LuAG:Ce as a function of photon energy from 1 KeV to 1000 KeV. b RL spectra of Cs3TbCl6 and LuAG:Ce at dose rate of 6.6 mGy s−1. c RL integral intensity of Cs3TbCl6 and LuAG:Ce as a function of X-ray dose rate. d Comparison of LY and synthesis temperature of Cs3TbCl6 MCs in this work with the reported Ln-based metal halides scintillators. e The RL intensity of Cs3TbCl6 MCs as a function of cumulative dose. f Schematic of the X-ray imaging system. The photographs of (g) a smartphone, (h) a headset, and (i) a wireless network interface controller under the visible light and X-ray at a dose rate of 1.2 mGy s−1. j The photograph of the standard line-pair card under the visible light (top) and the corresponding X-ray image of the standard line-pair card (bottom)
Discussion
In summary, we have prepared a series of Cs3LnCl6 MCs through a facile recrystallization method at room temperature for the first time. Especially, based on this method, large-scale production at one time and multiple recyclable recrystallizations of single-element Cs3LnCl6 MCs and the preparation of high-entropy five-element Cs3{TbDyHoErTm}1Cl6 metal halide crystals could also be realized. Based on DFT calculations, low energy for 4f → 5d transitions or Cl → Ln charge transfer transitions could overcome the 4f → 4f parity forbidden transitions of Ln3+ and bring excellent absorption ability and great PL performance of Cs3LnCl6 MCs. Especially, Cs3TbCl6 with 4f → 5d transitions absorption band possesses the highest PLQY of 90.8% among these Cs3LnCl6 MCs. Under X-ray irradiation, Cs3TbCl6 MCs show excellent X-ray scintillation performance with a high LY of ~51800 photons MeV-1 and the as-fabricated Cs3TbCl6@PDMS thin films possess promising X-ray imaging ability and preferable spatial resolution (12 lp mm−1). This work displays a novel recrystallization method for ultrafast and mild preparation of Ln-based metal halides and highlights their excellent potential as scintillators for X-ray imaging.
Materials and methods
Chemicals
Cesium chloride (CsCl, 99.99%), Cerium chloride heptahydrate (CeCl3·7H2O, 99%), praseodymium chloride hydrate (PrCl3·xH2O, 99.9%), neodymium chloride (NdCl3, 99.9%), samarium chloride hydrate (SmCl3·xH2O, 99.99%), Europium chloride hydrate (EuCl3·xH2O, 99.9%), gadolinium chloride hexahydrate (GdCl3·6H2O, 99.9%), terbium chloride hexahydrate (TbCl3·6H2O, 99.99%), dysprosium chloride hydrate (DyCl3·xH2O, 99.99%), holmium chloride hexahydrate (HoCl3·6H2O, 99.9%), erbium chloride hydrate (ErCl3·xH2O, 99.99%), thulium chloride hydrate (TmCl3·xH2O, 99.9%), ytterbium chloride hydrate (YbCl3·xH2O, 99.9%), lutetium chloride hexahydrate (LuCl3·6H2O, 99.9%) were purchased from Alfa Aesar. Methanol (MeOH, AR) and ethanol (EtOH, AR) were purchased from XiLONG SCIENCE. Polydimethylsiloxane (PDMS, SYLGARD® 184) was purchased from Dow Corning. All the chemicals were commercially purchased and used without further purification.
Preparation of Cs3LnCl6 MCs
0.6 mmol CsCl was first dissolved in 4 mL of methanol under ultrasound. 0.2 mmol LnCl3·xH2O was dissolved in 1 mL of methanol. Then, the above two solutions were mixed evenly. After introducing 5 mL of ethanol into the mixture, white precipitate formed immediately. After standing for 2 min, the supernatant was discarded, the collected precipitation was dried at 60 °C for 10 min to obtain Cs3LnCl6 MCs.
Preparation of Cs3{TbDyHoErTm}1Cl6 high entropy crystals
0.6 mmol CsCl was first dissolved in 4 mL of methanol under ultrasound. 0.04 mmol TbCl3·6H2O, 0.04 mmol DyCl3·xH2O, 0.04 mmol HoCl3·6H2O, 0.04 mmol ErCl3·xH2O and 0.04 mmol TmCl3·xH2O were dissolved in 1 mL of methanol. Then, the above two solutions were mixed evenly. Subsequently, an appropriate amount of ethanol was slowly diffused into the methanol solution for 12 h to form the transparent white crystals. Then, the supernatant was discarded, and the collected precipitation was dried at 60 °C for 10 min to obtain Cs3{TbDyHoErTm}1Cl6 high entropy crystals.
Preparation of Cs3TbCl6@PDMS flexible thin film
First, 2.270 g of PDMS base resin and 0.2300 g of curing agent were mixed in a beaker. Then, 0.1880 g of Cs3TbCl6 powder was dispersed in the above PDMS precursor with stirring for 30 min. After curing at 100 °C for 60 min, the Cs3TbCl6@PDMS flexible thin film was obtained.
Data availability
Data will be available when the paper is officially published.
References
Chen, Q. S. et al. All-inorganic perovskite nanocrystal scintillators. Nature 561, 88–93 (2018).
Mi, Z. H. et al. Real-time single-proton counting with transmissive perovskite nanocrystal scintillators. Nat. Mater. 23, 803–809 (2024).
Yi, L. Y. et al. A double-tapered fibre array for pixel-dense gamma-ray imaging. Nat. Photonics 17, 494–500 (2023).
Cho, S. et al. Hybridisation of perovskite nanocrystals with organic molecules for highly efficient liquid scintillators. Light 9, 156 (2020).
Pang, J. C. et al. Vertical matrix perovskite X-ray detector for effective multi-energy discrimination. Light 11, 105 (2022).
Jin, T. et al. Self-wavelength shifting in two-dimensional perovskite for sensitive and fast gamma-ray detection. Nat. Commun. 14, 2808 (2023).
Lian, H. W. et al. Aqueous-based inorganic colloidal halide perovskites customizing liquid scintillators. Adv. Mater. 35, 2304743 (2023).
Marshall, K. P. et al. Enhanced stability and efficiency in hole-transport-layer-free CsSnI3 perovskite photovoltaics. Nat. Energy 1, 16178 (2016).
Park, Y. et al. Designing zero-dimensional dimer-type all-inorganic perovskites for ultra-fast switching memory. Nat. Commun. 12, 3527 (2021).
Luo, J. J. et al. Efficient and stable emission of warm-white light from lead-free halide double perovskites. Nature 563, 541–545 (2018).
Saikia, S., Ghosh, A. & Nag, A. Broad dual emission by codoping Cr3+(d→d) and Bi3+(s→p) in Cs2Ag0.6Na0.4InCl6 double perovskite. Angew. Chem. Int. Ed. 62, e202307689 (2023).
Yang, H. X. et al. A universal hydrochloric acid-assistant powder-to-powder strategy for quick and mass preparation of lead-free perovskite microcrystals. Light 12, 75 (2023).
Li, H. W. et al. Efficient multi-luminescence covering the visible to near-infrared range in antimony and lanthanide co-doped indium-based zero-dimensional perovskites nanocrystals. Adv. Opt. Mater. 11, 2300429 (2023).
Chen, J. K. et al. Ultrafast and multicolor luminescence switching in a lanthanide-based hydrochromic perovskite. J. Am. Chem. Soc. 144, 22295–22301 (2022).
Jin, S. L. et al. Compact ultrabroadband light-emitting diodes based on lanthanide-doped lead-free double perovskites. Light 11, 52 (2022).
Li, H. W. et al. Double perovskite Cs2NaInCl6 nanocrystals with intense dual-emission via self-trapped exciton-to-Tb3+ dopant energy transfer. J. Mater. Chem. C 10, 10609–10615 (2022).
Li, H. W. et al. Multiple energy transfer channels in rare earth doped multi-exciton emissive perovskites. Adv. Sci. 11, 2307354 (2024).
Wang, X. J. et al. Nearly-unity quantum yield and 12-hour afterglow from a transparent perovskite of Cs2NaScCl6:Tb. Angew. Chem. Int. Ed. 61, e202210853 (2022).
Dang, P. P. et al. Red-NIR luminescence in rare-earth and manganese ions Codoped Cs4CdBi2Cl12 vacancy-ordered quadruple perovskites. Chem. Mater. 35, 1640–1650 (2023).
Wang, H. Y. et al. Simultaneously achieving multicolor emission of down-shifting and up-conversion in Yb3+, Er3+‐Codoped Cs2NaGdCl6 double perovskites. Adv. Opt. Mater. 11, 2300694 (2023).
Shi, Y. H. et al. Pure green upconversion from a multicolor downshifting perovskite crystal. Adv. Opt. Mater. 11, 2202704 (2023).
Zhang, N. et al. X‐ray-activated long afterglow double-perovskite scintillator for detection and extension imaging. Adv. Opt. Mater. 11, 2300187 (2023).
Li, H. et al. A solar-blind perovskite scintillator realizing portable X-ray imaging. ACS Energy Lett. 7, 2876–2883 (2022).
Kachhap, S. et al. Expanding the emission of CsPbBr3 nanocrystals in the blue region. ACS Appl. Opt. Mater. 1, 1974–1986 (2023).
Zeng, Z. C. et al. Rare-earth-based perovskite Cs2AgScCl6:Bi for strong full visible spectrum emission. Adv. Funct. Mater. 32, 2204780 (2022).
Sun, L. H. et al. Efficient and stable multicolor emissions of the coumarin-modified Cs3LnCl6 lead-free perovskite nanocrystals and LED application. Adv. Mater. 36, 2310065 (2024).
Zhou, W. et al. Sb‐doped Cs3TbCl6 nanocrystals for highly efficient narrow-band green emission and X‐ray imaging. Adv. Mater. 36, 2302140 (2024).
Han, J. H. et al. Highly stable zero-dimensional lead-free metal halides for X-ray imaging. ACS Energy Lett. 8, 545–552 (2023).
Han, J. H. et al. Intense hydrochromic photon upconversion from lead-free 0D metal halides for water detection and information encryption. Adv. Mater. 35, 2302442 (2023).
Reddy, M. M. & Hoch, A. R. Calcite crystal growth rate inhibition by polycarboxylic acids. J. Colloid Interface Sci. 235, 365–370 (2001).
Luo, Y. B. et al. High thermoelectric performance in the new cubic semiconductor AgSnSbSe3 by high-entropy engineering. J. Am. Chem. Soc. 142, 15187–15198 (2020).
Deng, Z. H. et al. Semiconducting high-entropy chalcogenide alloys with ambi-ionic entropy stabilization and ambipolar doping. Chem. Mater. 32, 6070–6077 (2020).
Hu, Q. S. et al. X-ray scintillation in lead-free double perovskite crystals. Sci. China Chem. 61, 1581–1586 (2018).
Wang, L. et al. Exploration of nontoxic Cs3CeBr6 for violet light-emitting diodes. ACS Energy Lett. 6, 4245–4254 (2021).
Luo, J. J. et al. Efficient blue light emitting diodes based on europium halide perovskites. Adv. Mater. 33, 2101903 (2021).
Chen, B. et al. Multiexcitonic emission in zero-dimensional Cs2ZrCl6:Sb3+ perovskite crystals. J. Am. Chem. Soc. 143, 17599–17606 (2021).
Wu, R. Y. et al. Red-emitting perovskite variant Cs2PtCl6 phosphor: material design, luminous mechanism, and application in high-color-rendering white light-emitting diodes. Adv. Opt. Mater. 10, 2201081 (2022).
Kong, Q. K. et al. Phase engineering of cesium manganese bromides nanocrystals with color-tunable emission. Angew. Chem. Int. Ed. 60, 19653–19659 (2021).
Wei, J. H. et al. All-inorganic lead-free heterometallic Cs4MnBi2Cl12 perovskite single crystal with highly efficient orange emission. Matter 3, 892–903 (2020).
Han, K. et al. Promoting single channel photon emission in Copper(I) halide clusters for X-ray detection. Adv. Opt. Mater. 10, 2200865 (2022).
Han, K. et al. Seed-crystal-induced cold sintering toward metal halide transparent ceramic scintillators. Adv. Mater. 34, 2110420 (2022).
Zhou, W. et al. Bright green-emitting all-inorganic terbium halide double perovskite nanocrystals for low-dose X-ray imaging. J. Phys. Chem. Lett. 14, 8577–8583 (2023).
Michail, C. et al. Measurement of the luminescence properties of Gd2O2S:Pr,Ce,F powder scintillators under X-ray radiation. Radiat. Meas. 70, 59–64 (2014).
He, T. Y. et al. High-performance copper-doped perovskite-related silver halide X-ray imaging scintillator. ACS Energy Lett. 7, 2753–2760 (2022).
Jin, J. C. et al. Zn2+ doping in organic manganese(II) bromide hybrid scintillators toward enhanced light yield for X‐ray imaging. Adv. Opt. Mater. 11, 2300330 (2023).
Wei, H. T. et al. Sensitive X-ray detectors made of methylammonium lead tribromide perovskite single crystals. Nat. Photonics 10, 333–339 (2016).
Acknowledgements
This work was supported by financial aid from the National Natural Science Foundation of China (22271273).
Author information
Authors and Affiliations
Contributions
H. L., J. F., S. S. and H. Z. designed the experiments, interpreted the data, and co-wrote the paper. H. L. carried out the syntheses, characterization studies, and data analyses. K. L. interpreted the theoretical results. Z. L., X. F., Q. Y. and N. W. participated in the measurement and data analyses. K. L. and X. W. gave suggestions on writing the paper. J. F., S. S. and H. Z. discussed the results and commented on the paper.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, H., Li, K., Li, Z. et al. Lanthanide-based metal halides prepared at room temperature by recrystallization method for X-ray imaging. Light Sci Appl 14, 195 (2025). https://doi.org/10.1038/s41377-025-01839-5
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41377-025-01839-5