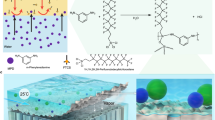
Abstract
Optical probes hold great promise for temperature sensing owing to their attractive properties including rapid response, high spatial resolution, and remote non-invasive detection. However, the exploration of thermometric probes is hindered by their low relative sensitivity (Sr) or poor structural stability in water. Herein, we propose the first example of organic-inorganic metal halides based on TPP3Cu2Br2 (TPP = triphenylphosphine) that simultaneously present excellent water resistance and sensitive temperature-dependent photoluminescence lifetime in water. Benefiting from the soft lattice induced by the organic molecule of TPP, giant thermal expansion and great lattice distortion were achieved with increasing temperature. As such, the self-trapped exciton luminescence lifetime of TPP3Cu2Br2 can be shortened to 1.9% of the initial value from 280 to 380 K, resulting in the highest Sr of 12.82% K−1 among the undoped metal halides based luminescent thermometers. Significantly, TPP3Cu2Br2 displayed extraordinary water stability with emission intensity remaining nearly unchanged after immersing in water for 15 days. Moreover, high-precision luminescence lifetime based thermal sensing in water environment was successfully conducted, which proved to be inert to the detection depth in water with a small read-out error. This work offers new routes in the exploration of novel metal halides for highly sensitive thermometric probes toward versatile application scenarios.
Similar content being viewed by others
Introduction
Optical thermometer is widely employed for temperature sensing by monitoring optical parameters like temperature-dependent changes in photoluminescence (PL) intensity1,2,3,4,5,6,7 or PL lifetime8,9,10. Nevertheless, intensity-based optical thermometers like infrared thermal imager are susceptible to various factors including light source, sample concentration, photobleaching, scattering, and absorption at different wavelengths11,12,13,14. PL lifetime proves advantageous for precise temperature sensing as it remains unaffected by the aforementioned factors. As such, PL lifetime-based thermometry fundamentally circumvents the limitations inherent to the conventional infrared thermal imager15. Currently, temperature sensing based on PL lifetime mainly employs Ln3+ ions and ns2 ions doped phosphors. For Ln3+-doped phosphors, the 4f-4f transitions of Ln3+ ions are not sensitive to surroundings due to the shielding of 5s5p shell, therefore exhibiting a small PL lifetime variation with temperature and a low temperature sensing sensitivity16. Although ns2 ions doped phosphors tend to display higher temperature sensing sensitivity, their lifetimes are generally located in the ns range17,18,19,20. Such a small PL lifetime requires sophisticated instrumentation and short-pulsed lasers, which is not feasible for most practical scenarios. Therefore, there is an urgent need to develop novel materials with large PL lifetime variation ability for sensitive temperature sensing.
In the past decade, metal halides (MHs) have received increasing attention for PL lifetime-based temperature sensing21,22,23,24,25,26. Particularly, some organic-inorganic metal halides (OIMHs) have significant temperature-dependent structural properties. At high temperatures, the thermal movement of organic molecules causes dramatic expansion of the crystal cells. Such crystal expansion induces the formation of defects, leading to a temperature-dependent nature of the PL lifetime. These OIMHs can provide sensitive temperature sensing based on self-trapped exciton (STE) luminescence with microseconds or longer PL lifetime, making them very suitable for applications in PL lifetime-based thermometry27,28,29,30. However, their instability in water severely limits their application in humid environments31,32,33. Despite substantial advancements being made in OIMHs, it remains challenging to explore OIMHs that are water-stable and highly temperature-sensitive in PL lifetime.
One possible strategy to address this challenge is introducing hydrophobic organic molecules into the OIMHs. Herein, we propose a novel kind of OIMH based on TPP3Cu2Br2 (TPP = Triphenylphosphonium), presenting exceptional water stability as sensitive luminescence lifetime thermometers in water environment. Upon excitation with ultraviolet (UV) light, TPP3Cu2Br2 emitted bright green STE luminescence with a PL quantum yield (PLQY) of 41.5%. Benefitting from its excellent temperature-dependent PL lifetime, a maximal relative sensitivity (Sr) of 12.82% K−1 at 380 K for temperature sensing was achieved. Moreover, TPP3Cu2Br2 exhibited excellent water stability, whose PL intensity remained 97.3% of the initial value after 15 days of soaking in the water. We revealed that such excellent luminescent stability was owing to the protective effect of the hydrophobic organic molecules. Furthermore, we demonstrated the application of TPP3Cu2Br2 for high-precision thermal sensing in the water environment.
Results
Crystal Structure
TPP3Cu2Br2 was synthesized by a simple solvothermal method from the precursors of TPP-HBr and CuBr in a mixed solvent of N,N-Dimethylformamide and H3PO2, resulting in subcentimeter transparent acicular crystals with green emission upon 365 nm excitation (Fig. S1, Supporting Information). The crystal structure of TPP3Cu2Br2 was obtained by resolving single-crystal X-ray diffraction (SCXRD) data (Fig. S2 and Tables S1–S4, Supporting Information). It was determined that TPP3Cu2Br2 crystallized in a monoclinic phase with P21/n space group, where a = 19.0348(2) Å, b = 9.87200(10) Å and c = 26.1822(3) Å. The [Cu2Br2] dimer is covalently bonded to the P atom in the TPP molecule via the Cu atom, which isolates the [Cu2Br2] dimer and constitutes the 0D structure (Fig. 1a, b). The bond lengths of Cu-P and Cu-Br are distributed in the range of 2.193–2.563 Å, whereas the Cu-Cu distance in the nearest dimer is 8.370 Å. Such a large distance also elucidated the isolated 0D structure of TPP3Cu2Br2. Powder X-ray diffraction (PXRD) pattern of the as-synthesized crystals agreed well with the simulated structure from single crystal data (Fig. 1c). Meanwhile, X-ray photoelectron spectroscopy (XPS) was used to probe the chemical nature of TPP3Cu2Br2. The characteristic peaks of C, P, Cu, and Br were identified, and the peaks of Cu 2p1/2 (952.4 eV) and 2p3/2 (932.6 eV) confirmed the monovalent state of Cu (Fig. 1d and Fig. S3, Supporting Information). Scanning electron microscopy (SEM) showed that the crystals displayed an acicular shape. Energy dispersive X-ray (EDX) spectrum mapping confirmed a uniform distribution of the elements Cu, P, and Br (Fig. S4, Supporting Information). In addition, the characteristic peaks located at 3053 and 1434 cm−1 in the Fourier transform infrared (FTIR) spectrum were attributed to C-H stretching vibrations and C = C vibrations of benzene, indicating that the organic groups were distributed in TPP3Cu2Br2 (Fig. S5, Supporting Information). Through thermogravimetric analysis (TGA) and differential thermal analysis (DTA), it was observed that the decomposition of TPP3Cu2Br2 started at a temperature up to 230 °C. Such good thermal stability favors their applications in high-temperature environments (Fig. 1e).
a Single crystal structure of TPP3Cu2Br2. b Schematic diagram of the structure of TPP3Cu2Br2. Inset: Micrograph of the TPP3Cu2Br2 single crystal under ambient light, schematic diagram of the structure of triphenylphosphine and [Cu2Br2] dimer. c PXRD pattern of TPP3Cu2Br2 and simulated pattern from the single crystal. d High-resolution XPS spectrum of Cu 2p in TPP3Cu2Br2. e TGA and DTA curves for the TPP3Cu2Br2 powder
Photoluminescence properties and mechanisms
According to the absorption spectroscopy (Fig. 2a), TPP3Cu2Br2 displayed broad absorption across the UV region (200–400 nm) with a peak of 310 nm. The bandgap was calculated to be 3.26 eV using the Tauc plot method (Fig. S6, Supporting Information). Upon excitation at 355 nm, TPP3Cu2Br2 exhibited broad green emission peaking at 524 nm, with a full width at half maximum (FWHM) of 120 nm and a PLQY of 41.5% (Fig. 2b and Fig. S7, Supporting Information). The PL lifetime of TPP3Cu2Br2 was determined to be 32.95 μs based on mono-exponential fitting (Fig. 2c). We measured the excitation wavelength-dependent (280–360 nm) emission spectra and the emission wavelength-dependent (450–620 nm) excitation spectra, which indicated that both the normalized PL emission and excitation spectra were essentially unchanged with different wavelengths, demonstrating a single source of emission (Fig. 2d and Fig. S8, Supporting Information). Note that TPP3Cu2Br2 emitted strong broadband green PL while TPPBr exhibited negligible emission (Fig. S9, Supporting Information), revealing that the TPP group in TPP3Cu2Br2 may not act as a luminescent center.
a UV-vis absorption spectrum of TPP3Cu2Br2. b Steady-state PL excitation (left) and PL emission (right) spectra of TPP3Cu2Br2. c PL decays of TPP3Cu2Br2 by monitoring the emission at 524 nm (λex = 355 nm). d Excitation wavelength-dependent emission spectra with the excitation wavelength from 280 to 360 nm. e Temperature-dependent emission spectra ranging from 80 to 300 K (λex = 355 nm). f Integral intensity of temperature-dependent emission spectra in the temperature range 80 to 300 K. g FWHM as a function of temperature. Data are fitted based on Eq. (1)
In order to gain a deep understanding of the photophysical mechanism of TPP3Cu2Br2, we acquired temperature-dependent steady-state PL spectra from 80 to 300 K (Fig. 2e, f). It was discovered that the PL intensity anomalously increased with elevating the temperature from 80 to 280 K. The FWHM of the emission became progressively wider with the rising of temperature. The FWHM variation with the temperature can be fitted to derive the Huang-Ryhs factor (S) and the phonon frequency (ℏω) by using the following equation34,35:
Correspondingly, S and ℏω were determined to be 20.68 and 46.7 meV, respectively (Fig. 2g). Such a large S revealed the strong electron-phonon coupling in TPP3Cu2Br236.
Furthermore, we performed density functional theory (DFT) calculations to shed more light on the PL mechanism. As indicated by the projected density of states (DOS), TPP3Cu2Br2 has a direct bandgap (Fig. 3a). From the charge density plots of the valence band maximum (VBM) and the conduction band minimum (CBM), it can be observed that the VBM was mainly composed of the Cu 3d and Br 4p orbitals, and the CBM was composed of TPP (Fig. 3b). The majority of the electron cloud was distributed around the TPP, with a small portion distributed on [Cu2Br2] dimer (Fig. S10, Supporting Information). In addition, the charge density plots of the excited state were also modeled. Compared to the ground state, the electron cloud in the excited state was drastically concentrated towards [Cu2Br2], leading to the distortion of [Cu2Br2] dimer (Fig. 3c)37. Through the excited state simulation of TPP3Cu2Br2, theoretical lattice parameters were calculated (Table S4, Supporting Information), confirming no significant change in the lattice parameters of a and c compared to the ground state. Nevertheless, the lattice parameter b was significantly prolonged from 9.87 Å in the ground state to 9.95 Å in the excited state. As such, the lattice was distorted in the excited state, thus resulting in the emission of STE (Fig. 3d)38,39,40.
a Calculated band structure, b total and partial DOS of TPP3Cu2Br2 based on the DFT method. c Diagram of the partial charge density for the VBM and CBM for the excited state of TPP3Cu2Br2 crystals, wherein isosurfaces are electron cloud distributions. d Schematic mechanism of TPP3Cu2Br2. GS ground state; ES excited state; STE self-trapped exciton
Temperature sensing performance
Organic molecular TPP endows TPP3Cu2Br2 with a soft lattice structure, which is sensitive to temperature change. At low temperature, the rigidity of organic molecules was strengthened, which would inhibit the deformation of [Cu2Br2] dimer and suppress the creation of the self-trapped state41,42. Consequently, the PL intensity of TPP3Cu2Br2 was substantially boosted with the temperature increased from 80 to 280 K (Fig. 2e). Meanwhile, it was found that the lattice greatly expanded with lattice volume increased by 3.6% (from 4818.3 to 4994.0 Å3) from 300 to 380 K (Fig. S11 and Table S5, Supporting Information), which is much higher than that (< 0.5%) of typically MHs19,20. The giant thermal expansion of lattice was also confirmed by the temperature-dependent PXRD of TPP3Cu2Br2, as evidenced by the shifting of diffraction peaks towards smaller angles with the rising of temperature (Fig. 4a). The dramatic lattice expansion with elevating temperature would introduce more defects within the crystal and thus quenching the luminescence. We compared the temperature dependence of PL intensity and lifetime from 280 to 380 K. It was discovered that the PL intensity decreased steadily as temperature increased, eventually dropping to 10.8% of its original value at 380 K (Fig. 4b). Intriguingly, the PL lifetime of TPP3Cu2Br2 displayed a much wider range of variation from 51.2 μs (280 K) to 0.97 μs (380 K), which was only 1.9% of the initial value (Fig. 4c, d). The PL lifetime (τ) and temperature (T) can be fitted with the following empirical exponential equation19:
where A, t, τ0 are constants. Furthermore, the temperature sensing performance can be evaluated with absolute sensitivity (Sa) and Sr based on the following equations:
a Temperature-dependent PXRD patterns of TPP3Cu2Br2 with temperature from 300 to 380 K. b Temperature-dependent PL spectra of TPP3Cu2Br2 with temperature from 280 to 380 K (λex = 355 nm). c Temperature-dependent decays of TPP3Cu2Br2 with temperature from 280 to 380 K (λex = 355 nm, λem = 524 nm). d Integrated PL intensity and PL lifetime of TPP3Cu2Br2 at different temperatures. e Calculated Sr values based on the PL lifetime of TPP3Cu2Br2 at different temperatures. f) PL lifetime of TPP3Cu2Br2 at 280 K and 380 K through multiple heating and cooling cycles
Accordingly, the maximum Sa value was determined to be 1.33 μs K−1 (280 K) (Fig. S12, Supporting Information), and the maximum Sr value was calculated to be 12.82% K−1 (380 K) (Fig. 4e). Note that the Sr value is the highest for temperature sensing among the undoped MHs. For comparison, the maximum values of Sa and Sr based on PL intensity were only 1.39% K−1 and 6.46% K−1, respectively (Figs. S13, S14, Supporting Information). Besides, the repeatability of the thermometer is a decisive parameter for its application in temperature sensing. After 11 test cycles, the PL lifetime of the TPP3Cu2Br2 remained essentially unchanged, confirming its good repeatability in temperature sensing (Fig. 4f). The temperature uncertainty δT can be calculated by using the following equation19:
where δτ/τ is the uncertainty of lifetime measurements. Accordingly, it was determined that TPP3Cu2Br2 exhibited small δT values (≤ 0.47 K) in the range of 280–380 K, which is beneficial for high-precision temperature sensing (Fig. S15, Supporting Information).
Water Stability Performance
The PXRD pattern of TPP3Cu2Br2 remained in the pure phase even after immersing it in water for 15 days (Fig. 5a), indicating its excellent structural stability in water. Correspondingly, TPP3Cu2Br2 exhibited almost no alteration in both PL photographs and PL spectra (Fig. 5b, c). Specifically, the PL intensity was 97.3% of the initial value after 15 days, indicative of the extraordinary photostability of TPP3Cu2Br2 in water (Fig. 5d). To investigate the impact of TPP, we compared the surface hydrophobicity of hybrid TPP3Cu2Br2 and all-inorganic Cs3Cu2Br5. Through characterization of static-contact angle, the degree of water infiltration on TPP3Cu2Br2 and Cs3Cu2Br5 was investigated (Fig. 5e). A larger contact angle of 66° was detected for TPP3Cu2Br2 crystals, which greatly surpassed that of 23° for the Cs3Cu2Br5 counterpart. Such a high contact angle indicated that the crystal surface of TPP3Cu2Br2 was highly hydrophobic, thus protecting the matrix from permeability and diffusion of water molecules. Through DFT calculations, the binding energy of TPP3Cu2Br2 and water molecules was determined to be 0.223 eV (Fig. 5f), which is much lower than that of Cs3Cu2Br5 and water molecules (0.882 eV), revealing weaker hydrophilicity of TPP3Cu2Br2 than Cs3Cu2Br5.
a PXRD patterns, b photographs, c PL emission spectra and d integral intensity of PL emission spectra of TPP3Cu2Br2 soaking in water for different days upon excitation at 365 nm. e Contact angle of water molecule on crystal surfaces of TPP3Cu2Br2 and Cs3Cu2Br5. f Theoretically calculated surface structures of H2O interacted with Cs3Cu2Br5 (up) and TPP3Cu2Br2 (down), wherein gray balls are C atoms, white balls are H atoms, blue balls are Cu atoms, dark red balls are Br atoms, orange balls are P atoms, purple balls are Cs atoms, red balls are O atoms. The simulation model is constructed based on water molecules on the (001) surface of TPP3Cu2Br2 and (040) surface of Cs3Cu2Br5
Moreover, the interaction force between atoms was reported to be proportional to their distance43. The rigid molecular conformation of the TPP molecule and the large steric hindrance limit the movement of water molecules, inhibiting water molecules from directly contacting with the hydrophilic [Cu2Br2] dimer in TPP3Cu2Br2. The closest contact distance between water molecule and TPP3Cu2Br2 was calculated to be 6.583 Å for the H-Br bond length, which is much longer than the H-Br distance of 2.335 Å between water molecule and Cs3Cu2Br5 (Fig. S16, Supporting Information). The longer contact distance between water molecule and hydrophilic [Cu2Br2] dimer endowed superior water-resistance stability. In addition, the bond type in the matrix also significantly affects the stability of the crystal44. The strong Cu-P covalent bond in TPP3Cu2Br2 results in a higher energy barrier for ionization in water, facilitating the structural stability of TPP3Cu2Br2. By contrast, the ionic bonds in Cs3Cu2Br5 are more susceptible to ionization in water, which might lead to structural disruption. Hence, the impressive resistance to water of TPP3Cu2Br2 renders it an ideal material as the luminescence thermometer in water environment. To the best of our knowledge, such water-resistance MHs based luminescence lifetime thermometers have never been reported before. Note that the micron-scale dimensions of TPP3Cu2Br2 crystals enable spatial resolution in microdomain thermal mapping, favoring diverse sensing scenarios like mapping microzone-temperature variations in water.
Temperature sensing in water environment
As a proof-of-concept experiment, we applied TPP3Cu2Br2 for temperature sensing in aqueous solution. The PL lifetime was independently monitored three times at 37 °C (Fig. 6a). The average PL lifetime was determined to be 19.83 μs (Fig. S17, Supporting Information). Meanwhile, the PL emission spectra were monitored under the same conditions (Fig. S18, Supporting Information). The temperature can be calculated by using Eqs. 2 and 6 as standard curves, respectively.
where A, t, and I0 are constants. Specifically, temperatures of 38.57 ± 0.13 and 31.31 ± 0.36 °C were determined on the basis of PL lifetime and PL intensity with errors of 1.57 and 5.69 °C, respectively. Therefore, PL lifetime-based temperature sensing provides higher accuracy than PL intensity-based temperature sensing.
a Schematic illustration of luminescence temperature-sensing based on TPP3Cu2Br2 soaking in water. b PL decays of the TPP3Cu2Br2 at different depths of water by monitoring the emission at 524 nm (λex = 355 nm) at 37 °C. c PL emission spectra of the TPP3Cu2Br2 at different depths of water upon 355 nm excitation at 37 °C. d Comparison of calculated temperatures at different depths of water from multiple experiments based on PL intensity and PL lifetime, where data were presented as average ± standard deviation calculated from three independently measured PL emission and PL lifetime spectra. e Error values between calculated and actual temperatures (37 °C) at different water depths
Moreover, the absorption and scattering of light may significantly reduce the accuracy of intensity measurements at varying depths, which is unavoidable in practical applications. To this regard, we placed TPP3Cu2Br2 in cuvettes filled with water at varying depths to illustrate the impact of water on light interference. We monitored the PL lifetime and PL intensity of TPP3Cu2Br2 in different depths at 37 °C (Fig. 6b, c). The accuracy of the temperature measurements was then calculated (Fig. 6d, e). As the depth of the water increased, the PL intensity quenched significantly, while the PL lifetime remained essentially unchanged. When the water depth reached 2 mm, the error value based on PL intensity reached 14.88 °C while the value based on PL lifetime remained essentially small (1.61 °C). When the sample was immersed in 10 mm water, the detection error based on PL intensity was elevated to 30.78 °C, significantly larger than the read-out error based on PL lifetime (1.09 °C). These results highlighted that TPP3Cu2Br2 has great potential for accurate temperature sensing applications in water environment, offering promising opportunities for monitoring microzone-temperature variations beneath the water surface.
Discussion
In summary, we have developed a new class of zero-dimensional hybrid cuprous halide of TPP3Cu2Br2, which presents excellent water resistance for sensitive temperature sensing. Specifically, TPP induced the soft lattice of TPP3Cu2Br2, enabling substantial lattice distortion and significant reduction of STE luminescence lifetime to 1.9% of the initial value from 280 to 380 K. Such remarkable temperature-dependent PL lifetime favored highly sensitive optical temperature sensing, exhibiting a Sr of 12.82% K−1, representing the highest value based on the undoped MHs. Furthermore, we successfully applied TPP3Cu2Br2 for thermal sensing with a small read-out error of 1.09 °C at a water depth of 10 mm, demonstrating the superiority of PL lifetime strategy relative to the conventional PL intensity approach. Our work reveals the great promise of TPP3Cu2Br2 for temperature detection in water environment, which may accelerate the exploitation of novel MHs for thermometry applications in versatile scenarios.
Materials and methods
Chemicals and materials
Triphenylphosphine hydrobromide (C18H16BrP, 97%) and phosphinic acid (H3PO2, 50 wt.%) were purchased from Adamas-beta Ltd. Copper(I) bromide (CuBr, 99.9%) was purchased from Aladdin (Shanghai, China). N,N-Dimethylformamide (DMF, 99.5%), and isopropanol (C3H8O, 99.7%) were purchased from Sinopharm Chemical Reagent Co (Shanghai, China). All chemicals were used without any further purification.
Synthesis of TPP3Cu2Br2 single crystal
TPP3Cu2Br2 single crystals were prepared by a saturated crystallization method. In a typical synthesis, 1 mmol C18H16BrP and 1 mmol CuBr were dissolved in 4 mL DMF and 1 mL H3PO2 at 95 °C to form a colorless transparent solution. Then the solution was cooled to room temperature slowly. After that, the transparent crystals of TPP3Cu2Br2 were filtered off, washed with isopropanol to remove the solvent from the crystal surface, and dried in an oven at 60 °C. Finally, the crystals were ground into powder for further utilization.
Data availability
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
References
Wang, X. D., Wolfbeis, O. S. & Meier, R. J. Luminescent probes and sensors for temperature. Chem. Soc. Rev. 42, 7834–7869 (2013).
Xue, K. et al. A sensitive and reliable organic fluorescent nanothermometer for noninvasive temperature sensing. J. Am. Chem. Soc. 143, 14147–14157 (2021).
Li, Q. et al. A wide range photoluminescence intensity-based temperature sensor developed with BN quantum dots and the photoluminescence mechanism. Sens. Actuators B: Chem. 304, 127353 (2020).
He, Z. L. et al. Reversible human-temperature-responsive luminescence switching in a Mn(II)-based metal halide. J. Mater. Chem. C. 11, 1251–1257 (2023).
Gao, M. et al. Ultra-sensitive luminescent ratiometric thermometry based on matrix energy transfer in Dy3+ doped CaWO4 phosphors. J. Lumin. 263, 120102 (2023).
Zhu, J. et al. Improving the up/down-conversion luminescence via cationic substitution and dual-functional temperature sensing properties of Er3+ doped double perovskites. Chem. Eng. J. 471, 144550 (2023).
Rao, Z. H. et al. Understanding and effective tuning of red-to-green upconversion emission in ho-based halide double perovskite microcrystals. Adv. Funct. Mater. 34, 2311568 (2024).
Qiu, L. T. et al. Cr3+-doped InTaO4 phosphor for multi-mode temperature sensing with high sensitivity in a physiological temperature range. Inorg. Chem. Front. 9, 3187–3199 (2022).
Yılmaz, M. & Alp, E. Low-cost Zn2(OH)BO3:Pb2+ phosphor for large-scale thermometric applications. J. Alloy. Compd. 934, 167865 (2023).
Wang, Y. Z., Liu, G. C. & Xia, Z. G. NIR‐II luminescence in Cr4+ activated CaYGaO4 toward non‐invasive temperature sensing and composition detection. Laser Photonics Rev. 18, 2300717 (2024).
Zheng, T. et al. Mechanoluminescence and photoluminescence heterojunction for superior multimode sensing platform of friction, force, pressure, and temperature in fibers and 3D‐printed polymers. Adv. Mater. 35, 2304140 (2023).
Parker, D., Fradgley, J. D. & Wong, K. L. The design of responsive luminescent lanthanide probes and sensors. Chem. Soc. Rev. 50, 8193–8213 (2021).
Yu, S. H. et al. A dual‐excitation decoding strategy based on NIR hybrid nanocomposites for high‐accuracy thermal sensing. Adv. Sci. 7, 2001589 (2020).
Li, X. R. et al. A ratiometric optical thermometer with dual-color emission based on Eu2+-doped CsCu2I3 microcrystals. J. Rare Earths 42, 1429–1436 (2024).
Li, K. J. et al. Li-based nanoprobes with boosted photoluminescence for temperature visualization in NIR imaging-guided drug release. Nano Lett. 25, 776–785 (2025).
Zhao, S. et al. Luminescent enhancement and multi-mode optical thermometry of erbium doped halide Cs2(Na/Ag)BiCl6 microcrystals. J. Rare Earths 42, 2018–2026 (2024).
Wei, J. H. et al. Te4+-doped Cs2InCl5·H2O single crystals for remote optical thermometry. Sci. China Mater. 65, 764–772 (2022).
Wu, L. K. et al. Te4+-doping rubidium scandium halide perovskite single crystals enabling optical thermometry. J. Phys. Chem. C. 126, 21689–21698 (2022).
Li, G. Q. et al. Regulating exciton de-trapping of Te4+-doped zero-dimensional scandium-halide perovskite for fluorescence thermometry with record high time-resolved thermal sensitivity. Adv. Mater. 35, 2305495 (2023).
Benin, B. M. et al. The Rb7Bi3−3xSb3xCl16 family: a fully inorganic solid solution with room‐temperature luminescent members. Angew. Chem. Int. Ed. 59, 14490–14497 (2020).
Zhou, H. L. et al. Efficient blue emission in organic–inorganic metal halide (TEA)2InCl5 triggered by Bi3+‐doping. Adv. Mater. Technol. 10, 2400777 (2025).
Yao, J. D. et al. Boosting photoluminescence of rare-earth-based double perovskites by isoelectronic doping of ns2 metal ions. Small 21, e2405724 (2025).
Hou, A. et al. Zero-dimensional halides with ns2 electron (Sb3+) activation to generate broad photoluminescence. Inorg. Chem. 62, 12501–12509 (2023).
Wang, Y. Z. et al. Efficient X-ray luminescence imaging with ultrastable and eco-friendly copper(I)-iodide cluster microcubes. Light Sci. Appl. 12, 155 (2023).
Han, K. et al. Hybrid Eu(II)-bromide scintillators with efficient 5d-4f bandgap transition for X-ray imaging. Light Sci. Appl. 13, 222 (2024).
Zhang, Y. Y., Zhao, T. Y. & Chen, G. Y. Recent progress in lanthanide ions doped inorganic metal halide perovskites. J. Rare Earths 42, 237–250 (2024).
Yao, J. S. et al. Modulation of metal halide structural units for light emission. Acc. Chem. Res. 54, 441–451 (2021).
Liu, Y. et al. Near‐infrared light emitting metal halides: materials, mechanisms, and applications. Adv. Mater. 36, 2312482 (2024).
Bai, Y. L. et al. Temperature-dependent self-trapped exciton emission in Cu(I) doped zinc-based metal halides from well-resolved excited state structures. Nano Res. 17, 7768–7775 (2024).
Li, M. Z. & Xia, Z. G. Recent progress of zero-dimensional luminescent metal halides. Chem. Soc. Rev. 50, 2626–2662 (2021).
Zhou, L., Liao, J. F. & Kuang, D. B. An overview for zero‐dimensional broadband emissive metal‐halide single crystals. Adv. Optical Mater. 9, 2100544 (2021).
Lv, W. Z. et al. Improving the stability of metal halide perovskite quantum dots by encapsulation. Adv. Mater. 31, 1900682 (2019).
Cao, J. J. et al. Interactions between H2O and lead halide perovskites: recent progress and applications. Matter 7, 3728–3755 (2024).
Quan, M. Z. et al. Structural diversity and photoluminescence enhancement of indium-based hybrid metal halides. J. Alloy. Compd. 972, 172818 (2024).
Li, C. L. et al. Excitation-dependent correlated color temperature manipulation of white-light emission in Sb3+-doped PMA2ZrCl6 single crystals. J. Lumin. 269, 120513 (2024).
Blasse, G. & Grabmaier, B. C. Luminescent Materials. (Berlin, Heidelberg: Springer, 1994).
Zhang, Y. et al. Strong self‐trapped exciton emissions in two‐dimensional Na‐In halide perovskites triggered by antimony doping. Angew. Chem. Int. Ed. 60, 7587–7592 (2021).
Zhou, L. et al. A highly red‐emissive lead‐free indium‐based perovskite single crystal for sensitive water detection. Angew. Chem. Int. Ed. 58, 5277–5281 (2019).
Tan, Z. F. et al. Highly efficient blue‐emitting Bi‐doped Cs2SnCl6 perovskite variant: photoluminescence induced by impurity doping. Adv. Funct. Mater. 28, 1801131 (2018).
Luo, J. J. et al. Efficient and stable emission of warm-white light from lead-free halide double perovskites. Nature 563, 541–545 (2018).
Meng, X. et al. Organic–inorganic hybrid cuprous‐based metal halides for warm white light‐emitting diodes. Adv. Sci. 9, 2203596 (2022).
Zhang, X. T. et al. Bright orange electroluminescence from lead-free two-dimensional perovskites. ACS Energy Lett. 4, 242–248 (2019).
Fronzi, M. & Nolan, M. First-principles analysis of the stability of water on oxidised and reduced CuO(111) surfaces. RSC Adv. 7, 56721–56731 (2017).
Hei, X. Z. & Li, J. All-in-one: a new approach toward robust and solution-processable copper halide hybrid semiconductors by integrating covalent, coordinate and ionic bonds in their structures. Chem. Sci. 12, 3805–3817 (2021).
Acknowledgements
This work is supported by the National Natural Science Foundation of China (Nos. 22135008, 22275188, U22A20398, 12104455), Natural Science Foundation of Fujian Province (Nos. 2023I0032, 2022J05092). The authors also thank Dr Feng Xu (Fujian University of Technology) for his helpful discussions on single-crystal structure analysis.
Author information
Authors and Affiliations
Contributions
C.L. Li, L.P. Wang and D.T. Tu conceived the intial concept. C.L. Li and L.P. Wang prepared the sample. C.L. Li, L.P. Wang and X.Y. Shang processed the data. C.L. Li wrote the paper. L.P. Wang, D.T. Tu and X.Y. Chen supervised the work and revised the paper. All authors discussed and edited the paper.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Supplementary information
41377_2025_1910_MOESM1_ESM.pdf
Supplementary Information for Luminescence Lifetime Thermometers Based on Hybrid Cuprous Halides with Exceptional Water Resistance and Giant Thermal Expansion
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, C., Wang, L., Tu, D. et al. Luminescence lifetime thermometers based on hybrid cuprous halides with exceptional water resistance and giant thermal expansion. Light Sci Appl 14, 224 (2025). https://doi.org/10.1038/s41377-025-01910-1
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41377-025-01910-1
This article is cited by
-
Defect and crystal field engineering enables high efficiency and anti-thermal quenching in Cr3+ doped rigid garnet phosphors for multifunctional optoelectronics
Science China Materials (2026)
-
Hybrid cuprous halides enable high-sensitivity luminescence lifetime thermometry with exceptional water resistance
Light: Science & Applications (2025)