Abstract
Ultrasonic power and data transfer is a promising technology for implantable medical devices because of its non-invasiveness, deep penetration depth, and potential for a high-power transmission rate with a low specific absorption rate. However, ultrasound-powered implantable devices still suffer from low power transfer efficiency due to beam misalignment and are limited to short-term use due to the bulkiness of the transmitting transducers. Here, we report the first proof of concept for adaptive positioning and targeting of ultrasound-based implantable devices through ultrasound image guidance. A lightweight patch-type ultrasonic transducer array is fabricated to enable ultrasound imaging and beam-forming during long-term operation. The uniform performance of the array is established through the silicon micromachining process. We demonstrate the complete scheme of imaging, positioning, and targeted power transfer in an ex vivo environment, achieving precise targeting of moving implanted devices through real-time ultrasound imaging. Enhanced power transfer efficiency through the use of patch-type ultrasonic transducers can enhance patient comfort and minimize invasive procedures, opening new applications for ultrasonic-powered implantable devices.

Similar content being viewed by others
Introduction
Implantable medical devices (IMDs) are a promising technology designed to support, monitor, and restore critical functions of human organs. By placing the device directly at the targeted tissue interface, IMDs enable precise and controlled therapy1,2,3. For patients with complex regional pain syndromes (CRPS), an implantable electrical stimulator can effectively modulate spinal cord nerves to help control chronic pain4. Another condition requiring continuous monitoring and real-time treatment is arrhythmia, which is managed by implanting an artificial pacemaker in the heart. Artificially generated electrical signals are applied to the myocardium to induce regular heart contractions, maintain a normal heart rhythm, and support effective blood pumping4,5. In addition to stimulation, IMDs are used for continuous monitoring of physiological signals that are challenging to measure from outside, such as bladder pressure6. Now widely used in clinical practice, IMDs are essential in sustaining the lives of patients in need by providing continuous monitoring and targeted stimulation from within the body6,7.
Although most IMDs are designed to consume low power (typically less than several hundred μW), high-capacity batteries commonly used in IMDs still have limited lifespans, necessitating additional surgeries for battery replacement8,9. To overcome the need for re-surgery, recharging batteries via wireless power transmission (WPT) has emerged as a potential solution for continuously powering the implanted devices. Two widely adopted WPT methods are inductive power transfer and capacitive power transfer. While capacitive coupling allows high-power transmission above 100 mW without requiring precise alignment, the high specific absorption rate of the electric field in the human body limits its penetration depth (<1 cm). In contrast, inductive power transfer that delivers in the range of 20 mW enables power transmission at greater depths (over several tens of millimeters), though the inductive method requires precise alignment between the transmission and reception devices.
A new modality is required to deliver power to deeper structures with high efficiency, which is essential in monitoring signals from deep organs such as gastric waves and cardiac functions. Due to its long penetration depth and well-established safety profile, ultrasound has emerged as a promising modality for transferring power and data to devices that are implanted in deep tissues10. Ultrasound has achieved high-power transmission efficiency (above several hundred μW), which is sufficient to operate commercial pacemakers11. In addition, since the specific absorption rate of ultrasound is much smaller than that of electromagnetic waves, the penetration depth of ultrasound is in the order of several centimeters, extending its use beyond subdermal applications12. Despite the promise, the ultrasonic transfer method also faces challenges. Power transfer efficiency highly depends on the alignment between the ultrasound beam and the device13. Device placement is also constrained by ultrasound attenuation in various structures, such as surrounding tissues and bones14. Furthermore, long-term and continuous operation remains challenging as most ultrasonic transducers are designed as handheld probes, which require another person to hold the devices during the power and data transfer.
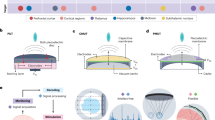
Here, we present long-term ultrasound power transfer with adaptive alignment of the ultrasound beam through the development of the patch-type ultrasonic transducer array (Fig. 1a). While the position of IMDs is continuously monitored through ultrasound imaging, a focused beam of ultrasound for power transmission could be adaptively modulated to target the device correctly15. Several studies have demonstrated power and signal transfer using ultrasonic localization. However, most have relied on bulk transducers for ultrasound transmission (Table S1). Conventional handheld transducers often face challenges such as displacement from the skin surface during motion, leading to difficulties in maintaining ultrasound imaging and power delivery over long-term operation. To overcome these limitations and enable long-term operation, we developed an attachable patch composed of a 1D capacitive micromachined ultrasonic transducer (CMUT) array with sufficient acoustic performance to achieve both ultrasound imaging and beam-forming to monitor and power an IMD. Empowered by its wide bandwidth and compact packaging without a backing layer, CMUT is a suitable choice for the patch-type ultrasound transducers compared to the other transducer types (Table. S2). Adaptively targeted power transfer was demonstrated in an ex vivo environment using a chicken breast phantom, exhibiting a power level of 166 nW. In addition to the adaptive ultrasound power transfer, we demonstrated the first proof of concept of the long-term, adaptive targeting of power transfer via ultrasound. Enhanced patient comfort and improved power transfer efficiency using patch-type ultrasonic transducers open up new applications of ultrasonic-powered implantable devices for long-range and long-term monitoring.
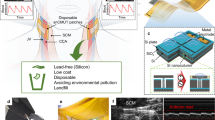
Overview of adaptive ultrasonic power and data transfer using a patch-type CMUT array a Application scenario of focused acoustic power transfer using a conventional handheld ultrasound transducer and a patch-type CMUT. b Photograph of the patch-type CMUT array attached to a human hand. c A conceptual schematic showing the components of the patch-type CMUT array. d k-Wave simulation of a focused ultrasound beam generated from a 1D array composed of different aperture sizes. e The −6 dB beam width of simulated beams measured at a depth of 10 cm
Results and discussion
Design of patch-type CMUTs
We developed a patch composed of a rigid silicon-based CMUT array (Fig. 1b). The patch-type package that can be readily attached to the skin was devised to allow long-term operation. Two key components of patch-type ultrasonic transducers are the transducer array itself and the coupling medium between the transducer and the skin. While flexible ultrasonic transducers adhere conformally to skin and require no coupling layer, they are prone to inevitable beam misalignment during body movements16,17. In addition, patch-type ultrasound transducers do not experience significant curvature changes since most targets are soft tissues with smooth curvature, such as the abdominal and thoracic cavities. Thus, we chose a rigid transducer to avoid misalignment and provide stable imaging. A suitable coupling medium must be free of air to minimize the impedance mismatch, exhibit low ultrasound attenuation to maximize the transmission, and exhibit adhesive characteristics18. We used the calcium (Ca+)-modified silk adhesive as the coupling layer, which offers high adhesive characteristics and high ultrasound transmission (Fig. 1c)19. The hydrophilic silk patch absorbs moisture from the skin surface and fills the cavity between the transducer and skin wrinkles. To prevent an electrical short between the electrode on the transducer and the silk patch, a thin PDMS layer was coated on the surface of the transducer as an encapsulation layer. Considering the thickness, acoustic impedance, and attenuation rate, the transmission signal degradation of 23.17 dB was estimated (Fig. S1 and Table S3). Lastly, the transducer was implemented in a 1D array configuration to enable adaptive tracking of an IMD location through ultrasound B-mode imaging.
Since the aperture size is inversely proportional to the beam width at the focus, a larger aperture is beneficial for ultrasonic power and signal transfer applications (Fig. S2). For the rigid transducer, the aperture size should be sufficiently small to withstand the movement of the human body while sufficiently large to allow long imaging depth. To investigate the optimal size, k-Wave simulation was conducted for three aperture sizes (4.8, 9.6, and 14.4 mm) of 1D ultrasound arrays composed of 16, 32, and 48 channels (Fig. 1d). Since the chest depth of male humans ranges from 20 cm to 30 cm20,21, we obtained the lateral resolution at 10 cm, close to the midway of a typical upper torso. The resolution was characterized by obtaining −6 dB full width at half maximum (FWHM) on the pressure field22. Since the lateral resolutions of 32 and 48 channels at 10 cm (3.12 and 2.12 mm, respectively) were significantly higher than that of 16 channels but comparable to each other, we chose the design of 32 channels to minimize the susceptibility to body movements (Fig. 1e).
Characterization of packaged 1D CMUT arrays
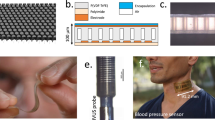
We designed and fabricated 1D CMUT arrays with 32 channels for ultrasound imaging using the standard CMUT fabrication process based on the wafer-bonding method (Fig. S3). The 32 channels were arranged in a 1D linear configuration with a pitch of 300 μm. Each element was composed of 336 circular cells with a membrane radius of 18 μm and a thickness of 1.9 μm, which was suspended on a vacuum cavity with a gap height of 120 nm and 120-nm-thick silicon dioxide (SiO2) insulation layer (Fig. S4). The cross-sectional scanning electron microscope (SEM) images (S-4800, Hitachi, Japan) of a single cell confirmed that the structural dimensions of the fabricated CMUTs matched well with the design (Fig. 2a and Fig. S5). A clear deflection of the membranes was observed using a 3D optical profiler (ContourGT, Brucker, USA), confirming the successful electrostatic actuation of the CMUT membrane (Fig. 2b). To analyze the deflection range of a single cell, the maximum static deflection was measured as a function of DC bias voltage using an optical profiler (Fig. S6). The maximum applicable voltage was determined by the breakdown voltage of the insulator. At 95 V, the membrane exhibited a maximum deflection of approximately 24.73 nm, which corresponds to 20.6% of the gap height. The fabricated CMUT was packaged into a 4-mm-thick patch where the size of the contact area with the skin was 13.0 mm by 11.5 mm (Fig. 2c).
Characterization of the fabricated CMUTs a A cross-sectional SEM image of a single CMUT cell. Scale bar = 1 μm. b A 3D optical profile of the deflections of circular cells in a single element of CMUT. c A photograph of the packaged CMUT patch. d Electrical input impedance of a single channel of the CMUT array. e 6-element modeling of electrical impedance at a bias voltage of 70 V. f Uniformity of center frequencies of 32 channels in air. g Pitch-catch impulse response of 70 V-biased single CMUT channel. h Transmit and receive sensitivity of a single channel. i Transmit pressures before and after the encapsulation
The electrical characteristics of the 1D CMUT array were measured to confirm its operation in air. A clear spring softening effect where resonant frequency decreased at higher DC bias voltages was observed (Fig. 2d). The electrical impedance of CMUT in air was modeled using a six-element Butterworth-Van Dyke (BVD) model, which consists of series contact impedances (Rs, Cs), mechanical impedance components (Rx, Lx, Cx), and electrical capacitance of the device (C0)23. The values of Rs, Cs, Rx, Lx, Cx, and C0 were 2.14 kΩ, 70.59 pF, 334.8 Ω, 14.85 mH, 843.1 fF, and 43.39 pF, respectively (Fig. 2e). The electromechanical coupling coefficient (kT2), which indicates conversion efficiency between electrical and acoustic energy, was estimated to be 2.6 × 10−3. kT2 was derived using the following equation where the capacitance of a single CMUT element was measured in an oil-immersed environment (Fig. S7) 24.
Uniform distribution of resonant frequencies with a center frequency of 14.7 MHz in the air was observed across all channels with a standard deviation of 85.2 kHz (Fig. 2f). Finally, we characterized the acoustic characteristics of a single channel, including impulse response, transmit sensitivity, and receive sensitivity. A pitch-catch measurement showed that the center frequency and the −6 dB fractional bandwidth of the single channel were 8.86 MHz in water and 109%, respectively (Fig. 2g). At a fixed bias voltage of 70 V, transmit sensitivity was about 528 Pa/V for AC voltage while the receive sensitivity was 13 μV/kPa (Fig. 2h). The effect of the encapsulation material on the acoustic transmission was also evaluated (Fig. 2i). After the encapsulation, the pressure was decreased to 45.2% of the initial pressure.
B-mode imaging and targeted ultrasound transmission
Based on the successful pitch-catch measurement, the imaging performance of the patch-type 1D CMUT array was evaluated using a custom-built string phantom (Fig. 3a). We conducted a linear B-mode imaging to obtain point responses from the string phantom located at a depth of 1 cm, 2 cm, and 3 cm (Fig. 3b). The axial and lateral resolution and signal-to-noise ratio at different depths was measured. As the FWHM of the point spread function is known to be the resolution of the point25, the resolution was determined from the point spread function of the string by depth. The FWHM of axial and lateral direction for 3 cm depth was 4.26 mm and 2.26 mm, respectively, which indicated sufficient image resolution to locate the position since most IMDs should be larger than 2.26 mm (Fig. 3c)26. In addition, a stable signal-to-noise ratio above 16.76 dB was maintained up to 3 cm (Fig. 3d). To further support the data, a simulation was conducted using Field II to analyze the relationship between the peak intensity of the point spread function and detection distance (Fig. S8). The simulation assumed a homogeneous environment with an attenuation coefficient of 1.43 dB/cm/MHz, which corresponds to the attenuation rate of the human chest27. According to the results, the peak intensity decreases beyond 4 cm. Since the aperture size is limited for patch-type transducers, phased array operation is beneficial in order to achieve a wider region of interest (ROI). Although we initially adopted linear operation for higher resolution and sensitivity, additional analysis of phased array operation was conducted using Field II simulation. A simulated transducer array with the same specifications as the proposed design was implemented in the simulation field. A single-wire phantom was placed at a depth of 4 cm, and steering angles ranged from −40° to 40° (Fig. S9). The dynamic range was set to 60 dB, and the scan lines varied up to 128. Within the extended ROI, the resolution of the wire phantom remained within 3 mm from −18° to 18°, which is sufficient for detecting breast cancer lesions 28.
Ultrasound imaging, beam steering, and data transfer using the patch-type CMUT a Profile of the string phantom. b B-mode images of the string phantom located at the depths of 1 cm, 2 cm, and 3 cm. c Lateral and axial resolution of the point spread function at different depths. d Signal-to-noise of the point spread function at different depths. e Beam profiles of the 1D CMUT array during beam steering. f Impulse response of the 1D CMUT array, which shows the bit-rate of the system. g Acoustic pressure measured at the focal point at 1 cm depth. h Transmitted ultrasound signals received by the hydrophone
To evaluate the performance of targeted ultrasound transmission for both power and data transfer, dynamically focused beams from the patch-type devices were analyzed through acoustic beam profile measurement. The time delay was applied to each channel to form a focus at the 1 cm depth. Beam profile measurements confirm successful steering from +10° to −10° in 5° increments with focus at 1 cm axially away from the surface of the transducer (Fig. 3e). The characteristics of the ultrasound transmission for power transfer were evaluated by analyzing the impulse response of the transducer. The center frequency of 8.61 MHz was observed, which decides the operational frequency for power transfer (Fig. 3f). The ability of the power transmission was also evaluated by measuring the maximum pressure at the focal point (Fig. 3g). The pressure level was measured as 65.3 kPa on the focus with a center frequency of 8.61 MHz, which implies the ultrasonic intensity of 156 mW/cm2. Actual power received by the IMD is determined by the area of the energy-harvesting component of the device, which is a product of the intensity and the area 29.
In a typical communication system utilizing pulsed signal transmission, the width of a single pulse or the bandwidth determines the bit-rate of the system30. The impulse response of a single channel showed a wide −3 dB fractional bandwidth of 85.7% without any additional backing layer (Fig. 3f)31,32. As the single pulse width exhibits a single-bit transmission period, the bit-rate was calculated as a reciprocal of the pulse width. The width of the single pulse was demonstrated to be 142 ns, which indicates a bit-rate of 7.04 MHz. The wide fractional bandwidth implies the ability to transmit ultrasound in a broad operating frequency range. Adaptive control of the operating frequency, tailored to specific applications, is achievable with systems offering a wide frequency range. For instance, if the device is implanted near highly attenuative tissue, the center frequency for signal transmission can be adjusted to a lower frequency, which exhibits reduced attenuation. The penetration depth can also be adjusted by modifying the frequency, allowing systems with wide operational frequency ranges to cover a broader region of interest. Thus, since the attenuation rate is a strong function of the transmission signal frequency, the signal transmission can be adaptively controlled based on the attenuation characteristics of the target tissue around the implanted device33. We demonstrated the ability to transmit signals over the wide bandwidth of the device by using our patch to transmit signals with a carrier frequency from 6 MHz to 13 MHz (Fig. 3h). To transmit signals with ultrasound, focused targeted transmission of the signal is necessary to achieve a high bit-rate. The transferred impulse signal from the focus and off-focused points was compared to justify the significance of targeted transmission (Fig. S10). Due to the mismatch of time of flight, the bandwidth of the received signal from the off-focused point was decreased to 3.29 MHz.
Long-term and mechanical stability
We evaluated both the long-term and mechanical stability of the patch-type CMUTs to validate their ability to support reliable long-term and continuous operation. First, the stability of the coupling layer was evaluated by measuring the FWHM of the pressure field focused at 2 cm depth. The long-term stability of silk gel compared to the conventional ultrasonic gel was observed after 12 h (Fig. S11). After 12 h, the FWHM of the patch-type CMUTs with silk coupling material was within the error of 0.27%, which maintained a constant level of performance compared to the ultrasonic gel (error of 4.6%). After confirming the long-term use of a coupling medium, a focused ultrasound beam profile was measured for three sessions with an interval of 12 h to monitor the long-term stability of beam-forming. During the total measurement sessions, the device was attached through the silk adhesive coupling layer to the 2-mm-thick polymethylpentene (TPX) plate, which is known to exhibit similar acoustic impedance to human tissue34. The focused ultrasound beam pattern was successfully maintained throughout the whole session; the FWHM was maintained within an average and standard deviation of 598 μm and 57.6 μm, respectively (Fig. 4a, b). In addition, B-mode ultrasound imaging was conducted at 12-h intervals throughout the day to evaluate the long-term stability of imaging performance (Fig. 4c). The point spread function of the string phantom at 2 cm depth was observed throughout the total session. Lateral and axial resolutions below 3.1 mm and 0.98 mm, respectively, were observed over 24 h (Fig. 4d). The resolution of the ultrasonic image degraded after 12 h due to electrode damage caused by humidity-induced swelling of the PDMS layer.
Imaging stability of the patch-type CMUT a Long-term stability of focused ultrasound beam at 12-hour intervals over one day. b The −3 dB full width at half maximum measured at a depth of 2 cm over one day. c Long-term B-mode imaging of the IMD at 12-hour intervals over one day. d Lateral and axial resolution of the point spread function at 2 cm depth over one day
As the proposed patch is designed to adhere to the skin, it must endure the potential strain caused by skin movement and deformation (Fig. S12). To evaluate the mechanical durability of the device, a specially designed phantom was constructed with gelatin. The CMUT patch was attached to the top of the phantom, and the strain was applied in three orthogonal directions: along the roll, pitch, and yaw axes, twisting along the axial direction. The signal-to-noise ratio of the point spread function for roll, pitch, and yaw axes was decreased by 2.56 dB, 1.91 dB, and 0.84 dB, respectively (Fig. S13).
Ex vivo targeted power transfer assisted by continuous image monitoring
To validate the concept of adaptive power transfer, an ultrasound imaging session followed by the power transmission was conducted on the implanted devices on the chicken breast phantom (Fig. 5a). As a piezoelectric disk was inserted on the 1 cm depth of the chicken breast, the patch-type CMUT was attached to the top of the tissue to mimic the wearable applications. The electrical impedance of the inserted piezoelectric disk transducer was analyzed to obtain the center frequency and equivalent circuit model (Fig. 5b, c). The equivalent circuit model was analyzed using the Butterworth-Van Dyke (BVD) model including the parallel parasitic components35, which were attributed to the parallel interconnection from the soldered regions on the PZT. The resonant frequency of the piezoelectric transducer was 10.8 MHz, while C0, Rx, Lx, and Cx were 31.8 pF, 13.1 Ω, 2.74 μH, and 68.9 pF, respectively (Fig. 5c). Equivalent series components were added to the model to adequately describe the parasitic components of the piezoelectric transducer. LESL, RESR, CESC, and REPR were 511 nH, 1.6 Ω, 1.10 nF, and 733 Ω, respectively. Since the piezoelectric transducer was directly connected to the oscilloscope, the equivalent circuit model consisted of the piezoelectric transducer connected in parallel to the parasitic capacitance (Cp) and the input resistance (Rp) of the oscilloscope, which was 13 pF and 50 Ω, respectively (Fig. 5b).
Ex vivo power and data transfer using the patch-type CMUT on the chicken breast a A schematic diagram of ultrasound-guided power transmission. b Equivalent circuit modeling of the proposed ultrasound system. c Electrical input impedance of the implanted piezoelectric disk that is used to receive ultrasound signal. d Ultrasound B-mode image of the implanted piezoelectric device. e Transmitted waveform and received signals for power transfer. f Bit-coded ultrasound signal received by the implanted piezoelectric device for data transfer
The encapsulated piezoelectric disk inside the chicken breast was identified through the ultrasound B-mode imaging session (Fig. 5d). Then, the focused ultrasound beam was transmitted along the scanline of B-mode imaging with a focus at 1 cm depth. To evaluate the power transmission rate, the patch-type CMUT biased at 70 V was driven by a waveform consisting of 15 cycles of a square wave at the center frequency of the transducer (10.8 MHz) (Fig. 5e), and the transmitted signal was received by the embedded piezoelectric transducer. The total induced power through this ultrasound transmission was calculated based on the measured voltage signals and using the equivalent circuit model. The measured root mean square (RMS) of the voltage signal was 2.76 mV, and the power was calculated by dividing the real part of the impedance by the square of the RMS voltage. As the total impedance of the system, including the oscilloscope, was 33.67–20i Ω, the average received power for the total system was calculated to be 166 nW. Lastly, the data transmission was demonstrated by transmitting 7 cycles of square signals at a carrier frequency of 10.8 MHz. The 7 cycles were utilized as a single-bit, and thus a coded signal of ‘101’ was successfully transmitted to the targeted site (Fig. 5f).
Discussion
In this work, a concept of ultrasound-guided powering of ultrasound-based implantable devices was demonstrated with a miniaturized patch-type ultrasonic transducer. To enable long-term operation for monitoring and powering an IMD, a patch-type device was designed and fabricated. By utilizing CMUT as the ultrasound transducer, the requirements for additional layers, such as backing and matching layers, were eliminated, resulting in the lightweight form factor. The high-yield wafer-scale fabrication enabled uniform distribution of the micromachined transducer array and thus stable performance across the elements. Image-guiding performance was demonstrated by conducting B-mode imaging of a string phantom with a high acoustic impedance, which simulated IMDs constructed with materials with high acoustic impedance36. Sufficient beam-forming performance, steering performance, and suitable power were demonstrated with patch-type CMUTs. The CMUT patch demonstrated stable transmission and reception performance over the long-term sessions, which supports its potential to be used for long-term monitoring of deep tissue via implantable devices. In addition, stable imaging of a phantom was also observed using the CMUT patch under strains at various directions, which emulated the atypical movement of skin underneath the device.
While we demonstrated the proof of concept of using a wearable ultrasound patch to achieve both power and data transmission through accurate alignment using B-mode imaging, there remains room for several improvements. First, the CMUT can be further optimized for imaging performance. Even if CMUT is not the best choice for high transmission, there are possible methods to improve its performance to better suit the application. If a CMUT is developed for transmission applications, a higher Q-factor of the structure is preferred to generate high-power transmission, which leads to a higher spring constant and membrane thickness37. A thick vacuum gap is also preferred for transmission applications since the maximum displacements of the device are limited by the gap height. However, since the receive sensitivity of the CMUT is inversely proportional to the effective gap height, CMUT designs to achieve high-power transmission are unfavorable for ultrasound imaging applications that also require excellent receive sensitivity38. In this work, since the effective gap height was higher than that of the previous CMUTs for the imaging application (Table S4), the imaging sensitivity of the transducer was not optimal, and the detection objects were limited to highly reflective objects such as metal wires. Another approach is to use separate elements in the array for transmission and reception39. Each element can be optimized for transmission and reception, which increases the efficiency of the overall system.
If the sensitivity is further improved, complex imaging schemes can be used for the patch-type CMUTs. In this work, we use the CMUTs for linear B-mode imaging. If the sensitivity is improved, the transducer can be used as a sector probe, which increases the field of view with the limited aperture size40, enhancing the advantage of wearable devices with a compact form factor. Furthermore, since ultrasound imaging facilitates distinguishing slight differences between flexible materials via quantitative imaging, the same system can also monitor IMDs with flexible materials.
Materials and methods
Fabrication of 1D capacitive micromachined ultrasonic transducer array
1D CMUT arrays were fabricated using the conventional wafer-bonding process (Fig. S3)41. A 4-inch silicon-on-insulator (SOI) wafer with a 1.9-μm-thick silicon device layer was oxidized using a thermal wet oxidation process to form a 120-nm-thick silicon oxide layer. Next, circular cavities with a radius of 18 μm were patterned through the photolithography process (MA/BA6 Gen4, SÜSS MicroTec, Germany) and wet etching using buffered-oxide-etch (BOE) 6:1 solution at room temperature. Another highly conductive silicon wafer was thermally oxidized to form a 120-nm-thick SiO2 layer, which served as an insulation layer inside the cavity to prevent breakdown. After cleaning two wafers with piranha solution (3:1 (v/v) mixture of 98% of H2SO4 and 34% of H2O2 (aq)) at 70 °C for 10 min, the surface of two wafers was hydrophilized with the RCA1 process for 10 min. The RCA1 solution was prepared by a 5:1:1 (v/v) mixture of deionized water, 34% ammonium hydroxide (NH4OH) (aq) solution, and 34% hydrogen peroxide (H2O2) (aq) at 70 °C. Immediately after the cleaning process, two wafers were directly bonded in a high vacuum chamber under 3 × 10−5 mbar (SB8E, SÜSS MicroTec, Germany), followed by 4 h of annealing at 1050 °C in N2 atmosphere to achieve permanent covalent bonding at the SiO2-SiO2 interface.
After the bonding, the handle layer of the SOI wafer was removed through a Si chemical mechanical polishing (CMP), followed by silicon wet etching with 25% of tetramethylammonium hydroxide (aq) solution at 65 °C. After removing the buried-oxide layer with 49% hydrogen fluoride (HF) (aq) solution, the exposed silicon membrane was isolated by a reactive ion etching (RIE) to prevent crosstalk between adjacent elements. The oxide layer was patterned and opened using RIE to access the heavily boron-doped substrate, which is used as shared bottom electrodes for the CMUT array. Top and bottom electrodes were formed by depositing and wet etching a 200-nm-thick gold layer with an adhesion layer of a 10-nm-thick chrome film. Finally, each array was isolated using a saw-dicing process.
Patch packaging
A rigid-flexible printed circuit board was custom-designed and manufactured to provide an electrical connection to a CMUT die. A rigid rectangular stiffener board about 11 mm by 10 mm was applied to the backside of the CMUT die to achieve successful wire bonding. After attaching the CMUT die to the rigid-flexible printed circuit board, every channel (top electrodes) and ground electrodes were wire bonded using a 75 μm thick gold wire (TPT HB05, TPT Wire Bonder GmbH & Co. KG, Germany). After molding the wires with epoxy resin, the whole structure of CMUT was encapsulated by PDMS (Sylgard 184, Dow Inc., USA). Using the epoxy to form a dam around the device, the mixture of PDMS and curing agent with a ratio of 10:1 (w/w) was drop cast on the upper side of CMUT and cured in a 60 °C oven overnight (Fig. 1c). To enhance ultrasound transmission between the packaged CMUT and skin, a Ca+-modified silk fibroin adhesive layer was applied. The silk patch was prepared following the method of previously reported work19. Finally, a 50 mm by 50 mm polyethylene film for medical use (Tegaderm, 3 M, USA) was placed over the patch-type CMUT to strengthen the attachment of the patch to the skin.
Input impedance characterization
The input impedance of the CMUT array was measured through an impedance analyzer in air (E4990A, Keysight Technologies, USA). The bias voltage from 13.5 V to 77.0 V was applied to each channel. The resonant frequency of each channel was extracted to validate the uniformity of channels in the array.
Acoustic characterization
Impulse response, transmit sensitivity, and ultrasound beam profile were measured to characterize and evaluate the acoustic properties of the developed patch-type CMUTs. The impulse response was measured by the one-way method using a needle hydrophone (NHO500, Precision Acoustics, United Kingdom). The patch-type CMUT was positioned in the soybean oil tank at a distance of 10 mm from the observing point. A single channel of the array was driven by a 10 V unipolar pulse signal with 50 ns in width. The frequency responses were obtained by performing a fast Fourier transform of the measured impulse transient signal.
Using the same setup, the transmit sensitivity of a single channel was observed. Three cycles of 8.61 MHz sine signal were applied to the device, and the acoustic pressure was measured using the needle hydrophone. The CMUT element was biased at 70 V DC bias voltage superimposed with an AC peak-peak voltage increasing from 1 V to 10 V through a bias-T structure to characterize the transmit sensitivity. The voltage signal from the hydrophone was amplified through a preamplifier and a DC coupler and measured using an oscilloscope. Acoustic pressure was converted from the obtained voltage signal based on the pre-calculated sensitivity of the hydrophone. The focused ultrasound beam profile was measured by scanning the needle hydrophone over a 12-mm-by-15-mm plane. 1.5 cycles of pulses at a center frequency were transmitted from 32 channels where each channel was driven with a phase delay to form a focus at 2 cm depth. Acoustic intensity was calculated using the following equation, with a density of 0.9175 g/cm³ and a speed of sound of 1483.4 m/s, respectively 42,43,44.
The receive sensitivity of the transducer was measured in a pitch-catch setup. The CMUT array was immersed in the oil chamber to transmit from a channel and receive from a neighboring channel. The oil-air interface served as a reflector, which was 10 mm away from the transducer surface. A single channel of the transducer was driven by the 3-cycle sine wave at the center frequency of the device with an AC voltage sweep from 1 V to 10 V, and the reflected signal was received by an adjacent channel. The received signal was amplified through a low-noise voltage amplifier (LNA-HF, Ciprian, France) with a gain of 60 dB.
B-mode ultrasound imaging
B-mode ultrasound imaging was conducted on the custom-built string phantom using the patch-type CMUT. A Ca+ -modified silk patch was applied between the device and the surface of the phantom to attach the CMUT device to the top of the phantom. Film dressing (Tegaderm, 3 M Company, USA) was attached to the top to support the whole structure. The custom-built string phantom consisted of a gelatin block and aluminum wires. 150 ml of deionized water was applied to the 45 g of gelatine powder, and the mixture was heated to 80 °C on the hotplate to completely dissolve the gelatine. The gelatine solution was stirred a rate of 250 rpm for 2 h to degas the bubbles completely. A 5 cm plastic cubic mold with holes on the side walls was 3D printed with a stereolithography printer (Form 3, Formlabs, USA). 100-μm-thick copper wires were inserted at depths of 1, 2, and 3 cm of the cube. Next, the gelatine solution was poured into the mold. The phantom mold was stored at 30 °C and 60% relative humidity in a thermo-hydrostat (TH-ME-025, JEIO TECH, South Korea).
B-mode linear imaging of the string phantom with a depth of 5 cm was conducted using the diagnostic ultrasound system (Biopson, Barreleye, Korea). The system was specially modified to allow the application of a DC bias voltage (70 V) to CMUTs. To evaluate ultrasound imaging performance, the point spread function of the strings at each depth was extracted from the linear B-mode image. The brightness of the point image for axial and lateral directions was measured and compared against the noise level.
Long-term imaging test
Using the same imaging setup, B-mode imaging sessions were conducted over one day for every 12 h. The patch attached to the phantom was stored at 30 °C and 60% relative humidity inside the thermo-hydrostat. For every session, the full width at half maximum of the axial and lateral resolution was calculated from the point spread function of the string phantom at 1 cm depth to evaluate the long-term operation of the patch-type CMUT. The stability of the ultrasound beam was also measured by obtaining a beam profile every 12 h over one day. The single CMUT channel was driven under the same conditions as the conditions used for B-mode imaging.
Imaging using CMUTs under strain
To emulate the wearable environment where stress is applied to the device due to the body movement, the patch-type CMUT was attached to another gelatine phantom (5 cm wide, 4 cm tall, and 1 cm thick) with a wire placed at 1 cm depth. The phantom was bent in three orthogonal directions and twisted back and forth. The point spread function was obtained from the B-mode image to compare the signal-to-noise ratio for each case.
Ex vivo experiment setup
With the aforementioned setup of phantom imaging, the patch-type CMUT was attached to the surface of the chicken breast phantom. A PZT die with a thickness of 0.2 mm was coated with silicon elastomer (KWIK-SILTM silicone adhesive, WPI, USA) and implanted at a depth of 1 cm depth within the chicken breast phantom. A pair of wires from the PZT dice was directly connected to an oscilloscope (DSOX2022A, Keysight Technologies, USA) to read out the voltage signal induced by transmitted ultrasound.
References
Chappel, E. In Drug Delivery Devices and Therapeutic Systems. 129–156 (Elsevier, 2021).
Faro Barros, J., Sahraoui, P. F., Kalia, Y. N. & Lapteva, M. In Targeted Drug Delivery, 349–387 (Wiley, 2022).
Afari, M. E., Syed, W. & Tsao, L. Implantable devices for heart failure monitoring and therapy. Heart Fail. Rev. 23, 935–944 (2018).
Fatima, K. et al. Long-term efficacy of spinal cord stimulation for chronic primary neuropathic pain in the contemporary era: a systematic review and meta-analysis. J. Neurosurg. Sci. 68, 128–139 (2023).
Feiner, R. & Dvir, T. A ray of light for treating cardiac conduction disorders. Proc. Natl Acad. Sci. USA 116, 347–349 (2019).
McShane, M. J., Zavareh, A. T. & Jeevarathinam, A. S. In Encyclopedia of Sensors and Biosensors: Volume 1-4, First Edition 115-132 (Elsevier, 2022).
Khan, K. et al. Innovations in cardiac implantable electronic devices. Cardiovasc. Drugs Ther. 36, 763–775 (2022).
Salmi‐Belmihoub, S., Frank, R., Bessière, F., Delinière, A. & Chevalier, P. Forty years of longevity for a cardiac pacemaker: What can top that? J. Cardiovasc. Electrophysiol. 31, 3359–3360 (2020).
Zhang, J. et al. Battery‐free and wireless technologies for cardiovascular implantable medical devices. Adv. Mater. Technol. 7, 2101086 (2022).
Miller, D. L. et al. Overview of therapeutic ultrasound applications and safety considerations. J. Ultrasound Med. 31, 623–634 (2012).
Yi, X. et al. Wireless power transmission for implantable medical devices using focused ultrasound and a miniaturized 1-3 piezoelectric composite receiving transducer. IEEE Transactions on Ultrasonics, Ferroelectrics, and Frequency Control 68, 3592–3598 (2021).
Hoang, T., Rosinski, B. & Felix, N. In 2022 IEEE International Ultrasonics Symposium (IUS). 1–3 (IEEE).
Zheng, Y. et al. Enhancing ultrasound power transfer: efficiency, acoustics, and future directions. Adv. Mater. In press, https://doi.org/10.1002/adma.202407395 (2024).
Jaafar, B., Neasham, J. & Degenaar, P. What ultrasound can and cannot do in implantable medical device communications. IEEE Rev. Biomed. Eng. 16, 357–370 (2021).
Meng, M. & Kiani, M. In 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC). 364–367 (IEEE, 2019).
Yu, C. C. et al. A conformable ultrasound patch for cavitation‐enhanced transdermal cosmeceutical delivery. Adv. Mater. 35, 2300066 (2023).
Chen, W. et al. Flexible ultrasound transducer with embedded optical shape sensing fiber for biomedical imaging applications. IEEE Trans. Biomed. Eng. 70, 2841–2851 (2023).
Casarotto, R. A., Adamowski, J. C., Fallopa, F. & Bacanelli, F. Coupling agents in therapeutic ultrasound: acoustic and thermal behavior. Arch. Phys. Med. Rehabil. 85, 162–165 (2004).
Lee, S.-M. et al. Calcium-modified silk patch as a next-generation ultrasound coupling medium. ACS Appl. Mater. Interfaces 13, 55827–55839 (2021).
Borkan, G., Glynn, R., Bachman, S., Bosse, R. & Weiss, S. Relationship between cigarette smoking, chest size and body size in health-screened adult males. Ann. Hum. Biol. 8, 153–160 (1981).
Beall, C. M. A comparison of chest morphology in high altitude Asian and Andean populations. Hum. Biol. 54, 145–163 (1982).
Gohari, H. J. Focusing of Ultrasound Beams. Master's thesis, University of Oslo (1997).
Lee, H. J. et al. A low-noise oscillator based on a multi-membrane CMUT for high sensitivity resonant chemical sensors. Proc IEEE Micr. Elect. 761–764. https://doi.org/10.1109/Memsys.2009.4805494 (2009).
Yaralioglu, G. G., Ergun, A. S., Bayram, B., Haeggström, E. & Khuri-Yakub, B. T. Calculation and measurement of electromechanical coupling coefficient of capacitive micromachined ultrasonic transducers. IEEE T Ultrason Ferr. 50, 449–456 (2003).
Viessmann, O., Eckersley, R., Christensen-Jeffries, K., Tang, M.-X. & Dunsby, C. Acoustic super-resolution with ultrasound and microbubbles. Phys. Med. Biol. 58, 6447 (2013).
Kim, H., Rigo, B., Wong, G., Lee, Y. J. & Yeo, W.-H. Advances in wireless, batteryless, implantable electronics for real-time, continuous physiological monitoring. Nanomicro Lett. 16, 52 (2024).
Patterson, B. & Miller, D. L. Experimental measurements of ultrasound attenuation in human chest wall and assessment of the mechanical index for lung ultrasound. Ultrasound Med. Biol. 46, 1442–1454 (2020).
El Saghir, N. S. & Charara, R. International screening and early detection of breast cancer: resource-sensitive, age- and risk-specific guidelines. Breast Cancer Manag. 3, 397–407 (2014).
Mazzilli, F., Thoppay, P. E., Praplan, V. & Dehollain, C. in 2012 IEEE International Symposium on Circuits and Systems (ISCAS). 2865–2868 (IEEE, 2012).
Svilainis, L., Rodriguez-Martinez, A., Chaziachmetovas, A. & Aleksandrovas, A. Ultrasound transmission spectral compensation using arbitrary position and width pulse sets. IEEE Trans. Instrum. Meas. 67, 1778–1785 (2018).
Hou, C. et al. Optimized backing layers design for high frequency broad bandwidth ultrasonic transducer. IEEE Trans. Biomed. Eng. 69, 475–481 (2021).
Wang, Z. et al. Fabrication of 2-D capacitive micromachined ultrasonic transducer (CMUT) array through silicon wafer bonding. Micromachines 13, 99 (2022).
Rui, D. et al. Measurement and compensation of frequency-dependent attenuation in ultrasonic backscatter signal from cancellous bone. Acta Phys. Sinica 68, 184301–1 (2019).
Chen, P. et al. Acoustic characterization of tissue-mimicking materials for ultrasound perfusion imaging research. Ultrasound Med. Biol. 48, 124–142 (2022).
Shortt, D. J. In Proceedings, Fourth Annual IEEE Applied Power Electronics Conference and Exposition. 136–142 (IEEE, 2014).
Ashter, S. Selection of materials for construction of medical devices. applications of polymers and plastics in medical devices. 45–64 (Elsevier, 2022).
Wygant, I. O., Kupnik, M. & Khuri-Yakub, B. T. In 2016 IEEE International Ultrasonics Symposium (IUS). 1–4 (IEEE, 2016).
Merbeler, F., Wismath, S., Haubold, M., Bretthauer, C. & Kupnik, M. In 2022 IEEE International Ultrasonics Symposium (IUS). 1–4 (IEEE, 2022).
Liu, C., Djuth, F. T., Zhou, Q. & Shung, K. K. Micromachining techniques in developing high-frequency piezoelectric composite ultrasonic array transducers. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 60, 2615–2625 (2013).
Han, Z., Peng, H., Zhao, X., Chen, X. & Lu, P. Sector-scanning 3D ultrasound imaging in frequency domain with 1D array transducer. Ultrasonics 84, 1–8 (2018).
Huang, Y., Ergun, A. S., Haeggstrom, E., Badi, M. H. & Khuri-Yakub, B. T. Fabricating capacitive micromachined ultrasonic transducers with wafer-bonding technology. J. Microelectromechanical Syst. 12, 128–137 (2003).
Oliveira, P., Baesso, R., Alvarenga, A. & Costa-Félix, R. Evaluation of measurement uncertainty of speed of sound in soybean oil J. Phys. Conf. Ser. 012013 (IOP Publishing, 2021).
Velásquez Piñas, J. A. et al. Production and characterization of biodiesel from cotton oil as an alternative energy in substitution of soybean oil. J. Eng. Sci. Technol. Rev. 11, 182–186 (2018).
Tichy, J. Acoustic intensity measurements—a review. In 9th Aeroacoustics Conference. 2310 (ARC, 2012).
Acknowledgements
This work was supported by the Korea Medical Device Development Fund grant funded by the Korea government (the Ministry of Science and ICT, the Ministry of Trade, Industry and Energy, the Ministry of Health & Welfare, Republic of Korea, the Ministry of Food and Drug Safety) (202011B01, RS-2020-KD000007), by the K-Brain Project of the National Research Foundation (NRF) funded by the Korean government (MSIT) (RS-2023-00262568), by a grant of the Korea Dementia Research Project through the Korea Dementia Research Center(KDRC), funded by the Ministry of Health & Welfare and Ministry of Science and ICT, Republic of Korea (RS-2024-00355871), by Nanomedical Devices Development Project of NNFC (1711197701), and by Samsung Electronics.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Oh, C., Kim, YM., Lee, T. et al. Patch-type capacitive micromachined ultrasonic transducer for ultrasonic power and data transfer. Microsyst Nanoeng 11, 124 (2025). https://doi.org/10.1038/s41378-025-00967-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41378-025-00967-7