Abstract
Neural organoids are emerging as promising in vitro models, offering a unique platform to partially recapitulate the structural and functional complexity of the human nervous system. These three-dimensional (3D) constructs, which mimic key aspects of organ architecture, can be reliably derived from pluripotent stem cells (iPSCs) or embryonic stem cells (ESCs). Their ability to faithfully model neural development and disease pathogenesis has positioned them as indispensable tools in neuroscience research. However, to further unleash their potential, there is a pressing need for long-term and stable monitoring of their dynamic functions in a 3D context. This review provides a brief overview on diverse types of neural organoids and their induction protocols. We further highlight recent advancements in bioelectronic interfaces and sensors tailored for 3D culture. Finally, we discuss future directions aimed at advanced methodologies for real-time, multidimensional functional analysis, ultimately paving the way for breakthroughs in understanding neural development and pathology.

Similar content being viewed by others
Introduction
With the gradual progress of biomedical research and tissue engineering, animal models and 2D-cell models have emerged as powerful tools for exploring the development and pathology of the human nervous system. Nonetheless, the inherent species discrepancies between animal models and human beings, coupled with the limitations of simplicity in 2D human cell cultures, have cast doubt on the validity of these models1,2. To address these concerns and accurately replicate the intricacies of the human neural system, the advent of organoids, which are 3D self-organized structures derived from iPSCs and ESCs, has brought a new perspective3,4. The neural organoids by far have been established vary a lot in types, including specific areas of brain5,6,7,8, spinal cord9, retina10, inner ear11 and so on. Compared to conventional 2D culture models, the 3D neural cultures generated complex hierarchical structures that foster increased cellular communication12. Moreover, they can mimic part of the key structural and attributes of human organs13,14, which is crucial for advancing our understanding of disease progression15,16 and facilitating drug screening17,18 in the context of human neurological disorders. For example, disease manifestations such as the deposition and misfolding of proteins in Alzheimer's disease (AD), can only be accurately reflected in 3D in vitro models15. Further, to fully unleash the potential of neural organoids, long-term and stable characterization of their organization structure and physiological functions in a 3D manner is essential.
Establishing the structural and developmental congruence between organoids and human tissues constitutes the most compelling evidence to validate the applicability of organoid models. The image detection is a convenient method to achieve it. For example, fluorescent labeling of developmental stages and cell type-specific proteins, have demonstrated that the developmental trajectory of human brain organoids closely resembles that of the human brain, and under optical microscopy, brain organoids exhibit key structural features similar to the ventricular and subventricular zones of the human brain5,13. Enhanced clarity and granularity of information can be achieved through the application of advanced instruments, including scanning electron microscopy (SEM), which emphasizes the visualization of surface characteristic texture, and transmission electron microscopy (TEM), which is adept at capturing the internal ultrastructural details19. To adapt to the intricate complexity of the 3D structure of organoids, noninvasive optical sectioning techniques, such as confocal imaging, multiphoton microscopy and light-sheet technology have enabled the acquisition of more detailed 3D information from intact organoids20. Further, the escalating demand for high-resolution dynamic observation has led to the emergence of 4D live cell imaging using light-sheet microscopy as a gentle and rapid imaging method on organoids21,22. However, the traditional imaging methodologies are constrained in their capacity to capture a comprehensive spectrum of features, especially in the key features of discharge characteristics and metabolic activities. Additionally, the developmental trajectory of organoids is protracted and intricate, and the traditional terminal detection techniques exhibit inherent limitations when assessing this process.
To address the limitations of traditional methods, there is a pressing need for bioelectronic interfaces and sensors capable of continuous surveillance23. These tools are essential for monitoring the morphological and functional evolution of organoids during development. Furthermore, due to the substantial potential of organoid models in realms such as regenerative medicine, disease modeling, and personalized therapeutics, there is also a need for bioelectronic interfaces and sensors to dissect the functional alterations of organoids under pathological conditions and accurately simulate the disease progression24,25. New materials and advanced manufacturing methods are progressively breaking through the constraints of biosensors. High-density multi-electrode arrays can improve the spatiotemporal resolution26 while compact multi-well arrays have made high-throughput drug screening feasible24. Although several commercialized bioelectronic technologies have satisfied partial requirements, there remains some deficiencies (Table 1). The prevalent bioelectronic devices, being planar in configuration, can hardly accommodate the 3D architecture of organoids. Many researchers adapt the organoids to these flat devices either by directly contacting or slicing26,27, which inevitably results in the loss of a large amount of valuable information. With the development of materials science and manufacturing technology, the neural interfaces are increasingly evolving towards miniaturization, enhanced flexibility, and improved biocompatibility28,29. In addition, the complexity of organoids requires monitoring from diverse perspectives, including changes in morphology, electrophysiology and molecular levels. The development of multimodal bioelectronic interfaces and sensors, offering a more comprehensive characterization of organoid functions, represents an emerging frontier in the field30,31,32.
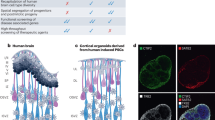
In this review, we mainly focused on the cutting-edge advancements in neural organoids and evolution of bioelectronic interfaces and sensors for long-time dynamic monitoring. As illustrated in Fig. 1, neural organoids have been successfully developed to recapitulate key physiological characteristics of the nervous system in vivo. We provide a concise overview on the progress achieved in the induction technology of neural organoids over the past decade, which mainly encompasses brain, spinal cord, retina, inner ear and olfactory organoids. Various types of neural interfaces and sensors have been employed to reflect the comprehensive physiological characteristics of these neural organoids. This review critically evaluates the application of advanced neural interfaces for in vitro functional assessment of neural organoids, with particular emphasis on technological innovation of 3D biosensors for long-term dynamic detection in small 3D cultures (such as neural organoids). The enhanced structural conformity of these 3D interfaces with organoids facilitate more durable, real-time, high-throughput and stable monitoring capabilities. These technological breakthroughs hold substantial translational potential, with future applications anticipated in human physiological and pathological modeling, pharmaceutical screening, regenerative medicine and other related fields.
Different types of neural organoids
Brain organoids
The human brain is such a complex and unique system that its protracted development and genetic characteristics cannot be commendably described by animal models1. The establishment of brain organoids has cast light on our understanding of our human brain5. Different from neuro-spheres, the unique advantages of organoids lie not only in 3D structure but also in the capability to replicate the cellular composition, intercellular tissue structure and developmental trajectory of the human brain.
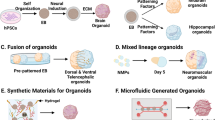
In general, the generation of brain organoids typically commences with the formation of an embryoid body (EB) or a spheroid, which is subsequently subjected to neural induction media to direct differentiation towards a neuroectodermal lineage5,33. Two principal methodologies are employed in the generation of brain organoids: unguided and guided approaches (Fig. 2). Unguided methods, represented by the protocol of Lancaster in 2013, rely on the self-assembly and intrinsic differentiation capacities of iPSCs and ESCs5. The EBs are embedded in the extracellular matrix (ECM) with few factors and a spinning bioreactor is needed for continuous maturity and long-term cultivation5,34,35. The products, also called cerebral organoids, which can achieve diameters of up to 4 mm, encompass a diverse array of cellular constituents, including neural progenitors, mature neurons, astrocytes and so on5,13,36. Encouragingly, the lumen can be observed in the cerebral organoids, and cells are organized into polarized radial structures, forming a ventricular zone, subventricular zone, and cortical plate orderly, which is reminiscent of the early developmental process of newborns34,37. However, unrestricted induction brings about unpredictable product sizes and heterogeneous internal arrangements, posing challenges for reproducibility in experimental settings. In contrast, guided methods involve the addition of specific exogenous components during the early stages of differentiation, which can constrain the self-patterning of organoids and lead to specific brain regions, such as the forebrain38,39, midbrain40,41, cerebellum42,43, hippocampus44,45, thalamus46,47, hypothalamus8,48, striatum49,50 and choroid plexus51,52 (Table 2). Thanks to the establishment of region-specific organoids, customized organoids for different regions can be formed separately before being assembled, forming fused organoids. The method embraces various cell lineages from different brain regions, offering a controlled and reproducible framework for investigating the intricate intercellular interactions53,54. So far, researches on fused brain organoids have primarily focused on cellular migration55,56, nerve distal projection46,49, regulation of downstream tissues57,58 and so on.
Overall, the brain organoids with hierarchical structures and organizational microenvironments have emerged as invaluable tools for modeling human neurological disorders, including autism59, AD16, Parkinson's disease (PD)60 and so on. However, due to the maturity limitation, the current models excel in recapitulating the early embryonic neurodevelopment as well as in the screening of safe drugs during pregnancy61,62. The establishment of complex neuronal circuitry and description of system aging remain the weaknesses. To compensate these shortcomings, researchers have incorporated elderly primary monocytes into the model, which enhanced the appearance of aging phenotypes in brain organoids successfully63. With the development of technology and the continuous optimization of modeling schemes, brain organoids are poised to complement other models, offering a more comprehensive and realistic reflection of human brain.
Spinal cord organoids
The spinal cord, another pivotal component of the central nervous system, serves for information exchange between the brain and the periphery, and it is also responsible for the generation of motor activity64. The spinal motor neurons and sensory neurons within are of vital importance for the maintenance of physiological functions and damage to these neurons can lead to motor neuron diseases, including amyotrophic lateral sclerosis (ALS) and spinal muscular atrophy (SMA)65.
The first cerebral organoids established in 2013 have opened a new chapter for in vitro 3D modeling of the central nervous system, subsequently igniting interest in the development of hindbrain and spinal cord organoids with refine protocols66. Similarly, embryoid bodies are formed before the neural induction and maturation process. The incorporation of specific factors during these stages guides towards a more precise cellular fate, mitigating the cellular heterogeneity derived from unrestricted self-differentiation. Specifically, the inhibition of the SMAD signaling pathway, in conjunction with the coordinated roles of retinoic acid (RA), WNT and fibroblast growth factor (FGF) signaling pathways, facilitates the differentiation and axial extension of neuro-mesodermal progenitors (NMPs) (Fig. 2)9,67. Recent studies have demonstrated that human iPSC-derived gastrula can elongate into distinctive tubular structures, with axial symmetry disruption stimulated by WNT signaling and the formation of NMPs close to a signaling center similar to a tail bud, following the initial spherical configuration68,69. The mature spinal cord organoids exhibit a range of cell types and structures along the dorsal-ventral and rostral-caudal axes within a single entity, overcoming the limitation of traditional 2D cultures and addressing the significant challenge of simulating the intricate tubular architecture of the spinal cord in vitro70.
Motor neuron diseases pose significant health risks and organoid models have proven instrumental in mitigating these risks71. The organoids derived from patient-specific iPSCs are instrumental in elucidating the underlying molecular mechanisms of neurodegeneration and further serve as a drug screening platform72, facilitating personalized medical interventions. Numerous studies have focused on ALS73 and SMA74, investigating the pathogenesis and potential therapeutics. In addition, stem cell-based therapies have shown potential in replacing lost spinal cord neurons, with some animal and clinical trials reporting that the transplantation of spinal cord-derived neural stem cells can ameliorate disease progression75.
Retinal organoids
The retina, an integral component of the peripheral nervous system, originates from the diencephalon during brain development, where a region transforms into a pair of optic vesicles that ultimately differentiate into the retina, the neural tissue directly interfacing with the external environment76,77. Relying on the substantial correlation between the retina and brain development process, Elke Gabriel et al. reconstructed the induction process of brain organoids to incorporate optic vesicle-containing brain organoids, thereby achieving the functional integration of retinal structure within brain organoids78. While more studies induced retinal organoids individually by using iPSCs, retinal progenitor cells (RPCs), and other types of stem cells79,80. The induction protocols for retinal organoids exhibit variability, yet they generally emulate the developmental trajectory of the human retina, with critical guidance on the optic vesicle's fate80,81,82. Initially, stem cells are aggregated or induced to form EBs at the beginning, which can be continuously sustained in suspension or added into ECM for further induction and cultivation. Some key signaling pathways, such as WNT and Notch, are of vital importance in cell fate determination83,84, necessitating the inclusion of exogenous factors such as BMP4, DKK1 and RA in the induction protocols (Fig. 2). With the progress of development, neural retina progenitors differentiate into retinal ganglion cells, amacrine cells, and horizontal cells, followed by photoreceptor cells85. After the neural induction and retinal differentiation procedure, the organoids are continuously induced for over 100 days. Ultimately, the mature retinal organoids encompass a spectrum of retinal neuronal cell types arranged in a laminated structure, with photoreceptor cells (PRCs) located in the outer layer and retinal ganglion cells (RGCs) in the inner layer of organoids86,87,88.
Beyond their significance in developmental theory and therapeutic strategies, the retinal organoids have accelerated advancements in regenerative medicine. Patients with advanced retinal degeneration, often hindered by a scarcity of donor sources, may benefit from retinal organoids, which could potentially alleviate the transplantation gap. The high degree of cellular and structural similarity between organoids and the human retina allows for the extraction and subsequent transplantation of corresponding segments into diseased ocular regions. This surgical approach has demonstrated promising results in animal models, offering a significant breakthrough for patients with visual impairments89,90,91.
Inner ear organoids
The vertebrate inner ear, which originates from the otic placode, is responsible for balance maintenance and undertakes the function of the auditory system92. The sensory cells in the inner ear, also known as hair cells, work as transducers to convert mechanical stimuli into electrical signals. However, these cells are incapable of self-duplication and their absence can result in irreversible hearing impairment and balance disorders93,94. With the deepening of stem cell biology, the inner ear organoids are promising to become new donors for transplantation, offering a potential therapeutic avenue for individuals with hearing impairments95. The generation of inner ear organoids commences with the establishment of EBs, which serve as the foundation for a 3D structural framework. The key branch point of inner ear fate depends on the different orientations of otic placodes, with the Sonic Hedgehog and WNT signaling pathways being pivotal in fate selection (Fig. 2). A suite of key growth factors, including TGF, FGF, and BMP are implicated in the regulation of the pathways mentioned above96,97. Upon completion of the differentiation process, the inner ear organoids successfully generate the sensory epithelia, consisting of functional hair cells, support cells and spiral ganglion neurons97,98.
The inner ear organoids have broad prospects in the exploration of developmental and pathological mechanisms, as well as in drug development. Stem cell therapy has been increasingly mentioned in the treatment of hearing loss while the transplanted stem cells often encounter impediments in normal differentiation and migration96. To address this challenge, organoids can provide a more differentiated population of neural progenitor cells for delivery into the body, presenting a promising alternative approach99.
Olfactory organoids
The initiation of olfactory perception transpires within the olfactory epithelium, where odorant molecules interact with corresponding receptors on olfactory sensory neurons (OSNs). The odor information can be subsequently transmitted to the olfactory bulb and olfactory cortex by OSNs100. Our team has devoted a lot to establishing the olfactory organoids model based on the regenerative ability of basal cells in the olfactory epithelium101,102. To cultivate olfactory organoids, mouse olfactory epithelial tissue is meticulously segregated and digested to obtain single basal cells. Within a Matrigel matrix, the basal cells capable of self-differentiating into olfactory organoids, facilitated by factors that modulate the WNT and Notch signaling pathways (Fig. 2)103.For the regeneration of olfactory basal cells, the acquisition of olfactory organoids is more convenient and time-saving. Investigating olfactory disorders holds the potential to aid in the early detection of certain neurodegenerative conditions, such as AD103. Furthermore, the acute olfactory acuity of biological systems has enabled the development of biomimetic sensors that incorporate biomaterials, such as olfactory organoids, as sensing components. These sensors are capable of closely replicating the biological sensory system in vitro with a high degree of stability and reproducibility104. Nonetheless, interspecies disparities still present a challenge in the accurate reconstruction of human sensory perception in vitro.
In general, with further exploration in developmental biology, researchers have successfully constructed neural organoids of the human species, including the brain, spinal cord, retina and inner ear. The models emulate the early developmental stages of human embryos, thereby providing a novel avenue for probing the intricacies of early neurodevelopment and associated pathologies. The emergence of organoid models has significantly broadened our investigative repertoire, offering a complementary approach to the limitations inherent in animal and cell models. The induction process of neural organoids is conceptually similar to that of 2D neural cultures, yet it requires the preservation of a 3D architecture through supportive systems such as gel culture or other scaffold frameworks. For instance, biomaterials such as Matrigel, natural hydrogels105 and hyaluronic acid hydrogels106,107 have played an important role in the development of neural organoids due to their good biocompatibility and capacity to incorporate neurotrophic factors. Despite these advancements, the current modeling methods for neural organoids are still in their early stages. There are still substantial disparities between these models and living organisms in terms of morphological characteristics, architectural configurations and cellular composition.
The lack of vascularization represents a common problem in the modeling of various neural organoids. Some large neural organoids, such as brain and retina organoids, can reach a diameter of over 1 mm. In the absence of a vascular network, the normal transportation of nutrients and the elimination of metabolic waste are impeded within such a large volume, ultimately leading to the death of organoids in the core108. Common strategies to address this issue include transplantation into a host organism109,110 or co-culture with vascular tissues111,112. On this basis, engineering solutions, such as organ-on-chip technology, with microfluidic devices, have been employed to further refine the available methodologies. These devices are capable of simulating the process of vascular invasion into neural tissue during embryonic angiogenesis and can facilitate the targeted transport of molecules to specific locations via microfluidic channels113. Numerous studies have reported the advantages of vascularized neural organoids, including enhanced maturation and improved functional outcomes114,115.
In addition, the formation of organoids relies on the self-assembly of stem cells, which inherently introduces substantial variability in cell type, size, and morphology among individual organoids within the same batch116. This intrinsic heterogeneity makes it difficult to achieve reproducibility and consistency in research. To address these limitations, several strategies have been proposed. Firstly, reducing the operational variability during the formation process and transitioning towards automated cultivation platforms for organoids can enhance reproducibility and standardization117,118,119. Secondly, controlling the quality of intermediate products during critical induction steps is essential. Specifically, most reported protocols for neural organoid induction initiate with stem cell aggregates known as EBs, and recent studies have made effort in achieving consistent EB states to mitigate variability120,121,122. Additionally, 3D bioprinting has emerged as a promising technology for the mass production of highly consistent neural organoids. It can precisely print various cell types within defined 3D architectures with bioinks, thereby offering a scalable and uniform approach to neural organoid fabrication123,124.
Multi-electrode array (MEA) for electrophysiological recording of neural organoids
The traditional biochemical analysis and imaging techniques that primarily delineate the cellular constituents and anatomical characteristics of neural organoids capture only a limited aspect of the multifaceted functions of these models. The core contribution of these neural organoids, however, lies in their capacity to simulate functionally active neurons within the human nervous system and establish interconnected neural networks. Indeed, in some neurological diseases including but not limited to epilepsy125, AD126, the early stage of retinitis pigmentosa127 and the early stage of spinal neurodegenerative diseases128, clinical observations often fail to reveal substantial structural alterations. Consequently, it proposes new challenges for researchers to identify the subtle yet critical impairments in the neural circuitry, which underlie these neurological disorders. To ensure the accurate assessment of neuronal firing and the functionality neural networks, bioelectrical interfaces and sensors are needed to acquire electrophysiological data of these neural organoids. The MEA allows for the simultaneous recording of electrical signals across multiple sites, providing insights into the spatiotemporal dynamics of neural network activity and facilitating a more comprehensive evaluation of the functional integrity and maturation of neural organoids129. Here, we shall present an overview on various types of MEAs that are frequently employed on neural organoids, along with their structural adaptations designed to address the novel challenges posed by the 3D architecture of organoids.
Planer MEA
The traditional electrode technology, which employs rigid substrate materials, continues to play a fundamental role in the field of neurophysiological research. This technology has been widely applied across various contexts, ranging from in vivo neurons130 to stem cell-derived 2D neurons131, and more recently, in neural organoids37. Specifically, neural organoids are positioned on these electrodes to establish neural interfaces. The encapsulated metal electrodes can then be connected to an amplification system, which enables the continuous and long-term recording of the discharge patterns of these organoids after the preprocessing and analysis of computer. The signals detected by bioelectronic interfaces encompass extracellular action potentials (EAPs), commonly referred to as "spikes," from individual neurons, as well as local field potentials (LFP), which aggregate synaptic currents within a specific region132. When the interconnected neural network is formed, synchronous bursts of spike and flourish of LFP can be observed simultaneously, providing compelling evidence for the maturity and credible function of neural organoids37,133. Take human brain organoids for example, numerous studies have documented the progressive enhancement of electrical activity and characteristic network dynamics across a 10-month period37,133. These investigations have consistently reported that electrical activity in human brain organoids is initially detected after approximately one month of cultivation, coinciding with the early stages of neurogenesis within brain organoids. Following two months of development, the brain organoids exhibit a gradual increase in the firing rate and amplitude of spike, accompanied by highly synchronized network activity. In the advanced stages of development, the proliferation of inhibitory interneurons within organoids leads to a coordinated balance between inhibitory and excitatory neurons, signifying the maturation of functional neural network communication (Fig. 3a–c). The complex oscillatory waves emerging from brain organoids are similar to that of human infants recorded by EEG devices, affirming the proficiency of brain organoids in mimicking the early developmental stages of the human brain37. The technology has since been extended to explore the electrophysiological characteristics of other neural organoids, such as spinal cord organoids134 and olfactory organoids104.
a, b Spontaneous electrical activity can be detected from brain organoids using a MEA system. a A transferred brain organoid on an MEA probe during acquisition of spontaneous electrical activity. b brain organoid recording setup with 64 planar microelectrodes133. c Detection of spontaneous electrical activity in day-34 brain organoids and approximately every month thereafter shows a progressive increase in and complexity of EP properties133. (with permission of Elsevier). d Left, picture of a brain organoid at 2 months, scale bar is 1 mm. Right, organoids (4–6 months old) were embedded in 10% agarose and sectioned into 500 μm thick slices27. e Organoid slices were then placed on high-density CMOS microelectrode arrays27 (with permission of Springer Nature)
The functional neural networks within the human body are highly precise and intricate, yet they remain vulnerable. Upon being compromised by pathological conditions, it may not exhibit overt anatomical alterations, yet they often manifest significant electrophysiological aberrations, which compromise their functional integrity135. The human brain, which serves as the central hub for the collection and processing of vast amounts of neuronal information within the central nervous system, has been the focus of numerous studies, which employ neural interfaces to explore the pathogenesis of various neurological disorders. These disorders encompass neurodevelopmental conditions such as Rett syndrome136 and autism spectrum disorder137, neurodegenerative diseases like AD138, Parkinson's disease139 and amyotrophic lateral sclerosis140, as well as epilepsy141 (Table 3). By leveraging key electrophysiological features, such as firing rate and network bust rate, these studies aim to replicate the pathogenesis of human neurological disorders in vitro. The approach also provides a reliable platform for clinically relevant drug screening, for instance, hyaluronan has been shown to inhibit the overexcitability of neural and exert a mitigating effect on autism spectrum disorders in the 3D spheroid model142.
In addition, drugs can also induce neurotoxicity, resulting in damage to the nervous system. Fetal brains, in contrast to adult brains, exhibit heightened susceptibility to such detrimental impacts143. Since the brain organoid can exhibit the morphological and functional properties of the early embryonic human brain more accurately61, it demonstrates superior efficacy in the screening of neurotoxic compounds. For example, the combination of rigid bioelectrode interfaces with brain organoids has been utilized in the evaluation of medication-assisted treatment in opioid use disorder during gestation62,144. Beyond their well-established applications in modeling neurodevelopmental processes and investigating disease mechanisms, neural organoids have emerged as promising candidates for cell replacement therapy through tissue transplantation. For example, recent reports have demonstrated that rigid bioelectronic interfaces serve as essential tools for evaluating the functional integration of transplanted human retinal organoids in rats, thereby facilitating improvements in visual function90,145.
Although the current electrode technology can fulfill the fundamental requirements for detecting electrophysiological networks in neural organoids, there remain many aspects requiring improvement. One of the limitations lies in the restricted number of channels, which leads to insufficient spatiotemporal resolution37. The utilization of Complementary Metal Oxide Semiconductor (CMOS) technology has emerged as a promising solution to these limitations. It demonstrates remarkable compatibility with various rigid substrate materials, including silicon and glass146. The incorporation of CMOS technology has facilitated the development of electrode arrays with enhanced channel density and greater integration, thereby significantly improving detection probability and the richness of acquired information27,147. When the number of electrodes reaches 26,400, subcellular spatial resolution becomes attainable, which is a representative commonly used CMOS-MEA148. Further, the CMOS-MEA designed by Ikuro Suzuki et al. has reached an impressive 236880 channels and has been successfully implemented in various neural tissue models, including mouse brain slices, 2D neural cell cultures, peripheral neurons, and human brain organoids26. The acquisition of high temporal resolution is crucial for accurately capturing rapidly fluctuating electrophysiological signals. This capability not only enables expeditious data collection but also facilitates the comprehensive analysis of dynamic properties within neural networks. For instance, it opens a new avenue for investigating the correlation between cell waveform clusters and specific cell types149. Furthermore, the high-density electrode configuration provides extensive coverage of larger measurement areas, thereby enhancing the capacity to monitor propagation patterns across different regions of large-scale neural organoids, including the modeling of multiple brain region interactions26.
Apart from that, similar to electroencephalography (EEG) or electrocorticography (ECoG), the detection depth of planar MEA is limited to the immediate 100 µm vicinity of the electrodes150, while the diameter of neural organoids can often reach the millimeter level5. Consequently, a large amount of valid information, especially the discharge situation in the core parts of organoids and global electrophysiological network connection, remains beyond effective characterization. To obtain deeper activity records, the slicing of tissues or organoids has been employed to accommodate the planar MEA designed for 2D cultures (Fig. 3d-e)27,151. Although the slicing of organoids facilitates long-term cultivation and real-time monitoring, the challenges associated with delicate slicing procedures and the inevitable damage incurred still present certain obstacles36. Most critically, regardless of the increase in electrode numbers or the supplement of slicing technology, the data acquired remains confined to a single 2D plane, thus failing to fully exploit the holistic advantages of 3D models132.
3D MEA
The organoid model serves as a valuable repository of information, and the application of commercialized planer electrodes has partially harnessed its potential in neural development, disease modeling, drug screening and so on. However, the structural constraints of planar electrodes limit their capacity to access deep tissue regions that generate specific local signals. Biological signal generation within organisms typically exhibits regional specificity, for instance, distinct brain regions possess unique roles and functions, and their communication and interconnectivity collectively underpin the holistic functionality of the brain152. To fully exploit the potential of this 3D organization, various 3D MEA technologies are emerging.
The traditional rigid probes used to contribute to understanding the electrophysiological activity of the brain in vivo153 and some studies have also attempted to apply it to 2D or 3D neural cultures154. For example, Hyogeun Shin et al. designed a 3D high-density MEA, and their design concept involves sequentially stacking three 2D MEAs of varying dimensions, which were previously employed for in vivo neural recording, to construct a 3D configuration. The 3D MEA, integrated with 63 recording microelectrodes and multifunction of optical stimulation and drug delivery, can record the developing neural network of neuron culture nearby daily. Although the culture analyzed is not as complex as organoids, the spatial mapping of active neurons has been preliminarily established155. However, this approach has not been widely researched and applied for the reason that these electrodes, originally developed for in vivo transplantation, have poor adaptability to small organoids in terms of size. Also, the invasive implantation process with destructive effects causes damage to the neural network connections of organoids, hindering the long-term stable signal sources. The emergence of increasingly sensitive and flexible detection devices tailored for organoids are becoming viable alternatives.
Microneedle electrode
Compared to the aforementioned invasive approaches of sliced or rigid-implanted electrodes, microneedle electrode represents a comparatively non-invasive form of 3D interfaces, which does not compromise the tissue integrity of organoids. A pivotal advantage of this configuration lies in its enhanced signal transduction efficiency at the tissue-electrode interface156. The fidelity of electrophysiological recordings from organoids is critically dependent on the quality of this interface. For planar electrodes, the thickness of the interstitial gap between the cells and electrode surface is about 150 nm under optimal contact conditions and the situation is more pessimistic for organoids due to its 3D characteristic157. In contrast, when organoids are interfaced with microneedle electrodes, the mechanical compliance of the cellular membrane facilitates its deformation and subsequent encapsulation of the electrode surface. This phenomenon significantly improves both the sealing impedance and effective contact area, thereby optimizing interfacial coupling and signal transduction efficiency158.
A typical microneedle electrode was developed by Oranny Phouhetlintong et al., utilizing the principle of accumulated stress within multi-layer thin films to fabricate a microelectrode array featuring protruding cantilevers (Fig. 4a–c)159. This configuration comprises 60 vertically aligned electrodes arranged in a uniform square array across four quadrants, with precise spatial distribution. These electrodes, extending several hundred micrometers from the substrate surface, enable the monitoring of intrinsic electrophysiological activity within brain organoids. Notably, the tight interfacial coupling between the organoids and electrode array significantly enhanced signal amplitude, thereby facilitating reliable detection and analysis. Additionally, other microneedle electrode with diverse geometries, such as mushroom-shaped, wire, pillar, tube, volcanic shapes and coronal designs have demonstrated distinct advantages in 2D cellular electrophysiological recordings and hold significant potential for application in 3D organoid studies160.
a Technological cross section of the MEA showing the layer stack before and after sacrificial layer etching and beam displacement159. b Schematic of the placement of a cerebral organoid on top of a conventional planar MEA showing the cleft layer between cells and electrodes and a schematic representation of an organoid inserted on a spiky microelectrode159. c 3D schematic representation of a cerebral organoid impaled on the MEA159. (with permission of Royal Society of Chemistry). d Schematic illustrations of the pneumatically controlled direct printing stage with high precision163. e Schematic illustrations of microelectrode array with 3D liquid metal electrodes for retinal organoids with various heights163. (with permission of Wiley). f Configuration of 3D liquid metal MEA165. g Schematic illustration of magnetically reshapable 3D liquid metal MEA165 (with permission of Springer Nature). h SEM image of a 4 by 4 grid of μ-needles printed in different heights with print durations ranging from 60 to 300 s onto a gold-covered wafer166. i Bright-field microscopy image of a μ-needle MEA166. j Photograph of the experimental setup. Four organoids were immobilized in 4 wells on the μ-needle MEA, which was inserted in the headstage of the amplifier system. After being filled with medium, the glass ring chamber on the MEA was sealed with a PDMS lid equipped with an Ag/AgCl reference electrode166. k Time traces of signals detected via a μ-needle MEA from a brain organoid166 (with permission of American Chemical Society)
Traditional fabrication of microneedle electrodes predominantly relies on conventional photolithography techniques, which impose stringent requirements on the manufacturing processes and consequently entail substantial costs160,161. Also, the traditional method often struggles to accommodate the variable sizes of different neural organoids, thereby limiting their adaptability. In contrast, 3D printing has emerged as a transformative fabrication paradigm, offering enhanced simplicity and exceptional customization capabilities162. The research team led by Jang-Ung Park has made significant contributions in the development of 3D-printed liquid metal microneedle electrodes. Utilizing high-precision 3D printing technology, the team has developed flexible liquid metal microelectrodes capable of precise targeting to the inner layer of retinal organoids. These electrodes exhibit remarkable structural compatibility with retinal organoids, thereby mitigating mechanical trauma and preserving tissue integrity. The team further demonstrated the utility of this platform in monitoring the development of RGCs within retinal organoids, which exhibit striking morphological and functional similarities to early-stage fetal retina (Fig. 4d–e)163. Moreover, the printed electrodes can be integrated into an electrical stimulation system, enabling precise spatial modulation of retinal neural activity and concurrent electrophysiological recording of both pre- and post-stimulation responses164. Besides, the research team designed a reconfigured 3D MEA using liquid metal direct printing technology for the purpose of recording electrophysiological signals from brain organoids. This innovative approach capitalizes on the magnetic field-assisted reconfiguration capability of the flexible microneedle electrodes, enabling the establishment of multiple recording sites through a single electrode unit. Such spatial reconfiguration significantly enhances recording site density without proportional increases in electrode quantity, thereby optimizing the spatial resolution of neural activity mapping (Fig. 4f–g)165. Sabine Zips et al. employed another 3D printing paradigm, aerosol jet printing, to readily achieve rapid 3D patterning of functional inks with high aspect ratios and varying heights. The μ-needle electrodes fabricated enable long-term and stable application on neurospheres and brain organoids derived from iPSCs (Fig. 4h–k)166.
In addition, microneedle electrodes offer a significant advantage in accessing the intracellular membrane potential of individual cells167,168. While traditional patch-clamp methods can provide high-fidelity electrophysiological recordings at the single-cell level, they are inherently limited by their manual operation and low-throughput characteristics14. In contrast, microneedle electrodes can be synergistically integrated with auxiliary methodologies, including electroporation169 and optoporation170, to enable precise localization of single-cell membrane penetration, thereby facilitating the acquisition of high-throughput and stable intracellular electrophysiological signals. To date, the application has primarily been confined to 2D cell cultures while its potential extension to 3D organoid systems represents a promising direction. Overall, the microneedle electrodes demonstrate the dual capability of precisely monitoring individual cellular activities while simultaneously capturing the collective electrophysiological network dynamics within organoids to a certain depth.
Flexible interface and stretchable mesh
The advent of flexible bioelectronics has ushered in a new era of neural interfaces, wherein flexible interfaces and scaffolds demonstrate superior integration capabilities with biological tissues, potentially mitigating the adverse effects frequently associated with foreign material integration on organoid development and function. These flexible substrates, primarily fabricated from polymers such as SU-8, polyimide and parylene C, are engineered through advanced microfabrication techniques including spin coating, photolithography, and reactive ion etching to create precisely patterned functional layers28,29. In contrast to microneedle electrode, which primarily target deep electrophysiology signals, flexible MEAs exhibit exceptional compatibility with the 3D architecture of organoid surfaces. This design expands the contact area with the neural organoids, thereby enabling a macroscopic assessment of the overall neural network formation and signal propagation within the organoids. Remarkably, the system also compatible with a commercially 256-channel MEA electrophysiological hardware system, which means the convenience of functional characteristics catching and widespread use (Fig. 5a)171,172. For the sake of a wider range of applications, the authors further developed improved computational analytical tools to increase the spatial precision, especially in the Z dimension of the 3D tissue, making the engineered 3D neural organoids a visualized and trustworthy model in vitro173.
a Light micrograph of a 3D flexible MEA with vertical electrodes171. (with permission of Royal Society of Chemistry). b Schematic of a brain organoid encapsulated in the 3D self-rolled MEA174. (with permission of IOP Publishing Ltd.). c Schematic of the self-folding 3D shell MEA175. (with permission of Wiley). d FEM snapshots showing organoids of different sizes (400 to 600 μm) fitting in tailored shell electrodes176. e Corresponding SEM images of 3D shell electrodes with different levels of folding. Scale bar, 100 μm176. (with permission of AAAS). f Optical image of a brain organoid enclosed in a 3D mesh MEA177. g 3D illustration of the positions of the microelectrodes across the surface of the spheroid177. (with permission of AAAS). h Photos of the whole device and a single well with a suspended mesh MEA178. i Waveforms overlaid at electrode positions of the mesh MEA178. (with permission of Wiley)
Nevertheless, the electrodes mentioned above remain constrained to specific regions of organoid surfaces. The limitation has prompted ongoing research efforts to develop optimized flexible interfaces and scaffolds with diverse geometries to maximize tissue-electrode contact and enable comprehensive reflection of the overall network activity. For instance, the 3D self-rolled array developed by Kalmykov et al. is capable of achieving complete spatial envelopment of spherical organoid structures, driven by the residual mismatch stress between the constituent layers (Fig. 5b)174. Similarly, Cools et al. designed a 3D self-rolled shell MEA, which mimics the anatomical configuration of electroencephalogram caps. The array, with good biocompatibility and a higher signal-to-noise ratio, can perform fluorescence imaging along with signal recording for its optical transparency (Fig. 5c)175. Addressing the inherent size variability in spontaneously differentiated brain organoids, QI HUANG et al. engineered an adaptable shell MEA with tunable folding characteristics through precise photolithographic parameter modulation, enabling robust electrophysiology recording of organoids ranging from 400 to 600 μm (Fig. 5d–e)176. Furthermore, the buckled MEA designed by Park et al. utilizes mechanically designed compressive buckling to transform 2D films into 3D structures. This multifunctional neural interface not only facilitates the investigation of synchronous burst events on the surface of individual brain organoid spheres but also enables the study of the cascading processes involved in the formation and regeneration of the overall electrical activity network between assembloids (Fig. 5f–g)177.
However, the aforementioned neural interfaces are primarily more suitable for acute detection rather than long-term monitoring for that the long-term growth and survival of organoids necessitate adequate oxygenation and nutrient supply. To achieve sustained electrophysiological recordings from neural organoids, researchers have developed innovative strategies for maintaining suspended state of these organoids in culture medium while establishing stable neural interface connections. Mesh scaffolds represent an effective solution, offering several critical advantages for prolonged organoid maintenance. These scaffolds facilitate free floating of organoids, eliminating undesirable mechanical constraints associated with solid substrates. This design not only supports unhindered 3D growth of organoids but also preserves the integrity of their intrinsic electrophysiological networks, thereby enabling reliable long-term signal acquisition and monitoring (Fig. 5h–i)178.
The growth and development of neural organoids typically involve extended periods, rendering chronic electrophysiological monitoring indispensable for the comprehensive assessment of growth trajectories, functional characterization and pharmacological intervention efficacy in these 3D models. To accommodate the 3D growth and volume expansion inherent in the development of organoids, an ideal long-term sensing device should possess not only sufficient bending stiffness to adapt to the curved boundaries of 3D cultures and form a stable electrical interface, but also a stretchable design that enables the device to extend in response to forces generated by the organoids themselves.
The stretchable mesh MEA reported by Thomas L. Li et al. incorporates an elastic polymer, styrene-ethylene-butylene-styrene (SEBS), in the selection of substrate and encapsulation materials. The electrodes facilitate the establishment of enduring neural interfaces with freely suspended organoid cultures, demonstrating exceptional mechanical resilience with tolerance to 50% compressive and tensile strain (Fig. 6a–b)179. Yan Wu et al. have also developed a highly stretchable MEA employing a gallium indium (GaIn) alloy integrated with elastic polymers (thermoplastic polyurethane TPU, and polyurethane, PU). The electrodes can freely deform and fold at any angle when the organoids float and grow within the culture medium, exhibiting stretchability of up to 500% (Fig. 6c–d)45. Furthermore, the Kirigami Electronics reported by Xiao Yang et al. feature a highly innovative structural design. Drawing inspiration from the principles of Kirigami art, researchers have devised an ultrathin 2D spiral pattern that spontaneously transforms into a 3D basket-like structure upon suspension. The neural organoid is subsequently embedded within the concentrated area of the bottom electrodes. Owing to the unique Kirigami pattern design, the electronics exhibit remarkable deformability, enabling long-term recording on freely floating organoids for at least 120 days without compromising their spherical morphology and differentiation processes (Fig. 6e–f)180.
a An illustration of the stretchable mesh MEA and its fabrication process179. b Photographs of a mesh electrode with organoids being manipulated by metal forceps. The left image is of an undisturbed mesh, and the right image is the mesh being deformed by the forceps179. (with permission of Elsevier). c Schematic diagram of coupling the highly stretchable MEA with the brain organoid45. d The bottom view captured by a microscope of the stretchable MEA with the brain organoid45. (with permission of Springer Nature). e Schematics of the generation of neural organoids from hiPS cells, the concept of vertically deformable Kirigami Electronics and the integration of neural organoids with electronics180. f The spiral Kirigami Electronics design consists of concentric rings connected by spiral latches with 32 electrodes (gold circles) in the central area180. (with permission of Springer Nature). g Schematics illustrating the stepwise integration of stretchable mesh nanoelectronics into 3D human iPSC-derived neural tissues through cell self-organization and brain organoids through organogenesis182. h Optical photograph of stretchable mesh nanoelectronics182. i Optical photograph of a 2 × 2 devices well, with a single culture chamber for four cyborg brain organoids cultured simultaneously182. (with permission of Wiley)
The stretchable mesh MEAs discussed previously primarily focus on monitoring neural activity at the surface of organoids, leaving a gap in the detection of deep internal signals within 3D structures. This limitation has been partly addressed by the cyborg system developed by Jia Li’s research team. The soft and stretchable mesh nanoelectronics employed in this system exhibit physicochemical properties that closely resemble those of tissue scaffolds, thereby achieving excellent biocompatibility and tissue-like bending stiffness. The implementation of serpentine mesh architecture further enhances its stretchable performance, enabling seamless integration with developing organoids. Through precise engineering modifications, the researchers successfully incorporated this system into both brain and cardiac organoid models. Specifically, the mesh-like nanoelectronics were transferred into 2D stem cells in advance and stretched continuously along with the 2D-to-3D reconfiguration of organogenesis. The engineered organoids ultimately form a 3D spherical morphology, with a uniform spatial distribution of the embedded sensors throughout the entire sphere. The tissue-like stretchable mesh nanoelectronics do not disturb the typical development of organoids and provide long-term stable electrophysiological recording of single cells with millisecond spatiotemporal resolution (Fig. 6g–i)181,182.
In conclusion, the organoid model has emerged as a highly promising tool for exploring the intricacies of the human nervous system. To fully decode the intricate biological phenomena within these 3D structures, it is imperative to establish comprehensive electrophysiological monitoring capabilities that span from single-cell resolution activity to functional network dynamics. While planar electrodes, which have been extensively studied and applied, continue to play a significant role in organoid research, there remains an unmet need for MEAs specifically optimized for 3D architectures. The practice of organoid slicing and implantation of rigid electrodes, though effective in providing access to internal neural activity, is constrained by the introduction of inevitable mechanical damage. As an alternative, microneedle electrodes mitigate mechanical disruption and enable precise detection of electrical signals at specific depths within organoids, yet they sacrifice global coverage of the organoid. The advent of flexible and stretchable electronics, coupled with innovative engineering concepts, has redefined the trajectory of 3D MEA development. The emerging 3D MEAs is expected to focus on minimizing interference with the physiological activities of organoids while enabling long-term and stable data acquisition and current 3D MEAs have already demonstrated promising capabilities in spatial mapping of organoid activity. Moreover, given the long history and in-depth research on brain organoids, most optimized neural interfaces have thus far been tailored primarily for brain organoids. As investigations expand to diverse neural organoids, these advanced 3D MEAs will undoubtedly be adapted and refined to address the specific requirements of various neural models, thereby broadening their application scope in neuroscience research.
Impedance biosensors for the physiological recording of neural organoids
Electric cell-substrate impedance sensing (ECIS) is an assay to measure the dielectric properties of cells based on the principles of electrochemical impedance spectroscopy (EIS)183. The dielectric properties can demonstrate cell behaviors like proliferation, metabolism, and viability. The impedance measurements have the advances of being non-invasive and label-free and can detect and record data sensitively and quantitatively. Nowadays, impedance measurement devices have been applied for cell research184, cancer research185, drug screening186, environmental pollution187, food safety188, and so on.
In terms of neuroscience research, the ECIS system works multiple uses. For example, neurodegenerative diseases are common nowadays, causing a major threat to human health. Neurodegenerative disease is affecting all ages, ranging from congenital leukodystrophies in childhood to AD and Parkinson’s disease (PD) during aging. The diseases have genetic, environmental, or complex etiologies, resulting in obstacles in clinical treatment189. As a result, it is critical to develop new tools for facilitating disease diagnosis and treatment. Tau hyperphosphorylation was reported implicated in a group of neurodegenerative diseases190. The ECIS system is expected to monitor tau hyperphosphorylation and retrograde neurite degeneration. An early study conducted by Robitzki et al. applied impedance spectroscopy to measure the hyperphosphorylated tau protein in human neuroblastoma cell line SH-SY5Y for evaluating the pathological risk potential of AD191. To verify the prospect in vivo, Robitzki et al. induced tau hyperphosphorylation in excised organotypic hippocampal slice and monitored the real-time change with electrical impedance spectroscopy technique192. Researchers successfully detected two distinct phases of neurite degeneration that were reflected by different impedance data.
The distinguishing feature of neural organoid is the 3D structure and complex composition. The ECIS system has been applied to organoids for drug screening and the results showed higher drug resistance in 3D models193,194. Due to poor adhesion between 3D organoids and planar electrodes, the expandable application of impedance sensors to 3D cultures still has many limitations. Our team has made a pioneering work to explore the ECIS system to 3D application. We developed a biosensing platform that involves a vertical impedance electrode array and a multi-channel detection system where 3D cultured cells were planted between a pair of gold electrodes for anti-cancer drugs testing195,196 (Fig. 7a). The platform can record 3D cell impedance in the vertical direction and with the penetration of drugs, the survival rate of cells will exhibit temporal and positional dependencies. In addition, we’ve miniaturized the biosensing chip to a silicon chip, resulting in a multi-dimensional microgroove impedance sensor (MGIS)197 (Fig. 7b). Similar to our work, Lei et al. reported a simple paper-based ECIS system in a 3D in vitro system to monitor cell viability by measuring impedance between 0.1 to 100 kHz198. Although most available articles focus on cancer organoids, the ECIS system has broad application prospects on neural organoids due to the similar features.
a Schematics of the vertical impedance electrode array196. (with permission of Elsevier). b Schematics of the MGIS chip197. (with permission of Springer Nature). c Schematic illustration of ECIS system based real-time imaging and synchronous electrophysiological monitoring for OE organoids and the principle of impedance detection103. (with permission of Elsevier)
Recently, we implemented an innovative strategy to investigate olfactory dysfunction in the early stages of neurodegenerative diseases using the ECIS system103. The ECIS system serves as a non-invasive tool for monitoring olfactory epithelial organoids derived from basal stem cells. By integrating a live imaging system, we performed a real-time spatiotemporal analysis of the morphological and physiological features of olfactory epithelium organoids. To examine the early pathological changes associated with AD, we established olfactory epithelial organoids from APP/PS1 transgenic mice and observed their aberrant growth in both spatial and temporal dynamics. Abnormal pathogenic protein aggregation and accumulation was found in AD organoids and increased as disease progressed. This multimodal monitor strategy could help investigate the onset mechanisms of olfactory dysfunction, which would shed new light on early diagnosis and treatment of neurodegenerative disease (Fig. 7c).
The impedance sensing strategy is valuable in neural organoid research, although there is a limited number of published articles on the subject. A distinctive advantage of the ECIS system is its ability to enable researchers to monitor organoids in a real-time and non-invasive manner. This innovative monitoring tool is crucial for advancing neuroscience studies, particularly in the area of drug development. In the foreseeable future, the importance of the impedance biosensing strategy is expected to become increasingly evident.
Electrochemical biosensors for biomarker detection of neural organoids
In drug discovery studies involving organoid systems, tracking the metabolic activities of tissue-engineered organoids is crucial. Soluble biomolecules secreted by cells serve as indicators for assessing the functionality of ex vivo organoids and evaluating their response to drug compounds or toxic chemicals199. Quantitative detection of biomarkers from complex biological media in ex vivo organoids holds great promise for monitoring cell status and the efficacy and toxicity of drugs directly200. Electrochemical sensors, which detect analytes by measuring changes in the electrical properties induced by chemical reactions on the electrode surface, play a key role in assessing the functionality of ex vivo neural organoids and their response to drug compounds or chemicals. Their outstanding characteristics include excellent detection limits, label-free detection capability, wide linear response range, portability, and suitability for continuous monitoring.
The detection of disease biomarkers in neural organoids is crucial for studying corresponding neurological disease models, while neurotransmitter release is essential for understanding synaptic connectivity and activity in neural organoid models. Therefore, electrochemical detection in neural organoids mainly focuses on detecting neurotransmitters or disease biomarkers. Taking Parkinson's disease as an example, α-synuclein is one of its biomarkers. Hung-Yin Lin et al. successfully developed a sensor based on a molecularly imprinted electrochemical polymer to detect increased levels of α-synuclein in brain organoid culture medium from Parkinson's disease patients (Fig. 8a)201. They later improved the sensor's sensitivity by optimizing the template peptide sequence202. Dopamine, an important neurotransmitter, and biomarker for Parkinson's disease, was successfully monitored in a human midbrain organoid culture medium using an electrochemical sensor strategy based on self-assembled 3-mercaptopropionic acid monolayer-modified electrodes and cyclic voltammetry signals (Fig. 8b)203. Serotonin is also a neurotransmitter, and its chemical similarity to dopamine makes its discrimination crucial. Tomi Laurila et al. successfully achieved selective detection of dopamine and serotonin at the nanomolar level in human brain organoid culture medium using a single-walled carbon nanotube-based electrochemical sensor, validating the healthy development of dopamine neurons in the midbrain on this sensor204. Glutamate is closely related to neural development, and Mirella Dottori et al. successfully detected glutamate release from human ESCs-derived brain organoids in different brain regions using a functionalized borosilicate glass capillary-based electrochemical biosensor205.
a Preparation of Peptide-Imprinted Poly(hydroxy 3,4-ethylenedioxythiophene)-Coated Electrodes and Their Recognition of α-Synuclein201. (with permission of American Chemical Society). b Schematic of the improved electrochemical dopamine detection method203. (with permission of Royal Society of Chemistry)
These electrochemical sensors exhibit distinct sensitivity and selectivity limits in neurotransmitter detection. For instance, a single-walled carbon nanotube sensor further demonstrates nanomolar selectivity for dopamine and serotonin by optimizing detection potentials, addressing their close oxidation potentials204. Glutamate detection via a nanostructured microelectrode shows an LOD of 5.6 μM, with selectivity enhanced by enzyme-specific catalysis and polymeric film barriers, though interference from structural analogs remains205. Sensitivity limitations primarily arise from matrix fouling and competitive adsorption, while selectivity relies on electrochemical potential tuning, surface modification, and molecular imprinting strategies—approaches that have improved α-synuclein detection to 4.0 pM via peptide-imprinted nanotubes201. These studies highlight that sensitivity is constrained by matrix fouling and competitive adsorption, while selectivity relies on electrochemical potential tuning, surface modification, and molecular recognition elements, with ongoing challenges in long-term stability and multi-analyte discrimination in complex environments.
Overall, electrochemical sensors for neural organoids are currently mainly used to detect chemical substances in the culture environment. Future directions may involve integrating them into organoid-on-a-chip platforms to achieve real-time monitoring of multiple biomolecules to assess organoid function and their response to drug compounds or chemicals. However, this field still faces various challenges, such as the biocompatibility of electrode materials, ultra-low detection limits, long-term continuous monitoring, simultaneous detection of multiple biomarkers, compatibility with microfluidic technologies, and highly complex culture environments.
Multi-model biosensors for comprehensive evaluation of neural organoids
The utilization of neural interfaces, with diverse morphologies, materials and functionalities, offers a perspective into specific aspects of neural organoids, facilitating a more detailed understanding of their structural and functional attributes. To further achieve a comprehensive and synchronous detection of the diverse attributes of neural organoids, the development of dual-mode and multimodal electronic interfaces and sensors represents an emerging frontier in the field.
The image-based detection method is a classic method for the visualization of internal spatial structure and assessment of developmental stages of organoids. The morphological and compositional similarity between various organoids and their corresponding human organs is one of the most direct evidence supporting their efficacy as a valid model in vitro. The morphological examination accompanied the growth process of neural organoids is instrumental in ascertaining the regulation of their normative growth patterns and in validating the efficacy of supplementary physiological data recorded. Certain widely utilized biocompatible substrate materials, including parylene, SU-8 and PDMS, exhibit inherent optical transparency characteristics, enabling the seamless integration of optical imaging modalities with electrophysiological recording techniques in neural interface systems. This unique material property facilitates the establishment of a novel dual-mode monitoring platform that concurrently captures both optical and electrophysiological neural activity with high spatial and temporal resolution28. For instance, the optically transparent graphene microelectrode developed by Madison N. Wilson et al. serves as a sophisticated tool for chronically monitoring the developmental progression, maturation and functional integration of neural networks between human brain organoids implanted within the retrosplenial cortex of a mouse brain and the host's neural tissue. The transparent electrode can capture the propagation of neural activity at the interface between the organoid and the cortex and it can also provide a detailed visualization of the vascular network's integration of organoids within the host, assisted by two-photon imaging. This approach offers a comprehensive and multimodal assessment of the interaction between implanted organoids and the host brain, enhancing our understanding of the complex dynamics involved in neural transplantation and integration109. Additionally, with the development of MEMS technology, micro-platform with compact structure has been designed for long-term monitoring on organoids models. Based on this, our team integrates the image detection and impedance detection into an e-plate, achieving the dynamic multimodal spatiotemporal monitoring of olfactory organoids in vitro103. In addition, fluorescence imaging using cell-type-specific protein markers is a classic method for revealing the cellular composition within organoids. It facilitates the observation of the developmental process from stem cells to fully mature organoids at the cellular level, as well as subsequent apoptotic processes206. The combination of electrical recording and cell-type-specific imaging can accurately measure the electrophysiological changes on the surface of neural organoids to the single-cell level and relate the electrical signals with cell-type-specific information207,208.
Neurons, as the fundamental structural and functional units of the nervous system, play a crucial role in sensing stimuli and conducting excitatory signals209. The communication between neurons relies on complex patterns of electrical and chemical signals210. Specifically, electrophysiological signals reflect the excitability of neurons and their activity within neural networks, while chemical signals reveal the release and metabolic dynamics of neurotransmitters and other neurochemicals. These two signaling modes are interdependent and jointly regulate the operation of neural networks. Therefore, the development of dual-mode microelectrodes capable of simultaneously detecting electrophysiological and chemical signals is of great significance for understanding the functional mechanisms and pathological processes of the nervous system. In recent years, with the rapid advancement of materials science and microfabrication technologies, dual-mode microelectrodes have been widely applied in neuroscience research211,212. For instance, Mao et al. successfully achieved simultaneous detection of ascorbic acid (AA) and electrophysiological signals in the rat brain by integrating carbon fiber microelectrodes with glass micropipette electrodes, providing a powerful tool for studying the relationship between neurochemistry and electrical activity213. Additionally, Cai et al. designed a silicon-based microelectrode array (MEA) modified with platinum nanoparticles (PtNPs) and glutamate oxidase (Gluox), enabling the concurrent detection of glutamate (Glu) and electrophysiological signals in the rat hippocampus214. This approach offers a novel methodology for exploring the dynamics of neurotransmitters and their correlation with electrophysiological activity. Furthermore, Anita A. Disney et al. developed a multi-channel ceramic-based MEA capable of recording electrophysiological and chemical signals from multiple brain regions simultaneously, providing critical technical support for investigating the spatiotemporal characteristics of neural networks215. Although these studies primarily focus on in vivo experiments, their design principles and technical methodologies can also serve as valuable references for research on in vitro neural organoids. However, the application of dual-mode microelectrodes in neural organoid research still faces several challenges. First, the three-dimensional (3D) structure of neural organoids imposes higher demands on the spatial resolution of electrodes. Achieving high-resolution electrochemical detection in such complex 3D environments remains an area requiring further exploration216. Second, the long-term stability of electrodes is crucial for ensuring the reliability of experimental data, particularly in the dynamic growth environment of organoids. The biocompatibility and mechanical stability of electrode materials need further optimization217. Additionally, the interface design between electrodes and neural organoids requires improvement to reduce interfacial impedance and enhance signal transmission efficiency. By continuously optimizing electrode design and fabrication processes, dual-mode microelectrodes are expected to play a more significant role in functional studies, disease modeling, and drug screening of neural organoids, thereby advancing the field of neuroscience.
In conclusion, the image detection has high compatibility with other sensing techniques. The requirement for material transparency ensures the quality of image detection and the integration will not compromise the integrity of other sensing modalities, thus preserving the accuracy and reliability of the data collected. In the realm of multimodal sensing, the image detection serves as a foundational component, providing a visual interface that complements other analytical methods, enhancing the overall functionality of the system. Electrophysiology, as the most important domain in the study of neural organoids, is often assisted by electrochemical and electrical impedance sensing to enrich the dataset and investigate the fundamental principles governing the dynamics of change. The current research on dual-mode and multimodal electronic interfaces and sensors are predominantly concentrated on in vivo electrodes, which can be easily extended to neural organoids to obtain the inside information. Nevertheless, there are still some challenges. Signal interference presents a significant obstacle, as it can compromise the integrity of the data collected. The complexity of data analysis is also a considerable factor, as it requires sophisticated algorithms and computational resources to discern different signal sources. Additionally, there are inherent limitations in the manufacturing processes and associated costs, which can impede the widespread adoption and scalability of these multimodal sensing platforms. Achieving complete synchronization of signal acquisition and subsequent separate analysis remains an aspirational goal that necessitates further research and development. It is anticipated that with ongoing advancements in materials science, signal processing and manufacturing techniques, these challenges will be progressively addressed, leading to more refined and effective multimodal sensing devices capable of providing comprehensive understanding of the intricate organoid models.
Conclusion and outlook
Neural organoids, as an ideal in vitro model, have shown great potential in deciphering neural circuit connections and understanding the human body. To fully utilize this potential and promote the development of neurobiology, technologies need to be developed to reflect the architectures and functionalities of the organoids. In this review, we have summarized the latest advances in bioelectronic interfaces and sensors, focusing on their innovation in adapting to the 3D structure of organoids. The long-term, real-time, and accurate spatiotemporal description of 3D neural networks ensures the continuously expanding application scope of neural organoids in basic research and clinical treatment in the future.
The emerging bioelectronics has offered a unique biological interface with neural organoids. These technologies, each with its unique focus, encompass a comprehensive range of applications, extending from the surface to the interior of neural organoids and from the detection of fine neuronal activity to the characterization of the entire global physiological network of the organoid. Functionally integrated devices that combine existing technologies, including sequencing, imaging and computing, with electrical recording may further promote the development and fully excavate the potential of organoids model103,208. Despite substantial advancements, current neural interface technologies still face notable challenges. For instance, the prolonged growth and development period of neural organoids complicates the establishment of a long-term stable neural interface without disrupting their natural developmental processes. The advent of flexible and adaptive materials offers promising solutions, as optimized electrodes can be designed to better mimic the properties of biological tissues, thereby minimizing mechanical mismatches and resultant damage218,219. However, the long-term introduction of electrodes is inevitably associated with some degree of damage. Strategies such as employing biodegradable materials as electrodes or utilizing highly biocompatible and corrosion-resistant coatings can effectively minimize the adverse effects of foreign substances on biological systems, ensuring the sustained stability and functionality of neural interfaces220,221,222.
In recent years, numerous researchers have focused their attention on incorporating localized and precisely controllable physical and chemical stimuli into neural interfaces, aiming to manipulate neural activity and investigate the underlying mechanisms of neural circuit dynamics. The closed-loop modulation and sensing system, which integrates both stimulation and sensing capabilities, is capable of not only delivering controllable electrical, optical, magnetic, and chemical signal stimulation to neurons but also detecting the electrical activity of target neurons in real time. Based on the feedback from neuronal responses, the stimulation parameters can be dynamically adjusted to achieve optimal modulation32. Electrical stimulation has garnered widespread attention due to its involvement in various key biological processes, including cell proliferation, differentiation, and migration223. For instance, commercially available closed-loop brain stimulation systems have been used for the treatment of neurological disorders such as PD and epilepsy224,225. With the ongoing miniaturization of devices, these systems show promising potential for applications in safe and effective preclinical research with neural organoids. Optogenetic stimulation enables precise spatial and temporal control of neural activity through the targeted expression of light-sensitive proteins in specific neuronal populations226. For instance, opto-electronic neural probes have been developed to facilitate the analysis and high-resolution recording of signals from individual target neurons227. Additionally, chemical modulation, including drug delivery, ultrasound modulation and magnetic modulation techniques, have attracted significant research interest as potential therapeutic approaches for neurological disorders31,228. This closed-loop regulatory system is beneficial for modulating neural plasticity, replacing damaged neurons and establishing new circuits within the nervous system. While current implementations primarily rely on implantable electrodes, with the increasing maturation of neural organoid models, this stimulus-sensing closed-loop regulatory system is likely to emerge as a prominent trend in the development of next-generation neural interface technologies for organoid applications.
The clinical translational potential of organoid technology has garnered increasing attention as an effective preclinical platform for evaluating and guiding personalized therapeutic strategies. In particular, patient-derived stem cell organoids have emerged as an importance tool in advancing personalized medicine. These organoids can faithfully recapitulate the histological architecture and mutational profiles of the original pathology, and neural interfaces can further visualize these disease-associated features229. Accumulating evidence from clinical trials demonstrates the successful application in cancer treatment and fetal congenital disorders230,231. The synergistic combination of organoid platforms with neural interfaces holds significant promise for optimizing therapeutic regimens through high-throughput drug and combination screening. This approach not only enhances clinical decision-making but also contributes to improved patient outcomes and reduced healthcare expenditures. The limited maturity of current organoid systems remains a critical barrier to their broader scalability and clinical translation, particularly due to the absence of key physiological features such as functional vasculature and tissue-specific microenvironmental cues232. The development of more sophisticated models that recapitulate these essential components will be crucial for achieving clinically relevant phenotypes. The organoid technology also holds significant promise for transplantation therapy through the use of patient-derived stem cell products. A notable advantage of this alternative approach is the absence of immune rejection, a feature that has encouraged ongoing clinical trials with considerable therapeutic potential. Nevertheless, due to technical and ethical limitations, comprehensive preclinical validation is necessary prior to clinical implementation230,231. In this regard, implantable electrodes have made significant contributions to validating the functional connections formed between organoids and the host following transplantation109.
References
Tsilidis, K. K. et al. Evaluation of excess significance bias in animal studies of neurological diseases. PLoS Biol. 11, e1001609, https://doi.org/10.1371/journal.pbio.1001609 (2013).
Leenaars, C. H. C. et al. Animal to human translation: a systematic scoping review of reported concordance rates. J. Transl. Med. 17, 223. https://doi.org/10.1186/s12967-019-1976-2 (2019).
Li, M., Gao, L., Zhao, L., Zou, T. & Xu, H. Toward the next generation of vascularized human neural organoids. Med. Res. Rev. 43, 31–54, https://doi.org/10.1002/med.21922 (2023).
Lee, J. H. & Sun, W. Neural organoids, a versatile model for neuroscience. Mol. Cells 45, 53–64, https://doi.org/10.14348/molcells.2022.2019 (2022).
Lancaster, M. A. et al. Cerebral organoids model human brain development and microcephaly. Nature 501, 373–379, https://doi.org/10.1038/nature12517 (2013).
Nickels, S. L. et al. Reproducible generation of human midbrain organoids for in vitro modeling of Parkinson's disease. Stem Cell Res. 46, 101870, https://doi.org/10.1016/j.scr.2020.101870 (2020).
Gomes, A. R. et al. Modeling rett syndrome with human patient-specific forebrain organoids. Front Cell Dev. Biol. 8, 610427, https://doi.org/10.3389/fcell.2020.610427 (2020).
Huang, W. K. et al. Generation of hypothalamic arcuate organoids from human induced pluripotent stem cells. Cell Stem Cell 28, 1657–1670.e1610, https://doi.org/10.1016/j.stem.2021.04.006 (2021).
Zhou, G., Pang, S., Li, Y. & Gao, J. Progress in the generation of spinal cord organoids over the past decade and future perspectives. Neural Regen. Res. 19, 1013–1019, https://doi.org/10.4103/1673-5374.385280 (2024).
Kandoi, S. & Lamba, D. A. Retinal organoids: a human model system for development, diseases, and therapies. Adv. Exp. Med. Biol. 1415, 549–554, https://doi.org/10.1007/978-3-031-27681-1_80 (2023).
Roccio, M. & Edge, A. S. B. Inner ear organoids: new tools to understand neurosensory cell development, degeneration and regeneration. Development 146, https://doi.org/10.1242/dev.177188 (2019).
Kapałczyńska, M. et al. 2D and 3D cell cultures—a comparison of different types of cancer cell cultures. Arch. Med Sci. 14, 910–919, https://doi.org/10.5114/aoms.2016.63743 (2018).
Kelava, I. & Lancaster, M. A. Dishing out mini-brains: current progress and future prospects in brain organoid research. Dev. Biol. 420, 199–209, https://doi.org/10.1016/j.ydbio.2016.06.037 (2016).
Passaro, A. P. & Stice, S. L. Electrophysiological analysis of brain organoids: current approaches and advancements. Front Neurosci. 14, 622137, https://doi.org/10.3389/fnins.2020.622137 (2020).
Barak, M. et al. Human iPSC-derived neural models for studying Alzheimer's disease: from neural stem cells to cerebral organoids. Stem Cell Rev. Rep. 18, 792–820, https://doi.org/10.1007/s12015-021-10254-3 (2022).
Raja, W. K. et al. Self-organizing 3D human neural tissue derived from induced pluripotent stem cells recapitulate Alzheimer's disease phenotypes. PLoS One 11, e0161969, https://doi.org/10.1371/journal.pone.0161969 (2016).
Badr-Eldin, S. M., Aldawsari, H. M., Kotta, S., Deb, P. K. & Venugopala, K. N. Three-dimensional in vitro cell culture models for efficient drug discovery: progress so far and future prospects. Pharmaceuticals (Basel) 15, https://doi.org/10.3390/ph15080926 (2022).
Langhans, S. A. Three-dimensional in vitro cell culture models in drug discovery and drug repositioning. Front Pharm. 9, 6, https://doi.org/10.3389/fphar.2018.00006 (2018).
Maharjan, S. et al. Advanced 3D imaging and organoid bioprinting for biomedical research and therapeutic applications. Adv. Drug Deliv. Rev. 208, 115237, https://doi.org/10.1016/j.addr.2024.115237 (2024).
Jamieson, P. R. et al. Derivation of a robust mouse mammary organoid system for studying tissue dynamics. Development 144, 1065–1071, https://doi.org/10.1242/dev.145045 (2017).
Rios, A. C. & Clevers, H. Imaging organoids: a bright future ahead. Nat. Methods 15, 24–26, https://doi.org/10.1038/nmeth.4537 (2018).
Reynolds, D. E. et al. Live organoid cyclic imaging. Adv. Sci. (Weinh.) 11, e2309289, https://doi.org/10.1002/advs.202309289 (2024).
Liu, S. et al. Biosensors integrated 3D organoid/organ-on-a-chip system: a real-time biomechanical, biophysical, and biochemical monitoring and characterization. Biosens. Bioelectron. 231, 115285, https://doi.org/10.1016/j.bios.2023.115285 (2023).
Kim, D., Kang, H. & Nam, Y. Compact 256-channel multi-well microelectrode array system for in vitro neuropharmacology test. Lab Chip 20, 3410–3422, https://doi.org/10.1039/d0lc00384k (2020).
Wang, Y., Wang, L., Guo, Y., Zhu, Y. & Qin, J. Engineering stem cell-derived 3D brain organoids in a perfusable organ-on-a-chip system. RSC Adv. 8, 1677–1685, https://doi.org/10.1039/c7ra11714k (2018).
Suzuki, I. et al. Large-area field potential imaging having single neuron resolution using 236 880 electrodes CMOS-MEA technology. Adv. Sci. (Weinh.) 10, e2207732, https://doi.org/10.1002/advs.202207732 (2023).
Sharf, T. et al. Functional neuronal circuitry and oscillatory dynamics in human brain organoids. Nat. Commun. 13, 4403. https://doi.org/10.1038/s41467-022-32115-4 (2022).
Fekete, Z., Zátonyi, A., Kaszás, A., Madarász, M. & Slézia, A. Transparent neural interfaces: challenges and solutions of microengineered multimodal implants designed to measure intact neuronal populations using high-resolution electrophysiology and microscopy simultaneously. Microsyst. Nanoeng. 9, 66, https://doi.org/10.1038/s41378-023-00519-x (2023).
Zeng, Q. & Huang, Z. Challenges and opportunities of implantable neural interfaces: from material, electrochemical and biological perspectives. Adv. Funct. Mater. 33, 2301223, https://doi.org/10.1002/adfm.202301223 (2023).
Chae, U. et al. A neural probe for concurrent real-time measurement of multiple neurochemicals with electrophysiology in multiple brain regions in vivo. Proc. Natl Acad. Sci. USA 120, e2219231120, https://doi.org/10.1073/pnas.2219231120 (2023).
Malekoshoaraie, M. H. et al. Fully flexible implantable neural probes for electrophysiology recording and controlled neurochemical modulation. Microsyst. Nanoeng. 10, 91, https://doi.org/10.1038/s41378-024-00685-6 (2024).
Li, L. et al. Multimodal technologies for closed-loop neural modulation and sensing. Adv. Health. Mater. 13, e2303289, https://doi.org/10.1002/adhm.202303289 (2024).
Sidhaye, J. & Knoblich, J. A. Brain organoids: an ensemble of bioassays to investigate human neurodevelopment and disease. Cell Death Differ. 28, 52–67, https://doi.org/10.1038/s41418-020-0566-4 (2021).
Qian, X. et al. Brain-region-specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell 165, 1238–1254, https://doi.org/10.1016/j.cell.2016.04.032 (2016).
Lancaster, M. A. & Knoblich, J. A. Generation of cerebral organoids from human pluripotent stem cells. Nat. Protoc. 9, 2329–2340, https://doi.org/10.1038/nprot.2014.158 (2014).
Quadrato, G. et al. Cell diversity and network dynamics in photosensitive human brain organoids. Nature 545, 48–53, https://doi.org/10.1038/nature22047 (2017).
Trujillo, C. A. et al. Complex oscillatory waves emerging from cortical organoids model early human brain network development. Cell Stem Cell 25, 558–569.e557, https://doi.org/10.1016/j.stem.2019.08.002 (2019).
Gabriel, E. et al. Generation of iPSC-derived human forebrain organoids assembling bilateral eye primordia. Nat. Protoc. 18, 1893–1929, https://doi.org/10.1038/s41596-023-00814-x (2023).
Shimada, H. et al. A next-generation iPSC-derived forebrain organoid model of tauopathy with tau fibrils by AAV-mediated gene transfer. Cell Rep. Methods 2, 100289, https://doi.org/10.1016/j.crmeth.2022.100289 (2022).
Jo, J. et al. Midbrain-like organoids from human pluripotent stem cells contain functional dopaminergic and neuromelanin-producing neurons. Cell Stem Cell 19, 248–257, https://doi.org/10.1016/j.stem.2016.07.005 (2016).
Frattini, E. et al. Lewy pathology formation in patient-derived GBA1 Parkinson's disease mid. Brain Organoids. Brain 148, 1242–1257, https://doi.org/10.1093/brain/awae365 (2025).
Atamian, A. et al. Human cerebellar organoids with functional Purkinje cells. Cell Stem Cell 31, 39–51.e36, https://doi.org/10.1016/j.stem.2023.11.013 (2024).
Atamian, A., Birtele, M., Hosseini, N. & Quadrato, G. Generation and long-term culture of human cerebellar organoids from pluripotent stem cells. Nat Protoc. https://doi.org/10.1038/s41596-024-01093-w (2024).
Sakaguchi, H. et al. Generation of functional hippocampal neurons from self-organizing human embryonic stem cell-derived dorsomedial telencephalic tissue. Nat. Commun. 6, 8896. https://doi.org/10.1038/ncomms9896 (2015).
Wu, Y. et al. Three-dimensional liquid metal-based neuro-interfaces for human hippocampal organoids. Nat. Commun. 15, 4047. https://doi.org/10.1038/s41467-024-48452-5 (2024).
Xiang, Y. et al. hESC-derived thalamic organoids form reciprocal projections when fused with cortical organoids. Cell Stem Cell 24, 487–497.e487, https://doi.org/10.1016/j.stem.2018.12.015 (2019).
Kiral, F. R. et al. Generation of ventralized human thalamic organoids with thalamic reticular nucleus. Cell Stem Cell 30, 677–688.e675, https://doi.org/10.1016/j.stem.2023.03.007 (2023).
Sarrafha, L. et al. Novel human pluripotent stem cell-derived hypothalamus organoids demonstrate cellular diversity. iScience 26, 107525, https://doi.org/10.1016/j.isci.2023.107525 (2023).
Miura, Y. et al. Generation of human striatal organoids and cortico-striatal assembloids from human pluripotent stem cells. Nat. Biotechnol. 38, 1421–1430, https://doi.org/10.1038/s41587-020-00763-w (2020).
Chen, X. et al. Human striatal organoids derived from pluripotent stem cells recapitulate striatal development and compartments. PLoS Biol. 20, e3001868, https://doi.org/10.1371/journal.pbio.3001868 (2022).
Pellegrini, L. et al. Human CNS barrier-forming organoids with cerebrospinal fluid production. Science 369, https://doi.org/10.1126/science.aaz5626 (2020).
Muok, L. et al. Inflammatory response and exosome biogenesis of choroid plexus organoids derived from human pluripotent stem cells. Int. J. Mol. Sci. 24, https://doi.org/10.3390/ijms24087660 (2023).
Bagley, J. A., Reumann, D., Bian, S., Lévi-Strauss, J. & Knoblich, J. A. Fused cerebral organoids model interactions between brain regions. Nat. Methods 14, 743–751, https://doi.org/10.1038/nmeth.4304 (2017).
Xiang, Y. et al. Fusion of regionally specified hPSC-derived organoids models human brain development and interneuron migration. Cell Stem Cell 21, 383–398.e387, https://doi.org/10.1016/j.stem.2017.07.007 (2017).
Birey, F. et al. Assembly of functionally integrated human forebrain spheroids. Nature 545, 54–59, https://doi.org/10.1038/nature22330 (2017).
Birey, F. et al. Dissecting the molecular basis of human interneuron migration in forebrain assembloids from Timothy syndrome. Cell Stem Cell 29, 248–264.e247, https://doi.org/10.1016/j.stem.2021.11.011 (2022).
Pang, W. et al. Generation of human region-specific brain organoids with medullary spinal trigeminal nuclei. Cell Stem Cell 31, 1501–1512.e1508, https://doi.org/10.1016/j.stem.2024.08.004 (2024).
Andersen, J. et al. Generation of functional human 3D cortico-motor assembloids. Cell 183, 1913–1929.e1926, https://doi.org/10.1016/j.cell.2020.11.017 (2020).
Wang, Y. et al. Modeling human telencephalic development and autism-associated SHANK3 deficiency using organoids generated from single neural rosettes. Nat. Commun. 13, 5688. https://doi.org/10.1038/s41467-022-33364-z (2022).
Smits, L. M. et al. Modeling Parkinson's disease in midbrain-like organoids. NPJ Parkinsons Dis. 5, 5, https://doi.org/10.1038/s41531-019-0078-4 (2019).
Qian, X., Song, H. & Ming, G. L. Brain organoids: advances, applications and challenges. Development 146, https://doi.org/10.1242/dev.166074 (2019).
Yao, H. et al. Buprenorphine and methadone differentially alter early brain development in human cortical organoids. Neuropharmacology 239, 109683, https://doi.org/10.1016/j.neuropharm.2023.109683 (2023).
Ao, Z. et al. Understanding immune-driven brain aging by human brain organoid microphysiological analysis platform. Adv. Sci. (Weinh.) 9, e2200475, https://doi.org/10.1002/advs.202200475 (2022).
Goulding, M. Circuits controlling vertebrate locomotion: moving in a new direction. Nat. Rev. Neurosci. 10, 507–518, https://doi.org/10.1038/nrn2608 (2009).
Quinn, C. & Elman, L. Amyotrophic lateral sclerosis and other motor neuron diseases. Contin. (Minneap. Minn.) 26, 1323–1347, https://doi.org/10.1212/con.0000000000000911 (2020).
Lippmann, E. S. et al. Deterministic HOX patterning in human pluripotent stem cell-derived neuroectoderm. Stem Cell Rep. 4, 632–644, https://doi.org/10.1016/j.stemcr.2015.02.018 (2015).
Hor, J. H. et al. Cell cycle inhibitors protect motor neurons in an organoid model of Spinal Muscular Atrophy. Cell Death Dis. 9, 1100. https://doi.org/10.1038/s41419-018-1081-0 (2018).
Libby, A. R. G. et al. Axial elongation of caudalized human organoids mimics aspects of neural tube development. Development 148, https://doi.org/10.1242/dev.198275 (2021).
Buchner, F., Dokuzluoglu, Z., Grass, T. & Rodriguez-Muela, N. Spinal cord organoids to study motor neuron development and disease. Life (Basel) 13, https://doi.org/10.3390/life13061254 (2023).
Iyer, N. R. & Ashton, R. S. Bioengineering the human spinal cord. Front Cell Dev. Biol. 10, 942742, https://doi.org/10.3389/fcell.2022.942742 (2022).
Dharmadasa, T. et al. Motor neurone disease: progress and challenges. Med J. Aust. 206, 357–362, https://doi.org/10.5694/mja16.01063 (2017).
Zheng, Y., Zhang, F., Xu, S. & Wu, L. Advances in neural organoid systems and their application in neurotoxicity testing of environmental chemicals. Genes Environ. 43, 39. https://doi.org/10.1186/s41021-021-00214-1 (2021).
Pereira, J. D. et al. Human sensorimotor organoids derived from healthy and amyotrophic lateral sclerosis stem cells form neuromuscular junctions. Nat. Commun. 12, 4744. https://doi.org/10.1038/s41467-021-24776-4 (2021).
Grass, T. et al. An isogenic human iPSC model unravels neurodevelopmental abnormalities in SMA. bioRxiv, 2023.2001.2002.522499, https://doi.org/10.1101/2023.01.02.522499 (2023).
Sironi, F., De Marchi, F., Mazzini, L. & Bendotti, C. Cell therapy in ALS: an update on preclinical and clinical studies. Brain Res. Bull. 194, 64–81, https://doi.org/10.1016/j.brainresbull.2023.01.008 (2023).
Hollenberg, M. J. & Spira, A. W. Early development of the human retina. Can. J. Ophthalmol. 7, 472–491 (1972).
Provis, J. M. et al. Development of the human retinal vasculature: cellular relations and VEGF expression. Exp. Eye Res. 65, 555–568, https://doi.org/10.1006/exer.1997.0365 (1997).
Gabriel, E. et al. Human brain organoids assemble functionally integrated bilateral optic vesicles. Cell Stem Cell 28, 1740–1757.e1748, https://doi.org/10.1016/j.stem.2021.07.010 (2021).
O'Hara-Wright, M. & Gonzalez-Cordero, A. Retinal organoids: a window into human retinal development. Development 147, https://doi.org/10.1242/dev.189746 (2020).
Capowski, E. E. et al. Reproducibility and staging of 3D human retinal organoids across multiple pluripotent stem cell lines. Development 146, https://doi.org/10.1242/dev.171686 (2019).
Zhong, X. et al. Generation of three-dimensional retinal tissue with functional photoreceptors from human iPSCs. Nat. Commun. 5, 4047. https://doi.org/10.1038/ncomms5047 (2014).
Eiraku, M. et al. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature 472, 51–56, https://doi.org/10.1038/nature09941 (2011).
Capowski, E. E. et al. Loss of MITF expression during human embryonic stem cell differentiation disrupts retinal pigment epithelium development and optic vesicle cell proliferation. Hum. Mol. Genet 23, 6332–6344, https://doi.org/10.1093/hmg/ddu351 (2014).
Chew, S. H., Martinez, C., Chirco, K. R., Kandoi, S. & Lamba, D. A. Timed notch inhibition drives photoreceptor fate specification in human retinal organoids. Invest Ophthalmol. Vis. Sci. 63, 12, https://doi.org/10.1167/iovs.63.10.12 (2022).
Li, X., Zhang, L., Tang, F. & Wei, X. Retinal organoids: cultivation, differentiation, and transplantation. Front Cell Neurosci. 15, 638439, https://doi.org/10.3389/fncel.2021.638439 (2021).
Nakano, T. et al. Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell 10, 771–785, https://doi.org/10.1016/j.stem.2012.05.009 (2012).
Ohlemacher, S. K., Iglesias, C. L., Sridhar, A., Gamm, D. M. & Meyer, J. S. Generation of highly enriched populations of optic vesicle-like retinal cells from human pluripotent stem cells. Curr. Protoc. Stem Cell Biol. 32, 1 h.8.1–1 h.8.20, https://doi.org/10.1002/9780470151808.sc01h08s32 (2015).
Chichagova, V. et al. Differentiation of retinal organoids from human pluripotent stem cells. Curr. Protoc. Stem Cell Biol. 50, e95, https://doi.org/10.1002/cpsc.95 (2019).
Ahmad, I., Teotia, P., Erickson, H. & Xia, X. Recapitulating developmental mechanisms for retinal regeneration. Prog. Retin Eye Res 76, 100824, https://doi.org/10.1016/j.preteyeres.2019.100824 (2020).
McLelland, B. T. et al. Transplanted hESC-derived retina organoid sheets differentiate, integrate, and improve visual function in retinal degenerate rats. Invest Ophthalmol. Vis. Sci. 59, 2586–2603, https://doi.org/10.1167/iovs.17-23646 (2018).
Hayashi, R. et al. Co-ordinated ocular development from human iPS cells and recovery of corneal function. Nature 531, 376–380, https://doi.org/10.1038/nature17000 (2016).
Streit, A. Origin of the vertebrate inner ear: evolution and induction of the otic placode. J. Anat. 199, 99–103, https://doi.org/10.1046/j.1469-7580.2001.19910099.x (2001).
Lim, R. & Brichta, A. M. Anatomical and physiological development of the human inner ear. Hear Res. 338, 9–21, https://doi.org/10.1016/j.heares.2016.02.004 (2016).
Moeinvaziri, F., Zarkesh, I., Pooyan, P., Nunez, D. A. & Baharvand, H. Inner ear organoids: progress and outlook, with a focus on the vascularization. FEBS J. 289, 7368–7384, https://doi.org/10.1111/febs.16146 (2022).
Tang, P. C., Hashino, E. & Nelson, R. F. Progress in modeling and targeting inner ear disorders with pluripotent stem cells. Stem Cell Rep. 14, 996–1008, https://doi.org/10.1016/j.stemcr.2020.04.008 (2020).
Bergman, J. E. et al. Advancements in stem cell technology and organoids for the restoration of sensorineural hearing loss. J. Am. Acad. Audio. 32, 636–645, https://doi.org/10.1055/s-0041-1728677 (2021).
Koehler, K. R. et al. Generation of inner ear organoids containing functional hair cells from human pluripotent stem cells. Nat. Biotechnol. 35, 583–589, https://doi.org/10.1038/nbt.3840 (2017).
Nie, J. & Hashino, E. Generation of inner ear organoids from human pluripotent stem cells. Methods Cell Biol. 159, 303–321, https://doi.org/10.1016/bs.mcb.2020.02.006 (2020).
Chen, J. et al. Differentiation and transplantation of human induced pluripotent stem cell-derived otic epithelial progenitors in mouse cochlea. Stem Cell Res. Ther. 9, 230, https://doi.org/10.1186/s13287-018-0967-1 (2018).
Lledo, P. M., Gheusi, G. & Vincent, J. D. Information processing in the mammalian olfactory system. Physiol. Rev. 85, 281–317, https://doi.org/10.1152/physrev.00008.2004 (2005).
Ren, W. et al. Expansion of murine and human olfactory epithelium/mucosa colonies and generation of mature olfactory sensory neurons under chemically defined conditions. Theranostics 11, 684–699, https://doi.org/10.7150/thno.46750 (2021).
Chen, X., Fang, H. & Schwob, J. E. Multipotency of purified, transplanted globose basal cells in olfactory epithelium. J. Comp. Neurol. 469, 457–474, https://doi.org/10.1002/cne.11031 (2004).
Liu, M. et al. Multimodal spatiotemporal monitoring of basal stem cell-derived organoids reveals progression of olfactory dysfunction in Alzheimer's disease. Biosens. Bioelectron. 246, 115832, https://doi.org/10.1016/j.bios.2023.115832 (2024).
Jiang, N. et al. in 2022 IEEE International Symposium on Olfaction and Electronic Nose (ISOEN) 1–3.
Liu, H. et al. Advances in hydrogels in organoids and organs-on-a-chip. Adv. Mater. 31, e1902042, https://doi.org/10.1002/adma.201902042 (2019).
Shin, J. et al. Three-dimensional electroconductive hyaluronic acid hydrogels incorporated with carbon nanotubes and polypyrrole by catechol-mediated dispersion enhance neurogenesis of human neural stem cells. Biomacromolecules 18, 3060–3072, https://doi.org/10.1021/acs.biomac.7b00568 (2017).
Hong, S. et al. Hyaluronic acid catechol: a biopolymer exhibiting a ph-dependent adhesive or cohesive property for human neural stem cell engineering. Adv. Funct. Mater. 23, 1774–1780, https://doi.org/10.1002/adfm.201202365 (2013).
Yu, J. Vascularized organoids: a more complete model. Int J. Stem Cells 14, 127–137, https://doi.org/10.15283/ijsc20143 (2021).
Wilson, M. N. et al. Multimodal monitoring of human cortical organoids implanted in mice reveal functional connection with visual cortex. Nat. Commun. 13, 7945. https://doi.org/10.1038/s41467-022-35536-3 (2022).
Dong, X. et al. Human cerebral organoids establish subcortical projections in the mouse brain after transplantation. Mol. Psychiatry 26, 2964–2976, https://doi.org/10.1038/s41380-020-00910-4 (2021).
Ahn, Y. et al. Human blood vessel organoids penetrate human cerebral organoids and form a vessel-like system. Cells 10, https://doi.org/10.3390/cells10082036 (2021).
Shi, Y. et al. Vascularized human cortical organoids (vOrganoids) model cortical development in vivo. PLoS Biol. 18, e3000705, https://doi.org/10.1371/journal.pbio.3000705 (2020).
Salmon, I. et al. Engineering neurovascular organoids with 3D printed microfluidic chips. Lab Chip 22, 1615–1629, https://doi.org/10.1039/d1lc00535a (2022).
Jeong, E., Choi, S. & Cho, S. W. Recent advances in brain organoid technology for human brain research. ACS Appl Mater. Interfaces 15, 200–219, https://doi.org/10.1021/acsami.2c17467 (2023).
Cakir, B. et al. Engineering of human brain organoids with a functional vascular-like system. Nat. Methods 16, 1169–1175, https://doi.org/10.1038/s41592-019-0586-5 (2019).
Hernández, D. et al. Culture variabilities of human iPSC-derived cerebral organoids are a major issue for the modelling of phenotypes observed in Alzheimer's disease. Stem Cell Rev. Rep. 18, 718–731, https://doi.org/10.1007/s12015-021-10147-5 (2022).
Renner, H. et al. A fully automated high-throughput workflow for 3D-based chemical screening in human midbrain organoids. Elife 9, https://doi.org/10.7554/eLife.52904 (2020).
Rezaei, B. et al. Modular 3D printed platform for fluidically connected human brain organoid culture. Biofabrication 16, https://doi.org/10.1088/1758-5090/ad0c2c (2023).
Sun, X. et al. One-stop assembly of adherent 3D retinal organoids from hiPSCs based on 3D-printed derived PDMS microwell platform. Biofabrication 15, https://doi.org/10.1088/1758-5090/acc761 (2023).
Cui, K. et al. Neurodevelopmental impairment induced by prenatal valproic acid exposure shown with the human cortical organoid-on-a-chip model. Microsyst. Nanoeng. 6, 49, https://doi.org/10.1038/s41378-020-0165-z (2020).
Zhu, Y. et al. In situ generation of human brain organoids on a micropillar array. Lab Chip 17, 2941–2950, https://doi.org/10.1039/c7lc00682a (2017).
Wu, H. W., Hsiao, Y. H., Chen, C. C., Yet, S. F. & Hsu, C. H. A PDMS-based microfluidic hanging drop chip for embryoid body formation. Molecules 21, https://doi.org/10.3390/molecules21070882 (2016).
Gu, Q., Tomaskovic-Crook, E., Wallace, G. G. & Crook, J. M. 3D bioprinting human induced pluripotent stem cell constructs for in situ cell proliferation and successive multilineage differentiation. Adv. Healthc. Mater. 6, https://doi.org/10.1002/adhm.201700175 (2017).
Zhou, L. et al. Lipid-bilayer-supported 3d printing of human cerebral cortex cells reveals developmental interactions. Adv. Mater. 32, e2002183, https://doi.org/10.1002/adma.202002183 (2020).
Stam, C. J. Modern network science of neurological disorders. Nat. Rev. Neurosci. 15, 683–695, https://doi.org/10.1038/nrn3801 (2014).
Palop, J. J. & Mucke, L. Network abnormalities and interneuron dysfunction in Alzheimer disease. Nat. Rev. Neurosci. 17, 777–792, https://doi.org/10.1038/nrn.2016.141 (2016).
Deng, W. L. et al. Gene correction reverses ciliopathy and photoreceptor loss in iPSC-derived retinal organoids from retinitis pigmentosa patients. Stem Cell Rep. 10, 1267–1281, https://doi.org/10.1016/j.stemcr.2018.02.003 (2018).
Huang, R. et al. Progress in spinal cord organoid research: advancing understanding of neural development, disease modelling, and regenerative medicine. Biomater. Transl. 5, 355–371, https://doi.org/10.12336/biomatertransl.2024.04.003 (2024).
Lv, S. et al. Using human-induced pluripotent stem cell derived neurons on microelectrode arrays to model neurological disease: a review. Adv. Sci. (Weinh.) 10, e2301828, https://doi.org/10.1002/advs.202301828 (2023).
Fu, T. M. et al. Stable long-term chronic brain mapping at the single-neuron level. Nat. Methods 13, 875–882, https://doi.org/10.1038/nmeth.3969 (2016).
Ko, S. et al. Electrode potential influences the reversibility of lithium-metal anodes. Nat. Energy 7, 1217–1224, https://doi.org/10.1038/s41560-022-01144-0 (2022).
Pelkonen, A. et al. Functional characterization of human pluripotent stem cell-derived models of the brain with microelectrode arrays. Cells 11, https://doi.org/10.3390/cells11010106 (2021).
Fair, S. R. et al. Electrophysiological maturation of cerebral organoids correlates with dynamic morphological and cellular development. Stem Cell Rep. 15, 855–868, https://doi.org/10.1016/j.stemcr.2020.08.017 (2020).
Ao, Z. et al. Human spinal organoid-on-a-chip to model nociceptive circuitry for pain therapeutics discovery. Anal. Chem. 94, 1365–1372, https://doi.org/10.1021/acs.analchem.1c04641 (2022).
Zhou, J. et al. Divergent network connectivity changes in behavioural variant frontotemporal dementia and Alzheimer's disease. Brain 133, 1352–1367, https://doi.org/10.1093/brain/awq075 (2010).
Samarasinghe, R. A. et al. Identification of neural oscillations and epileptiform changes in human brain organoids. Nat. Neurosci. 24, 1488–1500, https://doi.org/10.1038/s41593-021-00906-5 (2021).
Mossink, B. et al. Cadherin-13 is a critical regulator of GABAergic modulation in human stem-cell-derived neuronal networks. Mol. Psychiatry 27, 1–18, https://doi.org/10.1038/s41380-021-01117-x (2022).
Ghatak, S. et al. NitroSynapsin ameliorates hypersynchronous neural network activity in Alzheimer hiPSC models. Mol. Psychiatry 26, 5751–5765, https://doi.org/10.1038/s41380-020-0776-7 (2021).
Lebedeva, O. S. et al. The morphofunctional properties of induced pluripotent stem cells derived from human skin fibroblasts and differentiated to dopaminergic neurons. Neurochem. J. 7, 207–214, https://doi.org/10.1134/S1819712413030082 (2013).
Perkins, E. M. et al. Altered network properties in C9ORF72 repeat expansion cortical neurons are due to synaptic dysfunction. Mol. Neurodegener. 16, 13, https://doi.org/10.1186/s13024-021-00433-8 (2021).
Tukker, A. M., Wijnolts, F. M. J., de Groot, A. & Westerink, R. H. S. Applicability of hiPSC-derived neuronal cocultures and rodent primary cortical cultures for in vitro seizure liability assessment. Toxicol. Sci. 178, 71–87, https://doi.org/10.1093/toxsci/kfaa136 (2020).
Wilson, E., Knudson, W. & Newell-Litwa, K. Hyaluronan regulates synapse formation and function in developing neural networks. Sci. Rep. 10, 16459. https://doi.org/10.1038/s41598-020-73177-y (2020).
Vasistha, N. A. & Khodosevich, K. The impact of (ab)normal maternal environment on cortical development. Prog. Neurobiol. 202, 102054, https://doi.org/10.1016/j.pneurobio.2021.102054 (2021).
Yao, H. et al. Methadone interrupts neural growth and function in human cortical organoids. Stem Cell Res. 49, 102065, https://doi.org/10.1016/j.scr.2020.102065 (2020).
Thomas, B. B. et al. Co-grafts of human embryonic stem cell derived retina organoids and retinal pigment epithelium for retinal reconstruction in immunodeficient retinal degenerate royal college of surgeons rats. Front Neurosci. 15, 752958, https://doi.org/10.3389/fnins.2021.752958 (2021).
Perna, A., Angotzi, G. N., Berdondini, L. & Ribeiro, J. F. Advancing the interfacing performances of chronically implantable neural probes in the era of CMOS neuroelectronics. Front Neurosci. 17, 1275908, https://doi.org/10.3389/fnins.2023.1275908 (2023).
Berdondini, L. et al. Active pixel sensor array for high spatio-temporal resolution electrophysiological recordings from single cell to large scale neuronal networks. Lab Chip 9, 2644–2651, https://doi.org/10.1039/b907394a (2009).
Ballini, M. et al. A 1024-channel CMOS microelectrode array with 26,400 electrodes for recording and stimulation of electrogenic cells in vitro. IEEE J. Solid-State Circuits 49, 2705–2719, https://doi.org/10.1109/jssc.2014.2359219 (2014).
Schröter, M. et al. Functional imaging of brain organoids using high-density microelectrode arrays. MRS Bull. 47, 530–544, https://doi.org/10.1557/s43577-022-00282-w (2022).
Obien, M. E. J., Hierlemann, A. & Frey, U. Accurate signal-source localization in brain slices by means of high-density microelectrode arrays. Sci. Rep. 9, 788. https://doi.org/10.1038/s41598-018-36895-y (2019).
Giandomenico, S. L. et al. Cerebral organoids at the air-liquid interface generate diverse nerve tracts with functional output. Nat. Neurosci. 22, 669–679, https://doi.org/10.1038/s41593-019-0350-2 (2019).
Thiebaut de Schotten, M. & Forkel, S. J. The emergent properties of the connected brain. Science 378, 505–510, https://doi.org/10.1126/science.abq2591 (2022).
Jun, J. J. et al. Fully integrated silicon probes for high-density recording of neural activity. Nature 551, 232–236, https://doi.org/10.1038/nature24636 (2017).
Tang-Schomer, M. D. et al. Bioengineered functional brain-like cortical tissue. Proc. Natl. Acad. Sci. USA 111, 13811–13816, https://doi.org/10.1073/pnas.1324214111 (2014).
Shin, H. et al. 3D high-density microelectrode array with optical stimulation and drug delivery for investigating neural circuit dynamics. Nat. Commun. 12, 492. https://doi.org/10.1038/s41467-020-20763-3 (2021).
Cho, Y. H., Park, Y. G., Kim, S. & Park, J. U. 3D Electrodes for Bioelectronics. Adv. Mater. 33, e2005805, https://doi.org/10.1002/adma.202005805 (2021).
Zeck, G. & Fromherz, P. Repulsion and attraction by extracellular matrix protein in cell adhesion studied with nerve cells and lipid vesicles on silicon chips. Langmuir 19, 1580–1585, https://doi.org/10.1021/la0263209 (2003).
Xiang, Y. et al. AcTive Micro-nano-collaborative Bioelectronic Device For Advanced Electrophysiological Recording. Nanomicro Lett. 16, 132, https://doi.org/10.1007/s40820-024-01336-1 (2024).
Phouphetlinthong, O. et al. Protruding cantilever microelectrode array to monitor the inner electrical activity of cerebral organoids. Lab Chip 23, 3603–3614, https://doi.org/10.1039/d3lc00294b (2023).
Zheng, J. et al. Micronano synergetic three-dimensional bioelectronics: a revolutionary breakthrough platform for cardiac electrophysiology. ACS Nano 18, 15332–15357, https://doi.org/10.1021/acsnano.4c00052 (2024).
Aoyagi, S., Izumi, H., Isono, Y., Fukuda, M. & Ogawa, H. Laser fabrication of high aspect ratio thin holes on biodegradable polymer and its application to a microneedle. Sens. Actuators A Phys. 139, 293–302, https://doi.org/10.1016/j.sna.2006.11.022 (2007).
Yuk, H. et al. 3D printing of conducting polymers. Nat. Commun. 11, 1604. https://doi.org/10.1038/s41467-020-15316-7 (2020).
Lee, S. et al. Electrophysiological analysis of retinal organoid development using 3D microelectrodes of liquid metals. Adv. Mater. 36, e2404428, https://doi.org/10.1002/adma.202404428 (2024).
Chung, W. G. et al. Liquid-metal-based three-dimensional microelectrode arrays integrated with implantable ultrathin retinal prosthesis for vision restoration. Nat. Nanotechnol. 19, 688–697, https://doi.org/10.1038/s41565-023-01587-w (2024).
Kim, E. et al. Magnetically reshapable 3D multi-electrode arrays of liquid metals for electrophysiological analysis of brain organoids. Nat. Commun. 16, 2011. https://doi.org/10.1038/s41467-024-55752-3 (2025).
Zips, S. et al. Aerosol jet-printed high-aspect ratio micro-needle electrode arrays applied for human cerebral organoids and 3d neurospheroid networks. ACS Appl. Mater. Interfaces 15, 35950–35961, https://doi.org/10.1021/acsami.3c06210 (2023).
Qing, Q. et al. Free-standing kinked nanowire transistor probes for targeted intracellular recording in three dimensions. Nat. Nanotechnol. 9, 142–147, https://doi.org/10.1038/nnano.2013.273 (2014).
Zhao, Y. et al. Scalable ultrasmall three-dimensional nanowire transistor probes for intracellular recording. Nat. Nanotechnol. 14, 783–790, https://doi.org/10.1038/s41565-019-0478-y (2019).
Desbiolles, B. X. E. et al. Nanovolcano microelectrode arrays: toward long-term on-demand registration of transmembrane action potentials by controlled electroporation. Microsyst. Nanoeng. 6, 67, https://doi.org/10.1038/s41378-020-0178-7 (2020).
Dipalo, M. et al. Intracellular action potential recordings from cardiomyocytes by ultrafast pulsed laser irradiation of fuzzy graphene microelectrodes. Sci. Adv. 7, https://doi.org/10.1126/sciadv.abd5175 (2021).
Soscia, D. A. et al. A flexible 3-dimensional microelectrode array for in vitro brain models. Lab Chip 20, 901–911, https://doi.org/10.1039/c9lc01148j (2020).
Lam, D., Fischer, N. O. & Enright, H. A. Probing function in 3D neuronal cultures: A survey of 3D multielectrode array advances. Curr. Opin. Pharm. 60, 255–260, https://doi.org/10.1016/j.coph.2021.08.003 (2021).
Lam, D. et al. Spatiotemporal analysis of 3D human iPSC-derived neural networks using a 3D multi-electrode array. Front Cell Neurosci. 17, 1287089, https://doi.org/10.3389/fncel.2023.1287089 (2023).
Kalmykov, A. et al. Bioelectrical interfaces with cortical spheroids in three-dimensions. J. Neural Eng. 18, https://doi.org/10.1088/1741-2552/abf290 (2021).
Cools, J. et al. A micropatterned multielectrode shell for 3d spatiotemporal recording from live cells. Adv. Sci. (Weinh.) 5, 1700731, https://doi.org/10.1002/advs.201700731 (2018).
Huang, Q. et al. Shell microelectrode arrays (MEAs) for brain organoids. Sci. Adv. 8, eabq5031, https://doi.org/10.1126/sciadv.abq5031 (2022).
Park, Y. et al. Three-dimensional, multifunctional neural interfaces for cortical spheroids and engineered assembloids. Sci. Adv. 7, https://doi.org/10.1126/sciadv.abf9153 (2021).
McDonald, M. et al. A mesh microelectrode array for non-invasive electrophysiology within neural organoids. Biosens. Bioelectron. 228, 115223, https://doi.org/10.1016/j.bios.2023.115223 (2023).
Li, T. L. et al. Stretchable mesh microelectronics for the biointegration and stimulation of human neural organoids. Biomaterials 290, 121825, https://doi.org/10.1016/j.biomaterials.2022.121825 (2022).
Yang, X. et al. Kirigami electronics for long-term electrophysiological recording of human neural organoids and assembloids. Nat. Biotechnol. https://doi.org/10.1038/s41587-023-02081-3 (2024).
Li, Q. et al. Cyborg organoids: implantation of nanoelectronics via organogenesis for tissue-wide electrophysiology. Nano Lett. 19, 5781–5789, https://doi.org/10.1021/acs.nanolett.9b02512 (2019).
Le Floch, P. et al. Stretchable mesh nanoelectronics for 3D single-cell chronic electrophysiology from developing brain organoids. Adv. Mater. 34, e2106829, https://doi.org/10.1002/adma.202106829 (2022).
Morgan, H., Sun, T., Holmes, D., Gawad, S. & Green, N. G. Single cell dielectric spectroscopy. J. Phys. D Appl. Phys. 40, 61, https://doi.org/10.1088/0022-3727/40/1/S10 (2007).
Nguyen, T. A., Yin, T.-I., Reyes, D. & Urban, G. A. Microfluidic chip with integrated electrical cell-impedance sensing for monitoring single cancer cell migration in three-dimensional matrixes. Anal. Chem. 85, 11068–11076, https://doi.org/10.1021/ac402761s (2013).
Hong, J., Kandasamy, K., Marimuthu, M., Choi, C. S. & Kim, S. Electrical cell-substrate impedance sensing as a non-invasive tool for cancer cell study. Analyst 136, 237–245, https://doi.org/10.1039/C0AN00560F (2011).
Pick, H. et al. Monitoring proliferative activities of hormone-like odorants in human breast cancer cells by gene transcription profiling and electrical impedance spectroscopy. Biosens. Bioelectron. 50, 431–436, https://doi.org/10.1016/j.bios.2013.06.052 (2013).
Otero-González, L., Sierra-Alvarez, R., Boitano, S. & Field, J. A. Application and validation of an impedance-based real time cell analyzer to measure the toxicity of nanoparticles impacting human bronchial epithelial cells. Environ. Sci. Technol. 46, 10271–10278, https://doi.org/10.1021/es301599f (2012).
Yang, L. & Bashir, R. Electrical/electrochemical impedance for rapid detection of foodborne pathogenic bacteria. Biotechnol. Adv. 26, 135–150, https://doi.org/10.1016/j.biotechadv.2007.10.003 (2008).
Armstrong, R. What causes neurodegenerative disease?. Folia Neuropathol.58, 93–112, https://doi.org/10.5114/fn.2020.96707 (2020).
Arendt, T., Stieler, J. T. & Holzer, M. Tau and tauopathies. Brain Res. Bull. 126, 238–292, https://doi.org/10.1016/j.brainresbull.2016.08.018 (2016).
Jahnke, H.-G. et al. An impedimetric microelectrode-based array sensor for label-free detection of tau hyperphosphorylation in human cells. Lab Chip 9, 1422–1428, https://doi.org/10.1039/B819754G (2009).
Jahnke, H.-G. et al. Impedance spectroscopy based measurement system for quantitative and label-free real-time monitoring of tauopathy in hippocampal slice cultures. Biosens. Bioelectron. 32, 250–258, https://doi.org/10.1016/j.bios.2011.12.026 (2012).
Wu, Q. et al. Bionic 3D spheroids biosensor chips for high-throughput and dynamic drug screening. Biomed. Microdevices 20, 82. https://doi.org/10.1007/s10544-018-0329-x (2018).
Ouahoud, S., Giugliano, F. P. & Muncan, V. Monitoring intestinal organoid-derived monolayer barrier functions with electric cell-substrate impedance sensing (ECIS). Bio Protoc. 14, e4947, https://doi.org/10.21769/BioProtoc.4947 (2024).
Pan, Y. et al. 3D cell-based biosensor for cell viability and drug assessment by 3D electric cell/matrigel-substrate impedance sensing. Biosens. Bioelectron. 130, 344–351, https://doi.org/10.1016/j.bios.2018.09.046 (2019).
Qiu, Y. et al. Vertical impedance electrode array for spatiotemporal dynamics monitoring of 3D cells under drug diffusion effect. iScience 26, 107962, https://doi.org/10.1016/j.isci.2023.107962 (2023).
Pan, Y. et al. 3D microgroove electrical impedance sensing to examine 3D cell cultures for antineoplastic drug assessment. Microsyst. Nanoeng.6, 23, https://doi.org/10.1038/s41378-020-0130-x (2020).
Lei, K. F., Liu, T.-K. & Tsang, N.-M. Towards a high throughput impedimetric screening of chemosensitivity of cancer cells suspended in hydrogel and cultured in a paper substrate. Biosens. Bioelectron. 100, 355–360, https://doi.org/10.1016/j.bios.2017.09.029 (2018).
Luo, X. & Davis, J. J. Electrical biosensors and the label free detection of protein disease biomarkers. Chem. Soc. Rev. 42, 5944–5962, https://doi.org/10.1039/c3cs60077g (2013).
Duan, X. et al. Quantification of the affinities and kinetics of protein interactions using silicon nanowire biosensors. Nat. Nanotechnol. 7, 401–407, https://doi.org/10.1038/nnano.2012.82 (2012).
Lee, M.-H. et al. Peptide-imprinted poly(hydroxymethyl 3,4-ethylenedioxythiophene) nanotubes for detection of α synuclein in human brain organoids. ACS Appl. Nano Mater. 3, 8027–8036, https://doi.org/10.1021/acsanm.0c01476 (2020).
Lee, M. H. et al. Epitope imprinting of alpha-synuclein for sensing in Parkinson's brain organoid culture medium. Biosens. Bioelectron. 175, 112852, https://doi.org/10.1016/j.bios.2020.112852 (2021).
Zanetti, C. et al. Monitoring the neurotransmitter release of human midbrain organoids using a redox cycling microsensor as a novel tool for personalized Parkinson's disease modelling and drug screening. Analyst 146, 2358–2367, https://doi.org/10.1039/d0an02206c (2021).
Rantataro, S., Parkkinen, I., Airavaara, M. & Laurila, T. Real-time selective detection of dopamine and serotonin at nanomolar concentration from complex in vitro systems. Biosens. Bioelectron. 241, 115579, https://doi.org/10.1016/j.bios.2023.115579 (2023).
Nasr, B. et al. Self-organized nanostructure modified microelectrode for sensitive electrochemical glutamate detection in stem cells-derived brain organoids. Biosensors (Basel) 8, https://doi.org/10.3390/bios8010014 (2018).
Mishin, A. S. & Lukyanov, K. A. Live-cell super-resolution fluorescence microscopy. Biochemistry (Moscow) 84, S19–s31, https://doi.org/10.1134/s0006297919140025 (2019).
Zafeiriou, M. P. et al. Developmental GABA polarity switch and neuronal plasticity in Bioengineered Neuronal Organoids. Nat. Commun. 11, 3791. https://doi.org/10.1038/s41467-020-17521-w (2020).
Wang, X. et al. Three-dimensional intact-tissue sequencing of single-cell transcriptional states. Science 361, https://doi.org/10.1126/science.aat5691 (2018).
Xu, Y., Jia, Y., Ma, J., Hayat, T. & Alsaedi, A. Collective responses in electrical activities of neurons under field coupling. Sci. Rep. 8, 1349. https://doi.org/10.1038/s41598-018-19858-1 (2018).
Pereda, A. E. Electrical synapses and their functional interactions with chemical synapses. Nat. Rev. Neurosci. 15, 250–263, https://doi.org/10.1038/nrn3708 (2014).
Zhang, S. et al. Real-time simultaneous recording of electrophysiological activities and dopamine overflow in the deep brain nuclei of a non-human primate with Parkinson’s disease using nano-based microelectrode arrays. Microsyst. Nanoeng.4, 17070, https://doi.org/10.1038/micronano.2017.70 (2018).
Hejazi, M. A. et al. Hybrid diamond/ carbon fiber microelectrodes enable multimodal electrical/chemical neural interfacing. Biomaterials 230, 119648, https://doi.org/10.1016/j.biomaterials.2019.119648 (2020).
Cheng, H. et al. Simultaneous in vivo ascorbate and electrophysiological recordings in rat brain following ischemia/reperfusion. J. Electroanal. Chem. 781, 90–96, https://doi.org/10.1016/j.jelechem.2016.10.024 (2016).
Li, Z. et al. Bio-electrochemical microelectrode arrays for glutamate and electrophysiology detection in hippocampus of temporal lobe epileptic rats. Anal. Biochem 550, 123–131, https://doi.org/10.1016/j.ab.2018.04.023 (2018).
Disney, A. A., McKinney, C., Grissom, L., Lu, X. & Reynolds, J. H. A multi-site array for combined local electrochemistry and electrophysiology in the non-human primate brain. J. Neurosci. Methods 255, 29–37, https://doi.org/10.1016/j.jneumeth.2015.07.009 (2015).
Xu, M. et al. Recent development of neural microelectrodes with dual-mode detection. Biosensors (Basel) 13, https://doi.org/10.3390/bios13010059 (2022).
Monteiro, T. et al. Microelectrode sensor for real-time measurements of nitrite in the living brain, in the presence of ascorbate. Biosensors (Basel) 11, https://doi.org/10.3390/bios11080277 (2021).
Yang, W., Gong, Y. & Li, W. A review: electrode and packaging materials for neurophysiology recording implants. Front Bioeng. Biotechnol. 8, 622923, https://doi.org/10.3389/fbioe.2020.622923 (2020).
Araki, T. et al. Flexible neural interfaces for brain implants-the pursuit of thinness and high density. Flexible Printed Electron. 5, https://doi.org/10.1088/2058-8585/abc3ca (2020).
Hu, Z., Niu, Q., Hsiao, B. S., Yao, X. & Zhang, Y. Bioactive polymer-enabled conformal neural interface and its application strategies. Mater. Horiz. 10, 808–828, https://doi.org/10.1039/d2mh01125e (2023).
Redolfi Riva, E. & Micera, S. Progress and challenges of implantable neural interfaces based on nature-derived materials. Bioelectron. Med. 7, 6, https://doi.org/10.1186/s42234-021-00067-7 (2021).
Woeppel, K. M. & Cui, X. T. Nanoparticle and biomolecule surface modification synergistically increases neural electrode recording yield and minimizes inflammatory host response. Adv. Health. Mater. 10, e2002150, https://doi.org/10.1002/adhm.202002150 (2021).
O'Hara-Wright, M., Mobini, S. & Gonzalez-Cordero, A. Bioelectric potential in next-generation organoids: electrical stimulation to enhance 3D structures of the central nervous system. Front Cell Dev. Biol. 10, 901652, https://doi.org/10.3389/fcell.2022.901652 (2022).
Kang, W. et al. Closed-loop direct control of seizure focus in a rodent model of temporal lobe epilepsy via localized electric fields applied sequentially. Nat. Commun. 13, 7805. https://doi.org/10.1038/s41467-022-35540-7 (2022).
Scangos, K. W. et al. Closed-loop neuromodulation in an individual with treatment-resistant depression. Nat. Med 27, 1696–1700, https://doi.org/10.1038/s41591-021-01480-w (2021).
Yizhar, O., Fenno, L. E., Davidson, T. J., Mogri, M. & Deisseroth, K. Optogenetics in neural systems. Neuron 71, 9–34, https://doi.org/10.1016/j.neuron.2011.06.004 (2011).
Xu, Q. et al. An opto-electrophysiology neural probe with photoelectric artifact-free for advanced single-neuron analysis. ACS Nano 18, 25193–25204, https://doi.org/10.1021/acsnano.4c07379 (2024).
Li, X. H. et al. Low-intensity ultrasound ameliorates brain organoid integration and rescues microcephaly deficits. Brain 147, 3817–3833, https://doi.org/10.1093/brain/awae150 (2024).
Fujii, M. & Sato, T. Somatic cell-derived organoids as prototypes of human epithelial tissues and diseases. Nat. Mater. 20, 156–169, https://doi.org/10.1038/s41563-020-0754-0 (2021).
Verstegen, M. M. A. et al. Clinical applications of human organoids. Nat. Med 31, 409–421, https://doi.org/10.1038/s41591-024-03489-3 (2025).
Tang, X. Y. et al. Human organoids in basic research and clinical applications. Signal Transduct. Target Ther. 7, 168, https://doi.org/10.1038/s41392-022-01024-9 (2022).
Andrews, M. G. & Kriegstein, A. R. Challenges of organoid research. Annu Rev. Neurosci. 45, 23–39, https://doi.org/10.1146/annurev-neuro-111020-090812 (2022).
Zhang, W. et al. Human forebrain organoid-based multi-omics analyses of PCCB as a schizophrenia associated gene linked to GABAergic pathways. Nat. Commun. 14, 5176. https://doi.org/10.1038/s41467-023-40861-2 (2023).
Seo, K. et al. Symmetry breaking of human pluripotent stem cells (hPSCs) in micropattern generates a polarized spinal cord-like organoid (pSCO) with dorsoventral organization. Adv. Sci. (Weinh. Baden.-Wurtt., Ger.) 10, e2301787, https://doi.org/10.1002/advs.202301787 (2023).
Norrie, J. L. et al. Retinoblastoma from human stem cell-derived retinal organoids. Nat. Commun. 12, 4535. https://doi.org/10.1038/s41467-021-24781-7 (2021).
van der Valk, W. H. et al. A single-cell level comparison of human inner ear organoids with the human cochlea and vestibular organs. Cell Rep. 42, 112623, https://doi.org/10.1016/j.celrep.2023.112623 (2023).
Gameiro, J. G. et al. Quiescent horizontal basal stem cells act as a niche for olfactory neurogenesis in a mouse 3D organoid model. Cell Rep. Methods 5, 101055, https://doi.org/10.1016/j.crmeth.2025.101055 (2025).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 82330064, 32250008, 62271443), Zhejiang Provincial Natural Science Foundation of China (No. LQ24H090008), Key Project of Zhejiang Province (2024C03146, 2023C03104), the Fundamental Research Funds for the Central Universities (226-2024-00059).
Author information
Authors and Affiliations
Contributions
Qifei Wang, Deming Jiang and Shichao Tian conceptualized the work, searched the literature and finalized the original draft. Yong Qiu, Yuxuan Zhu, Shunuo Shang and Yajie Zhang searched the literature. Yong Qiu, Jianguo Wu, Xin Dong, Ping Wang and Liujing Zhuang conceptualized, supervised and reviewed the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, Q., Dong, X., Jiang, D. et al. Bioelectronic Interfaces and Sensors for Neural Organoids. Microsyst Nanoeng 11, 172 (2025). https://doi.org/10.1038/s41378-025-01038-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41378-025-01038-7