Abstract
TP53 alteration in chronic lymphocytic leukemia indicates a high-risk disease that is usually refractory to chemotherapy. It may be caused by deletion of 17p involving the loss of TP53 gene, which occurs in low percentage of patients at diagnosis but can be acquired as the disease progresses. Since patients may harbor TP53 mutation without chromosome 17p deletion, consensus recommendations call for both cytogenetic and PCR mutation analysis of TP53 in chronic lymphocytic leukemia. We conducted a single-institution retrospective study to investigate the clinicopathologic features of chronic lymphocytic leukemia with TP53 alterations as well as the utility of different diagnostic modalities to identify p53 alterations. Forty percent of chronic lymphocytic leukemia patients with TP53 alterations demonstrated atypical lymphocytes with cleaved/irregularly shaped nuclei and/or large atypical lymphoid cells with abundant cytoplasm in the peripheral blood. Progression was also observed in lymph node and bone marrow samples (21% with Richter transformation; 33% with findings suggestive of “accelerated phase” of chronic lymphocytic leukemia including prominent proliferation centers and/or increased numbers of prolymphocytes). However, the presence of the morphologic features suggestive of “accelerated phase” had no effect on overall survival within the chronic lymphocytic leukemia group with TP53 abnormalities (p > 0.05). As previously reported by others, a subset of patients with TP53 alterations were only identified by either PCR mutation analysis (12%) or cytogenetic studies (14%). p53 immunostain positivity was only identified in approximately half of the patients with TP53 alterations identified by either method, and it failed to identify any additional patients with p53 abnormalities. In summary, chronic lymphocytic leukemia patients with TP53 alterations frequently show atypical morphologic features. Use of multiple modalities to identify p53 abnormalities is recommended to ensure optimal sensitivity and specificity.
Similar content being viewed by others
Background
Chronic lymphocytic leukemia demonstrates heterogeneity in morphology, immunophenotype, chromosomal abnormalities, and genetic mutations. Among the commonly encountered genetic abnormalities identified in chronic lymphocytic leukemia, TP53 alterations have long been considered as a poor prognostic marker [1,2,3,4]. Deletion of 17p involving the loss of TP53 gene and/or the mutations in TP53 are identified in 4–10% of patients at diagnosis [5, 6] but can be acquired throughout the disease course, with an estimated prevalence of 42–45% in refractory chronic lymphocytic leukemia [7]. Even in cases when this abnormality only occurs in a small fraction of the neoplastic cells, the presence of subclonal TP53 alterations have been shown to carry poor prognosis [8, 9]. These findings highlight the importance of identifying patients with TP53 abnormalities and led to the recommendation to include evaluation for TP53 abnormalities into routine workup for chronic lymphocytic leukemia [10,11,12]. Since a subset of patients harboring TP53 mutations demonstrate no chromosome 17p deletion by metaphase-based karyotype or fluorescence in-situ hybridization studies [9, 10], the importance of utilizing mutation analysis of TP53 to complement the traditional cytogenetic approaches has been emphasized in many studies. Historically, in addition to the cytogenetic and molecular evaluation, p53 immunohistochemical stain has also been used as a quick method to identify potential TP53 abnormalities in both hematologic malignancies and non-hematopoietic neoplasms [11,12,13]. The value of different diagnostic modalities in the identification of p53 alterations in chronic lymphocytic leukemia as well as setting up a testing algorithm involving different platforms with variable sensitivity and specificity, taking costs and benefits into consideration, has long been a topic of great interest.
To date, the reports regarding the morphologic findings associated with chronic lymphocytic leukemia carrying TP53 abnormalities are scanty. Morphologic features such as excess of prolymphocytes in the peripheral blood (as seen in the so-called prolymphocytoid transformation) have been previously investigated [13, 14]. Preliminary results have shown a potential link between TP53 abnormalities and prolymphocytoid transformation in chronic lymphocytic leukemia, although the occurrence of prolymphocytoid transformation appeared to behave in a very heterogeneous fashion and has not been shown to carry a uniformly poor prognosis [15]. On the other hand, the presence of expanded proliferation centers in chronic lymphocytic leukemia has been shown to represent a distinct disease subgroup with more aggressive clinical behavior [16, 17]. These cases were shown to be associated with cytogenetic abnormalities including 17p deletion, IgH translocation, and trisomy 12 in one study [16], although their TP53 mutation status was not characterized. Recognition of the potential morphologic findings associated with the subgroup of chronic lymphocytic leukemia patients carrying TP53 abnormalities may facilitate the optimization of the testing algorithm, especially in a setting of limited resources or limited access to the appropriate testing platforms. In this study, we carefully outlined the clinicopathologic features of chronic lymphocytic leukemia patients with TP53 alterations and examined the value of different diagnostic modalities, including p53 immunohistochemistry.
Materials and methods
Patients and materials
Specimens (including peripheral blood, bone marrow, and lymph node) diagnosed as chronic lymphocytic leukemia were identified through natural language search of the pathology archive at Weill Cornell Medical College from 1993 to 2013. All cases had to meet the requirements for a diagnosis of chronic lymphocytic leukemia by the 2016 revised World Health Organization Classification. Cases were included in the study if they had evidence of TP53 alterations detected by cytogenetic, molecular, or immunohistochemical analysis. Clinical information was retrieved from the electronic medical records. This study was approved by the Institutional Review Board at Weill Cornell Medicine.
Cytogenetic and fluorescence in-situ hybridization (FISH) studies
Conventional cytogenetic analysis was performed on G-banded metaphase cells prepared from bone marrow aspirate or lymph node cultures using standard techniques. Twenty metaphases were analyzed and the results were reported using the International System for Human Cytogenetic Nomenclature. FISH studies for detecting abnormalities including 6q- [SEC63 (6q21), MYB (6q23)], ATM (11q22.3), TP53 (17p13.1), Trisomy 12 (Cen 12), 13q-/-13 (13q14, 13q34), and IgH rearrangement (Abbott Molecular, Des Plaines, IL, USA) were performed in cases with available material.
Immunohistochemical staining for p53 protein
Immunohistochemical staining for p53 protein was performed with the monoclonal antibody (clone 1801, BioGenex, San Ramon, CA, USA) at a dilution of 1:150 on a TechMate 500 automated stainer (Ventana, Tucson, AZ, USA). The p53 staining was scored based on the intensity of nuclear staining and the number of cells stained. The quantity of cells stained was recorded as percentage of cells stained positive in the sample. A positive staining is defined as cells with strong positivity accounting for >5% of the total cells.
Mutation sequencing
Samples that underwent single gene mutation analysis for TP53 had genomic DNA extracted from either peripheral blood or unfractionated bone marrow aspirate sample. The extracted DNA was amplified by PCR using primer sets flanking exons 5–9 of TP53. Amplification products are sequenced bi-directionally by Sanger sequencing. Mutations including insertion/deletion and non-synonymous variants were identified.
Targeted next-generation sequencing mutation testing for genes commonly mutated in lymphoma was performed using a targeted hybrid-capture procedure on genomic DNA extracted from peripheral blood or unfractionated bone marrow aspirate sample. A probe panel targeting coding regions of the 75 genes recurrently mutated in lymphoma was used (Supplemental Table 1). Non-synonymous variants and insertions/deletions were reported.
Morphologic and histologic assessment
Peripheral blood smears, bone marrow and lymph node biopsies from all patients were reviewed. The cytologic and histologic criteria for morphologic evaluation were defined as follows: for the peripheral blood samples, at least 200 lymphocytes were counted in the first available smear(s) at the time of or after identifying TP53 mutation. Percentage of prolymphocytes and the presence of atypical lymphocytes were recorded. Atypical lymphocytes were defined as >10% of the lymphocytes with marked nuclear irregularities (including cleaved nuclei, binucleate cells, cells with micronuclei) or lymphocytes that were large in size with less condensed chromatin, small or absent nucleoli, and moderate-to-abundant cytoplasm. Regarding the bone marrow samples, the presence of proliferation centers and the presence of increased large cells including prolymphocytes and paraimmunoblasts were recorded. The lymph node samples were divided into three groups: (1) Typical chronic lymphocytic leukemia morphology; (2) Richter transformation, defined as confluent sheets of large transformed lymphoid cells, consistent with large cell lymphoma, or the presence of classic Hodgkin lymphoma; and (3) “Accelerated phase” chronic lymphocytic leukemia, defined as expanded proliferation centers exceeding ×20 field, and/or increased number of large cells including prolymphocytes and paraimmunoblasts, as previously defined in prior publications [12, 17,18,19,20]. The classification of the lymph node samples was agreed upon by the three hematopathologists (JG, YL, and AO). All morphologic review was blinded to the clinical, cytogenetic, and molecular findings.
Statistical analyses
For continuous variables, data were reported as median and range. For nominal variables, data were reported as the number of patients unless otherwise specified. Disease-specific survival was calculated from the day of diagnosis to the last follow-up or death attributed to chronic lymphocytic leukemia (or related disease progression). Kaplan–Meier estimator was used to estimate survival probability. Survival difference between groups was tested by log-rank test. Fisher’s exact and Chi-square tests were used for categorical comparisons. The statistical test was two-sided and an alpha level of 0.05 was used to determine the statistical significance.
Results
Clinical characteristics and outcome in patients with TP53 abnormalities
We identified 95 chronic lymphocytic leukemia patients with TP53 abnormalities. The male/female ratio in the group was 2:1 (63 males and 32 female). The median age of these patients was 62 years old (range: 34–84 years old). With a median follow-up of 7 years from the initial diagnosis of chronic lymphocytic leukemia (range: <1 year to 21 years), 32/95 patients (34%) died while 60/95 patients (63%) were alive with disease. The status of TP53 alteration at the time of diagnosis was available in more than half of the patients (54/95; 57%). Among them, 35/54 patients (65%) demonstrated TP53 alterations at the time of diagnosis while 35% demonstrated detectable TP53 alteration only in follow-up sample(s). Median follow-up after the detection of TP53 alterations in our study was 3.8 years (range: 1 month to 12 years). Patients received treatment following a similar management algorithm. The overall survival in the patients with TP53 abnormalities who died of chronic lymphocytic leukemia or related disease progression in our study was 168 months, which was significantly different from the chronic lymphocytic leukemia cohort with no TP53 abnormalities diagnosed and treated in the same institution (Weill Cornell Medicine, 311 months; Supplemental Fig. 1).
Peripheral blood: morphologic review of chronic lymphocytic leukemia patients with TP53 alterations
One or more peripheral blood smears were available for review in 70/95 chronic lymphocytic leukemia patients with TP53 alterations. The average percentage of prolymphocytes in these patients was 5% (median: 2.5%; range: 1–88%) (Fig. 1a). The one patient with 88% prolymphocytes with well-documented history of chronic lymphocytic leukemia diagnosed 4 years prior to the finding, was considered to have prolymphocytic transformation. A significant proportion of the patients (28/70; 40%) demonstrated atypical lymphocytes accounting for >10% of the lymphocytes. Of these 28, 71% had atypical lymphocytes with marked nuclear irregularities (including cleaved nuclei, binucleate cells, and cells with micronuclei) (Fig. 1b–d). In the remaining 8 cases (29%), the atypical lymphocytes displayed large size, less condensed nuclear chromatin, small or absent nucleoli, and moderate-to-abundant cytoplasm (Fig. 1e, f). However, the presence of circulating atypical lymphocytes was not prognostically relevant when compared to other patients with TP53-mutated chronic lymphocytic leukemia (p = 0.1).
Peripheral blood findings in chronic lymphocytic leukemia patients carrying p53 alterations. Most of the chronic lymphocytic leukemia patients carrying p53 alterations demonstrate a small percentage of prolymphocytes in the peripheral blood (a). The atypical lymphocytes identified in the peripheral blood smear in chronic lymphocytic leukemia patients carrying p53 alterations demonstrate atypical cytologic features, including (b) binucleate cells, (c) cells with micronuclei, and (d) cells with cleaved nuclei. Some chronic lymphocytic leukemia cases carrying p53 alterations demonstrate (e, f) “prolymphocytoid cells” that are larger in size with less condensed chromatin, small nucleoli, and moderate cytoplasm
Lymph node: morphologic review of chronic lymphocytic leukemia patients with TP53 alterations
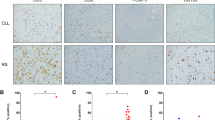
We identified 32 lymph node biopsy specimens in 24/95 chronic lymphocytic leukemia patients with TP53 alterations. Of these 24 patients, 5 showed typical morphologic features of chronic lymphocytic leukemia, 9 showed “accelerated phase” of chronic lymphocytic leukemia, while 10 (42%) demonstrated evidence of Richter transformation. The cases with Richter transformation include 9 cases of diffuse large B cell lymphoma (1/9 with concurrent histiocytic sarcoma) and 1 case of classic Hodgkin lymphoma. Of note, a chronic lymphocytic leukemia patient with lymph node biopsy showing diffuse large B cell lymphoma also demonstrated plasmablastic lymphoma in the skin. Another patient demonstrated diffuse large B cell lymphoma in the spleen. All the cases with Richter/plasmablastic transformation appeared to be Epstein–Barr virus (EBV)-negative by EBV in-situ hybridization. Of note, 4/10 patients with follow-up lymph node samples showing Richter transformation had prior lymph node biopsies demonstrating morphologic evidence suggestive of “accelerated phase” chronic lymphocytic leukemia. Nine patients with no evidence of Richter transformation demonstrated findings consistent with “accelerated phase” of chronic lymphocytic leukemia (Fig. 2). All of the “accelerated phase” chronic lymphocytic leukemia samples were characterized by either (1) expanded proliferation centers (72%) or (2) increased number of prolymphocytes and/or medium- and/or large-sized lymphoid cells without expansion of proliferation canters (“paraimmunoblastic variant”) (28%). The mean Ki67 proliferation rate in these samples was estimated to be 30% (range: 10–70%). Immunostain for p53 was positive in all cases in ~50% of the cells. All the cases with evidence suggestive of “accelerated phase” of chronic lymphocytic leukemia were also negative by EBV in-situ hybridization study. In a univariate survival analysis, the presence of morphologic findings suggestive of an “accelerated phase” of chronic lymphocytic leukemia was not prognostically relevant compared to other chronic lymphocytic leukemia patients harboring TP53 abnormalities (p > 0.05).
Morphologic findings in the lymph nodes involved by chronic lymphocytic leukemia carrying p53 alterations. (a) Expanded proliferation centers or (b) increased numbers of medium- and/or large-sized lymphoid cells with no expansion of proliferation centers (“paraimmunoblastic variant”) can be seen in lymph nodes involved by chronic lymphocytic leukemia carrying p53 alterations
Bone marrow: morphologic review of chronic lymphocytic leukemia patients with TP53 alterations
Bone marrow samples were available for review in 63/95 chronic lymphocytic leukemia patients with TP53 alterations. Of these, 5 patients demonstrated evidence of Richter transformation and 18 showed evidence of “accelerated phase” chronic lymphocytic leukemia (Fig. 3). The patients with Richter transformation comprised three cases of diffuse large B cell lymphoma and two cases of classic Hodgkin lymphoma. As in the lymph nodes, cases with evidence of “accelerated phase” chronic lymphocytic leukemia were characterized by either prominent proliferation centers (11/63 bone marrow samples; 17%) or increased number of medium- to large-sized cells without expanded proliferation centers (“paraimmunoblastic variant”) (7/63 bone marrow samples; 11%). The presence of “accelerated phase” of chronic lymphocytic leukemia did not appear to have a significant impact on the patients’ survival compared to patients with TP53-mutated chronic lymphocytic leukemia with routine morphology (p > 0.05).
Summary: morphologic review
Altogether, tissue samples (lymph node, spleen, skin, or bone marrow biopsies) were available for review in 67/95 chronic lymphocytic leukemia patients with TP53 alterations, a significant portion of which demonstrated morphologic evidence of disease progression (36/67 cases; 54%). Among them, 14/67 patients (21%) demonstrated evidence of Richter transformation, including 12 cases of diffuse large B cell lymphoma and 2 cases of classic Hodgkin lymphoma. In addition, 22/67 chronic lymphocytic leukemia patients with TP53 alterations (33%) demonstrated evidence suggestive of “accelerated phase” of chronic lymphocytic leukemia without Richter transformation. The overall patient survival was not significantly different among chronic lymphocytic leukemia patients with typical morphology, “accelerated phase” morphology, or Richter transformation (p = 0.35), though patients with Richter transformation received chemotherapy regimen corresponding to their diagnoses.
Identification of p53 alterations in chronic lymphocytic leukemia patients
In our study, multiple modalities including Sanger sequencing, FISH, and immunohistochemical staining were used to identify p53 alterations. Among the 95 chronic lymphocytic leukemia patients with TP53 abnormalities, the vast majority of the patients demonstrated deletion of 17p by FISH studies (81/89 patients; 91%) while 46/55 patients (84%) demonstrated TP53 mutations through Sanger sequencing. Approximately half of the chronic lymphocytic leukemia patients with TP53 abnormalities demonstrated positive staining for p53 immunohistochemical stain (39/80; 49%). Conventional karyotyping was performed in 39 patients: 27 had demonstrable 17p loss in the context of a complex karyotype; 11 had a normal karyotype; and 1 had del(13q) as a sole abnormality. The patient with del(13q)as a sole abnormality demonstrated 17p loss by FISH in 7% of the cells. Of the 11 patients with a normal karyotype, 8 had 17p loss by FISH in variable percentage of cells examined (3–93% of the cells), while 3 had normal FISH studies.
Focusing on the 49 chronic lymphocytic leukemia patients who were evaluated by sequencing, FISH, and immunohistochemistry, approximately 26% of the patients (13/49) demonstrated p53 alteration detected by only one testing method: 6 PCR alone, 7 FISH alone. No patient with p53 alteration was identfiied by immunohistochemistry alone. The majority of the patients demonstrated abnormalities in TP53 identified by multiple modalities: 21/49 (43%) of cases were PCR+/FISH+/immunohistochemistry+, 12/49 (24%) of cases were PCR+/FISH+/immunohistochemistry−, 2/49 (4%) of cases were PCR−/FISH+/immunohistochemistry+, and 1/49 (2%) of cases were PCR+/FISH−/immunohistochemistry+ (Fig. 4a). Thus, we determined that p53 immunostain is specific for TP53 alterations identified by either Sanger sequencing or FISH testing; it is not sensitive as it was negative in approximately half of our cases.
Identification of p53 alterations in chronic lymphocytic leukemia patients. (a) Venn diagram demonstrating the relationships between positive FISH, sequencing, and immunohistochemical evaluation among patients tested by all three modalities. (b, c) TP53 mutations identified by Sanger sequencing. The majority of the mutations identified represents missense mutations and demonstrates a distribution similar to that seen in other studies characterizing chronic lymphocytic leukemia
Among the 46 patients with TP53 mutations identified by Sanger sequencing, 75% of the patients carried missense mutations, 6% carried nonsense mutations, 5% carried frame-shift insertion/deletions, and 6% of the patients carried in-frame insertion/deletions. Another 8% of the patients carried mutations involving the splicing sites (Fig. 4b). The distribution of the mutations identified in our study was similar to that seen in other studies characterizing chronic lymphocytic leukemia (Fig. 4c). Twenty-eight patients carrying TP53 mutations identified by Sanger Sequencing had follow-up samples subjected to targeted next-generation sequencing for genes that were recurrently mutated in lymphoid neoplasms (Table 1). Concurrent mutations in genes implicated to be chronic lymphocytic leukemia-driver genes [8] occurred in 15/28 cases (54% of cases), involving the mitogen-activated protein kinases/extracellular signal–regulated kinases (MAPK/ERK) pathway (BRAF, KRAS), B cell activity-related pathway/inflammatory pathway (BIRC3), DNA damage control (POT1), Notch signaling pathway (NOTCH1), chromatin modification (ZMYM3), and RNA/ribosomal processing pathways (SF3B1, XPO1, DDX3X). Mutations involving B cell receptor signaling pathway (BTK, PLCG2, ITPKB) were also seen in 4/28 cases (14.3%).
Discussion
TP53 abnormality is one of the most important prognostic factors in chronic lymphocytic leukemia and its presence, even in a subclonal fashion, predicts worse survival [1,2,3,4, 8, 9]. How to incorporate the identification of p53 alteration in routine chronic lymphocytic leukemia workup with a robust testing algorithm ensuring an adequate evaluation has been an important topic for this common disease entity [10,11,12]. The discussions mainly involve the timing (e.g., at diagnosis or at the time of treatment) as well as the platforms of these tests. The combination of FISH and PCR testing is recommended for optimal sensitivity. The data collected in our retrospective study confirmed the importance of utilizing multiple modalities in identifying p53 alterations.
In our study conducted at a tertiary medical center, a significant portion of the patients presented with a diagnosis of chronic lymphocytic leukemia established elsewhere. They were referred to our institution because of progressive clinical symptoms, treatment concerns, or with refractory disease requiring further management. By examining the peripheral blood, bone marrow, and lymph node samples, we frequently observed that p53 alterations were associated with morphologic and architectural abnormalities that were atypical for chronic lymphocytic leukemia, including a high proportion of prolymphocytes or atypical lymphocytes in peripheral blood as well as findings suggestive of an “accelerated phase” of chronic lymphocytic leukemia in the bone marrow or lymph node. A high percentage of cases demonstrated evidence of progression, including Richter transformation. Although sampling bias is a consideration, as clinicians are more inclined to biopsy patients they perceive to be at high risk of progression, we feel this would only affect lymph node sampling, as bone marrow and peripheral blood evaluation are standard of care at our institution. Our data also suggest that peripheral blood smear review for increased prolymphocytes and/or atypical lymphocytes in patients with chronic lymphocytic leukemia may be helpful for identifying patients with a higher pretest probability of TP53 abnormalities, which may be helpful in resource-limited settings. In addition, testing for TP53 gene abnormalities may be warranted in chronic lymphocytic leukemia patients with prominent proliferation centers, increased number of prolymphocytes, atypical lymphocytes and/or medium/large cells, or in the presence of high proliferation rate of the lymphoid cells. This constellation of atypical morphologic findings likely represents the hallmark of all cases with TP53 gene abnormalities, as these morphologic findings are not correlated with more aggressive behavior when compared to patients with p53 alterations and “typical” morphology. However, it is worth noting that cases with subclonal TP53 abnormalities may not demonstrate similar morphologic findings.
In relation to the detection of p53 abnormalities, the cases carrying TP53 mutations in our series were characterized by Sanger sequencing, a technical approach with a sensitivity threshold of roughly 20%. In addition, using an immunohistochemistry cut-off for p53 positivity based on the presence of strongly positive cells accounting for >5% of the lymphoid cells, immunostaining for p53 was only positive in roughly half of the cases with TP53 abnormalities and was negative in all cases that were negative by cytogenetics or Sanger sequencing studies. Immunohistochemical staining for p53 with the cut-off used in our case study appears specific but has low sensitivity for chronic lymphocytic leukemia with TP53 abnormalities.
It is known that there are no well-established criteria for p53 immunostain predicting genetic alterations in different disease entities; and different cut-offs were applied in studies investigating the association between p53 expression and prognosis [21,22,23,24,25,26,27]. Different methodologies including imaging analysis have been applied to the evaluation of p53 expression, which highlights the difficulties in establishing a consensus cut-off due to variation in the intensity of the staining. We speculate that the variable staining pattern of p53 immunohistochemistry may potentially reflect the tumor heterogeneity, as well as effects of decalcification in the bone marrow trephine biopsies and the technical artifact in the peripheral blood cell block preparation.
It has been suggested that “accelerated phase” chronic lymphocytic leukemia, characterized by expanded proliferation centers (broader than a ×20 field) and high proliferation rate in the lymph node, demonstrates more aggressive clinical behavior [6, 7]. Though these cases were shown to be associated with 17p deletion [6], the TP53 mutation status in these cases was not well characterized. Recently, the presence of proliferation centers in bone marrows involved by chronic lymphocytic leukemia was also reported to demonstrate a more aggressive clinical behavior and an association with complex karyotype and TP53 disruption [28]. The presence of morphologic findings suggestive of “accelerated phase” did not appear to carry a statistically significant impact on survival in our retrospective study; instead it seems to represent a feature associated with p53 alteration.
The long clinical follow-up in a single institution with standardized management guidelines contributes to the strength of our study. It is worth noting that a significant portion of the patients included in this study received ibrutinib, which is a small molecule BTK inhibitor, first approved by Food and Drug Administration (FDA) in 2014 [29]. One recent report demonstrated no significantly reduced overall survival or progression-free survival with ibrutinib treatment in previously treated chronic lymphocytic leukemia patients with 17p deletion [30]. However, the investigation of TP53 abnormalities in chronic lymphocytic leukemia remains critical and is currently recommended by the International Workshop on Chronic Lymphocytic Leukemia prior to initiation of therapy [31]. Those harboring mutations should avoid conventional chemotherapy (such as fludarabine-containing regimens) in light of proven inferior outcome and should consider targeted therapies utilizing BTK or BCL2 inhibitor [32]. Venetoclax, a selective inhibitor of BCL2, was approved by FDA in 2016 for chronic lymphocytic leukemia patients with TP53 abnormality who have received prior therapy with a B cell receptor inhibitor or who are not suitable for B cell receptor inhibitors. A recent study of venetoclax demonstrated no significant association between 17p deletion and/or TP53 mutation in patients with disease progression on venetoclax [33].
Though morphologic evaluation appears to demonstrate frequent atypical findings in chronic lymphocytic leukemia with p53 abnormalities, the potential mechanisms leading to atypical morphology in these patients are not clear. Studies utilizing RNA sequencing and 3′-end sequencing on transcriptome in chronic lymphocytic leukemia have uncovered new abnormalities in chronic lymphocytic leukemia [34,35,36]. Recent studies on chronic lymphocytic leukemia with trisomy 12, another chronic lymphocytic leukemia subtype characterized by atypical features, demonstrated upregulation of specific pathways such as the nuclear factor of activated T-cells (NFAT) signaling pathways and integrin signaling that appeared modulated by mutations in NOTCH1 [37, 38]. Similar studies on chronic lymphocytic leukemia with TP53 abnormalities may help shed light on the link between the morphologic findings and genetic alteration as well as reveal more potential therapeutic targets.
In summary, we demonstrate that a significant subset of patients with chronic lymphocytic leukemia with p53 abnormalities have reproducible morphologic features. Presence of atypical morphology should trigger an investigation of patient’s p53 status. A multimodality approach with use of sequencing, PCR, and FISH allows for optimal sensitivity and specificity. Immunohistochemical staining with p53 antibody has high specificity but low sensitivity, thus negative results should be interpreted with caution.
References
Seiler T, Dohner H, Stilgenbauer S. Risk stratification in chronic lymphocytic leukemia. Semin Oncol. 2006;33:186–94.
The International CLL-IPI Working Group. An international prognostic index for patients with chronic lymphocytic leukaemia (CLL-IPI): a meta-analysis of individual patient data. Lancet Oncol. 2016;17:779–90.
Takahashi K, Hu B, Wang F, Yan Y, Kim E, Vitale C, et al. Clinical implications of cancer gene mutations in patients with chronic lymphocytic leukemia treated with lenalidomide. Blood. 2018;131:1820–32.
Houldsworth J, Guttapalli A, Thodima V, Yan XJ, Mendiratta G, Zielonka T, et al. Genomic imbalance defines three prognostic groups for risk stratification of patients with chronic lymphocytic leukemia. Leuk Lymphoma. 2014;55:920–8.
Dohner H, Stilgenbauer S, Benner A, Leupolt E, Krober A, Bullinger L, et al. Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med. 2000;343:1910–6.
Buccheri V, Barreto WG, Fogliatto LM, Capra M, Marchiani M, Rocha V. Prognostic and therapeutic stratification in CLL: focus on 17p deletion and p53 mutation. Ann Hematol. 2018;97:2269–78.
Furman RR, Sharman JP, Coutre SE, Cheson BD, Pagel JM, Hillmen P, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med. 2014;370:997–1007.
Landau DA, Tausch E, Taylor-Weiner AN, Stewart C, Reiter JG, Bahlo J, et al. Mutations driving CLL and their evolution in progression and relapse. Nature. 2015;526:525–30.
Nadeu F, Delgado J, Royo C, Baumann T, Stankovic T, Pinyol M, et al. Clinical impact of clonal and subclonal TP53, SF3B1, BIRC3, NOTCH1, and ATM mutations in chronic lymphocytic leukemia. Blood. 2016;127:2122–30.
Ladetto M, Buske C, Hutchings M, Dreyling M, Gaidano G, Le Gouill S, et al. ESMO consensus conference on malignant lymphoma: general perspectives and recommendations for prognostic tools in mature B-cell lymphomas and chronic lymphocytic leukaemia. Ann Oncol. 2016;27:2149–60.
Ladetto M, Buske C, Hutchings M, Dreyling M, Gaidano G, Le Gouill S, et al. ESMO consensus conference on malignant lymphoma: general perspectives and recommendations for prognostic tools in mature B-cell lymphomas and chronic lymphocytic leukaemia. Ann Oncol. 2018;29:525.
Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick HR, et al. Proposals for the classification of chronic (mature) B and T lymphoid leukaemias. French-American-British (FAB) Cooperative Group. J Clin Pathol. 1989;42:567–84.
Lens D, Dyer MJ, Garcia-Marco JM, De Schouwer PJ, Hamoudi RA, Jones D, et al. p53 abnormalities in CLL are associated with excess of prolymphocytes and poor prognosis. Br J Haematol. 1997;99:848–57.
Cordone I, Masi S, Mauro FR, Soddu S, Morsilli O, Valentini T, et al. p53 expression in B-cell chronic lymphocytic leukemia: a marker of disease progression and poor prognosis. Blood. 1998;91:4342–9.
Stark AN, Limbert HJ, Roberts BE, Jones RA, Scott CS. Prolymphocytoid transformation of CLL: a clinical and immunological study of 22 cases. Leuk Res. 1986;10:1225–32.
Ciccone M, Agostinelli C, Rigolin GM, Piccaluga PP, Cavazzini F, Righi S, et al. Proliferation centers in chronic lymphocytic leukemia: correlation with cytogenetic and clinicobiological features in consecutive patients analyzed on tissue microarrays. Leukemia. 2012;26:499–508.
Gine E, Martinez A, Villamor N, Lopez-Guillermo A, Camos M, Martinez D, et al. Expanded and highly active proliferation centers identify a histological subtype of chronic lymphocytic leukemia (“accelerated” chronic lymphocytic leukemia) with aggressive clinical behavior. Haematologica. 2010;95:1526–33.
Bonato M, Pittaluga S, Tierens A, Criel A, Verhoef G, Wlodarska I, et al. Lymph node histology in typical and atypical chronic lymphocytic leukemia. Am J Surg Pathol. 1998;22:49–56.
Frater JL, McCarron KF, Hammel JP, Shapiro JL, Miller ML, Tubbs RR, et al. Typical and atypical chronic lymphocytic leukemia differ clinically and immunophenotypically. Am J Clin Pathol. 2001;116:655–64.
Pugh WC, Manning JT, Butler JJ. Paraimmunoblastic variant of small lymphocytic lymphoma/leukemia. Am J Surg Pathol. 1988;12:907–17.
Cooper K, Haffajee Z. bcl-2 and p53 protein expression in follicular lymphoma. J Pathol. 1997;182:307–10.
Greiner TC, Moynihan MJ, Chan WC, Lytle DM, Pedersen A, Anderson JR, et al. p53 mutations in mantle cell lymphoma are associated with variant cytology and predict a poor prognosis. Blood. 1996;87:4302–10.
Kobel M, Rahimi K, Rambau PF, Naugler C, Le Page C, Meunier L, et al. An immunohistochemical algorithm for ovarian carcinoma typing. Int J Gynecol Pathol. 2016;35:430–41.
Kobel M, Reuss A, du Bois A, Kommoss S, Kommoss F, Gao D, et al. The biological and clinical value of p53 expression in pelvic high-grade serous carcinomas. J Pathol. 2010;222:191–8.
Le Page C, Kobel M, de Ladurantaye M, Rahimi K, Madore J, Babinszky S, et al. Specimen quality evaluation in Canadian biobanks participating in the COEUR repository. Biopreserv Biobank. 2013;11:83–93.
Xu-Monette ZY, Wu L, Visco C, Tai YC, Tzankov A, Liu WM, et al. Mutational profile and prognostic significance of TP53 in diffuse large B-cell lymphoma patients treated with R-CHOP: report from an International DLBCL Rituximab-CHOP Consortium Program Study. Blood. 2012;120:3986–96.
Yemelyanova A, Vang R, Kshirsagar M, Lu D, Marks MA, Shih Ie M, et al. Immunohistochemical staining patterns of p53 can serve as a surrogate marker for TP53 mutations in ovarian carcinoma: an immunohistochemical and nucleotide sequencing analysis. Mod Pathol. 2011;24:1248–53.
Garces S, Khoury JD, Kanagal-Shamanna R, Salem A, Wang SA, Ok CY, et al. Chronic lymphocytic leukemia with proliferation centers in bone marrow is associated with younger age at initial presentation, complex karyotype, and TP53 disruption. Hum Pathol. 2018;82:215–31.
Woyach JA, Johnson AJ, Byrd JC. The B-cell receptor signaling pathway as a therapeutic target in CLL. Blood. 2012;120:1175–84.
Brown JR, Hillmen P, O’Brien S, Barrientos JC, Reddy NM, Coutre SE, et al. Extended follow-up and impact of high-risk prognostic factors from the phase 3 RESONATE study in patients with previously treated CLL/SLL. Leukemia. 2018;32:83–91.
Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Dohner H, et al. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood. 2018;131:2745–60.
Zenz T, Eichhorst B, Busch R, Denzel T, Habe S, Winkler D, et al. TP53 mutation and survival in chronic lymphocytic leukemia. J Clin Oncol. 2010;28:4473–9.
Anderson MA, Tam C, Lew TE, Juneja S, Juneja M, Westerman D, et al. Clinicopathological features and outcomes of progression of CLL on the BCL2 inhibitor venetoclax. Blood. 2017;129:3362–70.
Lee SH, Singh I, Tisdale S, Abdel-Wahab O, Leslie CS, Mayr C. Widespread intronic polyadenylation inactivates tumour suppressor genes in leukaemia. Nature. 2018;561:127–31.
Wang L, Brooks AN, Fan J, Wan Y, Gambe R, Li S, et al. Transcriptomic characterization of SF3B1 mutation reveals its pleiotropic effects in chronic lymphocytic leukemia. Cancer Cell. 2016;30:750–63.
Wang L, Fan J, Francis JM, Georghiou G, Hergert S, Li S, et al. Integrated single-cell genetic and transcriptional analysis suggests novel drivers of chronic lymphocytic leukemia. Genome Res. 2017;27:1300–11.
Abruzzo LV, Herling CD, Calin GA, Oakes C, Barron LL, Banks HE, et al. Trisomy 12 chronic lymphocytic leukemia expresses a unique set of activated and targetable pathways. Haematologica. 2018;103:2069–78.
Riches JC, O’Donovan CJ, Kingdon SJ, McClanahan F, Clear AJ, Neuberg DS, et al. Trisomy 12 chronic lymphocytic leukemia cells exhibit upregulation of integrin signaling that is modulated by NOTCH1 mutations. Blood. 2014;123:4101–10.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Liu, YC., Margolskee, E., Allan, J.N. et al. Chronic lymphocytic leukemia with TP53 gene alterations: a detailed clinicopathologic analysis. Mod Pathol 33, 344–353 (2020). https://doi.org/10.1038/s41379-019-0356-z
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41379-019-0356-z