Abstract
Gastric-type mucinous carcinoma (GAS) is a recently established variant of endocervical mucinous adenocarcinoma that is characterized as being unrelated to HPV and having aggressive behavior and chemoresistance. GAS has a distinct morphology resembling nonneoplastic gastric glands or pancreaticobiliary adenocarcinoma, and their possible genetic similarity has been posed. In this study, next-generation sequencing was performed in 21 GAS cases using a customized panel including 94 cancer-associated genes. A total of 54 nonsynonymous somatic mutations were detected with an average mutation rate of 2.6 per lesion (range: 0–9). The most frequently mutated gene was TP53 (11/21, 52.4%), followed by STK11, HLA-B, PTPRS (4/21, 19.0%), FGFR4 (3/21, 14.3%), GNAS, BRCA2, ELF3, ERBB3, KMT2D, SLX4 (2/21, 9.5%), CDH1, EPCAM, KRAS, MLH1, RNF43, SNAI1, TWIST1, ZEB1, ZEB2, and so on (1/21, 4.8%). The mutated genes were mostly involved in signal transduction, DNA damage repair, and epithelial–mesenchymal transition (EMT). Correlation of TP53 mutation and p53 protein expression demonstrated that 31.3% with abnormal p53 expression harbored wild-type TP53. Compared to genetic features of gastric and pancreaticobiliary adenocarcinoma, TP53 mutations were frequent in both GAS and gastrointestinal adenocarcinoma. While KMT2D, ERBB3, and RNF43 mutations were shared between GAS and gastric adenocarcinoma, highly mutated genes in pancreatic ductal adenocarcinoma such as KRAS, SMAD4, and CDKN2A were rarely mutated in GAS. Of frequently mutated genes in cholangiocarcinoma, BAP1 and HLA-B were identified in GAS. Frequent EMT-related gene mutations suggested a possible role of EMT-related pathways in tumor dissemination and chemoresistance of GAS. In addition, GAS shared some genetic features with gastrointestinal adenocarcinoma. These findings provide a clue in understanding the biological basis of GAS.
Similar content being viewed by others
Introduction
Endocervical adenocarcinoma accounts for 20–25% of invasive cervical carcinomas, and its incidence is gradually increasing [1, 2]. As tumor etiology, i.e., human papillomavirus (HPV) infection, becomes an important factor for predicting the clinical outcome of endocervical adenocarcinoma, the International Endocervical Adenocarcinoma Criteria and Classification, a recent proposal for endocervical adenocarcinoma classification, divided endocervical adenocarcinoma into HPV-related and HPV-unrelated types [3]; HPV-unrelated types include gastric-type mucinous carcinoma (GAS), clear cell carcinoma, and mesonephric carcinoma. Of HPV-unrelated endocervical adenocarcinoma, GAS is the most common subtype, which accounts for ~10% of endocervical adenocarcinoma [3]. GAS is considered a distinct and clinically important entity as it is unrelated to HPV, has aggressive behavior and worse clinical outcomes than usual type endocervical adenocarcinoma, and exhibits chemoresistance [4,5,6,7,8]. Histologically, GAS is defined as a mucinous carcinoma with voluminous eosinophilic cytoplasm and distinct cell borders with glands resembling nonneoplastic gastric glands or pancreaticobiliary adenocarcinoma [9]. In addition, GAS typically shows widespread involvement and advanced stage at initial diagnosis [10]. Immunohistochemically, GAS shows positivity for HIK1083 and/or MUC6, which are components of pyloric-type mucin [11, 12]. In addition, aberrant expression of p53 is frequently observed in GAS with 41.3–64.3%, while p16 block positivity is rarely found despite HPV negativity [3, 13, 14]. While distinct morphology of GAS poses a possible genetic similarity to gastric or pancreaticobiliary cancers, the potential relationship has not been investigated.
To understand the pathogenesis of GAS and identify available therapeutic targets, its genetic characteristics and underlying pathogenic mechanisms need to be investigated. According to TCGA data of cervical cancer, the HPV-negative group showed higher epithelial–mesenchymal transition (EMT) mRNA score and significantly lower promoter-methylation level compared to HPV-positive cancer group [15]. In addition, they proposed a unique HPV-negative subgroup, which might include a considerable number of GAS cases, harboring frequent KRAS, ARID1A, and PTEN mutations similar to endometrial cancer. Recently, few studies reported targeted sequencing results of GAS. Murali et al. identified that TP53, GNAS, and SMAD4 were recurrently mutated in eight GAS cases; such mutations are also frequently identified in pancreaticobiliary or gastrointestinal cancers [16]. Garg et al. analyzed genetic alterations of 14 GAS cases and reported that known genetic variants of endometrial cancers, such as POLE, ARID1A, KRAS, and FBXW7, were enriched in GAS [13]. These findings suggest that the genetic characteristics of GAS are distinct from those of HPV-positive cervical cancers.
In this study, we performed targeted deep sequencing in 21 GAS cases to identify their genetic characteristics. Immunohistochemistry of p16, p53, and E-cadherin was performed to support the diagnosis of GAS or identify EMT-related alteration. We also compared their genetic features to those of cervical squamous cell carcinoma, endocervical adenocarcinoma, endometrial carcinoma, and gastric and pancreaticobiliary adenocarcinoma in an effort to increase the understanding of GAS pathogenesis.
Materials and methods
Patients
A total of 138 patients were diagnosed with endocervical adenocarcinoma at Yonsei Severance Hospital between June 2015 and April 2019. The cases of endocervical adenocarcinoma included 99 usual type (71.7%), 23 GAS (16.7%), 8 intestinal type mucinous carcinoma (5.8%), 3 clear cell carcinoma (2.2%), 2 mesonephric carcinoma (1.4%), 1 signet-ring cell type mucinous carcinoma (0.7%), 1 villoglandular carcinoma (0.7%), and 1 invasive stratified mucin-producing carcinoma (iSMILE) (0.7%). Among 23 patients with GAS, 21 patients with available formalin-fixed paraffin-embedded (FFPE) tissue and informed consent were investigated. Twenty-one tumor samples and 17 matched available normal samples were analyzed. Clinicopathologic data were obtained from medical records and pathology reports. This study was approved by the Institutional Review Board of Severance Hospital (IRB no. 4-2019-0538).
Histologic analysis
Hematoxylin and eosin-stained slides were reviewed by two gynecologic pathologists (EP and NHC) based on the 2014 WHO classification [17]. All of the enrolled cases showed at least focal areas with typical histology of GAS: (1) voluminous, clear, or pale eosinophilic cytoplasm; (2) distinct cell borders; and (3) moderate to marked nuclear atypia.
Immunohistochemistry
Immunohistochemistry was used to assess p16, p53, and E-cadherin expressions. Four-micrometer FFPE slices were deparaffinized and rehydrated with xylene and alcohol solutions. Immunostaining was performed using an automatic immunostaining instrument (Ventana Benchmark XT; Ventana Medical Systems, Tucson, AZ, USA) according to the manufacturer’s recommendations. The p16 (prediluted, clone E6H4; Ventana), p53 (1:300, clone DO-7; Novocastra, Leica Biosystems, Newcastle Upon Tyne, UK), and E-cadherin (1:100, clone NCH-38; Dako, Carpinteria, CA, USA) were used as primary antibodies. The p16 immunostaining pattern was interpreted as block positivity when strong, nuclear, or nuclear plus cytoplasmic staining of horizontal continuous cells was identified. For p53, at least 90% intense nuclear staining was defined as an overexpression pattern, no staining was defined as a complete absence pattern, and focal weak positive staining was defined as wild-type pattern [18, 19]. The loss of membranous expression was evaluated for E-cadherin.
HPV genotyping
HPV genotyping was performed with polymerase chain reaction (PCR)-based microarray analysis using an HPV 9G DNA chip (Biometrix Technology Inc., Chuncheon, South Korea), which detects 14 high-risk (types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68) and 5 low-risk (types 6, 11, 34, 40, and 42) HPV types. The test was performed according to the manufacturer’s protocol [20]. Briefly, genomic HPV DNA was extracted and amplified via PCR. The PCR product was mixed with hybridization solution and loaded on the HPV 9G DNA chip. DNA chip was incubated at 23–26 °C for 30 min and washed at room temperature (25 °C). Array images of the final product were automatically scanned and analyzed using a fluorescent scanner (ScanArray GX Microarray Scanner; PerkinElmer Life and Analytical Sciences, Waltham, MA, USA).
Next-generation sequencing (NGS) analysis
For DNA extraction, ten 5 µm-thick sections from each FFPE tissue were manually microdissected or laser microdissected (LMD6500, Leica Microsystems). Genomic DNA was then purified and isolated from paired tumor and normal tissue samples using the GeneRead DNA FFPE Kit (Qiagen) according to the manufacturer’s instructions.
A 468.654-kbp custom capture probe was created with the SureSelect DNA Advanced Design Wizard (Agilent Technologies, Santa Clara, CA, USA) including exons of 96 genes. The panel was based on frequently altered genes in cervical carcinoma, endometrial carcinoma, gastric, and pancreaticobiliary adenocarcinoma, recently reported mutated genes in GAS, and EMT-related genes [13, 15, 16, 21,22,23,24,25,26]. The gene list of the custom-designed panel is shown in Supplementary Table 1.
Target enrichment was performed using the SureSelect Target Enrichment protocol for the Illumina paired-end sequencing library (Agilent Technologies, ver. B.3, San Diego, CA, USA). Briefly, 200 ng of input DNA was extracted from FFPE tissue. DNA quantification and quality were measured using the PicoGreen dsDNA quantitation assay (Molecular Probes, Eugene, OR, USA). DNA was diluted in elution buffer and fragmented using Adaptive Focused Acoustic technology (AFA; Covaris, Woburn, MA, USA) according to the manufacturer’s recommendations. Fragmented DNA was repaired, adapter ligated, and PCR amplified. For exome capture, 250 ng of DNA library was mixed with hybridization buffers, blocking mixes, RNase block, and 5 µl of SureSelect all exon capture library according to the standard Agilent SureSelect Target Enrichment protocol. Hybridization to capture baits was conducted at 65 °C using a heated thermal cycler lid option at 105 °C for 24 h on the PCR machine. Captured DNA was then washed and amplified. The final purified product was quantified using quantitative PCR with the KAPA Library Quantification kit (Kapa Biosystems, Wilmington, MA, USA), and qualified using the TapeStation DNA ScreenTape D1000 (Agilent Technologies). Sequencing was performed with the HiSeq™ 2500 platform (Illumina, San Diego, CA, USA).
For data analysis, all reads were aligned to the human genome (hg19) with Burrows–Wheeler Aligner. Further data processing, variant calling, and annotation were performed according to the Genome Analysis Toolkit (GATK) best practice guidelines using Picard, GATK, and SnpSift. According to the 1000 Genomes, dbSNP, and ESP6500SI-V2 databases, variants with population frequency over 1% were grouped as SNPs and excluded. For data comparison with other cancer types, mutational data of The Cancer Genome Atlas (TCGA) dataset including 291 cervical carcinoma, 24 endocervical adenocarcinoma, 248 endometrial carcinoma, 295 gastric adenocarcinoma, 150 pancreatic ductal adenocarcinoma, and 35 cholangiocarcinoma cases were retrieved from cBioPortal.
Statistical analysis
A χ2 test or Fisher’s exact test was used to evaluate the statistical significance between two variables, and Kaplan–Meier method was used for survival analysis. Age distribution was evaluated with Shapiro–Wilk test. p < 0.05 was considered statistically significant for all analyses, and all analyses were two-tailed. All data were analyzed using R software (version 3.6.1).
Results
Patient characteristics
Among 21 patients with GAS, the median patient age was 56.6 years, and their age ranged from 32 to 90 years. FIGO stage was IB in seven (33.3%) patients, II in five (23.8%) patients, III in four (19.0%) patients, and IV in five (23.8%) patients. The median follow-up period was 16.3 months. Twenty patients (95.2%) received total hysterectomy, while one patient (4.8%) received punch biopsy for the diagnosis and was transferred for palliative care due to old age and advanced stage. At the time of initial diagnosis, p16 and p53 immunohistochemistry and HPV genotyping were performed in all cases. Seventeen (81.0%) cases were p16-negative, while four (19.0%) cases showed block positivity. The p53 staining result included 13 (61.9%) overexpression, 3 (14.3%) complete absence, and 5 (23.8%) wild-type pattern. All cases showed negative HPV genotyping results. NGS-tested specimens included 17 (81.0%) radical hysterectomy, 1 (4.8%) large loop excision of the transformation zone, and 3 (14.3%) punch biopsy cases. Patient characteristics are summarized in Table 1, and detailed clinicopathologic information of patients is shown in Table 2.
Among a total of 138 patients with endocervical adenocarcinoma, 110 patients (79.7%) were classified as HPV-related subtypes—usual type endocervical adenocarcinoma, villoglandular carcinoma, intestinal type mucinous carcinoma, signet-ring cell type mucinous carcinoma, and iSMILE; while 28 patients (20.3%) were classified as HPV-unrelated subtypes—GAS, clear cell carcinoma, and mesonephric carcinoma. Compared with HPV-related endocervical adenocarcinoma, GAS showed significantly higher initial stage, higher frequency of distant metastasis, and worse overall survival (Table 3 and Fig. 1).
Histologic characteristics
All cases harbored at least focal areas of classic GAS histology with abundantly clear to pale eosinophilic cytoplasm and distinct cell borders. Among them, 14.3% (3/21) of cases were entirely composed of well-differentiated tubular glands which could be designated to minimal deviation adenocarcinoma. Well-differentiated GAS resembled nonneoplastic gastric glands (Fig. 2a–c). GAS was also similar to gastric tubular adenocarcinoma and pancreatic ductal adenocarcinoma (Fig. 2d–f). Small tubular, angulated, cystic, or cribriform glands and single infiltrating tumor cells were identified (Fig. 3). Cytologically, tumor cells had voluminous, clear, foamy, or pale eosinophilic cytoplasm with mild to severe nuclear atypia. Goblet cells and abortive mucin production were not infrequently identified. In addition, GAS showed diffuse infiltration to deep cervical stroma and/or wide involvement to ectocervix and endometrium. At the invasive front of the tumor, single infiltrating tumor cells were frequently identified, and the single dyscohesive cells showed decreased expression of E-cadherin (Supplementary Fig. 1).
Well-differentiated GAS resembles nonneoplastic gastric glands (a gastric-type mucinous carcinoma, b nonneoplastic gastric glands, c a high-power view of gastric pyloric glands). Moderately differentiated gastric-type mucinous carcinoma shows histologic similarity to gastric and pancreatic ductal adenocarcinoma (d gastric-type mucinous carcinoma, e gastric tubular adenocarcinoma, f pancreatic ductal adenocarcinoma).
a Infiltrating small tubular glands with abundantly clear to pale eosinophilic cytoplasm and distinct cell borders. b Dilated glands with micropapillary infolding and goblet cells. c Cribriform pattern of tumor cells with desmoplastic stromal reaction. d Single infiltrating tumor cells with severe nuclear atypia and eosinophilic cytoplasm at the invasive front of the tumor.
Genetic characteristics
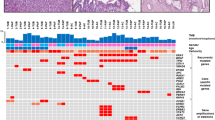
Overall, 55 nonsynonymous somatic mutations, including 41 missense, 1 nonsense, 4 inframe indel, 8 frameshift, and 1 splice site mutations, were detected in 21 GAS samples. The average mutation rate was 2.6 mutations per lesion (range: 0–9). VAF ranged from 5.8 to 75.3%, with an average read depth of 1285.60×. The most frequently mutated gene was TP53 (11/21, 52.4%), followed by serine-threonine kinase 11 (STK11/LKB1), HLA-B, PTPRS (4/21, 19.0%), FGFR4 (3/21, 14.3%), GNAS, BRCA2, ELF3, ERBB3, KMT2D, SLX4 (2/21, 9.5%), CDH1, EPCAM, KRAS, MLH1, RNF43, SNAI1, TWIST1, ZEB1, ZEB2, etc. (1/21, 4.8%) (Fig. 4). According to functional annotation, major mutant genes were categorized as involved in signal transduction, DNA damage repair, and EMT. In EMT-related genes, the mutational frequency of GAS was compared to that of cervical squamous cell carcinoma (n = 291) and endocervical adenocarcinoma (n = 24) TCGA datasets. Of eight EMT-related genes (CDH1, ELF3, EPCAM, SNAI1, SNAI2, TWIST1, ZEB1, and ZEB2), the total mutational frequency was 33.4% in GAS, while it was 9.8% and 8.4% in cervical carcinoma and endocervical adenocarcinoma, respectively (Supplementary Table 2).
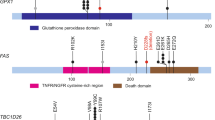
Of TP53 mutations, 90.9% (10/11) was located in DNA-binding domain (residues 94–312) and 9.1% (1/11) was located in carboxy (C)-terminal domain (residues 355–393). With respect to the correlation between p53 immunohistochemistry and TP53 mutation status, 68.8% (11/16) of cases with abnormal p53 expression harbored correlated TP53 missense or nonsense mutations or pathogenic splice site mutation. The remaining 31.3% of cases (5/16) showed p53 overexpression, however, TP53 mutation was not identified (Supplementary Table 3 and Supplementary Fig. 2).
We compared the genetic features of GAS with frequently mutated genes of cervical squamous cell carcinoma, endocervical adenocarcinoma, endometrial carcinoma, and gastric and pancreaticobiliary adenocarcinoma based on TCGA data. The results are summarized in Fig. 5, Supplementary Fig. 3, and Supplementary Table 4–9.
Representative frequently mutated genes in cervical squamous cell carcinoma, endocervical adenocarcinoma, endometrial carcinoma, gastric adenocarcinoma, pancreatic ductal adenocarcinoma, and biliary adenocarcinoma were listed in the each bar graph. Their mutational frequencies were compared with those of gastric-type mucinous carcinoma.
Discussions
In this study, we investigated genetic characteristics of 21 GAS cases to understand the biological basis of GAS. TP53 was the most frequently mutated gene, and mutated genes were mostly involved in signal transduction, DNA damage repair, and EMT. In addition, GAS shared some genetic features with gastric and pancreaticobiliary adenocarcinoma.
Histologically, pyloric gland mucin is a characteristic feature of GAS tumor cells. Although it is normally secreted from gastric pyloric glands and mucous neck cells, pyloric metaplasia could be identified in other organs as a protective process toward chronic injury or inflammation [27]. In addition, pyloric metaplasia could have a sensitizing effect toward oncogenic stimuli, which increases the risk of malignancy [28]. In uterine cervix, the possible link between benign pyloric metaplastic lesions and GAS had been suggested [29]. If GAS develops from benign metaplastic lesions, pyloric gland phenotype of GAS might be retained from the benign precursors. On the other hand, pyloric differentiation could be a manifestation of stem cell-like properties of GAS. Cancer stem cells could transdifferentiate to other cell types to support the tumor’s aggressive behavior [30]. In our GAS cases, genes associated with stem cell properties, such as CDH1, EPCAM, SNAI1, TWIST1, ZEB1, and ZEB2, were frequently altered. Although the underlying mechanism remains unknown, the pyloric phenotype of GAS may result from complex genetic or epigenetic mechanisms and further study is needed.
In our analysis, the majority of mutated genes were involved in signal transduction and DNA damage repair, which were commonly identified mutations in most malignant tumors. In addition, a considerable number of EMT-related genes were mutated in GAS, which was higher than those of the cervical squamous cell carcinoma and endocervical adenocarcinoma TCGA dataset. Alterations of EMT-related genes are frequently identified in multiple cancers and regarded to contribute to EMT phenomenon [31]. EMT is crucial for tumor dissemination and chemoresistance [32,33,34]. In this regard, EMT-related pathways might have a role in aggressive behavior and chemoresistance of GAS [8]. TCGA mRNA data of the HPV-negative group also showed higher EMT mRNA score compared to the HPV-positive group, in line with our results [15].
In our GAS cases, 76.2% showed p53 protein overexpression; however, 31.3% (5/16) of these cases harbored wild-type TP53. Likewise, Garg et al. reported three GAS cases with p53 overexpression and wild-type TP53 [13]. The accumulation of wild-type p53 had been identified in various tumors and regarded as a response to a variety of cellular stresses [35,36,37,38,39]. DNA damaging agents such as ultraviolet light radiation and chemotherapeutic agents could induce accumulation of wild-type p53 through posttranslational modifications. In addition, oncogenic stimuli could activate and accumulate p53 by inducing DNA damage or abnormal interaction with MDM2 [40]. These cellular stresses may accelerate the rate of mutation accumulation and tumor progression in malignant tumor.
In this study, we compared the genetic features of GAS to those of other anatomically or histologically related cancers. Compared to frequently mutated genes of cervical squamous cell carcinoma, 9.5% of GAS cases harbored KMT2D mutations [15]. Regarding frequently mutated genes in endocervical adenocarcinoma, KMT2D, ERBB3, and KRAS were mutated in 9.5%, 9.5%, and 4.8% of GAS cases, respectively. Of frequently mutated genes in endometrial carcinoma, TP53 was mutated in GAS (52.4%), followed by KMT2D (9.5%) and KRAS (4.8%) [26]. Other well-known endometrial carcinoma-related genes, such as PTEN, PIK3CA, ARID1A, PIK3R1, CTNNB1, FBXW7, and POLE, were not mutated in GAS. On the contrary, Garg et al. observed frequent endometrial cancer-related gene mutations (POLE, ARID1A, KRAS, and FBXW7) in GAS [13]. One possible reason for such discrepancy is the different ethnicity of the two study populations. Although Caucasians were the majority of the patient cohort of Garg et al., our 21 GAS cases consisted exclusively of Korean population. In addition, the different genetic features between the two studies might imply genetic heterogeneity of GAS.
In comparison with gastric and pancreaticobiliary adenocarcinoma, TP53 mutations were frequently identified, near 50%, in both GAS and gastrointestinal cancers. In addition, of frequently mutated genes of gastric adenocarcinoma, KMT2D, ERBB3, and RNF43 mutations were identified in 9.5%, 9.5%, and 4.8% of GAS cases, respectively [22]. In gastric adenocarcinoma, KMT2D, ERBB3, and RNF43 mutations were associated with poor prognosis [41]. These overlapping genetic features might be a clue to understand the distinct behavior of GAS. On the other hand, highly mutated genes of pancreatic ductal adenocarcinoma such as KRAS, SMAD4, and CDKN2A were rarely mutated in GAS [21]. These genetic differences suggest that GAS and pancreatic ductal adenocarcinoma are genetically distinct entities, despite their histologic similarities. Of frequently mutated genes in cholangiocarcinoma, BAP1, HLA-B, and TP53 mutations were identified in GAS with frequencies of 4.8%, 19.0%, and 52.4%, respectively [25]. Of these, BAP1 mutations were reported to be associated with better prognosis; however, it was correlated to small duct type cholangiocarcinoma, a subtype that is histologically dissimilar to GAS, that it was difficult to attach much meaning [42, 43].
In conclusion, this study identified a considerable number of EMT-related gene mutations in GAS, which suggest a possible role of EMT-related pathways in tumor dissemination and chemoresistance of GAS. Compared to gastric and pancreaticobiliary adenocarcinoma, TP53 mutations were frequent in both GAS and gastrointestinal cancers; KMT2D, ERBB3, and RNF43 mutations were shared between GAS and gastric adenocarcinoma, while highly mutated genes in pancreatic ductal adenocarcinoma were rarely mutated in GAS. These findings would provide clues to the biological basis of GAS.
References
Smith HO, Tiffany MF, Qualls CR, Key CR. The rising incidence of adenocarcinoma relative to squamous cell carcinoma of the uterine cervix in the United States—a 24-year population-based study. Gynecol Oncol. 2000;78:97–105.
Bray F, Carstensen B, Møller H, Zappa M, Žakelj MP, Lawrence G, et al. Incidence trends of adenocarcinoma of the cervix in 13 European countries. Cancer Epidemiol Biomark Prev. 2005;14:2191–9.
Stolnicu S, Barsan I, Hoang L, Patel P, Terinte C, Pesci A, et al. International endocervical adenocarcinoma criteria and classification (IECC): a new pathogenetic classification for invasive adenocarcinomas of the endocervix. Am J Surg Pathol. 2018;42:214–26.
Nishio S, Mikami Y, Tokunaga H, Yaegashi N, Satoh T, Saito M, et al. Analysis of gastric-type mucinous carcinoma of the uterine cervix—an aggressive tumor with a poor prognosis: a multi-institutional study. Gynecol Oncol. 2019;153:13–9.
Park KJ, Kiyokawa T, Soslow RA, Lamb CA, Oliva E, Zivanovic O, et al. Unusual endocervical adenocarcinomas: an immunohistochemical analysis with molecular detection of human papillomavirus. Am J Surg Pathol. 2011;35:633–46.
Kusanagi Y, Kojima A, Mikami Y, Kiyokawa T, Sudo T, Yamaguchi S, et al. Absence of high-risk Human Papillomavirus (HPV) detection in endocervical adenocarcinoma with gastric morphology and phenotype. Am J Pathol. 2010;177:2169–75.
Pirog EC, Lloveras B, Molijn A, Tous S, Guimerà N, Alejo M, et al. HPV prevalence and genotypes in different histological subtypes of cervical adenocarcinoma, a worldwide analysis of 760 cases. Mod Pathol. 2014;27:1559–67.
Kojima A, Shimada M, Mikami Y, Nagao S, Takeshima N, Sugiyama T, et al. Chemoresistance of gastric-type mucinous carcinoma of the uterine cervix: a study of the sankai gynecology study group. Int J Gynecol Cancer. 2018;28:99–106.
Kojima A, Mikami Y, Sudo T, Yamaguchi S, Kusanagi Y, Ito M, et al. Gastric morphology and immunophenotype predict poor outcome in mucinous adenocarcinoma of the uterine cervix. Am J Surg Pathol. 2007;31:664–72.
Pirog EC, Park KJ, Kiyokawa T, Zhang X, Chen W, Jenkins D, et al. Gastric-type adenocarcinoma of the cervix: tumor with wide range of histologic appearances. Adv Anat Pathol. 2019;26:1–12.
Ishii K, Katsuyama T, Ota H, Watanabe T, Matsuyama I, Tsuchiya S, et al. Cytologic and cytochemical features of adenoma malignum of the uterine cervix. Cancer Cytopathol. 1999;87:245–53.
Utsugi K, Hirai Y, Takeshima N, Akiyama F, Sakurai S, Hasumi K. Utility of the monoclonal antibody HIK1083 in the diagnosis of adenoma malignum of the uterine cervix. Gynecol Oncol. 1999;75:345–8.
Garg S, Nagaria TS, Clarke B, Freedman O, Khan Z, Schwock J, et al. Molecular characterization of gastric-type endocervical adenocarcinoma using next-generation sequencing. Mod Pathol. 2019;32:1823–33.
Carleton C, Hoang L, Sah S, Kiyokawa T, Karamurzin YS, Talia KL, et al. A detailed immunohistochemical analysis of a large series of cervical and vaginal gastric-type adenocarcinomas. Am J Surg Pathol. 2016;40:636–44.
Burk RD, Chen Z, Saller C, Tarvin K, Carvalho AL, Scapulatempo-Neto C, et al. Integrated genomic and molecular characterization of cervical cancer. Nature. 2017;543:378–84.
Murali R, Filippo MD, Weigelt BWPK. Genomic characterization of gastric-type endocervical adenocarcinomas (abstract). Mod Pathol. 2016;31:1185.
Kurman RJ, Carcangiu ML, Herrington CS, Young RH. WHO classification of tumours of female reproductive organs. 4th ed. Lyon: International Agency for Research on Cancer; 2014. p. 307.
Köbel M, Reuss A, Du Bois A, Kommoss S, Kommoss F, Gao D, et al. The biological and clinical value of p53 expression in pelvic high-grade serous carcinomas. J Pathol. 2010;222:191–8.
Yemelyanova A, Vang R, Kshirsagar M, Lu D, Marks MA, Shih IM, et al. Immunohistochemical staining patterns of p53 can serve as a surrogate marker for TP53 mutations in ovarian carcinoma: an immunohistochemical and nucleotide sequencing analysis. Mod Pathol. 2011;24:1248–53.
An H, Song KS, Nimse SB, Kim J, Nguyen VT, Ta VT, et al. HPV 9G DNA chip: 100% clinical sensitivity and specificity. J Clin Microbiol. 2012;50:562–8.
Raphael BJ, Hruban RH, Aguirre AJ, Moffitt RA, Yeh JJ, Stewart C, et al. Integrated genomic characterization of pancreatic ductal adenocarcinoma. Cancer Cell. 2017;32:185–203.
Bass AJ, Thorsson V, Shmulevich I, Reynolds SM, Miller M, Bernard B, et al. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202–9.
Ojesina AI, Lichtenstein L, Freeman SS, Pedamallu CS, Imaz-Rosshandler I, Pugh TJ, et al. Landscape of genomic alterations in cervical carcinomas. Nature. 2014;506:371–5.
Hodgson A, Park KJ. Cervical adenocarcinomas: a heterogeneous group of tumors with variable etiologies and clinical outcomes. Arch Pathol Lab Med. 2019;143:34–46.
Farshidfar F, Zheng S, Gingras MC, Newton Y, Shih J, Robertson AG, et al. Integrative genomic analysis of cholangiocarcinoma identifies distinct IDH-mutant molecular profiles. Cell Rep. 2017;18:2780–94.
Getz G, Gabriel SB, Cibulskis K, Lander E, Sivachenko A, Sougnez C, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497:67–73.
Goldenring JR. Pyloric metaplasia, pseudopyloric metaplasia, ulcer-associated cell lineage and spasmolytic polypeptide-expressing metaplasia: reparative lineages in the gastrointestinal mucosa. J Pathol. 2018;245:132–7.
Yuan S, Norgard RJ, Stanger BZ. Cellular plasticity in cancer. Cancer Discov. 2019;9:837–51.
Mikami Y, Kiyokawa T, Hata S, Fujiwara K, Moriya T, Sasano H, et al. Gastrointestinal immunophenotype in adenocarcinomas of the uterine cervix and related glandular lesions: a possible link between lobular endocervical glandular hyperplasia/pyloric gland metaplasia and “adenoma malignum”. Mod Pathol. 2004;17:962–72.
Shekhani MT, Jayanthy AS, Maddodi N, Setaluri V. Cancer stem cells and tumor transdifferentiation: implications for novel therapeutic strategies. Am J Stem Cells. 2013;2:52–61.
Zhao M, Kong L, Liu Y, Qu H. DbEMT: an epithelial-mesenchymal transition associated gene resource. Sci Rep. 2015;5:1–14.
Thiery JP, Acloque H, Huang RYJ, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–90.
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–15.
Singh A, Settleman J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene. 2010;29:4741–51.
Lavin MF, Gueven N. The complexity of p53 stabilization and activation. Cell Death Differ. 2006;13:941–50.
Wang YC, Lin RK, Tan YH, Chen JT, Chen CY, Wang YC. Wild-type p53 overexpression and its correlation with MDM2 and p14ARF alterations: an alternative pathway to non-small-cell lung cancer. J Clin Oncol. 2005;23:154–64.
Lim SO, Park YM, Kim HS, Quan X, Yoo JE, Park YN, et al. Notch1 differentially regulates oncogenesis by wildtype p53 overexpression and p53 mutation in grade III hepatocellular carcinoma. Hepatology. 2011;53:1352–62.
Guillou L, Estreicher A, Chaubert P, Hurlimann J, Kurt AM, Metthez G, et al. Germ cell tumors of the testis overexpress wild-type p53. Am J Pathol. 1996;149:1221–8.
Rubio MP, Von Deimling A, Yandell DW, Wiestler OD, Gusella JF, Louis DN. Accumulation of wild type p53 protein in human astrocytomas. Cancer Res. 1993;53:3465–7.
Jimenez GS, Khan SH, Stommel JM, Wahl GM. p53 regulation by post-translational modification and nuclear retention in response to diverse stresses. Oncogene. 1999;18:7656–65.
Ge S, Li B, Li Y, Li Z, Liu Z, Chen Z, et al. Genomic alterations in advanced gastric cancer endoscopic biopsy samples using targeted next-generation sequencing. Am J Cancer Res. 2017;7:1540–53.
Misumi K, Hayashi A, Shibahara J, Arita J, Sakamoto Y, Hasegawa K, et al. Intrahepatic cholangiocarcinoma frequently shows loss of BAP1 and PBRM1 expression, and demonstrates specific clinicopathological and genetic characteristics with BAP1 loss. Histopathology. 2017;70:766–74.
Andrici J, Goeppert B, Sioson L, Clarkson A, Renner M, Stenzinger A, et al. Loss of BAP1 expression occurs frequently in intrahepatic cholangiocarcinoma. Medicine. 2016;95:1–8.
Acknowledgements
This study was supported by a faculty research grant of Yonsei University College of Medicine (6-2019-0108 to EP), and by a Mid-Career Researcher Program through a grant from the National Research Foundation of Korea (2019R1A2B5B01069934 to NHC).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Park, E., Kim, S.W., Kim, S. et al. Genetic characteristics of gastric-type mucinous carcinoma of the uterine cervix. Mod Pathol 34, 637–646 (2021). https://doi.org/10.1038/s41379-020-0614-0
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41379-020-0614-0
This article is cited by
-
Cervical HPV-independent adenosquamous carcinoma: report of a case series
Virchows Archiv (2026)
-
Gastric-type endocervical adenocarcinoma in situ as the presenting feature in a mosaic STK11 pathogenic variant carrier with a Peutz-Jeghers syndrome child
Familial Cancer (2025)
-
Bit1 promotes the progression of gastric cancer by facilitating epithelial–mesenchymal transition and alleviating apoptosis
Medical Oncology (2025)
-
Endocervical adenocarcinoma with a micropapillary component: a clinicopathologic analysis in the setting of current WHO classification
Virchows Archiv (2025)
-
Clinicopathological characterization of female genital tract cancers with STK11 alterations: redefining the spectrum beyond STK11 adnexal tumors
Virchows Archiv (2025)