Abstract
Desmoplastic small round cell tumor (DSRCT) is a high-grade round cell sarcoma that typically arises in the abdominopelvic cavity of young males, co-expresses keratins and desmin, and carries a pathognomonic EWSR1-WT1 gene fusion. The EWSR1-WT1 gene fusion is generally considered specific for DSRCT, although there are two reports of this fusion in tumors otherwise lacking features of DSRCT. We report three female genital tract tumors with EWSR1-WT1 fusions but showing morphologic and immunohistochemical features incompatible with DSRCT. The tumors occurred in the uterine cervix, uterine corpus/ovaries, and vagina, respectively, of 46, 30, and 20-year-old women. Two tumors consisted of a sheet-like to fascicular proliferation of relatively uniform spindled to occasionally more epithelioid cells arrayed about thick-walled, hyalinized, and capillary-sized vessels, with distinctive areas of pseudovascular change, and absence of desmoplastic stroma. The third tumor resembled a monomorphic spindle cell sarcoma with necrosis. All had diffuse desmin and variable but more limited keratin expression, two of three expressed smooth muscle actin, and all were negative for h-caldesmon, CD10, estrogen receptor, myogenin, N-terminus WT-1, and S100 protein. One patient received neoadjuvant chemotherapy and radiation therapy followed by resection and is disease-free 42 months after diagnosis. Another patient was managed by resection only and is disease-free 9 months after initial diagnosis. The remaining patient recently underwent resection of multifocal pelvic disease. Comprehensive differential gene expression analysis on two tumors compared to two classic DSRCTs with known EWSR1-WT1 fusions resulted in 1726 genes that were differentially expressed (log2 fold change >2 or < −2) and statistically significant (FDR < 5%). In combination with previous reports, our findings suggest pleiotropy of the EWSR1-WT1 fusion is possible and not limited to DSRCT. Subsets of non-DSRCT EWSR1-WT1 positive tumors may represent discrete entities, but further study is necessary.
Similar content being viewed by others
Introduction
Recurrent gene fusions, first described in soft tissue tumors by Delattre et al. in Ewing sarcoma (EWSR1-FLI1) [1], are present in a broad and ever-increasing assortment of neoplasms. Despite early optimism that these genetic events might prove specific and defining, it has become clear many are not. For example, the ETV6-NTRK3 fusion, identified in infantile fibrosarcoma in 1998 by Knezevich and colleagues [2], is now known to also occur in cellular mesoblastic nephroma [3], acute myelogenous leukemia [4] and secretory carcinoma of the breast [5]. Similarly, the EWSR1-ATF1 fusion, thought particular to clear cell sarcoma of soft parts [6], is also in malignant gastrointestinal neuroectodermal tumors, indolent angiomatoid fibrous histiocytomas, unusual myxoid tumors of the lung and central nervous system, and even clear cell salivary gland carcinomas [7]. Rather than standing in isolation, the presence or absence of gene fusions represents one of many data points integrated by the surgical pathologist in establishing a diagnosis.
Desmoplastic small round cell tumor (DSRCT) is a highly aggressive round cell sarcoma, which typically occurs in the abdominal cavity of male children and adolescents, co-expresses keratins, desmin and vimentin, and is characterized at the genetic level by t(11;22)(p13;q12), with in-frame fusion of the first 7 exons of EWSR1 (22q12.2) with exons 8–10 of WT1 (11p13) [8,9,10]. This EWSR1-WT1 fusion is considered among the most entity-specific; there is no mention of it in tumors other than DSRCT in either the most recent WHO Classification of Soft Tissue and Bone Tumors [11] or the latest edition of Enzinger and Weiss’s Soft Tissue Tumors [12].
However, there are two prior reports of the EWSR1-WT1 fusion in tumors deviating significantly from DSRCT. The first, by Alaggio and colleagues in 2007, described two leiomyosarcoma-like spindle cell tumors of the abdominal cavity in adolescent males with favorable outcome [13]. Eight years later, Ud Din et al. described an indolent glomoid tumor of the L4 spinal nerve roots sharing this same gene fusion.
We present the clinicopathologic, immunohistochemical, and molecular genetic features of three unusual spindle cell neoplasms with EWSR1-WT1 fusions that involved the gynecologic tract, and discuss their distinction from DSRCT as well as more common spindle cell neoplasms in these locations.
Materials and methods
Case selection
All available slides and blocks from three consultation cases with a known EWSR1-WT1 fusion and deviating significantly from DSRCT by morphology (see below) were retrieved from our archives. Formalin-fixed paraffin-embedded (FFPE) tissue blocks were processed by standard protocols for hematoxylin and eosin staining, preparation of immunohistochemical studies and next generation sequencing. Patient history and outcome were retrieved from institutional medical records and managing healthcare providers.
Immunohistochemistry
FFPE sections from cases 1 and 2 were stained for keratins (clone OSCAR, 1:100, Covance, Princeton, NJ, and clones AE1/AE3, 1:100, Dako, Carpinteria, CA), estrogen receptor alpha (clone SP1, prediluted, Ventana Medical Systems, Tucson, AZ), desmin (clone DER11, 1:100. Leica Biosystems, Buffalo Grove, IL), smooth muscle actin (clone 1A4, 1;3000, Dako), h-Caldesmon (clone H-CALD, 1:500, ThermoFisher), CD10 (clone 56C6, Leica Biosystems), N-terminus WT-1 (clone WT49, 1:75, Leica Biosystems), myogenin (clone F5D, 1:50, Biocare), myoD1 (clone EP212, prediluted, Ventana Medical Systems) and S100 protein (polyclonal, 1:750, Leica Biosystems), using routine laboratory protocols and the Ultraview DAB, Refine DAB or Optiview DAB detection systems.
FFPE sections from case 3 were stained for keratins (clone CAM5.2, 1:50, BD Biosciences, Woburn, MA), estrogen receptor (clone SP1, 1:40, Fisher Scientific, Hampton, NH), desmin (clone DE-U-10, 1:5000, Sigma, St. Louis, MO), smooth muscle actin (clone 1A4, 1:20 000, Sigma), h-Caldesmon (clone h-CD, 1:300, Dako), CD10 (clone 56C6, 1:20, Cell Marque, Rocklin, CA), N-terminus WT-1 (clone 6F-H2, 1:75, Dako), C-terminus WT-1 (polyclonal, 1:1000, ThermoFisher), myogenin (clone EP162, 1:200, Cell Marque), myoD1 (clone EP212, 1:100, Cell Marque), CD99 (clone O13, 1:150, BioLegend, Dedham, MA), and S100 protein (polyclonal, 1:3000, Dako).
Next generation sequencing
Cases 1 and 2 were evaluated by the Illumina TruSeq RNA Exome assay that targets over 20,000 genes and includes gene fusion targets as well as common BCOR internal tandem duplications. Details of this assay have been previously published [14]. RNA integrity number (RIN) and DV200 values were determined using the Agilent Fragment Analyzer and satisfied laboratory quality thresholds. cDNA libraries were prepared using 200–400 ng of total RNA according to the manufacturer’s instructions for the TruSeq® RNA Exome Library Prep Kit. The concentration and size distribution of the final libraries were determined on an Agilent Bioanalyzer DNA 1000 chip (Santa Clara, CA). A final quantification, using Qubit fluorometry (Invitrogen, Carlsbad, CA), was performed to confirm sample concentration.
Samples were sequenced on an Illumina HiSeq 4000 instrument using the 200 cycle Rapid v2 Reagent Kit (Illumina). Base-calling was performed using Illumina’s RTA version 2.7.7. Raw data were processed through an automated in-house bioinformatics pipeline [15] with clinically validated scripts [16] for annotating gene fusions in sarcomas. Differentially expressed genes were identified using bioinformatics R package edgeR 2.6.2 [17], and reported with magnitude of change (log2 scale) and level of significance (False Discovery Rate, FDR < 5%). Canonical pathway analysis was performed using the Ingenuity pathway analysis software IPA (Ingenuity® Systems, www.ingenuity.com).
Case 3 was evaluated using an RNA sequencing platform, the details of which have been previously published [18]. Briefly, the Illumina TruSight RNA fusion panel (Illumina) was used to create libraries of 507 target genes, which were then sequenced on an Illumina MiSeq instrument. Raw data were analyzed using an in-house pipeline and both the STAR aligner and Manta fusion caller and JAFFA fusion caller and BOWTIE2 aligner.
Results
Clinical findings
Table 1 summarizes the clinicopathological features of the three cases that comprise our report as well as three previously reported tumors with EWSR1-WT1 fusions lacking characteristic features of DSRCT.
Case 1: A 46-year-old woman was referred for biopsy after identification of a cervical mass by physical examination and diagnosis of atypical glandular cells by cervical Papanicolaou smear. This biopsy showed a spindle cell tumor thought to possibly represent a leiomyoma with bizarre nuclei, and she was referred for further surgery. A total hysterectomy, bilateral salpingo-oophorectomy, and bilateral pelvic sentinel lymph node biopsies were performed. A 2.2 × 1.6 × 1.6 cm indurated, yellow-tan to orange-brown mass was confined to the cervix. The remaining specimens were negative for disease. The tumor was diagnosed as an unusual low grade spindle cell sarcoma with EWSR1-WT1 gene fusion. Four months after resection, physical examination and CT scans of the chest, abdomen, and pelvis showed no recurrent or metastatic disease. A second series of postoperative surveillance CT scans was negative 9 months after diagnosis.
Case 2: A 30-year-old woman experienced sudden onset left-sided abdominal pain and was found by CT imaging to have large ovarian and uterine masses, numerous retroperitoneal cystic and solid masses, and multiple indeterminate pulmonary nodules. She underwent total hysterectomy, bilateral salpingo-oophorectomy, left obturator lymph node dissection and resection of the retroperitoneal, and peritoneal masses. At the time of surgery, she was noted to have multifocal disease involving the left ovary (9.5 × 9.2 × 0.8 cm), myometrium (7.1 × 7 × 5.8 cm), retroperitoneum (18.5 × 14.5 × 11 cm), peritoneum (21 × 12 × 10 cm), uterine serosa (1.7 × 1.4 cm), and small soft tissue deposit in the region of left obturator lymph nodes. The tumors were described as solid and multicystic, variably hemorrhagic and necrotic, and brown-tan (Fig. 2A). The tumor was diagnosed as an unusual low grade spindle cell sarcoma with EWSR1-WT1 gene fusion. Seven months following diagnosis, CT abdomen and pelvis identified two sites of subcentimeter lymph node metastases, one biopsy-proven, in the regions of the right anterior kidney and anterior to the inferior vena cava at the level of the pancreatic head. The patient is considering stereotactic body radiotherapy.
Case 3: A 20-year-old woman presented for evaluation of a vaginal mass. Her clinical history was significant for a remote history of Ewing sarcoma of the toe metastatic to the pleura with membranous CD99 expression and EWSR1-ERG gene fusion for which she received adjuvant chemo/radiotherapy. She also had a germline heterozygous BRIP1 c.2098-3 T > C variant of uncertain significance. At the time of surgery, a 5.4 cm mass was centered in the left vaginal fornix, abutting the cervix, bladder, and rectum. Biopsies from the vaginal mass showed a malignant neoplasm with spindle cell and epithelioid features that were difficult to classify. This patient received neoadjuvant chemotherapy followed by radiation therapy, with partial vaginectomy, total hysterectomy, and left salpingectomy 8 months after initial diagnosis. Residual tumor grossly measured 2.8 × 2.1 × 1.4 cm. The tumor was diagnosed as an unclassifiable spindled and epithelioid sarcoma, morphologically high grade. Nearly three and a half years after diagnosis, the patient is alive without evidence of disease.
Morphologic findings
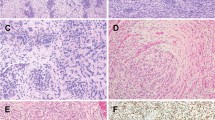
The morphologic features of Cases 1 (Figs. 1A–F) and 2 (Fig. 2B–F) were similar. At low power magnification, the tumors permeated surrounding cervical stroma and myometrium reminiscent of endometrial stromal sarcoma with multiple tongues and nests of tumor cells percolating fibroconnective tissue and smooth muscle (Figs. 1A and 2B). The neoplastic cells generally formed sheets and fascicles with areas of nested and corded architecture and occasional cystic change (Figs. 1B and 2C). The tumor cells were for the most part spindled with a modest amount of eosinophilic cytoplasm, indistinct cell borders, and elongated nuclei with blunt or tapered ends (Fig. 1C). In some portions, the tumor cells were smaller and more epithelioid (Figs. 1D and 2D). Scattered pleomorphic cells were present. Numerous blood vessels were intermixed, but vessels resembling spiral arterioles were not. The mitotic index ranged from 1 to 4 figures per 10 high power fields. Cystic change was seen in two tumors and necrosis was identified in one tumor. In one tumor, a combination of cystic change and pseudopapillary growth produced in one area a somewhat “cotyledonoid” pattern. Classic features of DSRCT including primitive round cells in a desmoplastic stroma were absent in both tumors.
The tumor in Case 1 permeated cervical stroma in multiple tongues and nests (A) and had a mixture of sheet-like and fascicular architecture with scattered cystic change (B). The tumor cells were mostly spindled with a modest amount of eosinophilic cytoplasm, indistinct cell borders and elongated nuclei with blunt or tapered ends (C). Some portions of tumor had more epithelioid cells (D). Desmin expression was diffuse (E) whereas keratin AE1/AE3 was limited to a few clusters of cells (F).
Case 2’s patient presented with multifocal disease that partly included the ovary and uterus as solid brown-tan masses that were variably hemorrhagic and necrotic (A). Like Case 1, Case 2 permeated myometrium in a manner resembling an endometrial stromal sarcoma (B), and was composed of sheets and fascicles of cells with cystic change (C, D). Thick and thin-walled blood vessels, occasionally associated with perivascular hyaline, were present (C). Desmin was diffusely positive (E). Keratin AE1/AE3 was more extensively expressed by tumor cells (F, correlative of C).
In contrast, Case 3 was a fascicular tumor composed of uniform spindle cells with scant lightly eosinophilic cytoplasm, hyperchromatic nuclei, up to 13 mitotic figures/10 high power fields, and scattered foci of necrosis (Fig. 3A–D). The tumor resembled monophasic synovial sarcoma or malignant peripheral nerve sheath tumor, but alternating zones of hyper- and hypocellularity, accentuated perivascular cellularity, staghorn blood vessels and wiry collagen were lacking. Desmoplastic stroma was also not seen.
The tumor in Case 3 was predominantly fascicular (A, B) and composed of a uniform population of spindle cells with scant pale eosinophilic cytoplasm and hyperchromatic nuclei. In contrast to Cases 1 and 2, mitotic activity in Case 3 was more frequent at up to 13 mitotic figures/10 high power fields and there were foci of necrosis (C, D).
Immunohistochemical findings
Table 1 summarizes immunohistochemical results. Diffuse expression of desmin was seen in all tumors (Figs. 1E and 2E). Keratin was expressed by all tumors, but it was more limited and varied by antibody (Figs. 1E and 2E). Smooth muscle actin was positive in 2 of 3 tumors; all were h-Caldesmon negative. CD10, estrogen receptor, N-terminus WT-1, myogenin, myoD1 and S100 protein were negative in all tumors. Case 3 was negative for CD99 and showed nuclear positivity for WT-1 directed against the C-terminus.
Next generation sequencing findings
An EWSR1-WT1 gene fusion was identified in each tumor. The fusion transcript in Cases 1 and 2 had a 5’ gene partner breakpoint at exon 9 and 3’ gene partner breakpoint at exon 8. For Case 3, the EWSR1 breakpoint was at exon 10 (of 18) and the WT1 breakpoint at exon 7; in addition, WT1 had a 12 bp in-frame deletion.
Comprehensive differential gene expression analysis between Cases 1 and 2 and two cases of classic DSRCTs with known EWSR1-WT1 fusions in adult males resulted in 1,726 genes that were differentially expressed (log2 fold change >2 or < −2) and statistically significant (FDR < 5%). About 40% of these genes were upregulated in Cases 1 and 2 (Fig. 4). Canonical pathway analysis indicated activated immune response and negative regulation of cell cycle signaling pathways in Cases 1 and 2.
Discussion
Inclusive of the 3 tumors in our report, we are aware of 6 tumors with EWSR1-WT1 fusions that are something other than DSRCT (Table 1). Although these non-DSRCT EWSR1-WT1 tumors share some clinical and immunohistochemical features, they are morphologically heterogeneous, and are unlikely to represent a single discrete entity.
Perhaps most critical is whether these unusual EWSR1-WT1 tumors are variants of DSRCT. We interpret the morphologic, immunohistochemical and clinical evidence to suggest they are not. DSRCT may occasionally show minor morphologic variation such as glands, papillae, or slightly spindled cells, but the majority of these tumors have nests of malignant primitive round cells embedded in a richly vascular desmoplastic background [19, 20]. The morphologic features of our 3 cases are different, with none having desmoplastic stroma, two consisting of bland ovoid to spindle cells in association with hyalinized blood vessels and pseudovascular spaces, and one resembling a monomorphic spindle cell sarcoma. Furthermore, while our cases co-express keratins and desmin, similar to DSRCT, they also express other markers of smooth muscle differentiation, unlike DSRCT [10]. Co-expression of keratins and desmin is not specific to DSRCT and may be found in conventional leiomyosarcomas and rhabdomyosarcomas, among others [12]. In addition, that these unusual EWSR1-WT1 tumors affect both males and females and are relatively indolent significantly differs from the male predilection and markedly aggressive behavior of DSRCT [21,22,23]. On a transcriptomic level, profiling of two EWSR1-WT1 tumors and two classic DSRCTs with EWSR1-WT1 fusions demonstrated significant differences in gene expression. Finally, DSRCT of the female genital tract is exceptionally rare, with only two reported examples: a genetically confirmed case of the uterine corpus in a 46-year-old woman [21] and a morphologically and immunohistochemically typical example in the cervicovaginal region of a 19-year-old woman [24].
Data are insufficient to definitively address whether non-DSRCTs with EWSR1-WT1 fusions represent discrete entities––as in tumors with EWSR1/FUS-ATF1/CREB1 fusions -- or are an assortment of unrelated tumors that share a single molecular genetic event. The distinctive morphologic and immunohistochemical features of Cases 1 and 2 suggest these two tumors may represent a discrete entity within the family of EWSR1-WT1 neoplasia. Likewise, the tumors reported by Alaggio et al (Table 1, Cases 5 and 6) appear homogenous and do not clearly correspond to another well-defined soft tissue entity [13]. No conclusions can yet be formed about Cases 3 and 4 (Table 1) which have singular features.
One patient in our series (Case 3) had a history of Ewing sarcoma (EWSR1-ERG positive) and was known to carry a germline single nucleotide variant in intron 14 of BRIP1. Although her particular VUS is not present in population databases and has not, to the best of our knowledge, been reported in patients with known BRIP1-related neoplasms, hereditary mutations of homologous recombination repair genes such as BRIP1, both pathogenic and likely pathogenic variants, have been linked to Ewing sarcoma predisposition and may be related to development of other EWSR1 rearranged neoplasms [25]. One might attribute the occurrence of two different EWSR1 tumors in a young patient as somehow related to her underlying BRIP1 VUS. However, the morphologic, immunohistochemical, and molecular genetic features of her vaginal mass were not those of recurrent Ewing sarcoma.
The differential diagnosis for these non-DSRCT EWSR1-WT1 tumors includes endometrial stromal neoplasms, smooth muscle tumors and, for Case 3, other monomorphic spindle cell sarcomas such as synovial sarcoma. Low grade endometrial stromal sarcomas classically permeate as separate nests of monotonous spindle cells resembling endometrial stroma with intermixed spiral arterioles and occasional hyaline plaques [26, 27]. They often express CD10 and hormone receptors, but can be positive for smooth muscle markers, particularly in tumors with smooth muscle differentiation [28]. Several gene rearrangements have been described in low grade endometrial stromal sarcomas, most commonly involving JAZF1 and PHF1 [26]. Some low grade endometrial stromal sarcomas are accompanied by a high grade component that has a solid, permeative, or frankly infiltrative growth of small round cells [29]. The high grade round cell component typically diffusely expresses cyclin D1 and BCOR, often expresses KIT, and is negative for CD10 and estrogen and progesterone receptors [30, 31]. Genetically defined high grade endometrial stromal sarcomas can also develop without a low grade component as pure YWHAE-NUTM2A/B fused tumors or as myxoid spindled to epithelioid neoplasms resembling myxoid smooth muscle tumors that have ZC3H7B-BCOR fusions or internal tandem duplications of BCOR [32, 33]. BCOR-altered high grade endometrial stromal sarcomas are often positive for CD10, cyclin D1, BCOR, and SATB2 while variably positive for muscle markers and hormone receptors [32,33,34]. Cases 1 and 2 from our series had some morphologic features of low grade endometrial stromal tumors, but lacked CD10 and hormone receptor expression as well as endometrial stromal tumor-related molecular genetic alterations.
The morphologic and immunohistochemical findings of Cases 1 and 2 also prompted consideration of a gynecologic smooth muscle tumor. However, these tumors lacked characteristic eosinophilic cytoplasm, elongated nuclei and perinuclear vacuoles often seen in smooth muscle neoplasms, as well as the cleft-like spaces and hydropic change found in some gynecologic smooth muscle tumors [35,36,37]. The immunohistochemical features of Cases 1 and 2 overlap with gynecologic smooth muscle tumors, but absent expression of hormone receptors and h-Caldesmon is unusual for the latter group.
In addition to leiomyosarcoma, the differential diagnosis for Case 3 included other monomorphic spindle cell sarcomas: synovial sarcoma, spindle cell rhabdomyosarcoma, malignant peripheral nerve sheath tumor, and others. Careful morphologic study, a panel of immunostains to include keratins, S100 protein, SOX10, H3K27me3, myogenin, myoD1 and molecular genetic evaluation for events such as synovial sarcoma-specific SS18/SS18L1-SSX1/2/4 gene fusion should allow differentiation of these considerations from rare EWSR1-WT1 spindle cell sarcomas.
In summary, we present the clinicopathologic, immunohistochemical, and molecular genetic findings of three unusual neoplasms involving the female genital tract with EWSR1-WT1 fusions. The features of these tumors deviate from those of DSRCT, and, in combination with prior reports, strongly suggest this fusion is not specific to a single entity. Whether the presence of EWSR1-WT1 fusions defines other discrete entities remains to be established.
Data availability
Data generated or analyzed during this study are included in the manuscript.
References
Delattre O, Zucman J, Plougastel B, Desmaze C, Melot T, Peter M, et al. Gene fusion with an ETS DNA-binding domain caused by chromosome translocation in human tumours. Nature. 1992;359:162–5.
Knezevich SR, McFadden DE, Tao W, Lim JF, Sorensen PH. A novel ETV6-NTRK3 gene fusion in congenital fibrosarcoma. Nat Genet. 1998;18:184–7.
Knezevich SR, Garnett MJ, Pysher TJ, Beckwith JB, Grundy PE, Sorensen PH. ETV6-NTRK3 gene fusions and trisomy 11 establish a histogenetic link between mesoblastic nephroma and congenital fibrosarcoma. Cancer Res. 1998;58:5046–8.
Kralik JM, Kranewitter W, Boesmueller H, Marschon R, Tschurtschenthaler G, Rumpold H, et al. Characterization of a newly identified ETV6-NTRK3 fusion transcript in acute myeloid leukemia. Diagn Pathol. 2011;6:19.
Tognon C, Knezevich SR, Huntsman D, Roskelley CD, Melnyk N, Mathers JA, et al. Expression of the ETV6-NTRK3 gene fusion as a primary event in human secretory breast carcinoma. Cancer Cell. 2002;2:367–76.
Zucman J, Delattre O, Desmaze C, Epstein AL, Stenman G, Speleman F, et al. EWS and ATF-1 gene fusion induced by t(12;22) translocation in malignant melanoma of soft parts. Nat Genet. 1993;4:341–5.
Thway K, Fisher C. Tumors with EWSR1-CREB1 and EWSR1-ATF1 fusions: the current status. The. Am J surgical Pathol. 2012;36:e1–e11.
Gerald WL, Miller HK, Battifora H, Miettinen M, Silva EG, Rosai J. Intra-abdominal desmoplastic small round-cell tumor. Report of 19 cases of a distinctive type of high-grade polyphenotypic malignancy affecting young individuals. Am J Surg Pathol. 1991;15:499–513.
Ladanyi M, Gerald W. Fusion of the EWS and WT1 genes in the desmoplastic small round cell tumor. Cancer Res. 1994;54:2837–40.
Ordonez NG. Desmoplastic small round cell tumor: II: an ultrastructural and immunohistochemical study with emphasis on new immunohistochemical markers. Am J Surg Pathol. 1998;22:1314–27.
Soft Tissue and Bone Tumours. 5th edn, (International Agency for Research on Cancer: Lyon, France, 2020).
Goldblum, JR, Folpe, AL & Weiss, SW Enzinger & Weiss’s Soft Tissue Tumors. 7. edn, (Elsevier: Philadelphia, 2019).
Alaggio R, Rosolen A, Sartori F, Leszl A, d’Amore ES, Bisogno G, et al. Spindle cell tumor with EWS-WT1 transcript and a favorable clinical course: a variant of DSCT, a variant of leiomyosarcoma, or a new entity? Report of 2 pediatric cases. Am J Surg Pathol. 2007;31:454–9.
Martinez AP, Fritchie KJ, Weiss SW, Agaimy A, Haller F, Huang H-Y, et al. Histiocyte-rich rhabdomyoblastic tumor: rhabdomyosarcoma, rhabdomyoma, or rhabdomyoblastic tumor of uncertain malignant potential? A histologically distinctive rhabdomyoblastic tumor in search of a place in the classification of skeletal muscle neoplasms. Mod Pathol. 2019;32:446–57.
Kalari KR, Nair AA, Bhavsar JD, O’Brien DR, Davila JI, Bockol MA, et al. MAP-RSeq: Mayo Analysis Pipeline for RNA sequencing. BMC Bioinforma. 2014;15:224.
Winters JL, Davila JI, McDonald AM, Nair AA, Fadra N, Wehrs RN, et al. Development and verification of an RNA sequencing (RNA-Seq) assay for the detection of gene fusions in tumors. J Mol Diagn. 2018;20:495–511.
Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–40.
Dickson BC, Childs TJ, Colgan TJ, Sung YS, Swanson D, Zhang L, et al. Uterine tumor resembling ovarian sex cord tumor: a distinct entity characterized by recurrent NCOA2/3 gene fusions. Am J Surg Pathol. 2019;43:178–86.
Ordonez NG. Desmoplastic small round cell tumor: I: a histopathologic study of 39 cases with emphasis on unusual histological patterns. Am J Surg Pathol. 1998;22:1303–13.
Lae ME, Roche PC, Jin L, Lloyd RV, Nascimento AG. Desmoplastic small round cell tumor: a clinicopathologic, immunohistochemical, and molecular study of 32 tumors. Am J Surg Pathol. 2002;26:823–35.
Al-Ibraheemi A, Broehm C, Tanas MR, Horvai AE, Rubin BP, Cheah AL, et al. Desmoplastic small round cell tumors with atypical presentations: a report of 34 cases. Int J Surg Pathol. 2019;27:236–43.
Parkash V, Gerald WL, Parma A, Miettinen M, Rosai J. Desmoplastic small round cell tumor of the pleura. Am J Surg Pathol. 1995;19:659–65.
Farhat F, Culine S, Lhomme C, Duvillard P, Soulie P, Michel G, et al. Desmoplastic small round cell tumors: results of a four-drug chemotherapy regimen in five adult patients. Cancer. 1996;77:1363–6.
Khalbuss WE, Bui M, Loya A. A 19-year-old woman with a cervicovaginal mass and elevated serum CA 125. Desmoplastic small round cell tumor. Arch Pathol Lab Med. 2006;130:e59–61.
Brohl AS, Patidar R, Turner CE, Wen X, Song YK, Wei JS, et al. Frequent inactivating germline mutations in DNA repair genes in patients with Ewing sarcoma. Genet Med. 2017;19:955–8.
Chang KL, Crabtree GS, Lim-Tan SK, Kempson RL, Hendrickson MR. Primary uterine endometrial stromal neoplasms. A clinicopathologic study of 117 cases. Am J Surg Pathol. 1990;14:415–38.
Masand RP, Euscher ED, Deavers MT, Malpica A. Endometrioid stromal sarcoma: a clinicopathologic study of 63 cases. Am J Surg Pathol. 2013;37:1635–47.
Oliva E, Young RH, Amin MB, Clement PB. An immunohistochemical analysis of endometrial stromal and smooth muscle tumors of the uterus: a study of 54 cases emphasizing the importance of using a panel because of overlap in immunoreactivity for individual antibodies. Am J Surg Pathol. 2002;26:403–12.
Lee CH, Marino-Enriquez A, Ou W, Zhu M, Ali RH, Chiang S, et al. The clinicopathologic features of YWHAE-FAM22 endometrial stromal sarcomas: a histologically high-grade and clinically aggressive tumor. Am J Surg Pathol. 2012;36:641–53.
Chiang S, Lee CH, Stewart CJR, Oliva E, Hoang LN, Ali RH, et al. BCOR is a robust diagnostic immunohistochemical marker of genetically diverse high-grade endometrial stromal sarcoma, including tumors exhibiting variant morphology. Mod Pathol. 2017;30:1251–61.
Lee CH, Hoang LN, Yip S, Reyes C, Marino-Enriquez A, Eilers G, et al. Frequent expression of KIT in endometrial stromal sarcoma with YWHAE genetic rearrangement. Mod Pathol. 2014;27:751–7.
Lewis N, Soslow RA, Delair DF, Park KJ, Murali R, Hollmann TJ, et al. ZC3H7B-BCOR high-grade endometrial stromal sarcomas: a report of 17 cases of a newly defined entity. Mod Pathol. 2018;31:674–84.
Marino-Enriquez A, Lauria A, Przybyl J, Ng TL, Kowalewska M, Debiec-Rychter M, et al. BCOR Internal Tandem Duplication in High-grade Uterine Sarcomas. Am J Surg Pathol. 2018;42:335–41.
Kao YC, Sung YS, Zhang L, Jungbluth AA, Huang SC, Argani P, et al. BCOR Overexpression Is a Highly Sensitive Marker in Round Cell Sarcomas With BCOR Genetic Abnormalities. Am J Surg Pathol. 2016;40:1670–8.
Oliva E. Practical issues in uterine pathology from banal to bewildering: the remarkable spectrum of smooth muscle neoplasia. Mod Pathol. 2016;29:S104–120. Suppl 1
Sayeed S, Xing D, Jenkins SM, Weisman PS, Buehler D, Warmke L, et al. Criteria for Risk Stratification of Vulvar and Vaginal Smooth Muscle Tumors: An Evaluation of 71 Cases Comparing Proposed Classification Systems. Am J Surg Pathol. 2018;42:84–94.
Swanson AA, Howitt BE, Schoolmeester JK. Criteria for risk stratification of vulvar and vaginal smooth muscle tumors: a follow-up study with application to leiomyoma variants, smooth muscle tumors of uncertain malignant potential, and leiomyosarcomas. Hum Pathol. 2020;103:83–94.
Funding
RNA seq for Case 3 was supported by the Panov 2 Research Fund. The authors otherwise received no specific funding for this work
Author information
Authors and Affiliations
Contributions
The study was conducted with approval by institutional review boards. All authors contributed and approved submission and resubmission of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval
The study was conducted in compliance with the authors’ institutional ethics committees.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Schoolmeester, J.K., Folpe, A.L., Nair, A.A. et al. EWSR1-WT1 gene fusions in neoplasms other than desmoplastic small round cell tumor: a report of three unusual tumors involving the female genital tract and review of the literature. Mod Pathol 34, 1912–1920 (2021). https://doi.org/10.1038/s41379-021-00843-5
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41379-021-00843-5
This article is cited by
-
The role of the transcription factor PATZ1 in tumorigenesis and metabolic regulation
Journal of Cancer Research and Clinical Oncology (2025)
-
“Putting the cart before the horse”: an update on promiscuous gene fusions in soft tissue tumors
Virchows Archiv (2025)
-
Emerging round cell sarcomas in children
Virchows Archiv (2025)
-
Durable response to treatment of an atypical desmoplastic small round cell tumor with enfortumab-vedotin
Journal of Cancer Research and Clinical Oncology (2025)
-
Small round cell sarcomas
Nature Reviews Disease Primers (2022)