Abstract
Low-grade oncocytic tumor (LOT) has been recently proposed as a unique renal tumor. However, we have encountered tumors with more oncocytoma-like morphology that show diffuse keratin 7 reactivity, which we sought to characterize molecularly. Eighteen tumors with a diffuse keratin 7 positive and KIT negative pattern were identified from 184 with predominantly oncocytoma-like histology. These tumors were subjected to detailed immunohistochemical evaluation and 14 were evaluated using the Illumina® HiSeq 4000 platform for 324 cancer-associated genes. Patients’ ages ranged from 39 to 80 (median = 59.5 years) with a male to female ratio of 1.25:1. Morphology was predominantly oncocytoma-like with discrete nests, compared to the solid and edematous patterns described in LOT. Other than positive keratin 7 and negative KIT, the tumor cells were positive for PAX8, E-cadherin, AE1/AE3, Ber-EP4, AMACR, CD10, and MOC31, and were negative for other studied markers. FH and INI1 were normal. Eleven of 14 harbored genomic abnormalities, likely sporadic, primarily involving the MTOR pathway (73%). Overall, the alterations included MTOR activating mutation (n = 1), TSC1 inactivating mutation (n = 1), TSC2 mutation (p.X534 splice site, n = 1), STK11 (a negative regulator of the MTOR pathway) mutation (n = 1), both STK11 and TSC1 mutations (n = 1), biallelic loss of PTEN and TSC1 deletion (n = 1), and MET amplification and TSC1 inactivating mutation (n = 1). Amplification of FGFR3 was identified in one additional tumor. Other alterations included FOXP1 loss (n = 1), NF2 E427 homozygous loss (n = 1), and PI3KCA activating mutation (n = 1). At a median follow-up of 68 months (2–147 months) for 15 patients, all were alive without disease. Oncocytic renal tumors with diffuse keratin 7 labeling show frequent alterations in the TSC/MTOR pathway, despite more oncocytoma-like morphology than initially described in LOT, likely expanding the morphologic spectrum of the latter.
Similar content being viewed by others
Introduction
Oncocytic renal tumors remain a common diagnostic challenge for the pathologist1,2,3,4,5. Whereas previously these included primarily oncocytoma and eosinophilic variant chromophobe renal cell carcinoma (ChrRCC), recent work has identified several recently established and emerging diagnostic entities based on key morphologic attributes, immunohistochemistry (IHC) and genomic profiles, and biologic behavior6,7,8,9,10,11,12,13. In general, low-grade oncocytic renal tumors are not aggressive neoplasms. They mostly portend an indolent clinical course with a good prognosis14. However, it has been recently recognized that some oncocytic renal neoplasms that have more ominous implications, such as succinate dehydrogenase-deficient RCC (which is hereditary) and rare examples of fumarate hydratase-deficient RCC that are deceptively low-grade with oncocytic features (which is hereditary and potentially more aggressive)15,16,17. Sometimes, the distinction between various categories of low-grade oncocytic renal neoplasm is not straightforward based on the morphology alone2. Keratin 7 and KIT are among the most common IHC markers employed to resolve this diagnostic dilemma. Although both oncocytoma and ChrRCC are usually KIT immunoreactive neoplasms, diffuse keratin 7 positivity would typically strongly favor ChrRCC. In contrast, keratin 7 is usually restricted to only occasional groups of cells in oncocytoma, constituting a very small percentage of the tumor cells1. Recently, low-grade oncocytic tumor (LOT) has been proposed as a potential entity in renal neoplasia that exhibits diffuse keratin 7 positivity with a negative result for KIT18, differing from the expected phenotype of oncocytoma and showing more diffuse keratin 7 labeling than usually encountered in eosinophilic ChrRCC18,19,20. These have been found to have a distinct set of macroscopic and microscopic features characterized by a solitary, unilateral, unencapsulated, and well-circumscribed tumor mass with low-grade oncocytic cells arranged in a wide variety of architectural patterns. These tumors have some oncocytoma-like features and some features that argue against the diagnosis of oncocytoma. Oncocytoma-like features include lack of a well-formed capsule, diffuse solid growth pattern, compact nests at the periphery of the tumor, and tumor cells with uniformly abundant eosinophilic/oncocytic cytoplasm, and smooth contoured round to oval nuclei lacking significant nuclear irregularities. Other features including mosaic growth patterns and rarefied connective tissue stroma with loosely arranged neoplastic cells distinct from the hypocellular zones of oncocytoma (compact cell islands and nests in the central area, described as archipelago-like pattern) and are sharply distinct from the solid areas, and the presence of delicate perinuclear halo/clearing argue against a diagnosis of oncocytoma. Since the number of cases of LOT studied to date is small, and these have likely been classified in the past under various terms, such as eosinophilic ChrRCC, oncocytic renal tumor, NOS, unclassified or LOT, hybrid oncocytoma-ChrRCC, borderline/uncertain/low malignant potential tumors, and so on, it is unclear what their true biologic behavior may be. Therefore, caution should be exercised in diagnosing oncocytoma when the typical features are not present.
We were particularly interested to study the TSC/MTOR (tuberous sclerosis complex/mammalian target of rapamycin) pathway in these neoplasms, as there is emerging evidence to suggest that LOT may be characterized by alterations in this pathway. For example, the Cancer Genome Atlas study of ChrRCC included at least 2 tumors with predominantly eosinophilic morphology that harbored mutations in MTOR21. Likewise, a few recent studies have found alterations in this pathway in neoplasms likely fitting the diagnostic criteria for LOT22,23,24,25. This is a key regulator pathway for wide array of vital cellular functions- cell metabolism, growth, proliferation, and survival26,27,28. The molecular weight of MTOR is 289 kDa and it belongs to the phosphoinositide 3-kinase related kinase family. It nucleates two multi-protein complexes MTORC1 and MTORC2. MTORC1 complex acts as one of the master regulators of cell growth and metabolism. Its activity is regulated by various growth factor pathways, environmental stress, nutritional, and oxygen status in the cell. The most important regulator is the TSC, which is a heterodimer of TSC1 (hamartin) and TSC2 (tuberin)26. TSC is a GTPase activating protein (GAP). The GTPase protein RHEB regulates MTOR by increasing its activity. TSC inactivates RHEB and in turn downregulates MTOR activity29,30,31. Activation of MTORC1 represses the PI3K-AKT pathway. Environmental stress results in the activation of AMP activated protein kinase (AMPK) by serine threonine kinase 11 (STK11). AMPK activates TSC1/TSC2 complex, which in turn regulates the activity of MTOR. MTORC2 depletion inhibits AKT, leading to decreased phosphorylation of forkhead box protein (FOXO1) and FOXO3A and their activation (Fig. 1).
Its activity is regulated by the tuberous sclerosis complex (TSC). TSC is a GTPase activating protein (GAP). The GTPase protein RHEB regulates MTOR by increasing its activity. TSC inactivates RHEB and in turn downregulates MTOR activity. AMPK activates TSC complex, which in turn regulates the activity of MTOR. AKT signaling cascade is activated by receptor tyrosine kinases which induce production of phosphatidylinositol (3,4,5) trisphosphates (PIP3) by phosphoinositide 3-kinase (PI3K). NF1 protein, neurofibromin 1, negatively regulates RAS proteins through GTPase activity. Ras is an activator of the phosphatidylinositol-3-kinase (PI3K)-AKT pathway. The tumor suppressor phosphatase and tensin homolog (PTEN) inhibits AKT activity by dephosphorylating PIP3. AKT regulates cell growth through its effects on the TSC1/TSC2 complex and MTORC signaling.
We sought to study the morphologic and genetic characteristics of oncocytic tumors having low-grade features and keratin 7-positive/KIT-negative IHC profile from the archives of a single institution.
Materials and methods
Case selection criteria
This study was initiated on approval from the institutional review board. Tumors with predominantly renal oncocytoma-like morphology or subtle features of ChrRCC were included in the study. A total of 184 cases over a period of 9 years (January 2011 to April 2020) were selected. Among them, 18 renal tumors showing ISUP/WHO low-grade oncocytic morphology and diffuse positivity for keratin 7 and negativity for KIT were identified. Demographics, surgical procedure involved, type of specimen, macroscopic and microscopic features, and follow-up information were recorded in these cases.
All tissues were formalin-fixed and paraffin embedded (FFPE). Hematoxylin and eosin-stained sections were reviewed by three genitourinary pathologists (SKMo, SRW, and AS) to confirm the diagnosis.
Histochemical and immunohistochemical studies
Muller-Mowry colloidal iron stain was performed in 11 cases. The IHC stains performed were as follows: keratin 7 (Ventana Medical Systems: Clone SP52), KIT (Abcam, Polyclonal), PAX8 (Cell Marque; Polyclonal), alpha methyacyl CoA racemase (AMACR) (Cell Marque; Clone: 13H4), CD10 (Ventana Medical Systems; Clone: SP67), E-cadherin (Leica Biosystems; Clone: 36B5), keratin 20 (Biocare Medical, Clone: Ks20.8), carbonic anhydrase (CA) 9 (LS Bio; Clone: 303123), keratin AE1/AE3 (Ventana Medical Systems; Clone: AE1/AE3), vimentin (Ventana Medical Systems; Clone: V9), EPCAM (Biocare Medical; Clone: Ber-EP4), MOC31 (Biocare Medical; Clone: MOC31), Ki-67 (Biocare Medical; Clone: MIB 1), keratin 5/6 (Ventana Medical Systems; Clone: D5), melanosome (Ventana Medical Systems; Clone: HMB-45), melan A (Ventana Medical Systems; Clone: A103), INI1 (SMARCB1, Ventana Medical Systems; Clone: MRQ-27), CD15 (Ventana Medical Systems; Clone: MMA), inhibin (Cell Marque; Clone: MRQ-63), FH (Santa Cruz Biotechnology; Clone: J-13), p63 (Ventana Medical Systems; Clone: 4A4), and PD-L1 (Ventana Medical Systems; Clone: SP263). Four micrometer sections were prepared for IHC. Ventana Benchmark ULTRA autoimmunostainer. Cell conditioning 1 at high pH (pH = 8) was used for the antigen retrieval buffer solution and UltraView DAB Detection Kit was used the detection of the IHC product. Appropriate positive and negative controls were performed with each antibody. The results of the IHC stains were recorded in a semiquantitative fashion as described below.
Positive staining was defined as cytoplasmic and membranous (keratin 7, KIT, AMACR, CD10, E-cadherin, keratin 20, CA9, pan keratin, vimentin, Ber-EP4, MOC31, HMB-45, melan A, CD15, inhibin, PD-L1 [membranous]) or nuclear (PAX8 and p63) staining pattern in the tumor cells, which can be easily observed at low power magnification (×<40). Scant fine granular background staining of the tumor cells, which cannot be seen at low power magnification, or no staining at all was considered negative. For each case, immunoreactivity was interpreted as follows: negative ≤ 5% tumor cell staining; positive = ≥ 5% tumor cell staining. The percentages of immunoreactivity for these markers were evaluated in a semiquantitative fashion as follows: 0 ≤ 5% tumor cell staining; focal = 5–10% tumor cell staining; multifocal = 11–50% tumor cell staining; diffuse ≥ 50% tumor cell staining. The intensity of immunoreactivity was graded as weak, moderate, and strong.
MIB1 (Ki-67) demonstrated nuclear immunoreactivity and aided in evaluation of the proliferation index. The results were expressed in a semiquantitative manner as estimated percentage of tumor cells immunoreactive with the antibodies. A MIB1 (Ki-67) proliferation index (% positivity in 100 cells counted) was ascertained for each case. INI1 depicted nuclear positivity. Approximate percentage of INI1 positive nuclei were recorded in each case. It was considered as ‘retained’ (normal) if the majority of the nuclei showed positivity. FH showed cytoplasmic positivity. Percentage of cells retaining this IHC was noted for each case.
Molecular analysis
Targeted NGS panel
FFPE tumor tissue blocks of the selected tumor specimens were used for molecular evaluation using targeted NGS-based panel to detect small nucleotide variants/substitutions, small indels (insertions and/or deletions), and copy number variations in 324 cancer-associated genes (including genes involved in PI3K/AKT/MTOR pathway). The panel also detects selected gene rearrangements as well as genomic signatures including microsatellite instability and tumor mutational burden. The panel was based on Illumina® HiSeq 4000 platform.
Pathological evaluation of clinical specimens
Paraffin sections from the FFPE blocks were stained with H&E-stain for pathological evaluation, and FFPE tissue blocks that had at least 15% of tumor content were selected. Molecular analysis was performed in 14 cases.
DNA extraction
DNA was extracted from the selected FFPE blocks using the GeneREAD DNA FFPE kit (Qiagen) following the manufacturer’s instruction. Then DNA was quantified by using Qubit 1XdsDNA high sensitivity assay.
Construction of libraries
DNA (50–1000 ng) was used for whole-genome shotgun library construction and hybridization-based capture of all coding exons from 309 cancer-related genes, one promoter region, one non-coding (ncRNA), and select intronic regions from 34 commonly rearranged genes, 21 of which also include the coding exons. In total, the assay detects alterations in a total of 324 genes.
Sequencing
Using the Illumina® HiSeq 4000 platform, hybrid capture-selected libraries were sequenced to high uniform depth (targeting ×500 median coverage with 99% of exons at coverage ×100).
Sequence data processing
Sequence data was then processed using a customized analysis pipeline. Low-quality data was filtered out and the output BAM files were aligned to the human reference genome hg19. Variant calling pipeline was then called all the variants that has passed various QC checks, including strand bias, minor allele frequency (MAF), Q score, depth of coverage (×500), Phred quality score, noise in the surrounding region etc. The variants were then segregated on the basis of their genomic location and variant effects (missense, non-frameshift, and frameshift insertions, deletions, and base substitutions). Variant annotation was performed using the Association for Molecular Pathology classification.
Results
Clinical features
There were 18 patients that satisfied the criteria and were included in the study. The patients’ ages ranged from 39 years to 80 years (mean = 56.5 years; median = 59.5 years) at the time of diagnosis. A slight male preponderance was observed with 56% of the total patients (10/18) being male. Unilateral solitary renal mass was observed in every patient (right, n = 10; left, n = 8). No other mass was present concurrently in any of the patients. There was neither any notable family or personal history of malignancy, nor any syndromic association. The patients had undergone either partial nephrectomy (n = 3) or total nephrectomy (n = 15) (Table 1).
Macroscopic features
On gross examination, the maximum dimension of the tumors ranged from 14 to 107 mm (median = 29.5 mm; mean = 37.1 mm). The tumors were unilateral, solitary, and unencapsulated but well-circumscribed. The cut surfaces were homogeneous and tan-brown in all tumors. One tumor had focal tan-white area and two had focal areas of hemorrhage. No cystic, tan-yellow/yellow, or overtly friable necrotic areas was observed in any of the tumors (Table 1).
Microscopic features
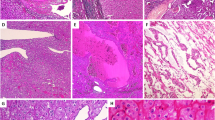
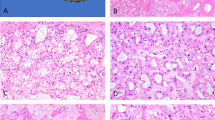
The tumors were unencapsulated but well-circumscribed. Predominant morphology was oncocytoma-like with frequent and repetitive nested appearance. A variety of other growth patterns was observed that include solid, nested, reticular, sheets, tubular, trabecular, and cord-like. The stroma was edematous and was sharply demarcated from the cellular tumor areas. Fibrous stroma was also observed. The neoplastic cells were round to polygonal. They had homogenous abundant fine granular eosinophilic cytoplasm with round to oval nuclei. No frank nuclear envelope irregularity or perinuclear clearing was identified, except for two tumors where mildly wrinkled (raisinoid) nuclear membrane was observed. Degenerative nuclear atypia was seen in three tumors. The nucleoli were small and delicate to conspicuous. The ISUP/WHO nuclear grades were as follows: grade 2, n = 7; grade 3, n = 11 (Figs. 2 and 3 and Table 1).
These included: abundant fine granular eosinophilic cytoplasm with round to oval nuclei, resembling what has been described in LOT (A). Occasional tumors showed minor perinuclear clearing (B). Minor nuclear membrane irregularity was noted in a subset of cells (C), and some tumors showed degenerative nuclear atypia, as seen in some oncocytomas (D).
Immunohistochemical features
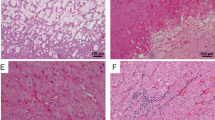
All the tumors were positive for keratin 7 and negative for KIT by the study selection criteria (Fig. 4). The keratin 7 expression was diffuse and strong in all 18 tumors. Other positive stains were as follows: PAX8 (16/16), E-cadherin (16/16), keratin AE1/AE3(18/18), Ber-EP4 (14/14), and MOC31 (16/16). The expression ranged from moderate to strong in the intensity and focal to diffuse in the extent. Three of eighteen tumors showed weak and focal AMACR expression. CD10 was expressed in a moderate to focal manner in 3 of 18 tumors. keratin 20 (0/18), CA9 (0/18), vimentin (0/18), keratin 5/6 (0/18), HMB-45 (0/17), melan A (0/18), CD15 (0/18), inhibin (0/10), and p63 (0/17) were negative. FH (12/12) and INI1 (18/18) were retained/normal in the tumor cells. Ki-67 labeling index varied between 2 and 7%. PD-L1 was performed in six tumors and all were negative. Colloidal iron Muller-Mowry stain depicted a negative result (0/11) when performed (Table 2a and b).
Molecular analysis features
Eleven of fourteen tumors harbored genomic abnormalities, as shown in Table 3 and Fig. 5. The mutations were primarily involving the MTOR pathway in eight tumors (73%). TSC1 inactivating mutation and deletion was identified in two and one tumors, respectively. TSC2 mutation (p.X534 splice site) was seen in one tumor. STK11, a negative regulator of the MTOR pathway, was mutated in two tumors. Amplifications of MET and FGFR3 genes were identified in one tumor each. Other mutations observed were biallelic loss of PTEN (n = 1), FOXP1 loss (n = 1), NF2 E427 homozygous loss (n = 1), and PI3KCA activating mutation (n = 1). One tumor had both STK11 and TSC1 mutations and another tumor had biallelic loss of PTEN and TSC1 deletion. MET amplification and TSC1 inactivating mutation was seen in one tumor (Table 3 and Fig. 5). Comparison to the adjacent normal tissue was performed in all the 14 tumors, in which the alterations were identified in tumor tissue only, arguing against germline alterations.
Staging, follow-up, and survival information
Assuming application of the 8th edition AJCC TNM system to these tumors, as they do not meet definite criteria for oncocytoma, most of the tumors would be regarded as low-stage: 72% of the tumors (13/18) were of stage pT1a, 11% (2/18) pT1b, 6% (1/18) each was pT2a, pT2b, and pT3a. Follow-up data was available in 15 cases. The follow-up period ranged from 2 months to 147 months, with a median of 68 months. All the patients were alive without evidence of recurrent renal tumor (Table 1).
Discussion
Classifying oncocytic renal tumors into one of two categories—ChrRCC and oncocytoma—remains challenging. In general, the anticipated IHC pattern of oncocytoma is membranous KIT positivity, sometimes weak, and either a negative or focal/occasional keratin 7 positive profile. However, LOT has been recently described as a potentially distinct group, being negative for KIT, yet with diffuse positivity for keratin 718. In this study, we hypothesized that LOT may be characterized by MTOR pathway alterations; however, we found diffuse keratin 7 reactivity and enriched somatic mutation of the TSC/MTOR pathway genes in a broader than expected spectrum of tumors with oncocytoma-like morphology.
According to previous reports, LOT exhibits morphology that primarily includes a solid sheet-like pattern, compact nested appearance, trabecular arrangement, tubule formation, reticular growth, and various intermediate areas with variable cellularity. A key helpful clue to recognition of this entity is that the stroma is often loose and edematous, with tumor cells that appear “stretched” or loosely connected to one another, contrasting to the discrete round nests typical of oncocytoma18. However, in this series, we encountered diffuse keratin 7 labeling in a cohort of tumors with predominantly nested oncocytoma-like morphology. Worrisome features pointing towards a diagnosis of malignancy, such as pleomorphism, mitotic activity, and/or coagulative necrosis were lacking. Most of the tumors in our cohort were small and confined to the kidney.
The molecular alterations in LOTs have not been studied in detail in the existing literature. Molecularly, oncocytomas generally have minimal/rare chromosomal abnormalities. They usually have diploid karyotype. In some other instances, they show loss of chromosome 1, loss of Y, or rearrangement of 11q131. On the other hand, eosinophilic ChrRCCs have significantly greater extent of chromosomal aberrations characterized frequently by multiple losses of chromosomes1,32. Both oncocytoma and ChrRCC display mitochondrial gene mutations33. Reported “hybrid” tumors may show a mixture gains and losses of the chromosomes34. Initial studies of LOT suggest different karyotypic patterns, including a similar karyotype to that of oncocytoma. Our study sought to find out any unique abnormality at the genomic level.
Genomic analyses were performed in 14 tumors, in which alterations in the TSC/MTOR pathway was enriched. Various genetic alterations found are depicted in the Table 3 and Fig. 5. Abnormal MTOR signaling can result from the genetic aberrations in various levels, both upstream and downstream effectors. Increased activity of the MTOR results in cellular proliferation and metabolism that can lead to tumorigenesis. Theoretically, this tumor subtype could potentially be targeted by MTOR inhibitors, assuming that malignant behavior necessitates it, or large tumor size becomes worrisome in a patient that is not a candidate for surgery; however, to date these tumors appear nonaggressive, making necessity of targeted therapy likely rare.
MTOR pathway aberration is not unique to this group of renal neoplasms. Various other renal neoplasms such as eosinophilic solid and cystic RCC and eosinophilic vacuolated tumor have also shown these molecular alterations35. This common molecular alteration has made us wonder, if the tumors studied herein should be considered part of the spectrum of LOT, according to their shared genomics, despite differing morphologic patterns. At present, it seems that this should expand the spectrum of LOT, to include architecture that is more oncocytoma-like. As such, it would likely be prudent to ascertain the keratin 7 and KIT status in low-grade oncocytic neoplasms including possible oncocytomas, given the overlapping histomorphology of LOT. This would aid in differentiating a well characterized benign neoplasm from LOT, which is less clearly understood and requires further study. Nonetheless, it is likely that the behavior of all these oncocytic neoplasms is highly favorable, given that there remains considerable debate in optimal diagnostic criteria and most of these have almost certainly been historically diagnosed as variations of oncocytoma or eosinophilic ChrRCC in the past.
Data availability
Raw data is available from the corresponding author upon reasonable request.
References
Wobker, S. E. & Williamson, S. R. Modern pathologic diagnosis of renal oncocytoma. J. Kidney Cancer VHL 4, 1–12 (2017).
Williamson, S. R. et al. Diagnostic criteria for oncocytic renal neoplasms: a survey of urologic pathologists. Hum. Pathol. 63, 149–156 (2017).
Ng, K. L. et al. A systematic review and meta-analysis of immunohistochemical biomarkers that differentiate chromophobe renal cell carcinoma from renal oncocytoma. J. Clin. Pathol. 69, 661–671 (2016).
Ng, K. L. et al. Differentiation of oncocytoma from chromophobe renal cell carcinoma (RCC): can novel molecular biomarkers help solve an old problem? J. Clin. Pathol. 67, 97–104 (2014).
Kryvenko, O. N., Jorda, M., Argani, P. & Epstein, J. I. Diagnostic approach to eosinophilic renal neoplasms. Arch. Pathol. Lab. Med. 138, 1531–1541 (2014).
Mehra, R. et al. Somatic bi-allelic loss of TSC genes in eosinophilic solid and cystic renal cell carcinoma. Eur. Urol. 74, 483–486 (2018).
Palsgrove, D. N. et al. Eosinophilic solid and cystic (ESC) renal cell carcinomas harbor TSC mutations: molecular analysis supports an expanding clinicopathologic spectrum. Am. J. Surg. Pathol. 42, 1166–1181 (2018).
Parilla, M. et al. Are sporadic eosinophilic solid and cystic renal cell carcinomas characterized by somatic tuberous sclerosis gene mutations? Am. J. Surg. Pathol. 42, 911–917 (2018).
Trpkov, K. et al. Eosinophilic, Solid, and Cystic Renal Cell Carcinoma: Clinicopathologic Study of 16 Unique, Sporadic Neoplasms Occurring in Women. Am. J. Surg. Pathol. 40, 60–71 (2016).
Chen, Y. B. et al. Somatic mutations of TSC2 or MTOR characterize a morphologically distinct subset of sporadic renal cell carcinoma with eosinophilic and vacuolated cytoplasm. Am. J. Surg. Pathol. 43, 121–131 (2019).
He, H. et al. “High-grade oncocytic renal tumor”: morphologic, immunohistochemical, and molecular genetic study of 14 cases. Virchows Arch. 473, 725–738 (2018).
Siadat, F. & Trpkov, K. ESC, ALK, HOT and LOT: Three Letter Acronyms of Emerging Renal Entities Knocking on the Door of the WHO Classification. Cancers 12, 168 (2020).
Trpkov, K. & Hes, O. New and emerging renal entities: a perspective post-WHO 2016 classification. Histopathology 74, 31–59 (2019).
Richard, P. O. et al. Active surveillance for renal neoplasms with oncocytic features is safe. J. Urol. 195, 581–586 (2016).
Smith, S. C. et al. A distinctive, low-grade oncocytic fumarate hydratase-deficient renal cell carcinoma, morphologically reminiscent of succinate dehydrogenase-deficient renal cell carcinoma. Histopathology 71, 42–52 (2017).
Gill, A. J. Succinate dehydrogenase (SDH)-deficient neoplasia. Histopathology 72, 106–116 (2018).
Gill, et al. Succinate dehydrogenase-deficient renal carcinoma. In WHO Classification of Tumours of the Urinary System and Male Genital Organs Vol. 8 (eds Moch, H., Humphrey, P. A., Ulbright T. M., & Reuter, V. E.) 35–36 (International Agency for Research on Cancer, Lyon, 2016).
Trpkov, K. et al. Low-grade oncocytic tumour of kidney (CD117-negative, cytokeratin 7-positive): a distinct entity? Histopathology 75, 174–184 (2019).
Kravtsov, O. et al. Low-Grade Oncocytic Tumor of Kidney (CK7-Positive, CD117-Negative): Incidence in a single institutional experience with clinicopathological and molecular characteristics. Hum. Pathol. 114, 9–18 (2021).
Guo, Q. et al. Characterization of a distinct low-grade oncocytic renal tumor (CD117-negative and cytokeratin 7-positive) based on a tertiary oncology center experience: the new evidence from China. Virchows Arch. 478, 449–458 (2021).
Davis, C. F. et al. The somatic genomic landscape of chromophobe renal cell carcinoma. Cancer Cell 26, 319–330 (2014).
Tjota, M. et al. Eosinophilic Renal Cell Tumors With a TSC and MTOR Gene Mutations Are Morphologically and Immunohistochemically Heterogenous: Clinicopathologic and Molecular Study. Am. J. Surg. Pathol. https://doi.org/10.1097/PAS.0000000000001457 (2020).
Tjota, M. Y., Wanjari, P., Segal, J. & Antic, T. TSC/MTOR-mutated eosinophilic renal tumors are a distinct entity that is CK7+/CK20-/vimentin-: a validation study. Hum. Pathol. 115, 84–95 (2021).
Tong, K. & Hu, Z. FOXI1 expression in chromophobe renal cell carcinoma and renal oncocytoma: a study of The Cancer Genome Atlas transcriptome-based outlier mining and immunohistochemistry. Virchows Arch. 478, 647–658 (2021).
Skala, S. L. et al. Next-generation RNA Sequencing-based Biomarker Characterization of Chromophobe Renal Cell Carcinoma and Related Oncocytic Neoplasms. Eur. Urol. 78, 63–74 (2020).
Saxton, R. A. & Sabatini, D. M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 168, 960–976 (2017).
Zoncu, R., Efeyan, A. & Sabatini, D. M. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat. Rev. Mol. Cell Biol. 12, 21–35 (2011).
Kim, L. C., Cook, R. S. & Chen, J. mTORC1 and mTORC2 in cancer and the tumor microenvironment. Oncogene 36, 2191–2201 (2017).
Huang, J. & Manning, B. D. The TSC1-TSC2 complex: a molecular switchboard controlling cell growth. Biochem. J. 412, 179–190 (2008).
Tee, A. R., Manning, B. D., Roux, P. P., Cantley, L. C. & Blenis, J. Tuberous sclerosis complex gene products, Tuberin and Hamartin, control mTOR signaling by acting as a GTPase-activating protein complex toward Rheb. Curr. Biol. 13, 1259–1268 (2003).
Dibble, C. C. & Cantley, L. C. Regulation of mTORC1 by PI3K signaling. Trends Cell Biol. 25, 545–555 (2015).
Paner, G., Amin, M. B., Moch, H. & Störkel, S. Chromophobe renal cell carcinoma. In WHO Classification of Tumours of the Urinary System and Male Genital Organs Vol. 8 (eds Moch, H., Humphrey, P. A., Ulbright, T. M., & Reuter, V. E.) 27–28 (International Agency for Research on Cancer, Lyon, 2016).
Hes, O., Moch, H. & Reuter, V. Oncocytoma. In WHO Classification of Tumours of the Urinary System and Male Genital Organs Vol. 8 (eds Moch, H., Humphrey, P. A., Ulbright T. M., & Reuter, V. E) 43–44 (International Agency for Research on Cancer, Lyon, 2016).
Petersson, F., Gatalica, Z., Grossmann, P., Perez Montiel, M. D., Alvarado Cabrero, I. & Bulimbasic, S. et al. Sporadic hybrid oncocytic/chromophobe tumor of the kidney: a clinicopathologic, histomorphologic, immunohistochemical, ultrastructural, and molecular cytogenetic study of 14 cases. Virchows Arch. 456, 355–365 (2010).
Trpkov, K. et al. Novel, emerging and provisional renal entities: The Genitourinary Pathology Society (GUPS) update on renal neoplasia. Mod. Pathol. 34, 1167–1184 (2021).
Author information
Authors and Affiliations
Contributions
Contributions of the authors were as follows: drafting the manuscript: SKMo, SRW. Critical revision and final approval of the manuscript: all authors. Data collection, analysis, and interpretation: all authors. Conception/design: SKMo, SRW.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval/consent to participate
The study was approved by the Institutional Review Board of the Advanced Medical Research Institute. Informed consent was waived due to the retrospective nature of the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mohanty, S.K., Satapathy, A., Aggarwal, A. et al. Oncocytic renal neoplasms with diffuse keratin 7 immunohistochemistry harbor frequent alterations in the mammalian target of rapamycin pathway. Mod Pathol 35, 361–375 (2022). https://doi.org/10.1038/s41379-021-00969-6
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41379-021-00969-6
This article is cited by
-
Whole exome sequencing identified mutations of forkhead box I 1 (FOXI1), keratin 6 C (KRT6C) and gap junction protein delta 2 (GJD2) in a low-grade oncocytic tumor of the kidney: a case report
Diagnostic Pathology (2025)
-
Endobronchialer Tumor bei einem 40-jährigen Mann
Zeitschrift für Pneumologie (2024)
-
Clinical utility of keratin 14 expression measurement in reflecting the tumor properties and prognosis in patients with renal cell carcinoma: a study with long-term follow-up
International Urology and Nephrology (2024)
-
Low-grade oncocytic tumor of the kidney: imaging features of a novel tumor entity
Abdominal Radiology (2024)
-
Evaluation of an institutional series of low-grade oncocytic tumor (LOT) of the kidney and review of the mutational landscape of LOT
Virchows Archiv (2023)