Abstract
Chronic stress exerts profound negative effects on cognitive and emotional behaviours and is a major risk factor for the development of neuropsychiatric disorders. However, the molecular links between chronic stress and its deleterious effects on neuronal and synaptic function remain elusive. Here, using a combination of in vitro and in vivo approaches, we demonstrate that the upregulation of miR-186-5p triggered by chronic stress may be a key mediator of such changes, leading to synaptic dysfunction. Our results show that the expression levels of miR-186-5p are increased both in the prefrontal cortex (PFC) of mice exposed to chronic stress and in cortical neurons chronically exposed to dexamethasone. Additionally, viral overexpression of miR-186-5p in the PFC of naïve mice induces anxiety- and depressive-like behaviours. The upregulation of miR-186-5p through prolonged glucocorticoid receptor activation in vitro, or in a mouse model of chronic stress, differentially affects glutamatergic and GABAergic synaptic transmission, causing an imbalance in excitation/inhibition that leads to altered neuronal network activity. At glutamatergic synapses, we observed both a reduction in synaptic AMPARs and synaptic transmission, whereas GABAergic synaptic transmission was strengthened. These changes could be rescued in vitro by a miR-186-5p inhibitor. Overall, our results establish a novel molecular link between chronic glucocorticoid receptor activation, the upregulation of miR-186-5p and the synaptic changes induced by chronic stress, that may be amenable to therapeutic intervention.
Similar content being viewed by others
Introduction
Stress is a normal, adaptive response that helps organisms cope with situations challenging their survival [1, 2]. It is primarily mediated by the release of glucocorticoids by the hypothalamic–pituitary–adrenal (HPA) axis and the activation of mineralocorticoid (MRs) and glucocorticoid receptors (GRs). This response is limited in time and subsides once homeostasis is re-established. However, the prolonged activation of the HPA axis, associated with chronic stress, has long-term deleterious effects on cognitive and emotional processes and is a major risk factor for neuropsychiatric disorders such as major depressive disorder (MDD), anxiety and schizophrenia [3,4,5,6]. In the prefrontal cortex (PFC), a brain region involved in emotional, social and cognitive control [7], chronic stress exerts complex and profound effects on the synaptic structure and function of pyramidal neurons [8,9,10,11], leading to reduced AMPAR and NMDAR-mediated synaptic transmission and reduced receptor expression [12]. This weakens glutamatergic projections from the PFC pyramidal neurons to downstream brain regions, such as the hippocampus and amygdala, causing aberrant functional connectivity that results in emotional dysregulation [13, 14]. Stress can also impact the GABAergic system, causing dysfunction in downstream limbic structures controlling emotions [15, 16]. However, the molecular pathways linking a sustained elevation in stress hormones to synaptic dysfunction are poorly understood.
Numerous molecules have been proposed as mediators of the effects of chronic stress. Among these, microRNAs (miRNAs) may play a prominent role in the stress response by regulating the translation of large pools of transcripts [17,18,19]. Indeed, several miRNAs have been found dysregulated in human and rodent studies in the context of neuropsychiatric disorders [20, 21]. In patients with MDD, miR-186-5p is found increased in exosomes obtained from the peripheral blood [22]. Its high expression was also detected in different brain regions and serum after prolonged exposure to high levels of corticosterone, triggered by different protocols of chronic stress in rodents [22,23,24,25,26,27]. We previously showed that this neuron-enriched miRNA regulates synaptic function and homeostatic plasticity by targeting the GluA2 subunit of AMPAR [28]. Additionally, its DNA coding sequence is localized to an intronic region of the ZRANB2 gene, which is under transcriptional control of glucocorticoid response elements (GREs) [29,30,31], altogether making it a likely candidate to mediate the synaptic effects of chronic stress.
In this work, we find that both chronic stress in mice and prolonged GR activation in vitro cause an upregulation of miR-186-5p expression. This leads to altered glutamatergic and GABAergic synaptic transmission, which can be rescued by expressing a miR-186-5p inhibitor. Importantly, overexpression of miR-186-5p in the PFC of mice induces anxiety and depressive-like behaviours. Our results establish that upregulated expression of miR-186-5p acts as the molecular link between chronic GR activation and the synaptic dysfunction induced by chronic stress.
Materials and methods
DNA and lentiviral constructs
DNA constructs encoding pre-miR-186, a miR-186-5p inhibitor, or a scramble control were purchased from GeneCopoeia (Rockville, MD, USA). Transfection efficiency was monitored by the co-expression of EGFP (pre-miR-186) and mCherry (miR-186-5p inhibitor). Lentiviral vectors from these plasmids were produced at ViraVector (University of Coimbra, Portugal), as previously described [32].
Animals
Eighty-three young adult C57Bl/6J (10 weeks-old) and thirteen post-natal-day (PND) 2 mice were used (Charles River Laboratories, Saint-Germain-sur-l’Arbresle, France). Mice were housed in groups of four per cage and maintained on a 12 h light-dark cycle with food and water ad libitum. Behavioural testing was conducted during the light phase, after habituation to the testing room. E17–19 Wistar rat embryos were used to prepare primary neuronal cultures.
All procedures and quantifications were performed by researchers blinded to the experimental group. Sample size estimates were based on previous literature. No randomization was applied.
Stress protocols
The maternal separation and maternal unpredictable stress (MS-US) protocol was performed as previously described [33]. Ten-week-old male mice were submitted to 4–6 weeks of chronic unpredictable stress (CUS), as previously described [34]. At the end of the protocol, blood was collected at 8 AM and 8 PM from the submandibular vein and the animals were sacrificed for tissue collection. Body weight gain and serum corticosterone levels (ELISA assay; Abcam, Cambridge, United Kingdom) were evaluated as indicators of the stress protocol efficacy.
Stereotaxic procedures
Stereotaxic injections of lentiviral vectors were performed in the medial PFC (mPFC) of 10-week-old mice. Briefly, mice were administered with analgesia and 15 min later were deeply anaesthetized with medetomidine (1 mg/kg) and ketamine (75 mg/kg; i.p.). After complete loss of reflexes, the head was fixed in a stereotaxic apparatus and 200 ng lentivirus (750 nl) were bilaterally infused at a rate of 250 nl/min (coordinates: anteroposterior +1.9 mm, mediolateral ±0.5 mm, dorsoventral −1.9 mm). Animals were awakened with atipamezole (2 mg/kg, i.p.) and administered meloxicam (1 mg/kg, s.c.) for 48-72 h after surgery. Histological confirmation of the injection sites was performed at the end of the behavioural protocols. To evaluate miR-186-5p levels, brain coronal sections (10 µm) were mounted on membrane slides and EGFP-expressing cell groups were laser micro-dissected and pressure-catapulted using a P.A.L.M. Laser Dissecting Microscope (Carl Zeiss, Jena, Germany) with a 5×/0.25 NA Fluar objective (Carl Zeiss).
Behaviour analyses
In the elevated plus maze (EPM; 5 × 30 × 15 cm) and open field (OF; 41 × 41 cm) tests, animals were placed in the centre of the arena and allowed to explore in a 5 min and 10 min session, respectively, under dim light. The open arms of the EPM and the centre of the OF were illuminated with 100 lux. The total distance travelled, time spent in the open and closed arms of the EPM, and in the OF arena centre were analysed with Ethovision XT software (Noldus, Wageningen, Netherlands). Spontaneous alternation behaviour during a single session in a Y-maze was assessed as previously described [35]. Same-arm return was defined as visiting the same arm repeatedly. In the tail suspension test (TST), mice were video recorded for 6 min while suspended upside-down from a metal bar by the tail using lab tape, at a height of approximately 40 cm. The duration of immobility was assessed manually. All quantifications were performed by researchers blinded to animal experimental conditions.
Primary cortical neuron cultures, transfection, transduction and imaging
Primary neuronal cultures were prepared from cortices of E17-19 Wistar rat embryos, as previously described [28, 36]. For multi-electrode array (MEA) recordings, 1.143 × 105 cells/cm2 were plated onto coated 24 well plates (M384-tMEA-24W; Axion Biosystems, Atlanta, GA, USA). Neurons were transfected or transduced at DIV8-9 with 3-4 µg of DNA using the calcium phosphate method, or 150 ug lentivirus, respectively [37]. Immunocytochemistry, immunohistochemistry and image analysis were performed blind, as previously described [28].
Electrophysiology
Whole-cell voltage-clamp recordings were performed on DIV15 rat cortical neurons, as described [28], and from layer 5-6 neurons of the mPFC of 14 week-old mice at a holding potential of −70 mV using a Multiclamp 700B amplifier. Data were filtered at 2.8 kHz, digitized at 20 kHz (Digidata 1550 A, Molecular Devices, San Jose, CA, USA), and acquired using Clampex 10.7 software (Molecular Devices). Recordings were analysed in Clampfit (Molecular Devices) using a template search method to detect events.
Simultaneous extracellular recordings from 16 extracellular electrodes were collected from DIV17 neurons, using the Maestro MEA system (Axion Biosystems). Data were digitized at 12.5 kHz and analysed using Axion Integrated Studio software. Network activity was detected using the Envelope algorithm. All electrophysiology experiments and analyses were done blind to the experimental condition.
RNA quantification
RNA was extracted from rat cultured neurons and mouse brain tissue using the Total RNA Purification Plus Kit (Norgen Biotek Corp., Thorold, Canada) and miRNeasy Tissue/Cells Advanced MiniKit (Qiagen, Hilden, Germany), respectively. Complementary DNA was synthesized by reverse transcription (miRCURY LNA RT, Qiagen), followed by DNA amplification (miRCURY LNA SYBR Green PCR Kits, Qiagen). Fluorescence was detected in a Bio-Rad CFX96 Touch Real-Time PCR Detection System (Hercules, CA, USA). MicroRNA levels were normalized to miR-99b-5p (which was not changed in the examined samples) for each sample and the fold-change in expression was determined using GenEx software. Experiments and analyses were done blind to the experimental condition.
Bioinformatic analysis
Mus musculus ZRANB2 gene sequence was downloaded from NCBI (https://www.ncbi.nlm.nih.gov/datasets/gene/id/53861/). The sequence was imported to R using the readDNAStringSet function from the Biostrings library [38]. The function vmatchPattern from the same library was used to find GRE within the ZRANB2 sequence. The GRE sequence was defined as “AGAACANNNTGTTCT” and a max.mismatch was set to 5 (3 mismatch correspond to the variable elements, represented by “NNN” and the extra 2 will be freely set).
Published datasets of miR-186-5p – target interactions in mouse neocortex identified with CLEAR-CLIP method [39] were retrieved for gene ontology enrichment analysis. The list of miR-186-5p – precipitated targets was imported to the Gene Ontology Consortium knowledgebase (version: release 2019-12-09, [40]) and PantherDB (Version 14.1, [41]) and ontological classes with biological significance were selected manually. Venn diagram visualization was generated using Jvenn [42]. Graphical representation and Venn diagram contain statistically enriched, non-redundant, ontology classes associated with synaptic plasticity and neuronal function.
Statistical analysis
Results are expressed either as mean ± SEM of n experiments, as boxes showing percentiles and median values, or as frequency distribution plots, as indicated in figure legends. Data was checked for normality with Anderson-Darling, D’Agostino & Pearson, Shapiro–Wilk and Kolmogorov–Smirnov test. According to this evaluation parametric or non-parametric tests were used, as described in the figure legends. For all tests, p ≤ 0.05 was considered statistically significant. Statistical significance of bioinformatic analysis data was evaluated with Binominal test with Sidak’s, Dunn’s and Dunnett’s T3 multiple comparison tests. Outliers were identified and removed from biochemical, electrophysiological and behavioural analysis using the ROUT test. Statistical analyses were performed using Prism 8.2.1 (GraphPad software). Details concerning the number of independent experiments, replicates, statistical tests used, and p-values can be found in Supplementary Tables S4–11.
Detailed description of all methodologies can be found in the Supplementary Information.
Results
MiR-186-5p expression levels are increased by chronic stress or chronic activation of GRs
Previous observations indicate elevated levels of miR-186-5p in the brain and serum of animals subjected to chronic stress [22,23,24,25, 27], and in the blood of MDD patients [22]. Since genomic binding sites for GRs are present in the miR-186-5p host gene (ZRANB2) [29,30,31] (Fig. 1a and Supplementary Table 3), we assessed whether overexpression of miR-186-5p in the PFC region is a shared feature of distinct chronic stress paradigms and whether the direct and prolonged activation of GRs can mimic the changes in miR-186-5p expression in cortical neurons (Fig. 1). We first evaluated miR-186-5p levels in the PFC of neonate animals (PND10) subjected to maternal separation and maternal unpredictable stress (MS-US) during the first postnatal days (PND2-10), which drives enhanced risk-taking, depressive-like and social subordinate behaviours in adulthood [33]. These animals showed increased levels of miR-186-5p in the PFC compared to control animals (Fig. 1b). We then assessed whether chronic unpredictable stress (CUS) in young adult animals affects miR-186-5p expression in the PFC. We implemented a previously validated protocol of CUS [34] that leads to depressive-like behaviour and impairs working memory (Supplementary Fig. 1), and found that 4 weeks of CUS transiently increased miR-186-5p levels in the PFC (Fig. 1c, Supplementary Fig. S1k). Finally, we found that direct and prolonged activation of GRs (250 nM dexamethasone for 8 days) augmented miR-186-5p levels both in young (DIV7-14) and mature (DIV14-21) cultured cortical neurons compared to non-treated cells (Fig. 1d). Contrarily, acute (20 min) GR activation decreased the levels of miR-186-5p in cultured neurons (Fig. 1d). These results show that both chronic stress in neonate and young adult animals, as well as the direct, prolonged activation of GRs in neurons, upregulate the expression of miR-186-5p.
a miR-186 is encoded in an intronic region of the ZRANB2 gene. A total of 5 GRE sequences were found in the ZRANB2 gene. The GREs are labelled in the gene structure overview. b When compared to naïve animals, PND10 mice subjected to a MS-US protocol showed increased miR-186-5p levels in the PFC (n = 6–7 animals per condition; Unpaired t-test with Welch’s corrections: #p ≤ 0.05). c Mice exposed to four weeks of CUS showed significantly increased miR-186-5p levels in the PFC compared to naïve mice (n = 5–6 animals per condition; Mann–Whitney test: ##p ≤ 0.01). d Chronic exposure to dexamethasone (250 nM), either from DIV7 to 14 or from DIV14 to 21, induced an increase in miR-186-5p levels in cultured cortical neurons compared to control neurons (n = 6–7 independent experiments; One sample t-test and Wilcoxon test: #p ≤ 0.05; ##p ≤ 0.01). Modelling acute stress in vitro (100 nM dexamethasone for 20 min) caused a significant reduction in miR-186-5p levels in DIV14 cortical neurons compared to control (n = 6 independent experiments; One sample t-test and Wilcoxon test: ##p ≤ 0.01). Results are presented as mean ± SEM.
MiR-186-5p overexpression in the mPFC of naïve mice induces anxiety- and depressive-like behaviours
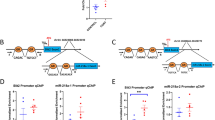
Given that both chronic stress and chronic GR activation induced upregulation of miR-185-5p expression, we explored a potential role for elevated miR-186-5p to causally drive phenotypes relevant to chronic stress-induced behaviour alterations. This involved lentiviral overexpression of either the precursor form of miR-186-5p (pre-miR-186) or a scramble (control) sequence in the mPFC of 10-week-old C57BL/6J naïve mice (Fig. 2). Effective lentivirus-mediated expression in the mPFC was validated by detecting EGFP (Fig. 2a), and qPCR analysis showed that miR-186-5p was increased by more than two-fold in the mPFC area transduced with the pre-miR-186 lentiviral vector in comparison to scramble-infected neurons (Fig. 2b). Analyses for both anxiety- and depressive-like behaviours were performed after 4 and 6 weeks of lentivirus expression. Four weeks of enhanced pre-miR-186 expression in the mPFC of young adult mice markedly decreased the total distance travelled with no significant difference in the percentage of distance travelled in the open arms (Fig. 2c, e) and induced anxiety-like behaviour, as seen by the reduced time spent in the open arms (Fig. 2c, f) in the elevated plus maze (EPM) test. However, no changes were detected in the time that the animals spent immobile in the tail suspension test (TST, Fig. 2g), suggesting that behaviour despair is not present at this time point. However, at 6 weeks of pre-miR-186 expression, there was no longer evidence of anxious behaviour in the open field test (OFT, Fig. 2h–j); nevertheless, a significant increase in the immobile time in the TST was now observed (Fig. 2k), indicating increased learned helplessness behaviour. These findings show that selectively elevating miR-186-5p levels in the mPFC triggers anxiety- and depressive-like behaviours similar to those observed in mice exposed to CUS.
a Left – schematic illustration and right – representative image of the injection site in the mPFC with lentivirus encoding a scramble or the pre-miR-186 sequence, together with EGFP. b The infected cells were laser micro-dissected and the subsequent real-time PCR detected that lentiviral-mediated pre-miR-186 expression increased miR-186-5p levels in the PFC of injected animals (n = 3–7 animals per condition; Unpaired t-test with Welch’s correction: ##p ≤ 0.05). c, h Cumulative occupancy heatmaps in the c EPM and h OFT of scramble- or pre-miR-186-expressing mice. Warmer colours represent areas where longer time was spent. d–f Animals expressing pre-miR-186 for 4 weeks d showed reduced total travelled distance with no differences in e the open arms travelled distance, and f spent less time in the open arms (n = 14–19 animals per condition; Unpaired t-test with Welch’s correction: ns p > 0.05; #p ≤ 0.05). g The time that the animals spent immobile during the TST was not significantly different between experimental groups at four weeks after lentiviral injection (n = 14–19 animals per condition; Unpaired t-test with Welch’s correction: ns p > 0.05). i The total distance travelled and j the time spent in the centre of the OFT were not different between scramble- and pre-miR-186-injected mice after 6 weeks of lentiviral expression (n = 13–19 animals per condition; Mann–Whitney test: ns p > 0.05). k Six weeks of miR-186-5p overexpression increased the immobility time in the TST (n = 14–18 animals per condition; Mann–Whitney test: #p ≤ 0.05). b Results are presented as mean ± SEM. d–k Boxes show 25th and 75th percentiles, whiskers show the range (minimum to maximum values) and the horizontal line shows the median value.
Chronic GR activation differently impacts excitatory and inhibitory synaptic transmission
One of the hallmarks of the chronically stressed brain is altered excitatory and inhibitory synaptic transmission [10, 11]. However, the molecular mechanisms linking chronic stress to synaptic changes have remained elusive. We previously demonstrated that miR-186-5p is an important modulator of glutamatergic synaptic transmission by directly targeting AMPAR expression [28]. Since miR-186-5p levels are increased upon prolonged GR activation, we hypothesized that it could trigger changes in synaptic activity under chronic stress conditions. To test this premise, we first evaluated structural and functional changes in both excitatory and inhibitory synapses in cultured cortical neurons submitted to prolonged GR activation (Figs. 3 and 4).
a Representative whole-cell current traces of AMPAR-mediated mEPSCs recorded from DIV15 control cortical neurons or neurons exposed to dexamethasone (250 nM, DIV7-15). b Long-term exposure to dexamethasone decreased the amplitude of AMPAR-mediated mEPSCs (n = 7 independent experiments, 20–25 cells per condition; Nested t-test: #p ≤ 0.05). c The cumulative probability curve of mEPSC amplitudes of neurons treated with dexamethasone presented a leftward shift (smaller amplitudes; n = 7 independent experiments, 100 events/cell, 20–25 cells per condition). d Representative images of cortical neurons submitted to prolonged GR activation with dexamethasone (250 nM, DIV7-16) or under control conditions and labelled for surface AMPARs (GluA), PSD95, VGluT1 and MAP2. e Chronic dexamethasone treatment decreased the intensity of synaptic AMPARs (n = 3 independent experiments, 30–32 cells per condition; Nested t-test: ns p > 0.05; ##p ≤ 0.01). f Representative whole-cell current traces of AMPAR-mediated mEPSCs recorded from the mPFC of mice after 4 weeks of CUS. g The CUS protocol decreased the amplitude of AMPAR-mediated mEPSCs (n = 3 animals per condition, 18–19 cells per condition; Nested t-test: #p ≤ 0.05). h The cumulative probability curve of mEPSC amplitudes of neurons from CUS animals presented a leftward shift (smaller amplitudes; 3 animals per condition, 50 events/cell, 18–19 cells per condition). i Representative images of layer 5–6 mPFC region from young naïve or CUS mice labelled for GluA2 and VGluT1. j Intensity and k area of GluA2 clusters decreased in the mPFC region of CUS mice (n = 3–4 animals per condition, 8 fields of view/animal). e, j, k Boxes show 25th and 75th percentiles, whiskers show the range (minimum to maximum values) and the horizontal line shows the median value. b, g Results are presented as mean ± SEM.
a Left – gene ontology analysis of miR-186-5p target transcripts, identified from endogenous AGO–miRNA–mRNA complexes in the mouse brain [39], revealed enrichment of targets relevant for glutamatergic transmission but also for GABAergic synapse function. Scale = fold enrichment. Right – examples of gene targets of miR-186-5p with a role in synapse assembly, chemical synaptic transmission, glutamatergic and GABAergic synapses. b Representative images of cortical neurons submitted to prolonged activation of GRs with dexamethasone (250 nM, DIV7-16) or under control conditions and labelled for gephyrin, VGAT and MAP2. c Chronic GR activation increased the intensity of gephyrin puncta that colocalize with VGAT (n = 5 independent experiments, 47–48 cells per condition; Nested t-test: #p ≤ 0.05). d Representative whole-cell current traces of mIPSCs recorded from DIV15 control cortical neurons or neurons incubated with dexamethasone (DIV7-15). e Long-term exposure of cortical neurons to dexamethasone augmented the frequency of GABAAR-mediated mIPSCs (n = 7 independent experiments, 21–27 cells per condition; Nested t-test: #p ≤ 0.05). f The cumulative probability curve of mIPSC inter-event intervals of scramble-expressing neurons treated with dexamethasone presented a leftward shift (higher frequencies; n = 7 independent experiments, 100 events/cell, 21–27 cells per condition). g Representative images of PFC slices from young naïve or CUS mice labelled for gephyrin and VGAT. h The intensity of gephyrin puncta in the mPFC region of CUS animals was significantly increased (n = 3 animals per condition, 8 fields of view/animal; Nested t-test: #p ≤ 0.05). c, h Boxes show 25th and 75th percentiles, whiskers show the range (minimum to maximum values) and the horizontal line shows the median value. e Results are presented as mean ± SEM.
At excitatory synapses, this treatment decreased both the area and intensity, but not the number (Supplementary Fig. 2a, b), of clusters of the postsynaptic protein PSD95 colocalized with the presynaptic vesicular glutamate transporter VGluT1. Functionally, sustained GR activation resulted in a significantly decreased amplitude of AMPAR-mediated miniature excitatory postsynaptic currents (mEPSCs) (Fig. 3a–c), without affecting their frequency (Supplementary Fig. 2c). To examine if the reduced excitatory transmission could be accounted for by altered synaptic AMPAR content, we labelled AMPAR subunits in non-permeabilized neurons and evaluated synaptic AMPA receptor clusters colocalized with PSD95/VGluT1 (Fig. 3d). We found chronic GR activation specifically reduced the labelling intensity of cell surface synaptic AMPAR clusters (Fig. 3e, Supplementary Fig. 2d).
Additionally, we tested whether the alterations in excitatory transmission caused by direct GR prolonged activation in cultured neurons could be similarly found in the PFC of young adult mice submitted to CUS for 4 weeks. The amplitude of AMPAR-mediated mEPSCs recorded from layer 5-6 mPFC neurons from chronically stressed mice was also decreased when compared with aged-matched naïve animals (Fig. 3f–h). However, an increase in the frequency of mEPSCs was observed in the CUS condition (Supplementary Fig. 2e), in parallel with the augmented number of VGluT1-positive PSD95 clusters, and total PSD95 and VGluT1 clusters detected in the same brain region (Supplementary Fig. 2f–i). Overall, these results show that prolonged GR activation through direct activation with dexamethasone as well as exposure to a CUS protocol both decrease the amplitude of cortical AMPAR-mediated currents.
The functional properties of AMPARs are determined by their synaptic content and subunit composition [43, 44] and miR-186-5p impacts AMPAR subunit composition by targeting GluA2 expression [28]. Since the expression of miR-186-5p is increased upon chronic GR activation (Fig. 1d), we tested whether this treatment affects the subunit content of AMPARs by labelling total and synaptic GluA2- and GluA1-containing AMPARs in non-permeabilized cortical neurons (Supplementary Fig. 3). The number and intensity of GluA2 clusters were decreased (Supplementary Fig. 3a–c), while synaptic and total surface GluA1 clusters remained unchanged (Supplementary Fig. 3d–f) in neurons chronically treated with dexamethasone. To test whether AMPAR content is changed in the PFC of mice submitted to CUS, we labelled GluA2 in the PFC of stressed mice and compared it with aged-matched naïve animals (Fig. 3i). In line with the results from GR activation on cultured cortical neurons, four weeks of CUS induced a dramatic decrease in the intensity (~63%) and area of GluA2 clusters (Fig. 3i–k). Therefore, the decreased AMPAR-mediated synaptic transmission upon chronic GR activation or CUS may be due to overall reduced GluA2 expression levels caused by upregulation of miR-186-5p.
Dysregulation of the GABAergic system, overlapped with a weak glutamatergic system, has been linked to cognitive and emotional changes associated with neuropsychiatric diseases like schizophrenia, attention-deficit hyperactivity disorder and depression triggered by stress exposure [45,46,47,48]. We performed gene ontology enrichment analysis of miR-186-5p-target chimeras previously identified in the mouse brain [39] and, in addition to transcripts encoding proteins critical for glutamatergic synapse function, we found target genes for proteins regulating inhibitory synaptic transmission (Fig. 4a). To directly investigate if prolonged GR activation also causes alterations in GABAergic transmission, we labelled inhibitory synapses (colocalized gephyrin/VGAT clusters) and recorded GABAAR-mediated miniature inhibitory postsynaptic currents (mIPSCs) in cultured cortical neurons (Fig. 4b–f). Contrary to the effects seen at excitatory synapses, prolonged GR activation increased the intensity of VGAT-colocalized gephyrin clusters (Fig. 4b, c), but not their number or area (Supplementary Fig. 4a). Concomitantly, we found that the frequency of mIPSCs (Fig. 4d–f), but not their amplitude (Supplementary Fig. 4b), increased after sustained GR activation. Interestingly, the levels of total surface and synaptic γ2-containing GABAARs were not affected by chronic GR activation (Supplementary Fig. 4c–e). To determine whether these alterations caused by the direct activation of GR could be recapitulated in vivo under chronic stress conditions, the number and intensity of gephyrin and VGAT puncta were analysed in layer 5-6 of the mPFC of naïve and chronically stressed mice (Fig. 4g, h). Similar to the results in cultured neurons, four weeks of CUS increased the intensity of gephyrin labelling in the mPFC, when compared with naïve animals, whereas the number of gephyrin and VGAT clusters did not change (Supplementary Fig. 4f, g). Thus, chronic GR activation or CUS both potentiated the GABAergic system by enhancing the accumulation of gephyrin at synaptic sites and increasing the frequency of GABAAR-mediated mIPSCs, without affecting their amplitude.
MiR-186-5p inhibition prevents GR-induced weakening of excitatory synaptic transmission
Considering the functional role of miR-186-5p in regulating glutamate receptor expression and excitatory synaptic transmission [28], its upregulation through prolonged GR activation (Fig. 1d), and its role in driving anxiety- and depressive-like phenotypes (Fig. 2), we hypothesized that miR-186-5p could be a main contributor to the synaptic alterations induced by long-term exposure to dexamethasone (Fig. 3). If so, inhibition of miR-186-5p should normalize AMPAR-mediated synaptic transmission under such conditions. To test for this, we expressed a miR-186-5p inhibitor or a scramble sequence in cortical neurons that were then chronically exposed to dexamethasone (from DIV7-16). Remarkably, prolonged dexamethasone exposure affected neither the intensity of GluA clusters (Fig. 5a, b) nor the amplitude of AMPAR-mediated mEPSCs (Fig. 5c–e) in neurons expressing a miR-186-5p inhibitor. No significant effects of miR-186-5p inhibition were found on GluA levels (Supplementary Fig. 5b–d) or on mEPSC properties (Supplementary Fig. 5e–g) in basal conditions. These results therefore establish the upregulation of miR-186-5p as a molecular link between chronic GR activation and the reduction in excitatory synaptic transmission; repressing this upregulation rescues the synaptic transmission deficits triggered by chronic dexamethasone treatment.
a Representative images of DIV16 cortical neurons expressing either a miR-186-5p inhibitor or a scramble sequence, under control conditions or exposed to dexamethasone (250 nM, DIV7-16). Neurons were labelled for surface AMPARs (GluA), VGluT1 and MAP2. b MiR-186-5p inhibition rescued the intensity of synaptic GluA clusters in dexamethasone treated neurons to control levels (n = 4 independent experiments, 45–48 cells per condition; Nested One-way ANOVA: p = 0.0041, followed by Tukey’s multiple comparisons test: ns p > 0.05; ##p ≤ 0.001). c Representative whole-cell current traces of AMPAR-mediated mEPSCs recorded from DIV15 cortical neurons expressing a scramble sequence or a miR-186-5p inhibitor, under control conditions or treated with dexamethasone (250 nM, DIV7-15). d Amplitudes of mEPSCs of neurons expressing the miR-186-5p inhibitor under chronic GR activation are not different from those of scramble-expressing neurons under control conditions (n = 5 independent experiments, 18–21 cells per condition; Nested One-way ANOVA: p = 0.0267, followed by Tukey’s multiple comparisons test: #p ≤ 0.05). e The cumulative probability curve of mEPSC amplitudes of scramble-expressing neurons treated with dexamethasone presented a leftward shift (smaller amplitudes) in comparison with control scramble-expressing neurons; this was prevented by miR-186-5p inhibition (n = 5 independent experiments, 100 events/cell, 18–21 cells per condition). b Boxes show 25th and 75th percentiles, whiskers show the range (minimum to maximum values) and the horizontal line shows the median value. d Results are presented as mean ± SEM.
MiR-186-5p inhibition normalizes GABAergic transmission and network activity upon prolonged GR activation
MiR-186-5 targets relevant transcripts for GABAergic synapse function (Fig. 4a), hinting that its upregulation could also contribute to the maladaptation of the GABAergic system caused by sustained GR activation (Fig. 4b–h). Therefore, we tested whether inhibiting miR-186-5p could also prevent the changes in inhibitory transmission in neurons chronically exposed to dexamethasone. The expression of the miR-186-5p inhibitor normalized the frequency of mIPSCs in dexamethasone treated neurons (Fig. 6a–c), while the amplitude of mIPSCs was not affected by dexamethasone exposure or miR-186-5p inhibition (Supplementary Fig. 6a, c). However, expression of the miR-186-5p inhibitor alone caused a tendency to increase the frequency of mIPSCs in non-stimulated neurons (Supplementary Fig. 6b, d). These results demonstrate that increased expression of miR-186-5p upon GR activation enhances inhibitory transmission by targeting a pool of transcripts specifically present in neurons that were chronically exposed to dexamethasone; inhibiting miR-186-5p overexpression in these conditions can rescue inhibitory transmission.
a Representative whole-cell current traces of GABAAR-mediated mIPSCs from DIV15 cortical neurons expressing a scramble sequence or a miR-186-5p inhibitor, under control conditions or treated with dexamethasone (250 nM, DIV7-15). b Expressing a miR-186-5p inhibitor rescued the frequency of mIPSCs in dexamethasone treated neurons to control levels (n = 5 independent experiments, 11–19 cells per condition; Nested One-way ANOVA: p = 0.0288, followed by Tukey’s multiple comparisons test: #p ≤ 0.05). c The cumulative probability curve of mIPSC inter-event intervals of scramble-expressing neurons treated with dexamethasone presented a leftward shift (higher frequencies) in comparison with control scramble-expressing neurons. This shift was prevented by miR-186-5p inhibition (n = 5 independent experiments, 100 events/cells, 11–19 cells per condition). d Representative raster plots from a 16-electrode array showing 60 s of spontaneous activity in cortical neurons expressing a scramble sequence or a miR-186-5p inhibitor, under control conditions or chronically exposed to dexamethasone (250 nM, DIV7-17). Spikes are represented in blue, spike bursts in grey/green and network bursts are represented by black boxes. Ten days of dexamethasone treatment increased the coefficient of variation of the (e) IBI and (f) network IBI activity in scramble-expressing neurons. MiR-186-5p inhibition abolished these effects (n = 3 independent experiments, 15-16 wells per condition; Nested One-way ANOVA: p = 0.0353 (e), p = 0.0011 (f) followed by Tukey’s multiple comparison test: ns p > 0.05; #p ≤ 0.05; ##p ≤ 0.01). b Results are presented as mean ± SEM. e, f Boxes show 25th and 75th percentiles, whiskers show the range (minimum to maximum values) and the horizontal line shows the median value.
Balanced neuronal activity results from the interplay of an intricate network of glutamatergic and GABAergic neurons [49]. We hypothesized that the impairment in excitatory and inhibitory synaptic transmission caused by prolonged GR activity could be reflected at the network level and, therefore, tested whether inhibition of miR-186-5p could normalize neuronal network activity in neurons exposed to dexamethasone. Spontaneous electrophysiological population activity was recorded in scramble- or miR-186-5p inhibitor-expressing neurons cultured on microelectrode array plates under control or chronic GR activation conditions. In scramble-expressing neurons, ten days of GR activation had no significant effect on the mean firing rate, burst frequency, or network burst frequency (Supplementary Fig. 7a–c). However, it dramatically increased the coefficient of variation of the inter-burst interval (IBI) and network IBI, a measure of burst and network burst rhythmicity (Fig. 6d–f). These observations suggest that prolonged dexamethasone treatment increased the temporal irregularity of burst and network activity. Strikingly, expression of the miR-186-5p inhibitor prevented the impact of prolonged GR activation on the coefficient of variation of the IBI or network IBI, normalizing the rhythmicity of bursts and network bursts (Fig. 6d–f). In unstimulated conditions, miR-186-5p inhibition increased the IBI coefficients of variation, without changing the network IBI coefficients of variation, mean firing rate, burst frequency or network burst frequency (Supplementary Fig. 7d–i), again suggesting that, in conditions of chronic GR activation, miR-186-5p is implicated in the regulation of a specific set of stress-associated transcripts. Overall, these results demonstrate that the aberrant pattern of neuronal network activity triggered by long-term GR activation can be rescued by specifically inhibiting miR-186-5p.
Discussion
The molecular mechanisms associating chronic stress to its deleterious effects on synaptic and neuronal function have remained elusive. This study identifies miR-186-5p as a novel molecular link between chronic stress conditions – and prolonged GR activation – and neuronal and behavioural phenotypes associated with chronic stress. We found that miR-186-5p is elevated in the PFC of both neonate mice subjected to early life stress and transiently in young adult mice subjected to chronic stress, as well as in cultured cortical neurons upon direct and prolonged GR activation. Additionally, viral overexpression of miR-186-5p in the mPFC of young adult animals leads to anxiety- and depressive-like behaviours, suggesting that elevated miR-186-5p levels can trigger behaviour phenotypes reminiscent of those observed in chronically stressed animals. Consistent with these results, miR-186-5p inhibition in neurons prevents the changes in synaptic transmission and neuronal network activity induced by prolonged GR activation.
Our results provide insights into the molecular mechanisms linking GR-induced miR-186-5p overexpression to its detrimental effects in excitatory and inhibitory neuronal transmission, and consequent disturbed behavioural outputs related to neuropsychiatric disorders. Using a simplified in vitro chronic stress model allowed us to directly assess the impact of prolonged GR activation on synaptic function and neuronal network activity, and to dissect the contribution of elevated miR-186-5p levels to these changes.
MiR-186-5p upregulation and its relevance to chronic stress-induced brain pathology
Our data show that the levels of miR-186-5p are elevated in the PFC of rodents in two different models of repetitive stress, as well as in cultured cortical neurons subjected to prolonged (7 days) GR activation. These results are in agreement with numerous other studies that found changed miR-186-5p levels in the brain and serum of animals submitted to chronic stress [22,23,24,25, 27, 50], as well as in the serum of MDD patients [22]. Of note, miR-186-5p is encoded in an intron within the ZRANB2 gene [51], which is under the transcriptional control of GREs [29,30,31]. This suggests that its expression could be directly controlled by GR-regulated transcription. Interestingly, while prolonged GR activation increased miR-186-5p expression in cortical neurons, modelling acute stress in vitro by a short 20 min exposure to dexamethasone had the opposite effect. This response aligns with previous findings in the serum of mice subjected to a single episode of restraint stress [50]. Although a straightforward explanation for this duality of effects is unclear, the short-term activation of GRs could interfere with other steps in the miR-186-5p biogenesis process besides the transcription of the miR-186-5p-encoding ZRANB2 gene, and/or impact the machinery that operates to degrade miRNAs, but these hypotheses remain to be tested. Nonetheless, these results show that different temporal scales of GR activation oppositely affect miR-186-5p expression, suggesting its direct implication in the neuronal responses to stress. Indeed, we demonstrated that miR-186-5p overexpression in the mPFC of naïve mice was sufficient to induce anxiety- and depressive-like behaviours, supporting its relevance in mediating behavioural changes akin to those observed in animals exposed to chronic stress.
Maladaptation of cortical synaptic and network activity upon chronic GR activation
Changes in the balance between excitatory and inhibitory drive impact the activity of neuronal circuits and, ultimately, compromise neuronal and network function. Our data show that prolonged GR activation by direct GR stimulation in cultured neurons, or chronic stress in young adult mice, decrease excitatory synaptic strength in cortical neurons, while inhibitory synaptic transmission is enhanced, suggesting an imbalance in excitation/inhibition. Additionally, decreased AMPAR-mediated synaptic transmission, triggered by chronic GR activation, is accompanied by postsynaptic density protein changes, and by a reduction in synaptic AMPARs. These results support a role for GR activation in mediating the synaptic effects of chronic stress on cortical excitatory synapses, as previously described [12, 52, 53]. Altogether, our data support the hypothesis that chronic stress impairs the functioning of neuronal circuits at least partially by changing the function of excitatory synapses, giving rise to pathological behavioural alterations (reviewed in ref. [54]).
In parallel with dampened excitatory responses, both CUS in mice and prolonged activation of GR in cultured neurons reduce GluA2-containing AMPAR clusters at synapses. In cultured neurons GluA1-containing AMPARs remain unchanged. These observations support a switch in synaptic AMPAR subunit composition triggered by chronic GR activation, similar to what was observed in orbitofrontal cortex to basolateral amygdala synapses in a model of repetitive tail-shock stress [13], and in human post-mortem nucleus accumbens tissue from depressed individuals [55]. On the other hand, upregulation of GluA2 has been linked to resilience to chronic stress maladaptation [55, 56] and accumulating evidence shows the importance of GluA2-containing AMPARs during facilitation of learning and memory formation by stress [57,58,59,60,61]. Our results suggest that miR-186-5p-mediated changes in GluA2 expression levels could be correlated with the cognitive and behavioural outcomes of chronic stress, possibly by modifying synaptic transmission and altering plasticity mechanisms.
Contrary to the effects seen in excitatory neurotransmission, sustained GR activation and chronic response to stress cause an enrichment in gephyrin, and prolonged treatment of cultured cortical neurons with dexamethasone increases the frequency of mIPSCs, similar to what was reported in PFC pyramidal neurons of chronically stressed animals [62] and of animals submitted to early life stress [33]. However, the number of inhibitory synapses is not altered, suggesting either presynaptic changes in synaptic vesicle release or an elevated number of functional inhibitory synapses. Together, these results show that chronic GR activation causes an increase in inhibitory drive accompanied by decreased excitation, which should negatively impact neuronal network activity. Indeed, cortical neuron cultures, which show typical synchronous network activity at DIV17 [63, 64], exhibit a more irregular pattern of burst and network burst activity upon prolonged GR activation. The network phenotypes may be secondary to increased inhibitory transmission in neurons subjected to prolonged GR activation, which can influence network burst activity [65]. However, intrinsic neuronal properties may also be impacted by dexamethasone exposure, similar to what has been shown in diverse models of chronic stress [66,67,68].
Overall, our results demonstrate that sustained GR activation in cortical neurons causes an imbalance between excitatory and inhibitory transmission, accompanied by increased temporal irregularity of neuronal and network bursting. Additionally, the glutamatergic system undergoes adaptive mechanisms through loss of GluA2 and resulting in decreased synaptic strength. Together, these alterations supply a mechanism for cortical brain hypoactivity and loss of connectivity triggered by chronic exposure to stress hormones, which contribute to emotional disturbances like those seen in depression, anxiety and PTSD [49].
Targeting miR-186-5p expression to correct the E/I imbalance induced by chronic GR activation
Whereas chronic GR activation leads to a reduction in synaptic AMPARs and AMPAR-mediated synaptic transmission, and to a concomitant increase in GABAergic transmission, inhibiting the binding of miR-186-5p to its targets prevents these effects. This demonstrates that the synaptic effects of prolonged GR activation are at least partially mediated by upregulated miR-186-5p levels. The overall set of relevant transcripts implicated in this mechanism is currently unknown. However, since our previous work showed that miR-186-5p directly targets the Gria2 mRNA and reduces synaptic GluA2-containing AMPARs [28], inhibiting miR-186-5p under chronic GR activation is likely to prevent decreased excitatory transmission by normalizing GluA2 levels. The analysis of miR-186-5p targets, previously described in the mouse brain [39], identified additional transcripts encoding for proteins with a role at both excitatory and inhibitory synapses. It will thus be important to further investigate which targets are relevant for the miR-186-5p-dependent regulation of GABAergic synaptic transmission, particularly because both miR-186-5p inhibition and chronic GR activation increase the frequency of mIPSCs. However, preventing the upregulation of miR-186-5p triggered by GR overactivation precludes the increase in mIPSC frequency. This intriguing result suggests that miR-186-5p targets different pools of transcripts expressed in control neurons and in neurons exposed to dexamethasone, and highlights the complex effects of miRNAs in the regulation of neuronal activity.
Other miRNAs that are involved in the neuronal processes targeted by chronic stress have been found altered in several patients and brain regions of mouse models of affective disorders [19, 21, 69,70,71]. Nevertheless, the finding that normalizing the levels of miR-186-5p prevents the effects of chronic exposure to dexamethasone on excitatory and inhibitory synaptic transmission, and restores neuronal network activity, gives further support to a major role for miR-186-5p in mediating the adverse effects of stress on synaptic function.
Our study does not specifically address the cell-type specific synaptic responses to chronic dexamethasone exposure or to CUS, nor whether increased miR-186-5p levels play cell-type specific roles. Given recent data emphasizing the significance of changes in PFC interneurons in response to chronic stress paradigms, and suggesting a key role for these changes in promoting adaptation and driving pathological conditions (reviewed in ref. [72]), it would be relevant to specifically address chronic stress-induced synaptic alterations in interneurons.
It is also important to acknowledge that the results of this study pertain to cultured cortical neurons (derived from embryonic rat brains) subjected to prolonged dexamethasone treatment, and to young adult mice submitted to CUS. Discrepancies between in vitro and in vivo results may be accounted for by the early maturation state of neuronal cultures and species differences in the rat and mouse response to stress. In addition, the PFC is still undergoing significant development during the period at which the CUS paradigm was performed, and alterations in the maturation of the circuitry could occur that potentiate or underestimate synaptic alterations. Therefore, our results may not fully translate to the effects of stress on a fully mature PFC and further studies should be performed on adult animals, where the neural circuitry construction is less vulnerable.
In conclusion, we unveil a molecular mechanism linking GR-induced miR-186-5p overexpression to the detrimental effects of chronic stress in excitatory and inhibitory synaptic transmission, contributing to their imbalance and to the disturbed behavioural outputs related to neuropsychiatric disorders. The work described here provides proof of concept that normalizing miR-186-5p levels can be effective in preventing the adverse synaptic effects of sustained GR activation. Besides the direct effects of miR-186-5p expression that our results show on basal excitatory and inhibitory synaptic transmission, altered homeostatic plasticity, controlled in part by miR-186-5p [28], could also contribute to anxiety and depressive-like behaviour. Therefore, our results suggest that miR-186-5p could be a promising candidate as a novel biomarker associated with the vulnerability to neurological alterations linked to stress, and as a possible therapeutic target to prevent the synaptic alterations associated with chronic stress.
Data availability
The data that support the findings of this study are available from the corresponding authors upon request.
References
Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10:397–409.
Russell G, Lightman S. The human stress response. Nat Rev Endocrinol. 2019;15:525–34.
Davis MT, Holmes SE, Pietrzak RH, Esterlis I. Neurobiology of Chronic Stress-Related Psychiatric Disorders: Evidence from Molecular Imaging Studies. Chronic Stress. 2017;1:2470547017710916.
Tafet GE, Nemeroff CB. The links between stress and depression: Psychoneuroendocrinological, genetic, and environmental interactions. J Neuropsychiatry Clin Neurosci. 2016;28:77–88.
Liu Q, Zhang Z, Zhang W. Optogenetic Dissection of Neural Circuits Underlying Stress-Induced Mood Disorders. Front Psychol. 2021;12:1–7.
Woo E, Sansing LH, Arnsten AFT, Datta D. Chronic Stress Weakens Connectivity in the Prefrontal Cortex: Architectural and Molecular Changes. Chronic Stress. 2021;5:24705470211029254.
Chini M, Hanganu-Opatz IL. Prefrontal Cortex Development in Health and Disease: Lessons from Rodents and Humans. Trends Neurosci. 2021;44:227–40.
McEwen BS, Morrison JH. The Brain on Stress: Vulnerability and Plasticity of the Prefrontal Cortex over the Life Course. Neuron. 2013;79:16–29.
Arnsten AFT. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 2009;10:410–22.
Sanacora G, Yan Z, Popoli M. The stressed synapse 2.0: pathophysiological mechanisms in stress-related neuropsychiatric disorders. Nat Rev Neurosci. 2022;23:86–103.
Perez-Rando M, Carceller H, Castillo-Gomez E, Bueno-Fernandez C, García-Mompó C, Gilabert-Juan J, et al. Impact of stress on inhibitory neuronal circuits, our tribute to Bruce McEwen. Neurobiol Stress. 2022;19:100460.
Yuen EY, Wei J, Liu W, Zhong P, Li X, Yan Z. Repeated Stress Causes Cognitive Impairment by Suppressing Glutamate Receptor Expression and Function in Prefrontal Cortex. Neuron. 2012;73:962–77.
Kuniishi H, Yamada D, Wada K, Yamada M, Sekiguchi M. Stress induces insertion of calcium-permeable AMPA receptors in the OFC–BLA synapse and modulates emotional behaviours in mice. Transl Psychiatry. 2020;10:154.
Wei J, Zhong P, Qin L, Tan T, Yan Z. Chemicogenetic Restoration of the Prefrontal Cortex to Amygdala Pathway Ameliorates Stress-Induced Deficits. Cereb Cortex. 2018;28:1980–90.
Shepard R, Page CE, Coutellier L. Sensitivity of the prefrontal GABAergic system to chronic stress in male and female mice: Relevance for sex differences in stress-related disorders. Neuroscience. 2016;332:1–12.
Ghosal S, Hare BD, Duman RS. Prefrontal cortex GABAergic deficits and circuit dysfunction in the pathophysiology and treatment of chronic stress and depression. Curr Opin Behav Sci. 2017;14:1–8.
Gray JD, Kogan JF, Marrocco J, McEwen BS. Genomic and epigenomic mechanisms of glucocorticoids in the brain. Nat Rev Endocrinol. 2017;13:661–73.
Dubes S, Favereaux A, Thoumine O, Letellier M. miRNA-Dependent Control of Homeostatic Plasticity in Neurons. Front Cell Neurosci. 2019;13:1–11.
Musazzi L, Mingardi J, Ieraci A, Barbon A, Popoli M. Stress, microRNAs, and stress-related psychiatric disorders: an overview. Mol Psychiatry. 2023. https://doi.org/10.1038/s41380-023-02139-3.
Narayanan R, Schratt G. miRNA regulation of social and anxiety-related behaviour. Cell Mol Life Sci. 2020;77:4347–64.
Martins HC, Schratt G. MicroRNA-dependent control of neuroplasticity in affective disorders. Transl Psychiatry. 2021;11:263.
Jiang M, Gu Yfang, Cai Jfen, Wang A, He Y, Feng Y. ling. MiR-186-5p Dysregulation Leads to Depression-like Behavior by De-repressing SERPINF1 in Hippocampus. Neuroscience. 2021;479:48–59.
Balakathiresan NS, Chandran R, Bhomia M, Jia M, Li H, Maheshwari RK. Serum and amygdala microRNA signatures of posttraumatic stress: Fear correlation and biomarker potential. J Psychiatr Res. 2014;57:65–73.
Sun X, Song Z, Si Y, Wang JH. microRNA and mRNA profiles in ventral tegmental area relevant to stress-induced depression and resilience. Prog Neuro Psychopharmacology Biol Psychiatry. 2018;86:150–65.
Fang K, Xu JX, Chen XX, Gao XR, Huang LL, Du AQ, et al. Differential serum exosome microRNA profile in a stress-induced depression rat model. J Affect Disord. 2020;274:144–58.
Torres-Berrío A, Morgunova A, Giroux M, Cuesta S, Nestler EJ, Flores C. miR-218 in Adolescence Predicts and Mediates Vulnerability to Stress. Biol Psychiatry. 2021;89:911–9.
Babenko O, Golubov A, Ilnytskyy Y, Kovalchuk I, Metz GA. Genomic and epigenomic responses to chronic stress involve miRNA-mediated programming. PLoS One. 2012;7:e29441.
Silva MM, Rodrigues B, Fernandes J, Santos SD, Carreto L, Santos MAS, et al. MicroRNA-186-5p controls GluA2 surface expression and synaptic scaling in hippocampal neurons. Proc Natl Acad Sci USA. 2019;116:5727–36.
Van Weert LTCM, Buurstede JC, Mahfouz A, Braakhuis PSM, Polman JAE, Sips HCM, et al. NeuroD factors discriminate mineralocorticoid from glucocorticoid receptor DNA binding in the male rat brain. Endocrinology. 2017;158:1511–22.
Polman JAE, De Kloet ER, Datson NA. Two populations of glucocorticoid receptor-binding sites in the male rat hippocampal genome. Endocrinology. 2013;154:1832–44.
Howrylak JA, Moll M, Weiss ST, Raby BA, Wu W, Xing EP. Gene expression profiling of asthma phenotypes demonstrates molecular signatures of atopy and asthma control. J Allergy Clin Immunol. 2016;137:1390–97.e6.
Rufino-Ramos D, Albuquerque PR, Leandro K, Carmona V, Martins IM, Fernandes R, et al. Extracellular vesicle-based delivery of silencing sequences for the treatment of Machado-Joseph disease/spinocerebellar ataxia type 3. Mol Ther. 2023;31:1275–92.
Franco LO, Carvalho MJ, Costa J, Ferreira PA, Guedes JR, Sousa R, et al. Social subordination induced by early life adversity rewires inhibitory control of the prefrontal cortex via enhanced Npy1r signaling. Neuropsychopharmacology. 2020;45:1438–47.
Lopes S, Teplytska L, Vaz-Silva J, Dioli C, Trindade R, Morais M, et al. Tau deletion prevents stress-induced dendritic atrophy in prefrontal cortex: Role of synaptic mitochondria. Cereb Cortex. 2017;27:2580–91.
Hiramatsu M, Inoue K. Effects of nocistatin on nociceptin-induced impairment of learning and memory in mice. Eur J Pharmacol. 1999;367:151–5.
Santos SD, Iuliano O, Ribeiro L, Veran J, Ferreira JS, Rio P, et al. Contactin-associated protein 1 (Caspr1) regulates the traffic and synaptic content of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)-type glutamate receptors. J Biol Chem. 2012;287:6868–77.
Jiang M, Deng L, Chen G. High Ca2+-phosphate transfection efficiency enables single neuron gene analysis. Gene Ther. 2004;11:1303–11.
Pagès H, Aboyoun P, Gentleman R, DebRoy S. Biostrings: Efficient manipulation of biological strings. R package version 2.72.1, 2023. https://bioconductor.org/packages/Biostrings.
Moore MJ, Scheel TKH, Luna JM, Park CY, Fak JJ, Nishiuchi E, et al. MiRNA-target chimeras reveal miRNA 3′-end pairing as a major determinant of Argonaute target specificity. Nat Commun. 2015;6:8864.
Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, et al. Gene Ontology: tool for the unification of biology. Nat Genet. 2000;25:25–29.
Thomas PD, Ebert D, Muruganujan A, Mushayahama T, Albou LP, Mi H. PANTHER: Making genome-scale phylogenetics accessible to all. Protein Sci. 2022;31:8–22.
Bardou P, Mariette J, Escudié F, Djemiel C, Klopp C. SOFTWARE Open Access jvenn: an interactive Venn diagram viewer. BMC Bioinformatics. 2014;15:1–7.
Isaac JTR, Ashby M, McBain CJ. The Role of the GluR2 Subunit in AMPA Receptor Function and Synaptic Plasticity. Neuron. 2007;54:859–71.
Henley JM, Wilkinson KA. Synaptic AMPA receptor composition in development, plasticity and disease. Nat Rev Neurosci. 2016;17:337–50.
Klune CB, Jin B, Denardo LA. Linking mpfc circuit maturation to the developmental regulation of emotional memory and cognitive flexibility. Elife. 2021;10:1–33.
Czéh B, Vardya I, Varga Z, Febbraro F, Csabai D, Martis LS, et al. Long-term stress disrupts the structural and functional integrity of GABAergic neuronal networks in the medial prefrontal cortex of rats. Front Cell Neurosci. 2018;12:1–21.
Coghlan S, Horder J, Inkster B, Mendez MA, Murphy DG, Nutt DJ. GABA system dysfunction in autism and related disorders: From synapse to symptoms. Neurosci Biobehav Rev. 2012;36:2044–55.
Gonzalez-Burgos G, Hashimoto T, Lewis DA. Alterations of cortical GABA neurons and network oscillations in schizophrenia. Curr Psychiatry Rep. 2010;12:335–44.
Page CE, Coutellier L. Prefrontal excitatory/inhibitory balance in stress and emotional disorders: Evidence for over-inhibition. Neurosci Biobehav Rev. 2019;105:39–51.
Solich J, Kuśmider M, Faron-Górecka A, Pabian P, Dziedzicka-Wasylewska M. Restraint stress in mice alters SET of 25 miRNAs which regulate stress-and depression-related mrnas. Int J Mol Sci. 2020;21:1–13.
Antoniou A, Mastroyiannopoulos NP, Uney JB, Phylactou LA. miR-186 Inhibits Muscle Cell Differentiation through Myogenin Regulation. J Biol Chem. 2014;289: 3923–35.
Kvarta MD, Bradbrook KE, Dantrassy HM, Bailey AM, Thompson SM. Corticosterone mediates the synaptic and behavioral effects of chronic stress at rat hippocampal temporoammonic synapses. J Neurophysiol. 2015;114:1713–24.
Kallarackal AJ, Kvarta MD, Cammarata E, Jaberi L, Cai X, Bailey AM, et al. Chronic stress induces a selective decrease in AMPA receptor-mediated synaptic excitation at hippocampal temporoammonic-CA1 synapses. J Neurosci. 2013;33:15669–74.
Thompson SM. Plasticity of synapses and reward circuit function in the genesis and treatment of depression. Neuropsychopharmacology. 2023;48:90–103.
Vialou V, Robison AJ, Laplant QC, Covington HE, Dietz DM, Ohnishi YN, et al. ΔfosB in brain reward circuits mediates resilience to stress and antidepressant responses. Nat Neurosci. 2010;13:745–52.
Elhussiny MEA, Carini G, Mingardi J, Tornese P, Sala N, Bono F, et al. Modulation by chronic stress and ketamine of ionotropic AMPA/NMDA and metabotropic glutamate receptors in the rat hippocampus. Prog Neuro Psychopharmacol Biol Psychiatry. 2021;104:110033.
Conboy L, Sandi C. Stress at learning facilitates memory formation by regulating ampa receptor trafficking through a glucocorticoid action. Neuropsychopharmacology. 2010;35:674–85.
Aubry AV, Serrano PA, Burghardt NS. Molecular mechanisms of stress-induced increases in fear memory consolidation within the amygdala. Front Behav Neurosci. 2016;10:1–10.
Martin S, Henley JM, Holman D, Zhou M, Wiegert O, van Spronsena M, et al. Corticosterone alters AMPAR mobility and facilitates bidirectional synaptic plasticity. PLoS One. 2009;4:1–8.
Groc L, Choquet D, Chaouloff F. The stress hormone corticosterone conditions AMPAR surface trafficking and synaptic potentiation. Nat Neurosci. 2008;11:868–70.
Gourley SL, Kedves AT, Olausson P, Taylor JR. A history of corticosterone exposure regulates fear extinction and cortical NR2B, GluR2/3, and BDNF. Neuropsychopharmacology. 2009;34:707–16.
McKlveen JM, Morano RL, Fitzgerald M, Zoubovsky S, Cassella SN, Scheimann JR, et al. Chronic Stress Increases Prefrontal Inhibition: A Mechanism for Stress-Induced Prefrontal Dysfunction. Biol Psychiatry. 2016;80:754–64.
Martens MB, Frega M, Classen J, Epping L, Bijvank E, Benevento M, et al. Euchromatin histone methyltransferase 1 regulates cortical neuronal network development. Sci Rep. 2016;6:1–11.
Chiappalone M, Vato A, Berdondini L, Koudelka-Hep M, Martinoia S. Network dynamics and synchronous activity in cultured cortical neurons. Int J Neural Syst. 2007;17:87–103.
Mossink B, van Rhijn JR, Wang S, Linda K, Vitale MR, Zöller JEM, et al. Cadherin-13 is a critical regulator of GABAergic modulation in human stem-cell-derived neuronal networks. Mol Psychiatry. 2022;27:1–18.
Urban KR, Valentino RJ. Age- And sex-dependent impact of repeated social stress on intrinsic and synaptic excitability of the rat prefrontal cortex. Cereb Cortex. 2017;27:244–53.
Nawreen N, Baccei ML, Herman JP. Single Prolonged Stress Reduces Intrinsic Excitability and Excitatory Synaptic Drive Onto Pyramidal Neurons in the Infralimbic Prefrontal Cortex of Adult Male Rats. Front Cell Neurosci. 2021;15:1–11.
Kim J, Lei Y, Lu XY, Kim CS. Glucocorticoid-glucocorticoid receptor-HCN1 channels reduce neuronal excitability in dorsal hippocampal CA1 neurons. Mol Psychiatry. 2022. https://doi.org/10.1038/s41380-022-01682-9.
Allen L, Dwivedi Y. MicroRNA mediators of early life stress vulnerability to depression and suicidal behavior. Mol Psychiatry. 2020;25:308–20.
Daskalakis NP, Provost AC, Hunter RG, Guffanti G. Noncoding RNAs: Stress, Glucocorticoids, and Posttraumatic Stress Disorder. Biol Psychiatry. 2018;83:849–65.
Dwivedi Y, Roy B, Lugli G, Rizavi H, Zhang H, Smalheiser NR. Chronic corticosterone-mediated dysregulation of microRNA network in prefrontal cortex of rats: Relevance to depression pathophysiology. Transl Psychiatry. 2015;5:e682–11.
McKlveen JM, Moloney RD, Scheimann JR, Myers B, Herman JP. “Braking” the Prefrontal Cortex: The Role of Glucocorticoids and Interneurons in Stress Adaptation and Pathology. Biol Psychiatry. 2019;86:669–81.
Acknowledgements
The authors wish to thank support from the MICC Imaging facility of CNC-UC, and to all Ana Luísa Carvalho’s laboratory members for technical assistance and for indispensable discussion of the work.
Funding
This work was funded by La Caixa Iniciativa Ibérica and FCT 4b initiative under the project LCF/PR/HP21/52310010 “microSTRESS”, and by the European Regional Development Fund (ERDF), through the COMPETE 2020 — Operational Programme for Competitiveness and Internationalisation and Portuguese national funds via FCT, under projects UIDB/04539/2020, UIDP/04539/2020, LA/P/0058/2020, 2022.04018.PTDC and CENTRO01-0145-FEDER-022095. BR was supported by SFRH/BD/131812/2017 and 10.54499/COVID/BD/151947/2021 scholarships funded via FCT. AT received support from a European Society for Neurochemistry (ESN) scholarship.
Author information
Authors and Affiliations
Contributions
Conceptualization, ALC, PP, BR, RAL; Investigation, BR, RAL, MS, AT, MSilva, ASI, MA, RJN, JC, ALCardoso, JP, LPA, PP, ALC; Bioinformatics: BR, MSilva, MA, IM; Statistical analyses: BR, RAL, MS, BO; Writing – Original Draft, ALC, BR, PP; Funding Acquisition, ALC, PP; Supervision, ALC, PP. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All methods were performed in accordance with the relevant guidelines and regulations. All animal procedures were approved by the Institute’s animal welfare commission (ORBEA; permit 311_2022/28012022) and Direção Geral da Alimentação e Veterinária (DGAV, Lisbon, Portugal; permit 0421/000/000/2022).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Rodrigues, B., Leitão, R.A., Santos, M. et al. MiR-186-5p inhibition restores synaptic transmission and neuronal network activity in a model of chronic stress. Mol Psychiatry 30, 1034–1046 (2025). https://doi.org/10.1038/s41380-024-02715-1
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41380-024-02715-1
This article is cited by
-
Opposing roles of microglial and macrophagic C3ar1 signaling in stress-induced synaptic and behavioral changes
Molecular Psychiatry (2025)
-
Unveiling the enigma of anxiety disorders and depression: from pathogenesis to treatment
Science China Life Sciences (2025)