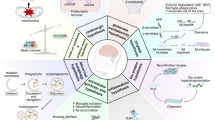
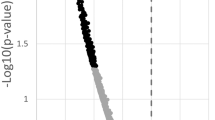
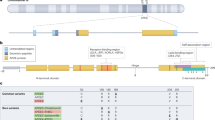
Abstract
Preservation of dendritic spines is a putative mechanism of protection against cognitive impairment despite development of Alzheimer Disease (AD)-related pathologies. Aging, the chief late-onset AD risk factor, is associated with dendritic spine loss in select brain areas. However, no study to our knowledge has observed this effect in precuneus, an area selectively vulnerable to early accumulation of AD-related pathology. We therefore quantified dendritic spine density in precuneus from 98 subjects without evidence of neurocognitive decline, spanning ages 20–96, and found a significant negative correlation between age and large dendritic spine density. In these same subjects, we conducted liquid chromatography–tandem mass spectrometry of >5000 proteins and identified 203 proteins which statistically mediate the effect of age on large dendritic spine density. Using computational pharmacology, we identified ten drugs which are predicted to target these mediators, informing future studies designed to test their effects on age-related dendritic spine loss and cognitive decline.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE [45] partner repository with the dataset identifier PXD057115 and 10.6019/PXD057115. All other data are available from the corresponding author (or other sources, as applicable) on reasonable request.
References
Penzes P, Cahill ME, Jones KA, VanLeeuwen JE, Woolfrey KM. Dendritic spine pathology in neuropsychiatric disorders. Nat Neurosci. 2011;14:285–93.
Dickstein DL, Weaver CM, Luebke JI, Hof PR. Dendritic spine changes associated with normal aging. Neuroscience. 2013;251:21–32.
Walker CK, Herskowitz JH. Dendritic spines: mediators of cognitive resilience in aging and Alzheimer’s disease. Neuroscientist. 2021;27:487–505.
Boros BD, Greathouse KM, Gearing M, Herskowitz JH. Dendritic spine remodeling accompanies Alzheimer’s disease pathology and genetic susceptibility in cognitively normal aging. Neurobiol Aging. 2019;73:92–103.
Boros BD, Greathouse KM, Gentry EG, Curtis KA, Birchall EL, Gearing M, et al. Dendritic spines provide cognitive resilience against Alzheimer’s disease. Ann Neurol. 2017;82:602–14.
Penzes P, Rafalovich I. Regulation of the actin cytoskeleton in dendritic spines. Adv Exp Med Biol. 2012;970:81–95.
Colangeli S, Boccia M, Verde P, Guariglia P, Bianchini F, Piccardi L. Cognitive reserve in healthy aging and Alzheimer’s disease: a meta-analysis of fMRI studies. Am J Alzheimers Dis Other Demen. 2016;31:443–9.
Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–83.
Yokoi T, Watanabe H, Yamaguchi H, Bagarinao E, Masuda M, Imai K, et al. Involvement of the precuneus/posterior cingulate cortex is significant for the development of Alzheimer’s disease: a PET (THK5351, PiB) and resting fMRI Study. Front Aging Neurosci. 2018;10:304.
Aghakhanyan G, Vergallo A, Gennaro M, Mazzarri S, Guidoccio F, Radicchi C, et al. The precuneus - a witness for excessive abeta gathering in Alzheimer’s disease pathology. Neurodegener Dis. 2018;18:302–9.
Sperling RA, Laviolette PS, O’Keefe K, O’Brien J, Rentz DM, Pihlajamaki M, et al. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron. 2009;63:178–88.
Sheline YI, Raichle ME, Snyder AZ, Morris JC, Head D, Wang S, et al. Amyloid plaques disrupt resting state default mode network connectivity in cognitively normal elderly. Biol Psychiatry. 2010;67:584–7.
Koch G, Casula EP, Bonni S, Borghi I, Assogna M, Minei M, et al. Precuneus magnetic stimulation for Alzheimer’s disease: a randomized, sham-controlled trial. Brain. 2022;145:3776–86.
MacDonald ML, Alhassan J, Newman JT, Richard M, Gu H, Kelly RM, et al. Selective loss of smaller spines in schizophrenia. Am J Psychiatry. 2017;174:586–94.
Otsu N. A threshold selection method from gray-level histograms. IEEE Trans Syst Man Cybern. 1979;9:62–66.
McKinney BC, MacDonald ML, Newman JT, Shelton MA, DeGiosio RA, Kelly RM, et al. Density of small dendritic spines and microtubule-associated-protein-2 immunoreactivity in the primary auditory cortex of subjects with schizophrenia. Neuropsychopharmacology. 2019;44:1055–61.
Dumrongprechachan V, Salisbury RB, Soto G, Kumar M, MacDonald ML, Kozorovitskiy Y. Cell-type and subcellular compartment-specific APEX2 proximity labeling reveals activity-dependent nuclear proteome dynamics in the striatum. Nat Commun. 2021;12:4855.
Plubell DL, Wilmarth PA, Zhao Y, Fenton AM, Minnier J, Reddy AP, et al. Extended multiplexing of tandem mass tags (TMT) labeling reveals age and high fat diet specific proteome changes in mouse epididymal adipose tissue. Mol Cell Proteom. 2017;16:873–90.
Krivinko JM, Erickson SL, Ding Y, Sun Z, Penzes P, MacDonald ML, et al. Synaptic proteome compensation and resilience to psychosis in Alzheimer’s disease. Am J Psychiatry. 2018;175:999–1009.
MacDonald ML, Ding Y, Newman J, Hemby S, Penzes P, Lewis DA, et al. Altered glutamate protein co-expression network topology linked to spine loss in the auditory cortex of schizophrenia. Biol Psychiatry. 2015;77:959–68.
Zhang Z. Multivariable fractional polynomial method for regression model. Ann Transl Med. 2016;4:174.
Langfelder P, Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinform. 2008;9:559.
Liao Y, Wang J, Jaehnig EJ, Shi Z, Zhang B. WebGestalt 2019: gene set analysis toolkit with revamped UIs and APIs. Nucleic Acids Res. 2019;47:W199–W205.
Keenan AB, Jenkins SL, Jagodnik KM, Koplev S, He E, Torre D, et al. The library of integrated network-based cellular signatures NIH program: system-level cataloging of human cells response to perturbations. Cell Syst. 2018;6:13–24.
Qi X, Shen M, Fan P, Guo X, Wang T, Feng N, et al. The performance of gene expression signature-guided drug-disease association in different categories of drugs and diseases. Molecules. 2020;25:2776.
O’Donnell C, Nolan MF, van Rossum MC. Dendritic spine dynamics regulate the long-term stability of synaptic plasticity. J Neurosci. 2011;31:16142–56.
Pchitskaya E, Bezprozvanny I. Dendritic spines shape analysis-classification or clusterization? Perspective. Front Synaptic Neurosci. 2020;12:31.
Matsuzaki M, Ellis-Davies GC, Nemoto T, Miyashita Y, Iino M, Kasai H. Dendritic spine geometry is critical for AMPA receptor expression in hippocampal CA1 pyramidal neurons. Nat Neurosci. 2001;4:1086–92.
Hayashi Y, Majewska AK. Dendritic spine geometry: functional implication and regulation. Neuron. 2005;46:529–32.
Spacek J, Harris KM. Three-dimensional organization of smooth endoplasmic reticulum in hippocampal CA1 dendrites and dendritic spines of the immature and mature rat. J Neurosci. 1997;17:190–203.
Hao J, Rapp PR, Janssen WG, Lou W, Lasley BL, Hof PR, et al. Interactive effects of age and estrogen on cognition and pyramidal neurons in monkey prefrontal cortex. Proc Natl Acad Sci USA. 2007;104:11465–70.
Dumitriu D, Hao J, Hara Y, Kaufmann J, Janssen WG, Lou W, et al. Selective changes in thin spine density and morphology in monkey prefrontal cortex correlate with aging-related cognitive impairment. J Neurosci. 2010;30:7507–15.
Young ME, Ohm DT, Dumitriu D, Rapp PR, Morrison JH. Differential effects of aging on dendritic spines in visual cortex and prefrontal cortex of the rhesus monkey. Neuroscience. 2014;274:33–43.
Hara Y, Park CS, Janssen WG, Punsoni M, Rapp PR, Morrison JH. Synaptic characteristics of dentate gyrus axonal boutons and their relationships with aging, menopause, and memory in female rhesus monkeys. J Neurosci. 2011;31:7737–44.
Schikorski T, Stevens CF. Quantitative ultrastructural analysis of hippocampal excitatory synapses. J Neurosci. 1997;17:5858–67.
Schikorski T, Stevens CF. Quantitative fine-structural analysis of olfactory cortical synapses. Proc Natl Acad Sci USA. 1999;96:4107–12.
Ettcheto M, Busquets O, Cano A, Sanchez-Lopez E, Manzine PR, Espinosa-Jimenez T, et al. Pharmacological strategies to improve dendritic spines in Alzheimer’s disease. J Alzheimers Dis. 2021;82:S91–S107.
Pushpakom S, Iorio F, Eyers PA, Escott KJ, Hopper S, Wells A, et al. Drug repurposing: progress, challenges and recommendations. Nat Rev Drug Discov. 2019;18:41–58.
Waring MJ, Arrowsmith J, Leach AR, Leeson PD, Mandrell S, Owen RM, et al. An analysis of the attrition of drug candidates from four major pharmaceutical companies. Nat Rev Drug Discov. 2015;14:475–86.
Krishnamurthy N, Grimshaw AA, Axson SA, Choe SH, Miller JE. Drug repurposing: a systematic review on root causes, barriers and facilitators. BMC Health Serv Res. 2022;22:970.
Bennett DA, Schneider JA, Arvanitakis Z, Kelly JF, Aggarwal NT, Shah RC, et al. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 2006;66:1837–44.
Boyle PA, Yu L, Wilson RS, Leurgans SE, Schneider JA, Bennett DA. Person-specific contribution of neuropathologies to cognitive loss in old age. Ann Neurol. 2018;83:74–83.
Boyle PA, Wang T, Yu L, Wilson RS, Dawe R, Arfanakis K, et al. To what degree is late life cognitive decline driven by age-related neuropathologies? Brain. 2021;144:2166–75.
Lang L, Clifford A, Wei L, Zhang D, Leung D, Augustine G, et al. Prevalence and determinants of undetected dementia in the community: a systematic literature review and a meta-analysis. BMJ Open. 2017;7:e011146.
Perez-Riverol Y, Bai J, Bandla C, Garcia-Seisdedos D, Hewapathirana S, Kamatchinathan S, et al. The PRIDE database resources in 2022: a hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res. 2022;50:D543–D552.
Acknowledgements
Subject tissue was obtained from the University of Pittsburgh Brain Tissue Donation Program and the NIH NeuroBioBank at the University of Pittsburgh Brain Tissue Donation Program.
Author information
Authors and Affiliations
Contributions
All authors reviewed and approved the manuscript. Specific contributions are as follows: JMK—manuscript writing, study conceptualization and design, data analyses and interpretation; PF and LW—study design, computational pharmacology studies and analyses, manuscript writing; ZS and YD—study design, statistical analyses, manuscript writing; CaH—identification of precuneus, proteomics studies and analyses; CH and JN—IHC and microscopy studies, data analyses; JG—data analyses. MDI—study design, identification of precuneus; BCM—study conceptualization and design, postmortem cohort assembly, identification of precuneus; RAS—project conceptualization and design, data analyses and interpretation, manuscript writing; MLM—project conceptualization and design, data analyses and interpretation, manuscript writing.
Corresponding author
Ethics declarations
Competing interests
The University of Pittsburgh holds a Physician-Scientist Institutional Award from the Burroughs Wellcome Fund (JMK). This work was supported in part by NIH grants: AG027224 and MH116046 (RAS), MH125235 and MH118497 (MLM), and P01AG14449 (MDI). RAS, JMK, PF, BCM, LW, and MLM are listed inventors on pending patent 63404994 filed by the University of Pittsburgh Innovation Institute. The methods of treating, preventing, or reducing dendritic spine density loss in a subject by administering one or a combination of the ten drugs listed in Fig. 4, or a pharmaceutically acceptable salt, prodrug, or derivative thereof, are protected by the patent.
Ethics approval and consent to participate
All methods were conducted in accordance with the University of Pittsburgh’s Committee for Oversight in Research Involving Decedents (ID 474) and the International Review Board for Biomedical Research (ID 19080015). Consent was obtained for all subjects from next of kin.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Krivinko, J.M., Fan, P., Sui, Z. et al. Age-related loss of large dendritic spines in the precuneus is statistically mediated by proteins which are predicted targets of existing drugs. Mol Psychiatry 30, 2059–2067 (2025). https://doi.org/10.1038/s41380-024-02817-w
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41380-024-02817-w