Abstract
Exposure to stress during sensitive developmental periods comes with long term consequences for neurobehavioral outcomes and increases vulnerability to psychopathology later in life. While we have advanced our understanding of the mechanisms underlying the programming effects of early-life stress (ES), these are not yet fully understood and often hard to target, making the development of effective interventions challenging. In recent years, we and others have suggested that nutrition might be instrumental in modulating and possibly combatting the ES-induced increased risk to psychopathologies and neurobehavioral impairments. Nutritional strategies are very promising as they might be relatively safe, cheap and easy to implement. Here, we set out to comprehensively review the existing literature on nutritional interventions aimed at counteracting the effects of ES on neurobehavioral outcomes in preclinical and clinical settings. We identified eighty six rodent and ten human studies investigating a nutritional intervention to ameliorate ES-induced impairments. The human evidence to date, is too few and heterogeneous in terms of interventions, thus not allowing hard conclusions, however the preclinical studies, despite their heterogeneity in terms of designs, interventions used, and outcomes measured, showed nutritional interventions to be promising in combatting ES-induced impairments. Furthermore, we discuss the possible mechanisms involved in the beneficial effects of nutrition on the brain after ES, including neuroinflammation, oxidative stress, hypothalamus-pituitary-adrenal axis regulation and the microbiome-gut-brain axis. Lastly, we highlight the critical gaps in our current knowledge and make recommendations for future research to move the field forward.
Similar content being viewed by others
Introduction
Early-life is a period of rapid central nervous system (CNS) development and of unique sensitivity, during which environmental factors, for example stress and nutrition, can profoundly influence brain structure and function long-term [1,2,3]. There is increasing evidence from preclinical and clinical studies that exposure to stress during this sensitive developmental period lastingly affects neurobehavioral outcomes and increases vulnerability to psychopathology later in life [4,5,6,7]. Early-life stress (ES) includes a wide range of exposures, amongst others physical stress (e.g. pain, physical abuse or malnutrition) and emotional stress (e.g. parental neglect, parental separation or emotional abuse). Despite major advances in the field concerning the neurobiological substrates underlying the ES-induced increased risks for adverse neurobehavioral outcomes and psychopathology, the underlying mechanisms remain complex, multi-faceted, not yet completely understood and often hard to target, rendering the development of effective interventions challenging [8, 9].
Given (i) that during fetal and early postnatal life, the brain is a fast growing organ, thus very high in energy and nutrient demand [10,11,12,13]; (ii) the observed similarities in neurocognitive, mental and behavioral outcomes between children exposed to perinatal malnutrition and to ES [14,15,16,17]; and (iii) the converging mechanisms and interplay between the regulation of the stress and food intake, it has been suggested that nutrition is instrumental in mediating and a potential target for combatting the ES-induced (long-term) impairments [8, 9, 18].
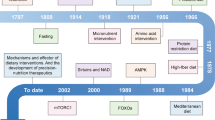
The aim of this paper is to comprehensively review the existing literature on nutritional interventions aimed at targeting the effects of ES on neurobehavioral outcomes in preclinical and clinical settings, discuss the possible mechanisms involved (Fig. 1) and highlight the critical gaps in our current knowledge to move the field forward. Understanding how ES influences brain development and if and how nutrition affects this process is essential for the development of effective nutritional therapies to improve long-term (mental) health in children exposed to ES. Nutritional strategies hold much potential as they are relatively safe, cheap and easy to implement.
Materials and methods
Search strategy
A comprehensive literature search was performed in the database PubMed. The aim of the search was to identify papers on the effect of nutrition/diet on the (long-term) neurobehavioral/cognitive consequences of ES from human and rodent studies. The timeframe within the database(s) was from inception to 29th of November 2024 and the search was conducted by GLB. The search included keywords and free text terms for (synonyms of) ‘diet’ combined with (synonyms of) ‘Early-Life Stress’. A full overview of the search strategy can be found in the Supplementary Information (see Supplementary table 1). No limitations on date or language were applied in the search.
Definitions and inclusion/exclusion criteria
Concerning ES: ES was defined as stress during early-life, from conception up to up to 18 years of age (human studies) or weaning (preclinical studies). ES exposure for human studies included stress exposure in both the (pregnant/lactating) mother or in the child (see Supplementary table 2A): (maternal) perceived stress, (maternal) anxiety, (maternal) depression and other forms of stress (e.g. bereavement, violence, a disaster, low socioeconomic status, hospital admission etc.). For the preclinical studies, papers were included that employed an early-life stressor of a physical or psychological nature (e.g. prenatal restraint, variable stress, maternal separation or the limited nesting and bedding material paradigm, see Supplementary table 2B). Papers that used a nutrition-, metabolism- or inflammation-related stressor were excluded.
Concerning nutritional intervention (i.e. specific nutrients /diets and time window of intervention): Nutrition or diet was defined as the administration, consumption, supplementation or omission of a nutrient, pre-/pro-/synbiotic or diet at any point in time (before or after stressor, and before or after the manifestation of ES-related symptoms) by the infant/offspring or the pregnant/lactating mother/dam. There were no limitations on route of administration (e.g. per os (including oral gavage, tube feeding, intramuscular or intravenous).
During screening, only articles with a functional neurocognitive or behavioral outcome were included. For the preclinical studies outcomes were subdivided in the following domains: depressive-like behavior, anxiety-like behavior, cognition and social behavior. See Supplementary table 3A for outcomes in human studies and Supplementary table 3B for outcomes in preclinical studies. For human studies, all types of studies were included.
Studies addressing the effects of ES as well as nutritional interventions in both males and females were included, however the literature to date mostly fails to test whether the efficacy of the nutritional intervention is sex-specific.
Exclusion criteria were defined as follows:
-
Known nutritional-, metabolism- or inflammation-related stressors
-
Premature birth as a stressor
-
Nutrition only described as a variable in the statistical model of a human cohort study (and not in main research question of study)
-
Intervention with pharmacological extracts/molecules that are not typically nutrient-derived
-
Studies that did not include non-stressed controls
-
Only a structural or mechanistic outcome measure
-
Reviews
Considerations for interpretation of neurobehavioral outcomes in response to ES and nutritional intervention
Throughout this review we will refrain from assigning positive or negative connotations concerning to neurobehavioral consequences of ES and nutritional interventions as these can be adaptive or maladaptive contingent on the specifics of environment and setting where these are expressed [19, 20]. In addition, it is important to acknowledge that the interpretations of the behavioral tests used in preclinical research aimed to test specific traits and phenotypes of complex and multifaceted conditions like depression, anxiety are often debated and poses some challenges. For example, the FST and TST, commonly used to test depression-like behavior, measure immobility, often interpreted as “behavioral despair”. However, there is substantial debate about whether immobility truly reflects a depressive state or rather an adaptive coping strategy such as energy conservation [21, 22]. In this review, we have relied on the interpretations provided by the original authors of the referenced studies while synthesizing the results, however, we acknowledge the limitations of these paradigms and the need for caution in their interpretation.
Data collection
Figure 2 shows the flowchart of the review process according to the PRISMA-statement. Through database searching in PubMed 3446 records were identified. After removal of duplicates, 3443 records were screened for title and abstract. All studies were written in English. One-hundred-and-nine of these articles were eligible for full text screening, out of which 96 articles were included in the current review (Table 1). Snowball searching did not result in new inclusions.
Results
Evidence of effect of nutritional interventions on ES-induced behavioral deficits
Of the 96 included articles (For an overview of all articles, see Table 1 and Supplementary Table. 4), ten studies included human subjects, while the other 86 studies were performed in rodents. Below, for the preclinical studies, we will first describe the effect of ES on behavioral outcomes across the various domains and thereafter describe the effect of nutrition on the ES-induced alterations divided by nutrient group. In Supplementary table 4B, we calculate the percentage of effective studies per nutrient group to get a general idea of effectiveness of the specific nutrient on ES-induced outcomes.
ES affects behavior in rodent studies
Within the 86 preclinical studies that were included in the review, the effect of ES on a specific behavioral domain was investigated 150 times, as several studies investigated the effect of ES on multiple behavioral domains. Overall, the ES paradigms affected behavior in 82.00% of the cases, this was consistent across the behavioral domains assessed (depressive-like behavior 82.35%, anxiety-like behavior 78.57%, cognitive deficits 85.00% and social deficits 80.00% and other ES-induced behaviors 90.00%). This percentage reflects the expected effect of ES on rodent behavior as described in previous reviews [23]. See Supplementary table 4A for details. Some studies reported no effects of ES on any of the measured cognitive domains [24,25,26,27,28,29].
The effect of nutritional interventions on ES-induced behavioral deficits
For preclinical studies, the effect of nutrition will only be discussed for studies where an effect of ES was found on any of the behavioral domains studied as without an ES effect, the research question could not be answered. Below, the effect of a nutritional component/diet on ES-induced outcomes will be discussed per nutrient (i.e. fatty acids (FA), polyphenols, pre-and pro-biotics, micronutrients, combination preparates, diet/nutritional programs, other nutritional interventions) first in the human studies, followed by the preclinical studies. For an overview of the effects of nutritional effects on ES-induced behavioral deficits, see Supplementary table 4B, visualized in Fig. 3.
Fatty acids
Fatty acids are important macronutrients and have multiple critical functions in the (developing) brain and body [30, 31]. There are three different classes of FA (saturated FAs (SFAs), monounsaturated FAs (MUFAs) and polyunsaturated FAs (PUFAs)) [32]. PUFAs are considered essential nutrients as they cannot be produced by the body itself and can only be ingested through the diet. The most important PUFAs are the omega-6 (N-6) (linoleic acid, LA) and omega-3 (N-3) PUFAs (e.g. α-linolenic acid (ALA), docosahexaenoic acid (DHA), eicosapentaenoic acid (EPA), docosapentaenoic acid (DPA) and eicosatetraenoic acid (ETA)) [33]. Rather than individual concentrations, the proportion of N-3 to N-6 PUFAs is key for the effects on health, where a lower concentration of N-6 PUFAs and a higher concentration of N-3 PUFAs is considered healthy [34]. N-3 PUFAs are well known for their critical role in development, structure and function of the brain [35] and play a critical role in supporting the healthy regulation of cellular inflammation [36]. Most of the nutritional strategies described below were with PUFAs as well as one intervention with sodium butyrate, a short chain (saturated) fatty acid. Sodium butyrate can be produced in the gut and is well known for shaping the microbiome [37] and as an important metabolite for gut-brain-axis signaling [37, 38].
Human studies
Two studies investigated the effects of PUFA supplementation/intake on child development after ES (low socioeconomic status [39] and maternal prenatal negative life events [40] respectively). More specifically, in a randomized controlled trial in pregnant women (n = 64) with a low socioeconomic status, prenatal supplementation of PUFAs (DHA (450 mg), DPA (40 mg), ETA (40 mg) and EPA (90 mg) and Vitamin E (10 mg) for six weeks had no effect on child behavioral and cognitive development at three months of age as measured by the Bayley Scales of Infant Development (BSID-III) [39]. In an observational study (n = 255) the association between maternal negative life events (NLE), infant temperament (Infant Behavior Questionnaire Revised (IBQ-r)) and PUFA intake (habitual intake ratio of N-3 and N-6 PUFAs as measured by a food frequency questionnaire) in black, white and Hispanic women was addressed. At 6 month of age there was a negative association between NLEs and Orienting and Regulation in black women only, which was attenuated by higher maternal N-3/N-6 PUFA intake ratio [40].
Rodent studies
Twelve preclinical studies investigated the effect of FA supplementation on the later-life consequences of ES, out of which seven studies found an effect of ES and will be described below.
Depressive-like behavior - In male rats, supplementation with N-3 PUFAs (postnatal day (P)41-61) [41] or sodium butyrate (P60-67) [42] mitigated the postnatal stress (POS)-induced depressive-like behavior in the forced swim test (FST) at two months of age [41] and at P67 [42]. In male mice, supplementation with EPA (P22-49) alleviated the effect of POS-induced depressive-like behavior in the FST, the sucrose preference test (SPT) and the tail-suspension test (TST) at P49 [43].
Anxiety-like behavior – In mice, supplementing the diet with a high N-6/N-3 PUFA ratio (from breeding until P14) led to increased prenatal stress (PRS)-induced anxiety-like behavior in the elevated plus maze (EPM) at two months of age [44].
Cognitive impairments – In rats, supplementation with DHA (for two weeks before breeding) ameliorated the PRS-induced cognitive impairments in the Morris water maze (MWM) at P30 [45]. In male mice, increasing the availability of N-3 PUFAs (P2-42) restored the POS-induced cognitive impairments in the novel object location test (OLT) [14, 46] novel object recognition test (ORT) [14] and MWM [14] at four months of age.
Other outcomes – In rats, supplementation with proprionic acid (at P40, P43 and P74) ameliorated the POS-induced changes in the pre-pulse inhibition test at P40, P43 and P74 [47].
In conclusion, clinical evidence on FA supplementation on behavioral outcomes after ES is scarce. However, the two studies included state that prenatal N-3 PUFA supplementation does not seem to modulate ES-induced behavioral problems, however it can be speculated that effects are different in mother-infant dyads from different ethical backgrounds. In rodents, N-3 PUFA supplementation ameliorated ES-induced depressive-like behavior and cognitive impairments. An excess of N-6 PUFAs aggravated the ES-induced anxiety-like behavior while deficiency in N-3 PUFAs did not further worsen the ES-induced depressive-like behavior.
Polyphenols
Polyphenols are naturally occurring plant metabolites available for consumption in many fruits, vegetables, coffee, tea and wine. They have shown to have a wide range of potential health benefits [48]. Chemically, polyphenols are phenolic compounds classified based on their structure and substituents [49]. The mechanisms underpinning their effects on brain functioning are not fully understood, but the general consensus attributes their benefit to antioxidant capabilities [50]. More than 8000 polyphenolic compounds have been identified in various plant species. Polyphenols may be classified into different groups as a function of the number of phenol rings that they contain and on the basis of structural elements that bind these rings [51]. Polyphenols used in the included studies belong to the main polyphenol classes of the phenolic acids (ferulic acid), flavonoids (proanthocyanidins, xanthohumol, quercetin, kolaviron, catechins), tyrosols (hydroxytyrosol), coumarins (auraptene), tannins (phlorotannins) and the stilbenes (resveratrol).
Human studies
Only one observational study investigated the effect of polyphenol supplementation on ES-induced behavior. In a longitudinal cohort study (n = 6404) the relationship between adverse childhood events (ACEs), flavonoid intake and depressive symptoms in adulthood was investigated. A higher habitual flavonoid intake as measured by a food frequency questionnaire buffered the association between perceived stress and depressive symptoms after ACEs. Depressive symptoms were lower for those that consumed more flavonoids [52].
Rodent studies
Thirteen preclinical studies investigated the effect of polyphenol supplementation on the later-life consequences of ES. All thirteen studies found an effect of ES on at least one behavioral domain and will be described below.
Depressive-like behavior – In rats, supplementation with proanthocyanidins to females (P21-P30) [53] or ferulic acid to males (P60-P88) [54], ameliorated PRS-induced depressive-like behavior as measured in the FST and SPT at one month and at three months of age respectively [53, 54]. Supplementation with resveratrol (P51-62) [55] and rosmarinic acid (P35-55) [56] improved POS-induced depressive-like behavior measured in rats in the FST and SPT at P62-65 and P39-42 respectively.
Moreover, in rats, supplementation with either phlorotannins, xanthohumol or quercetin (Week (W) 8-16), reversed POS-induced depressive like behavior in the FST at W12-13 [57].
Anxiety-like behavior – In rats, supplementation with ferulic (P60-88) [54] or quercetin (Gestational day (G)14-19) [58] improved PRS-induced anxiety-like behavior in males in the OFT at P89-95 [54] and in males, but not in females in the light-dark box (LDB) and novelty suppressed feeding (NSF) at P35-45 [58]. In rats, supplementation with either phlorotannins, xanthohumol and quercetin (W8-16) [57], kolaviron (P21-35) [59], resveratrol (P51-62) [55] or quercetin (P21-42) [60] dampened the effects of POS-induced anxiety-like behavior as measured in the open field test (OFT) at W12-13 [57], at P35 [59], at P62-65 [55], and at P39-42 [60] and in the LDB at P62-65 [55]. However, in male rats, supplementation with catechins (P21-80) did not modulate the POS-induced anxiety- like behavior in the OFT, EPM and hot plate test (HPT) at P81-90 [61]. In mice, supplementation with auraptene (P45-60) and umbelliprennin in males (for 7 days between P51-60) [62] prevented POS-induced anxiety-like behavior in OFT and EPM at two months of age [63] and in the EPM immediately after treatment [62].
Cognitive impairments – In rats, supplementation with hydroxytyrosol to males (2 weeks before breeding [64]), resveratrol (G1-P1) [65] or quercetin (G14-19) [58] ameliorated the PRS-induced cognitive impairments in MWM and the T-maze (TM) at one month [64, 65] and only in females in the ORT [58]. In rats, supplementation with catechins to males (P21-80) [61] or kolaviron (P21-35) [59] alleviated the POS-induced cognitive impairments in the HPT and ORT at P81-90 [61] and the MWM and Y-maze (YM) at a not specified age [59]. In male mice, umbelliprenin supplementation for 7 days between P51-60 alleviated POS-induced cognitive impairments in the shuttleboxtest (SBT) immediately after treatment [62].
Social impairment – In male mice and rats, umbelliprenin (for 7 days between P51-60) [62] and quercetin (P21-42) [60] supplementation lead to reductions in social impairments in POS-exposed offspring immediately after treatment in social approach (SA) [62] and at P39-42 in the social interaction (SI) test [60].
Other outcomes – In male rats, supplementing with resveratrol (P51-62) [55] led to less aggressive behavior in POS-offspring in the resident intruder test immediately at P62-65. In male mice, supplementing umbelliprenin (for 7 days between P51-60) to POS-offspring lead to less repetitive behavior immediately after treatment [62].
In conclusion, there is not enough data to suggest a modulating role of polyphenol supplementation after ES in humans. However, in rodents, in 95.83% of the cases, supplementation with a large variety of polyphenols during and after PRS and POS mostly ameliorates ES-induced changes in behavior across the various domains. In addition, there seems to be evidence for sex-specific responses to polyphenols in the context of ES.
Pre-, pro- and synbiotics
The gut microbiome comprises of the trillions of bacteria residing in the gut, metabolizing components of the food ingested by the host and providing essential gastrointestinal ecosystem services. In the last decades both preclinical and clinical research has also pointed towards microbial regulation of brain function and behavior [66] and is incorporated as a key node within the framework of the gut-brain axis. Key initial studies showed that ES induces changes in gut microbiome composition later in life [67]. Furthermore, the seminal study by de Palma and colleagues showed that an intact microbiota is necessary to induce some effects of ES [68]. Germ-free mice (i.e. animals without a gut microbiome) exposed to maternal separation do not show changes in anxiety-like and depression-like behavior [68]. Hence, the microbiota can be a promising new target to treat the consequences of ES. The most common dietary interventions directly targeting the gut microbiome are pre- and probiotics. Prebiotics are substrates selectively utilized by host microorganisms conferring a health benefit. These can be digestible fibers that act as nutrients for the beneficial bacteria in the gut and their degradation products are short-chain fatty acids (e.g. butyrate) that are released into the circulation, affecting overall health. Fructo-oligosaccharides and galacto-oligosaccharides are the two main groups of prebiotics studied for beneficial effects on health. Probiotics are live microorganisms that, when administered in adequate amounts, confer a health benefit on the host. Modulation of the gut microbiome exerts health benefits via various routes including microbial metabolites [69], immune system [70], neuroendocrine system [71], the enteric nervous system, and the vagus nerve [72]. Probiotics exert their effects usually in the gastrointestinal tract, where they may influence the intestinal microbiota and exert health effects by nonspecific, species-specific, and strain-specific mechanisms [73]. The probiotics that are most frequently used for beneficial health effects are strains from the Lactobacillus and Bifidobacterium genera, which are also most frequently used in the included studies as described below.
Human studies
One clinical study investigated the effect of pro- and prebiotic supplementation on ES-induced behavior in the offspring. A randomized, double-blind, controlled trial was carried out in 190 healthy mothers divided in 2 groups (one taking a supplement containing Limosilactobacillus reuteri PBS072 and Bifidobacterium breve BB077 and a control group). Symptoms related to maternal depression were evaluated at day 45 and 90. At both timepoints, the score obtained from the Edinburgh Postnatal Depression Scale questionnaire was lower in the supplemented group. This led to less crying and fussing events during the treatment in the offspring [74].
Rodent studies
Eighteen preclinical studies investigated the effects of pre- and/or probiotic supplementation on ES-induced behavioral deficits. Out of these eighteen studies, seventeen found an effect of ES on at least one of the behavioral domains and will be discussed below.
Depressive-like behavior – In rats, supplementation with L. Paracasei (P2-16) [75], with B. Infantis to males (P50-P95) [76], or B. infantis to both sexes [77] restored the POS-induced depressive like behavior in the TST at 1 month of age [75], and in the FST at P21 (in females), 41(in males) and 61 (in females) [77] at 3 months of age [76]. In mice, prenatal supplementation with B. breve CCFM1025 (G0-delivery) [78] or postnatal supplementation with L. Plantarum (P29-57) [79], L. Paracasei (P28-56) [80], prevented the POS-induced depressive-like behaviors in FST and TST at P57-63 [79] and in the FST at P49 [78] and P54-56 [80].
Anxiety-like behavior – In rats, lifetime supplementation with B. Trisporus [81] or with a mixture of L. Acidophilus, L. Fermentum and B. Lactis (G1-G14 or P31-45) [82] ameliorated PRS-induced anxiety-like behavior in the EPM at P45-46 [82] and at P60-70 [81]. In male rats, supplementation with L. Paracasei (P2-16) [75], a mix of L. Helveticus, B. Longum, L. Lactis and S. Thermophilus (W6-15) [83], a mix of Polydextrose + Galactooligosaccharide and/or L. Rhamnosus (P21-49) [84] or a mix of L. Rhamnosus and L. Helveticus (P2-14) [85], ameliorated the POS-induced anxiety-like behavior in the OFT 2 weeks after supplementation [75] or in the OFT [83, 84], EPM [85] and LDB [85] right after the supplementation period. However, in male rats supplementation with a mix of L. Helveticus, B. Longum, L. Lactis and S. Thermophilus (W6-15) [83], L. Rhamnosus and L. Helveticus (P2-14) [86] or a mix of L. Rhamnosus and L. Helveticus (P2-14) [85], reduced the POS-induced anxiety like behavior in the EPM [83, 86], LDB [83], novelty seeking (NS) [83] an OFT [85] right after the supplementation period. In male mice, supplementation with B. pseudocatenulatum (P2-21) and L. reuteri (P21-P63) ameliorated the POS-induced anxiety-like behavior in the EPM at P42 [87] at P63-P70 respectively and in the OFT at P63-P70 [88]. However, supplementation with L. Paracasei (P28-56) [80] or L. Plantarum (P29-57) [79] did not affect the POS-induced anxiety-like behavior in the EPM at P54-56 [80] and at P57-63 [79].
Cognitive impairments – In rats, lifetime supplementation with B. Trisporus [81] or supplementation with a mixture of L. Acidophilus, L. Fermentum and B. Lactis (G1-G14 or P31-45) [82] ameliorated PRS-induced cognitive impairments in the ORT and Barnes maze (BM) at P60-70 [81], and the MWM at P45-46 [82]. In male rats, supplementation with L. Rhamnosus and L. Helveticus (P2-14) [85, 86, 89], Polydextrose + Galactooligosaccharide (P21-W13/W14) [90], or a mix of Polydextrose + Galactooligosaccharide and L. Rhamnosus (P21-49) [84], ameliorated the POS-induced cognitive impairments in fear conditioning (FC) at P17-24 [85, 86, 89] and the MWM at W7-11 [84] and at W7-13 but not the ORT at W7-13 [90].
Social impairment – In male mice, supplementation with L. Reuteri (P21-P63) reversed the POS-induced social impairment in SA at P63-P70 [88].
Other outcomes – In male POS-rat offspring, prebiotic Polydextrose + Galactooligosaccharide (P21-W13) did not affect pain behavior at 7 to 13 weeks of age [90]. In mice, supplementation with L. Reuteri (P5-14) normalized POS-induced alterations in calling behavior [91].
In conclusion, supplementation with both pre- and/or pro- and synbiotics alleviates ES-induced depressive-like behavior and cognitive impairments in 100% of the cases. Regarding the domain of anxiety-like behavior, in rats subjected to PRS, pre- and/or probiotic supplementation demonstrates anxiolytic effects. However, results were conflicting in rats exposed to POS. This variability in results can be attributed to differences in the timing of pre- and/or probiotic supplementation relative to the stressor or the timing of outcome measurements. In mice, it is noteworthy that supplementation during the stress period modulated the POS-induced alterations in behavior, whereas supplementation after the stress period did not. Interestingly, pre- and/or probiotic supplementation during POS ameliorates the ES-induced deficits in cognition, however, supplementation after POS required supplementation with both pre- and probiotics, as a synbiotic.
Micronutrients
Micronutrients are essential dietary elements, required to orchestrate a range of physiological functions important for development and maintenance of a healthy brain [92]. Micronutrients can be subdivided in different categories, such as vitamins and minerals. Therefore, below we will describe the effect of supplementation with amino acids, vitamins, minerals, carotenoids and other micronutrients on ES-induced behavioral deficits.
Amino Acids
Amino acids are important micronutrients and are the building blocks for proteins and critical for almost all body processes and especially for neurodevelopment [93]. In their free form, some amino acids also work as signaling molecules and some, such as tryptophan, are important precursors for the synthesis of neuroactives [93, 94]. Most amino acids, including cysteine and tyrosine, can be synthesized by the body under normal circumstances. However, nine amino acids, are not synthesized (e.g. methionine and tryptophan) or synthesized in too low concentrations (n-acetylcysteine (NAC)) by mammals and are therefore dietary essential nutrients [93]. Interestingly, the studies that we will describe next have only supplemented NAC in the contex of ES, but no other amino acids have been studied yet in this context.
Rodent studies: Three preclinical studies investigated the effects of amino acid supplementation on ES-induced behavioral deficits. All studies found an effect of ES on at least one of the behavioral domains and will be discussed below.
Depressive-like behavior – In male rats, supplementation with NAC (P41 to P61), ameliorated the POS-induced depressive like behavior in the FST at two months of age [41]. In male mice, NAC supplementation (5 weeks pre-mating until G16) did not have an effect on PRS-induced depressive-like behavior at P33-50 in the FST [95].
Anxiety-like behavior - In mice, supplementation with NAC from G12- P1 [96] or pre-mating until G16 [95] did not have an effect on PRS-induced anxiety-like behavior in the EPM at W10-14 [96] or in the OFT and EPM at P33-50 [95].
Social impairment – In mice, supplementation with NAC (G12-P1) did not ameliorate the PRS-induced social impairments in SA at two months of age [96].
Vitamins
Vitamins are essential micronutrients that are required in small quantities for a variety of physiological functions in the body, including for those in the brain [12, 92]. There are several different vitamins that are important for the brain, each of them playing unique roles in for example antioxidative mechanisms and the production of neurotransmitters [97, 98]. The studies below describe supplementation with several different vitamins. For example, folic acid is important for functioning of the nervous system at all ages and is well known for its relation with neural tube defects [99]. Vitamin A is essential for the developing CNS where it affects neurogenesis and neuronal patterning, but keeps playing an important role in the adult brain by regulating neuroplasticity in cerebral structures [100]. Vitamin C and E are, amongst having other functions, vital antioxidant molecules in the brain studies [101, 102].
Human studies: One clinical study investigated the effect of vitamin supplementation/intake on ES-induced outcomes. An observational cohort study (n = 137) found that maternal prenatal stress, measured as NLEs during pregnancy, was associated with higher scores in the infant temperament domain of negative affectivity (Early Childhood Behavior Questionnaire-Very Short form). A trend towards mitigation of this relationship by a higher maternal habitual intake of vitamin A and C, but not E, was found [103].
Rodent studies: Four preclinical studies investigated the effect of vitamin supplementation on ES-induced behavioral deficits. All studies found an effect of ES on at least one of the behavioral domains and will be discussed below.
Depressive-like behavior – In male rodents, supplementation with vitamin E (P31-45) [104] or folic acid (P41-61) [41] reversed the POS-induced depressive-like behavior measured in the FST at P60—61 [41] and at P45-47 [104], but not in the splash test (ST) at P45-47 [104].
Anxiety-like behavior – In male rats, supplementation with leucine (P20-60), did not reduce POS-induced anxiety-like behavior in the OFT at P60 [105]. In male mice, supplementations with vitamin E (P31-45) protected against POS-induced anxiety-like behavior in the EPM at P45-47 [104].
Cognitive impairments – In male POS-offspring, both vitamin PP (P56-85) [106] and Leucine (P20-60) [105] led to improvement in ES-induced cognitive impairments in the ORT and the BM at an unknown age [106] and in the MWM at 2 months of age [105]. Other outcomes - In male POS-offspring, vitamin PP (P56-85) reversed stress-induced changes in pre-pulse inhibition in adulthood [106].
Minerals
Minerals are micronutrients and inorganic substances that cannot be synthesized by organisms, but are ingested through the diet, including for example magnesium, calcium, zinc and selenium. They play key roles in oxygen transport, the synthesis of neurotransmitters and signaling between neurons. In addition, they can have antioxidant activities.
Human study: One clinical study was identified that investigated the effect of mineral supplementation on ES-induced outcomes. An observational cohort study (n = 137) found that maternal prenatal stress, measured as negative life events during pregnancy, was associated with higher scores in the infant temperament domain of negative affectivity (Early Childhood Behavior Questionnaire-Very Short form). This association was mitigated by a higher maternal habitual intake of zinc and selenium, but not magnesium [103].
Rodent studies: One rodent study was included that investigated the effect of mineral supplementation on ES-induced behavioral outcomes.
In female rat offspring of dams that have experienced PRS, zinc supplementation (G0-19) lead to reductions in stress-induced depressive like behavior measured in the FST and stress-induced anxiety-like behavior as measured in the OFT and EPM at P25-27 [107].
Carotenoids
Carotenoids are a group of micronutrients and are organic pigments naturally found in plants, algae and bacteria. Carotenoids have powerful antioxidant capacities and anti-inflammatory functions. In addition, they seem to assist the preservation of cognitive function, independent of ES [108].
Human study: One clinical study was identified that investigated the effect of carotenoid supplementation on ES-induced outcomes. An observational cohort study (n = 137) found that maternal prenatal stress, measured as negative life events during pregnancy, was associated with higher scores in the infant temperament domain of negative affectivity (Early Childhood Behavior Questionnaire-Very Short form). This association was not affected by a higher maternal habitual intake of beta-carotene [103].
Rodent study: Two rodent studies were identified that investigated the effect of carotenoid supplementation on ES-induced behavioral deficits. Both studies reported stress-induced behavioral effects.
Anxiety-like behavior - In mice, supplementation with astaxanthin (G12-P1) had no effect on the PRS-induced anxiety-like behavior as measured in the OFT and the EPM at W10-14 [96], but supplementation with lutein (late gestation-W9) led to a reduction in stress-induced anxiety-like behavior in the EPM at 9 weeks of age [109].
Other micronutrients
The studies below describe supplementation with several micronutrients that do not officially fall in the categories of the amino acids, vitamins, minerals or carotenoids. For example, choline and carnitine are both quaternary ammonium compounds and both important for brain development [110, 111]. Where choline mostly is important for membrane structure and the production of acetylcholine and thus neurotransmission [110], carnitine is critical in fatty acid oxidation and therefore energy production [111]. Another study will describe supplementation with taurine, a derivative of the amino acid cysteine, which has been linked to development of the CNS and the immune system [112,113,114].
Rodent studies: Six preclinical studies were identified that investigated the effect of supplementation with these ‘other’ micronutrients on ES-induced behavioral deficits, out of which five found an effect of the nutrients on ES-induced behavioral deficits on at least one of the behavioral domains investigated and will be described below.
Depressive-like behavior - In male mice, supplementation with acetyl-L-carnitine (P21-56 or P49-56) reduced the PRS-induced depressive like behavior in the FST at W8-13, the effect only lasted for a week [115].
Anxiety-like behavior – In rats, supplementation with choline (G0-P21) ameliorated the PRS-induced anxiety-like behavior in the EPM in females at P79-106, but not in the OFT [116].
Cognitive impairment – In rats, supplementation with a high, but not a low, dose of taurine, (P21-30), ameliorated the PRS-induced cognitive deficits in the MWM at P31 [117]. In male rats, supplementation with choline (P21-60) [118] or choline chloride (P1-14 or P15-28) [119] reversed the POS-induced cognitive impairments in the ORT and OLT at P90 [118] and in the avoidance learning task (ALT) at P80 and P180 [119].
Social impairment – In rats, choline supplementation (G0-P21) ameliorated the PRS–induced social impairments in the SI test in males only at P79-106 [116].
Overall, the micronutrient categories, vitamin (85.71%) and mineral supplementation (100% - 1 study) seem to ameliorate the ES-induced behavioral deficits. While in both humans and rodents, carotenoids (33%) and most amino acids (25%) did not seem convincing in mitigating ES-induced deficits. Supplementation with nutrients from the ‘other micronutrients’ category (carnitine, choline and taurine) did reverse the ES-induced behavioral deficits (100%). However, due to the small number of studies, the different nutrients used and heterogeneity in study designs, it is impossible to draw any conclusions.
Combination Preparations
Several studies investigated the effects of the mix of multiple nutrients rather than on individual nutrient groups on the outcomes after ES. These combination preparations differ from containing a combination of two different nutrients to a combination of seven different nutrients.
Rodent studies
Eight preclinical studies investigated the effect of supplementation with a combination of different nutrients on ES-induced behavioral deficits, out of which six studies found an effect of ES on at least one of the behavioral domains investigated and those will be described below.
Depressive-like behavior - In male rats, supplementation with fish oil (G0-P21) exacerbated the PRS-induced decrease in depressive-like behavior in FST at three months of age [120]. In addition, supplementation with a combination of folic acid, vitamin B12, betaine and choline (G14-P21) improved the PRS-induced depressive-like behavior in males in the FST between one and two months of age [121]. Supplementation with a combination of fish oil containing FAs and vitamins and minerals to males (W8-16) had no effect on POS-induced depressive-like behavior in the FST at W10-16 [122], while in females supplemented with choline, betaine, folic acid and vitamin B12 (P60-P186) the POS-induced depressive-like behavior was ameliorated in the FST at P165-186 [123].
Anxiety-like behavior – In male rats, supplementation with a combination of fish oil containing FAs and vitamins and minerals (W8-16) improved the POS-induced anxiety-like behavior in the EPM and the OFT at W10-16 [122].
Cognitive impairments – In rats, supplementation with folic acid, vitamin B12, betaine and choline (G14-P21) reduced the PRS-induced cognitive impairments in the MWM and in the ORT, only in aged females, at month (M)19-20 [121]. In male rats submitted to POS, supplementation with milk fat globule membrane (MFGM) combined with polydextrose and galacto-oligosaccharides (weaning-W13/14) resulted in improvement in the MWM, but not the ORT at W7-13 [90]. A different study submitting male rats to POS, a mix of EPA, DHA and vitamin A (P25-P76) reduced the cognitive impairments in adolescence (P46-51) and adulthood (P70-P76) as measured in FC and ORT [124]. In female rats, supplementation with choline, betaine, folic acid and vitamin B12 (P60-186) had no effect on the POS-induced cognitive impairments as measured in the ORT at P165-186 [123]. In male mice, supplementation with folic acid, vitamin B6 + B12, choline, methionine and zinc (P2-P9) reversed POS-induced cognitive deficits in the MWM and the ORT, but not the OLT at four months of age [15].
Other – In rats, supplementation with a combination of the fatty acids LA and ALA and probiotic B. Breve (P28-77) had no effect on POS-induced changes in pain behavior at P77 [125], but MFGM combined with polydextrose and galacto-oligosaccharides (P21-W13/14) decreased pain behavior at W7-13 [90].
Although some of the above described combination preparations seemed to be effective in the context of reducing ES-induced impairments, due to the variety in nutrients, stressors and supplementation duration/period, no definite conclusions can be drawn.
Diets/Nutritional Programs
Several studies investigated the effect of a complete diet, for example a high-fat diet, or the implementation of a nutritional program (human studies) on the outcomes after ES. In these diets/programs, the exact nutrient intakes are not specified, but a certain diet is provided over a given time period.
Human studies
Five human studies investigated the effect of specific diets/nutritional programs on ES-induced outcomes.
A community-based intervention trial (n = 240) showed that an integrated nutrition rehabilitation intervention (supplementation of the diet with shredded liver, fish and anchovy twice weekly for 6 months) had benefits on the socioemotional development (BSID-III) in ≥24-month-old Earthquake survivors. There were no effects of the intervention on the other BSID-III outcome domains [126].
Moreover, a large longitudinal cohort (n = 6979) provided evidence that maternal depression symptoms during pregnancy were associated with both more unhealthy and less healthy diets as measured by a food frequency questionnaire during pregnancy and postpartum. This was in turn prospectively associated with reduced child cognitive function at eight years of age. This suggests that maternal depression symptoms in pregnancy can affect child development via a less healthy nutritional environment [127]. A longitudinal cohort study (n = 1503), including women-infant dyads with low socioeconomic status, found no positive effect of The Special Supplemental Nutrition Program for Women, Infants, and Children (a program in which nutritional education and healthy supplemental foods are provided [128]) on child competence or problem behaviors between 12 and 24-months of age as measured by the Brief Infant Toddler Social Emotional Assessment (BITSEA) [129]. Lastly, a longitudinal cohort study (n = 6404) showed that plant-based dietary intake frequency measured by a questionnaire, was related to a reduction in the association of more than four ACEs with later-life mental health outcomes at any age [130]. Finally, a prospective cohort study including 7438 mother-child pairs, investigated whether a maternal anti-inflammatory diet reduced the risk of prenatal environmental adversity (PEA)-induced neurodevelopmental delay. Diets with a low inflammatory score were protective for an increased risk of PEA-related neurodevelopmental delay [131].
Rodent studies
Thirteen preclinical studies investigated the effect of supplementation with a complete diet on ES-induced behavioral deficits, out of which twelve studies found an effect of the diet on at least one of the behavioral domains investigated and those will be described below.
Depressive-like behavior – In rats, supplementation with a high fat diet (HFD)(G14-P21) aggravated the PRS-induced depressive-like behavior in old males in the FST at M19-20 [121]. Supplementation with an olive oil rich diet (G1-P21) [132], a highly palatable food diet in females (P28-65) [133], a highly palatable food diet in both sexes (P20-84) [134] or a HFD in males (P20-84) [135] reversed the POS-induced depressive-like behavior in the FST in males at P80-87 [132] and at W10-12 [134] and in females at P54-59 [133], and in the SPT in males at P34-84 [135]. In contrast, supplementation with a highly palatable food diet in males (P22-59) [136] and an olive oil rich diet (G1-P21) [132] did not have an effect on POS-induced depressive like behavior as measured in the FST at P54-59 [136] and in the SPT at P80-87 [132].
Anxiety-like behavior – In rats, supplementation with HFD (G0-P21 [137] and P20-84 [134], a high-fat-high-sugar diet (P21-91) [138], palatable diet (P21-P60) [139] or highly palatable food diet (P28-56 [133] and P22-59 [136] and P20-84 [135]) reversed the POS-induced anxiety-like behavior as measured in the OFT at M4-7 [137] and P54-59 [133, 136], in the EPM between W10-12 [134, 138], P60-P67 [139] and at P54-59 [133] and in the LDB at W10-12 [134] and at P34-84 in females [135]. Supplementation with highly palatable food diet (P22-59) did not have an effect on POS-induced anxiety like behavior in the EPM at P54-59 [136].
In male mice, supplementation with Western-pattern diet (G14-weaning and G14-P80/83) after PRS improved anxiety-like behavior as measured in the OFT, but in females only the time window of G14-P80/83 accomplished this effect [140].
Cognitive impairments – In rats, a HFD (G14-P21) did not have an effect on the PRS-induced cognitive impairments as measured in the MWM at M19-20 and the ORT at both M1-2 and M19-20 [121]. In male rats, a HFD (G0-P21) reduced POS-induced cognitive impairments as measured in the MWM at M4-7 [137] and the conditioned odor preference (COP) at M6 [141].
Social behavior – In male rats, a high-fat-diet (G0-P21) or a palatable diet (P21-P75/76) reduced POS-induced social impairments as measured in SI at M4-7 [137], at P61-P64 [142] and at P30-P37 and P60-P67 [139].
In conclusion, a HFD and a highly palatable diet seemed to be effective in reducing ES-induced depressive and anxiety like behaviors. However, for the cognitive domain there is too little evidence that points in the same direction to draw firm conclusions.
Other nutritional interventions
Several studies researched supplementation interventions with nutrients that do not fall into the categories as described above. Most of these are herb-, plant- or berry-derived and sometimes used in alternative medicine, for example bacopa monnieri, acacia gum, acai seed extract and wolfberry preparation, but also for example the alkaloid trigonelline.
Rodent studies
Fourteen preclinical studies investigated the effect of supplementation with one of these ‘other’ nutrients on ES-induced behavioral deficits. All of these studies found an effect of the diet on at least one of the behavioral domains investigated and will be described below.
Depressive-like behavior – In male rats, supplementation with Euterpe oleracea Mart. (açaí) seed extract (P76-110) ameliorated the POS-induced depressive like behavior in the FST at P106-108 [143]. In female rats, supplementation with spirulina platensis (P41-P55) reversed the POS-induced depressive-like behavior in the FST at P60-P70 [144]. In male mice, supplementation with Trigonelline (P31-45/47) ameliorated the POS-induced depressive like behavior in the FST and the ST at P45-47 [104].
Anxiety-like behavior – In male rats, supplementation with Bacopa monnieri and acacia gum (G10-P23 and P15-30) [145] or Bacopa monnieri and acacia gum or L-carnosine (G10-P23) [146] or herbal medicine (G1-P0) [147] ameliorated the PRS-induced anxiety-like behavior in the EPM at P30-32 [145], the LDB at P31-33 and P84-86 [146], and the OFT at P25 [147]. In male rats, supplementation with Euterpe oleracea Mart. (açaí) seed extract (P76-110) [143], capsaicin (P56-70) [148] and vanillic acid (VA) (P46-60) [149], reduced POS-induced anxiety-like behavior in the OFT at P106-108 [143], P63-70 [148] and P60 [149] and in the EPM at P60 [149]. In female rats, supplementation with spirulina platensis (P41-P55) reversed the POS-induced anxiety-like behavior measured in the OFT and the EPM at P60-P70 [144]. In mice, supplementation with Trigonelline in males (P31-45/47) and 2’Fucosyllactose (Weaning-W7) [150] reversed the POS-induced anxiety-like behavior as measured in the EPM at P45-47 [104] and at W7 [150].
Cognitive impairments – In rats, supplementation with Bacopa monnieri and acacia gum (G10-P23 and P15-30) [145] to males and with milk-based wolfberry preparation (2 weeks before breeding) [151] to females ameliorated PRS-induced cognitive impairments in the YM at P30-32 [145] and in the MWM at P30 [151]. In male rat POS-offspring, supplementation with capsaicin (P56-70) [148], VA(P46-60) [149], MFGM (P21-W13/14) [90] or quinoa supplemented food (P21-P52/53) [152] improved ES-induced cognitive impairment at P63-70 in the ORT and BM [148] and in the SBT at P60 [149], the MWM, but not the ORT at W7-13 [90] and in the YM at P52-53 [152]. In female rats, supplementing the diet with spirulina platensis (P41-P55) reversed the POS-induced cognitive impairments measured in AL and the MWM at P60-P70 [153].
Other – In male rats, supplementations with MFGM G19/21-P100 [154] and from P21-W13/14 [90] reduced the POS-induced alterations in pain behavior at P70-77 [154], but not at W7-13 [90]. In addition, in male rats, supplementations with capsaicin (P56-70) [148] and VA (P46-60) [149] reduced ES-induced changes in prepulse inhibition (PPI) at P63-70 [148] and repetitive behavior at P60 [149].
In general, the above described studies show that supplementation with Bacopa monnieri, acacia gum, acai seed extract, wolfberry preparation, milk fat globule and trigonelline was able to mitigate the ES-induced alterations in behavior. Due to the diversity of nutrients and the relative scarcity of studies pertinent to each, it remains impossible to draw any conclusions at this point.
Potential mechanisms underlying the modulatory effects of nutrition
In the previous section, we described the effect of nutritional interventions and their effectiveness in modulating the effect of ES on various behavioral domains. It remains of importance to understand not only if, but also how they modulate the ES-induced effects and which are the specific neurobiological processes mediating the effects of nutrients on the brain in the context of ES. This poses a significant challenge considering that most of the nutrients will have a broad impact on the brain as they are essential building blocks as well as signaling molecules, acting often as co-factors in biochemical processes in the various cell types in our brain [12, 155] rather than targeting a specific brain region, or cell type. In addition, to add an even further layer of complexity, several of them act on converging pathways and there is ample cross talk between the various mechanisms that are modulated by specific nutrients. In the next section, we will discuss key processes and their potential crosstalk that have been implicated in the long-term effect of ES and how the above- described nutrients might contribute to or modulate these processes. This section is based on the literature identified resulting from primary search in our review, which addressed the impact of early-life stress and nutritional interventions but takes into consideration all papers independent on whether behavioral outcomes were addressed.
Neurogenesis and neurotrophic factors
Adult hippocampal neurogenesis is the process in which new neurons are generated in the hippocampus, a brain region critical for cognitive functioning, anxiety and depression-related behavior [156, 157]. Several preclinical studies have investigated immediate and lasting effects of adverse early-life experience on hippocampal neurogenesis and the associated behavioral alterations using different models for ES [158]. Both PRS as well as POS lead to learning deficits associated with a decrease in neurogenesis [159,160,161,162]. Interestingly, Increasing the bio-availability of N-3 PUFAs [14], but not enriching the diet with a combination of micronutrients [15] early in life protected against the ES-induced reduction in neuronal survival. How N-3 PUFAs specifically affected neuronal survival, how key the specific time window is within which nutrients are supplemented and whether other nutrients or the combination thereof might also modulate adult hippocampal neurogenesis in the context of ES remains to be determined.
Neurotrophic factors (e.g. brain derived neurotrophic factor (BDNF)) are a family of molecules that support the growth, survival and differentiation of both developing and mature neurons. Several studies point to the importance of BDNF in pathways of adult neurogenesis [163], suggesting it might contribute to the effect of nutrients on among others the adult neurogenic process. For example, that stress inhibits the pathway that leads to production of BDNF [164] and that following dietary restriction upregulation of BDNF is required for the antidepressant treatment-induced increase in survival of newborn granule cells [165]. Hydroxytyrosol [64], resveratrol [65], as well as DHA [45] supplementation increased BDNF expression in the brain of the ES-exposed offspring, potentially contributing to the beneficial effects on the ES-induced cognitive decline observed. Similar increases in BDNF levels were observed in the plasma of ES-exposed mice after supplementing with other polyphenols [57]. The specific mechanism via which these nutrients modulate BDNF and to what extents the role of BDNF is key for their effect on neurogenesis remain to be determined.
Furthermore, neurogenesis can be influenced by various other biological pathways, such as for example the gut microbiome [166] and neuroinflammation [9], later discussed in this review. Therefore, it is most likely that dietary factors may also indirectly affect neurogenesis not only via BDNF, but rather via a synergistic action through modulation of various pathways.
Apoptosis
Apoptosis is a tightly regulated process of programmed cell death and plays a crucial role in shaping the developing CNS. Notably, neurons are particularly vulnerable to programmed cell death, as a significant proportion of newly generated neurons are eliminated in specific brain regions during development [167]. A slight perturbation in this developmental trajectory by, for example ES, tipping the balance towards increased apoptosis, might be detrimental. Indeed, there is evidence that ES affects apoptotic pathways in the rodent brain. For example, POS leads to an immediate increase in hippocampal apoptosis [168, 169] associated with later-life cognitive deficits [168].
Nutrients could modulate apoptosis for example by inhibiting pro-apoptotic BAX and (pro-)caspases and stimulating anti-apoptotic Bcl-2 [75, 81, 117, 145]. Supplementation with DHA [45], B. trisporus [81], L. paracasei [75] and Bacopa monnieri [145] reversed the ES-induced increase of apoptotic markers BAX [45, 81, 117], caspase 3 [75, 81, 117, 145], pro-caspase 3 [45] and pro-caspase 9 [45] and decrease in anti-apoptotic marker Bcl-2 [81, 117].
There is evidence that neuroinflammation, oxidative stress and mitochondrial functioning might also be involved in the modulation of apoptosis and its modulation by nutrients and stress. For example, dysregulation of the NLRP3-Caspase 1 signaling pathway caused by ES, was reversed by nutritional interventions wit proanthocyanidins [53]. This signaling pathways is associated with an inflammatory-programmed cell death [170].
Synaptic plasticity
Synaptic plasticity refers to activity-dependent modification of the strength or efficacy of synaptic transmission at pre-existing synapses in response to experiences [171]. There is ample evidence that ES leads to long-lasting changes in synaptic plasticity influencing both the pre- and post-synapse [172, 173]. For example, ES reduced synaptophysin (SYP) and post-synaptic density (PSD)-95 expression, important in the formation and maintenance of synapses and their transmission [174, 175]. Nutritional interventions could increase synapse maintenance and stability by enhancing these neural proteins. Indeed, supplementation with polyphenols [64] or a diet enriched with Bacopa monnieri [145] and a diet rich in olive oil [132] were able to reverse the ES-induced decrease in SYP and PSD-95.
The plastic cellular process underlying learning and memory, long-term potentiation (LTP), and the herein essential glutamate ionotropic receptor (GLuR) and N-methyl-D-aspartate (NMDA) receptors (glutamatergic), are affected by ES [173]. There is evidence that nutrients might be able to modulate LTP via modulation of these receptors. For example, resveratrol supplementation [65] was able to reverse the ES-induced increase of NMDA receptors and supplementation with L. paracasei [75] reversed the ES-induced increase in GluR1, GluR2 and NMDA receptors.
Similarly, inhibitory Gamma-aminobutyric acid (GABA)-ergic synapses have been implicated in ES [176] and a combination of pre- and probiotics supplemented after POS [84] was able to reverse the increased number of GABA-A2 receptors [177].
Synaptic plasticity, closely linked to neurogenesis, plays a pivotal role in integrating newly formed neurons into existing neural circuits. Nutritional interventions that promote synaptic plasticity may enhance neurogenesis. Additionally, chronic neuroinflammation can impair synaptic plasticity, while nutritional interventions with anti-inflammatory properties may alleviate neuroinflammation and facilitate synaptic plasticity, that might mediate beneficial effects on cognition. Pro-inflammatory circumstances might also lead to aberrant mitochondrial functioning, thereby leading to oxidative stress, detrimental to synaptic plasticity.
Neuroinflammation
Neuroinflammation is defined as an inflammatory response within the CNS, in which the key players are microglia and astrocytes, endothelial cells and peripherally derived immune cells. This inflammation is mediated by induction of cyto- and chemokines, reactive oxygen species (ROS), and secondary messengers [178]. In addition, peripheral inflammation can lead to the release of inflammatory molecules that can cross the blood-brain barrier (BBB) and contribute to neuroinflammation [178]. ES can have a lasting impact on both peripheral and central immune systems. For instance, there is both clinical [179] and pre-clinical [180] evidence that ES leads to increased circulating levels of pro-inflammatory cytokines and pre-clinical evidence for a similar increase also in the brain later in life [181]. In addition, ES has been shown to modulate microglia directly after the stress paradigm [182], as well as lastingly into adulthood [9, 23], possibly exerting effects on key developmental processes like synaptic pruning [183].
There are several pathways via which nutrients can influence neuroinflammation: a more direct impact, with nutrient-derived messengers crossing the BBB and thereby exerting their effects directly in the CNS or a more indirect pathway, with nutrients modulating peripheral inflammation, that can in turn exert its effect on the central immune system.
One group of interventions that has shown to be effective in reducing ES-induced (neuro)inflammation, are the probiotics. Bifidobacteria [83] were potent in reducing ES-induced peripheral [83], neuro- [81] and gut inflammation [87] potentially contributing to the modulatory effects on some of the behavioral domains. Supplementation with Lactobacilli led to a reverse in the ES-induced increase in pro-inflammatory cytokines and decrease in anti-inflammatory cytokines [80]. Interestingly, strains from these genera often converge at the functional level in terms of production of short-chain fatty acids (SCFAs), such as propionate, acetate and butyrate that may exert anti-inflammatory effects [184]. Peripherally, SCFAs influence systemic inflammation via inhibiting histone deacetylases, thereby inhibiting Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) activation [185]. Importantly, SCFAs can cross the BBB via monocarboxylate transporters located on endothelial cells and influence BBB integrity by upregulating the expression of tight junction proteins [186] and thereby potentially exert direct effects on microglia.
Anti-inflammatory capacities are also reported after supplementing with N-3 PUFAs. Indeed, N-3 PUFA derivatives (e.g. oxylipins) are able to directly influence microglia, by modulating their phagocytic capacity, motility and their capacity to produce inflammatory factors [23, 187].
For example, increasing N-3 PUFAs bioavailability which was protective against the ES-induced cognitive decline, also reduced the ES-induced increase in cluster of differentiation (CD)68 expression (a marker for activated microglia) [14]. Interestingly, there is evidence that ES modulates the brain lipidome and oxylipin profile long lastingly and that these profiles depend on early life N-3 PUFA availability in the diet [188]. Another study reported an increased bioavailability of N-3 PUFAs in the frontal cortex after supplementation with fish oil [120, 122]. This increase in bioavailability of N-3 PUFAs could lead to a more anti-inflammatory environment [189] in these brain regions, potentially modulating microglia. Finally, nutritional supplements can exert anti-inflammatory effects via inhibition of NF-κB. For example, supplementing with ferulic acid suppresses hippocampal NF-κB, potentially contributing to a decrease in cytokine expression in the ES-exposed offspring [54].
While it becomes thus clear that several nutrients exert modulatory effects on (neuro)inflammatory processes, the specific pathway via which they exert these effects remains to be understood and are most likely a combination of direct and indirect processes potentially involving for example the gut microbiome [166] and the hypothalamus-pituitary-adrenal (HPA)-axis [190].
Mitochondrial & Oxidative stress
Mitochondria have been receiving increasing attention for their involvement in the stress response [191]. Energy demands increase during stress due to the “fight or flight” response and allostatic biological systems, both of which rely on adenosine triphosphate (ATP) as an energy source. Mitochondria play a crucial role in meeting this energy demand by increasing cellular energy production, promoting cellular adaptation through signal generation, and undergoing biogenesis [191, 192]. During the production of ATP, ROS and reactive nitrogen species (RNS) are formed. When the production of ROS and RNS exceeds the antioxidant defenses, oxidative stress occurs, leading to damage to cellular components including lipids, proteins and deoxyribonucleic acid (DNA). Clinical studies have addressed the potential involvement of mitochondria in the context of ES. For example, individuals exposed to ES exhibit an increased mitochondrial DNA copy number content in leukocytes [193] and in saliva [194] indicating that this effect might be widespread throughout the body and have its origin in childhood. In addition, there is evidence from preclinical studies that ES (POS in particular) affects mitochondria in the brain. For example, ES-exposed offspring exhibited increased ROS [195], decreased ATP production [195, 196], higher oxidative stress [197] and decreased antioxidant levels [196] and altered mitochondrial gene expression [198] in the hippocampus when compared to controls.
Some of the nutritional interventions might exert their beneficial effects in the context of ES via modulating mitochondria. For example, hydroxytyrosol (a polyphenol) [64], DHA [45] and taurine [117] supplementation in ES offspring all led to an increased mitochondrial metabolism that potentially contributed to cognitive improvement. Polyphenols have the capacity to modulate mitochondria via various pathways. For example, polyphenols have multiple hydroxyl groups within their structure [199] rendering them exceptional in buffering excess ROS in the CNS. Indeed, supplementation of hydroxytyrosol increased mitochondrial function and decreased oxidative stress [64]. Such buffering could for example be mediated by activation of the Nrf2-Keap1-ARE pathway, which has been shown to be increased after polyphenol supplementation which in turn induces the expression of phase II detoxifying enzymes, responsible for reducing endogenous toxic metabolites [65].
In addition to having a direct impact on mitochondria, nutrients might also influence ROS levels via decreasing production of ROS, increasing its breakdown or by mitigating downstream effects of ROS.
For example, supplementing with proanthocyanidins (a polyphenolic compound) [53] reduced ROS in ES offspring. Playing a crucial role in breakdown of ROS are superoxide dismutases (SOD), which inhibit superoxide radicals; and catalase, which inhibit free diffusion of hydrogen peroxide among cells. SOD and catalase were found to be increased after the supplementation of amino acids (specifically: NAC [41] and taurine [117] and diets rich in fatty acids [132] (specifically N-3 PUFAs and MUFAs) in ES rat offspring, related in turn to increased cognitive performance and a reduction in depressive-like symptoms.
Additionally, taurine supplementation has been shown to increase mitochondrial membrane potential, which in turn could lead to increased respiratory chain enzymatic activity. This increases ATP, which supplies for the increased energy demand during stress. This could in turn increase the production of ROS that might be mitigated by the increase that is seen in SOD1 [117].
N-3 PUFAs-induced beneficial effects [41, 45] might modulate production of micelles with scavenger-free radicals, thus reducing the production of ROS [200]. (Semi)vitamins, amino acids and FAs had strong effects in mitigating the downstream effects of ROS. For instance, protein carbonylation (a post-translational modification in proteins exposed to oxidative stress) was found to be reduced in ES-exposed offspring supplemented with folic acid [41]. Another downstream effect of ROS is lipid peroxidation, which leads to an increase in malondialdehyde (MDA), which can react with DNA and proteins. The supplementation with Vitamin E [104], Folic acid [41] and Auraptene [63] successfully inhibited the increased lipid peroxidation and thereby the expression of MDA caused by ES. N-3 PUFAs were only partially inhibiting this increase, potentially because N-3 PUFA supplementation could lead to exaggerated sensitivity to lipid peroxidation [201].
The involvement of mitochondrial functioning in the efficacy of nutritional interventions could be dependent on cross-talking pathways. For example, in response to acute stress exposure, mitochondria respond dynamically to cues from stress-signaling pathways enacted by glucocorticoids [192], to meet increased energy demands [202]. In addition, Myeloperoxidase (MPO) can affect mitochondrial function by oxidizing mitochondrial proteins and lipids [203], which can lead to mitochondrial damage and dysfunction. MPO has been found to be expressed in microglia and has been found to be reduced by PRS [41]. Thus, the decrease found in the study could be potentially related to changes in neuroinflammation which have been reported by other studies after ES exposure.
Finally, pro-caspase 9 has been shown to get activated by mitochondrial cytochrome c (which has been shown to be increased in ES) [204] also linking oxidative stress and mitochondrial functioning to apoptosis.
The intricate interaction between the HPA axis, neuroinflammation, mitochondrial functioning and oxidative stress might be crucial in mechanisms underlying the beneficial effects of the nutritional interventions in the context of ES.
HPA-axis regulation
The HPA axis is the neuroendocrine stress axis orchestrating the release of the stress hormones such as glucocorticoids. Upon stress, corticotropin-releasing factor is released from the hypothalamus, which leads to adrenocorticotropic hormone release from the pituitary which stimulates the production of glucocorticoids (cortisol in human and corticosterone in rodents) in the adrenal gland. Under basal levels of glucocorticoids, negative feedback is mediated mainly through the mineralocorticoid receptors (MR) in the hippocampus. The less sensitive glucocorticoid receptor (GR) comes into play in the hippocampus, hypothalamus, and pituitary gland under stress and therefore high glucocorticoid concentrations. The balance in these MR- and GR-mediated effects on the stress system is of crucial importance to the functioning the of HPA axis. Evidence from clinical and preclinical studies suggests that disruption of the HPA axis and changes in GR and MR balance are involved in the ES-induced behavioral alterations and the increased risk to develop psychopathologies later in life [162, 205,206,207,208].
HPA-axis regulation is one of the most addressed as a potential mechanism through which nutritional intervention could work. Nutritional supplementations with ferulic acid [54] hydroxytyrosol [64], Lactobacilli and Bifidobacteria [75, 79, 80, 82, 84, 85], micronutrients [15] and “comfort foods” (e.g. HFD [137], a high fat high sugar diet [134], or highly palatable food [136]) have all been proposed to ameliorate the ES-induced effects at least partly via modulation of the HPA axis (i.e. reduction of ES-induced corticosterone and modulation of GR expression).
Additionally, as already discussed above, none of the discussed mechanisms is acting in solo but rather through well-orchestrated interactions. There are indications that the HPA axis can be activated by the microbiome as a result of increased permeability of the intestinal barrier and a microbiota-driven proinflammatory state [209]. Moreover, glucocorticoids act on almost all types of immune cells and perform salient immunosuppressive and anti-inflammatory functions through genomic and non-genomic mechanisms [190].
Monoamine regulation
Monoamines are neurotransmitters that are derived from aromatic amino acids and include for example serotonin, dopamine (DA), and norepinephrine (NE) [210]. The serotonergic system has an important role in development, functioning in regulation of neurogenesis, synaptogenesis, neural connectivity, myelination and synaptic remodeling [211]. There are four major dopaminergic pathways, the mesolimbic and the mesocortical (important for reward-related cognition and executive functions [212]), the nigrostriatal (known for its role in motor function [213]) and the tuberoinfundibular pathway (for the regulation of prolactin secretion [214]). The dopaminergic system undergoes essential remodeling and maturation early in life. Perturbation in its signaling early in life has been associated with several neuropsychiatric disorders [215, 216]. For example, ES has been shown to lead to an enhanced adult 5-HT2-mediated function [217, 218] and elevated dopaminergic function [219]. Lastly, NE is a neurotransmitter that plays an important role in the body’s “fight or flight” response to stress. There is evidence that NE is affected by ES [75, 87, 91].
5-HT, DA and NE are considered key neurotransmitters that participate in the brain-gut axis. Indeed, supplementing with the probiotics consisting of Lactobacilli [75, 79] modulated the ES-induced alterations in the serotonergic system. SCFA-producing bacteria in the gut influence the expression of tryptophan hydroxylase and thereby 5-HT synthesis and release [220], potentially affecting 5-HT levels in the brain [75, 79].
Similarly, supplementation with Lactobacillus [79] and Bifidobacterium [87] modulated DA in the prefrontal cortex [79] and in the gut [87] as well as NA [87].
Several authors found a beneficial effect of supplementing “comfort foods” on behavioral deficits found in ES [133,134,135,136, 138]. It could be that these comfort foods have influence via the ventral tegmental area-nucleus accumbens reward network, emphasizing the monoaminergic system to be involved as well [221].
Gut Microbiome
The gut and the brain are in constant bidirectional communication. With the emergence of the gut microbiome in modulating host behavior via various routes (metabolites, neuronal, endocrine, immune system), the microbiota-gut-brain axis has become a key player in the research of different psychopathologies. Furthermore, the gut microbiome has a functional role in the development of the brain and can determine host behavior. Illustrated by the use of germ-free mice, it was shown that the microbiome is necessary to induce at least some of the neuropsychiatric effects observed after maternal separation [68]. There is also a bidirectional relationship between stress and the gut microbiome, and stress exposures often leaves an impact on the gut microbiome. Multiple studies have thus shown a link between ES and subsequent changes in gut microbiota compositional configurations that persist into adulthood [67, 83]. However, the time of initial arise and the trajectory of microbiota perturbations are unclear. Furthermore, how these changes contribute to neurodevelopmental changes leading to psychiatric disorders remains to be elucidated, but there are substantial overlaps between the assembly of the gut microbiome postnatally and important neurodevelopmental time windows [222, 223]. A growing body of research has shown that nutritional interventions including PUFAs, polyphenols, and HFDs modulate both the microbiome and brain. However, it is unclear how much diet-induced changes in microbiota contributes to the effects on the brain per se [224]. Indeed, there are a number of important pathways by which the gut microbiota can modulate behavior.
The most classical way of interaction between the gut microbiota and the host is via the metabolites the bacteria produce. The most commonly investigated metabolites are SCFA, the product of host-indigestible dietary fibers fermented by bacteria.
SCFA have been shown to have anti-inflammatory effects [225], epigenetic modulation capabilities and affect hormone secretion via G-protein coupled receptor binding [16]. It was shown that ES can affect the production of SCFA measured directly [122] or predicted based on the relative abundance of SCFA-producing bacteria [57]. Diet plays a major role in sculpting the composition and function of the gut microbiota such that these processes might be modifiable by the interventions under consideration here. For example, supplementation with polyphenols [57], increased propionate, while a diet containing 7% of fish oil [122] increased SCFA producers in ES-exposed animals. This shows that the effects induced by the intervention could either be due to the direct effects of the polyphenols or indirectly by modifying the configuration of the gut microbiota.
Importantly, in the last decade, a paradigm shift occurred in the field of the microbiota-gut-brain axis highlighting the need to move towards functional approaches for the assessment of gut microbes that goes beyond just compositional alterations [226]. In addition to the direct assessment of microbial metabolites discussed above, the genomic content of microbes can be analyzed and used to predict their metabolic capabilities of the gut microbiota in health and disease, as well as for specific bacterial strains. This concept evolved the field beyond compositional analysis into a functional analysis of the metabolic output of the gut population [227] with alterations in microbial metabolic pathways identified in stress-related disorders [228, 229]. These microbial metabolites include SCFA, but also monoamines and neurotransmitters including serotonin, DA, GABA and glutamate [228].
As discussed above, one of the key microbial pathways affecting host behavior is via bacteria-produced metabolites. One of the types of metabolites produced by the gut microbiome are monoamines and other neurotransmitters, most notably serotonin. The majority of serotonin in the body is produced and used in the gut from its precursor tryptophan. The concentration of serotonin can be modulated by the gut microbiome in multiple ways [230], most notably shown by altered concentration of serotonin and its metabolites in the hippocampus of germ-free animals [231]. This is most likely mediated via a humoral route based on increased availability of its precursor tryptophan in the plasma [231]. ES has been shown to affect central serotonin levels, which can be alleviated by the use of probiotics [75, 79]. The gut has multiple interactions with other involved pathways as mentioned before. An example is the influence of the gut on neurotransmitters and vice versa is the abovementioned monoaminergic system [76, 87, 232], where reduced DA and adrenaline in the gut, which led to a decrease in anxiety-like behavior, indicating probably a link of these neurotransmitters with behavior. Another important neurotransmitter produced by a wide range of Bifidobacterium and Lactobacillus strains, is GABA, the major inhibitory neurotransmitter of the CNS. Modulation of GABAergic neurotransmission, including receptor expression, by probiotics following ES-induced depression has been shown [72, 84]. While many studies provide evidence that the gut microbiota is engaged with in reciprocal crosstalk with the HPA-axis, the mechanism behind this interaction remains to be elucidated [71].
It has been proposed that levels of GABA and its receptors are one of the mechanisms by which the microbiome might modify HPA axis function by inhibiting the activation of CRH-neurons [233]. Changes in gut microbiota due to insults such as stress can affect the gut barrier integrity. This can have secondary implication on the host immune system due to potential increased translocation of bacteria and their metabolites [234]. This has been clearly demonstrated in multiple studies discussed here showing an increased immune activation in ES animals which is reversible by probiotics [80, 81, 87, 91]. Studies using similar probiotic cocktails [82, 235, 236] as discussed in this review link these changes to recovery of gut-barrier function, thus preventing inflammation via translocation across the barrier [237]. Beyond that, the precise mechanism resulting in a reduction of inflammation remains to be described.
Conclusion
In this comprehensive review, we set out to unravel the efficacy of nutritional strategies in mitigating effects of ES on later-life behavioral outcomes across clinical- and preclinical literature. From the included human studies, five studies were observational and only three studies were clinical trials. Out of these three clinical trials, only one study demonstrates an effect of a combined diet and behavioral program, making it impossible to disentangle the effects. The low number of clinical studies and the heterogeneous study designs significantly constrain our ability to draw conclusions regarding the effects of dietary interventions to modulate the detrimental effects of ES in human subjects and stresses the need to perform randomized clinical trials in humans.
However, in the included rodent studies, in 112 out of the 123 cases the ES induced later-life behavior could be mitigated by nutritional supplementations, underscoring the potential for such nutritional strategies. Indeed, despite large heterogeneity in the studies (i.e. the differences in timing and nature of the stressors, interventions and outcome measures), most of the interventions were able to modulate the ES-induced behaviors. However, an effect of (publication) bias cannot be ruled out and was not further assessed by this review. Notably, even though we included all the literature addressing the effects of ES and nutritional interventions in both males and females, the included studies mostly failed to test whether the efficacy of the nutritional supplementation might be sex-specific. The lack of addressing the impact of sex is not specific to the studies included in this review. In fact, even though sex and gender influence the prevalence, manifestation and progression of, as well as the treatment response to various diseases, progress in biomedical research remains slow in this domain. Indeed, most studies do not incorporate sex as a discovery variable, and thus, in only a small proportion of studies, sex is used as a predictor variable or a between-subject variable to analyse for main and interaction effects rather than as a covariate [238, 239]. Because of the key sex differences in the effect of ES [240,241,242,243] and nutrition [244] and general metabolisms [245] and disease prevalence and presentation [238, 239], it is key for future studies to consider both sexes when assessing efficacy of nutritional interventions in ES research and to include sex as a discovery variable.
Although mainly based on preclinical evidence and observational studies in humans, this review highlights the great potential of nutritional strategies for mental health of vulnerable population exposed to ES. Thus, nutrition is a very attractive element to target and to unlock its true potential and to develop an evidence-based tool for the prevention and treatment there are several barriers that we need to still overcome. In rodent studies, currently, the heterogeneity in the measured outcomes (different tasks), outcome age, and the stress models employed poses challenges to drawing strong conclusions and to pinpoint the specifics of the nutritional strategy. More studies are needed to determine which nutrients should be used in nutrition-based interventions (which exact nutrients/combinations/diets, dosages) and considering that vulnerability varies across the lifespan and between individuals, the optimal time window of opportunity (i.e. when we can best apply specific nutritional strategies for either prevention or intervention) remains to be addressed. Next to the points mentioned above, considering the very limited number of human studies, it will be key to increase clinical research in this area using randomized controlled trials and focusing on collecting prospective longitudinal data, including all appropriate control groups.
Finally, it will be key to further our insights into the biological mechanisms mediating the beneficial effect of nutrition on the brain. Through this review, the incredible complexity of these mechanisms becomes evident. While the studies presented in this review have made use of preclinical in vivo studies to explore the mechanisms underlying the biological mechanisms, an interesting avenue potentially aiding to gain further insight into the processes involved the effects of nutritional strategies in the context of ES and their relevance in the context of human biology, are humanised in vitro models. There are indeed first attempts to use cerebral organoids for exploring the effects of glucocorticoids on early human brain development, mimicking early-life stress exposures [246] as well as the use of human hippocampal progenitor cells to test the effects of specific nutrients [247, 248] and of how fatty acid exposure interacts with glucocorticoids [249] on the neurogenic process. There is a wide plethora of various nutrients, each with their broad impact on the brain, their dual nature of building blocks, energy source and signalling molecules, the intricate crosstalk between the biochemical cycles they are involved in and the often converging pathways they act upon rendering the unravelling of this complexity the next challenge to move the field forward.
References
Amieltison C, Pettigrew AG. Adaptive-changes in the developing brain during intrauterine stress. Brain Dev. 1991;13:67–76.
Lebel C, Deoni S. The development of brain white matter microstructure. Neuroimage. 2018;182:207–18.
Tau GZ, Peterson BS. Normal development of brain circuits. Neuropsychopharmacology. 2010;35:147–68.
Loman MM, Gunnar MR, Neurobehav EES. Early experience and the development of stress reactivity and regulation in children. Neurosci Biobehav Rev. 2010;34:867–76.
Alastalo H, von Bonsdorff MB, Raikkonen K, Pesonen AK, Osmond C, Barker DJP, et al. Early life stress and physical and psychosocial functioning in late adulthood. PLoS One. 2013;8:e69011.
Pesonen AK, Eriksson JG, Heinonen K, Kajantie E, Tuovinen S, Alastalo H, et al. Cognitive ability and decline after early life stress exposure. Neurobiol Aging. 2013;34:1674–9.
Daníelsdóttir HB, Aspelund T, Shen Q, Halldorsdottir T, Jakobsdóttir J, Song H, et al. Adverse childhood experiences and adult mental health outcomes. JAMA Psychiatry. 2024;81:586–94.
Lucassen PJ, Naninck EF, van Goudoever JB, Fitzsimons C, Joels M, Korosi A. Perinatal programming of adult hippocampal structure and function; emerging roles of stress, nutrition and epigenetics. Trends Neurosci. 2013;36:621–31.
Hoeijmakers L, Lucassen PJ, Korosi A. The interplay of early-life stress, nutrition, and immune activation programs adult hippocampal structure and function. Front Mol Neurosci. 2014;7:103.
Black MM. Impact of nutrition on growth, brain, and cognition. Nestle Nutr Inst Workshop Ser. 2018;89:185–95.
Cusick SE, Georgieff MK. The role of nutrition in brain development: the golden opportunity of the “first 1000 days”. J Pediatr. 2016;175:16–21.
Cusick SE, Barks A, Georgieff MK. Nutrition and brain development. Curr Top Behav Neurosci. 2022;53:131–65.
Georgieff MK. Early life nutrition and brain development: breakthroughs, challenges and new horizons. Proc Nutr Soc. 2023;82:104–12.
Yam KY, Schipper L, Reemst K, Ruigrok SR, Abbink MR, Hoeijmakers L, et al. Increasing availability of ω-3 fatty acid in the early-life diet prevents the early-life stress-induced cognitive impairments without affecting metabolic alterations. FASEB J. 2019;33:5729–40.
Naninck EF, Oosterink JE, Yam KY, de Vries LP, Schierbeek H, van Goudoever JB, et al. Early micronutrient supplementation protects against early stress-induced cognitive impairments. FASEB J. 2017;31:505–18.
van de Wouw M, Boehme M, Lyte JM, Wiley N, Strain C, O’Sullivan O, et al. Short-chain fatty acids: microbial metabolites that alleviate stress-induced brain-gut axis alterations. J Physiol. 2018;596:4923–44.
Cruz-Pereira JS, Moloney GM, Bastiaanssen TFS, Boscaini S, Tofani G, Borras-Bisa J, et al. Prebiotic supplementation modulates selective effects of stress on behavior and brain metabolome in aged mice. Neurobiol Stress. 2022;21:100501.
Lindsay KL, Buss C, Wadhwa PD, Entringer S. The Interplay between maternal nutrition and Stress during pregnancy: issues and considerations. Ann Nutr Metab. 2017;70:191–200.
Suri D, Vaidya VA. The adaptive and maladaptive continuum of stress responses - a hippocampal perspective. Rev Neurosci. 2015;26:415–42.
Daskalakis NP, Bagot RC, Parker KJ, Vinkers CH, de Kloet ER. The three-hit concept of vulnerability and resilience: toward understanding adaptation to early-life adversity outcome. Psychoneuroendocrinology. 2013;38:1858–73.
Molendijk ML, de Kloet ER. Forced swim stressor: trends in usage and mechanistic consideration. Eur J Neurosci. 2022;55:2813–31.
Cryan JF, Mombereau C, Vassout A. The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev. 2005;29:571–625.
Reemst K, Kracht L, Kotah JM, Rahimian R, van Irsen AAS, Congrains Sotomayor G, et al. Early-life stress lastingly impacts microglial transcriptome and function under basal and immune-challenged conditions. Transl Psychiatry. 2022;12:507.
Abbink MR, Schipper L, Naninck EFG, de Vos CMH, Meier R, van der Beek EM, et al. The effects of early life stress, postnatal diet modulation, and long-term western-style diet on later-life metabolic and cognitive outcomes. Nutrients. 2020;12:570.
Corriveau JA, Glenn MJ. Postnatal choline levels mediate cognitive deficits in a rat model of schizophrenia. Pharmacol Biochem Behav. 2012;103:60–8.
Mathieu G, Denis S, Lavialle M, Vancassel S. Synergistic effects of stress and omega-3 fatty acid deprivation on emotional response and brain lipid composition in adult rats. Prostaglandins Leukot Essent Fat Acids. 2008;78:391–401.
Mathieu G, Oualian C, Denis I, Lavialle M, Gisquet-Verrier P, Vancassel S. Dietary n-3 polyunsaturated fatty acid deprivation together with early maternal separation increases anxiety and vulnerability to stress in adult rats. Prostaglandins Leukot Essent Fat Acids. 2011;85:129–36.
Ferreira CF, Bernardi JR, Krolow R, Arcego DM, Fries GR, de Aguiar BW, et al. Vulnerability to dietary n-3 polyunsaturated fatty acid deficiency after exposure to early stress in rats. Pharmacol Biochem Behav. 2013;107:11–9.
Pusceddu MM, Kelly P, Ariffin N, Cryan JF, Clarke G, Dinan TG. n-3 PUFAs have beneficial effects on anxiety and cognition in female rats: Effects of early life stress. Psychoneuroendocrinology. 2015;58:79–90.
Saini RK, Keum YS. Omega-3 and omega-6 polyunsaturated fatty acids: Dietary sources, metabolism, and significance - A review. Life Sci. 2018;203:255–67.
de Carvalho CCCR, Caramujo MJ. The various roles of fatty acids. Molecules. 2018;23:2583.
Gunenc AN, Graf B, Stark H, Chari A. Fatty acid synthase: structure, function, and regulation. Subcell Biochem. 2022;99:1–33.
Calder PC. Functional roles of fatty acids and their effects on human health. J Parenter Enter Nutr. 2015;39:18s–32s.
Simopoulos AP. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed Pharmacother. 2002;56:365–79.
Simopoulos AP. Omega-3-fatty-acids in health and disease and in growth and development. Am J Clin Nutr. 1991;54:438–63.
Peng S, Peng Z, Qin M, Huang L, Zhao B, Wei L, et al. Targeting neuroinflammation: the therapeutic potential of ω-3 PUFAs in substance abuse. Nutrition. 2021;83:111058.
Stilling RM, van de Wouw M, Clarke G, Stanton C, Dinan TG, Cryan JF. The neuropharmacology of butyrate: the bread and butter of the microbiota-gut-brain axis? Neurochem Int. 2016;99:110–32.
O’Riordan KJ, Collins MK, Moloney GM, Knox EG, Aburto MR, Fülling C, et al. Short chain fatty acids: microbial metabolites for gut-brain axis signalling. Mol Cell Endocrinol. 2022;546:111572.
Keenan K, Hipwell A, McAloon R, Hoffmann A, Mohanty A, Magee K. The effect of prenatal docosahexaenoic acid supplementation on infant outcomes in African American women living in low-income environments: a randomized, controlled trial. Psychoneuroendocrinology. 2016;71:170–5.
Brunst KJ, Enlow MB, Kannan S, Carroll KN, Coull BA, Wright RJ. Effects of prenatal social stress and maternal dietary fatty acid ratio on infant temperament: does race matter? Epidemiology. 2014;4:1000167.
Reus GZ, Maciel AL, Abelaira HM, de Moura AB, de Souza TG, Dos Santos TR, et al. omega-3 and folic acid act against depressive-like behavior and oxidative damage in the brain of rats subjected to early- or late-life stress. Nutrition. 2018;53:120–33.
Valvassori SS, Resende WR, Budni J, Dal-Pont GC, Bavaresco DV, Reus GZ, et al. Sodium butyrate, a histone deacetylase inhibitor, reverses behavioral and mitochondrial alterations in animal models of depression induced by early- or late-life stress. Curr Neurovasc Res. 2015;12:312–20.
Zhao J, Ye L, Liu Z, Wu J, Deng D, An L, et al. The effects of early-life stress on liver transcriptomics and the protective role of EPA in a mouse model of early-life-stress-induced adolescent depression. Int J Mol Sci. 2023;24:13131.
Jones KL, Will MJ, Hecht PM, Parker CL, Beversdorf DQ. Maternal diet rich in omega-6 polyunsaturated fatty acids during gestation and lactation produces autistic-like sociability deficits in adult offspring. Behav Brain Res. 2013;238:193–9.
Feng Z, Zou X, Jia H, Li X, Zhu Z, Liu X, et al. Maternal docosahexaenoic acid feeding protects against impairment of learning and memory and oxidative stress in prenatally stressed rats: possible role of neuronal mitochondria metabolism. Antioxid Redox Signal. 2012;16:275–89.
Reemst K, Lopizzo N, Abbink MR, Engelenburg HJ, Cattaneo A, Korosi A. Molecular underpinnings of programming by early-life stress and the protective effects of early dietary ω6/ω3 ratio, basally and in response to LPS: integrated mRNA-miRNAs approach. Brain Behav Immun. 2024;117:283–97.
Wah DTO, Ossenkopp KP, Bishnoi I, Kavaliers M. Predator odor exposure in early adolescence influences the effects of the bacterial product, propionic acid, on anxiety, sensorimotor gating, and acoustic startle response in male rats in later adolescence and adulthood. Physiol Behav. 2019;199:35–46.
Rana A, Samtiya M, Dhewa T, Mishra V, Aluko RE. Health benefits of polyphenols: a concise review. J Food Biochem. 2022;46:e14264.
Gomez-Pinilla F, Nguyen TT. Natural mood foods: the actions of polyphenols against psychiatric and cognitive disorders. Nutr Neurosci. 2012;15:127–33.
Vauzour D. Dietary polyphenols as modulators of brain functions: biological actions and molecular mechanisms underpinning their beneficial effects. Oxid Med Cell Longev. 2012;2012:914273.
Manach C, Scalbert A, Morand C, Remesy C, Jimenez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr. 2004;79:727–47.
Tan A, Morton KR, Lee JW, Hartman R, Lee G. Adverse childhood experiences and depressive symptoms: protective effects of dietary flavonoids. J Psychosom Res. 2020;131:109957.
Sun Q, Jia N, Ren F, Li X. Grape seed proanthocyanidins improves depression-like behavior by alleviating oxidative stress and NLRP3 activation in the hippocampus of prenatally-stressed female offspring rats. J Histotechnol. 2021;44:90–8.
Zheng X, Cheng Y, Chen Y, Yue Y, Li Y, Xia S, et al. Ferulic acid improves depressive-like behavior in prenatally-stressed offspring rats via anti-inflammatory activity and HPA axis. Int J Mol Sci. 2019;20:493.
Shukla P, Akotkar L, Aswar U. Resveratrol attenuates early life stress induced depression in rats: Behavioural and neurochemical evidence. Neurosci Lett. 2024;820:137606.
Verma H, Bhattacharjee A, Shivavedi N, Nayak PK. Evaluation of rosmarinic acid against myocardial infarction in maternally separated rats. Naunyn Schmiedebergs Arch Pharmacol. 2022;395:1189–207.
Donoso F, Egerton S, Bastiaanssen TFS, Fitzgerald P, Gite S, Fouhy F, et al. Polyphenols selectively reverse early-life stress-induced behavioural, neurochemical and microbiota changes in the rat. Psychoneuroendocrinology. 2020;116:104673.
Toumi ML, Merzoug S, Tahraoui A. Effects of quercetin on predator stress-related hematological and behavioral alterations in pregnant rats and their offspring. J Biosci. 2016;41:237–49.
Omotoso GO, Mutholib NY, Abdulsalam FA, Bature AI. Kolaviron protects against cognitive deficits and cortico-hippocampal perturbations associated with maternal deprivation in rats. Anat Cell Biol. 2020;53:95–106.
Moghaddam AH, Eslami A, Jelodar SK, Ranjbar M, Hasantabar V. Preventive effect of quercetin-Loaded nanophytosome against autistic-like damage in maternal separation model: The possible role of Caspase-3, Bax/Bcl-2 and Nrf2. Behav Brain Res. 2023;441:114300.
Menezes J, Neves BH, Souza M, Mello-Carpes PB. Green tea protects against memory deficits related to maternal deprivation. Physiol Behav. 2017;182:121–7.
Karimi P, Ghahfarroki MS, Lorigooini Z, Shahrani M, Amini-Khoei H. Umbelliprenin via increase in the MECP2 and attenuation of oxidative stress mitigates the autistic-like behaviors in mouse model of maternal separation stress. Front Pharmacol. 2024;14:1300310.
Arabi M, Nasab SH, Lorigooini Z, Boroujeni SN, Mortazavi SM, Anjomshoa M, et al. Auraptene exerts protective effects on maternal separation stress-induced changes in behavior, hippocampus, heart and serum of mice. Int Immunopharmacol. 2021;93:107436.
Zheng A, Li H, Cao K, Xu J, Zou X, Li Y, et al. Maternal hydroxytyrosol administration improves neurogenesis and cognitive function in prenatally stressed offspring. J Nutr Biochem. 2015;26:190–9.
Cao K, Zheng A, Xu J, Li H, Liu J, Peng Y, et al. AMPK activation prevents prenatal stress-induced cognitive impairment: modulation of mitochondrial content and oxidative stress. Free Radic Biol Med. 2014;75:156–66.
Cryan JF, O’Riordan KJ, Cowan CSM, Sandhu KV, Bastiaanssen TFS, Boehme M, et al. The microbiota-gut-brain axis. Physiol Rev. 2019;99:1877–2013.
O’Mahony SM, Marchesi JR, Scully P, Codling C, Ceolho AM, Quigley EM, et al. Early life stress alters behavior, immunity, and microbiota in rats: implications for irritable bowel syndrome and psychiatric illnesses. Biol Psychiatry. 2009;65:263–7.
De Palma G, Blennerhassett P, Lu J, Deng Y, Park AJ, Green W, et al. Microbiota and host determinants of behavioural phenotype in maternally separated mice. Nat Commun. 2015;6:7735.
Olson CA, Vuong HE, Yano JM, Liang QY, Nusbaum DJ, Hsiao EY. The gut microbiota mediates the anti-seizure effects of the ketogenic diet. Cell. 2018;173:1728–41.e13.
Regen T, Isaac S, Amorim A, Nunez NG, Hauptmann J, Shanmugavadivu A, et al. IL-17 controls central nervous system autoimmunity through the intestinal microbiome. Sci Immunol. 2021;6:eaaz6563.
Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, Yu XN, et al. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol. 2004;558:263–75.
Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci USA. 2011;108:16050–5.
Butel MJ. Probiotics, gut microbiota and health. Med Mal Infect. 2014;44:1–8.
Vicariotto F, Malfa P, Torricelli M, Lungaro L, Caio G, De Leo V. Beneficial effects of limosilactobacillus reuteri PBS072 and bifidobacterium breve BB077 on mood imbalance, self-confidence, and breastfeeding in women during the first trimester postpartum. Nutrients. 2023;15:3513.
Karen C, Shyu DJH, Rajan KE. Lactobacillus paracasei supplementation prevents early life stress-induced anxiety and depressive-like behavior in maternal separation model-possible involvement of microbiota-gut-brain axis in differential regulation of MicroRNA124a/132 and glutamate receptors. Front Neurosci. 2021;15:719933.
Desbonnet L, Garrett L, Clarke G, Kiely B, Cryan JF, Dinan TG. Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience. 2010;170:1179–88.
Carlessi AS, Botelho MEM, Manosso LM, Borba LA, Maciel LR, Andrade NM, et al. Sex differences on the response to antidepressants and psychobiotics following early life stress in rats. Pharmacol Biochem Behav. 2022;220:173468.
Zhu H, Tian P, Qian X, Gu L, Zhao J, Wang G, et al. Perinatal transmission of a probiotic Bifidobacterium strain protects against early life stress-induced mood and gastrointestinal motility disorders. Food Funct. 2022;13:7520–8.
Liu YW, Liu WH, Wu CC, Juan YC, Wu YC, Tsai HP, et al. Psychotropic effects of Lactobacillus plantarum PS128 in early life-stressed and naive adult mice. Brain Res. 2016;1631:1–12.
Liao JF, Hsu CC, Chou GT, Hsu JS, Liong MT, Tsai YC. Lactobacillus paracasei PS23 reduced early-life stress abnormalities in maternal separation mouse model. Benef Microbes. 2019;10:425–36.
Huang Z, Zhang B, Liao L, Chen J, Zheng R, Cai D, et al. Probiotics improves abnormal behavior and hippocampal injury in pregnant-stressed offspring rats. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2022;47:443–52.
Hadizadeh M, Hamidi GA, Salami M. Probiotic supplementation improves the cognitive function and the anxiety-like behaviors in the stressed rats. Iran J Basic Med Sci. 2019;22:506–14.
Dauge V, Philippe C, Mariadassou M, Rue O, Martin JC, Rossignol MN, et al. A probiotic mixture induces anxiolytic- and antidepressive-like effects in fischer and maternally deprived long evans rats. Front Behav Neurosci. 2020;14:581296.
McVey Neufeld KA, O’Mahony SM, Hoban AE, Waworuntu RV, Berg BM, Dinan TG, et al. Neurobehavioural effects of Lactobacillus rhamnosus GG alone and in combination with prebiotics polydextrose and galactooligosaccharide in male rats exposed to early-life stress. Nutr Neurosci. 2019;22:425–34.
Peng HH, Tsai TC, Huang WY, Wu HM, Hsu KS. Probiotic treatment restores normal developmental trajectories of fear memory retention in maternally separated infant rats. Neuropharmacology. 2019;153:53–62.
Cowan CS, Callaghan BL, Richardson R. The effects of a probiotic formulation (Lactobacillus rhamnosus and L. helveticus) on developmental trajectories of emotional learning in stressed infant rats. Transl Psychiatry. 2016;6:e823.
Moya-Perez A, Perez-Villalba A, Benitez-Paez A, Campillo I, Sanz Y. Bifidobacterium CECT 7765 modulates early stress-induced immune, neuroendocrine and behavioral alterations in mice. Brain Behav Immun. 2017;65:43–56.
Zhu J, Zhong Z, Shi L, Huang L, Lin C, He Y, et al. Gut microbiota mediate early life stress-induced social dysfunction and anxiety-like behaviors by impairing amino acid transport at the gut. Gut Microbes. 2024;16:2401939.
Cowan CSM, Stylianakis AA, Richardson R. Early-life stress, microbiota, and brain development: probiotics reverse the effects of maternal separation on neural circuits underpinning fear expression and extinction in infant rats. Dev Cogn Neurosci. 2019;37:100627.
O’Mahony SM, McVey Neufeld K, Waworuntu RV, Pusceddu MM, Manurung S, Murphy K, et al. The enduring effects of early‐life stress on the microbiota–gut–brain axis are buffered by dietary supplementation with milk fat globule membrane and a prebiotic blend. Eur J Neurosci. 2020;51:1042–58.
Park ES, Freeborn J, Venna VR, Roos S, Rhoads JM, Liu Y. Lactobacillus reuteri effects on maternal separation stress in newborn mice. Pediatr Res. 2021;90:980–8.
Mattei D, Pietrobelli A. Micronutrients and brain development. Curr Nutr Rep. 2019;8:99–107.
Wu G. Amino acids: metabolism, functions, and nutrition. Amino Acids. 2009;37:1–17.
O’Mahony SM, Clarke G, Borre YE, Dinan TG, Cryan JF. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav Brain Res. 2015;277:32–48.
Musillo C, Creutzberg KC, Collacchi B, Ajmone-Cat MA, De Simone R, Lepre M, et al. Bdnf-Nrf-2 crosstalk and emotional behavior are disrupted in a sex-dependent fashion in adolescent mice exposed to maternal stress or maternal obesity. Transl Psychiatry. 2023;13:399.
Davis JL, O’Connor M, Erlbacher H, Schlichte SL, Stevens HE. The impact of maternal antioxidants on prenatal stress effects on offspring neurobiology and behavior. Yale J Biol Med. 2022;95:87–104.
Black MM. Effects of vitamin B12 and folate deficiency on brain development in children. Food Nutr Bull. 2008;29:S126–31.
Hanna M, Jaqua E, Nguyen V, Clay J. B vitamins: functions and uses in medicine. Perm J. 2022;26:89–97.
Shulpekova Y, Nechaev V, Kardasheva S, Sedova A, Kurbatova A, Bueverova E, et al. The concept of folic acid in health and disease. Molecules. 2021;26:3731.
Shearer KD, Stoney PN, Morgan PJ, McCaffery PJ. A vitamin for the brain. Trends Neurosci. 2012;35:733–41.
Hansen SN, Tveden-Nyborg P, Lykkesfeldt J. Does vitamin C deficiency affect cognitive development and function? Nutrients. 2014;6:3818–46.
Ramakrishna T. Vitamins and brain development. Physiol Res. 1999;48:175–87.
Lipton LR, Brunst KJ, Kannan S, Ni YM, Ganguri HB, Wright RJ, et al. Associations among prenatal stress, maternal antioxidant intakes in pregnancy, and child temperament at age 30 months. J Dev Orig Health Dis. 2017;8:638–48.
Lorigooini Z, Sadeghi Dehsahraei K, Bijad E, Habibian Dehkordi S, Amini-Khoei H. Trigonelline through the Attenuation of oxidative stress exerts antidepressant- and anxiolytic-like effects in a mouse model of maternal separation stress. Pharmacology. 2020;105:289–99.
Wang X, Wang X, Xie F, Sun Z, Guo B, Li F, et al. Leucine mediates cognitive dysfunction in early life stress-induced mental disorders by activating autophagy. Front Cell Neurosci. 2023;16:1060712.
Hao K, Chen F, Zhao L, Xu S, Xiong Y, Xu R, et al. Nicotinamide ameliorates mitochondria-related neuronal apoptosis and cognitive impairment via the NAD + /SIRT3 pathway. Schizophrenia. 2023;9:32.
Sameei P, Fatehfar S, Abdollahzadeh N, Chodari L, Saboory E, Roshan‐Milani S. The effects of forced exercise and zinc supplementation during pregnancy on prenatally stress‐induced behavioral and neurobiological consequences in adolescent female rat offspring. Dev Psychobiol. 2023;65:e22411.
Davinelli S, Ali S, Solfrizzi V, Scapagnini G, Corbi G. Carotenoids and cognitive outcomes: a meta-analysis of randomized intervention trials. Antioxidants. 2021;10:223.
YAJIMA M, MATSUMOTO M, HARADA M, HARA H, YAJIMA T. Effects of constant light during perinatal periods on the behavioral and neuronal development of mice with or without dietary lutein. Biomed Res. 2013;34:197–204.
Irvine N, England-Mason G, Field CJ, Dewey D, Aghajafari F. Prenatal folate and choline levels and brain and cognitive development in children: a critical narrative review. Nutrients. 2022;14:364.
Ferreira GC, McKenna MC. L-Carnitine and Acetyl-L-carnitine roles and neuroprotection in developing brain. Neurochem Res. 2017;42:1661–75.
Tochitani S. Functions of maternally-derived taurine in fetal and neonatal brain development. Adv Exp Med Biol. 2017;975:17–25.
Oja SS, Saransaari P. Significance of taurine in the brain. Adv Exp Med Biol. 2017;975:89–94.
Wu G. Important roles of dietary taurine, creatine, carnosine, anserine and 4-hydroxyproline in human nutrition and health. Amino Acids. 2020;52:329–60.
Alhassen S, Chen S, Alhassen L, Phan A, Khoudari M, De Silva A, et al. Intergenerational trauma transmission is associated with brain metabotranscriptome remodeling and mitochondrial dysfunction. Commun Biol. 2021;4:783.
Schulz KM, Pearson JN, Gasparrini ME, Brooks KF, Drake-Frazier C, Zajkowski ME, et al. Dietary choline supplementation to dams during pregnancy and lactation mitigates the effects of in utero stress exposure on adult anxiety-related behaviors. Behav Brain Res. 2014;268:104–10.
Jia N, Sun Q, Su Q, Dang S, Chen G. Taurine promotes cognitive function in prenatally stressed juvenile rats via activating the Akt-CREB-PGC1alpha pathway. Redox Biol. 2016;10:179–90.
Moreno Gudino H, Carias Picon D, de Brugada Sauras I. Dietary choline during periadolescence attenuates cognitive damage caused by neonatal maternal separation in male rats. Nutr Neurosci. 2017;20:327–35.
Tonjes R, Hecht K, Brautzsch M, Lucius R, Dorner G. Behavioural changes in adult rats produced by early postnatal maternal deprivation and treatment with choline chloride. Exp Clin Endocrinol. 1986;88:151–7.
Borsonelo EC, Suchecki D, Galduroz JC. Effect of fish oil and coconut fat supplementation on depressive-type behavior and corticosterone levels of prenatally stressed male rats. Brain Res. 2011;1385:144–50.
Bengoetxea X, Paternain L, Martisova E, Milagro FI, Martinez JA, Campion J, et al. Effects of perinatal diet and prenatal stress on the behavioural profile of aged male and female rats. J Psychopharmacol. 2017;31:356–64.
Egerton S, Donoso F, Fitzgerald P, Gite S, Fouhy F, Whooley J, et al. Investigating the potential of fish oil as a nutraceutical in an animal model of early life stress. Nutr Neurosci. 2022;25:356–78.
Paternain L, Martisova E, Campion J, Martinez JA, Ramirez MJ, Milagro FI. Methyl donor supplementation in rats reverses the deleterious effect of maternal separation on depression-like behaviour. Behav Brain Res. 2016;299:51–8.
Provensi G, Schmidt SD, Boehme M, Bastiaanssen TFS, Rani B, Costa A, et al. Preventing adolescent stress-induced cognitive and microbiome changes by diet. Proc Natl Acad Sci. 2019;116:9644–51.
Barrett E, Fitzgerald P, Dinan TG, Cryan JF, Ross RP, Quigley EM, et al. Bifidobacterium breve with alpha-linolenic acid and linoleic acid alters fatty acid metabolism in the maternal separation model of irritable bowel syndrome. PLoS One. 2012;7:e48159.
Fahmida U, AT H, AASI O, Suciyanti D, Pathurrahman P, Wangge G. Effectiveness of an integrated nutrition rehabilitation on growth and development of children under five post 2018 earthquake in East Lombok, Indonesia. Int J Env Res Public Health. 2022;19:2814.
Barker ED, Kirkham N, Ng J, Jensen SK. Prenatal maternal depression symptoms and nutrition, and child cognitive function. Br J Psychiatry. 2013;203:417–21.
(USDA) F and NS (FNS) of the USD of A. The special supplemental nutrition program for women, infants, and children (WIC). 1975. Available from: https://www.fns.usda.gov/wic.
Arons A, Bolbocean C, Bush NR, Tylavsky FA, LeWinn KZ. Participation in the special supplemental nutrition program for women, infants, and children is not associated with early childhood socioemotional development: Results from a longitudinal cohort study. Prev Med Rep. 2016;4:507–11.
Morton KR, Lee JW, Spencer-Hwang R. Plant-based dietary intake moderates adverse childhood experiences association with early mortality in an older Adventist cohort. J Psychosom Res. 2021;151:110633.
Wang H, Yin W, Ma S, Wang P, Zhang L, Li P, et al. Prenatal environmental adversity and child neurodevelopmental delay: the role of maternal low-grade systemic inflammation and maternal anti-inflammatory diet. Eur Child Adolesc Psychiatry. 2023;33:1771–81.
Machado AG, Silva Silveira AC, Peres AM, de Sa Couto-Pereira N, Trindade AA, Lucio JA, et al. Olive oil-rich diet during pregnancy/lactation attenuated the early life stress effects on depressive-like behavior and altered energy metabolism in the dorsal hippocampus in a sex-specific manner. Nutr Neurosci. 2022;25:2033–50.
Kim JY, Lee JH, Kim D, Kim SM, Koo J, Jahng JW. Beneficial effects of highly palatable food on the behavioral and neural adversities induced by early life stress experience in female rats. Int J Biol Sci. 2015;11:1150–9.
Maniam J, Morris MJ. Voluntary exercise and palatable high-fat diet both improve behavioural profile and stress responses in male rats exposed to early life stress: role of hippocampus. Psychoneuroendocrinology. 2010;35:1553–64.
Maniam J, Morris MJ. Palatable cafeteria diet ameliorates anxiety and depression-like symptoms following an adverse early environment. Psychoneuroendocrinology. 2010;35:717–28.
Lee JH, Kim JY, Jahng JW. Highly palatable food during adolescence improves anxiety-like behaviors and hypothalamic-pituitary-adrenal axis dysfunction in rats that experienced neonatal maternal separation. Endocrinol Metab. 2014;29:169–78.
Rincel M, Lepinay AL, Delage P, Fioramonti J, Theodorou VS, Laye S, et al. Maternal high-fat diet prevents developmental programming by early-life stress. Transl Psychiatry. 2016;6:e966.
Maniam J, Antoniadis CP, Le V, Morris MJ. A diet high in fat and sugar reverses anxiety-like behaviour induced by limited nesting in male rats: Impacts on hippocampal markers. Psychoneuroendocrinology. 2016;68:202–9.
MacKay JC, James JS, Cayer C, Kent P, Anisman H, Merali Z. Protracted effects of juvenile stressor exposure are mitigated by access to palatable food. PLoS One. 2014;9:e96573.
Clauss N, Allen KB, Billings KD, Tolliver MDM, Garza R, Byrd-Craven J, et al. The modification of offspring stress-related behavior and the expression of Drd1, Drd2, and Nr3c1 by a western-pattern diet in mus musculus. Int J Mol Sci. 2022;23:9245.
Rincel M, Lepinay AL, Janthakhin Y, Soudain G, Yvon S, Da Silva S, et al. Maternal high-fat diet and early life stress differentially modulate spine density and dendritic morphology in the medial prefrontal cortex of juvenile and adult rats. Brain Struct Funct. 2018;223:883–95.
Ali EF, MacKay JC, Graitson S, James JS, Cayer C, Audet MC, et al. Palatable food dampens the long-term behavioral and endocrine effects of juvenile stressor exposure but may also provoke metabolic syndrome in rats. Front Behav Neurosci. 2018;12:19.
de Bem GF, Okinga A, Ognibene DT, da Costa CA, Santos IB, Soares RA, et al. Anxiolytic and antioxidant effects of Euterpe oleracea Mart. (acai) seed extract in adult rat offspring submitted to periodic maternal separation. Appl Physiol Nutr Metab. 2020;45:1277–86.
Moradi-Kor N, Dadkhah M, Ghanbari A, Rashidipour H, Bandegi AR, Barati M, et al. Protective effects of Spirulina platensis, voluntary exercise and environmental interventions against adolescent stress-induced anxiety and depressive-like symptoms, oxidative stress and alterations of BDNF and 5HT-3 receptors of the prefrontal cortex in female rats. Neuropsychiatr Dis Treat. 2020;16:1777–94.
Sivasangari K, Rajan KE. Standardized bacopa monnieri extract ameliorates learning and memory impairments through synaptic protein, neurogranin, pro-and mature BDNF signaling, and HPA axis in prenatally stressed rat offspring. Antioxidants. 2020;9:1229.
Sivasangari K, Sivamaruthi BS, Chaiyasut C, Rajan KE. Maternal stress-induced changes in adolescent and adult offspring: Neurobehavioural improvement and telomere maintenance. Heliyon. 2023;9:e20385.
Zhang XG, Zhang H, Tan R, Peng JC, Liang XL, Liu Q, et al. Mechanism of earthquake simulation as a prenatal stressor retarding rat offspring development and chinese medicine correcting the retardation: hormones and gene-expression alteration. Evid Based Complement Altern Med. 2012;2012:670362.
Xu S, Hao K, Xiong Y, Xu R, Huang H, Wang H. Capsaicin alleviates neuronal apoptosis and schizophrenia-like behavioral abnormalities induced by early life stress. Schizophrenia. 2023;9:77.
Farzan M, Farzan M, Amini-Khoei H, Shahrani M, Bijad E, Anjomshoa M, et al. Protective effects of vanillic acid on autistic-like behaviors in a rat model of maternal separation stress: Behavioral, electrophysiological, molecular and histopathological alterations. Int Immunopharmacol. 2023;118:110112.
Araki R, Tominaga Y, Inoue R, Kita A, Yabe T. 2′-Fucosyllactose alleviates early weaning-induced anxiety-like behavior, amygdala hyperactivity, and gut microbiota changes. Biol Pharm Bull. 2023;46:b22-00803.
Feng Z, Jia H, Li X, Bai Z, Liu Z, Sun L, et al. A milk-based wolfberry preparation prevents prenatal stress-induced cognitive impairment of offspring rats, and inhibits oxidative damage and mitochondrial dysfunction in vitro. Neurochem Res. 2010;35:702–11.
Terreros G, Pérez MÁ, Muñoz-LLancao P, D’Espessailles A, Martínez EA, Dagnino-Subiabre A. The neuroprotective role of quinoa (Chenopodium quinoa, Wild) supplementation in hippocampal morphology and memory of adolescent stressed rats. Nutrients. 2024;16:381.
Moradi-Kor N, Ghanbari A, Rashidipour H, Yousefi B, Bandegi AR, Rashidy-Pour A. Beneficial effects of Spirulina platensis, voluntary exercise and environmental enrichment against adolescent stress induced deficits in cognitive functions, hippocampal BDNF and morphological remolding in adult female rats. Horm Behav. 2019;112:20–31.
Collins JM, Caputi V, Manurung S, Gross G, Fitzgerald P, Golubeva AV, et al. Supplementation with milk fat globule membrane from early life reduces maternal separation-induced visceral pain independent of enteric nervous system or intestinal permeability changes in the rat. Neuropharmacology. 2022;210:109026.
Goyal MS, Venkatesh S, Milbrandt J, Gordon JI, Raichle ME. Feeding the brain and nurturing the mind: Linking nutrition and the gut microbiota to brain development. Proc Natl Acad Sci USA. 2015;112:14105–12.
David DJ, Samuels BA, Rainer Q, Wang JW, Marsteller D, Mendez I, et al. Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron. 2009;62:479–93.
Terranova JI, Ogawa SK, Kitamura T. Adult hippocampal neurogenesis for systems consolidation of memory. Behav Brain Res. 2019;372:112035.
Korosi A, Naninck EF, Oomen CA, Schouten M, Krugers H, Fitzsimons C, et al. Early-life stress mediated modulation of adult neurogenesis and behavior. Behav Brain Res. 2012;227:400–9.
Lemaire V, Koehl M, Le Moal M, Abrous DN. Prenatal stress produces learning deficits associated with an inhibition of neurogenesis in the hippocampus. Proc Natl Acad Sci USA. 2000;97:11032–7.
Lajud N, Roque A, Cajero M, Gutierrez-Ospina G, Torner L. Periodic maternal separation decreases hippocampal neurogenesis without affecting basal corticosterone during the stress hyporesponsive period, but alters HPA axis and coping behavior in adulthood. Psychoneuroendocrinology. 2012;37:410–20.
Baek SB, Bahn G, Moon SJ, Lee J, Kim KH, Ko IG, et al. The phosphodiesterase type-5 inhibitor, tadalafil, improves depressive symptoms, ameliorates memory impairment, as well as suppresses apoptosis and enhances cell proliferation in the hippocampus of maternal-separated rat pups. Neurosci Lett. 2011;488:26–30.
Naninck EF, Hoeijmakers L, Kakava-Georgiadou N, Meesters A, Lazic SE, Lucassen PJ, et al. Chronic early life stress alters developmental and adult neurogenesis and impairs cognitive function in mice. Hippocampus. 2015;25:309–28.
Ribeiro FF, Xapelli S. Intervention of brain-derived neurotrophic factor and other neurotrophins in adult neurogenesis. Adv Exp Med Biol. 2021;1331:95–115.
Bondar NP, Merkulova TI. Brain-derived neurotrophic factor and early-life stress: multifaceted interplay. J Biosci. 2016;41:751–8.
Sairanen M, Lucas G, Ernfors P, Castrén M, Castrén E. Brain-derived neurotrophic factor and antidepressant drugs have different but coordinated effects on neuronal turnover, proliferation, and survival in the adult dentate gyrus. J Neurosci. 2005;25:1089–94.
Ogbonnaya ES, Clarke G, Shanahan F, Dinan TG, Cryan JF, O’Leary OF. Adult hippocampal neurogenesis is regulated by the microbiome. Biol Psychiatry. 2015;78:E7–9.
Meier P, Finch A, Evan G. Apoptosis in development. Nature. 2000;407:796–801.
Yang S, Li J, Han L, Zhu G. Early maternal separation promotes apoptosis in dentate gyrus and alters neurological behaviors in adolescent rats. Int J Clin Exp Pathol. 2017;10:10812–20.
Zhang LX, Levine S, Dent G, Zhan Y, Xing G, Okimoto D, et al. Maternal deprivation increases cell death in the infant rat brain. Brain Res Dev Brain Res. 2002;133:1–11.
Rathinam VAK, Fitzgerald KA. Inflammasome complexes: emerging mechanisms and effector functions. Cell. 2016;165:792–800.
Citri A, Malenka RC. Synaptic plasticity: multiple forms, functions, and mechanisms. Neuropsychopharmacology. 2008;33:18–41.
Chen YC, Baram TZ. Toward understanding how early-life stress reprograms cognitive and emotional brain networks. Neuropsychopharmacology. 2016;41:197–206.
Derks NAV, Krugers HJ, Hoogenraad CC, Jöels M, Sarabdjitsingh RA. Effects of early life stress on rodent hippocampal synaptic plasticity: a systematic review. Curr Opin Behav Sci. 2017;14:155–66.
Ganguly P, Holland FH, Brenhouse HC. Functional uncoupling NMDAR NR2A subunit from PSD-95 in the prefrontal cortex: effects on behavioral dysfunction and parvalbumin loss after early-life stress (vol 40, pg 2666, 2015). Neuropsychopharmacology. 2016;41:1188.
Wang AF, Zou XJ, Wu JJ, Ma QY, Yuan NJ, Ding FM, et al. Early-life stress alters synaptic plasticity and mTOR signaling: correlation with anxiety-like and cognition-related behavior. Front Genet. 2020;11:590068.
Hsu FC, Zhang GJ, Raol YSH, Valentino RJ, Coulter DA, Brooks-Kayal AR. Repeated neonatal handling with maternal separation permanently alters hippocampal GABA receptors and behavioral stress responses. Proc Natl Acad Sci USA. 2003;100:12213–8.
Karst H, Droogers WJ, van der Weerd N, Damsteegt R, van Kronenburg N, Sarabdjitsingh RA, et al. Acceleration of GABA-switch after early life stress changes mouse prefrontal glutamatergic transmission. Neuropharmacology. 2023;234:109543.
DiSabato DJ, Quan N, Godbout JP. Neuroinflammation: the devil is in the details. J Neurochem. 2016;139:136–53.
Carpenter LL, Gawuga CE, Tyrka AR, Lee JK, Anderson GM, Price LH. Association between plasma IL-6 response to acute stress and early-life adversity in healthy adults. Neuropsychopharmacology. 2010;35:2617–23.
Lumertz FS, Kestering-Ferreira E, Orso R, Creutzberg KC, Tractenberg SG, Stocchero BA, et al. Effects of early life stress on brain cytokines: a systematic review and meta-analysis of rodent studies. Neurosci Biobehav Rev. 2022;139:104746.
Dutcher EG, Pama EAC, Lynall ME, Khan S, Clatworthy MR, Robbins TW, et al. Early-life stress and inflammation: a systematic review of a key experimental approach in rodents. Brain Neurosci Adv. 2020;4:2398212820978049.
Bachiller S, Hidalgo I, Garcia MG, Boza-Serrano A, Paulus A, Denis Q, et al. Early-life stress elicits peripheral and brain immune activation differently in wild type and 5xFAD mice in a sex-specific manner. J Neuroinflammation. 2022;19:151.
Short AK, Baram TZ. Early-life adversity and neurological disease: age-old questions and novel answers. Nat Rev Neurol. 2019;15:657–69.
Cristofori F, Dargenio VN, Dargenio C, Miniello VL, Barone M, Francavilla R. Anti-Inflammatory and Immunomodulatory Effects of Probiotics in Gut Inflammation: a door to the body. Front Immunol. 2021;12:578386.
Li M, van Esch BCAM, Wagenaar GTM, Garssen J, Folkerts G, Henricks PAJ. Pro- and anti-inflammatory effects of short chain fatty acids on immune and endothelial cells. Eur J Pharmacol. 2018;831:52–9.
Silva YP, Bernardi A, Frozza RL. The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front Endocrinol. 2020;11:25.
Nadjar A, Leyrolle Q, Joffre C, Laye S. Bioactive lipids as new class of microglial modulators: when nutrition meets neuroimunology. Prog Neuropsychopharmacol Biol Psychiatry. 2017;79:19–26.
Reemst K, Broos JY, Abbink MR, Cimetti C, Giera M, Kooij G, et al. Early-life stress and dietary fatty acids impact the brain lipid/oxylipin profile into adulthood, basally and in response to LPS. Front Immunol. 2022;13:967437.
Joffre C, Rey C, Layé S. N-3 polyunsaturated fatty acids and the resolution of neuroinflammation. Front Pharmacol. 2019;10:1022.
Bellavance MA, Rivest STheHPA-. immune axis and the immunomodulatory actions of glucocorticoics in the brain. Front Immunol. 2014;5:136.
Picard M, Juster RP, McEwen BS. Mitochondrial allostatic load puts the “gluc” back in glucocorticoids. Nat Rev Endocrinol. 2014;10:303–10.
Manoli I, Alesci S, Blackman MR, Su YA, Rennert OM, Chrousos GP. Mitochondria as key components of the stress response. Trends Endocrinol Metab. 2007;18:190–8.
Tyrka AR, Parade SH, Price LH, Kao HT, Porton B, Philip NS, et al. Alterations of mitochondrial DNA Copy number and telomere length with early adversity and psychopathology. Biol Psychiatry. 2016;79:78–86.
Ridout KK, Parade SH, Kao HT, Magnan S, Seifer R, Porton B, et al. Childhood maltreatment, behavioral adjustment, and molecular markers of cellular aging in preschool-aged children: a cohort study. Psychoneuroendocrinology. 2019; 107:261–9.
Amini-Khoei H, Mohammadi-Asl A, Amiri S, Hosseini MJ, Momeny M, Hassanipour M, et al. Oxytocin mitigated the depressive-like behaviors of maternal separation stress through modulating mitochondrial function and neuroinflammation. Prog Neuropsychopharmacol Biol Psychiatry. 2017;76:169–78.
Masrour FF, Peeri M, Hosseini MJ, Azarbayjani MA. Exercise during adolescence attenuated depressive-like behaviors and hippocampal mitochondrial dysfunction following early life stress in adult male rats. Iran J Pharm Res. 2018;17:124–33.
Malcon LMC, Wearick-Silva LE, Zaparte A, Orso R, Luft C, Tractenberg SG, et al. Maternal separation induces long-term oxidative stress alterations and increases anxiety-like behavior of male Balb/cJ mice. Exp Brain Res. 2020;238:2097–107.
Ruigrok SR, Yim K, Emmerzaal TL, Geenen B, Stöberl N, den Blaauwen JL, et al. Effects of early-life stress on peripheral and central mitochondria in male mice across ages. Psychoneuroendocrinology. 2021;132:105346.
Quideau S, Deffieux D, Douat‐Casassus C, Pouységu L. Plant polyphenols: chemical properties, biological activities, and synthesis. Angew Chem Int Ed. 2011;50:586–621.
Giordano E, Visioli F. Long-chain omega 3 fatty acids: molecular bases of potential antioxidant actions. Prostaglandins Leukot Essent Fat Acids. 2014;90:1–4.
Zaloga GP. Narrative review of -3 polyunsaturated fatty acid supplementation upon immune functions, resolution molecules and lipid peroxidation. Nutrients. 2021;13:662.
Karatsoreos IN, Bhagat SM, Bowles NP, Weil ZM, Pfaff DW, McEwen BS. Endocrine and physiological changes in response to chronic corticosterone: a potential model of the metabolic syndrome in mouse. Endocrinology. 2010;151:2117–27.
Lefkowitz DL, Lefkowitz SS. Microglia and myeloperoxidase: a deadly partnership in neurodegenerative disease. Free Radic Biol Med. 2008;45:726–31.
Banqueri M, Gutiérrez-Menéndez A, Méndez M, Conejo NM, Arias JL. Early life stress due to repeated maternal separation alters the working memory acquisition brain functional network. Stress-Int J Biol Stress. 2021;24:87–95.
Gunnar MR, Morison SJ, Chisholm K, Schuder M. Salivary cortisol levels in children adopted from Romanian orphanages. Dev Psychopathol. 2001;13:611–28.
Essex MJ, Shirtcliff EA, Burk LR, Ruttle PL, Klein MH, Slattery MJ, et al. Influence of early life stress on later hypothalamic-pituitary-adrenal axis functioning and its covariation with mental health symptoms: a study of the allostatic process from childhood into adolescence. Dev Psychopathol. 2011;23:1039–58.
Vargas J, Junco M, Gomez C, Lajud N. Early life stress increases metabolic risk, HPA axis reactivity, and depressive-like behavior when combined with postweaning social isolation in rats. PLoS One. 2016;11:e0162665.
Juruena MF, Bourne M, Young AH, Cleare AJ. Hypothalamic-pituitary-adrenal axis dysfunction by early life stress. Neurosci Lett. 2021;759:136037.
de Punder K, Pruimboom L. Stress induces endotoxemia and low-grade inflammation by increasing barrier permeability. Front Immunol. 2015;6:223.
Ng J, Papandreou A, Heales SJ, Kurian MA. Monoamine neurotransmitter disorders-clinical advances and future perspectives. Nat Rev Neurol. 2015;11:567–84.
Whitaker-Azmitia PM. Serotonin and brain development: Role in human developmental diseases. Brain Res Bull. 2001;56:479–85.
Ikemoto S. Brain reward circuitry beyond the mesolimbic dopamine system: a neurobiological theory. Neurosci Biobehav Rev. 2010;35:129–50.
Reeves S, Bench C, Howard R. Ageing and the nigrostriatal dopaminergic system. Int J Geriatr Psychiatry. 2002;17:359–70.
Lyons DJ, Hellysaz A, Broberger C. Prolactin regulates tuberoinfundibular dopamine neuron discharge pattern: novel feedback control mechanisms in the lactotrophic axis. J Neurosci. 2012;32:8074–83.
Adjimann TS, Argañaraz CV, Soiza-Reilly M. Serotonin-related rodent models of early-life exposure relevant for neurodevelopmental vulnerability to psychiatric disorders. Transl Psychiatry. 2021;11:280.
Bonapersona V, Joëls M, Sarabdjitsingh RA. Effects of early life stress on biochemical indicators of the dopaminergic system: a 3 level meta-analysis of rodent studies. Neurosci Biobehav Rev. 2018;95:1–16.
Benekareddy M, Goodfellow NM, Lambe EK, Vaidya VA. Enhanced function of prefrontal serotonin 5-HT receptors in a rat model of psychiatric vulnerability. J Neurosci. 2010;30:12138–50.
Ohta K, Miki T, Warita K, Suzuki S, Kusaka T, Yakura T, et al. Prolonged maternal separation disturbs the serotonergic system during early brain development. Int J Dev Neurosci. 2014;33:15–21.
Rentesi G, Antoniou K, Marselos M, Syrrou M, Papadopoulou-Daifoti Z, Konstandi M. Early maternal deprivation-induced modifications in the neurobiological, neurochemical and behavioral profile of adult rats. Behav Brain Res. 2013;244:29–37.
Reigstad CS, Salmonson CE, Rainey JF, Szurszewski JH, Linden DR, Sonnenburg JL, et al. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. Faseb J. 2015;29:1395–403.
Dallman MF, Pecoraro N, Akana SF, la Fleur SE, Gomez F, Houshyar H, et al. Chronic stress and obesity: a new view of “comfort food”. Proc Natl Acad Sci USA. 2003;100:11696–701.
Borre YE, O’Keeffe GW, Clarke G, Stanton C, Dinan TG, Cryan JF. Microbiota and neurodevelopmental windows: implications for brain disorders. Trends Mol Med. 2014;20:509–18.
Codagnone MG, Spichak S, O’Mahony SM, O’Leary OF, Clarke G, Stanton C, et al. Programming bugs: microbiota and the developmental origins of brain health and disease. Biol Psychiatry. 2019;85:150–63.
Berding K, Vlckova K, Marx W, Schellekens H, Stanton C, Clarke G, et al. Diet and the microbiota-gut-brain axis: sowing the seeds of good mental health. Adv Nutr. 2021;12:1239–85.
Fung TC, Olson CA, Hsiao EY. Interactions between the microbiota, immune and nervous systems in health and disease. Nat Neurosci. 2017;20:145–55.
Eng A, Borenstein E. Taxa-function robustness in microbial communities. Microbiome. 2018;6:45.
Human Microbiome Project C. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–14.
Valles-Colomer M, Falony G, Darzi Y, Tigchelaar EF, Wang J, Tito RY, et al. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat Microbiol. 2019;4:623–32.
Spichak S, Bastiaanssen TFS, Berding K, Vlckova K, Clarke G, Dinan TG, et al. Mining microbes for mental health: determining the role of microbial metabolic pathways in human brain health and disease. Neurosci Biobehav Rev. 2021;125:698–761.
Gheorghe CE, Martin JA, Manriquez FV, Dinan TG, Cryan JF, Clarke G. Focus on the essentials: tryptophan metabolism and the microbiome-gut-brain axis. Curr Opin Pharmacol. 2019;48:137–45.
Clarke G, Grenham S, Scully P, Fitzgerald P, Moloney RD, Shanahan F, et al. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol Psychiatry. 2013;18:666–73.
Desbonnet L, Garrett L, Clarke G, Bienenstock J, Dinan TG. The probiotic bifidobacteria infantis: an assessment of potential antidepressant properties in the rat. J Psychiatr Res. 2008;43:164–74.
Tette FM, Kwofie SK, Wilson MD. Therapeutic anti-depressant potential of microbial GABA produced by lactobacillus rhamnosus strains for GABAergic signaling restoration and inhibition of addiction-induced HPA axis hyperactivity. Curr Issues Mol Biol. 2022;44:1434–51.
McPherson AC, Pandey SP, Bender MJ, Meisel M. Systemic immunoregulatory consequences of gut commensal translocation. Trends Immunol. 2021;42:137–50.
Barreau F, Ferrier L, Fioramonti J, Bueno L. Neonatal maternal deprivation triggers long term alterations in colonic epithelial barrier and mucosal immunity in rats. Gut. 2004;53:501–6.
Garcia-Rodenas CL, Bergonzelli GE, Nutten S, Schumann A, Cherbut C, Turini M, et al. Nutritional approach to restore impaired intestinal barrier function and growth after neonatal stress in rats. J Pediatr Gastroenterol Nutr. 2006;43:16–24.
Kelly JR, Kennedy PJ, Cryan JF, Dinan TG, Clarke G, Hyland NP. Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front Cell Neurosci. 2015;9:392.
Lee BH, Eid RS, Hodges TE, Barth C, Galea LAM. Leveraging research into sex differences and steroid hormones to improve brain health. Nat Rev Endocrinol. 2024;21:214–229.
Wierenga LM, Ruigrok A, Aksnes ER, Barth C, Beck D, Burke S, et al. Recommendations for a better understanding of sex and gender in the neuroscience of mental health. Biol Psychiatry Glob Open Sci. 2024;4:100283.
Bale TL, Epperson CN. Sex differences and stress across the lifespan. Nat Neurosci. 2015;18:1413–20.
Wilmes L, Caputi V, Bastiaanssen TFS, Collins JM, Crispie F, Cotter PD, et al. Sex specific gut-microbiota signatures of resilient and comorbid gut-brain phenotypes induced by early life stress. Neurobiol Stress. 2024;33:100686.
Parel ST, Peña CJ. Genome-wide signatures of early-life stress: influence of sex. Biol Psychiatry. 2022;91:36–42.
Reemst K, Ruigrok SR, Bleker L, Naninck EFG, Ernst T, Kotah JM, et al. Sex-dependence and comorbidities of the early-life adversity induced mental and metabolic disease risks: Where are we at? Neurosci Biobehav Rev. 2022;138:104627.
Christians JK, Reue K. The role of gonadal hormones and sex chromosomes in sex-dependent effects of early nutrition on metabolic health. Front Endocrinol. 2023;14:1304050.
Varlamov O, Bethea CL, Roberts CT. Sex-specific differences in lipid and glucose metabolism. Front Endocrinol. 2015;5:241.
Cruceanu C, Dony L, Krontira AC, Fischer DS, Roeh S, Di Giaimo R, et al. Cell-type-specific impact of glucocorticoid receptor activation on the developing brain: a cerebral organoid study. Am J Psychiatry. 2022;179:375–87.
Du Preez A, Lefèvre-Arbogast S, González-Domínguez R, Houghton V, de Lucia C, Low DY, et al. Impaired hippocampal neurogenesis in vitro is modulated by dietary-related endogenous factors and associated with depression in a longitudinal ageing cohort study. Mol Psychiatry. 2022;27:3425–40.
Houghton V, Du Preez A, Lefèvre-Arbogast S, de Lucia C, Low DY, Urpi-Sarda M, et al. Caffeine compromises proliferation of human hippocampal progenitor cells. Front Cell Dev Biol. 2020;8:806.
Borsini A, Stangl D, Jeffries AR, Pariante CM, Thuret S. The role of omega-3 fatty acids in preventing glucocorticoid-induced reduction in human hippocampal neurogenesis and increase in apoptosis. Transl Psychiatry. 2020;10:219.
Author information
Authors and Affiliations
Contributions
AK, conceptualized and supervised this study and reviewed and edited the manuscript. Literature search was performed and screened by JG and HGJ with support from GLB. LW contributed to the mechanisms related to microbiome. JG and HGJ analyzed the included papers, prepared Figs 2 and 3 and the tables and wrote the manuscript. KJOR prepared Fig. 1. AK, JG, HGJ, LW, SRdR, JBvG, GC, and JFC contributed to critically read and edit the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Geertsema, J., Juncker, H.G., Wilmes, L. et al. Nutritional interventions to counteract the detrimental consequences of early-life stress. Mol Psychiatry 30, 3269–3300 (2025). https://doi.org/10.1038/s41380-025-03020-1
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41380-025-03020-1