Abstract
Selective Serotonin Reuptake Inhibitors (SSRIs) are among the most common medications used for depression in postpartum and lactating people, who experience increased depression risk. However, there is a limited understanding of peripartum SSRI impacts on maternal neurobehavioral responses, and particularly those of sertraline, the most prescribed SSRI in United States (US) pregnancies. We administered C57Bl/6 females sertraline via a non-invasive, naturalistic approach (167 mg/L drinking water) from 2 weeks pre-conception through lactation (PND21) or for an equivalent duration in nonpregnant controls. We assessed behavior and molecular brain changes intrapartum and postpartum at ~1 year of age. Chronic sertraline reduced depressive- and anxiety-like behaviors. Pregnancy itself decreased anxiety-like and hedonic behaviors. RNA sequencing of maternal brain revealed only 52 differentially expressed genes (DEGs) in frontal cortex with sertraline. These DEGs over-represented functions related to immunity. In contrast, sertraline altered 962 targets in maternal hypothalamic paraventricular nucleus, with DEGs overrepresenting neurotransmission and neurodegeneration. We then discontinued sertraline and aged animals to approximately 1 year to test neurodegenerative phenotypes. Having one prior litter, regardless of peripartum sertraline, improved aged females' spatial learning and memory. Sertraline, regardless of postpartum status, improved working memory. Further, we found buffering of neurodegeneration-related gene network changes and increased excitatory synapse density in the hippocampus after peripartum sertraline. Peripartum sertraline alters maternal neurobiology and behavior in pregnancy and beyond, with long-term benefits to neurodegenerative processes. Pregnancy also exerts its own, lasting effects on learning and memory. These findings might be exploited in the future to abrogate neurodegenerative disease.
Similar content being viewed by others
Introduction
Perinatal mood and anxiety disorders (PMADs) impact approximately 1 in 5 pregnant and postpartum people [1, 2]. Rates have increased in recent years, and in particular since the COVID-19 pandemic era when PMAD prevalence more than doubled [3]. In 2022, mental health issues (suicide and overdose) were the leading cause of death in the first postpartum year [4] leading to the declaration of an Urgent Public Health Crisis in maternal health by The U.S. Department of Health and Human Services [5]. Selective serotonin reuptake inhibitors (SSRIs) are the most prescribed class of antidepressants, extensively utilized for their efficacy in treating mood disorders and PMADs [6]. SSRI use among people who are pregnant, postpartum, or lactating is common, with approximately 6–8% of pregnant people in the US prescribed an SSRI [7].
In response to the rising crisis of PMADs, antepartum (conception through delivery) SSRI use increased by nearly one-third over the early 2000’s in the US [8]. SSRIs are thought to modulate depressive symptoms by modifying serotonin uptake at the synapse. The serotonin transporter (SERT) takes up serotonin into the presynaptic neuron. SSRIs block SERT, preventing serotonin reuptake and allowing it to remain in the synaptic space where it continues to drive serotonin activity and thereby exerts therapeutic effects. Other SSRI mechanisms of action may also be involved in drug impacts. Recent work has revealed that neurotrophic, vascular, immunologic, microbiome, and other SSRI mechanisms are also involved [9,10,11,12,13,14,15,16]. Recent debates [17] have further emphasized a lack of clear understanding of SSRI mechanisms of action in the brain and periphery. Layered atop the dynamic neuroendocrine and metabolic changes of pregnancy, the neurobiological impacts of SSRIs in antepartum and postpartum become that much more unclear and in need of study [18].
The existent literature on SSRIs in peripartum has largely focused on impacts to offspring and potential teratogenicity [19,20,21,22,23]. Studies of peripartum SSRI often neglect to measure maternal biology (e.g., organ function, circulating drug levels, gestational outcomes), especially long-term, and are limited by confounds of intermittent blockade and administration stress (e.g. as with repetitive oral gavage or injection). These have their own, independent impacts on maternal-fetal biology and behavior, which are also related to serotonin-mediated phenotypes. For example, restraint stress alters mood-related phenotypes and is used to model depressive-like behavior and neurobiology in preclinical settings [24,25,26]. We address these limitations by maintaining a focus on maternal neurobiology and validating a noninvasive, naturalistic, chronic SSRI administration approach for use in pregnant mice: Ad libitum sertraline in drinking water throughout peripartum. While sertraline is the most prescribed SSRI used in US pregnancies [7], gestational sertraline is sparsely studied in preclinical models. Our approach non-invasively achieves a low dose of sertraline in murine pregnancy without impacts on gestational health to reveal effects on maternal brain and behavior in pregnancy and long-after, in the postpartum animal.
Our study goals were to examine the behavioral and molecular impacts of peripartum sertraline in a mouse model across cortical and subcortical brain regions involved in mood and stress response in both mice and humans [27]. We also explored gene expression related to neurodegeneration in young, pregnant animals after sertraline exposure. Subsequent, complementary behavioral studies in aged animals many months following a single pregnancy further examined learning and memory benefits of both pregnancy itself and of peripartum sertraline. Our work aimed to not only reveal immediate impacts of peripartum sertraline on brain health and function, but also to provide new insights into enduring consequences for maternal brain health across the lifespan.
Methods and materials
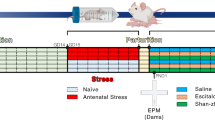
Female C57Bl6/J mice (Jackson Labs, Bar Harbor, ME) approximately 6–8 weeks of age were administered sertraline (167 mg/L oral suspension; NorthstarRx LLC Pharmaceuticals) or water ad libitum via in-cage bottles starting two weeks prior to breeding then for the duration of pregnancy and lactation, or for an equivalent duration (approximately 8 weeks) in non-pregnant animals. For neurodegeneration studies, animals were aged to approximately one year (n = 3–4/condition) prior to behavior, molecular, and histology studies.
Plasma and amniotic fluid sertraline were confirmed by liquid chromatography–mass spectrometry/mass spectrometry (LC-MS/MS), and circulating serotonin was measured by ELISA. Mice were used for intrapartum behavioral assessment (n = 5–8/condition) by the forced swim test, tail suspension test, elevated plus maze, open field test, hedonic assessment (chocolate:chow preference testing), barnes maze, and radial arm maze.
Tissues were collected by tissue punch from fresh frozen coronal brain sections for RNA sequencing (n = 3/condition/region; Illumina HiSeq platform) or qPCR studies of maternal brain (PVN and cortex) at gestational day 18 (GD18) (primers in Table 1). Complementary sections were immunostained for VGlut1 (1:1000 Millipore AB5905) and PSD-95 (1:200, Millipore AB9708) and synaptic density in the cortex, hippocampus, and corpus callosum quantified by ImageJ. Animal protocols received prior approval from the University of Iowa Institutional Animal Care and Use Committee (IACUC). Methods are fully detailed in the Supplement and outlined in Fig. 1A.
A Experimental timeline. Gestational day, GD; Experimental day, ED. B Oral sertraline (167 mg/L oral suspension) increases sertraline in plasma to approximately 9 ng/mL via LC-MS/MS and (C) in amniotic fluid after drinking water sertraline administration in pregnancy to approximately 0.7 ng/mL, both at GD18. Sertraline dosing began 2 weeks prior to breeding, and (D, E) weight gains were apparent after only 2 weeks of sertraline dosing (experimental day 0). Two weeks of sertraline pre-breeding increased weight of dams by GD or ED 0, which persisted across ED 12, 18 in nonpregnant animals (two-way repeated measures [R.M.] ANOVA main effect [M.E.] of sertraline: F[1,27] = 11.83, p = 0.002) and GD 12 and 18 in pregnant animals (two-way R.M. ANOVA M.E. of sertraline: F[1,21] = 29.60, p < 0.0001). F Plasma 5-HT is increased across pregnancy and in particular at GD18 by post-hoc Holm t-test (q = 0.014).
Results
Chronic oral sertraline permeates maternal and amniotic biofluids and promotes weight gain without disrupting obstetric health
Our experimental timeline (Fig. 1A) involved breeding, behavioral testing, and maternal molecular and histologic brain studies at multiple timepoints: in gestation and 38 weeks later, when animals were approximately one year of age, equivalent to a mature adult human with reduced fertility [28]. Treatment of drinking water with sertraline did not change consumption across two days of testing (Day 1: p = 0.92; Day 2: p = 0.39) (Fig. S1, C, D). Given this consumption rate and sertraline concentration in the water bottles, daily consumption was calculated to approximate a 1.28 mg daily dose of sertraline, roughly equivalent to a 25–50 mg human dose after accounting for mouse and pregnancy metabolism and drug clearance differences [29] (using animal equivalent dose calculation based on body surface area, as described previously [30, 31]: Km ratio of mouse/human= 3/37 × 25–50 mg daily dose= 2/3–4.05 mg/day x 0.5 pregnancy clearance adjustment= 1.015–2.025 mg/day). We further found that naturalistic administration of sertraline (167 mg/L oral suspension) ad libitum via in-cage drinking water throughout pregnancy achieves plasma sertraline levels at GD18 of approximately 9 ng/mL (Control: 0.038 ± 0.01 ng/mL, n = 3; SSRI: 8.952 ± 0.29, n = 4; p < 0.0001; Fig. 1B) and elevated amniotic fluid sertraline (Control: 0.020 ± 0.004 ng/mL, n = 3; SSRI: 0.74 ± 0.11, n = 4; p = 0.0023; Fig. 1C) via LC-MS/MS (Fig. S1A, B).
Two weeks of sertraline pre-breeding increased weight of dams by GD or ED 0, which persisted across ED 12, 18 in nonpregnant animals (two-way repeated measures [R.M.] ANOVA main effect [M.E.] of sertraline: F[1,27] = 11.83, p = 0.002; Fig. 1D) and GD 12 and 18 in pregnant animals (two-way R.M. ANOVA M.E. of sertraline: F[1,21] = 29.60, p < 0.0001; Fig. 1E). There were no differences in baseline animal weight by group (pregnant sertraline vs water: p = 0.54; non-pregnant sertraline vs water: p = 0.12; n = 11–21/group), nor weight gain interactions between sertraline treatment and pregnancy status (p = 0.44; Fig. 1D, E). Two weeks of sertraline pre-breeding significantly increased plasma 5-HT concentrations in sertraline treated animals, driven by GD18 (two-way ANOVA M.E of sertraline: F[1, 29] = 5.467, p = 0.027; post-hoc q = 0.014; Fig. 1F). Sertraline did not alter maternal GD16 urine protein (Fig. S1E), litter size (Fig. S1F), nor GD or ED 16–18 systolic blood pressure (Fig. S1G).
Pregnancy and sertraline exert differential effects on behavior
To further test our model of naturalistic sertraline administration for effects on mood, anxiety-like, and hedonic behaviors and for interactions with pregnancy, we assessed behavior in late pregnancy and in non-pregnant controls. The forced swim test revealed that sertraline increased swim time, an effect driven by the non-pregnant group (two-way ANOVA M.E. of sertraline: F[1, 20] = 5.017, p = 0.016 post-hoc difference by two-step Benjamini, Krieger, and Yekutieli (BKY) method in non-pregnant animals, q = 0.004; Fig. 2A).
A On the Forced Swim Test (FST), sertraline exposure significantly increased total swim time driven by non-pregnant animals (two-way ANOVA main effect (M.E) of SSRI: F[1, 20] = 5.017, †p = 0.037; post-hoc difference by two-step Bejamini, Krieger, and Yekutieli (BKY) method in non-pregnant animals, q = 0.004). B Total struggle time in the Tail Suspension Test (TST) was increased in non-pregnant animals only (two-way ANOVA M.E of pregnancy: F[1, 18] = 7.007, *p = 0.017) and (C) TST bouts of struggle were significantly increased in non-pregnant animals only (two-way ANOVA M.E of pregnancy, F[1, 18] = 32.04, *p < 0.0001). D Pregnant mice exhibited reduced open arm visits on the Elevated Plus Maze (EPM), driven by non-pregnant sertraline-treated animals (two-way ANOVA M.E of pregnancy: F[1, 18] = 29.77, *p < 0.0001; post-hoc difference by BKY method, q = 0.018), sertraline-treated animals had significantly increased open arm visits, driven by the non-pregnant group (two-way ANOVA M.E of sertraline: F[1, 18] = 7.077, †p = 0.0159, post-hoc difference by two-step BKY method, q = 0.018), and sertraline treatment increased open arm visits in the non-pregnant group only (ANOVA pregnancy x sertraline interaction: F[1, 18] = 4.814, ‡p = 0.0416). E Non-pregnant animals exhibited significantly less inner:outer time on the open field test, which was driven by non-pregnant animals (two-way ANOVA M.E of pregnancy: F[1, 18] = 6.218, *p = 0.022; post-hoc difference by two-step BKY method, q = 0.039). F Preference for a high-sugar treat (chocolate kisses) versus normal chow was increased in non-pregnant animals relative to pregnant animals regardless of sertraline status (two-way ANOVA M.E of pregnancy: F[1, 18] = 252.1, *p < 0.0001). † Main effect of sertraline by two-way ANOVA; * main effect of pregnancy by two-way ANOVA; ‡ Pregnancy-by-sertraline interaction by two-way ANOVA.
The tail suspension test revealed main effects of pregnancy: significantly less struggle time (two-way ANOVA M.E. of pregnancy: F[1, 18] = 7.007, p = 0.017; Fig. 2B) and fewer bouts of struggle among pregnant mice (F[1, 18] = 32.04, p < 0.0001; Fig. 2C).
On the elevated plus maze (EPM), pregnant animals made fewer visits to the open arms (two-way ANOVA pregnancy M.E.: F[1, 18] = 29.77, p < 0.0001; post-hoc q = 0.018; Fig. 2D) and sertraline animals made significantly more open arm visits, driven by the non-pregnant group (two-way ANOVA M.E. of sertraline: F[1, 18] = 7.077, p = 0.016, post-hoc q = 0.018; Fig. 2D).
On the open field test (OFT), non-pregnant animals spent significantly less time in the inner region of the OFT relative to the outer region (two-way ANOVA M.E. of pregnancy: F[1, 18] = 6.218, p = 0.022; Fig. 2E). Sertraline exposure interacted with pregnancy, decreasing time spent in the inner relative to outer region in non-pregnant animals only (interaction: F[1, 18] = 5.593, p = 0.024; post-hoc q = 0.039; Fig. 2E). There were no significant differences in total distance traveled in the OFT apparatus, regardless of pregnancy or sertraline status (Fig. S1H).
Hedonic behavior was assessed by measuring preference for a high-sugar treat (chocolate) versus standard chow. This unique alternative to the sucrose preference test allowed for continuous sertraline administration via in-cage water bottle. Non-pregnant animals, regardless of sertraline, consumed more chocolate vs chow compared to pregnant counterparts (two-way ANOVA M.E. of pregnancy: F[1, 18] = 252.1, p < 0.0001; Fig. 2F). Total chow or chocolate treat consumption revealed no change by sertraline status (Fig. S1I, J). Pregnancy suppressed appetite for both chow (two-way ANOVA main effect of pregnancy: F[1, 14] = 17.14; p = 0.001) and chocolate (F[1, 14] = 138.0; p < 0.0001) over 24 h in late gestation (Fig. S1I, J).
Collectively, these results demonstrate that sertraline reduced depressive- and anxiety-like behaviors, as measured by performance on the FST and EPM, respectively. Pregnancy obscured effects and drove main effects on the hedonic assay of chocolate consumption, TST, EPM, and OFT, where it interacted with sertraline status to decrease inner:outer time.
Transcriptomics of late-gestation maternal brain paraventricular nucleus and frontal cortex reveal neurodegeneration and neuroimmune changes, respectively, due to sertraline
We next tested whether sertraline led to cortical and subcortical changes in maternal brain. We compared sertraline to water-treated maternal brain transcriptomics from the last day of gestation (GD 18) in frontal cortex and in the hypothalamic paraventricular nucleus (PVN) (N = 3/condition/region). These brain regions were selected for their critical roles in the regulation and generation of affective and mood-related phenotypes [32]. Nonpregnant animal transcriptional changes with SSRI exposure have been described previously [33] and were beyond the scope of the present study, given our focus on neurobiology within pregnancy.
Differentially expressed gene (DEG) analyses revealed 506 down-regulated and 456 up-regulated genes in the sertraline-exposed maternal PVN and 34 down-regulated and 18 up-regulated genes in sertraline-exposed maternal frontal cortex (Fig. 3C, D; Supplementary Tables 1, 2; PCA: Fig. S6A, B). Of these, 11 were overlapping between regions, though only two in valence (downregulated in both; Fig. 3B). DEG enrichment for pathway-based gene sets was tested and revealed over-representation of neuronal and synaptic sets (e.g., Neuronal System, Transmission across Chemical Synapses, Synaptic Vesicle Pathway) in PVN and immunologic sets (e.g., Neutrophil degranulation, Immune System, IL-17 signaling pathway) in frontal cortex with sertraline (Fig. 3E, F; Tables 2, 3). Among the drivers of these changes were DEGs including synaptic genes Satb2 and Tbr1 in PVN and immune regulatory genes Mmp9 and Mmp8 in frontal cortex.
A Differentially expressed genes (DEG) in RNA sequencing datasets from hypothalamic paraventricular nucleus (PVN) and frontal cortex reveal changes in each region and overlapping changes. Top 10 DEGs with characterized protein products ranked by fold change (FC) with associated adjusted (adj) p values listed. PVN list contains top 5 up-regulated and down-regulated, by FC, respectively. Cortex includes top 10 by absolute FC. B Overlapping DEGs between PVN and frontal cortex datasets. C) Volcano plot visualization of global transcriptional changes in the PVN, with each data point representing a gene. DEGs with an adjusted p-value < 0.05 and a log2FC > 1 are indicated by red; log2FC < −1 indicated by green dots. D Volcano plot of DEGs in frontal cortex. E Select enriched pathway-based sets that are significantly over-represented among DEGs in PVN and (F) Cortex. Pathways are sourced from Reactome, Wikipathways, and KEGG via the Consensus Pathway Database (CPDB; Max Planck Institute). Details of pathway lists are provided in Tables 2, 3. G Significant overlap between PVN DEGs and Alzheimer’s Disease (AD) target genes, identified by the National Institute on Aging’s Accelerating Medicines Partnership in Alzheimer’s Disease (AMP-AD) consortium *** P < 0.0001 by chi-square. Cortical DEGs did not significantly overlap with Alzheimer’s (Alz.) genes. #P = 0.061 by chi-square. Created in BioRender. Gumusoglu, S. (2024) BioRender.com/d71t292. H Overlap between PVN DEGs and AD target genes included 92 genes; the top 5 most up-regulated and top 5 most down-regulated are listed by FC and detail target risk score (sum of multiomic and genetic risk scores, range 0–5), multiomic risk score (summary score of transcriptomic and proteomic studies supporting association of target gene with late-onset AD, range 0–2), and genetic risk score (summary score of genetic evidence supporting target gene’s association with late-onset AD drawn from genetic and phenotypic evidence from human and model organism studies, range 0–3). Target risk scores are gradient filled by magnitude, with darkest yellow being the highest score. Inferior frontal gyrus (IFG) expression in AD FC and P values are provided for comparison. AD scores and values all from https://agora.adknowledgeportal.org/.
PVN DEGs implicated in Alzheimer’s Disease (AD) were among the top hits, for example Synpo and Mef2c [34,35,36,37,38,39,40]. Significant overlap between PVN DEGs and AD genes more broadly was tested by over-representation analyses against the National Institute on Aging’s Accelerating Medicines Partnership in Alzheimer’s Disease (AMP-AD) consortium dataset (agora.adknowledgeporta.org). Cortical DEGs did not significantly overlap with AD genes (P = 0.061 by chi-square) but overlap between PVN DEGs and AD genes was significant (P < 0.0001 by chi-square) and included 92 DEGs (Fig. 3G). AD target risk scores of overlapping DEGs ranged from 1.19 (S100a8) to 4.03 (Cdh23), indicating strong genetic and phenotypic evidence of association with AD (Fig. 3H).
To examine specificity for AD versus depression genes, which might also be altered by chronic SSRI use, we also evaluated overlap with major depressive disorder (MDD) variants identified previously in three large genome-wide association studies [41,42,43]. No cortical DEGs and only two PVN DEGs were found to overlap with MDD intron variants: rs9530139 (mapped to B3GLCT) and rs112348907 (mapped to KCNQ5), both identified in a large study of loci associated with MDD in individuals of European descent [42, 44]. We further evaluated overlap with DEGs identified by transcriptomic profiling of 87 cerebral cortical samples from subjects with MDD (FDR < 0.05) [44]. Of these DEGs, none overlapped with cortical RNA seq hits but 11 overlapped with PVN RNA seq DEGs (see Supplementary Table 2, “Overlap with PMID: 29439242; FDR < 0.05” column). PVN DEGs did not significantly over-represent MDD DEGs from this dataset (P = 0.89 by chi-square).
Both postpartum status and perinatal sertraline improve learning and memory with age
Given our transcriptomic findings linking sertraline in pregnancy with AD-associated gene expression intrapartum, we additionally tested learning and memory behaviors after aging mice to approximately 1 year. We utilized a three-day Barnes maze and a one-day radial arm maze task [45, 46] to assess spatial learning/memory and working memory, respectively.
On day 1 of Barnes maze testing, there was no difference in overall baseline locomotion in either postpartum (trial 1, day 1: Water 2.677 + /− 2.051, SSRI 2.766 +/− 2.232, p = 0.952) or nonpostpartum animals (trial 1, day 1: Water 5.177 +/− 5.552, SSRI 9.082 +/− 6.024, p = 0.199). Postpartum status improved spatial learning and memory performance on day 1 (two-way ANOVA M.E of pregnancy: F[1, 21] = 8.661, p = 0.008; Fig. 4A) and day 2 (F[1, 20] = 5.485, p = 0.029; Fig. 4B). On day 3, postpartum animals had improved performance in control animals but not sertraline-treated animals (ANOVA interaction: F[1, 19] = 4.203, p = 0.034; post-hoc q = 0.034; Fig. 4C).
Barnes Maze was used to assess spatial memory in aged animals. A On the Barnes Maze, postpartum status significantly improved performance measured by distance to the target on day one (two-way ANOVA main effect (M.E) of pregnancy: F[1, 21] = 8.661, *p = 0.008) and (B) on day 2 (two-way ANOVA M.E of pregnancy: F[1, 20] = 5.485, *p = 0.029). C On day 3 of Barnes maze, postpartum status improved performance in control animals, but not sertraline-treated animals (ANOVA Interaction: F[1, 19] = 4.203, ‡p = 0.034; post-hoc difference by two-step Benjamin, Krieger, and Yekutieli (BKY) method, q = 0.034). D Control mice demonstrated that postpartum animals had significantly improved performance relative to non-postpartum animals(two-way ANOVA M.E of pregnancy: F[1, 11] = 12.92, *p = 0.004; post-hoc trend difference by two-step BKY method, q = 0.051). E Postpartum status was also sufficient to improve performance in sertraline treated mice (two-way ANOVA M.E of pregnancy: F[1, 10] = 5.244, *p = 0.045). F STARR Maze was used to assess working memory in aged animals. Sertraline treatment significantly reduced working memory errors, regardless of pregnancy status (two-way ANOVA M.E of sertraline: F[1, 20] = 9.094, †p = 0.016). † Main effect of sertraline by two-way ANOVA; * main effect of pregnancy by two-way ANOVA; ‡ Pregnancy-by-sertraline interaction by two-way ANOVA.
We next analyzed Barnes maze performance to understand postpartum impacts across test days. Among water controls, postpartum aged mice demonstrated significantly improved performance over all three days of testing (two-way ANOVA M.E. of pregnancy: F[1, 11] = 5.409, p = 0.002; day 3 post-hoc q = 0.051; Fig. 4D). Similarly, postpartum sertraline-treated animals demonstrated improved performance on the Barnes Maze over all three days of testing (F[1, 10] = 5.244, p = 0.040; Fig. 4E). No single day of testing drove these changes by post-hoc test.
Working memory was next assessed via a one-day radial arm maze test. Sertraline-treated females exhibited fewer errors on this task than controls, regardless of postpartum status (two-way ANOVA M.E. of sertraline: F[1, 20] = 9.094, p = 0.016; Fig. 4F).
Hippocampal and cortical neurodegeneration-related molecular phenotypes are buffered by peripartum sertraline and by postpartum status
Given buffering of learning and memory behaviors by sertraline and postpartum status, we next examined long-term molecular changes in brain regions subserving these functions in our aged animal cohort: the frontal cortex and hippocampus. Interaction analyses of DEGs from GD18 maternal brain RNAseq identified targets: RNAseq DEGs Grin2a, Grin2b, Itpr1, and Plcb1 strongly interact with Gsk3b, Psen1, and others in the “Alzheimer’s disease” (WikiPathways) network (Fig. S2). qPCR revealed disruption of genes by peripartum sertraline in this network in aged, postpartum cortex, including significantly decreased cortical Psen1 (p = 0.006), Csnk1a1 (p = 0.044), and Csnk2a2 (p = 0.020) (Fig. 5A). Contrary to this, only Synpo (p = 0.017) was significantly decreased by peripartum sertraline in aged, non-postpartum cortex (Fig. 5A). Also in aged mice, qPCR of postpartum hippocampus revealed no significant differences in gene expression with sertraline, while in non-postpartum animals’ expression of AD-related Psen1 (p = 0.008), Erk2 (p = 0.019), Fyn (p = 0.056), and Csnk2a2 (p = 0.012) were decreased or trend-decreased compared to water controls (Fig. 5B).
A In postpartum aged cortex, there was significantly decreased expression of Psen1 (two-tailed, unpaired t-test, p = 0.006), Csnk1a1 (p = 0.044), and Csnk2a2 (p = 0.020) with sertraline. In non-postpartum animals, there was trend-decreased Fyn expression (p = 0.10), and significantly increased Synpo expression (p = 0.017) compared to water-treated non-postpartum controls. B Analyses of aged hippocampus via qPCR revealed no significant differences in sertraline-treated postpartum animals, but decreased expression of Psen1 (two-tailed, unpaired t-test, p = 0.008), Fyn (p = 0.056), Erk2 (p = 0.019), and Csnk2a2 (p = 0.012) in non-postpartum sertraline-treated animals. C In postpartum, there were significantly increased colocalized Vglut1 and PSD-95 synaptic puncta in hippocampal CA3 among aged, sertraline-treated (vs water control) dams (two-tailed t-test, p = 0.030). D Representative images at 20x used to quantify CA3 synaptic density for water-treated animals E and sertraline-treated animals. Orange color indicates Vglut1 and PSD-95 overlap at a synapse. F Summary of main antepartum and long-term effects on neurobehavior of peripartum SSRI and/or pregnancy itself; postpartum (PP); Alzheimer’s Disease (AD). Created in BioRender. Gumusoglu, S. (2024) BioRender.com/d71t292.
Complementary studies in cortex and hippocampus evaluated whether AD network genes were also changed by chronic SSRI in late gestation (GD 18) or in non-pregnant females. Overall, we found more wide-spread changes in AD-related gene expression in late gestation than in aged brain; 8 genes significantly changed in GD 18 cortex by SSRI and 4 in nonpregnant cortex by SSRI (Fig. S5A). As in aged brain, hippocampal changes were subtle, with only Synpo (p = 0.017) and Csnk1a1 (p = 0.006) significantly decreased (Fig. S5B), as they were in aged, postpartum cortex (Fig. 5A).
AD genes shown to be differentially impacted by peripartum sertraline in postpartum vs non-postpartum females were also correlated with learning and memory behaviors. While there was no main effect of sertraline on day 3 Barnes maze performance (Fig. 4C), distance to the target on the final day of Barnes maze testing, day 3, when maximal learning has occurred, was significantly and negatively correlated with cortical Psen1 (linear regression, r2 = 0.598, p = 0.041; Fig. S4A) and Csnk2a2 (r2 = 0.743, p = 0.013; Fig. S4B), but not with Csnk1a1 expression (r2 = 0.419, p = 0.116; Fig S4C). These protein regulatory genes were significantly down-regulated in postpartum cortex with peripartum sertraline. No correlations were significant in non-postpartum mice (Fig. S4D–F), demonstrating a specific effect of peripartum sertraline in aged postpartum mice only.
In aged postpartum sertraline-treated animals, there was a significantly increased density of colocalized Vglut1 and PSD-95 double-positive excitatory synaptic puncta in hippocampal CA3 (p = 0.030; Fig. 5C–E). Volumetry studies of cortex, hippocampus including CA regions and dentate gyrus, and corpus callosum revealed no changes by pregnancy nor sertraline status (Fig. S3). Collectively, these results demonstrate buffering of AD-related changes by sertraline within postpartum animals only; this is evident in brain regions critical to learning and memory processes.
Discussion
While selective serotonin reuptake inhibitors (SSRIs) are widely prescribed during pregnancy, the literature on their acute and long-term impacts on intrapartum neurobiology remains limited. Here, we utilized a non-invasive, naturalistic preclinical model to investigate the consequences of chronic perinatal sertraline on maternal behavior, neurobiology, and neurodegeneration-associated phenotypes across the lifespan. We describe novel insights into neuroprotection by peripartum SSRIs and by pregnancy itself and reveal potential underlying molecular mechanisms relevant to female neurobiology across the lifespan.
We tested whether chronic, oral delivery of sertraline to mice achieves maternal neurobiological changes without obstetric disruptions. This approach avoids the chronic stress confounds of oral gavage [47] or repetitive injections [48], does not disrupt obstetric health, and achieves clinically-relevant levels of sertraline [30, 31] without major side-effects, as reported with clinical use of extended-release antidepressants [49].
Our behavior results indicate reduced sertraline modification of behavior in late gestation. However, pregnancy itself had significant impacts on locomotion (OFT), mood/anhedonia (TST, chocolate preference), and anxiety-like behaviors (EPM). Importantly, this is not simply a result of reduced activity, as demonstrated by animal locomotor behavior on the open field. Changes may be due to maternal brain biology, pharmacokinetics which reduce sertraline efficacy, an increased volume of distribution, or metabolism changes due to increased estrogen. For example, sertraline metabolism and clearance are increased from the second to third trimesters of pregnancy [29] as a function of hepatic cytochrome P450 activity, which varies greatly between individuals [50]. Our intrapartum behavior results support the conclusion that, as others have argued, SSRI dose adjustment should be considered to offset drug turnover and maintain pharmacotherapy in pregnancy [51, 52]. Sertraline, and indeed SSRI’s more broadly, have increased drug metabolism across pregnancy [51, 53] (as much as 143%) [29]. Furthermore, 50 mg/day represents only a starting, non-therapeutic dose in many cases. Future studies will test increased doses, as well as drug metabolism in this model across murine pregnancy and across periphery and brain. This is necessary to advance a translational understanding of drug impacts in peripartum, and to optimize the utility of animal models in pregnancy pharmacology.
Despite blunted changes to behavior in pregnancy, sertraline significantly changed cortical and hypothalamic gene expression in late pregnancy. To our knowledge, this is the first report of transcriptomic changes in late-gestation maternal brain after intrapartum sertraline. Changes were greater in the hypothalamic paraventricular nucleus (PVN) relative to frontal cortex. Prior reports have similarly described more pronounced PVN gene expression changes compared to the frontal cortex with SSRI use in non-pregnant animals [54]. Changes are not driven by SSRI-induced histone acetylation, implicating an alternate regulatory mechanism [55]. Hypothalamic cells actively sample cerebrospinal fluid and play a key role in environmental and stress responses [56]. Circulating immune, metabolic, steroidal, and other factors that are changed in depression and with SSRI use may act directly on regulatory elements to modify hypothalamic gene expression [57, 58]. For example, glucocorticoids directly repress cAM-stimulated CRF promotor activity in hypothalamic cells [59]. The neuroanatomy of the PVN supports its role in endocrine signaling and sensing of the periphery—retrograde signaling via posterior pituitary axons [60]; vagus-to-nucleus of the solitary tract (NTS) circuits [61]; and subfornical organ (SFO) and organum vasculosum of the lamina terminalis (OVLT) projections all enable peripheral neuroendocrine signaling to the PVN [62], which can drive central metabolic and transcriptional changes. Studies of these mechanisms in pregnancy, when blood volume and cerebrospinal fluid metabolites are significantly altered, remain absent, however.
Prior work has shown that SSRI use alters expression of AD-related genes [55]. Our work expands on this by including a pregnant versus non-pregnant comparison and, perhaps most unexpectedly, demonstrates that both postpartum status and perinatal sertraline may modify AD-relevant gene expression. We observed decreased expression of neurodegeneration-related genes (Fig. 5A) in cortex of aged postpartum dams after SSRI administration, and of GD 18 but not non-pregnant dams after SSRI administration (Fig. S5). Central nodes of AD gene interaction networks including cortical Psen1 and Csnk2a2 are down-regulated in postpartum relative to non-postpartum mice by peripartum sertraline and are inversely correlated with learning and memory performance. While we did find this interesting correlation, we not find a main effect of sertraline itself on day 3 maze performance. Given high variance in behavior, it is possible that we were under-powered to detect such a difference and future replication work will expand the cohort size. Psen1 mutation status has been shown to modify postpartum cognitive deficits in humans, which are correlated with immunosuppressive changes [63]. Our RNAseq studies further demonstrate immunologic changes in the cortex with peripartum sertraline use, in particular invoking IL-17 pathway dysregulation. Psen1 mutation status has been shown to regulate Th17 T cell effector responses and T cell differentiation via γ-secretase signals [64]. This mechanism of Psen1 neuroimmune modulation via IL-17 cascades may thereby underlie neuroimmune phenotypes driving some AD pathology. The immunologically dynamic events of pregnancy may further serve as a critical substrate for initiation of these processes [65], which requires additional investigation.
Beyond inflammation, our work implicates additional mechanisms of peripartum sertraline impacts on the ageing brain. SSRIs modulate neuroplasticity [66], synaptic function [67], and neurogenesis [68], which are crucial for learning and memory processes. Indeed, we find transcriptomic changes in neuroplasticity and synaptic genes after gestational sertraline administration, including to Plk2 [69], Nsmf [70], Shank2 [71], Shank3 [72], Mef2C [73], Grin2A [74], and others. Synaptic remodeling in pregnancy has been shown previously in both human and animal studies [75,76,77] and may be a critical mechanism for neuroprotection. Future work will need to sequence additional cohorts of mice and expand statistical power to validate these DEGs and identify additional ones. Greater power will also allow examination of DEGs and the functional pathways involved by valence, which will provide additional insights.
While parity and SSRI have both previously been associated with reduced neurodegeneration [78, 79], driving mechanisms remain unclear; our results offer a valuable first view. We confirm previous findings: SSRI’s may modify neurodegenerative mechanisms at the molecular and behavioral levels, implicating neuroimmune [80], plasticity [81], and synaptic processes [82]. Unlike this prior work, however, we explore these dynamics within a pregnancy and postpartum context. To our knowledge, this is the first study to examine pregnancy and postpartum changes in neurodegenerative pathways as they relate to chronic peripartum sertraline.
Future work is needed to further validate our study findings and expand them to explore SSRI rescue in depression-like models. While daily handling and behavioral assessments (especially tail suspension and forced swim) [83] have been shown to induce depression-like changes in murine models [84,85,86], we did not employ a traditional depression induction model concurrent with sertraline here. It will be important for future work to test the interactions of sertraline-induced brain and behavior changes we report with depression itself, and for extension of this work to non-pregnant animals to reveal pregnancy specificity of all molecular impacts (RNA sequencing was only done at GD 18 here). For example, our prior work in a chronic restraint model, which leads to depression-like changes in mice [87], implicates pro-inflammatory changes in dams [88]. These inflammatory changes may be modified by chronic sertraline administration, as we have suggested occurs in other pro-inflammatory conditions of pregnancy such as preeclampsia [11, 89, 90]. Our planned and ongoing studies will evaluate this potential given the promise of results demonstrated here.
As clinicians, the NIH, and others have begun to advocate with increasing fervor, the best way to enhance health in pregnancy is to “protect pregnant people through research not from research” [91]. Concerns about teratogenicity and other risks have restrained use and study of SSRIs in pregnancy, as discussed previously [92]. Despite the risks of untreated depression, multinational research finds that 75.4% of women discontinue antidepressants during pregnancy due to health concerns [93, 94]. Human cohort studies struggle to directly address these concerns; the causal, neurobiological effects of antidepressants in human pregnancy are difficult to ascertain due to study confounds and experimental and ethical limitations precluding randomized controlled trials. Observational studies are confounded by differences in co-medications, dosing, comorbidities and symptom severity, healthcare utilization and more [11]. These limitations argue for improved preclinical models to better determine SSRI impacts and reveal mechanistic insights. As we show here, preclinical work provides a powerful first step in understanding the impacts of antidepressants in pregnancy on brain health across the lifespan. Together, our results demonstrate that chronic, oral sertraline delivered via a naturalistic, non-invasive approach effectively modifies mood-related neurobiology and exerts lasting effects on maternal neurodegenerative processes, which may underlie dementia risk with advanced age. Interactions between SSRI exposure, pregnancy, and long-term brain health are complex and also require further study. We further find that pregnancy itself exerts long-term protective effects on neurodegenerative processes. If better understood, underlying mechanisms in pregnancy might be exploited to buffer against neurodegeneration. Our findings also highlight the need for additional, clinical studies to consider the long-term consequences of SSRI use and improve perinatal psychiatry guidelines.
Supplementary information is available at MP’s website.
Data availibility
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
Gaynes BN, Gavin N, Meltzer-Brody S, Lohr KN, Swinson T, Gartlehner G et al. Perinatal depression: prevalence, screening accuracy, and screening outcomes. Evid Rep Technol Assess (Summ). 2005:1–8. https://doi.org/10.1037/e439372005-001.
Lee AM, Lam SK, Sze Mun Lau SM, Chong CS, Chui HW, Fong DY. Prevalence, course, and risk factors for antenatal anxiety and depression. Obstet Gynecol. 2007;110:1102–12.
Davenport MH, Meyer S, Meah VL, Strynadka MC, Khurana R. Moms Are Not OK: COVID-19 and Maternal Mental Health. Front Glob Womens Health. 2020;1:1.
Trost SLBJ, Njie F, et al. Pregnancy-related deaths: data from maternal mortality review committees in 36 US States, 2017-2019. Centers for Disease Control and Prevention, US Department of Health and Human Services;2022.
House W Blueprint for addressing the maternal health crisis. 2022.
Brody D, Gu Q Antidepressant use among adults: United States, 2015–2018. NCHS Data Brief, no 377 Hyattsville, MD: National Center for Health Statistics 2020.
Anderson KN, Lind JN, Simeone RM, Bobo WV, Mitchell AA, Riehle-Colarusso T, et al. Maternal use of specific antidepressant medications during early pregnancy and the risk of selected birth defects. JAMA Psychiatry. 2020;77:1246–55.
Cornet MC, Wu YW, Forquer H, Avalos LA, Sriram A, Scheffler AW, et al. Maternal treatment with selective serotonin reuptake inhibitors during pregnancy and delayed neonatal adaptation: a population-based cohort study. Arch Dis Child Fetal Neonatal Ed. 2024;109:294–300.
Vignato JA, Gumusoglu SB, Davis HA, Scroggins SM, Hamilton WS, Brandt DS et al. Selective serotonin reuptake inhibitor use in pregnancy and protective mechanisms in preeclampsia. Reprod Sci. 2023;30:701–12.
Obermanns J, Flasbeck V, Steinmann S, Juckel G, Emons B. Investigation of the serotonergic activity and the serotonin content in serum and platelet, and the possible role of the serotonin transporter in patients with depression. Behav Sci (Basel). 2022;12:178.
Gumusoglu SB, Schickling BM, Vignato JA, Santillan DA, Santillan MK. Selective serotonin reuptake inhibitors and preeclampsia: a quality assessment and meta-analysis. Pregnancy Hypertens. 2022;30:36–43.
Hou R, Ye G, Liu Y, Chen X, Pan M, Zhu F, et al. Effects of SSRIs on peripheral inflammatory cytokines in patients with Generalized Anxiety Disorder. Brain Behav Immun. 2019;81:105–10.
Golyszny M, Obuchowicz E. Are neuropeptides relevant for the mechanism of action of SSRIs? Neuropeptides. 2019;75:1–17.
Więdłocha M, Marcinowicz P, Krupa R, Janoska-Jaździk M, Janus M, Dębowska W, et al. Effect of antidepressant treatment on peripheral inflammation markers - A meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2018;80:217–26.
Lopez-Vilchez I, Diaz-Ricart M, Navarro V, Torramade S, Zamorano-Leon J, Lopez-Farre A, et al. Endothelial damage in major depression patients is modulated by SSRI treatment, as demonstrated by circulating biomarkers and an in vitro cell model. Transl Psychiatry. 2016;6:e886.
Gur TL, Palkar AV, Rajasekera T, Allen J, Niraula A, Godbout J, et al. Prenatal stress disrupts social behavior, cortical neurobiology and commensal microbes in adult male offspring. Behav Brain Res. 2019;359:886–94.
Moncrieff J, Cooper RE, Stockmann T, Amendola S, Hengartner MP, Horowitz MA. The serotonin theory of depression: a systematic umbrella review of the evidence. Mol Psychiatry. 2022;28:3243–56.
Molenaar NM, Kamperman AM, Boyce P, Bergink V. Guidelines on treatment of perinatal depression with antidepressants: an international review. Aust N Z J Psychiatry. 2018;52:320–7.
Fischer Fumeaux CJ, Morisod Harari M, Weisskopf E, Eap CB, Epiney M, Vial Y, et al. Risk-benefit balance assessment of SSRI antidepressant use during pregnancy and lactation based on best available evidence - an update. Expert Opin Drug Saf. 2019;18:949–63.
Dubovicky M, Belovicova K, Csatlosova K, Bogi E. Risks of using SSRI / SNRI antidepressants during pregnancy and lactation. Interdiscip Toxicol. 2017;10:30–4.
Hviid A, Melbye M, Pasternak B. Use of selective serotonin reuptake inhibitors during pregnancy and risk of autism. N Engl J Med. 2013;369:2406–15.
Grzeskowiak LE, Gilbert AL, Morrison JL. Long term impact of prenatal exposure to SSRIs on growth and body weight in childhood: evidence from animal and human studies. Reprod Toxicol. 2012;34:101–9.
van Geffen EC, Hermsen JH, Heerdink ER, Egberts AC, Verbeek-Heida PM, van Hulten R. The decision to continue or discontinue treatment: experiences and beliefs of users of selective serotonin-reuptake inhibitors in the initial months–a qualitative study. Res Social Adm Pharm. 2011;7:134–50.
Ratajczak P, Martynski J, Zieba JK, Swilo K, Kopciuch D, Paczkowska A, et al. Comparative efficacy of animal depression models and antidepressant treatment: a systematic review and meta-analysis. Pharmaceutics. 2024;16:1144.
Normann C, Frase S, Haug V, von Wolff G, Clark K, Munzer P, et al. Antidepressants rescue stress-induced disruption of synaptic plasticity via serotonin transporter-independent inhibition of L-type calcium channels. Biol Psychiatry. 2018;84:55–64.
Schoenfeld TJ, McCausland HC, Morris HD, Padmanaban V, Cameron HA. Stress and loss of adult neurogenesis differentially reduce hippocampal volume. Biol Psychiatry. 2017;82:914–23.
McEwen BS, Gianaros PJ. Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. Ann N Y Acad Sci. 2010;1186:190–222.
Jackson SJ, Andrews N, Ball D, Bellantuono I, Gray J, Hachoumi L, et al. Does age matter? The impact of rodent age on study outcomes. Lab Anim. 2017;51:160–9.
George B, Lumen A, Nguyen C, Wesley B, Wang J, Beitz J, et al. Application of physiologically based pharmacokinetic modeling for sertraline dosing recommendations in pregnancy. NPJ Syst Biol Appl. 2020;6:36.
Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016;7:27–31.
DeVane CL, Liston HL, Markowitz JS. Clinical pharmacokinetics of sertraline. Clin Pharmacokinet. 2002;41:1247–66.
Kino T. Stress, glucocorticoid hormones, and hippocampal neural progenitor cells: implications to mood disorders. Front Physiol. 2015;6:230.
Baudat M, de Kort AR, van den Hove DLA, Joosten EA. Early-life exposure to selective serotonin reuptake inhibitors: long-term effects on pain and affective comorbidities. Eur J Neurosci. 2022;55:295–317.
Adriao A, Santana I, Ribeiro C, Cancela ML, Conceicao N, Grazina M. Identification of a novel mutation in MEF2C gene in an atypical patient with frontotemporal lobar degeneration. Neurol Sci. 2022;43:319–26.
Aloni E, Oni-Biton E, Tsoory M, Moallem DH, Segal M. Synaptopodin deficiency ameliorates symptoms in the 3xTg mouse model of Alzheimer’s disease. J Neurosci. 2019;39:3983–92.
Barker SJ, Raju RM, Milman NEP, Wang J, Davila-Velderrain J, Gunter-Rahman F, et al. MEF2 is a key regulator of cognitive potential and confers resilience to neurodegeneration. Sci Transl Med. 2021;13:eabd7695.
Ji C, Tang M, Zeidler C, Hohfeld J, Johnson GV. BAG3 and SYNPO (synaptopodin) facilitate phospho-MAPT/Tau degradation via autophagy in neuronal processes. Autophagy. 2019;15:1199–213.
Ren J, Zhang S, Wang X, Deng Y, Zhao Y, Xiao Y, et al. MEF2C ameliorates learning, memory, and molecular pathological changes in Alzheimer’s disease in vivo and in vitro. Acta Biochim Biophys Sin (Shanghai). 2022;54:77–90.
Sunderaraman P, Cosentino S, Schupf N, Manly J, Gu Y, Barral S. MEF2C common genetic variation is associated with different aspects of cognition in non-hispanic white and caribbean hispanic non-demented older adults. Front Genet. 2021;12:642327.
Zhang Z, Zhao Y. Progress on the roles of MEF2C in neuropsychiatric diseases. Mol Brain. 2022;15:8.
Wray NR, Ripke S, Mattheisen M, Trzaskowski M, Byrne EM, Abdellaoui A, et al. Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat Genet. 2018;50:668–81.
Howard DM, Adams MJ, Shirali M, Clarke TK, Marioni RE, Davies G, et al. Genome-wide association study of depression phenotypes in UK Biobank identifies variants in excitatory synaptic pathways. Nat Commun. 2018;9:1470.
Hyde CL, Nagle MW, Tian C, Chen X, Paciga SA, Wendland JR, et al. Identification of 15 genetic loci associated with risk of major depression in individuals of European descent. Nat Genet. 2016;48:1031–6.
Gandal MJ, Haney JR, Parikshak NN, Leppa V, Ramaswami G, Hartl C, et al. Shared molecular neuropathology across major psychiatric disorders parallels polygenic overlap. Science. 2018;359:693–7.
Weber MA, Kerr G, Thangavel R, Conlon MM, Gumusoglu SB, Gupta K, et al. Alpha-Synuclein Pre-Formed Fibrils Injected into Prefrontal Cortex Primarily Spread to Cortical and Subcortical Structures. J Parkinsons Dis. 2024;14:81–94.
Bertolli A, Halhouli O, Liu-Martinez Y, Blaine B, Thangavel R, Zhang Q et al. Renovating the Barnes maze for mouse models of Dementia with STARR FIELD: A 4-day protocol that probes learning rate, retention and cognitive flexibility. bioRxiv [Preprint]. 2024 https://doi.org/10.1101/2024.11.30.625516.
Seno S, Tomura S, Miyazaki H, Sato S, Saitoh D. Effects of selective serotonin reuptake inhibitors on depression-like behavior in a laser-induced shock wave model. Front Neurol. 2021;12:602038.
Mombereau C, Gur TL, Onksen J, Blendy JA. Differential effects of acute and repeated citalopram in mouse models of anxiety and depression. Int J Neuropsychopharmacol. 2010;13:321–34.
Jacobsen JPR, Oh A, Bangle R, Roberts WL, Royer EL, Modesto N, et al. Slow-release delivery enhances the pharmacological properties of oral 5-hydroxytryptophan: mouse proof-of-concept. Neuropsychopharmacology. 2019;44:2082–90.
Heinonen E, Blennow M, Blomdahl-Wetterholm M, Hovstadius M, Nasiell J, Pohanka A, et al. Sertraline concentrations in pregnant women are steady and the drug transfer to their infants is low. Eur J Clin Pharmacol. 2021;77:1323–31.
Sit DK, Perel JM, Helsel JC, Wisner KL. Changes in antidepressant metabolism and dosing across pregnancy and early postpartum. J Clin Psychiatry. 2008;69:652–8.
Schoretsanitis G, Spigset O, Stingl JC, Deligiannidis KM, Paulzen M, Westin AA. The impact of pregnancy on the pharmacokinetics of antidepressants: a systematic critical review and meta-analysis. Expert Opin Drug Metab Toxicol. 2020;16:431–40.
Stika CS, Wisner KL, George AL Jr., Avram MJ, Zumpf K, Rasmussen-Torvik LJ, et al. Changes in sertraline plasma concentrations across pregnancy and postpartum. Clin Pharmacol Ther. 2022;112:1280–90.
Conti B, Maier R, Barr AM, Morale MC, Lu X, Sanna PP, et al. Region-specific transcriptional changes following the three antidepressant treatments electro convulsive therapy, sleep deprivation and fluoxetine. Mol Psychiatry. 2007;12:167–89.
Rayan NA, Kumar V, Aow J, Rastegar N, Lim MGL, O’Toole N, et al. Integrative multi-omics landscape of fluoxetine action across 27 brain regions reveals global increase in energy metabolism and region-specific chromatin remodelling. Mol Psychiatry. 2022;27:4510–25.
Chaves T, Fazekas CL, Horvath K, Correia P, Szabo A, Torok B, et al. Stress adaptation and the brainstem with focus on corticotropin-releasing hormone. Int J Mol Sci. 2021;22:9090.
Qiu J, Yao S, Hindmarch C, Antunes V, Paton J, Murphy D. Transcription factor expression in the hypothalamo-neurohypophyseal system of the dehydrated rat: upregulation of gonadotrophin inducible transcription factor 1 mRNA is mediated by cAMP-dependent protein kinase A. J Neurosci. 2007;27:2196–203.
Shi Z, Pelletier NE, Wong J, Li B, Sdrulla AD, Madden CJ, et al. Leptin increases sympathetic nerve activity via induction of its own receptor in the paraventricular nucleus. Elife. 2020;9:e55357.
Kageyama K, Suda T. Regulatory mechanisms underlying corticotropin-releasing factor gene expression in the hypothalamus. Endocr J. 2009;56:335–44.
Herman JP, Tasker JG. Paraventricular hypothalamic mechanisms of chronic stress adaptation. Front Endocrinol (Lausanne). 2016;7:137.
Yuan PQ, Yang H. Neuronal activation of brain vagal-regulatory pathways and upper gut enteric plexuses by insulin hypoglycemia. Am J Physiol Endocrinol Metab. 2002;283:E436–48.
Jeong JK, Dow SA, Young CN. Sensory circumventricular organs, neuroendocrine control, and metabolic regulation. Metabolites. 2021;11:494.
Croese T, Ramos, JM, Castellani, G, Ferrera, S, Tzipora, FZ, Judith, AP, and Schwartz, M Pregnancy exacerbates early-onset familial Alzheimer’s disease. Neurology 2021;96:4075.
Cummings M, Arumanayagam ACS, Zhao P, Kannanganat S, Stuve O, Karandikar NJ, et al. Presenilin1 regulates Th1 and Th17 effector responses but is not required for experimental autoimmune encephalomyelitis. PLoS One. 2018;13:e0200752.
Li XY, Wang F, Chen GH, Li XW, Yang QG, Cao L, et al. Inflammatory insult during pregnancy accelerates age-related behavioral and neurobiochemical changes in CD-1 mice. Age (Dordr). 2016;38:59.
Pittenger C, Duman RS. Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology. 2008;33:88–109.
Johansen A, Armand S, Plaven-Sigray P, Nasser A, Ozenne B, Petersen IN, et al. Effects of escitalopram on synaptic density in the healthy human brain: a randomized controlled trial. Mol Psychiatry. 2023;28:4272–9.
Duan W, Peng Q, Masuda N, Ford E, Tryggestad E, Ladenheim B, et al. Sertraline slows disease progression and increases neurogenesis in N171-82Q mouse model of Huntington’s disease. Neurobiol Dis. 2008;30:312–22.
Seeburg DP, Feliu-Mojer M, Gaiottino J, Pak DT, Sheng M. Critical role of CDK5 and Polo-like kinase 2 in homeostatic synaptic plasticity during elevated activity. Neuron. 2008;58:571–83.
Spilker C, Grochowska KM, Kreutz MR. What do we learn from the murine Jacob/Nsmf gene knockout for human disease? Rare Dis. 2016;4:e1241361.
Wegener S, Buschler A, Stempel AV, Kang SJ, Lim CS, Kaang BK, et al. Defective synapse maturation and enhanced synaptic plasticity in Shank2 Deltaex7(-/-) Mice. eNeuro. 2018;5:ENEURO.0398-17.2018.
Monteiro P, Feng G. SHANK proteins: roles at the synapse and in autism spectrum disorder. Nat Rev Neurosci. 2017;18:147–57.
Barbosa AC, Kim MS, Ertunc M, Adachi M, Nelson ED, McAnally J, et al. MEF2C, a transcription factor that facilitates learning and memory by negative regulation of synapse numbers and function. Proc Natl Acad Sci USA. 2008;105:9391–6.
Farsi Z, Nicolella A, Simmons SK, Aryal S, Shepard N, Brenner K, et al. Brain-region-specific changes in neurons and glia and dysregulation of dopamine signaling in Grin2a mutant mice. Neuron. 2023;111:3378–96.e3379.
Ammari R, Monaca F, Cao M, Nassar E, Wai P, Del Grosso NA, et al. Hormone-mediated neural remodeling orchestrates parenting onset during pregnancy. Science. 2023;382:76–81.
Chan RW, Ho LC, Zhou IY, Gao PP, Chan KC, Wu EX. Structural and functional brain remodeling during pregnancy with diffusion tensor MRI and resting-state functional MRI. PLoS One. 2015;10:e0144328.
Hillerer KM, Jacobs VR, Fischer T, Aigner L. The maternal brain: an organ with peripartal plasticity. Neural Plast. 2014;2014:574159.
Zhou R, Liu HM, Zou LW, Wei HX, Huang YN, Zhong Q, et al. Associations of parity with change in global cognition and incident cognitive impairment in older women. Front Aging Neurosci. 2022;14:864128.
Bartels C, Wagner M, Wolfsgruber S, Ehrenreich H, Schneider A. Alzheimer’s disease neuroimaging I. Impact of SSRI therapy on risk of conversion from mild cognitive impairment to Alzheimer’s dementia in individuals with previous depression. Am J Psychiatry. 2018;175:232–41.
Rajkumar R. Resolving a paradox: antidepressants, neuroinflammation, and neurodegeneration. Explor Neuroprot Ther. 2024;4:11–37.
Reed MB, Vanicek T, Seiger R, Klobl M, Spurny B, Handschuh P, et al. Neuroplastic effects of a selective serotonin reuptake inhibitor in relearning and retrieval. Neuroimage. 2021;236:118039.
Sun DS, Gao LF, Jin L, Wu H, Wang Q, Zhou Y, et al. Fluoxetine administration during adolescence attenuates cognitive and synaptic deficits in adult 3xTgAD mice. Neuropharmacology. 2017;126:200–12.
Castagne V, Moser P, Roux S, Porsolt RD Rodent models of depression: forced swim and tail suspension behavioral despair tests in rats and mice. Curr Protoc Neurosci 2011; Ch. 8: Unit 8.10A.
Sensini F, Inta D, Palme R, Brandwein C, Pfeiffer N, Riva MA, et al. The impact of handling technique and handling frequency on laboratory mouse welfare is sex-specific. Sci Rep. 2020;10:17281.
Clarkson JM, Dwyer DM, Flecknell PA, Leach MC, Rowe C. Handling method alters the hedonic value of reward in laboratory mice. Sci Rep. 2018;8:2448.
Ueno H, Takahashi Y, Suemitsu S, Murakami S, Kitamura N, Wani K, et al. Effects of repetitive gentle handling of male C57BL/6NCrl mice on comparative behavioural test results. Sci Rep. 2020;10:3509.
Chiba S, Numakawa T, Ninomiya M, Richards MC, Wakabayashi C, Kunugi H. Chronic restraint stress causes anxiety- and depression-like behaviors, downregulates glucocorticoid receptor expression, and attenuates glutamate release induced by brain-derived neurotrophic factor in the prefrontal cortex. Prog Neuropsychopharmacol Biol Psychiatry. 2012;39:112–9.
Gumusoglu SB, Maurer SV, Stevens HE. Dataset describing maternal prenatal restraint stress effects on immune factors in mice. Data Brief. 2022;43:108348.
Gumusoglu S, Scroggins S, Vignato J, Santillan D, Santillan M. The serotonin-immune axis in preeclampsia. Curr Hypertens Rep. 2021;23:37.
Gumusoglu S, Meincke CR, Kiel M, Betz A, Nuckols V, DuBose L, et al. Low indoleamine 2, 3 dioxygenase (IDO) activity is associated with psycho-obstetric risk. Pregnancy Hypertens. 2024;35:12–8.
Mertens M Protect Pregnant Women ‘Through Research,’ Not ‘From Research,’ OB-GYNs Urge. npr.: Shots Health News From NPR, 2021.
Gumusoglu SB, Chilukuri ASS, Hing BWQ, Scroggins SM, Kundu S, Sandgren JA, et al. Altered offspring neurodevelopment in an arginine vasopressin preeclampsia model. Transl Psychiatry. 2021;11:79.
Zoega H, Kieler H, Norgaard M, Furu K, Valdimarsdottir U, Brandt L, et al. Use of SSRI and SNRI Antidepressants during Pregnancy: A Population-Based Study from Denmark, Iceland, Norway and Sweden. PLoS One. 2015;10:e0144474.
Wikman A, Skalkidou A, Wikstrom AK, Lampa E, Kramer MS, Yong EL, et al. Factors associated with re-initiation of antidepressant treatment following discontinuation during pregnancy: a register-based cohort study. Arch Womens Ment Health. 2020;23:709–17.
Acknowledgements
This work was supported by the Brain and Behavior Research Foundation (NARSAD to SG), the Burroughs Wellcome Fund (Next Gen Pregnancy to SG), that National Institutes of Health (R01 HD089940-01A1 to MS) and by the Department of Obstetrics and Gynecology (internal funds to SG).
Author information
Authors and Affiliations
Contributions
BB, MK, MR, BS, ML, YZ, AB, MAW, RT, SN, KP, LT, and SG performed experiments. BB, AB, MW, GA, and SG analyzed the data. SG and BB designed the study. BB, AB, BS, and GA wrote the manuscript. All authors reviewed and edited the manuscript. The authors wish to acknowledge helpful contributions from members of the Department of Obstetrics and Gynecology (Santillan Labs) and of the Iowa Neuroscience Institute (Aldridge, Stevens, and Gumusoglu Labs; Dr. Shane Heiney with the Neural Circuits and Behavior Core).
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
All methods were performed in accordance with the relevant guidelines and regulations. Animal protocols received prior approval from the University of Iowa Institutional Animal Care and Use Committee (IACUC) (protocol numbers 4062604, 3082339).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Blaine, B., Kamal, M., Roberts, M. et al. Peripartum sertraline impacts maternal neurobehavioral and neurodegenerative mechanisms in pregnant and postpartum mice. Mol Psychiatry 30, 5108–5120 (2025). https://doi.org/10.1038/s41380-025-03094-x
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41380-025-03094-x