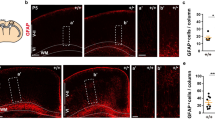
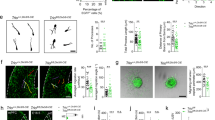
Abstract
Interneuron development is a crucial step of brain corticogenesis. When affected it often leads to brain dysfunctions like epilepsy, intellectual disabilities and autism spectrum disorder. Such defects are observed in the DYRK1A-haploinsufficiency syndrome, caused by mutations in DYRK1A, and commonly associated to cortical excitatory/inhibitory imbalance. However, how this imbalance is established in this syndrome remains elusive. Here, using mouse models and live imaging, we demonstrate that Dyrk1a specifically regulates the development of the cortical GABAergic system. We show that, unlike projection excitatory neurons, interneuron tangential migration relies on Dyrk1a dosage and kinase activity. We further reveal that Dyrk1a regulates actomyosin cytoskeleton remodeling during interneuron migration. Interestingly, mice with heterozygous inactivation of Dyrk1a in interneurons exhibited decreased interneuron density together with behavioral defects and epileptic activity, recapitulating phenotypes observed in human patients. Altogether, these data highlight the critical role of Dyrk1a in the development of the GABAergic system and the pathophysiology of DYRK1A-haploinsufficiency syndrome.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Ferguson BR, Gao WJ. Thalamic control of cognition and social behavior via regulation of gamma-aminobutyric acidergic signaling and excitation/inhibition balance in the medial prefrontal cortex. Biol Psychiatry. 2018;83:657–69.
Bezalel V, Paz R, Tal A. Inhibitory and excitatory mechanisms in the human cingulate-cortex support reinforcement learning: a functional proton magnetic resonance spectroscopy study. Neuroimage. 2019;184:25–35.
Fernandez F, Morishita W, Zuniga E, Nguyen J, Blank M, Malenka RC, et al. Pharmacotherapy for cognitive impairment in a mouse model of down syndrome. Nat Neurosci. 2007;10:411–3.
Gennaccaro L, Fuchs C, Loi M, Roncacè V, Trazzi S, Ait-Bali Y, et al. A GABAB receptor antagonist rescues functional and structural impairments in the perirhinal cortex of a mouse model of CDKL5 deficiency disorder. Neurobiol Dis. 2021;153:105304.
Sansone SM, Widaman KF, Hall SS, Reiss AL, Lightbody A, Kaufmann WE, et al. Psychometric study of the aberrant behavior checklist in fragile X syndrome and implications for targeted treatment. J Autism Dev Disord. 2012;42:1377.
Ferguson BR, Gao WJ. Pv interneurons: critical regulators of E/I balance for prefrontal cortex-dependent behavior and psychiatric disorders. Front Neural Circuits. 2018;12:37.
Fatemi SH, Halt AR, Stary JM, Kanodia R, Schulz SC, Realmuto GR. Glutamic acid decarboxylase 65 and 67 kDa proteins are reduced in autistic parietal and cerebellar cortices. Biol Psychiatry. 2002;52:805–10.
Chao HT, Chen H, Samaco RC, Xue M, Chahrour M, Yoo J, et al. Dysfunction in GABA signalling mediates autism-like stereotypies and Rett syndrome phenotypes. Nature. 2010;468:263–9.
Bozzi Y, Casarosa S, Caleo M. Epilepsy as a neurodevelopmental disorder. Front Psychiatry. 2012;3:19.
Van Bon BWM, Hoischen A, Hehir-Kwa J, De Brouwer APM, Ruivenkamp C, Gijsbers ACJ, et al. Intragenic deletion in DYRK1A leads to mental retardation and primary microcephaly. Clin Genet. 2011;79:296–9.
Van Bon BWM, Coe BP, Bernier R, Green C, Gerdts J, Witherspoon K, et al. Disruptive de novo mutations of DYRK1A lead to a syndromic form of autism and ID. Mol Psychiatry. 2016;21:126–32.
Courcet JB, Faivre L, Malzac P, Masurel-Paulet A, Lopez E, Callier P, et al. The DYRK1A gene is a cause of syndromic intellectual disability with severe microcephaly and epilepsy. J Med Genet. 2012;49:731–6.
O’Roak BJ, Vives L, Fu W, Egertson JD, Stanaway IB, Phelps IG, et al. Multiplex targeted sequencing identifies recurrently mutated genes in autism spectrum disorders. Science. 2012;338:1619–22.
Bronicki LM, Redin C, Drunat S, Piton A, Lyons M, Passemard S, et al. Ten new cases further delineate the syndromic intellectual disability phenotype caused by mutations in DYRK1A. Eur J Hum Genet. 2015;23:1482–7.
Ji J, Lee H, Argiropoulos B, Dorrani N, Mann J, Martinez-Agosto JA, et al. DYRK1A haploinsufficiency causes a new recognizable syndrome with microcephaly, intellectual disability, speech impairment, and distinct facies. Eur J Hum Genet. 2015;23:1473–81.
Ruaud L, Mignot C, Guët A, Ohl C, Nava C, Héron D, et al. DYRK1A mutations in two unrelated patients. Eur J Med Genet. 2015;58:168–74.
Luco SM, Pohl D, Sell E, Wagner JD, Dyment DA, Daoud H. Case report of novel DYRK1A mutations in 2 individuals with syndromic intellectual disability and a review of the literature. BMC Med Genet. 2016;17:15.
Courraud J, Chater-Diehl E, Durand B, Vincent M, del Mar Muniz Moreno M, Boujelbene I, et al. Integrative approach to interpret DYRK1A variants, leading to a frequent neurodevelopmental disorder. Genet Med. 2021;23:2150–9.
Tejedor FJ, Hämmerle B. MNB / DYRK1A as a multiple regulator of neuronal development. FEBS J. 2011;278:223–35.
Duchon A, Herault Y. DYRK1A, a dosage-sensitive gene involved in neurodevelopmental disorders, is a target for drug development in down syndrome. Front Behav Neurosci. 2016;10:104.
Atas-Ozcan H, Brault V, Duchon A, Herault Y. Dyrk1a from gene function in development and physiology to dosage correction across life span in down syndrome. Genes. 2021;12:1833.
Lochhead PA, Sibbet G, Morrice N. Activation-loop autophosphorylation is mediated by a novel transitional intermediate form of DYRKs. Cell. 2005;121:925–36.
Himpel S, Panzer P, Eirmbter K, Czajkowska H, Sayed M, Packman LC, et al. Identification of the autophosphorylation sites and characterization of their effects in the protein kinase DYRK1A. Biochem J. 2001;359:497–505.
Soundararajan M, Roos AK, Savitsky P, Filippakopoulos P, Kettenbach AN, Olsen JV, et al. Structures of down syndrome kinases, DYRKs, reveal mechanisms of kinase activation and substrate recognition. Structure. 2013;21:986–96.
Tejedor F, Zhu XR, Kaltenbach E, Ackermann A, Baumann A, Canal I, et al. minibrain: a new protein kinase family involved in postembryonic neurogenesis in Drosophila. Neuron. 1995;14:287–301.
Shindoh N, Kudoh J, Maeda H, Yamaki A, Minoshima S, Shimizu Y, et al. Cloning of a human homolog of the Drosophila minibrain/rat Dyrk gene from ‘the down syndrome critical region’ of chromosome 21. Biochem Biophys Res Commun. 1996;225:92–99.
Brault V, Nguyen TL, Flores-Gutiérrez J, Iacono G, Birling MC, Lalanne V, et al. Dyrk1a gene dosage in glutamatergic neurons has key effects in cognitive deficits observed in mouse models of MRD7 and down syndrome. PLoS Genet. 2021;17:1–34.
Hämmerle B, Elizalde C, Tejedor FJ. The spatio-temporal and subcellular expression of the candidate down syndrome gene Mnb/Dyrk1A in the developing mouse brain suggests distinct sequential roles in neuronal development. Eur J Neurosci. 2008;27:1061–74.
Telley L, Agirman G, Prados J, Amberg N, Fièvre S, Oberst P, et al. Temporal patterning of apical progenitors and their daughter neurons in the developing neocortex. Science. 2019;364:eaav2522.
Wegiel J, Kuchna I, Nowicki K, Frackowiak J, Dowjat K, Silverman WP, et al. Cell type- and brain structure-specific patterns of distribution of minibrain kinase in human brain. Brain Res. 2004;1010:69–80.
Fotaki V, Dierssen M, Alcantara S, Martinez S, Marti E, Casas C, et al. Dyrk1A haploinsufficiency affects viability and causes developmental delay and abnormal brain morphology in mice. Mol Cell Biol. 2002;22:6636–47.
Raveau M, Shimohata A, Amano K, Miyamoto H, Yamakawa K. DYRK1A-haploinsufficiency in mice causes autistic-like features and febrile seizures. Neurobiol Dis. 2018;110:180–91.
Benavides-Piccione R, Dierssen M, Ballesteros-Yáñez I, Martínez De Lagrán M, Arbonés ML, Fotaki V, et al. Alterations in the phenotype of neocortical pyramidal cells in the Dyrk1A+/- mouse. Neurobiol Dis. 2005;20:115–22.
Arqué G, Fotaki V, Fernández D, de Lagrán MM, Arbonés ML, Dierssen M. Impaired spatial learning strategies and novel object recognition in mice haploinsufficient for the dual specificity tyrosine-regulated kinase-1A (Dyrk1A). PLoS ONE. 2008;3:e2575.
Levy JA, LaFlamme CW, Tsaprailis G, Crynen G, Page DT. Dyrk1a mutations cause undergrowth of cortical pyramidal neurons via dysregulated growth factor signaling. Biol Psychiatry. 2021;90:1–12.
Arranz J, Balducci E, Arató K, Sánchez-Elexpuru G, Najas S, Parras A, et al. Impaired development of neocortical circuits contributes to the neurological alterations in DYRK1A haploinsufficiency syndrome. Neurobiol Dis. 2019;127:210–22.
Guedj F, Pereira PL, Najas S, Barallobre MJ, Chabert C, Souchet B, et al. DYRK1A: a master regulatory protein controlling brain growth. Neurobiol Dis. 2012;46:190–203.
Souchet B, Guedj F, Sahún I, Duchon A, Daubigney F, Badel A, et al. Excitation/inhibition balance and learning are modified by Dyrk1a gene dosage. Neurobiol Dis. 2014;69:65–75.
Park J, Oh Y, Yoo L, Jung MS, Song WJ, Lee SH, et al. Dyrk1A phosphorylates p53 and inhibits proliferation of embryonic neuronal cells. J Biol Chem. 2010;285:31895–906.
Yabut O, Domogauer J, D’Arcangelo G. Dyrk1A overexpression inhibits proliferation and induces premature neuronal differentiation of neural progenitor cells. J Neurosci. 2010;30:4004–14.
Najas S, Arranz J, Lochhead PA, Ashford AL, Oxley D, Delabar JM, et al. DYRK1A-mediated cyclin D1 degradation in neural stem cells contributes to the neurogenic cortical defects in down syndrome. EBioMedicine. 2015;2:120–34.
Monory K, Massa F, Egertová M, Eder M, Blaudzun H, Westenbroek R, et al. The endocannabinoid system controls key epileptogenic circuits in the hippocampus. Neuron. 2006;51:455–66.
López-Bendito G, Sturgess K, Erdélyi F, Szabó G, Molnár Z, Paulsen O. Preferential origin and layer destination of GAD65-GFP cortical interneurons. Cereb Cortex. 2004;14:1122–33.
Marín O, Valiente M, Ge X, Tsai LH. Guiding neuronal cell migrations. Cold Spring Harb Perspect Biol. 2010;2:a001834.
Stenman J, Toresson H, Campbell K. Identification of two distinct progenitor populations in the lateral ganglionic eminence: implications for striatal and olfactory bulb neurogenesis. J Neurosci. 2003;23:167–74.
Priya R, Paredes MF, Karayannis T, Yusuf N, Liu X, Jaglin X, et al. Activity regulates cell death within cortical interneurons through a calcineurin-dependent mechanism. Cell Rep. 2018;22:1695–709.
Jossin Y. Molecular mechanisms of cell polarity in a range of model systems and in migrating neurons. Mol Cell Neurosci. 2020;106:103503. https://doi.org/10.1016/j.mcn.2020.103503.
Kentrup H, Becker W, Heukelbach J, Wilmes A, Schürmann A, Huppertz C, et al. Dyrk, a dual specificity protein kinase with unique structural features whose activity is dependent on tyrosine residues between subdomains VII and VIII. J Biol Chem. 1996;271:3488–95.
Deau E, Lindberg MF, Miege F, Roche D, George N, George P, et al. Leucettinibs, a class of DYRK/CLK kinase inhibitors inspired by the marine sponge natural product leucettamine B. J Med Chem. 2023;66:10694–714.
Lindberg MF, Deau E, Miège F, Gréverie M, Roche D, George N, et al. Chemical, biochemical, cellular and physiological characterization of Leucettinib-21, a down syndrome and Alzheimer’s disease drug candidate. J Med Chem. 2023;66:15648–70.
Llorca A, Deogracias R. Origin, development, and synaptogenesis of cortical interneurons. Front Neurosci. 2022;16:986.
Mi D, Li Z, Lim L, Li M, Moissidis M, Yang Y. Early emergence of cortical interneuron diversity in the mouse embryo. Science. 2018;360:81–85.
Solecki DJ, Trivedi N, Govek EE, Kerekes RA, Gleason SS, Hatten ME. Myosin II motors and F-actin dynamics drive the coordinated movement of the centrosome and soma during CNS glial-guided neuronal migration. Neuron. 2009;63:63–80.
Martini FJ, Valdeolmillos M. Actomyosin contraction at the cell rear drives nuclear translocation in migrating cortical interneurons. J Neurosci. 2010;30:8660–70.
Huo HQ, Qu ZY, Yuan F, Ma L, Yao L, Xu M, et al. Modeling down syndrome with patient iPSCs reveals cellular and migration deficits of GABAergic neurons. Stem Cell Reports. 2018;10:1251–66.
Riedl J, Crevenna AH, Kessenbrock K, Yu JH, Neukirchen D, Bista M, et al. Lifeact: a versatile marker to visualize F-actin. Nat Methods. 2008;5:605–7.
Silva CG, Peyre E, Adhikari MH, Tielens S, Tanco S, Van Damme P, et al. Cell-intrinsic control of interneuron migration drives cortical morphogenesis. Cell. 2018;172:1063–78.e19.
Lim L, Mi D, Llorca A, Marín O. Development and functional diversification of cortical interneurons. Neuron. 2018;100:294–313.
Mayer C, Hafemeister C, Bandler RC, Machold R, Batista Brito R, Jaglin X, et al. Developmental diversification of cortical inhibitory interneurons. Nature. 2018;555:457–62.
Southwell DG, Paredes MF, Galvao RP, Jones DL, Froemke RC, Sebe JY, et al. Intrinsically determined cell death of developing cortical interneurons. Nature. 2012;491:109–13.
Bernstein BJ, Kendricks DR, Fry S, Wilson L, Koopmans B, Loos M, et al. Sex differences in spontaneous behavior and cognition in mice using an automated behavior monitoring system. Physiol Behav. 2024;283:114595.
Rusu A, Chevalier C, de Chaumont F, Nalesso V, Brault V, Hérault Y, et al. Day-to-day spontaneous social behaviours is quantitatively and qualitatively affected in a 16p11.2 deletion mouse model. Front Behav Neurosci. 2023;17:1294558.
Yang J, Yang X, Tang K. Interneuron development and dysfunction. FEBS J. 2022;289:2318–36.
Broix L, Asselin L, Silva CG, Ivanova EL, Tilly P, Gilet JG, et al. Ciliogenesis and cell cycle alterations contribute to KIF2A-related malformations of cortical development. Hum Mol Genet. 2018;27:224–38.
Morandell J, Schwarz LA, Basilico B, Tasciyan S, Dimchev G, Nicolas A, et al. Cul3 regulates cytoskeleton protein homeostasis and cell migration during a critical window of brain development. Nat Commun. 2021;12:3058.
Birey F, Andersen J, Makinson CD, Islam S, Wei W, Huber N, et al. Assembly of functionally integrated human forebrain spheroids. Nature. 2017;545:54–59. https://doi.org/10.1038/nature22330.
Viou L, Launay P, Rousseau V, Masson J, Pace C, Adelstein RS, et al. PAK3 controls the tangential to radial migration switch of cortical interneurons by coordinating changes in cell shape and polarity. BioRxiv. 2020.07.06.168179. https://doi.org/10.1101/2020.07.06.168179.
Marsh E, Fulp C, Gomez E, Nasrallah I, Minarcik J, Sudi J, et al. epilepsy mouse model and recapitulates the human phenotype in heterozygous females. 2009;2009. https://doi.org/10.1093/brain/awp107.
Hämmerle B, Ulin E, Guimera J, Becker W, Guillemot F, Tejedor FJ. Transient expression of Mnb/dyrk1a couples cell cycle exit and differentiation of neuronal precursors by inducing p27KIP1 expression and suppressing NOTCH signaling. Development. 2011;138:2543–54.
Dang T, Duan WY, Yu B, Tong DL, Cheng C, Zhang YF, et al. Autism-associated Dyrk1a truncation mutants impair neuronal dendritic and spine growth and interfere with postnatal cortical development. Mol Psychiatry. 2018;23:747–58.
Jiang X, Nardelli J. Cellular and molecular introduction to brain development. Neurobiol Dis. 2016;92:3–17.
Faheem M, Naseer MI, Rasool M, Chaudhary AG, Kumosani TA, Ilyas AM, et al. Molecular genetics of human primary microcephaly: an overview. BMC Med Genomics. 2013;8:24–7.
Godin JD, Thomas N, Laguesse S, Malinouskaya L, Close P, Malaise O, et al. p27(Kip1) is a microtubule-associated protein that promotes microtubule polymerization during neuron migration. Dev Cell. 2012;23:729–44.
Tielens S, Huysseune S, Godin JD, Chariot A, Malgrange B, Nguyen L. Elongator controls cortical interneuron migration by regulating actomyosin dynamics. Cell Res. 2016;26:1131–48.
Ben Zablah Y, Merovitch N, Jia Z. The role of ADF / cofilin in synaptic physiology and Alzheimer ’ s disease. Front Cell Dev Biol. 2020;8:594998.
Yin HL, Stossel TP. Control of cytoplasmic actin gel–sol transformation by gelsolin, a calcium-dependent regulatory protein. Nature. 1979;281:583–6.
Neuhaus E, Rea H, Jones E, Benavidez H, Miles C, Whiting A, et al. Shared and divergent mental health characteristics of ADNP-, CHD8- and DYRK1A-related neurodevelopmental conditions. J Neurodev Disord. 2024;16:15.
Bozzi Y, Provenzano G, Casarosa S. Neurobiological bases of autism–epilepsy comorbidity: a focus on excitation/inhibition imbalance. Eur J Neurosci. 2018;47:534–48.
Sohal VS, Rubenstein JLR. Excitation-inhibition balance as a framework for investigating mechanisms in neuropsychiatric disorders. Mol Psychiatry. 2019;24:1248.
Howell BW, Smith KM. Synaptic structural protein dysfunction leads to altered excitation inhibition ratios in models of autism spectrum disorder. Pharmacol Res. 2019;139:207–14.
Simonet JC, Sunnen CN, Wu J, Golden JA, Marsh ED. Conditional loss of arx from the developing dorsal telencephalon results in behavioral phenotypes resembling mild human ARX mutations. Cereb Cortex. 2015;25:2939–50.
Marsh ED, Nasrallah MP, Walsh C, Murray KA, Sunnen CN, Mccoy A, et al. Developmental interneuron subtype deficits after targeted loss of Arx. BMC Neurosci. 2016;17:35.
Lepiemme F, Silva CG, Nguyen L. Time lapse recording of cortical interneuron migration in mouse organotypic brain slices and explants. STAR Protoc. 2021;2:100467.
Leclech C, Métin C. In vitro models to analyze the migration of MGE-derived interneurons. Methods Mol Biol. 2018;1749:145–61.
Asselin L, Alvarez JR, Heide S, Bonnet CS, Tilly P, Vitet H, et al. Mutations in the KIF21B kinesin gene cause neurodevelopmental disorders through imbalanced canonical motor activity. Nat Commun. 2020;11:2441. https://doi.org/10.1038/s41467-020-16294-6.
Nguyen TL, Duchon A, Manousopoulou A, Loaëc N, Villiers B, Pani G, et al. Correction of cognitive deficits in mouse models of down syndrome by a pharmacological inhibitor of DYRK1A. Dis Model Mech. 2018;11:dmm035634.
Kim D, Langmead B, Salzberg SL. HISAT: a fast spliced aligner with low memory requirements. Nat Methods. 2015;12:357–60.
Anders S, Pyl PT, Huber W. HTSeq-A python framework to work with high-throughput sequencing data. Bioinformatics. 2015;31:166–9.
Wang L, Wang S, Li W. RSeQC: quality control of RNA-seq experiments. Bioinformatics. 2012;28:2184–5.
Dembélé D, Kastner P. Comments on: fold change rank ordering statistics: a new method for detecting differentially expressed genes. BMC Bioinformatics. 2016;17:462.
Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–504.
Ivanova EL, Gilet JG, Sulimenko V, Duchon A, Rudolf G, Runge K, et al. TUBG1 missense variants underlying cortical malformations disrupt neuronal locomotion and microtubule dynamics but not neurogenesis. Nat Commun. 2019;10:2129.
Acknowledgements
Gábor Szabó for providing the GAD65-EGFP mice. Laurent Meijer and Emmanuel Deau for providing LCTB-21 inhibitor and isoLCTB-21. Wojciech Krezel for providing the ASCL1 primary antibody. ICS animal facility, in particular Sophie Brignon and Milan Herrmann for their involvement in the project. All the staff of the IGBMC Platform of photonic microscopy, for their assistance in imaging experiments, and the IGBMC Genomeast platform, a member of the “France Génomique” consortium (ANR-10-INBS-0009), for the transcriptome studies. This work was funded by grants from the Fondation Jérôme-Lejeune (A.D and M-V.H, JLJ Postdoctoral fellowship and V.B for JLJ project N° 1647), the French state funds through the Agence Nationale de la Recherche under the project PRC DYRK-DOWN ANR-18-CE16-0020 (Y.H.; J.D.G), and support from the program Investissements d’Avenir labeled IdEx Unistra (ANR-10-IDEX-0002), a SFRI-STRAT’US project (ANR 20-SFRI-0012), EUR IMCBio (ANR-17-EURE-0023) to JDG and YH, INBS PHENOMIN (ANR-10-INBS-07 PHENOMIN) to YH, the ‘France Génomique’ consortium (ANR-10-INBS-0009) for the GenomeEast platform, and INSERM/CNRS and University of Strasbourg. P.T. and A.D. are, respectively, research assistants and research engineer at the University of Strasbourg. VN is an engineer assistant at the CNRS. VA is a PhD student at the ED414 Unistra. MdM and TLN were respectiveley a post-doct and PhD candidate at the CERBM GIE. VB and YH are respectively CNRS researcher and Principal investigator. J.D.G. is an INSERM investigator. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. All the members of Y.H and J.G research groups for their feedback.
Author information
Authors and Affiliations
Contributions
M-VH conceived and designed the experiments, performed and analyzed experiments, coordinated the study and wrote the manuscript. AD conceived and designed the experiments, performed and analyzed experiments. VA performed and analyzed time-lapse experiments with LCTB-21 treatment, and designed cartoons. GR performed and analyzed EEG studies. T-LN performed the kinase activity assay. PT performed in utero electroporations. MdeM performed bulk RNA-Seq analyses and VN further analyzed this data. M-CB developed and validated the Dyrk1aK188R/+ mouse model. JDG conceived and supervised in utero electroporation experiments. VB conceived, performed and analyzed behavioral studies and provided financial support. YH conceived, coordinated and supervised the study, and provided the financial support.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hinckelmann, M.V., Dubos, A., Artot, V. et al. Interneuron migration defects during corticogenesis contribute to Dyrk1a haploinsufficiency syndrome pathogenesis. Mol Psychiatry 30, 5227–5244 (2025). https://doi.org/10.1038/s41380-025-03109-7
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41380-025-03109-7