Abstract
Prenatal Δ9-tetrahydrocannabinol exposure (PTE) poses long-lasting neuropsychiatric risks, as evidenced by clinical and preclinical studies, yet the neurobiological mechanisms remain poorly defined. Emerging evidence implicates the neurolipidome, a critical mediator of neurodevelopment and endocannabinoid signaling, as a potential contributor. Here, we demonstrate that dietary omega-3 fatty acid supplementation sex-selectively ameliorates neurodevelopmental deficits induced by PTE in a Wistar rat model. Omega-3 supplementation reduced cognitive and emotional disturbances in male offspring and normalized many neuronal and neurochemical abnormalities in the prefrontal cortex, nucleus accumbens, and ventral hippocampus. However, lipidomic analyses, regardless of omega-3 supplementation, uncovered pronounced, sex-specific PTE-induced disruptions in pathways critical for synaptic integrity and neurodevelopment, including those related to the endocannabinoid system. These findings provide new insights into the interplay between lipid metabolism and the endocannabinoid system in the context of PTE.
Similar content being viewed by others
Introduction
Cannabis is one of the most commonly used substances during pregnancy, driven by expanding legalization and a widespread perception of its relative safety for managing pregnancy-related symptoms, though this trend varies geographically [1,2,3]. Contrary to this public perception, a growing body of evidence indicates that prenatal exposure to Δ9-tetrahydrocannabinol (THC), the primary psychoactive constituent of cannabis, in preclinical rodent models can adversely impact pregnancy outcomes and fetal neurodevelopment [2,3,4] and lead to subsequent behavioral abnormalities [5,6,7,8,9,10]. Prenatal THC exposure (PTE) is thus a significant developmental risk factor, and while complete abstinence from cannabis during pregnancy remains the primary clinical recommendation, the reality of continued use amongst some pregnant women necessitates the exploration of harm reduction strategies.
THC readily crosses the placental and fetal blood-brain barriers, exerting direct effects on the developing brain [3, 11]. The endocannabinoid system (ECS), comprised of cannabinoid receptors (CB1R and CB2R), endogenous ligands such as anandamide (AEA) and 2-arachidonoylglycerol (2-AG), and their metabolic enzymes, play a fundamental role in orchestrating neurodevelopmental events, including progenitor proliferation, migration, synaptogenesis, and circuit maturation [12, 13]. THC, through partial agonism at CB1R, disrupts this finely tuned signaling system. It induces receptor desensitization and downregulation, ultimately destabilizing ECS homeostasis and interfering with brain development [4, 14, 15]. Rodent studies consistently report that PTE leads to cognitive impairments, elevated anxiety- and depressive-like phenotypes, and deficits in social interaction [5,6,7,8,9,10, 16], implicating disruptions in the PFC-striatal-hippocampal network—regions enriched in CB1Rs and integral to emotional and cognitive function [17, 18]. These outcomes have clinical correlates: longitudinal human studies report elevated incidence of childhood psychopathologies, including attentional deficits, impulsivity, emotional dysregulation, social dysfunction, and reduced cognitive performance [18,19,20,21], with neuroimaging revealing structural and functional abnormalities in the PFC, hippocampus, and amygdala of exposed children [22, 23].
Beyond its direct neural impact, THC also interferes with placental development and function. The ECS is active in placental and uterine tissues, where it modulates trophoblast invasion, angiogenesis, and maternal-fetal nutrient exchange [11, 24, 25]. Exogenous cannabinoids such as THC disrupt these processes, leading to placental insufficiency. Rodent models reveal reduced placental weight, impaired vascularization, oxidative stress, and compromised oxygen and nutrient delivery to the fetus following PTE [5, 6, 11, 26,27,28]. Clinical findings parallel these observations, with PTE associated with increased rates of fetal growth restriction, preterm birth, and neonatal intensive care admissions [2, 8, 29,30,31,32]. While inconsistencies in birth weight outcomes exist, largely due to methodological and population-based variability, evidence from several large-scale studies support a link between cannabis use and impaired fetal growth [19, 29, 30, 32, 33]. Nevertheless, rodent models demonstrate postnatal compensatory catch-up growth following this fetal growth restriction, potentially exacerbating neurocognitive deficits [34, 35] and cardiometabolic dysfunction [11, 26, 27, 36] due to maladaptive resource allocation during development [37].
PTE also perturbs lipid metabolism; a critical mechanism contributing to neurodevelopmental disruption. The ECS is a lipid-based signaling system, and its endogenous ligands are derived from polyunsaturated fatty acids (PUFAs) such as arachidonic acid (AA), docosahexaenoic acid (DHA), and eicosapentaenoic acid (EPA). These fatty acids are essential for fetal brain development and depend upon maternal dietary intake for adequate fetal provision [18, 21, 26, 27]. Rodent models of PTE demonstrate symmetrical fetal growth restriction accompanied by altered placental structure, including reductions in placental weight and vascular development in the labyrinth zone, the key site of gas and nutrient (including lipid) exchange [5, 11, 26, 38,39,40,41]. THC-induced placental dysfunction compromises the delivery of PUFAs, oxygen, and other critical substrates, impairing both somatic and brain development. Recent work has highlighted persistent lipidomic abnormalities in PTE-exposed offspring, including reduced cerebral PUFA levels, altered lipid metabolism, and corresponding behavioral and electrophysiological disruptions [5, 6, 27, 42,43,44]. These findings suggest that disrupted lipid signaling may amplify ECS dysregulation, compounding the neurodevelopmental consequences of PTE that may otherwise be driven by THC’s activity on cannabinoid receptors.
Maternal dietray uptake of omega-3 fatty acids (particularly DHA and EPA) during the perinatal period critically supports fetal brain development given their fundamental roles in membrane structure and fluidity (effecting neurotransmitter release), neural circuit maturation, neurite outgrowth, dendritic spine morphology, synaptogenesis, and anti-inflammatory action [45,46,47,48,49]. Critically, deficiencies in maternal omega-3 intake is associated with suboptimal neural outcomes [50,51,52]. Given that the maternal diet is the primary source of PUFAs during fetal and neonatal development [48], dietary interventions targeting omega-3 PUFAs may serve as a promising preventive strategy [53, 54] against the adverse impacts of PTE.
In the present study, we investigated whether perinatal omega-3 supplementation can ameliorate the neurodevelopmental consequences of PTE in a Wistar rat model. Our findings reveal striking sex-specific outcomes: males exhibited rescue of behavioral deficits, synaptic protein dysregulation, and electrophysiological abnormalities, despite persistent lipidomic disruptions (fatty acids and lipids). Conversely, females showed partial improvements in social motivation and ventral hippocampal activity, but retained deficits in oscillatory dynamics, lipidomic disruptions (lipids only), and protein expression. These results highlight the therapeutic potential of omega-3 fatty acid dietary interventions in reducing neuropsychiatric risks associated with PTE. Furthermore, they highlight the need to further investigate the sex-specific mechanisms underlying these alterations within the PFC-striatal-hippocampal circuitry.
Materials and methods
Ethics approval
All experimental procedures adhered to Canadian Council of Animal Care guidelines and were approved by the Animal Care Committee and Veterinary Services Committee at the University of Western Ontario, protocol #2022-119 (for SRL) and # 2023-129 (for DBH).
Animals
Time-pregnant Wistar rat dams (Charles River, Quebec, Canada; n = 14–15 dams/treatment; n = 12–15 offspring/treatment/sex) arrived on gestational day 3 (GD3) and were randomly assigned to receive daily intraperitoneal (i.p.) injections of either vehicle (saline with cremophor) or THC (3 mg/kg; 2.6 mg/mL in saline with cremophor) from GD7 to GD22. Concurrently, beginning on GD7, dams were randomized to one of two dietary conditions: an omega-3 enriched diet containing DHA and EPA (ω3), or a matched control diet (ct). The sole compositional difference between the two diets was the inclusion of DHA and EPA in triglyceride (TAG) form in the ω3 group; detailed composition is provided in Supplemental Table 1.
Following weaning at postnatal day 21 (PD21), all offspring were transitioned to standard laboratory chow. This standard chow is nutritionally comparable to the control purified diet administered during gestation and lactation, though it is a closed formula subject to minor batch-to-batch variability. In contrast, the purified control diet ensured consistent composition throughout the treatment period.
Maternal and fetal parameters, including maternal weight gain, food intake, offspring birth weight, and offspring weight at PD21, are summarized in Supplemental Fig. 1. All experimental details are presented in detail in the Supplemental Materials. Supplemental Table 2 lists the tentatively assigned lipids with their mass-to-charge (m/z) values and ion that were analyzed with MALDI IMS.
Statistical analyses
Analyses were performed with three-way ANOVAs (sex × treatment × omega-3 diet) for PD1 and PD21 weights, behavioral assays, electrophysiological spiking activity, bursting activity, and for local field potential analyses. Follow-up analyses of significant main effects and interactions (p < 0.05) were accomplished using Tukey’s Honest Significant Difference (Tukey’s HSD) post hoc test (α = 0.05). For the matrix-assisted laser desorption/ionization imaging mass spectrometry (MALDI IMS) data, group differences were assessed using one-sample t-tests against a hypothetical mean of 1.0, representing the VEHct group and indicating no change from baseline. This relative quantification approach is standard for MALDI IMS data interpretation [5, 6, 55, 56]. Due to inherent experimental variability requiring all compared samples to be mounted on the same slide, direct statistical comparisons across treatment groups or between sexes were not feasible for MALDI IMS. Western blot data were analyzed using three-way ANOVAs (sex × treatment × omega-3 diet) with follow-up Fisher’s Least Significant Difference (LSD) post hoc test. We assessed the normality of data distributions using the Shapiro-Wilk test and applied suitable statistical adjustments for non-normal datasets; specifically, the logarithmic transformation of electrophysiological data. To identify and exclude statistically significant outliers, we utilized the Robust Regression and Outlier Removal (ROUT) method. Effect sizes for all three-way ANOVAs are reported as partial eta squared (η²p). Behavioral and electrophysiological datasets were analyzed offline and, where feasible, in a blinded manner to reduce experimenter bias. Western blot analyses were conducted blind to group assignment, although M.H.S. was not blinded to target proteins. For MALDI IMS, brain region selection was performed blinded to experimental conditions and analyzed using an automated MATLAB script developed by M.H.S., M.B., and S.L.C.; S.L.C. was not blinded to group assignment. Statistical analyses were performed using GraphPad Prism (version 9.0.0 for Windows 10), while R studio was used to calculate the effect sizes. Exact values ± SEM are reported.
Results
Prenatal THC exposure reduces birth weights in both sexes; omega-3 supplementation mitigates this effect without altering maternal or fetal outcomes
Maternal and fetal outcomes were evaluated for all cohorts. From gestational day 7 (GD7) to GD22, dams received vehicle (VEH; saline) or 3 mg/kg i.p., THC daily [5, 6, 11, 26, 28], in conjunction with either control (ct) or an omega-3 (ω3) diet (see Supplemental Table 1 for composition) from GD7 to postnatal day (PD) 21 (Fig. 1a; Supplemental Fig. 1).
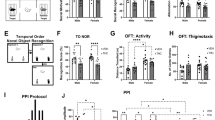
a Schematic representation of experimental timeline. b Impact of Prenatal THC on Birth Weights (8 pups/dam, approximately even sex distribution): Prenatal THC exposure (THCct) significantly reduced birth weights at postnatal day 1 (PD1) in both male and female pups (p < 0.05). Perinatal omega-3 supplementation (THCω3) prevented this reduction (p < 0.05), with a significant effect in females, but males, compared to VEHct. By PD21, only male VEHω3 offspring showed significant weight differences compared to THCct males. c–e, Anxiety-like behaviors assessed in adulthood (n = 12-20/treatment/sex) using EPM and LDB tests, while anxiety and motility was assessed using OFT: c Elevated Plus Maze (EPM): No significant anxiety-like behavior was observed in THCct males (p > 0.05). However, omega-3 supplementation resulted in reduced anxiety in both VEHω3 and THCω3 males (p < 0.05). d Light-Dark Box (LDB): THCct males exhibited increased anxiety-like behavior compared to VEHct males (p < 0.05). Omega-3 supplementation in THCω3 males normalized this effect (p < 0.05). e Open Field Test (OFT): No significant differences were found in anxiety (first 5 minutes of center entries) or locomotor activity over 30 minutes among the groups (p > 0.05). f Sensorimotor Processing Assessed by Prepulse Inhibition (PPI): Schematic illustrates the PPI protocol with a 100 ms interstimulus interval at prepulse intensities of 72 dB, 76 dB, and 80 dB. Baseline startle magnitudes: VEHω3 females > VEHct and THCω3; males > females (p < 0.05). No significant group differences were observed in PPI. g–i, Cognitive and Memory Assessments in Adulthood: g Three-Chamber Social Interaction Test: Schematic depicts the assessment of social motivation and recognition. THCct offspring showed impaired social motivation and memory compared to VEHct, VEHω3, and THCω3 groups (p < 0.05). Omega-3 supplementation rescued social motivation deficits in both sexes (p < 0.05) but improved social memory only in males (p < 0.05). h Y-Maze Spontaneous Alternation (SA) Test: Prenatal THC exposure led to deficits in spatial working memory in both sexes (p < 0.05). Omega-3 supplementation prevented these deficits in males but not females (p < 0.05). i Temporal Order Novel Object Recognition (TONOR) Test: THCct offspring exhibited impairments in object recognition memory in both sexes. Omega-3 supplementation ameliorated these deficits in males but not in females (p > 0.05). Estrous cycles were assessed for all female offspring, but no effect on estrous cycle and behavior were observed. Outliers were excluded using the ROUT method. Statistical analyses were conducted using three-way ANOVAs with factors of sex, THC treatment, and omega-3 diet, followed by Tukey’s HSD post hoc tests. Treatment effects are indicated with solid lines; sex effects with dashed lines. Significance levels are denoted as ****p < 0.0001, ***p < 0.001, **p < 0.01, *p < 0.05. Data are presented as individual points with mean±SEM.
Maternal outcomes
We assessed the impact of prenatal THC exposure and omega-3 fatty acid supplementation on several maternal variables, including gestational length, dietary intake, weight gain during pregnancy, litter size, and live birth index (Supplemental Fig. 1). Consistent with previous reports [5, 6, 11, 26, 28], no significant differences were observed in any of these parameters across the treatment groups. For live birth index, stillbirths were noted in only the THCct (two pups) and THCω3 (one pup) groups. Importantly, omega-3 supplementation did not affect any maternal outcomes, regardless of THC exposure.
Pup outcomes
To evaluate fetal growth restriction (FGR), we recorded body weights at birth/postnatal day 1 (PD1) and at PD21 and monitored survival up to PD4. At PD1, a three-way ANOVA revealed significant main effects of omega-3 diet (F(1528) = 49.33; p < 0.0001; η²p = 0.05) and THC treatment (F(1528) = 20.68; p < 0.0001; η²p = 0.03). Post-hoc comparisons revealed that male pups from THCct dams weighed significantly less than VEHct pups (p = 0.0018) and VEHω3 pups (p < 0.0001), but not THCω3 pups (p = 0.1353; Fig. 1b, Supplemental Fig. 1). Post-hoc comparisons also revealed female pups from THCct dams weighed significantly less than VEHct pups (p = 0.0018), VEHω3 pups (p < 0.0001), and THCω3 pups (p = 0.0130). Additionally, female VEHω3 weighed significantly more then THCω3 pups (p = 0.0198). Importantly, no differences were observed between male or female VEHct, and THCω3 groups (p > 0.05).
At PD21, three-way ANOVA revealed an effect of only omega-3 diet (F(1522) = 18.69; p < 0.0001; η²p = 0.04). Post-hoc comparisons revealed that VEHω3 male offspring weighed significantly more than male THCct (p = 0.0084) offspring (Fig. 1b, Supplemental Fig. 1). The body weight normalization of THCct at PD21, relative to VEHct, indicates postnatal catch-up growth, consistent with gestational THC exposure in rodents and humans [5, 8, 26]. Three pups were dead at birth; survival until PD4 was unaffected, except in n = 1 pup in one VEHω3 dam (Supplemental Fig. 1).
Omega-3 supplementation prevents PTE-induced anxiety-like phenotypes in early adulthood
Prior work has demonstrated male-specific anxiety-like phenotypes following PTE, with females largely unaffected [6, 7]. Thus, we assessed anxiety in both sexes using the elevated plus maze (EPM) and light dark box (LDB) and assessed general motility with the open field test (OFT) (Fig. 1a) to rule out motility-related effects of THC or the omega-3 diet that might confound the interpretation of anxiety (and cognitive) tasks.
Elevated plus maze
EPM data are presented in Fig. 1c. At adulthood, three-way ANOVA on times spent in open arms revealed a significant effect of sex (F(1,96) = 39.54, p < 0.0001; η²p = 0.34), THC treatment (F(1,96) = 19.04, p < 0.0001; η²p = 0.19), interaction Sex×omega-3 diet (F(1,96) = 5.831, p = 0.0176; η²p = 0.06) and Sex×THC (F(1,96) = 4.655, p = 0.0334; η²p = 0.05). Post-hoc analyses revealed a significant decrease in times spent in open arms in male THCct compared to VEHω3 (p < 0.0001) and THCω3 (p = 0.0074), but not VEHct (p = 0.4471), and a significant increase in male VEHω3 compared to male VEHct (p = 0.0178). No effect was observed in any treatment in females. Furthermore, males spent significantly less time in open arm vs. female counterparts in VEHct (p = 0.0068), THCct (p < 0.0001), and THCω3 (p = 0.0059).
Three-way ANOVA on open arm entries revealed significant effects of sex (F(1,95) = 48.43, p < 0.0001; η²p = 0.31) and interaction Sex×omega-3 Diet (F(1,95) = 6.838, p = 0.0104; η²p = 0.08). Post-hoc analysis revealed no within sex differences. However, we observed significantly lower entries of males vs. their female counterpart in THCct (p < 0.0001), and THCω3 (p = 0.0020).
Light/Dark box
LDB data are presented in Fig. 1d. At adulthood, three-way ANOVA on times spent on the light side revealed significant effects of sex (F(1,91) = 7.145, p = 0.00089; η²p = 0.09), omega-3 diet (F(1,91) = 24.71, p < 0.0001; η²p = 0.20), and THC treatment (F(1,91) = 21.93, p < 0.0001; η²p = 0.21). Post hoc analyses revealed a significant decrease in the time spent on the light side in the male THCct group compared to male VEHct (p = 0.0177), VEHω3 (p < 0.0001), and THCω3 (p = 0.0096). Female THCct also spent significantly less time on the light side compared to female VEHω3 (p = 0.0012). Three-way ANOVA on the latency to re-emerge from dark to light environment revealed significant effects of omega-3 diet (F(1,91) = 12.64, p = 0.0006; η²p = 0.14), interaction Sex× omega-3 diet (F(1,91) = 4.203, p = 0.0432; η²p = 0.05), Sex×THC (F(1,91) = 5.885, p = 0.0172; η²p = 0.07), THC×omega-3 diet (F(1,91) = 4.659, p = 0.0335; η²p = 0.06), and Sex×THC×omega-3 diet (F(1,91) = 9.270, p = 0.0030; η²p = 0.09). Post-hoc analyses revealed that male THCct rats transitioned to the light side later male VEHct (p < 0.0001), VEHω3 (p < 0.0001), and THCω3 (p < 0.0001). No effect was observed in the female progeny (p > 0.05). Furthermore, males THCct exhibited significantly greater latency vs. their female counterpart (p = 0.0018).
Open field test
OFT data are presented in Fig. 1e. For 5 min center entries (anxiety), three-way ANOVA revealed significant effects of sex (F(1,88) = 7.414, p = 0.0078; η²p = 0.07), THC treatment (F(1,88) = 4.827, p = 0.0306; η²p = 0.04), and interaction Sex×THC treatment (F(1,88) = 4.817, p = 0.0308; η²p = 0.04). Post-hoc analyses revealed no significant comparisons. For 30 min movement distance (motility), three-way ANOVA did not find any significant effects.
Omega-3 supplementation mitigates PTE-induced cognitive deficits in adulthood
Previous findings demonstrate that PTE induces cognitive and memory impairments in male/female adult offspring, while only males exhibited sensorimotor processing deficits [5, 6, 9, 10]. In this study, acoustic prepulse inhibition (PPI) of the startle reflex was performed to investigate sensorimotor gating. We also employed social interaction (SI) to examine social motivation and memory, spontaneous alternation (SA) to assess spatial working memory and temporal order novel object recognition (TONOR) to gauge object recognition memory.
Prepulse inhibition of the acoustic startle response
PPI data are presented in Fig. 1f. At adulthood, three-way ANOVA of startle baselines revealed a main effect of sex (F(1,93) = 78.75; p < 0.0001; η²p = 0.47), and significant interactions of THC×omega-3 diet (F(1,93) = 7.491; p = 0.0074; η²p = 0.06), and Sex×THC×omega-3 diet (F(1,93) = 4.930; p = 0.0288; η²p = 0.05). Post-hoc analysis revealed a significant significantly greater startle baselines in males vs. female counterparts in VEHct (p < 0.0001), THCct (p = 0.0140), and THCω3 (p < 0.0001), but not VEHω3 (p = 0.0766).
Three-way ANOVA of PPI revealed significant effects of sex (F(5279) = 3.785; p = 0.0025; η²p = 0.00336), and omega-3 diet (F(1279) = 7.641; p = 0.0061; η²p = 0.08). Post-hoc analysis revealed no significant group differences.
Social interaction
SI test data are presented in Fig. 1g. At adulthood three-way ANOVA on social motivation (phase 1) revealed main effects of THC treatment (F(1,96) = 9.246, p = 0.0030; η²p = 0.10), omega-3 diet (F(1,96) = 29.63, p < 0.0001; η²p = 0.25), and a significant interaction THC×omega-3 diet (F(1,96) = 11.25, p = 0.0011; η²p = 0.11). Post-hoc analyses revealed that the THCct male rats spent less time with social target over empty cage in male THCct compared to male VEHct (p < 0.0001), VEHω3 (p = 0.0008), and THCω3 (p = 0.0257). Similar effects were observed in the female group (THCct vs. VEHct p = 0.0013; THCct vs. VEHω3 p = 0.0036; and THCct vs. THCω3 p = 0.0386).
Moreover, at adulthood, three-way ANOVA on social memory (phase 2) revealed main effects of THC treatment (F(1,96) = 12.04, p = 0.0008; η²p = 0.13), omega-3 diet (F(1,96) = 37.60, p < 0.0001; η²p = 0.28), and significant interactions of THC×omega-3 diet (F(1,96) = 8.571, p = 0.0043; η²p = 0.09). Post-hoc analyses revealed a significant decrease in time spent interacting with novel target over the familiar target in the male THCct compared to male VEHct (p < 0.0001), VEHω3 (p = 0.0009), and THCω3 (p = 0.0002). Furthermore, female THCct exhibited a reduction in social memory compared to VEHct (p = 0.0007) and VEHω3 (p < 0.0001), but not THCω3 (p = 0.5079). Additionally, female VEHω3 exhibited significantly better performance then THCω3 (p = 0.0445).
Spontaneous alternation
Y-maze SA test data are presented in Fig. 1h. At adulthood, three-way ANOVA revealed main effects of omega-3 diet (F(1,98) = 52.52; p < 0.0001; η²p = 0.38), THC treatment (F(1,98) = 37.57, p < 0.0001; η²p = 0.31), and significant interactions of THC treatment×omega-3 diet (F(1,98) = 8.436; p = 0.0045; η²p = 0.11) and Sex×THC×omega-3 diet (F(1,98) = 14.66; p = 0.0002; η²p = 0.13). Post-hoc analyses revealed a significant decrease in spontaneous alternations score in the male THCct compared to male VEHct (p < 0.0001), VEHω3 (p < 0.0001), and THCω3 (p < 0.0001). Similarly, the female THCct had significantly lower scores compared to VEHct (p = 0.0447) and VEHω3 (p < 0.0001), while VEHω3 had better performance than VEHct (p = 0.0405) and THCω3 (p = 0.0052).
Temporal order novel object recognition
TONOR data are presented in Fig. 1i. At adulthood, three-way ANOVA revealed significant effects of omega-3 diet (F(1,87) = 14.62; p = 0.0002; η²p = 0.12), THC treatment (F(1,87) = 22.25; p < 0.0001; η²p = 0.22), and an interaction THC×omega-3 diet (F(1,87) = 8.205; p = 0.0052; η²p = 0.09). Post-hoc analyses revealed that male THCct had lower temporal recognition scores compared to male VEHct (p = 0.0198), VEHω3 (p = 0.0005), and THCω3 (p = 0.0001). For the females, the THCct group exhibited lower temporal recognition scores compared to VEHct (p = 0.0415), and VEHω3 (p = 0.0054), but not THCω3 (p = 0.0925).
Omega-3 supplementation prevents neuronal dysfunction in the male PFC and vHIPP, but only in the female vHIPP, of PTE offspring
PTE differentially dysregulates neuronal activity in the PFC and ventral hippocampus (vHIPP) of male and female offspring [9, 10]. To assess the impact of omega-3 supplementation on this circuit, we conducted in vivo extracellular electrophysiological recordings in the PFC and vHIPP of adult rats (Fig. 2).
a End track coordinates for recorded presumptive pyramidal cells in the PFC of both hemispheres for males and females. b Spontaneous Firing Rates in the PFC: Prenatal THC (THCct) significantly increases PFC spontaneous pyramidal cell firing frequency in male and female offspring at adulthood (cells/rat: males—VEHct n = 78/8, THCct n = 67/7, VEHω3 n = 62/8, THCω3 n = 82/8; females—VEHct n = 60/8, THCct n = 64/7, VEHω3 n = 57/8, THCω3 n = 81/8). Omega-3 supplementation prevents the hyper-glutamatergic phenotype in males (p < 0.05) and mitigates the effect in females THCω3 vs. VEHct. c Bursting Activity in the PFC: Neither prenatal THC exposure nor omega-3 supplementation significantly affected the bursting frequency of PFC pyramidal neurons. However, female PFC VEHct exhibit higher bursting rates then male counterparts. d End track coordinates for recorded presumptive pyramidal cells in the vHIPP of both hemispheres for males and females. e Spontaneous Firing Rates in the vHIPP: Prenatal THC induces significantly lower spontaneous pyramidal cell firing frequency in the male vHIPP and significantly higher firing frequency in the female vHIPP (cells/rat: males—VEHct n = 60/8, THCct n = 73/7, VEHω3 n = 54/8, THCω3 n = 60/8; females—VEHct n = 50/8, THCct n = 68/7, VEHω3 n = 70/8, THCω3 n = 47/8). Omega-3 intervention prevents these phenotypes in both males and females (p < 0.05). f Bursting Activity in the vHIPP: Omega-3 supplementation elevated bursting frequency in male THCω3 offspring compared to VEHct, THCct, and VEHω3 males (p < 0.05). Additionally, female THCct exhibited elevated bursting frequency compared to male counterparts (p < 0.05). This pattern suggests that while omega-3 fatty acids broadly confer resilience against prenatal THC-induced neuronal dysfunctions, they may also introduce sex-dependent neurophysiological alterations within hippocampal circuitry. Recordings outside the targeted brain regions were excluded based on histological confirmation. Outliers were removed using the ROUT method. Statistical analyses were conducted using three-way ANOVAs with factors of sex, THC treatment, and omega-3 diet, followed by Tukey’s HSD post hoc tests. Significance levels are indicated as ****p < 0.0001, ***p < 0.001, **p < 0.01, *p < 0.05. Log-normalized data are presented as individual points with mean±SEM.
Pyramidal cell activity
In PFC, three-way ANOVA comparing spontaneous firing frequency of putative pyramidal cells revealed significant effects of THC treatment (F(1544) = 7.747, p = 0.0056; η²p = 0.04) and omega-3 diet (F(1, 544) = 26.78, p < 0.0001; η²p = 0.01). Post-hoc analyses revealed significantly higher firing frequency in male THCct compared to VEHct (p = 0.0062), VEHω3 (p = 0.0051), and THCω3 (p = 0.0015). In females, the THCct group had significantly higher activity than the VEHct (p = 0.0347) and VEHω3 (p < 0.0001), but not THCω3 (p = 0.2475).
For bursting frequency in the PFC, three-way ANOVA revealed an effect of sex (F(1468) = 5.662, p = 0.0177; η²p = 0.01) and sex×omega-3 diet (F(1468) = 6.074, p = 0.0141; η²p = 0.01). Post-hoc analyses that female VEHct exhibited significantly higher bursting frequency then male VEHct (p = 0.0380).
In vHIPP, three-way ANOVA comparing spontaneous firing frequencies of putative pyramidal cells revealed significant interactions of Sex×omega-3 diet (F(1474) = 26.89, p < 0.0001; η²p = 0.06), Sex×THC treatment (F(1474) = 6.069, p = 0.0141; η²p = 0.01), and a Sex×THC×omega-3 diet (F(1474) = 19.60, p < 0.0001; η²p = 0.04). Post-hoc analyses revealed that male THCct rats had significantly lower activity compared to VEHct (p < 0.0001), VEHω3 (p = 0.0005) and THCω3 (p = 0.0213). On the other hand, female THCct group had significantly higher activity than the VEHct (p < 0.0001), VEHω3 (p = 0.0050), and THCω3 (p = 0.0073). Additionally, male VEHct vs. female VEHct exhibited a significantly lower firing rate (p = 0.0029), while female THCct vs. male THCct exhibited significantly elevated firing rates (p < 0.0001).
For bursting frequency in the vHIPP, three way ANOVA revealed an effect of sex (F(1469) = 4.702, p = 0.0306; η²p = 0.00729), omega-3 diet (F(1469) = 12.16, p = 0.0005; η²p = 0.03), THC treatment (F(1469) = 21.17, p < 0.0001; η²p = 0.05), Sex×THC treatment (F(1469) = 14.56, p = 0.0002; η²p = 0.03), THC×omega-3 diet (F(1469) = 9.130, p = 0.0027; η²p = 0.02) and sex×THC×omega-3 diet (F(1469) = 5.513, p = 0.0193; η²p = 0.01). Post-hoc analyses that male THCω3 exhibits higher burst frequencies than VEHct (p < 0.0001), VEHω3 (p < 0.0001) and THCct (p < 0.0001). Additionally, female THCct exhibited higher burst frequencies than the male THCct (p = 0.0175).
Local field potentials
Prenatal THC exposure induced alterations in oscillatory patterns within the PFC-vHIPP circuitry of both male and female offspring (Fig. 3; statistical analyses are presented in Supplemental Table 3). Male THCω3 normalized the THC-induced dysregulations in PFC LFP activity across the delta (0-4 Hz), theta (4-7 Hz), beta (14-30 Hz), and gamma (30-100 Hz), but not alpha bands (7-14 Hz) (Fig. 3b, c; Supplemental Table 3).
a Representative Spectrograms: Local field potential (LFP) recordings from male PFC and vHIPP across treatment groups. Spectrograms highlight small-amplitude, fast oscillations. Power values between 59–61 Hz (reflecting power line frequency) were excluded. b Normalized Power Spectra: Average power spectra of LFP recordings in the adult male and female PFC and vHIPP. Spectral analyses examined delta (0–4 Hz), theta (4–7 Hz), alpha (7–14 Hz), beta (14–30 Hz), and gamma (30–100 Hz) bands. c PFC Oscillations: Prenatal THC exposure and omega-3 supplementation induced significant changes in PFC oscillatory activity across sexes (recording sites/rat: males—VEHct n = 54/8; THCct n = 90/7; VEHω3 n = 75/8; THCω3 n = 85/8; females—VEHct n = 82/8; THCct n = 61/7; VEHω3 n = 88/8; THCω3 n = 68/8); see Supplemental Table 3 for statistical analyses. Males: THC exposure increased theta, alpha, and gamma power compared to VEHct. Male THCω3 did not exhibit significant differences from either VEHct or THCct; suggesting that some underlying THC-induced effects persist. Females: THC exposure increased only alpha power compared to VEHct. Omega-3 supplementation elevated delta, and reduced theta, beta, and gamma power in the PFC compared to VEHct. d vHIPP Oscillations: THC and omega-3 supplementation induced significant changes in vHIPP oscillations (recording sites/rat: males—VEHct n = 66/8; THCct n = 60/7; VEHω3 n = 77/8; THCω3 n = 60/8; females—VEHct n = 50/8; THCct n = 68/7; VEHω3 n = 76/8; THCω3 n = 48/8). Males: THC exposure elevated delta, and reduced theta, alpha, beta, and gamma oscillations. Omega-3 supplementation normalized delta, alpha, beta, and gamma oscillations to VEHct levels, compared to THCct, but had no effect on theta oscillations. Females: THC exposure increased alpha and gamma power compared to VEHct. Omega-3 supplementation significantly reduced total power across all frequency bands compared to THCct, except delta oscillations. Outliers were removed if failing the ROUT Outlier Test. Comparisons were made using Three-Way ANOVA with Sex, THC treatment, and omega-3 diet as factors, followed by post hoc Tukey’s HSD. ****p < 0.0001, ***p < 0.001, **p < 0.01, *p < 0.05; individual data points with mean ± SEM are shown.
Notably, the female THCω3 group exhibited a significant divergence in the both the PFC and vHIPP compared to VEHct in delta, theta, beta, and gamma oscillatory bands; as well as alpha in the vHIPP (Fig. 3c). In contrast, for males, in the vHIPP, male THCω3 exhibit disturbances compared to VEHct in only theta and gamma oscillatory bands, while vHIPP delta, alpha, and beta and all bands in the PFC exhibit control level LFP (Fig. 3c). This sex-specific difference may explain the behavioral phenotype that relies on normative PFC-vHIPP communication. The restoration of normal oscillatory patterns in male THCω3 rats may account for their improved cognitive performance relative to THCct males, while this is absent in females.
Omega-3 supplementation modifies lipidomic profile in PTE offspring’s PFC, NAc, and hippocampal formation
To assess whether perinatal omega-3 fatty acid supplementation can counteract THC-induced lipidomic alterations, we examined the impact of PTE on PUFAs and the broader phospholipid profiles in specific brain regions (Fig. 4). Our focus included the prelimbic (PRL) and infralimbic (IL) areas of the medial PFC, the core and shell of the NAc, and the ventral and dorsal subiculum, and the CA1 area, of the hippocampus, following established methodologies [5, 6]. Using MALDI IMS, we generated heatmaps representing the ratio of each treatment group’s area under the curve (AUC) to that of the VEHct groups AUC. Using one sample t test with a hypothetical mean set at 1.0, where 1.0 designates values at VEHct levels (statistical analyses are presented in Supplemental Table 4), MALDI-IMS analysis revealed significant changes in fatty acids due to prenatal THC exposure (THCct) (Fig. 4c–e); consistent with prior reports [5, 6, 26]. Perinatal omega-3 supplementation, both in the presence (THCω3) and absence (VEHω3) of THC exposure, also resulted in significant alterations in fatty acid profiles (Fig. 4c-e; Supplemental Table 4).
a Graphical representation illustrates the origin of the assessed fatty acids. The biosynthesis of PUFAs involves several enzymatic steps that largely take place within the endoplasmic reticulum of hepatocytes in the liver; a more limited capacity for fatty acid synthesis does exist within astrocytes in the brain. The ECS, through CB1R and PPAR, regulates fatty acid synthesis and glucose metabolism. These fatty acids and glycerol-3-phosphate (G3P) are then utilized in lipid formation (see Fig. 5). For omega-6 PUFAs, linoleic acid is converted to arachidonic acid (AA), while, for omega-3 PUFAs, alpha-linolenic acid is converted to EPA, and via a PPAR-mediated mechanism in peroxisomes, into DHA. These fatty acids are then incorporated into lipids within the brain. Their release from the membrane is mediated by the Land’s Cycle and various phospholipases (here, PLA2, which has a preference for AA cleavage), that then allow these fatty acids to be converted to inflammatory mediators, or endocannabinoids. b Representative male and female MALDI IMS images of DHA in each of the treatment groups. As mass spectrometry is subject to run-to-run variability, all experimental values are normalized as ratios to VEHct, which is treated as the value 1.0. Mass-to-charge (m/z) values are based on previously described mass target [55]. The assessed fatty acids and their m/z are detailed in Supplemental Table 2. c-e, Male and female offspring at PD21 (Male: n = 12/treatment, Female: n = 8/treatment) and PD120 (n = 10/treatment/sex, Female: n = 8/treatment) exposed to THC (THCct) exhibited significant age- and sex-specific fatty acid alterations in the (c) prelimbic and infralimbic regions of the PFC, (d) the NAc core (NAcc) and shell (NASh), and (e) the dorsal and ventral subiculum, and CA1 region, of the hippocampus. The omega-3 intervention (VEHω3 and THCω3) also led to significant changes in numerous fatty acids, notably AA and its metabolites. Female offspring exhibited largely normalized fatty acids by adulthood, while male THCct offspring showed substantial PFC, NAc, and hippocampal abnormalities. Area under the curve (AUC) ratio comparisons were conducted with one-sample t tests, with a hypothetical mean of 1.0; where 1.0 suggests no difference between the VEHct compared to each respective treatment’s offspring. Heatmaps present AUC ratio means, while statistical analyses, compared to VEHct, is presented in Supplemental Table 4.
Notably, males and female receiving omega-3 supplementation exhibited a normalized lipidome at PD21. However, the perinatal dietary supplement in males led to a downregulation of all assessed fatty acids by PD120 particularly in the PFC and NAc regions, with minimal disturbances in hippocampus. The female phenotype across all groups at PD120 was also largely in line with VEHct levels, normalized, and consistent with prior reports [5, 6].
While fatty acid levels provided some insights, a comprehensive analysis of the lipidome revealed significant anomalies not apparent from fatty acid data alone (Fig. 5). We examined several classes of phospholipids: lysophospholipids (signaling molecules with a cleaved fatty acid tail; Fig. 5a), sulfatide-bound phospholipids (sulfatides; associated with the myelin sheath or in transit to it; Fig. 5b), and membrane phospholipids (containing two fatty acid tails and are either part of the membrane or in transit to it; Fig. 5e). These lipid species are products of the hepatic fatty acid pathway (Fig. 4a) and glucose metabolism mediated by CB1Rs, produced via the Kennedy pathway and then remodeled via the Land’s Cycle either within the liver or the brain (Fig. 5a).
a The Kennedy Pathway and Land’s cycle are central in lipid formation. Once fatty acids and glyercol-3-phosphate (G3P) are formed they can be converted into a phosphatidic acid (PA). PA is the simplest phospholipid. PA is then converted into diacylglycerol; (DAG). DAG, central in the Kennedy Pathway, yields phosphatidylcholine (PC) through CDP-choline pathway, or phosphatidylethanolamine (PE) via the CDP-Eto pathway. PE and PC can be metabolized to form phosphatidylserine (PS), which are major synaptic phospholipids typically enriched with DHA. PA can also produce phosphatidylinositol (PI; a key fatty acid utilized for endocannabinoid production and other signaling pathways), phosphatidylglycerol (PG), and cardiolipin (CL; critical to mitochondrial membrane structure and function) via the CDP-DAG pathway. Land’s Cycle remodels brain phospholipids utilizing phospholipases, like PLA2, to modify fatty acyl chains; a process essential for membrane flexibility and endocannabinoid signaling. Lastly, a graphical representation of a neuron illustrates the potential origins of the assessed fatty acids and phospholipids: lysophospholipids are membrane phospholipids released by action of phospholipases and have a fatty acyl chain cleaved; membrane phospholipids, intact phospholipids with both fatty acid tails found within or being incorporated into membranes; and sulfatide-containing phospholipids (i.e., sulfatides), primarily located in myelin or in transit into myelin. b–d, Mass-to-charge (m/z) values are based on previously described mass target [55]. Following standard fatty acid and lipid nomenclature for the fatty acids, the brackets for each phospholipid indicate the carbon chain that constitutes the associated fatty acid. The assessed lipids and their m/z are detailed in Supplemental Table 2; statistical analyses are presented in Supplemental Table 5. Male and female offspring (PD21, n = 8/treatment/sex; PD120, n = 8/treatment/sex) exposed to THC (THCct) exhibited significant age- and sex-specific lipidomic alterations. The omega-3 intervention (VEHω3 and THCω3) also led to significant changes. Female THCω3 exhibited substantial alterations at PD21 and PD120 in the PFC, NAc, and hippocampal regions. On the other hand, male THCω3 exhibited disturbances in select phospholipids, particularly those containing AA (heatmaps present means and statistical significance compared to VEHct, see Supplemental Table 5 for statistical analyses). Area under the curve (AUC) ratio comparisons were conducted with one-sample t tests, with a hypothetical mean of 1.0; where 1.0 suggests no difference between the VEHct compared to each respective treatment’s offspring; ****p < 0.0001, ***p < 0.001, **p < 0.01, *p < 0.05, two-tailed.
Using one sample t test with a hypothetical mean set at 1.0, where 1.0 designates values at VEHct levels (see Supplemental Table 5 for all statistics) the omega-3 diet led to numerous region- and sex- specific alterations in membrane phospholipids in both THCω3 and VEHω3 groups, with lysophospholipids and sulfatides largely unaffected. Heatmaps exhibit ratios where each treatment groups AUC is divided by VEHct AUC. Interestingly, the lipid profiles in both THCω3 male and female offspring resembled those of THCct, at both postnatal days 21 and 120 in the hippocampus (Fig. 5, Supplemental Table 5). Severe deficits were observed in females within the PFC and NAc, particularly at PD120, effecting the lysophospholipids, membrane phospholipids, and sulfatides.
Both VEHω3 and THCω3 also exhibited disturbances in phospholipids associated with particularly AA or its related metabolites (Fig. 5, Supplemental Table 5). Alterations were also seen in the lysophospholipids, including lysophosphatidylethanolamines (lyso-PE), a critical precursor to the primary endocannabinoids AEA and 2-AG [57].
Omega-3 supplementation alters proteins involved in neural development, synaptic function, and fatty acid metabolism in the PFC, NAc, and vHIPP in a sex-dependent manner
We next examined biomarkers associated with neurodevelopment, neurotransmission, fatty acid metabolism and neuroinflammation. Targets examined in PFC, NAc, and vHIPP at PD21 and PD120 included dopamine D1R and D2R, NMDA receptor (GluN) 2 A and GluN2B, metabotropic glutamate receptor 2/3 (mGluR2/3), vesicular glutamate transporter (vGlut) 1 and vGlut2, synaptophysin (SYP), postsynaptic density protein 95 (PSD95), brain derived neurotropic factor (BDNF), GAD67, Gephyrin, peroxisome proliferator-activated receptor α (PPARα), and PPARγ (isoforms were not observed). At PD120, three-way ANOVA revealed that PTE induced alterations to several dopaminergic, GABAergic, and glutamatergic biomarkers, as well as proteins involved in synaptic organization and development (Fig. 6; see Supplemental Table 6 for statistical analyses). Most of these effects were absent in the THCω3 treated groups, however, some exceptions were observed in all categories of proteins examined (Fig. 6, Supplemental Table 6).
Western Blot protein analyses revealed sex- and region-specific changes in critical neural markers within the prefrontal cortex (PFC) and ventral hippocampus (vHIPP) at postnatal day 120 (PD120). Supplemental Fig. 2 presents nucleus accumbens (NAc) protein analyses. Detailed statistical analyses for the PFC, NAc, and vHIPP are provided in Supplemental Table 6. The proteins examined are key components of the dopamine, glutamate, and GABA neurotransmitter systems, synaptic organization, and lipid metabolism. a Protein bands of interest are shown for each treatment group, normalized to their respective α-tubulin controls and then to the vehicle control diet group (VEHct) for each sex. All blots displayed are PFC. b Dopamine Receptors (D1R and D2R): Dopamine D1R and D2R modulate excitatory and inhibitory responses, respectively, and have distinct roles in the PFC, NAc, and vHIPP affecting anxiety, reward processing, and cognition. c Glutamate Markers: NMDA receptor subunits GluN2A and GluN2B are critical for excitatory signaling, especially in the corticostriatal network. Metabotropic glutamate receptor 2/3 (mGluR2/3) inhibits glutamate release, helping maintain excitatory balance. Vesicular glutamate transporters vGlut1 and vGlut2 facilitate glutamate release, supporting excitatory neurotransmission. d Synaptic Organization Proteins: Synaptophysin (SYP), postsynaptic density protein 95 (PSD95), and brain-derived neurotrophic factor (BDNF) are essential for excitatory synapse integrity. BDNF promotes synaptic plasticity and neurogenesis, while PSD95 serves as a scaffolding protein at excitatory synapses. e GABAergic Markers: Glutamate decarboxylase 67 (GAD67) synthesizes GABA from glutamate, vital for maintaining baseline inhibitory neurotransmission. Gephyrin supports GABAA receptor clustering at inhibitory synapses. f Nuclear Receptors (PPARα and PPARγ): Peroxisome proliferator-activated receptors are involved in fatty acid metabolism(particularly DHA), contextual fear extinction, cognition, and inflammation. Outliers were removed if failing ROUT Outlier Test. Comparisons were made with Three-Way ANOVA with Sex, THC treatment, and the omega-3 diet as factors, with post hoc Fisher’s LSD, ****p < 0.0001, ***p < 0.001, **p < 0.01, *p < 0.05; individual data points with mean and ± SEM are presented.
Discussion
Our study uncovers various novel effects of PTE on neuropsychiatric biomarkers within the cortico-striatal-hippocampal network, encompassing cognitive, affective, neuronal, protein, and lipidomic alterations. We demonstrate that perinatal omega-3 fatty acid supplementation can mitigate many adverse outcomes of THC exposure, albeit with sex-specific efficacy. Male offspring exhibited significant improvements across behavioral, electrophysiological domains, effectively counteracting THC-induced deficits in all but protein and lipidomics. In stark contrast, female offspring exhibited improvements in only social behavior and neuronal activity but retained residual THC-related disturbances in their PFC, NAc, and hippocampus. These sex-dependent outcomes likely reflect variations in developmental responses to PTE, sensitivity to FGR, differential neuroinflammatory and metabolic pathways, and distinct reactions to omega-3 interventions. These findings also highlight the need to consider sex-specific neurobiological responses when designing interventions for ECS-related neurodevelopmental disorders.
We focused on the PFC, vHIPP, and NAc, regions central to cognition, affect, and reward [17, 58, 59] and highly sensitive to cannabinoid-induced disruptions [4,5,6, 10, 19, 60, 61]. These areas form the core of the cortico-striatal-hippocampal network and are modulated by dopaminergic VTA signaling [62,63,64,65]. A hallmark of rodent models of neurodevelopmental PTE is hyperdopaminergia in the VTA, which disrupts the balance of excitation and inhibition across downstream targets [6, 60, 61]. The ECS plays a central role in regulating this balance by modulating GABAergic and glutamatergic tone, particularly via CB1R-dependent signaling in GABAergic interneurons [57]. These regions, in turn, regulate dopamine release by providing inhibitory and/or excitatory tone to the VTA [66, 67]. Dysregulation of this system during development leads to enduring circuit-level perturbations [4,5,6, 9, 10, 60] and may impact crucial developmental processes like cell migration, axonal guidance, and synaptogenesis [4, 18, 57].
In the PFC, CB1Rs regulate GABAergic inhibitory control via parvalbumin (PV)- and cholecystokinin (CCK)-positive interneurons [68]. PFC cannabinoid signaling regulates inhibitory tone and influences anxiety and affective behaviors [62, 63]. Disruption of PFC PV+ interneuron function alters gamma oscillations and impairs cognitive processes such as attention and working memory [68, 69]. Interestingly, disrupting PFC PV+ neuronal activity disrupts normal social behaviors [70], consistent with the disturbed sociability phenotypes observed in our THC-treated cohorts, and other prenatal THC studies [71,72,73,74]. Our analyses also revealed dysregulation in PFC gamma power in THC-treated males, which was prevented by the omega-3 intervention, demonstrating a protective effect of omega-3 against THC-induced cortical disruptions. These findings align with reports of reduced PFC PV+ interneurons in rodent models of FGR-induced schizophrenia, specifically sociability dysfunction, and impaired sensorimotor gating [75], consistent with our sociability, but not PPI, findings. Importantly, during perinatal brain development, DHA directly modulates glutamatergic and GABAergic activity in the PFC and vHIPP by promoting normal synaptic maturation and fatty acid signaling [45, 76]. Thus, omega-3 supplementation restored gamma oscillations in males, potentially by normalizing PV+ interneuron activity. However, in females, this intervention failed to rescue PFC function, suggesting fundamental sex differences in the response to omega-3 supplementation. The impact of the supplementation on interneuron function was not examined and requires further exploration.
Sex-dependent differences extended to the vHIPP, a region critical for regulating anxiety and memory. vHIPP→PFC inputs activate CCK+ and PV+ interneurons, providing feed-forward inhibition of pyramidal cells [77, 78]. Optogenetic inhibition of PFC CCK+ interneurons has been shown to disrupt working memory [79]. Previous studies have reported male-specific reductions in CCK+ cells following PTE, a phenotype linked to spatial memory deficits [10, 80]. Consistent with these findings, male THC-treated (THCct) cohorts in our study displayed PFC hyperactivity and vHIPP hypoactivity, reflecting a disrupted balance within the PFC-vHIPP network. In contrast, females exhibited hyperactivity in both regions, suggesting divergent alterations in circuit excitability. Reduced vHIPP spiking activity and increased vGlut1 expression in males suggest impaired glutamatergic tone [5, 9, 10, 71, 80], whereas females showed no changes in vGlut1 or vGlut2 expression despite increased pyramidal cell activity. These findings indicate that omega-3 supplementation effectively normalized excitatory/inhibitory balance in males but failed to address similar disruptions in females. Male THCct offspring also exhibited increased mGluR2/3 expression in the PFC, a compensatory response to pyramidal cell hyperactivity, as mGluR2/3 suppresses local glutamate release [81]. Notably, this upregulation was absent in THCω3 males, consistent with normalized pyramidal cell activity in the omega-3-supplemented cohort. Both male and female THCω3 offspring displayed reduced PFC expression of NMDA receptor subunits GluN2A and GluN2B. A similar reduction was observed in VEHω3 groups, indicating a direct effect of omega-3 supplementation on NMDA receptor expression, consistent with previous studies [82]. However, the sustained decrease in DHA levels in the PFC of THCω3 males by PD120 may have contributed to this downregulation, highlighting the long-term limitations of early-life supplementation. Interestingly, while females are more efficient in DHA synthesis [5, 83], their NMDA receptor expression patterns in the PFC mirrored those of males, indicating complex sex-specific omega-3 interactions that warrant further study.
Functional vHIPP-PFC interactions are critical for anxiety regulation [84], sensorimotor gating and cognition [85,86,87]. PTE disrupts key axonal proteins required for normative hippocampal neuronal connectivity [15] and impedes DHA transfer to neural membranes [5, 6, 11, 26, 88, 89]. DHA is critical for hippocampal neurogenesis and functional connectivity, and supplementation during gestation can improve spatial learning, cognition, and anxiety behaviors [48, 53, 90, 91]. Gestational omega-3 deficiency impairs myelin integrity and hippocampus-PFC connectivity, further exacerbating network disruptions [92]. Synaptic phospholipids such as lysophosphatidic acid (Lyso-PA) are also implicated in cortical excitation/inhibition balance [93]. As the several classes of membrane phospholipids are significantly reduced at PD120, they may contribute to the observed oscillatory disruptions within the male THCct and female THCω3 offspring. These early-life phospholipid reductions, evident as early as PD21, suggest a disrupted neural architecture that persists into adulthood and highlights the limited efficacy of omega-3 supplementation in females [93, 94]. Persistent lipid disturbances in THCω3 offspring, however, may also reflect systemic effects of PTE, particularly in peripheral organs like the liver and pancreas that are also developing during gestation [42]. Both are sites of high CB1R expression and are vulnerable to cannabinoid-induced dysregulation of fatty acid metabolism [11, 28, 42, 95,96,97]. Hepatic dyslipidemia, resulting in reduced fatty acid transport to the brain, may contribute to persistent DHA deficits in males. Astrocytes, the primary sites of brain lipid remodeling, may also be affected, as even adolescent THC exposure disrupts astrocyte structure and function [98,99,100]. These effects, though speculative, provide a potential framework for understanding the sex-specific lipidomic alterations observed in this study. Further exploration is required of the gestational aspect of this intervention to understand how these effects persist to PD120.
The NAc, a target of dopaminergic VTA projections, mediates anxiety, motivation, and reward-related behaviors and is also regulated by CB1Rs [17, 101, 102]. Hyperactive VTA dopamine signaling, a well-documented phenotype of PTE [6, 60], may disrupt downstream circuits, including those involving the PFC and NAc [6, 60, 61]. Given the PFC’s regulatory influence on the NAc through direct and indirect pathways, alterations in PFC activity may also exert downstream effects on NAc functionality [58, 71,72,73,74]. Alterations in PFC gephyrin, mGluR2/3, and GluN2A/B expression and associated neurophysiological adaptations likely influence dopaminergic and glutamatergic signaling within the NAc, contributing to anxiety-related behaviors observed in male THCct cohorts [71, 103]. Importantly, omega-3 supplementation prevented many of these molecular changes and abolished anxiety phenotypes in male offspring, as assessed by the EPM and LDB. However, female offspring showed no significant changes in DHA levels, anxiety phenotypes, or dopaminergic markers in the PFC, NAc, or vHIPP, suggesting a sex-specific resilience of the dopaminergic system to PTE [7].
The vHIPP-NAc pathway, which preferentially targets D2 receptor (D2R)-expressing medium spiny neurons, plays a critical role in anxiety modulation [104]. Inhibition of the vHIPP produces anxiolytic effects, while local THC administration induces acute anxiogenesis in rodent models [105, 106]. Our findings suggest significant dysregulation of the vHIPP-NAc circuit following PTE, with distinct sex-specific alterations. For instance, both the vHIPP and NAc exhibited changes in GAD67 expression, a key marker of GABA synthesis. THC-treated (THCct) males showed reduced NAc GAD67 expression in these regions, while females displayed significant elevations in NAc GAD67 levels, potentially as a compensatory response to heightened excitatory input from the PFC and/or vHIPP. Regional variations in dopamine D2R expression further highlight the role of the vHIPP-NAc circuit, particularly in male offspring. In THCct males, significant increases in D2R expression were observed in the vHIPP, paired with pronounced downregulation in the NAc. These changes likely reflect disrupted signaling along the vHIPP-NAc axis, where D2R-expressing medium spiny neurons in the NAc shell integrate hippocampal inputs critical for regulating anxiety and reward-related behaviors. Interestingly, omega-3 supplementation normalized D2R expression in the vHIPP and PFC of THCω3 males but failed to rescue the deficits in the NAc. This outcome contrasts with reports of adult omega-3 supplementation, which is associated with decreased D2R expression, whereas prenatal deficiency correlates with increased D2R expression during early development [107, 108]. These findings suggest that early-life supplementation may offset some developmental disruptions, though its benefits appear incomplete.
By PD120, DHA levels were significantly reduced in the prelimbic and infralimbic PFC, the NAc core and shell, and showed trends toward reductions in the vHIPP of THCω3 males. This depletion of DHA, despite early-life omega-3 supplementation, may contribute to alterations in D2R expression, as DHA is critical for dopaminergic signaling and D2R function [108]. Furthermore, DHA influences ECS-mediated anxiety regulation [109]. In contrast, THCct-treated females did not exhibit reductions in DHA at PD120, nor did they exhibit anxiety or alterations in D2R expression in the PFC, NAc, or vHIPP. This sex-selective resilience suggests that omega-3 supplementation may target mechanisms specific to male dopaminergic vulnerability following PTE [6, 7, 60].
The pronounced sex differences observed in anxiety-related behavioral tests align with established sexual dimorphism in both baseline anxiety behaviors and endocannabinoid system function in rodents. Female rodents typically display lower baseline anxiety levels in tests such as the elevated plus maze and light/dark box, characterized by greater exploratory behavior and reduced thigmotaxis [110, 111]. This behavioral divergence is partly mediated by estrogenic modulation of the ECS, including elevated AEA levels and enhanced CB1R sensitivity in limbic regions [112, 113]. PTE appears to disrupt this endocannabinoid-mediated anxiety regulation more severely in males, potentially due to their lower baseline endocannabinoid tone and distinct developmental trajectories of ECS maturation [114, 115]. The differential response to omega-3 supplementation between sexes further supports this interpretation, as the restoration of appropriate membrane lipid composition may more effectively normalize endocannabinoid signaling in males, who exhibit more pronounced baseline disruptions following THC exposure [5, 6, 116, 117].
Our findings also highlight the potential of maternal omega-3 supplementation to mitigate PTE-induced FGR phenotypes, particularly cognitive deficits associated with FGR-related “catch-up” growth [34,35,36, 40]. Early nutritional interventions are known to improve growth and cognitive outcomes [118], suggesting that improving early childhood nutrition may counteract gestational nutritional imbalances [35]. Recent studies using similar omega-3 supplementation protocols in rodent models of autism support this hypothesis, demonstrating enhanced placental nutrient and fatty acid transfer, modulation of neuroinflammatory mediators, and improved fetal growth [53, 54, 119, 120]. This likely explains the increased birth weights observed in VEHω3 and the elevated PD21 weights of male offspring in our study. However, sex differences in FGR resilience may influence the efficacy of omega-3 supplementation. Female fetuses demonstrate greater resilience to FGR stressors, exhibiting superior placental function and adaptive responses to inflammation compared to males [121]. Despite their resilience to FGR, female offspring may remain particularly susceptible to direct THC-induced effects [5], likely due to higher CB1R expression in the hippocampus [122]. Consistent with this, while female THCω3 offspring performed comparably to VEHct in social motivation tasks, they retained deficits in hippocampus-dependent behaviors. These behavioral outcomes may also be influenced by maternal factors, as maternal socialization during early life shapes social motivation later in life. In males, preventing FGR alleviated cognitive deficits in THCct offspring, although protein and lipidomic deficits persisted. This suggests that omega-3 supplementation effectively targets certain developmental disruptions in males, but its benefits remain incomplete; lipidomic and protein abnormalities persisted in THCω3 males, even in the absence of overt neuropathology.
The mitigation of the male THCω3 phenotype may stem from preventing FGR, thus avoiding postnatal catch-up growth or reducing gestational neuroinflammation, as seen in other studies using this diet, particularly in the placenta [53, 54, 123]. Maternal cannabinoid use also reduces oxygen supply, alongside nutrients and fatty acids, potentially causing hypoxic damage and elevated inflammation [11, 124,125,126]. The upregulation of DHA and EPA, two fatty acids known to minimize hypoxic damage and enhance anti-inflammatory processes [127], might explain the long-term effects observed [53, 54, 119, 120]. DHA and EPA lower inflammation, inhibit neuronal cell death [46, 128, 129], with EPA playing a vital role in placental protective mechanisms against oxidative stress [130,131,132,133]. Neuroprotectin D1, a DHA derived mediator, is critical in preventing oxidative stress damage to the brain [130, 134]. In the NAc, PPARα was reduced in only the THCω3 females, while males exhibited elevations in PPARα and PPARɣ. This may suggest that the female ability to counteract inflammation may be impaired, while males upregulation may be a compensatory response to address inflammation. Sex differences in PPARα expression and activation by agonists have been previously reported to be more effective in males than females [135,136,137]. Prenatal hypoxia and adverse neuroinflammatory responses may better explain the sex-specificity of the observed outcomes and responses to THCω3, although more direct examination of neuroinflammation is required.
Notably, while cognitive and memory deficits are recognized outcomes of FGR, no direct association has been established between anxiety and placental anomaly-related FGR [38, 88, 138,139,140,141,142,143]. However, prenatal hypoxia, a co-incident occurrence with THC-induced FGR (i.e., reduced maternal oxygen transfer) [11, 31], is linked with changes in the hypothalamic-pituitary-adrenal (HPA) axis and heightened anxiety [144, 145] as well as impairments in spatial memory and exploration [146]. A series of studies by Howell and Pillai found that prenatal hypoxia particularly impacts blood flow to the hippocampus in mice [147, 148]. This diminished blood flow adversely affects reelin expression, a extracellular matrix protein essential for neuronal migration and cortical lamination [147,148,149]. Reelin deficiency itself leads to cortical dysgenesis and heightened anxiety phenotypes [150]. In our MALDI IMS data, adrenic acid—a metabolite of arachidonic acid critical for endothelial integrity and associated with reelin—was markedly reduced in both sexes at PD21, suggesting compromised cerebrovascular function that may exacerbate hypoxia’s impact on hippocampal development and later‐life anxiety [147,148,149, 151]. PTE likely perturbs HPA axis maturation both directly and indirectly. THC itself may alter HPA functionality of the developing fetus, but HPA levels in the placenta, that is, maternal responses to stressors (i.e., from the injection [152] or from THC itself [153]) can alter levels of corticotrophin-releasing hormones (CRH) [154, 155]. Elevated CRH can thin the developing cortex by dysregulating the release of hormones necessary for proper neural development from the hypothalamus [156]. Additionally, as maternal stressors can elevate corticosterone levels, this may in turn lead to elevated oxidative stress damage in the placenta [157], which has been associated with sex-specific neurobiological deficits [158, 159]. These include damage to hippocampal dendrite length, reductions in PV+ interneurons, and decreased GABAAR expression in the hippocampus and amygdala of male offspring; demonstrated in 2D cell culture studies [159, 160]. Prenatal stress is also thought to particularly impact GABAergic control within the corticolimbic circuit in a sex-dependent manner [161]. The prevailing phenotype observed from PTE offspring included disrupted glutamatergic activity, altered GABAergic markers, and modulation of dopaminergic signaling, with the hippocampus emerging as the most vulnerable brain region, in both males and females [5,6,7, 14, 60, 71, 162]. This hippocampal vulnerability aligns with findings from prenatal hypoxia models [146, 163,164,165], suggesting that mitigating hypoxic damage could potentially reverse or modulate the observed phenotype [159]. In the developing brain, reactive oxygen species (ROS) can disrupt critical neurodevelopmental processes such as synaptogenesis, neuronal differentiation, and myelination [166, 167]. The brain is particularly vulnerable to oxidative stress due to its high lipid content and relatively low antioxidant capacity [168]. Excessive ROS leads to lipid peroxidation, protein oxidation, and DNA damage, contributing to neuronal death and neural network disruption, and alterations in lipid levels beyond those caused by insufficient placental transfer [42, 169, 170]. Persistent disturbances to fatty acid levels, then, may be a consequence of not just glucose/lipid metabolic disturbances to the offspring liver [11, 26, 42, 153], but potentially stem from disturbed inflammatory balance within the fetal brain that persists into adulthood.
Interestingly, despite overall phenotype improvements, omega-3-supplemented groups continued to exhibit low fatty acid and phospholipid levels, at least at the time points measured. One possible explanation for the persistence of DHA, and notably AA and adrenic acid, reductions may be due to the timing of withdrawal of the dietary intervention, which allowed the subsequent emergence of underlying deficits in fatty acid levels previously masked by the omega-3 enriched diet (assessed at PD21). Temporary supplementation with DHA does not necessarily lead to permanent changes in the lifelong accumulation of these fatty acids in the brain. Additionally, considering the broader physiological impacts of THC, the brain’s vulnerability to both the indirect effects (i.e., FGR) and the direct effects of THC should be noted. For example, THC may cause lasting damage to fatty acid metabolic machinery outside the brain, such as the liver; the site where these large-chain polyunsaturated fatty acids, like DHA and AA, are produced and then trafficked to brain [11, 26,27,28]. In males, we found that phospholipids containing omega-3 PUFAs, especially DHA, were largely protected from PTE. Conversely, females are capable of upregulating endogenous fatty acid levels [83, 171], which may mean that levels observed at PD120 are less contingent on earlier life levels. Nevertheless, the neurophysiological relevance of fatty acid levels diminishes in favor of phospholipid levels with age, as they continue to be incorporated into cell and synaptic membranes, myelin sheath components, or become lysophospholipids and influence numerous signaling cascades.
Disruptions in lysophospholipid pools impose a potential “double hit” on the ECS by simultaneously depleting both primary endocannabinoid precursors and lipid mediators of neuroinflammation. Lyso-PE species serve as direct substrates for N-acyltransferase and subsequent N-acyl phosphatidylethanolamine-specific phospholipase D (NAPE-PLD) to generate AEA, while lyso-PC species feed into diacylglycerol lipase (DAGL) pathways for 2-AG synthesis [172]. In CB1R⁻/⁻ mice, loss of CB1R signaling produces heightened anxiety-like behavior and altered stress responsivity—phenotypes mirrored in our PTE cohorts with depleted lyso-PE and lyso-PC levels [173]. Moreover, AA, DHA, and EPA are precursors not only for endocannabinoids but also for cyclooxygenase- and lipoxygenase-derived eicosanoids and specialized pro-resolving mediators which temper neuroinflammation [57, 174]. The marked reductions in these lysophospholipid and PUFA pools—observed in male THCct, male THCω3, and female THCω3 offspring across the PFC, NAc, and vHIPP—suggest widespread PTE-induced ECS hypofunction and impaired resolution of inflammatory signaling. Such a state may perpetuate maladaptive neuroimmune crosstalk, locking neural circuits into heightened excitability and anxiety phenotypes.
Complementing these deficits, decreases in sulfatide-bound AA threaten myelin integrity and action potential fidelity. In cerebroside sulfotransferase (CST)⁻/⁻ mice, absence of sulfogalactolipids leads to myelin sheath decompaction, slowed nerve conduction, and elevated anxiety-like behaviors [175, 176]; phenotypes that parallel our electrophysiological findings of disrupted PFC-vHIPP oscillations. Sulfatide-AA constitutes one of the most abundant PUFA species in central myelin [177], and when these lipids are deficient, oligodendrocytes may incorporate alternative phospholipids with differing biophysical properties, altering membrane capacitance and ion channel gating [92, 177]. Concurrent reductions in DHA further compound membrane instability: DHA-rich lipid rafts are essential for clustering of NMDA and GABAA receptors, and their depletion compromises receptor trafficking, synaptic vesicle fusion, and ion channel conductance [116, 133, 178, 179]. Likewise, diminished PE and PC levels weaken membrane curvature and fusion competence, providing a mechanistic substrate for the altered bursting and aberrant synchrony we observed [180]. Importantly, these lipidomic signatures, and the associated downregulation of synaptic scaffolding proteins such as synaptophysin, PSD95, and gephyrin, map onto the sex-specific behavioral and electrophysiological phenotypes, with males exhibiting more severe disruptions in lipid species pivotal for synaptic plasticity within the PFC and NAc [116, 181]. The persistence of sulfatide deficits at PD120 in both sexes, despite omega-3 supplementation, indicates that early-life PTE triggers enduring alterations to lipid metabolic pathways that may require more targeted interventions to restore myelin and membrane function.
Our study has several limitations. First, we did not explore the neuroinflammatory effects, particularly during gestation when the dietary effects of omega-3 supplementation would be most evident. This limits our understanding of the maternal and placental impacts of the diet. Although omega-3 and omega-6 fatty acids are also known to play critical roles in neuroinflammation, and other studies have shown positive neuroinflammatory effects in the placenta leading to cognitive benefits [53, 54, 119, 120, 123], we did not directly assess these effects. Additionally, while the observed alterations in various fatty acid signaling pathways serve as reliable biomarkers for neuroinflammation, it is important to directly assay specific markers for neuroinflammation during key periods of gestation, within the placenta, and at the later-life timepoints, in future analyses. This will help elucidate the mechanisms by which omega-3 supplementation mitigates the neuropsychiatric risks associated with prenatal THC exposure. Of course, the supplementation protocol was prolonged and during THC exposure; we examined this extended GD6-PD21 period utilizing previously established protocols [53, 54, 119, 120, 123], and as such, is not directly translatable. Further research should focus on the gestational and maternal effects to provide a more comprehensive understanding of the intervention’s benefits. Our lab is presently pursuing these interventions.
While we demonstrated that omega-3 fatty acids can counteract many pathological impacts of prenatal THC exposure on behavior, molecular markers, and neuronal activity within the PFC-NAc-vHIPP network, the exact pathways remain unclear. Omega-3 supplementation potentially exerts its protective effects on the PFC-NAc-vHIPP network through several converging mechanisms that extend beyond simple replacement of deficient DHA. First, DHA/EPA remodel synaptic PE/PS composition, alter lipid-raft architecture, and thereby regulate GPCR (including CB1R) clustering and G-protein signaling [182,183,184,185,186]. By competing with AA during phospholipid remodeling, omega-3 s lower the immediate substrate pool used by DAGL and NAPE-PLD, thereby constraining the excess 2-AG and AEA formation that often follows PTE-induced phospholipase activation [117, 174, 187]. Second, enzymatic conversion of DHA and EPA also yields resolvins and protectins that dampen microglial NFκB signaling, downregulate IL-1β transcription, and bias macrophage polarization toward an M2 reparative phenotype; mechanisms shown to attenuate LPS-induced neuroinflammation in CB1R knockout and wild-type mice alike [47, 153]. Third, sex differences in both lipid metabolism and steroid signaling shape the therapeutic window: estrogen upregulates FADS1/2, MFSD2A, and several pro-resolving mediators, accelerating endogenous DHA synthesis and incorporation into dendritic spines [188,189,190,191]. Consequently, females enter gestation with higher baseline membrane DHA and greater CB1R reserve capacity, coupled with sexual dimorphism in ECS receptor density, signaling efficiency, and developmental trajectories, potentially render the marginal gains from supplementation smaller than those seen in males [113, 115, 190, 192,193,194]. Yet estrogen also increases synaptic ERβ signaling, which interacts with omega-3 derived pro-resolving mediators to stabilize PSD95 scaffolds and GAD67 expression, despite the observed lingering PFC lipid alterations [195,196,197,198]. Taken together, these complementary pathways highlight why omega-3’s partially, but not completely, normalized many PTE-related endophenotypes. They also reinforce that cannabis cessation remains the most reliable strategy for preventing long-term neurobehavioral morbidity, particularly given the dose, frequency, and potency‐dependent escalation of PTE risk [29, 32]. Future work should test brief but strategically timed supplementation windows, for example, during lactation or early adolescence, to determine whether these targeted interventions can match the efficacy of prolonged perinatal dosing while improving translational feasibility.
Perinatal omega-3 supplementation may exert its protective effects in part by attenuating placental inflammation, a known driver of both FGR and downstream neurodevelopmental deficits [199]. These conditions are characterized by elevated levels of pro-inflammatory cytokines such as IL-1β, IL-8, IL-6, and TNF-α [200,201,202,203]. THC disrupts trophoblast integrity via TLR4–MyD88 signaling (TLR4/MyD88/PI3K interactions) [204], triggering NFκB-mediated transcription of IL-1β, IL-6, and TNF-α in placental macrophages and endothelial cells. DHA and EPA counteract this cascade through several complementary pathways. First, DHA is converted by ALOX15 into resolvin D1 and protectin D1, which bind GPR32 and ALX/FPR2 on trophoblasts to inhibit IKKβ phosphorylation (NFκB pathway) and reduce COX-2 expression, thereby lowering PGE₂ synthesis in LPS-challenged murine placental eells [205, 206]. In parallel, EPA competes with AA for COX-2 and 5-LOX enzymes, again diminishing production of the pro-inflammatory PGE₂, and leukotriene B₄, in both primary human macrophages and rodent models [47, 207]. Critically, these mediators also enhance placental antioxidant defenses by upregulating Nrf2 target genes (e.g., HO-1, NQO1), which may neutralize ROS generated by THC smoke inhalation (e.g. cannabis smoke) or potentially through downstream signaling [208, 209]. In vitro, DHA supplementation of RAW264.7 macrophages reduces lipopolysaccharide-induced IL-1β and TNF-α secretion via NFκB inhibition, while EPA decreases IL-6 production through STAT3 modulation [47, 207, 210]. These anti-inflammatory shifts potentially restore placental expression of FATP2 and MFSD2A (lipid transporters critical for DHA and AA transfer), thus preserving fetal PUFA supply and preventing the placental labyrinth zone’s vascular rarefaction observed in THC-exposed rats [5, 11, 42, 125, 153]. The timing and duration of supplementation are pivotal: continuous DHA/EPA provision from GD7 through PD21 overlaps with trophoblast invasion, placental angiogenesis, and peak CRH expression, ensuring DHA/EPA-derived mediator levels remain sufficient to offset any potential THC-induced inflammatory response. Comparable perinatal omega-3 regimens have rescued placental cytokine profiles and fetal growth in models of maternal high-fat diet, gestational famine, alcohol exposure, and poly(I:C)–induced immune activation, each demonstrating positive outcomes, and, when assessed, reduced placental inflammatory activity [54, 123, 211,212,213,214]. Moreover, a recent study examining the cardiometabolic outcomes following PTE (3mg/kg i.p. in Wistar rats) with this same perinatal omega-3 supplementation model exhibited a promising prevention of both FGR and cardiac outcomes in the omega-3 supplemented groups, reinforcing placental immunomodulation as a potential mechanism [44]. Translating these insights to humans will require defining optimal dosing, formulation, and intervention windows. Nonetheless, the capacity of omega-3–derived mediators to curb placental NFκB activation and restore fatty acid transport emphasizes their potential as an adjunctive strategy—recognizing, however, that cessation of cannabis use remains the most direct and feasible approach to protect fetal development.
It is also important to consider that THC displays a canonical biphasic profile in rodents, with opposite behavioral and neurobiological consequences at the low and high ends of the dose–response curve [12, 215, 216]. Our choice of 3 mg/kg, situated in the midrange of published prenatal exposure paradigms [5, 6, 11, 26, 28, 153, 216], was intended to balance detectability of disruptions with preservation of fetal viability. Lower doses ( ≤ 1 mg/kg) preferentially engage CB1R on GABAergic interneurons, leaving glutamatergic pyramidal cells relatively unaffected [217, 218]; this selective engagement has been linked to enhanced neurogenesis and synaptic plasticity in the hippocampus [216, 219, 220]. In contrast, doses ≥ 4 mg/kg saturate CB1R on both interneurons and excitatory neurons, prolonging Gi/o coupled suppression of adenylate cyclase, dampening cAMP–PKA pathways, and downregulating CREB-dependent transcription [215,216,217,218]. As placental CB1R and FAAH are also dose-responsive, higher maternal doses intensify placental disruptions, alter ECS tone, and ultimately increase indices of fetal demise or growth restriction [32, 221, 222]. Dose (and method of adminstration) is likewise central when reconciling divergent findings across PTE models, and the potential for the omega-3 supplementation to limit the impact of PTE. The extent of placental labyrinth disruptions in other models are poorly characterized, but without such data, it will be difficult to assert that similar lipidomic disruptions occur within the fetal/adult brain. Defining a therapeutic window for any cotreatment therefore requires systematic dose–response work that integrates lipidomics, endocannabinoid quantification, and pathway specific readouts in both the placenta and fetal brain.
Our exploratory study highlights multiple divergent impacts of PTE on developing male and female brains, with differential treatment responses and long-term effects following dietary omega-3 intervention. However, several translational gaps must be resolved before recommending this strategy in obstetric practice. Foremost, the human‐equivalent dose, optimal formulation (triglyceride versus ethyl ester), and developmental window for supplementation remain undefined, and extrapolation from rodents is complicated by species differences in placental fatty acid transporter expression and fetal accretion of DHA [133, 223]. Moreover, the striking sex-specificity of both the pathological and protective effects observed highlights the necessity of integrating fetal sex as a variable in future intervention studies. Equally important, the incomplete rescue achieved in our model reinforces that omega‑3 s cannot replace cannabis cessation and should, at best, be integrated into a broader harm‑reduction framework that includes counselling, potency reduction, and monitoring of maternal cannabinoid biomarkers. Carefully powered prospective cohorts and ultimately randomized controlled trials will be required to link neonatal omega‑3 indices (for example, the erythrocyte DHA + EPA to total fatty acid ratio) with long‑term cognitive, emotional, and circuit‑level outcomes in cannabis‑exposed children. Only with such evidence can public‑health recommendations move beyond abstinence messaging to include targeted nutritional modulation for those pregnancies at highest risk.
Data availability
All data is available in the main text or the Supplementary Materials and Tables.
Code availability
MATLAB code used to calculate Area’s Under the Curve (AUC) for MALDI IMS is available at https://github.com/MHSarikahya/MALDI-IMS-Script.
References
Swenson K. Cannabis for morning sickness: areas for intervention to decrease Cannabis consumption during pregnancy. J Cannabis Res. 2023;5:22.
Young-Wolff KC, Sarovar V, Tucker L-Y, Ansley D, Goler N, Conway A, et al. Trends in Cannabis polysubstance use during early pregnancy among patients in a large health care system in Northern California. JAMA Netw Open. 2022;5:e2215418.
Rava A, Trezza V. Emerging roles of endocannabinoids as key lipid mediators for a successful pregnancy. Int J Mol Sci. 2023;24:5220.
Richardson KA, Hester AK, McLemore GL. Prenatal Cannabis exposure - the “first hit” to the endocannabinoid system. Neurotoxicol Teratol. 2016;58:5–14.
Sarikahya MH, Cousineau SL, De Felice M, Szkudlarek HJ, Wong KKW, DeVuono MV, et al. Prenatal THC exposure induces long-term, sex-dependent cognitive dysfunction associated with lipidomic and neuronal pathology in the prefrontal cortex-hippocampal network. Mol Psychiatry. 2023;28:4234–50.
Sarikahya MH, Cousineau S, De Felice M, Lee K, Wong KK, DeVuono MV, et al. Prenatal THC exposure induces sex-dependent neuropsychiatric endophenotypes in offspring and long-term disruptions in fatty-acid signaling pathways directly in the mesolimbic circuitry. eNeuro. 2022;9:ENEURO.0253-22.2022.
Traccis F, Serra V, Sagheddu C, Congiu M, Saba P, Giua G, et al. Prenatal thc does not affect female mesolimbic dopaminergic system in preadolescent rats. Int J Mol Sci. 2021;22:1–18.
Roncero C, Valriberas-Herrero I, Mezzatesta-Gava M, Villegas JL, Aguilar L, Grau-López L. Cannabis use during pregnancy and its relationship with fetal developmental outcomes and psychiatric disorders. A systematic review. Reprod Health. 2020;17:1–9.
Castelli V, Lavanco G, Feo S, D’Amico C, Micale V, Kuchar M, et al. Prenatal exposure to Δ9-Tetrahydrocannabinol affects hippocampus-related cognitive functions in the adolescent rat offspring: focus on specific markers of neuroplasticity. Pharmaceutics. 2023;15:692.
de Salas-Quiroga A, García-Rincón D, Gómez-Domínguez D, Valero M, Simón-Sánchez S, Paraíso-Luna J, et al. Long-term hippocampal interneuronopathy drives sex-dimorphic spatial memory impairment induced by prenatal THC exposure. Neuropsychopharmacology. 2020;45:877–86.
Natale BV, Gustin KN, Lee K, Holloway AC, Laviolette SR, Natale DRC, et al. Δ9-tetrahydrocannabinol exposure during rat pregnancy leads to symmetrical fetal growth restriction and labyrinth-specific vascular defects in the placenta. Sci Rep. 2020;10:544.
Hurd YL, Manzoni OJ, Pletnikov MV, Lee FS, Bhattacharyya S, Melis M. Cannabis and the developing brain: insights into its long-lasting effects. J Neurosci. 2019;39:8250–8.
MacCarrone M, Guzmán M, MacKie K, Doherty P, Harkany T. Programming of neural cells by (endo)cannabinoids: from physiological rules to emerging therapies. Nat Rev Neurosci. 2014;15:786–801.
Scheyer AF, Melis M, Trezza V, Manzoni OJJ. Consequences of perinatal Cannabis exposure. Trends Neurosci. 2019;42:871–84.
Tortoriello G, Morris CV, Alpar A, Fuzik J, Shirran SL, Calvigioni D, et al. Miswiring the brain: Δ9-tetrahydrocannabinol disrupts cortical development by inducing an SCG10/stathmin-2 degradation pathway. EMBO J. 2014;33:668–85.
Mulligan MK, Hamre KM. Influence of prenatal cannabinoid exposure on early development and beyond. Adv Drug Alcohol Res. 2023;3:10981.
Basavarajappa BS, Shivakumar M, Joshi V, Subbanna S. Endocannabinoid system in neurodegenerative disorders. J Neurochem. 2017;142:624–48.
Navarrete F, García-Gutiérrez MS, Jurado-Barba R, Rubio G, Gasparyan A, Austrich-Olivares A, et al. Endocannabinoid system components as potential biomarkers in psychiatry. Front Psychiatry. 2020;11:315.
Paul SE, Hatoum AS, Fine JD, Johnson EC, Hansen I, Karcher NR, et al. Associations between prenatal Cannabis exposure and childhood outcomes results from the ABCD study author audio interview supplemental content. JAMA Psychiatry. 2021;78:64–76.
Tadesse AW, Dachew BA, Ayano G, Betts K, Alati R. Prenatal Cannabis use and the risk of attention deficit hyperactivity disorder and autism spectrum disorder in offspring: a systematic review and meta-analysis. J Psychiatr Res. 2024;171:142–51.
Corsi DJ, Donelle J, Sucha E, Hawken S, Hsu H, El-Chaâr D, et al. Maternal Cannabis use in pregnancy and child neurodevelopmental outcomes. Nat Med. 2020;26:1536–40.
Fine JD, Moreau AL, Karcher NR, Agrawal A, Rogers CE, Barch DM, et al. Association of prenatal Cannabis exposure with psychosis proneness among children in the Adolescent Brain Cognitive Development (ABCD) study. JAMA Psychiatry. 2019;76:762–4.
Lichenstein SD, Manco N, Cope LM, Egbo L, Garrison KA, Hardee J, et al. Systematic review of structural and functional neuroimaging studies of Cannabis use in adolescence and emerging adulthood: evidence from 90 studies and 9441 participants. Neuropsychopharmacology. 2022;47:1000–28.
Banerjee S, Deacon A, Suter MA, Aagaard KM. Understanding the placental biology of tobacco smoke, nicotine, and marijuana (THC) exposures during pregnancy. Clin Obstet Gynecol. 2022;65:347.
Fonseca BM, Correia-da-Silva G, Taylor AH, Lam PMW, Marczylo TH, Konje JC, et al. Characterisation of the endocannabinoid system in rat haemochorial placenta. Reprod Toxicol. 2012;34:347–56.
Oke SL, Lee K, Papp R, Laviolette SR, Hardy DB. In utero exposure to ∆9-Tetrahydrocannabinol leads to postnatal catch-up growth and dysmetabolism in the adult rat liver. Int J Mol Sci. 2021;22:7502. https://doi.org/10.3390/ijms22147502
Lee K, Hardy DB. Metabolic consequences of gestational cannabinoid exposure. Int J Mol Sci. 2021;22:9528. https://doi.org/10.3390/ijms22179528
Gillies R, Lee K, Vanin S, Laviolette SR, Holloway AC, Arany E, et al. Maternal exposure to Δ9-tetrahydrocannabinol impairs female offspring glucose homeostasis and endocrine pancreatic development in the rat. Reprod Toxicol. 2020;94:84–91.
Young-Wolff KC, Adams SR, Alexeeff SE, Zhu Y, Chojolan E, Slama NE, et al. Prenatal Cannabis use and maternal pregnancy outcomes. JAMA Intern Med. 2024;184:1083–93. https://doi.org/10.1001/jamainternmed.2024.3270
Sorkhou M, Singla DR, Castle DJ, George TP. Birth, cognitive and behavioral effects of intrauterine Cannabis exposure in infants and children: a systematic review and meta-analysis. Addiction. 2024;119:411–37.
Brik M, Sandonis M, Gil J, Hernandez-Fleury A, Parramón-Puig G, Maiz N, et al. Intrauterine Cannabis exposure and fetal and maternal blood flow: a case–control study. Acta Obstet Gynecol Scand. 2022;101:1207–14.
Cupo L, Dominguez-Cancino KA, Nazif-Munoz JI, Chakravarty MM. Prenatal Cannabis exposure in the clinic and laboratory: what do we know and where do we need to go? Drug Alcohol Depend Rep. 2024;13:100282.
Dodge P, Nadolski K, Kopkau H, Zablocki V, Forrestal K, Bailey BA. The impact of timing of in utero marijuana exposure on fetal growth. Front Pediatr. 2023;11:1103749.
Casale D, Desmond C, Richter LM. Catch-up growth in height and cognitive function: why definitions matter. Econ Hum Biol. 2020;37:100853.
Leroy JL, Frongillo EA, Dewan P, Black MM, Waterland RA. Can children catch up from the consequences of undernourishment? Evidence from child linear growth, developmental epigenetics, and brain and neurocognitive development. Adv Nutr. 2020;11:1032–41.
Embleton ND, Skeath T. Catch-up growth and metabolic and cognitive outcomes in adolescents born preterm. Nestle Nutr Inst Workshop Ser. 2015;81:61–71. https://doi.org/10.1159/000365805
Gaccioli F, Lager S. Placental nutrient transport and intrauterine growth restriction. Front Physiol. 2016;7:40.
Ayonrinde OT, Ayonrinde OA, Rooyen DV, Tait R, Dunn M, Mehta S, et al. Association between gestational Cannabis exposure and maternal, perinatal, placental, and childhood outcomes. J Dev Orig Health Dis. 2021;12:694–703.
Crume TL, Juhl AL, Brooks-Russell A, Hall KE, Wymore E, Borgelt LM. Cannabis use during the perinatal period in a state with legalized recreational and medical marijuana: the association between maternal characteristics, breastfeeding patterns, and neonatal outcomes. J Pediatrics. 2018;197:90–96.
Miller SL, Huppi PS, Mallard C. The consequences of fetal growth restriction on brain structure and neurodevelopmental outcome. J Physiol. 2016;594:807–23.
Chassen SS, Zemski-Berry K, Raymond-Whish S, Driver C, Hobbins JC, Powell TL. Altered cord blood lipid concentrations correlate with birth weight and doppler velocimetry of fetal vessels in human fetal growth restriction pregnancies. Cells. 2022;11:3110.
Murru E, Carta G, Manca C, Verce M, Everard A, Serra V, et al. Impact of prenatal THC exposure on lipid metabolism and microbiota composition in rat offspring. Heliyon. 2024;10:e35637.
Ramírez-López MT, Vázquez M, Bindila L, Lomazzo E, Hofmann C, Blanco RN, et al. Maternal caloric restriction implemented during the preconceptional and pregnancy period alters hypothalamic and hippocampal endocannabinoid levels at birth and induces overweight and increased adiposity at adulthood in male rat offspring. Front Behav Neurosci. 2016;10:208.
Lee K, Sarikahya MH, Cousineau SL, Yeung KK-C, Lucas A, Loudon K, et al. Maternal dietary DHA and EPA supplementation ameliorates adverse cardiac outcomes in THC-exposed rat offspring. Sci Rep. 2025;15:8316.
McNamara RK, Vannest JJ, Valentine CJ. Role of perinatal long-chain omega-3 fatty acids in cortical circuit maturation: mechanisms and implications for psychopathology. World J Psychiatry. 2015;5:15.
Kidd PM. Omega-3 DHA and EPA for cognition, behavior, and mood: clinical findings and structural-functional synergies with cell membrane phospholipids. Altern Med Rev. 2007;12:207–27.
Calder PC. Omega-3 fatty acids and inflammatory processes. Nutrients. 2010;2:355–74.
Basak S, Mallick R, Banerjee A, Pathak S, Duttaroy AK. Maternal supply of both arachidonic and docosahexaenoic acids is required for optimal neurodevelopment. Nutrients. 2021;13:2061.
Martinat M, Rossitto M, Di Miceli M, Layé S. Perinatal dietary polyunsaturated fatty acids in brain development, role in neurodevelopmental disorders. Nutrients. 2021;13:1185.
Hibbeln JR, Gow RV. Omega-3 fatty acid and nutrient deficits in adverse neurodevelopment and childhood behaviors. Child Adolesc Psychiatr Clin N Am. 2014;23:555–90.
McNamara RK. DHA deficiency and prefrontal cortex neuropathology in recurrent affective disorders. J Nutr. 2010;140:864–8.
Maekawa M, Watanabe A, Iwayama Y, Kimura T, Hamazaki K, Balan S, et al. Polyunsaturated fatty acid deficiency during neurodevelopment in mice models the prodromal state of schizophrenia through epigenetic changes in nuclear receptor genes. Transl Psychiatry. 2017;7:1–11.
Schiavi S, Carbone E, Melancia F, Buzzelli V, Manduca A, Campolongo P, et al. Perinatal supplementation with omega-3 fatty acids corrects the aberrant social and cognitive traits observed in a genetic model of autism based on FMR1 deletion in rats. Nutritional Neurosci. 2022;25:898–911.
Pietropaolo S, Goubran M, Joffre C, Aubert A, Lemaire-Mayo V, Crusio WE, et al. Dietary supplementation of omega-3 fatty acids rescues fragile X phenotypes in Fmr1-Ko mice. Psychoneuroendocrinology. 2014;49:119–29.
Ibrahim H, Jurcic K, Wang JS, Whitehead SN, Yeung KK. 1,6-Diphenyl-1,3,5-hexatriene (DPH) as a novel matrix for MALDI MS imaging of fatty acids, phospholipids, and sulfatides in brain tissues. Anal Chem. 2017;89:48.
De Felice M, Chen C, Rodríguez-Ruiz M, Szkudlarek HJ, Lam M, Sert S, et al. Adolescent Δ-9-tetrahydrocannabinol exposure induces differential acute and long-term neuronal and molecular disturbances in dorsal vs. ventral hippocampal subregions. Neuropsychopharmacol. 2023;48:540–51.
Arturo IF, Fabiana P Endocannabinoidome. eLS. 2018;1–10. https://doi.org/10.1002/9780470015902.a0028301
Jackson ME, Moghaddam B. Amygdala regulation of nucleus accumbens dopamine output is governed by the prefrontal cortex. J Neurosci. 2001;21:676–81.
Soltani Zangbar H, Ghadiri T, Seyedi Vafaee M, Ebrahimi Kalan A, Fallahi S, Ghorbani M, et al. Theta oscillations through hippocampal/prefrontal pathway: importance in cognitive performances. Brain Connect. 2020;10:157–69.
Frau R, Miczán V, Traccis F, Aroni S, Pongor CI, Saba P, et al. Prenatal THC exposure produces a hyperdopaminergic phenotype rescued by pregnenolone. Nat Neurosci. 2019;22:1975–85.
Renard J, Rosen LG, Loureiro M, De Oliveira C, Schmid S, Rushlow WJ, et al. Adolescent cannabinoid exposure induces a persistent sub-cortical hyper-dopaminergic state and associated molecular adaptations in the prefrontal cortex. Cereb Cortex. 2017;27:bhv335–1310. https://doi.org/10.1093/cercor/bhv335
Draycott B, Loureiro M, Ahmad T, Tan H, Zunder J, Laviolette SR. Cannabinoid transmission in the prefrontal cortex bi-phasically controls emotional memory formation via functional interactions with the ventral tegmental area. J Neurosci. 2014;34:13096–109.
Laviolette SR, Grace AA. Cannabinoids potentiate emotional learning plasticity in neurons of the medial prefrontal cortex through basolateral amygdala inputs. J Neurosci. 2006;26:6458–68.
Belujon P, Grace AA. Critical role of the prefrontal cortex in the regulation of hippocampus–accumbens information flow. J Neurosci. 2008;28:9797–805.
Floresco SB, Todd CL, Grace AA. Glutamatergic afferents from the hippocampus to the nucleus accumbens regulate activity of ventral tegmental area dopamine neurons. J Neurosci. 2001;21:4915–22.
Lodge DJ, Grace AA. Hippocampal dysregulation of dopamine system function and the pathophysiology of schizophrenia. Trends Pharmacol Sci. 2011;32:507–13.
Loureiro M, Renard J, Zunder J, Laviolette SR. Hippocampal cannabinoid transmission modulates dopamine neuron activity: impact on rewarding memory formation and social interaction. Neuropsychopharmacology. 2015;40:1436–47. https://doi.org/10.1038/npp.2014.329
Durieux LJA, Gilissen SRJ, Arckens L. Endocannabinoids and cortical plasticity: CB1R as a possible regulator of the excitation/inhibition balance in health and disease. Eur J Neurosci. 2022;55:971–88.
Han Y, Dong Q, Peng J, Li B, Sun C, Ma C. Laminar distribution of cannabinoid receptor 1 in the prefrontal cortex of nonhuman primates. Mol Neurobiol. 2023;61:1–12. https://doi.org/10.1007/s12035-023-03828-4
Perez SM, Boley A, Lodge DJ. Region specific knockdown of parvalbumin or somatostatin produces neuronal and behavioral deficits consistent with those observed in schizophrenia. Transl Psychiatry. 2019;9:1–13.
Scheyer A, Borsoi M, Manzoni OJ. Perinatal THC exposure via lactation induces lasting alterations to social behavior and prefrontal cortex function in rats at adulthood. Neuropsychopharmacology. 2020;45:1826–33. https://doi.org/10.1101/2020.02.03.931733
Scheyer AF, Borsoi M, Pelissier-Alicot A-L, Manzoni OJJ. Maternal exposure to the cannabinoid agonist WIN 55,12,2 during lactation induces lasting behavioral and synaptic alterations in the rat adult offspring of both sexes. eNeuro. 2020;7:ENEURO.0144-20.2020.
Scheyer AF, Borsoi M, Wager-Miller J, Pelissier-Alicot A-L, Murphy MN, Mackie K, et al. Cannabinoid exposure via lactation in rats disrupts perinatal programming of the gamma-aminobutyric acid trajectory and select early-life behaviors. Biol Psychiatry. 2020;87:666–77.
Bara A, Manduca A, Bernabeu A, Borsoi M, Serviado M, Lassalle O, et al. Sex-dependent effects of in utero cannabinoid exposure on cortical function. eLife. 2018;7:e36234.
Dong J, Chen W, Liu N, Chang S, Zhu W, Kang J. NRG1 knockdown rescues PV interneuron GABAergic maturation deficits and schizophrenia behaviors in fetal growth restriction mice. Cell Death Discov. 2022;8:1–11.
Cao D, Kevala K, Kim J, Moon HS, Jun SB, Lovinger D, et al. Docosahexaenoic acid promotes hippocampal neuronal development and synaptic function. J Neurochem. 2009;111:510–21.
Anastasiades PG, Marlin JJ, Carter AG. Cell-type specificity of callosally evoked excitation and feedforward inhibition in the prefrontal cortex. Cell Rep. 2018;22:679–92.
Liu X, Dimidschstein J, Fishell G, Carter AG. Hippocampal inputs engage CCK+ interneurons to mediate endocannabinoid-modulated feed-forward inhibition in the prefrontal cortex. eLife. 2020;9:e55267.
Nguyen R, Venkatesan S, Binko M, Bang JY, Cajanding JD, Briggs C, et al. Cholecystokinin-expressing interneurons of the medial prefrontal cortex mediate working memory retrieval. J Neurosci. 2020;40:2314–31.
Vargish GA, Pelkey KA, Yuan X, Chittajallu R, Collins D, Fang C, et al. Persistent inhibitory circuit defects and disrupted social behaviour following in utero exogenous cannabinoid exposure. Mol Psychiatry. 2017;22:56–67.
Moussawi K, Kalivas PW. Group II metabotropic glutamate receptors (mGlu2/3) in drug addiction. Eur J Pharmacol. 2010;639:115–22.
Calon F, Lim GP, Morihara T, Yang F, Ubeda O, Salem N Jr, et al. Dietary n-3 polyunsaturated fatty acid depletion activates caspases and decreases NMDA receptors in the brain of a transgenic mouse model of Alzheimer’s disease. Eur J Neurosci. 2005;22:617–26.
Duttaroy AK, Basak S. Maternal fatty acid metabolism in pregnancy and its consequences in the feto-placental development. Front Physiol. 2022;12:787848.
Padilla-Coreano N, Bolkan SS, Pierce GM, Blackman DR, Hardin WD, Garcia-Garcia AL, et al. Direct ventral hippocampal-prefrontal input is required for anxiety-related neural activity and behavior. Neuron. 2016;89:857–66.
Adhikari A, Topiwala MA, Gordon JA. Synchronized activity between the ventral hippocampus and the medial prefrontal cortex during anxiety. Neuron. 2010;65:257–69.
Phillips ML, Robinson HA, Pozzo-Miller L. Ventral hippocampal projections to the medial prefrontal cortex regulate social memory. eLife. 2019;8:e44182.
Swerdlow NR, Shoemaker JM, Noh HR, Ma L, Gaudet I, Munson M, et al. The ventral hippocampal regulation of prepulse inhibition and its disruption by apomorphine in rats are not mediated via the fornix. Neuroscience. 2004;123:675–85.
Gagnon R. Placental insufficiency and its consequences. Eur J Obstet Gynecol Reprod Biol. 2003;110:S99–S107.
Sibley CP. Understanding placental nutrient transfer - why bother? New biomarkers of fetal growth. J Physiol. 2009;587:3431–40.
Coti Bertrand P, O’Kusky JR, Innis SM. Maternal dietary (n-3) fatty acid deficiency alters neurogenesis in the embryonic rat brain. J Nutr. 2006;136:1570–5.
Lozada LE, Desai A, Kevala K, Lee JW, Kim HY. Perinatal brain docosahexaenoic acid concentration has a lasting impact on cognition in mice. J Nutr. 2017;147:1624–30.
Leyrolle Q, Decoeur F, Dejean C, Brière G, Leon S, Bakoyiannis I, et al. N-3 PUFA deficiency disrupts oligodendrocyte maturation and myelin integrity during brain development. GLIA. 2022;70:50–70.
Thalman C, Horta G, Qiao L, Endle H, Tegeder I, Cheng H, et al. Synaptic phospholipids as a new target for cortical hyperexcitability and E/I balance in psychiatric disorders. Mol Psychiatry. 2018;23:1699–710.
Royo J, Villain N, Champeval D, Del Gallo F, Bertini G, Aujard F, et al. Effects of n-3 polyunsaturated fatty acid supplementation on cognitive functions, electrocortical activity and neurogenesis in a non-human primate, the grey mouse lemur (Microcebus murinus). Behav Brain Res. 2018;347:394–407.
Bazwinsky-Wutschke I, Zipprich A, Dehghani F. Endocannabinoid system in hepatic glucose metabolism, fatty liver disease, and cirrhosis. Int J Mol Sci. 2019;20:2516.
Chanda D, Kim Y-H, Kim D-K, Lee M-W, Lee S-Y, Park T-S, et al. Activation of cannabinoid receptor type 1 (Cb1r) disrupts hepatic insulin receptor signaling via cyclic AMP-response element-binding protein H (Crebh)-mediated induction of Lipin1 gene *. J Biol Chem. 2012;287:38041–9.
Elmes MW, Prentis LE, Mcgoldrick LL, Giuliano CJ, Sweeney JM, Joseph OM, et al. FABP1 controls hepatic transport and biotransformation of Δ 9-tHC. Sci Rep. 2019;9:7588. https://doi.org/10.1038/s41598-019-44108-3
Covelo A, Eraso-Pichot A, Fernández-Moncada I, Serrat R, Marsicano G. CB1R-dependent regulation of astrocyte physiology and astrocyte-neuron interactions. Neuropharmacology. 2021;195:108678.
Jouroukhin Y, Zhu X, Shevelkin AV, Hasegawa Y, Abazyan B, Saito A, et al. Adolescent Δ9-THC exposure and astrocyte-specific genetic vulnerability converge on NF-κB-COX-2 signaling to impair memory in adulthood. Biol Psychiatry. 2018;85:891–903.
Zamberletti E, Gabaglio M, Grilli M, Prini P, Catanese A, Pittaluga A, et al. Long-term hippocampal glutamate synapse and astrocyte dysfunctions underlying the altered phenotype induced by adolescent THC treatment in male rats. Pharmacol Res. 2016;111:459–70.
Covey DP, Yocky AG. Endocannabinoid modulation of nucleus accumbens microcircuitry and terminal dopamine release. Front Synaptic Neurosci. 2021;13:734975.
Marsicano G, Lafenêtre P Roles of the endocannabinoid system in learning and memory. In: Kendall D, Alexander S, editors. Behavioral neurobiology of the endocannabinoid system, Berlin, Heidelberg: Springer; 2009. pp. 201-30.
Hare BD, Duman RS. Prefrontal cortex circuits in depression and anxiety: contribution of discrete neuronal populations and target regions. Mol Psychiatry. 2020;25:2742–58.
Muir J, Tse YC, Iyer ES, Biris J, Cvetkovska V, Lopez J, et al. Ventral hippocampal afferents to nucleus accumbens encode both latent vulnerability and stress-induced susceptibility. Biol Psychiatry. 2020;88:843–54.
Bannerman DM, Rawlins JNP, McHugh SB, Deacon RMJ, Yee BK, Bast T, et al. Regional dissociations within the hippocampus—memory and anxiety. Neurosci Biobehav Rev. 2004;28:273–83.
Hudson R, Norris C, Szkudlarek HJ, Khan D, Schmid S, Rushlow WJ, et al. Anxiety and cognitive-related effects of Δ 9-tetrahydrocannabinol (THC) are differentially mediated through distinct GSK-3 vs. Akt-mTOR pathways in the nucleus accumbens of male rats. Psychopharmacology. 2022;239:509–24.
Ducrocq F, Walle R, Contini A, Oummadi A, Caraballo B, van der Veldt S, et al. Causal link between n-3 polyunsaturated fatty acid deficiency and motivation deficits. Cell Metab. 2020;31:755–72.e7.
Jobin M-L, De Smedt-Peyrusse V, Ducrocq F, Baccouch R, Oummadi A, Pedersen MH, et al. Impact of membrane lipid polyunsaturation on dopamine D2 receptor ligand binding and signaling. Mol Psychiatry. 2023;28:1960–9.
Larrieu T, Madore C, Joffre C, Layé S. Nutritional n-3 polyunsaturated fatty acids deficiency alters cannabinoid receptor signaling pathway in the brain and associated anxiety-like behavior in mice. J Physiol Biochem. 2012;68:671–81.
Donner NC, Lowry CA. Sex differences in anxiety and emotional behavior. Pflug Arch. 2013;465:601–26.
Kokras N, Dalla C. Sex differences in animal models of psychiatric disorders. Br J Pharmacol. 2014;171:4595–619.
Hill MN, Karacabeyli ES, Gorzalka BB. Estrogen recruits the endocannabinoid system to modulate emotionality. Psychoneuroendocrinology. 2007;32:350–7.
Farquhar CE, Breivogel CS, Gamage TF, Gay EA, Thomas BF, Craft RM, et al. Sex, THC, and hormones: effects on density and sensitivity of CB1 cannabinoid receptors in rats. Drug Alcohol Depend. 2019;194:20–27.
Viveros M, Llorente R, Suarez J, Llorente-Berzal A, López-Gallardo M, Rodriguez de Fonseca F. The endocannabinoid system in critical neurodevelopmental periods: sex differences and neuropsychiatric implications. J Psychopharmacol. 2012;26:164–76.
Rubino T, Parolaro D. Sexually dimorphic effects of cannabinoid compounds on emotion and cognition. Front Behav Neurosci. 2011;5:64.
Dyall SC. Long-chain omega-3 fatty acids and the brain: a review of the independent and shared effects of EPA, DPA and DHA. Front Aging Neurosci. 2015;7:52.
Lafourcade M, Larrieu T, Mato S, Duffaud A, Sepers M, Matias I, et al. Nutritional omega-3 deficiency abolishes endocannabinoid-mediated neuronal functions. Nat Neurosci. 2011;14:345–50.
Koshy B, Srinivasan M, Gopalakrishnan S, Mohan VR, Scharf R, Murray-Kolb L, et al. Are early childhood stunting and catch-up growth associated with school age cognition?—Evidence from an Indian birth cohort. PLOS ONE. 2022;17:e0264010.
Nolan SO, Hodges SL, Binder MS, Smith GD, Okoh JT, Jefferson TS, et al. Dietary rescue of adult behavioral deficits in the Fmr1 knockout mouse. PLoS One. 2022;17:e0262916.
Turpin V, Schaffhauser M, Thabault M, Aubert A, Joffre C, Balado E, et al. Mice prenatally exposed to valproic acid do not show autism-related disorders when fed with polyunsaturated fatty acid-enriched diets. Sci Rep. 2023;13:11235.
Broere-Brown ZA, Adank MC, Benschop L, Tielemans M, Muka T, Gonçalves R, et al. Fetal sex and maternal pregnancy outcomes: a systematic review and meta-analysis. Biol Sex Differ. 2020;11:26.
Castelli M, Fadda P, Casu A, Spano M, Casti A, Fratta W, et al. Male and female rats differ in brain cannabinoid CB1 receptor density and function and in behavioural traits predisposing to drug addiction: effect of ovarian hormones. Curr Pharm Des. 2014;20:2100–13.
Martin BS, Corbin JG, Huntsman MM. Deficient tonic GABAergic conductance and synaptic balance in the fragile X syndrome amygdala. J Neurophysiol. 2014;112:890–902.
Torres-Cuevas I, Corral-Debrinski M, Gressens P. Brain oxidative damage in murine models of neonatal hypoxia/ischemia and reoxygenation. Free Radic Biol Med. 2019;142:3–15.
Lee K, Laviolette SR, Hardy DB. Exposure to Δ9-tetrahydrocannabinol during rat pregnancy leads to impaired cardiac dysfunction in postnatal life. Pediatric Res. 2021;90:532–9. https://doi.org/10.1038/s41390-021-01511-9
Jang EA, Longo LD, Goyal R. Antenatal maternal hypoxia: criterion for fetal growth restriction in rodents. Front Physiol. 2015;6:176.
Giacobbe J, Benoiton B, Zunszain P, Pariante CM, Borsini A. The anti-inflammatory role of omega-3 polyunsaturated fatty acids metabolites in pre-clinical models of psychiatric, neurodegenerative, and neurological disorders. Front Psychiatry. 2020;11:122.
Kiecolt-Glaser JK, Belury MA, Andridge R, Malarkey WB, Hwang BS, Glaser R. Omega-3 supplementation lowers inflammation in healthy middle-aged and older adults: a randomized controlled trial. Brain Behav Immun. 2012;26:988–95.
Wärnberg J, Gomez-Martinez S, Romeo J, Díaz L-E, Marcos A. Nutrition, inflammation, and cognitive function. Ann N Y Acad Sci. 2009;1153:164–75.
Bazan NG. Neuroprotectin D1 (NPD1): A DHA-derived mediator that protects brain and retina against cell injury-induced oxidative stress. Brain Pathol. 2005;15:159–66.
Sun GY, Simonyi A, Fritsche KL, Chuang DY, Hannink M, Gu Z, et al. Docosahexaenoic acid (DHA): An essential nutrient and a nutraceutical for brain health and diseases. Prostaglandins Leukot Essent Fatty Acids. 2018;136:3–13.
Walczak R, Tontonoz P. PPARadigms and PPARadoxes: expanding roles for PPARγ in the control of lipid metabolism. J Lipid Res. 2002;43:177–86.
Lauritzen L, Brambilla P, Mazzocchi A, Harsløf LBS, Ciappolino V, Agostoni C. DHA effects in brain development and function. Nutrients. 2016;8:6.
Musto AE, Walker CP, Petasis NA, Bazan NG. Hippocampal neuro-networks and dendritic spine perturbations in epileptogenesis are attenuated by neuroprotectin D1. PLOS ONE. 2015;10:e0116543.
Dotson AL, Wang J, Chen Y, Manning D, Nguyen H, Saugstad JA, et al. Sex differences and the role of PPAR alpha in experimental stroke. Metab Brain Dis. 2016;31:539–47.
Pierrot N, Ris L, Stancu I-C, Doshina A, Ribeiro F, Tyteca D, et al. Sex-regulated gene dosage effect of PPARα on synaptic plasticity. Life Sci Alliance. 2019;2:e201800262.
Park H-J, Choi J-M. Sex-specific regulation of immune responses by PPARs. Exp Mol Med. 2017;49:e364–e364.
Beer HN, Lacey TA, Gibbs RL, Most MS, Hicks ZM, Grijalva PC, et al. Placental insufficiency improves when intrauterine growth-restricted fetal sheep are administered daily ω-3 polyunsaturated fatty acid infusions. Transl Anim Sci. 2021;5:S6–S10.
Cetin I, Giovannini N, Alvino G, Agostoni C, Riva E, Giovannini M, et al. Intrauterine growth restriction is associated with changes in polyunsaturated fatty acid fetal-maternal relationships. Pediatric Res. 2002;52:750–5.
Chen W, Liu N, Shen S, Zhu W, Qiao J, Chang S, et al. Fetal growth restriction impairs hippocampal neurogenesis and cognition via Tet1 in offspring. Cell Rep. 2021;37:109912.
Dall’Asta A, Brunelli V, Prefumo F, Frusca T, Lees CC. Early onset fetal growth restriction. Matern Health Neonatol Perinatol. 2017;3:2.
Vasiliadis H-M, Buka SL, Martin LT, Gilman SE. Fetal growth and the lifetime risk of generalized anxiety disorder. Depress Anxiety. 2010;27:1066–72.
Pettersson E, Larsson H, D’Onofrio B, Almqvist C, Lichtenstein P. Association of fetal growth with general and specific mental health conditions. JAMA Psychiatry. 2019;76:536–43.
Fan J-M, Chen X-Q, Jin H, Du J-Z. Gestational hypoxia alone or combined with restraint sensitizes the hypothalamic–pituitary–adrenal axis and induces anxiety-like behavior in adult male rat offspring. Neuroscience. 2009;159:1363–73.
Wang X, Meng F-S, Liu Z-Y, Fan J-M, Hao K, Chen X-Q, et al. Gestational hypoxia induces sex-differential methylation of Crhr1 linked to anxiety-like behavior. Mol Neurobiol. 2013;48:544–55.
Sab IM, Ferraz MMD, Amaral TAS, Resende AC, Ferraz MR, Matsuura C, et al. Prenatal hypoxia, habituation memory and oxidative stress. Pharmacol Biochem Behav. 2013;107:24–28.
Howell KR, Pillai A. Effects of prenatal hypoxia on schizophrenia-related phenotypes in heterozygous reeler mice: a gene×environment interaction study. Eur Neuropsychopharmacol. 2014;24:1324–36.
Howell KR, Pillai A. Long-term effects of prenatal hypoxia on schizophrenia-like phenotype in heterozygous reeler mice. Mol Neurobiol. 2016;53:3267–76.
Wijendran V, Lawrence P, Diau G-Y, Boehm G, Nathanielsz PW, Brenna JT. Significant utilization of dietary arachidonic acid is for brain adrenic acid in baboon neonates. J Lipid Res. 2002;43:762–7.
Lossi L, Castagna C, Granato A, Merighi A. The reeler mouse: a translational model of human neurological conditions, or simply a good tool for better understanding neurodevelopment? J Clin Med. 2019;8:2088.
Wang Z, Gao H, Ma X, Zhu D, Zhao L, Xiao W. Adrenic acid: a promising biomarker and therapeutic target (Review). Int J Mol Med. 2024;55:20.
Preez AD, Law T, Onorato D, Lim YM, Eiben P, Musaelyan K, et al. The type of stress matters: repeated injection and permanent social isolation stress in male mice have a differential effect on anxiety- and depressive-like behaviours, and associated biological alterations. Transl Psychiatry. 2020;10:325.
Black T, Baccetto SL, Barnard IL, Finch E, McElroy DL, Austin-Scott FVL, et al. Characterization of cannabinoid plasma concentration, maternal health, and cytokine levels in a rat model of prenatal Cannabis smoke exposure. Sci Rep. 2023;13:21070.
Weinstock M. The potential influence of maternal stress hormones on development and mental health of the offspring. Brain Behav Immun. 2005;19:296–308.
Kovács KJ. CRH: the link between hormonal-, metabolic- and behavioral responses to stress. J Chem Neuroanat. 2013;54:25–33.
Sandman CA, Curran MM, Davis EP, Glynn LM, Head K, Baram TZ. Cortical thinning and neuropsychiatric outcomes in children exposed to prenatal adversity: a role for placental CRH? AJP. 2018;175:471–9.
Sze Y, Brunton PJ. How is prenatal stress transmitted from the mother to the fetus? J Exp Biol. 2024;227:jeb246073.
Dowell J, Elser BA, Schroeder RE, Stevens HE. Cellular stress mechanisms of prenatal maternal stress: heat shock factors and oxidative stress. Neurosci Lett. 2019;709:134368.
Scott H, Phillips TJ, Sze Y, Alfieri A, Rogers MF, Volpato V, et al. Maternal antioxidant treatment prevents the adverse effects of prenatal stress on the offspring’s brain and behavior. Neurobiol Stress. 2020;13:100281.
Pham C, Thomson S, Chin S-T, Vuillermin P, O’Hely M, Burgner D, et al. Maternal oxidative stress during pregnancy associated with emotional and behavioural problems in early childhood: implications for foetal programming. Mol Psychiatry. 2023;28:3760–8.
Woodward EM, Coutellier L. Age- and sex-specific effects of stress on parvalbumin interneurons in preclinical models: relevance to sex differences in clinical neuropsychiatric and neurodevelopmental disorders. Neurosci Biobehav Rev. 2021;131:1228–42.
Beggiato S, Borelli AC, Tomasini MC, Morgano L, Antonelli T, Tanganelli S, et al. Long-lasting alterations of hippocampal GABAergic neurotransmission in adult rats following perinatal Δ9-THC exposure. Neurobiol Learn Mem. 2017;139:135–43.
Buonocore G, Perrone S, Muraca MC. Free radicals and brain damage in newborns with hypoxic-ischemic lesion. Ann Ist Super Sanita. 2001;37:527–35.
Golan MH, Mane R, Molczadzki G, Zuckerman M, Kaplan-Louson V, Huleihel M, et al. Impaired migration signaling in the hippocampus following prenatal hypoxia. Neuropharmacology. 2009;57:511–22.
Silvestro S, Calcaterra V, Pelizzo G, Bramanti P, Mazzon E. Prenatal hypoxia and placental oxidative stress: insights from animal models to clinical evidences. Antioxidants. 2020;9:414.
Guedes JR, Ferreira PA, Costa JM, Cardoso AL, Peça J. Microglia-dependent remodeling of neuronal circuits. J Neurochem. 2022;163:74–93.
Oswald MCW, Garnham N, Sweeney ST, Landgraf M. Regulation of neuronal development and function by ROS. FEBS Lett. 2018;592:679–91.
Salim S. Oxidative stress and the central nervous system. J Pharmacol Exp Ther. 2017;360:201–5.
Guo C, Sun L, Chen X, Zhang D. Oxidative stress, mitochondrial damage and neurodegenerative diseases. Neural Regen Res. 2013;8:2003–14.
Massaad CA, Klann E. Reactive oxygen species in the regulation of synaptic plasticity and memory. Antioxid Redox Signal. 2011;14:2013–54.
Wadhwani N, Patil V, Pisal H, Joshi A, Mehendale S, Gupte S, et al. Altered maternal proportions of long chain polyunsaturated fatty acids and their transport leads to disturbed fetal stores in preeclampsia. Prostaglandins Leukot Essent Fat Acids. 2014;91:21–30.
Ueda N, Tsuboi K, Uyama T. Metabolism of endocannabinoids and related N-acylethanolamines: canonical and alternative pathways. FEBS J. 2013;280:1874–94.
Haller J, Bakos N, Szirmay M, Ledent C, Freund TF. The effects of genetic and pharmacological blockade of the CB1 cannabinoid receptor on anxiety. Eur J Neurosci. 2002;16:1395–8.
Bosch-Bouju C, Layé S, Bosch-Bouju C, Layé S Dietary omega-6/omega-3 and endocannabinoids: implications for brain health and diseases. Cannabinoids in Health and Disease, IntechOpen; 2016. https://www.intechopen.com/chapters/50397
Palavicini JP, Wang C, Chen L, Ahmar S, Higuera JD, Dupree JL, et al. Novel molecular insights into the critical role of sulfatide in myelin maintenance/function. J Neurochem. 2016;139:40–54.
Marcus J, Honigbaum S, Shroff S, Honke K, Rosenbluth J, Dupree JL. Sulfatide is essential for the maintenance of CNS myelin and axon structure. Glia. 2006;53:372–81.
Barnes-Vélez JA, Aksoy Yasar FB, Hu J. Myelin lipid metabolism and its role in myelination and myelin maintenance. Innov. 2023;4:100360.
Wassall SR, Stillwell W. Polyunsaturated fatty acid-cholesterol interactions: domain formation in membranes. Biochim Biophys Acta. 2009;1788:24–32.
Wassall SR, Leng X, Canner SW, Pennington ER, Kinnun JJ, Cavazos AT, et al. Docosahexaenoic acid regulates the formation of lipid rafts: a unified view from experiment and simulation. Biochim Biophys Acta Biomembr. 2018;1860:1985–93.
Lauwers E, Goodchild R, Verstreken P. Membrane lipids in presynaptic function and disease. Neuron. 2016;90:11–25.
Bazinet RP, Layé S. Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat Rev Neurosci. 2014;15:771–85.
Serrano M, Saumell-Esnaola M, Ocerin G, García del Caño G, Puente N, Sallés J, et al. Impact of omega-3 on endocannabinoid system expression and function, enhancing cognition and behavior in male mice. Nutrients. 2024;16:4344.
Bari M, Battista N, Fezza F, Finazzi-Agrò A, Maccarrone M. Lipid rafts control signaling of type-1 cannabinoid receptors in neuronal cells. Implications for anandamide-induced apoptosis. J Biol Chem. 2005;280:12212–20.
Shaikh S, Rockett B, Salameh M, Carraway JK. Docosahexaenoic acid modifies the clustering and size of lipid rafts and the lateral organization and surface expression of MHC class I of EL4 cells. J Nutr. 2009;139:1632–9.
Deák F, Anderson RE, Fessler JL, Sherry DM. Novel cellular functions of very long chain-fatty acids: insight from ELOVL4 mutations. Front Cell Neurosci. 2019;13:428.
Kalkman HO, Hersberger M, Walitza S, Berger GE. Disentangling the molecular mechanisms of the antidepressant activity of omega-3 polyunsaturated fatty acid: a comprehensive review of the literature. Int J Mol Sci. 2021;22:4393.
Dyall SC. Interplay between n-3 and n-6 long-chain polyunsaturated fatty acids and the endocannabinoid system in brain protection and repair. Lipids. 2017;52:885–900.
Kim D, Choi JE, Park Y. Low-linoleic acid diet and oestrogen enhance the conversion of α-linolenic acid into DHA through modification of conversion enzymes and transcription factors. Br J Nutr. 2019;121:137–45.
Brann DW, Dhandapani K, Wakade C, Mahesh VB, Khan MM. Neurotrophic and neuroprotective actions of estrogen: basic mechanisms and clinical implications. Steroids. 2007;72:381–405.
Decsi T, Kennedy K. Sex-specific differences in essential fatty acid metabolism. Am J Clin Nutr. 2011;94:1914S–1919S.
Extier A, Langelier B, Perruchot M-H, Guesnet P, Van Veldhoven PP, Lavialle M, et al. Gender affects liver desaturase expression in a rat model of n−3 fatty acid repletion. J Nutr Biochem. 2010;21:180–7.
Rotarescu RD, Mathur M, Bejoy AM, Anderson GH, Metherel AH. Serum measures of docosahexaenoic acid (DHA) synthesis underestimates whole body DHA synthesis in male and female mice. J Nutr Biochem. 2024;131:109689.
Riebe CJN, Hill MN, Lee TTY, Hillard CJ, Gorzalka BB. Estrogenic regulation of limbic cannabinoid receptor binding. Psychoneuroendocrinology. 2010;35:1265–9.
Craft RM, Marusich JA, Wiley JL. Sex differences in cannabinoid pharmacology: a reflection of differences in the endocannabinoid system? Life Sci. 2013;92:476–81.
Serhan CN, Chiang N, Dalli J. The resolution code of acute inflammation: novel pro-resolving lipid mediators in resolution. Semin Immunol. 2015;27:200–15.
Akama KT, McEwen BS. Estrogen stimulates postsynaptic density-95 rapid protein synthesis via the Akt/Protein kinase B pathway. J Neurosci. 2003;23:2333–9.
Joh H-D, Searles RV, Selmanoff M, Alkayed NJ, Koehler RC, Hurn PD, et al. Estradiol alters only GAD67 mRNA levels in ischemic rat brain with no consequent effects on GABA. J Cereb Blood Flow Metab. 2006;26:518–26.
Slayo M, Rummel C, Singhaarachchi PH, Feldotto M, Spencer SJ. The role of n-3-derived specialised pro-resolving mediators (SPMs) in microglial mitochondrial respiration and inflammation resolution in Alzheimer’s disease. Mol Neurodegener. 2025;20:35.
Joo EH, Kim YR, Kim N, Jung JE, Han SH, Cho HY. Effect of endogenic and exogenic oxidative stress triggers on adverse pregnancy outcomes: preeclampsia, fetal growth restriction, gestational diabetes mellitus and preterm birth. Int J Mol Sci. 2021;22:10122.
Kemp MW, Saito M, Newnham JP, Nitsos I, Okamura K, Kallapur SG. Preterm birth, infection, and inflammation advances from the study of animal models. Reprod Sci. 2010;17:619–28.
Amarilyo G, Oren A, Mimouni FB, Ochshorn Y, Deutsch V, Mandel D. Increased cord serum inflammatory markers in small-for-gestational-age neonates. J Perinatol. 2011;31:30–32.
Kuzmicki M, Telejko B, Szamatowicz J, Zonenberg A, Nikolajuk A, Kretowski A, et al. High resistin and interleukin-6 levels are associated with gestational diabetes mellitus. Gynecol Endocrinol. 2009;25:258–63.
Jones ML, Mark PJ, Waddell BJ. Reproduction review maternal dietary omega-3 fatty acids and placental function. Reproduction. 2014;147:R143–R152. https://doi.org/10.1530/REP-13-0376
Fitzpatrick J-M, Minogue E, Curham L, Tyrrell H, Gavigan P, Hind W, et al. MyD88-dependent and -independent signalling via TLR3 and TLR4 are differentially modulated by Δ9-tetrahydrocannabinol and cannabidiol in human macrophages. J Neuroimmunol. 2020;343:577217.
Honda KL, Lamon-Fava S, Matthan NR, Wu D, Lichtenstein AH. Docosahexaenoic acid differentially affects TNFα and IL-6 expression in LPS-stimulated RAW 264.7 murine macrophages. Prostaglandins Leukot Essent Fatty Acids. 2015;97:27–34.
Honda KL, Lamon-Fava S, Matthan NR, Wu D, Lichtenstein AH. EPA and DHA exposure alters the Inflammatory response but not the surface expression of toll-like receptor 4 in macrophages. Lipids. 2015;50:121–9.
Calder PC. Omega‐3 polyunsaturated fatty acids and inflammatory processes: nutrition or pharmacology? Br J Clin Pharmacol. 2013;75:645–62.
Saha S, Buttari B, Panieri E, Profumo E, Saso L. An overview of Nrf2 signaling pathway and its role in inflammation. Molecules. 2020;25:5474.
Drolet J, Buchner-Duby B, Stykel MG, Coackley C, Kang JX, Ma DWL, et al. Docosahexanoic acid signals through the Nrf2–Nqo1 pathway to maintain redox balance and promote neurite outgrowth. Mol Biol Cell. 2021;32:511–20.
Williams-Bey Y, Boularan C, Vural A, Huang N-N, Hwang I-Y, Shan-Shi C, et al. Omega-3 free fatty acids suppress macrophage inflammasome activation by inhibiting NF-κB activation and enhancing autophagy. PLOS ONE. 2014;9:e97957.
Best KP, Sullivan TR, Gunaratne AW, Gould JF, Gibson RA, Collins CT, et al. Effect of Docosahexaenoic Acid (DHA) supplementation of preterm infants on growth, body composition, and blood pressure at 7-years corrected age: follow-up of a Randomized Controlled Trial. Nutrients. 2023;15:335.
Bozzatello P, Brignolo E, De Grandi E, Bellino S. Supplementation with omega-3 fatty acids in psychiatric disorders: a review of literature data. J Clin Med. 2016;5:67.
Carrié I, Clément M, Javel D, de, Francès H, Bourre J-M. Phospholipid supplementation reverses behavioral and biochemical alterations induced by n–3 polyunsaturated fatty acid deficiency in mice. J Lipid Res. 2000;41:473–80.
Dana K, Finik J, Koenig S, Motter J, Zhang W, Linaris M, et al. Prenatal exposure to famine and risk for development of psychopathology in adulthood: a meta-analysis. J Psychiatry Psychiatr Disord. 2019;3:227–40.
Galve-Roperh I, de Salas-Quiroga A, Simón Sánchez S, Guzmán M Chapter 13 - prenatal THC exposure interferes with the neurodevelopmental role of endocannabinoid signaling. In: Melis M, Manzoni OJJ, editors. Cannabis and the developing brain, Academic Press; 2022. pp. 259–82. https://www.sciencedirect.com/science/article/abs/pii/B9780128234907000137?via%3Dihub
Dinieri JA, Hurd YL. Rat models of prenatal and adolescent Cannabis exposure. Methods Mol Biol. 2012;829:231–42.
Chowdhury KU, Holden ME, Wiley MT, Suppiramaniam V, Reed MN. Effects of Cannabis on glutamatergic neurotransmission: the interplay between cannabinoids and glutamate. Cells. 2024;13:1130.
Stella N. THC and CBD: Similarities and differences between siblings. Neuron. 2023;111:302–27.
Suliman NA, Taib CNM, Moklas MAM, Basir R. Delta-9-Tetrahydrocannabinol (∆9-THC) induce neurogenesis and improve cognitive performances of male sprague dawley rats. Neurotox Res. 2018;33:402–11.
Pistis M, Ferraro L, Pira L, Flore G, Tanganelli S, Gessa GL, et al. Δ9-Tetrahydrocannabinol decreases extracellular GABA and increases extracellular glutamate and dopamine levels in the rat prefrontal cortex: an in vivo microdialysis study. Brain Res. 2002;948:155–8.
Nashed MG, Hardy DB, Laviolette SR. Prenatal cannabinoid exposure: emerging evidence of physiological and neuropsychiatric abnormalities. Front Psychiatry. 2021;11:624275.
Grant KS, Petroff R, Isoherranen N, Stella N, Burbacher TM. Cannabis use during pregnancy: pharmacokinetics and effects on child development. Pharmacol Ther. 2018;182:133–51.
Innis SM. Dietary (n-3) fatty acids and brain development. J Nutr. 2007;137:855–9.
Acknowledgements
The authors thank Algarithm Inc. for the generous donation of Omega-3 algal oil for the preparation of the Omega-3 enriched diets.
Funding
This work was supported by the Canadian Institutes of Health Research (CIHR) with grant #PJT-159586 to S.R.L, Canadian Institutes of Health Research Project Grant #R4228A28 to D.B.H, and the Natural Sciences and Engineering Research Council of Canada (NSERC) for MHS. Operational funding of the MALDI-MS Facility necessary for the acquisition of MS and MS/MS spectra, was provided by the Schulich School of Medicine and Dentistry and the Departments of Chemistry and Biochemistry, University of Western Ontario.
Author information
Authors and Affiliations
Contributions
Conceptualization: MHS, SRL. Methodology: MHS, SRL, SLC, MDF, HJS, KL SS, KK-CY, DBH, WJR. Investigation: MHS, SLC, MDF, HJS, KL, AD, AA, AD, KW, MVD, MB, SV, MY, KZ, HM. Visualization: MHS, SLC. Supervision: SRL, DBH, WJR, KKCY, SS. Writing—original draft: MHS, SRL. Writing—review & editing: MHS, SRL, DBH, SLC, MDF, HJS, MVD, KL.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sarikahya, M.H., Cousineau, S.L., De Felice, M. et al. Perinatal omega-3 sex-selectively mitigates neuropsychiatric impacts of prenatal THC in the cortico-striatal-hippocampal circuit. Mol Psychiatry (2025). https://doi.org/10.1038/s41380-025-03113-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41380-025-03113-x