Abstract
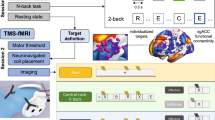
Small single-site studies found that transcranial magnetic stimulation (TMS) targets with better antidepressant response were more negatively functionally connected to the subgenual cingulate cortex (SGC). These led to “anti-subgenual” TMS targeting in recent clinical trials. We conducted a larger prospective multi-site observational study to test the robustness of this observation in more diverse clinical populations. Sixty-six treatment-seeking individuals with major depressive disorder (MDD) received 3–8 weeks of daily rTMS to the left dorsolateral prefrontal cortex using scalp-based targeting as part of standard clinical care. Stimulation sites were recorded with MRI neuronavigation on multiple days. Our primary outcome was the correlation between change in Beck Depression Inventory (BDI-II) score and connectivity of each individual’s TMS site to the SGC, computed using resting-state functional connectivity data from 1000 healthy individuals. Secondary (post hoc) analyses incorporated additional clinical covariates. No relationship was found between antidepressant response and normative connectivity of TMS site to SGC (r = 0.1, p = 0.39). This was not due to inconsistency in the location of the TMS sites, which showed smaller within- than between-individual variance (p < 0.0001). Post hoc analyses showed significant associations when adding clinical covariates (r = −0.27, p = 0.014). Baseline anxiety (p < 0.0001) and comorbid psychiatric conditions (p < 0.001) accounted for the most variance in response. Atlas-based connectivity of TMS site to the SGC accounted for minimal variance in antidepressant response in this diverse sample. The “anti-subgenual” target derived based on normative connectome may be suboptimal for MDD patients with high baseline anxiety or psychiatric comorbidities.
Trial registration
ClinicalTrials.gov Identifier: NCT03276793.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data for this study (R01MH113929-01) is available through the NIMH Data Archive (NDA), collection ID C2816. Access requires permission and can be requested at (https://nda.nih.gov).
Code availability
All custom MATLAB scripts used in this study are publicly available in the following GitHub repository: (https://github.com/sanazkhosravani/Khosravani_Fox_MPsy_2025_MATLAB_Scripts/tree/main). As an analogous normative connectome to the one used in this study [27], researchers may consider using the GSP connectome [85], which is openly accessible.
References
Connolly KR, Helmer A, Cristancho MA, Cristancho P, O’Reardon JP. Effectiveness of transcranial magnetic stimulation in clinical practice post-FDA approval in the United States: results observed with the first 100 consecutive cases of depression at an academic medical center. J Clin Psychiatry. 2012;73:e567–e573. https://doi.org/10.4088/JCP.11m07413
Trapp NT, Bruss J, King Johnson M, Uitermarkt BD, Garrett L, Heinzerling A, et al. Reliability of targeting methods in TMS for depression: beam F3 vs. 5.5 cm. Brain Stimul. 2020;13:578–81. https://doi.org/10.1016/j.brs.2020.01.010
Weigand A, Horn A, Caballero R, Cooke D, Stern AP, Taylor SF, et al. Prospective validation that subgenual connectivity predicts antidepressant efficacy of transcranial magnetic stimulation sites. Biol Psychiatry. 2018;84:28–37. https://doi.org/10.1016/j.biopsych.2017.10.028
Cash RFH, Zalesky A, Thomson RH, Tian Y, Cocchi L, Fitzgerald PB. Subgenual functional connectivity predicts antidepressant treatment response to transcranial magnetic stimulation: independent validation and evaluation of personalization. Biol Psychiatry. 2019;86:e5–e7. https://doi.org/10.1016/j.biopsych.2018.12.002
Fox MD, Buckner RL, White MP, Greicius MD, Pascual-Leone A. Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the subgenual cingulate. Biol Psychiatry. 2012;72:595–603. https://doi.org/10.1016/j.biopsych.2012.04.028
Cash RFH, Weigand A, Zalesky A, Siddiqi SH, Downar J, Fitzgerald PB, et al. Using brain imaging to improve spatial targeting of transcranial magnetic stimulation for depression. Biol Psychiatry. 2021;90:689–700. https://doi.org/10.1016/j.biopsych.2020.05.033
Siddiqi SH, Weigand A, Pascual-Leone A, Fox MD. Identification of personalized transcranial magnetic stimulation targets based on subgenual cingulate connectivity: an independent replication. Biol Psychiatry. 2021;90:e55–e56. https://doi.org/10.1016/j.biopsych.2021.02.015
Fox MD, Liu H, Pascual-Leone A. Identification of reproducible individualized targets for treatment of depression with TMS based on intrinsic connectivity. NeuroImage. 2013;66:151–60. https://doi.org/10.1016/j.neuroimage.2012.10.082
Cole EJ, Stimpson KH, Bentzley BS, Gulser M, Cherian K, Tischler C, et al. Stanford accelerated intelligent neuromodulation therapy for treatment-resistant depression. Am J Psychiatry. 2020;177:716–26. https://doi.org/10.1176/appi.ajp.2019.19070720
Cole EJ, Phillips AL, Bentzley BS, Stimpson KH, Nejad R, Barmak F, et al. Stanford neuromodulation therapy (SNT): a double-blind randomized controlled trial. Am J Psychiatry. 2021;179:132–41. https://doi.org/10.1176/appi.ajp.2021.20101429
Blumberger DM, Vila-Rodriguez F, Thorpe KE, Feffer K, Noda Y, Giacobbe P, et al. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): a randomised non-inferiority trial. Lancet. 2018;391:1683–92. https://doi.org/10.1016/S0140-6736(18)30295-2
Blumberger DM, Mulsant BH, Thorpe KE, McClintock SM, Konstantinou GN, Lee HH, et al. Effectiveness of standard sequential bilateral repetitive transcranial magnetic stimulation vs bilateral theta burst stimulation in older adults with depression: the FOUR-D randomized noninferiority clinical trial. JAMA Psychiatry. 2022;79:1065–73. https://doi.org/10.1001/jamapsychiatry.2022.2862
Yesavage JA, Fairchild JK, Mi Z, Biswas K, Davis-Karim A, Phibbs CS, et al. Effect of repetitive transcranial magnetic stimulation on treatment-resistant major depression in US Veterans: a randomized clinical trial. JAMA Psychiatry. 2018;75:884–93. https://doi.org/10.1001/jamapsychiatry.2018.1483
Mir-Moghtadaei A, Siddiqi SH, Mir-Moghtadaei K, Blumberger DM, Vila-Rodriguez F, Daskalakis ZJ, et al. Updated scalp heuristics for localizing the dorsolateral prefrontal cortex based on convergent evidence of lesion and brain stimulation studies in depression. Brain Stimul. 2022;15:291–5. https://doi.org/10.1016/j.brs.2022.01.013
Siddiqi SH, Taylor SF, Cooke D, Pascual-Leone A, George MS, Fox MD. Distinct symptom-specific treatment targets for circuit-based neuromodulation. Am J Psychiatry. 2020;177:435–46. https://doi.org/10.1176/appi.ajp.2019.19090915
Brakemeier EL, Luborzewski A, Danker-Hopfe H, Kathmann N, Bajbouj M. Positive predictors for antidepressive response to prefrontal repetitive transcranial magnetic stimulation (rTMS). J Psychiatr Res. 2007;41:395–403. https://doi.org/10.1016/j.jpsychires.2006.01.013
Fregni F, Marcolin MA, Myczkowski M, Amiaz R, Hasey G, Rumi DO, et al. Predictors of antidepressant response in clinical trials of transcranial magnetic stimulation. Int J Neuropsychopharmacol. 2006;9:641–54. https://doi.org/10.1017/S1461145705006280
Lisanby SH, Husain MM, Rosenquist PB, Maixner D, Gutierrez R, Krystal A, et al. Daily left prefrontal repetitive transcranial magnetic stimulation in the acute treatment of major depression: clinical predictors of outcome in a multisite, randomized controlled clinical trial. Neuropsychopharmacology. 2009;34:522–34. https://doi.org/10.1038/npp.2008.118
Liston C, Chen AC, Zebley BD, Drysdale AT, Gordon R, Leuchter B, et al. Default mode network mechanisms of transcranial magnetic stimulation in depression. Biol Psychiatry. 2014;76:517–26. https://doi.org/10.1016/j.biopsych.2014.01.023
Kimbrell TA, Little JT, Dunn RT, Frye MA, Greenberg BD, Wassermann EM, et al. Frequency dependence of antidepressant response to left prefrontal repetitive transcranial magnetic stimulation (rTMS) as a function of baseline cerebral glucose metabolism. Biol Psychiatry. 1999;46:1603–13. https://doi.org/10.1016/s0006-3223(99)00195-x
Paillère Martinot ML, Martinot JL, Ringuenet D, Galinowski A, Gallarda T, Bellivier F, et al. Baseline brain metabolism in resistant depression and response to transcranial magnetic stimulation. Neuropsychopharmacology. 2011;36:2710–9. https://doi.org/10.1038/npp.2011.161
Kalin NH. The critical relationship between anxiety and depression. Am J Psychiatry. 2020;177:365–7. https://doi.org/10.1176/appi.ajp.2020.20030305
American Psychiatric Association. Diagnostic and statistical manual of mental disorders (5th ed.). Arlington, VA: American Psychiatric Association; 2013.
Beam W, Borckardt JJ, Reeves ST, George MS. An efficient and accurate new method for locating the F3 position for prefrontal TMS applications. Brain Stimul. 2009;2:50–54. https://doi.org/10.1016/j.brs.2008.09.006
Mylius V, Ayache SS, Ahdab R, Farhat WH, Zouari HG, Belke M, et al. Definition of DLPFC and M1 according to anatomical landmarks for navigated brain stimulation: inter-rater reliability, accuracy, and influence of gender and age. NeuroImage. 2013;78:224–32. https://doi.org/10.1016/j.neuroimage.2013.03.061
McDonald WM, Durkalski V, Ball ER, Holtzheimer PE, Pavlicova M, Lisanby SH, et al. Improving the antidepressant efficacy of transcranial magnetic stimulation: maximizing the number of stimulations and treatment location in treatment-resistant depression. Depress Anxiety. 2011;28:973–80. https://doi.org/10.1002/da.20885
Yeo BTT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:1125–65. https://doi.org/10.1152/jn.00338.2011
Li J, Kong R, Liègeois R, Orban C, Tan Y, Sun N, et al. Global signal regression strengthens association between resting-state functional connectivity and behavior. NeuroImage. 2019;196:126–41. https://doi.org/10.1016/j.neuroimage.2019.04.016
Kong R, Li J, Orban C, Sabuncu MR, Liu H, Schaefer A, et al. Spatial topography of individual-specific cortical networks predicts human cognition, personality, and emotion. Cereb Cortex. 2019;26:2533–51. https://doi.org/10.1093/cercor/bhy123
Beck, AT, Steer, RA, & Brown, GK. Beck Depression Inventory–II (BDI-II). APA PsycTests; 1996. https://doi.org/10.1037/t00742-000
Fox MD. Mapping symptoms to brain networks with the human connectome. N Engl J Med. 2018;379:2237–45. https://doi.org/10.1056/NEJMra1706158
Kroenke, K, Spitzer, RL, & Williams, JBW. Patient Health Questionnaire-9 (PHQ-9). APA PsycTests; 1999. https://doi.org/10.1037/t06165-000
Rush AJ, Carmody T, Reimitz P-E. The Inventory of Depressive Symptomatology (IDS): clinician (IDS-C) and Self-Report (IDS-SR) ratings of depressive symptoms. Int J Methods Psychiatr Res. 2000;9:45–59. https://doi.org/10.1002/mpr.79
Jacobs, KM. Brodmann’s areas of the cortex. In JS Kreutzer, J DeLuca, & B Caplan (Eds.), Encyclopedia of clinical neuropsychology. Springer; 2011. https://doi.org/10.1007/978-0-387-79948-3_301
Siddiqi SH, Schaper FLWVJ, Horn A, Hsu J, Padmanabhan JL, Brodtmann A, et al. Brain stimulation and brain lesions converge on common causal circuits in neuropsychiatric disease. Nat Hum Behav. 2021;5:1707–16. https://doi.org/10.1038/s41562-021-01161-1
Oathes DJ, Zimmerman JP, Duprat R, Japp SS, Scully M, Rosenberg BM, et al. Resting fMRI-guided TMS results in subcortical and brain network modulation indexed by interleaved TMS/fMRI. Exp Brain Res. 2021;239:1165–78. https://doi.org/10.1007/s00221-021-06036-5
Thielscher, A, Antunes, A, & Saturnino, GB. Field modeling for transcranial magnetic stimulation: a useful tool to understand the physiological effects of TMS? In 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC) (pp. 222-5). IEEE; 2015. https://doi.org/10.1109/EMBC.2015.7318340
Elbau IG, Lynch CJ, Downar J, Vila-Rodriguez F, Power JD, Solomonov N, et al. Functional connectivity mapping for rTMS target selection in depression. Am J Psychiatry. 2023;180:230–40. https://doi.org/10.1176/appi.ajp.20220306
Van Essen DC, Ugurbil K, Auerbach E, Barch D, Behrens TE, Bucholz R, et al. The Human connectome project: a data acquisition perspective. NeuroImage. 2012;62:2222–31. https://doi.org/10.1016/j.neuroimage.2012.02.018
Glasser MF, Sotiropoulos SN, Wilson JA, Coalson TS, Fischl B, Andersson JL, et al. The minimal preprocessing pipelines for the Human Connectome Project. NeuroImage. 2013;80:105–24. https://doi.org/10.1016/j.neuroimage.2013.04.127
Fox MD, Zhang D, Snyder AZ, Raichle ME. The global signal and observed anticorrelated resting state brain networks. J Neurophysiol. 2009;101:3270–83. https://doi.org/10.1152/jn.90777.2008
Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM. FSL. NeuroImage. 2012;62:782–90. https://doi.org/10.1016/j.neuroimage.2011.09.015
Hoffmann M, Billot B, Greve DN, Iglesias JE, Fischl B, Dalca AV. SynthMorph: learning contrast-invariant registration without acquired images. IEEE Trans Med Imaging. 2021;41:543–58. https://doi.org/10.1109/TMI.2021.3116879
Iglesias JE. A ready-to-use machine learning tool for symmetric multi-modality registration of brain MRI. Sci Rep. 2023;13:6657 https://doi.org/10.1038/s41598-023-33781-0
Jenkinson M, Smith SM. A global optimization method for robust affine registration of brain images. Med Image Anal. 2001;5:143–56. https://doi.org/10.1016/s1361-8415(01)00036-6
Jenkinson M, Bannister PR, Brady JM, Smith SM. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17:825–41. https://doi.org/10.1016/s1053-8119(02)91132-8
Andersson, JLR, Jenkinson, M, & Smith, S Non-linear registration. FMRIB Technical Report TR07JA2.2010
Harrell FE Jr. Regression Modeling Strategies. New York: Springer-Verlag; 2001. https://doi.org/10.1007/978-1-4757-3462-1
James, G, Witten, D, Hastie, T, & Tibshirani, R. An introduction to statistical learning (8th ed.). New York: Springer Science+Business Media;2017. ISBN 978-1-4614-7138-7
Snee, R. Origins of the variance inflation factor as recalled by Cuthbert Daniel. Technical report. Snee Associates;1981.
Glasser GJ, Winter RF. Critical values of the coefficient of rank correlation for testing the hypothesis of independence. Biometrika. 1961;48:444–8. Oxford University Press. https://doi.org/10.2307/2332767
Spearman C. The proof and measurement of association between two things. Am J Psychol. 1904;15:72–101. https://doi.org/10.2307/1412159
James, G, Witten, D, Hastie, T, & Tibshirani, R An introduction to statistical learning (8th ed.). Springer Science+Business Media;2017.
James, G, Witten, D, Hastie, T, & Tibshirani, R. An introduction to statistical learning: with applications in R. New York, NY: Springer;2013. (Chapter 3)
Fox, J Applied regression analysis and generalized linear models (3rd ed.). Los Angeles, CA: Sage Publications;2015.
Agresti, A Categorical data analysis (3rd ed.). Hoboken, NJ: Wiley;2018.
Wright, DB, & London, K Modern regression techniques using R: A practical guide. Thousand Oaks, CA: Sage Publications;2009.
Akaike H. A new look at the statistical model identification. IEEE Trans Autom Control. 1974;19:716–23. https://doi.org/10.1109/TAC.1974.1100705
Meinshausen N, Bühlmann P. Stability selection. J R Stat Soc Ser B. 2010;72:417–73. https://doi.org/10.1111/j.1467-9868.2010.00740.x
Avesani P, McPherson B, Hayashi S, Caiafa CF, Henschel R, Garyfallidis E, et al. The open diffusion data derivatives: brain data upcycling via integrated publishing of derivatives and reproducible open cloud services. Sci Data. 2019;6:69 https://doi.org/10.1038/s41597-019-0073-y
Xia M, Wang J, He Y. BrainNet Viewer: a network visualization tool for human brain connectomics. PLOS ONE. 2013;8:e68910 https://doi.org/10.1371/journal.pone.0068910
Taylor S, Zvolensky MJ, Cox BJ, Deacon B, Heimberg RG, Ledley DR, et al. Robust dimensions of anxiety sensitivity: development and initial validation of the Anxiety Sensitivity Index-3. Psychol Assess. 2007;19:176–88. https://doi.org/10.1037/1040-3590.19.2.176
Herbsman T, Avery D, Ramsey D, Holtzheimer P, Wadjik C, Hardaway F, et al. More lateral and anterior prefrontal coil location is associated with better repetitive transcranial magnetic stimulation antidepressant response. Biol Psychiatry. 2009;66:509–15. https://doi.org/10.1016/j.biopsych.2009.04.034
Benster, L, Weissman, C, Suprani, F, Toney, K, Afshar, H, Stapper, N, et al. Predictive modeling of response to repetitive transcranial magnetic stimulation in treatment-resistant depression. Research Square. 2024. https://doi.org/10.21203/rs.3.rs-4396926/v1
Li CT, Su TP, Cheng CM, Chen MH, Bai YM, Tsai SJ. Factors associated with antidepressant responses to repetitive transcranial magnetic stimulation in antidepressant-resistant depression. Front Neurosci. 2022;16:1046920 https://doi.org/10.3389/fnins.2022.1046920
Zandberg LJ, Zang Y, McLean CP, Yeh R, Simpson HB, Foa EB. Change in obsessive-compulsive symptoms mediates subsequent change in depressive symptoms during exposure and response prevention. Behav Res Ther. 2015;68:76–81. https://doi.org/10.1016/j.brat.2015.03.005
Fox MD, et al. Resting-state networks link invasive and noninvasive brain stimulation across diverse psychiatric and neurological diseases. Proc Natl Acad Sci. 2014;111:E4367–E4375. https://doi.org/10.1073/pnas.1405003111
Baer L, Trivedi MH, Huz I, Rush AJ, Wisniewski SR, Fava M. Prevalence and impact of obsessive-compulsive symptoms in depression: a STARD report. J Clin Psychiatry. 2015;76:1668–74. https://doi.org/10.4088/JCP.14m09670
Sheng J, Liu S, Wang Y, Cui R, Zhang X. The link between depression and chronic pain: neural mechanisms in the brain. Neural Plast. 2017;2017:9724371 https://doi.org/10.1155/2017/9724371
Bijsterbosch JD, Ansari TL, Smith S, Gauld O, Zika O, Boessenkool S, et al. Stratification of MDD and GAD patients by resting state brain connectivity predicts cognitive bias. NeuroImage Clin. 2018;19:425–33. https://doi.org/10.1016/j.nicl.2018.04.033
Silk JS, Sequeira SS, Jones NP, Lee KH, Dahl RE, Forbes EE, et al. Subgenual anterior cingulate cortex reactivity to rejection vs. acceptance predicts depressive symptoms among adolescents with an anxiety history. J Clin Child Adolesc Psychol. 2023;52:659–74. https://doi.org/10.1080/15374416.2021.2019048
Tuescher O, Protopopescu X, Pan H, Cloitre M, Butler T, Goldstein M, et al. Differential activity of subgenual cingulate and brainstem in panic disorder and PTSD. J Anxiety Disord. 2011;25:251–7. https://doi.org/10.1016/j.janxdis.2010.09.010
Zhan C, Liu Y, Wu K, Gao Y, Li X. Structural and functional abnormalities in children with attention-deficit/hyperactivity disorder: a focus on subgenual anterior cingulate cortex. Brain Connect. 2017;7:106–14. https://doi.org/10.1089/brain.2016.0444
Osborne NR, Cheng JC, Rogachov A, Kim JA, Hemington KS, Bosma RL, et al. Abnormal subgenual anterior cingulate circuitry is unique to women but not men with chronic pain. Pain. 2021;162:97–108. https://doi.org/10.1097/j.pain.0000000000002016
Siddiqi SH, Fox MD. Targeting symptom-specific networks with transcranial magnetic stimulation. Biol Psychiatry. 2024;95:502–9. https://doi.org/10.1016/j.biopsych.2023.11.011
Siddiqi S, Philip NS, Palm S, Arulpragasam A, Barredo J, Bouchard H, et al. A potential neuromodulation target for PTSD in Veterans derived from focal brain lesions. Res Sq. 2024. https://doi.org/10.21203/rs.3.rs-3132332/v1
Brown JC, Kweon J, Sharma P, Siddiqi SH, Isserles M, Ressler KJ. Critically assessing the unanswered questions of how, where, and when to induce plasticity in the posttraumatic stress disorder network with transcranial magnetic stimulation. Biol Psychiatry. 2024;97:392–404. https://doi.org/10.1016/j.biopsych.2024.06.010
Isserles M, Tendler A, Roth Y, Bystritsky A, Blumberger DM, Ward H, et al. Deep transcranial magnetic stimulation combined with brief exposure for posttraumatic stress disorder: a prospective multisite randomized trial. Biol Psychiatry. 2021;90:721–8. https://doi.org/10.1016/j.biopsych.2021.04.019
Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092–7. https://doi.org/10.1001/archinte.166.10.1092
Ning L, Makris N, Camprodon JA, Rathi Y. Limits and reproducibility of resting-state functional MRI definition of DLPFC targets for neuromodulation. Brain Stimul. 2019;12:129–38. https://doi.org/10.1016/j.brs.2018.10.004
Cash RFH, Cocchi L, Lv J, Wu Y, Fitzgerald PB, Zalesky A. Personalized connectivity-guided DLPFC-TMS for depression: advancing computational feasibility, precision, and reproducibility. Hum Brain Mapp. 2021;42:4155–72. https://doi.org/10.1002/hbm.25330
Cash RFH, Cocchi L, Lv J, Fitzgerald P, Zalesky A. Functional magnetic resonance imaging-guided personalization of transcranial magnetic stimulation treatment for depression. JAMA Psychiatry. 2020;78:337–9. https://doi.org/10.1001/jamapsychiatry.2020.3794
Fang K, Niu L, Wen B, Liang L, Tian Y, Hou Y, et al. Individualized resting-state functional connectivity abnormalities unveil two major depressive disorder subtypes with contrasting abnormal patterns of abnormality. Transl Psychiatry. 2025;15:45 https://doi.org/10.1038/s41398-025-03268-9
Wang Q, Akram H, Muthuraman M, Gonzalez-Escamilla G, Sheth SA, Oxenford S, et al. Normative vs. patient-specific brain connectivity in deep brain stimulation. NeuroImage. 2021;224:117307 https://doi.org/10.1016/j.neuroimage.2020.117307
Cohen A, Soussand L, McManus P, Fox M. GSP1000 preprocessed connectome. Harv Dataverse. 2020. https://doi.org/10.7910/DVN/ILXIKS
Funding
This research was supported by a grant from the U.S. National Institutes of Health (R01 MH113929-01) awarded to MDF. Additional support was provided in part by the NIGMS-funded COBRE Center for Neuromodulation at Butler Hospital (P20GM130452). MDF has intellectual property on the use of brain connectivity imaging to analyze lesions and guide brain stimulation, is a consultant for Magnus Medical, Soterix, Abbott, and Boston Scientific, and has received research funding from Neuronetics. MDF was supported by grants from the NIH (R01MH113929, R21MH126271, R56AG069086, R21NS123813, R01NS127892, R01MH130666, UM1NS132358), Neuronetics, the Kaye Family Research Endowment, the Ellison / Baszucki Family Foundation, and the Manley Family. SHS is the owner of intellectual property involving the use of brain connectivity to target TMS, scientific consultant for Magnus Medical, investigator-initiated research funding from Neuronetics and Brainsway, speaking fees from Brainsway and Otsuka (for PsychU.org), shareholder in Brainsway (publicly traded) and Magnus Medical (not publicly traded). LLC reports clinical trials contracts with Brown University, Janssen, Neuronetics, and Neurolief; consulting income from Janssen, Neuronetics, Neurolief, Sage Therapeutics, Otsuka, Universal Brain, and Magnus Medical; and a research equipment loan from Nexstim. TB serves as a site Principal Investigator for the Magnus Medical Open Label Clinical Trial. APS is an employee of the Thermo Fisher Scientific with equity compensation. MS is the executive assistant to the CEO of the Nia Therapeutics, Inc.
Author information
Authors and Affiliations
Contributions
MDF conceptualized the study and secured funding. MDF, SHS, LLC, JJT, JCB, DP, APS, STP, WD, SF, CL, ET, LH contributed to data collection. MDF, SK, SHS, JJT, TB, STP, WD, SF, CL, AG, NC, EJ, DL conducted the data analysis. MDF, SK drafted the manuscript. All co-authors provided critical revisions to the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khosravani, S., Palm, S.T., Drew, W. et al. Predictive value of subgenual cingulate normative connectivity to TMS treatment site for antidepressant response in routine clinical practice: a prospective, multisite cohort study. Mol Psychiatry (2025). https://doi.org/10.1038/s41380-025-03153-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41380-025-03153-3