Abstract
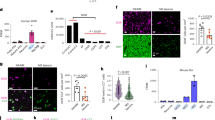
We have reported that mice in which the liver X receptor β (LXRβ) gene is inactivated lose dopaminergic neurons in the substantia nigra and motor neurons in the ventral horn of the spinal cord. These mice develop progressive hind limb paralysis starting at 6 months of age. Since LXRβ is not expressed in either dopaminergic neurons or motor neurons, we have focused on LXRβ-expressing cells whose function is essential for neuron survival. We now report defects in oligodendrocyte maturation in the absence of LXRβ. At 4 months of age, long before motor neuron loss occurs, there was reduction in expression of the four following genes in oligodendrocytes: The monocarboxylate transporter 1 (MCT1), which is essential for metabolic support of motor neurons; BDNF, a motor neuron trophic factor; 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase (HMGCR), a rate-limiting enzyme in cholesterol synthesis; glutamine synthetase (GS), an enzyme crucial for the elimination of neurotoxic glutamate from synapses. Differentiation of ES cells from WT and LXRβ−/− mice into motor neurons/oligodendrocytes revealed that LXRβ−/− cultures showed less arborization of motor neurons and a reduced proportion of mature oligodendrocytes. Our study suggests that defects in glial cells can have profound effects on neuronal survival and that early defective oligodendrocyte maturation can lead to motor neuron death. The expression of LXRβ in oligodendrocytes should be investigated as a target for preventing neuronal loss in diseases such as amyotrophic lateral sclerosis (ALS) and Parkinson’s disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary files. The datasets of the current study are available from the corresponding author on reasonable request.
References
Jakobsson T, Treuter E, Gustafsson JA, Steffensen KR. Liver X receptor biology and pharmacology: new pathways, challenges and opportunities. Trends Pharmacol Sci. 2012;33:394–404.
Kim HJ, Fan X, Gabbi C, Yakimchuk K, Parini P, Warner M, et al. Liver X receptor beta (LXRbeta): a link between beta-sitosterol and amyotrophic lateral sclerosis-Parkinson’s dementia. Proc Natl Acad Sci USA. 2008;105:2094–9.
Song XY, Wu WF, Gabbi C, Dai YB, So M, Chaurasiya SP, et al. Retinal and optic nerve degeneration in liver X receptor beta knockout mice. Proc Natl Acad Sci USA. 2019;116:16507–12.
Song XY, Wu WF, Dai YB, Xu HW, Roman A, Wang L, et al. Ablation of liver X receptor beta in mice leads to overactive macrophages and death of spiral ganglion neurons. Hear Res. 2022;422:108534.
Dodge JC, Jensen EH, Yu J, Sardi SP, Bialas AR, Taksir TV, et al. Neutral lipid cacostasis contributes to disease pathogenesis in amyotrophic lateral sclerosis. J Neurosci. 2020;40:9137–47.
Mouzat K, Chudinova A, Polge A, Kantar J, Camu W, Raoul C, et al. Regulation of brain cholesterol: what role do liver X receptors play in neurodegenerative diseases? Int J Mol Sci. 2019;20:3858.
Younger DS, Brown RH Jr. Amyotrophic lateral sclerosis. Handb Clin Neurol. 2023;196:203–29.
Xue YC, Ng CS, Xiang P, Liu H, Zhang K, Mohamud Y, et al. Dysregulation of RNA-Binding proteins in amyotrophic lateral sclerosis. Front Mol Neurosci. 2020;13:78.
Yang C, Wang H, Qiao T, Yang B, Aliaga L, Qiu L, et al. Partial loss of TDP-43 function causes phenotypes of amyotrophic lateral sclerosis. Proc Natl Acad Sci USA. 2014;111:E1121–9.
Blokhuis AM, Groen EJ, Koppers M, van den Berg LH, Pasterkamp RJ. Protein aggregation in amyotrophic lateral sclerosis. Acta Neuropathol. 2013;125:777–94.
Bonafede R, Mariotti R. ALS pathogenesis and therapeutic approaches: the role of mesenchymal stem cells and extracellular vesicles. Front Cell Neurosci. 2017;11:80.
Abdel-Khalik J, Yutuc E, Crick PJ, Gustafsson JA, Warner M, Roman G, et al. Defective cholesterol metabolism in amyotrophic lateral sclerosis. J Lipid Res. 2017;58:267–78.
Agrawal I, Lim YS, Ng SY, Ling SC. Deciphering lipid dysregulation in ALS: from mechanisms to translational medicine. Transl Neurodegener. 2022;11:48.
Godoy-Corchuelo JM, Fernandez-Beltran LC, Ali Z, Gil-Moreno MJ, Lopez-Carbonero JI, Guerrero-Sola A, et al. Lipid metabolic alterations in the ALS-FTD spectrum of disorders. Biomedicines. 2022;10:1105.
Wingo AP, Vattathil SM, Liu J, Fan W, Cutler DJ, Levey AI, et al. LDL cholesterol is associated with higher AD neuropathology burden independent of APOE. J Neurol Neurosurg Psychiatry. 2022;93:930–8.
Valenza M, Birolini G, Cattaneo E. The translational potential of cholesterol-based therapies for neurological disease. Nat Rev Neurol. 2023;19:583–98.
Cheon SY. Impaired cholesterol metabolism, neurons, and neuropsychiatric disorders. Exp Neurobiol. 2023;32:57–67.
Kang SH, Li Y, Fukaya M, Lorenzini I, Cleveland DW, Ostrow LW, et al. Degeneration and impaired regeneration of gray matter oligodendrocytes in amyotrophic lateral sclerosis. Nat Neurosci. 2013;16:571–9.
Lee Y, Morrison BM, Li Y, Lengacher S, Farah MH, Hoffman PN, et al. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature. 2012;487:443–8.
Alberti S, Schuster G, Parini P, Feltkamp D, Diczfalusy U, Rudling M, et al. Hepatic cholesterol metabolism and resistance to dietary cholesterol in LXRbeta-deficient mice. J Clin Invest. 2001;107:565–73.
Varshney MK, Inzunza J, Lupu D, Ganapathy V, Antonson P, Ruegg J, et al. Role of estrogen receptor beta in neural differentiation of mouse embryonic stem cells. Proc Natl Acad Sci USA. 2017;114:E10428–E37.
Song X, Wu W, Dai Y, Warner M, Nalvarte I, Antonson P, et al. Loss of ERbeta in aging LXRalphabeta knockout mice leads to colitis. Int J Mol Sci. 2023;24:12461.
Andersson S, Gustafsson N, Warner M, Gustafsson JA. Inactivation of liver X receptor beta leads to adult-onset motor neuron degeneration in male mice. Proc Natl Acad Sci USA. 2005;102:3857–62.
Nelissen K, Mulder M, Smets I, Timmermans S, Smeets K, Ameloot M, et al. Liver X receptors regulate cholesterol homeostasis in oligodendrocytes. J Neurosci Res. 2012;90:60–71.
Lee JH, Kim H, Park SJ, Woo JH, Joe EH, Jou I. Small heterodimer partner SHP mediates liver X receptor (LXR)-dependent suppression of inflammatory signaling by promoting LXR SUMOylation specifically in astrocytes. Sci Signal. 2016;9:ra78.
Bogie JFJ, Vanmierlo T, Vanmol J, Timmermans S, Mailleux J, Nelissen K, et al. Liver X receptor beta deficiency attenuates autoimmune-associated neuroinflammation in a T cell-dependent manner. J Autoimmun. 2021;124:102723.
Song X, Wu W, Warner M, Gustafsson JA. Liver X receptor regulation of glial cell functions in the CNS. Biomedicines. 2022;10:2165.
Dai YB, Wu WF, Huang B, Miao YF, Nadarshina S, Warner M, et al. Liver X receptors regulate cerebrospinal fluid production. Mol Psychiatry. 2016;21:844–56.
Halpain S, Spencer K, Graber S. Dynamics and pathology of dendritic spines. Prog Brain Res. 2005;147:29–37.
Huber N, Hoffmann D, Giniatullina R, Rostalski H, Leskela S, Takalo M, et al. C9orf72 hexanucleotide repeat expansion leads to altered neuronal and dendritic spine morphology and synaptic dysfunction. Neurobiol Dis. 2022;162:105584.
Fogarty MJ, Mu EWH, Lavidis NA, Noakes PG, Bellingham MC. Motor areas show altered dendritic structure in an amyotrophic lateral sclerosis mouse model. Front Neurosci. 2017;11:609.
Fogarty MJ, Mu EWH, Lavidis NA, Noakes PG, Bellingham MC. Size-dependent vulnerability of lumbar motor neuron dendritic degeneration in SOD1(G93A) mice. Anat Rec. 2020;303:1455–71.
Ferraiuolo L, Meyer K, Sherwood TW, Vick J, Likhite S, Frakes A, et al. Oligodendrocytes contribute to motor neuron death in ALS via SOD1-dependent mechanism. Proc Natl Acad Sci USA. 2016;113:E6496–E505.
Fisel P, Schaeffeler E, Schwab M. Clinical and functional relevance of the monocarboxylate transporter family in disease pathophysiology and drug therapy. Clin Transl Sci. 2018;11:352–64.
Philips T, Mironova YA, Jouroukhin Y, Chew J, Vidensky S, Farah MH, et al. MCT1 deletion in oligodendrocyte lineage cells causes late-onset hypomyelination and axonal degeneration. Cell Rep. 2021;34:108610.
Boucanova F, Pollmeier G, Sandor K, Morado Urbina C, Nijssen J, Medard JJ, et al. Disrupted function of lactate transporter MCT1, but not MCT4, in Schwann cells affects the maintenance of motor end-plate innervation. Glia. 2021;69:124–36.
Mouzat K, Molinari N, Kantar J, Polge A, Corcia P, Couratier P, et al. Liver X receptor genes variants modulate ALS phenotype. Mol Neurobiol. 2018;55:1959–65.
Xu Z, Lee A, Nouwens A, Henderson RD, McCombe PA. Mass spectrometry analysis of plasma from amyotrophic lateral sclerosis and control subjects. Amyotroph Lat Scler Frontotemporal Degener. 2018;19:362–76.
Raffaele S, Boccazzi M, Fumagalli M. Oligodendrocyte dysfunction in amyotrophic lateral sclerosis: mechanisms and therapeutic perspectives. Cells. 2021;10:565.
Funfschilling U, Supplie LM, Mahad D, Boretius S, Saab AS, Edgar J, et al. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature. 2012;485:517–21.
Ho WY, Chang JC, Lim K, Cazenave-Gassiot A, Nguyen AT, Foo JC, et al. TDP-43 mediates SREBF2-regulated gene expression required for oligodendrocyte myelination. J Cell Biol. 2021;220:e201910213.
Mou Y, Dong Y, Chen Z, Denton KR, Duff MO, Blackstone C, et al. Impaired lipid metabolism in astrocytes underlies degeneration of cortical projection neurons in hereditary spastic paraplegia. Acta Neuropathol Commun. 2020;8:214.
Byrne DJ, Garcia-Pardo ME, Cole NB, Batnasan B, Heneghan S, Sohail A, et al. Liver X receptor-agonist treatment rescues degeneration in a Drosophila model of hereditary spastic paraplegia. Acta Neuropathol Commun. 2022;10:40.
Wada T, Kang HS, Angers M, Gong H, Bhatia S, Khadem S, et al. Identification of oxysterol 7alpha-hydroxylase (Cyp7b1) as a novel retinoid-related orphan receptor alpha (RORalpha) (NR1F1) target gene and a functional cross-talk between RORalpha and liver X receptor (NR1H3). Mol Pharmacol. 2008;73:891–9.
Jia W, Wei M, Rajani C, Zheng X. Targeting the alternative bile acid synthetic pathway for metabolic diseases. Protein Cell. 2021;12:411–25.
Schule R, Siddique T, Deng HX, Yang Y, Donkervoort S, Hansson M, et al. Marked accumulation of 27-hydroxycholesterol in SPG5 patients with hereditary spastic paresis. J Lipid Res. 2010;51:819–23.
Xin W, Mironova YA, Shen H, Marino RAM, Waisman A, Lamers WH, et al. Oligodendrocytes support neuronal glutamatergic transmission via expression of glutamine synthetase. Cell Rep. 2019;27:2262–71.e5.
Ben Haim L, Schirmer L, Zulji A, Sabeur K, Tiret B, Ribon M, et al. Evidence for glutamine synthetase function in mouse spinal cord oligodendrocytes. Glia. 2021;69:2812–27.
Li X, Zhong H, Wang Z, Xiao R, Antonson P, Liu T, et al. Loss of liver X receptor beta in astrocytes leads to anxiety-like behaviors via regulating synaptic transmission in the medial prefrontal cortex in mice. Mol Psychiatry. 2021;26:6380–93.
Lv K, Luo Y, Liu T, Xia M, Gong H, Zhang D, et al. Inactivation of microglial LXRbeta in early postnatal mice impairs microglia homeostasis and causes long-lasting cognitive dysfunction. Proc Natl Acad Sci USA. 2025;122:e2410698122.
Warner M, Song X, Gustafsson JA. Liver X and thyroid hormone receptors in neurodegeneration. Genom Psychiatry. 2024;1:11.
Acknowledgements
J.-Å. G. acknowledges Robert A. Welch Foundation grant E-0004 and the Swedish Research Council.
Author information
Authors and Affiliations
Contributions
XS, MW, and JAG designed the research, analyzed the data, and wrote the manuscript. XS, WW, MV, AR, and MW performed the research. All authors read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All methods in this study were performed in accordance with the relevant guidelines and regulations. Ethical approval for this study was obtained from the local Animal Experimentation Ethics Committee for animal experimentation (University of Houston animal protocol 09-036). All participants provided written informed consent before participation.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Song, X., Wu, W., Varshney, M. et al. Role of LXRβ in oligodendrocytes in neuronal survival. Mol Psychiatry 31, 16–26 (2026). https://doi.org/10.1038/s41380-025-03278-5
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41380-025-03278-5