Abstract
The amygdala and nucleus accumbens are associated with emotion management in humans. Emotions play a central role in conditions such as depression and anxiety, where emotional states characterize the core symptoms. Effective emotion regulation can alleviate these symptoms, which highlights the importance of amygdala and nucleus accumbens in mental health. Functional magnetic resonance imaging studies that incorporate temporal characteristics of brain regions are limited. Therefore, this study examined the association of temporal variability of the limbic region, specifically the amygdala and nucleus accumbens, with worsening depressive or anxiety states mediated by negative emotions. The study included 1,080 healthy subjects from the Human Connectome Project dataset, and nine functional networks were extracted from the limbic region using group independent component analysis. Spectral clustering was performed on the temporal variability of these networks. Two distinct clusters were found in healthy adults according to the temporal variability of limbic networks. The association of increased temporal variability of functional networks with worsening affective states, including depression and anxiety, was partially mediated by the level of anger–physical aggression. These findings indicate that temporal variability in limbic regions might impact the emotion of anger and the level of anger, in turn, influences affective states, even in healthy participants.
Similar content being viewed by others
Introduction
Emotion is inherent in every individual’s life, influencing communication and decision making [1]. Individuals experiencing emotional dysfunction often encounter challenges in managing their daily lives and might receive diagnoses of mental illness. Emotions are central to depression and anxiety, as the core symptoms are characterized by emotional states, and these symptoms can be alleviated by regulating emotions [2]. Emotions are significant in human life and neuroimaging studies have revealed close associations between several brain regions and emotion processing [3]. The amygdala and nucleus accumbens stand out as prominent emotional brain regions. The amygdala, which processes emotional information, receives input from sensory regions and assesses the emotional significance of stimuli in terms of fear, threat, and reward [4]. This brain region modulates behavior and physiological arousal throughout emotional processes [5]. Likewise, the nucleus accumbens is a key brain region for processing emotion, particularly given that certain emotional experiences are associated with reward stimuli. The nucleus accumbens located deep within the ventral striatum, processes rewarding stimuli by integrating information from various brain regions involved in motivation, decision making, and reinforcement learning [6]. These processes are intertwined with emotional processing. Abnormal volumes and activities are exhibited in these emotional brain regions in depressive and anxious individuals [7].
Emotional processing and the associated brain regions are as complex as the emotions we experience. Each emotional process is not restricted to a single brain area but involves dynamic interaction, including receiving and transmitting information across various brain areas. Thus, investigating emotions requires assessing inter-regional functional connectivity (FC). Traditional FC is defined as statistical dependency between two brain areas over the entire scanning time, thereby obscuring temporal dynamics. However, time series scanning of resting-state functional magnetic resonance imaging (rs-fMRI) includes temporal observations of changes in brain networks over time. The time series data of the brain is divided into short windows (the specific number of scans), and the dynamic FC is calculated using coefficient between two distinct brain areas within each window [8]. Creating several dynamic functional connections across the entire time series enable the observation of the changing brain activity. Recently, these temporal snapshots have provided additional and meaningful insights into the brain–emotion relationship. Gaviria et al. (2021) reported that specific brain networks subsequently contribute to emotion elicitation and emotion regulation/restoration after negative events [9]. Meanwhile, many studies have used variability in dynamic FC as a variable. One study specifically observed changes in temporal variability in patients with depression [10]. Kaiser et al. showed that the group with major depressive disorder showed increased variability of dynamic resting-state FC between the medial prefrontal cortex and areas of the insula. Additionally, greater depression severity was associated with increased FC variability [11]. This dynamic approach using variability could reveal brain network patterns obscured by traditional rs-fMRI analysis. Therefore, this study applied a dynamic functional network to brain connections between emotional and whole brain regions to uncover brain activity related to emotion in healthy individuals. As brain functional network is changing over the scanning time, representative brain networks were extracted based on emotional brain connections and used to estimate FC variability in healthy individuals. We hypothesize that representative brain activity is associated with emotion and subsequently results in an affective symptom. To integrate all dynamic FC variabilities estimated from each emotional brain network, we clustered 1080 healthy individuals. This approach could help translate this research into clinical implications and provide further insight into affective symptoms. Subsequently, the directional associations among the clusters based on dynamic brain network variability, emotion, and affective symptoms were investigated. It holds significant potential to reveal valuable information about emotion by identifying representative brain network maps along the scanning time, defining clusters based on brain map variability, and examining the mediating effect of emotion-related brain activity, emotion, and affective symptoms in healthy individuals.
Methods
Participants
We used the Human Connectome Project (HCP) S1200 release dataset (http://www.humanconnectomeproject.org/), which comprise 1,206 healthy individuals. As HCP represents general population in terms of behavioral, ethnic, and socioeconomic diversity, the term “healthy” is broadly defined. It excluded individuals with severe neurodevelopmental disorders, documented neuropsychiatric disorders, or neurologic disorders through screening interviews. Additionally, those having illness such as diabetes or high blood pressure were excluded [12]. Out of 1,206 people, those without missing values in affective state and emotion metrics were selected; thus, 1080 individuals were included in the final study. The utilization of behavioral and imaging data from the HCP project received exempt approval from Ajou University Hospital’s Institutional Review Board and Ethics Committee (AJIRB-BMR-EXP-21-122).
Clinical assessment
The HCP data included results from a comprehensive set of clinical assessments of participants. Participants completed the Achenbach Adult Self Report (ASR) [13], which comprises eight syndromal scales—including Anxious/Depressed, Withdrawn, Somatic Complaints, Thought Problems, Attention Problems, Aggressive Behaviors, Rule-breaking Behaviors, and Intrusive Problems—and six DSM-oriented scales such as Depressive, Anxiety, Somatic, Avoidant Personality, Attention Deficit/Hyperactivity, and Antisocial Personality Problems. Each item on these scales is rated on a three-point scale: 0 (Not True), 1 (Somewhat or Sometimes True), and 2 (Very True or Often True). Higher raw scores indicate more problematic behavior, and these scores are converted into normalized T-scores adjusted for age and sex. ASR T-scores adjusted for age and sex were used with T > 69 that indicates a clinical diagnosis. Among the ASR scores, depressive and anxiety problems of DSM-oriented scales were prime areas of focus.
The NIH Toolbox Emotion Battery was employed to perform a comprehensive assessment of emotions [14]. This tool encompasses four primary domains—negative affect, psychological well-being, stress and self-efficacy, and social relationships—which are fundamental aspects of an individual’s emotional makeup. In this study, we analyzed all six subdomains from the negative affect domain at which each participant’s level of agreement was reported using a 5-point Likert scale. All NIH Toolbox Emotion measures provide T-scores, which are uncorrected standardized scores where a T-score of 50 represents the average for the US general population (based on the 2010 Census), and 10 T-score units equal one standard deviation.
To assess cognitive abilities, including executive function, cognitive flexibility, inhibition, and processing speed, we utilized the results from several tests included in the NIH Toolbox: the Picture Sequence Memory Test, List Sorting Working Memory Test, Dimensional Change Card Sort Test, Flanker Inhibitory Control and Attention Test, Oral Reading Recognition Test, Picture Vocabulary Test, and Pattern Comparison Processing Speed Test [15]. Participant scores were normed to the entire NIH Toolbox Normative Sample (ages 18 and older), regardless of age or other variables. A score of 100 represents performance at the national average, while scores of 115 and 85 correspond to performance 1 standard deviation above or below the national average, respectively.
MRI data acquisition
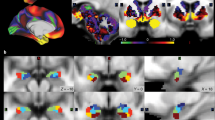
Figure 1 shows an overview of the study. The subcortical regions related to reward system (the amygdala and nucleus accumbens) were included as the regions of interest in this study (Fig. 1A). All participants were scanned on a customized Siemens 3 T Connectome Skyra with a standard 32-channel Siemens head coil at Washington University. Structural and rs-fMRI were acquired in 7 min 40 s and approximately 15 min, respectively. As this study focuses on dynamic FC, repetition time (TR) in rs-fMRI is a critical parameter, referring to the time interval between consecutive three-dimensional volume acquisitions. The TR of the obtained rs-fMRI is 720 ms and the total number of TRs is 1200. Supplementary Table 1 in the Supplementary Materials shows the details of the neuroimaging parameters. During the fMRI scanning, the participants were instructed to open eyes with relaxed fixation on a projected bright cross-hair on a dark background. This study included participants who met the quality control and assurance standards set by the HCP [16].
A Amygdala and nucleus accumbens were included as regions of interest in this study; B Specific functional networks were identified by group ICA; C Temporal variance values of the IC maps for each individual were obtained as characteristics of temporal variability; D Participants were clustered according to the temporal variability of their networks.
MRI data processing
The rs-fMRI data were preprocessed with canonical procedures using the statistical parametric mapping toolbox [17] (SPM12, http://www.fil.ion.ucl.ac.uk/spm/, Wellcome Trust Centre for Neuroimaging, London, UK) except for slice timing with considering the shortTR [18, 19]. Specifically, 14 scans corresponding to the initial 10 s were discarded for securing stable fMRI time series, and realignment was performed to correct the rigid head motions. Subsequently, the resulting fMRI data was coregistered to the gray matter map segment of individual T1-weighted structural MRI and sequentially normalized into a standard stereotactic Montreal Neurological Institutes space. Finally, the normalized fMRI data were smoothed using an isotropic 3D Gaussian kernel with a 6 mm full-width at half-maximum.
The preprocessed rs-fMRI time series were regionally averaged to the Schaefer 200 (for cortex) and the Brainnetome atlas (for subcortex). Each brain area in the Schaefer 200 was assigned to one of 17 functional networks such as language, control, and visual. Additional processing procedures included linear detrending; regressing out the effects of six rigid movements, their derivatives, and five principal components of the white matter and cerebrospinal fluid mask; spike detection and despiking with four times the median absolute deviation; and band-pass filtering (0.01 − 0.1 Hz) for estimating low-frequency fluctuation of rs-fMRI signals. Using the Pearson correlation coefficient, the functional network was calculated for the time series at two distinct regions and normalized using Fisher’s r-to-z transformation. The FC within the entire time series was used to extract the representative brain network.
Group independent component analysis to identify functional networks
Group independent component analysis (ICA) was utilized on the preprocessed functional network between emotional brain regions (the amygdala and nucleus accumbens) and the entire region to decompose the resting-state networks of the group into common spatially independent components. Specifically, this study used functional networks between the 200 regions from the Schaefer atlas and the amygdala from the Brainnetome atlas, the 200 regions and the nucleus accumbens from the Brainnetome atlas, and the amygdala and the nucleus accumbens. This method involved diminishing the redundancy of the group functional networks by performing a principal component analysis (PCA) prior to conducting the ICA [20]. Specifically, the functional network from each participant was concatenated over all subjects, and PCA decomposition was performed to reduce the dimension of the observed functional network matrix at the group level (Fig. 1B). The optimal number of principal components was determined using Laplace PCA [21]. Subsequently, a FastICA + ICASSO framework was applied. FastICA was executed 100 times on the reduced data, each time with different random initial values [22]. Following this, ICASSO was employed to perform hierarchical clustering based on the dissimilarities among the independent components (ICs) identified in each iteration [23]. Meaningful IC maps with a high reliability of over 0.8 were visually identified from the ICASSO output.
Clustering by variability of temporal dynamic functional networks
Temporal dynamic functional networks
As FC patterns exhibit substantial moment-to-moment changes over time, a sliding window framework was used to identify temporal dynamic functional networks [24]. The sliding window framework is designed to detect second-order changes by capturing FC “snapshots” from time windows of a set duration that are systematically moved across the entire fMRI time series [8]. Based on previous studies [25], a 30 s-fixed window size was set for the sliding window, which corresponded to 42 scans. Therefore, FC was calculated moving one scan at a time for every 42 scans, resulting in a total of 1,144 utilized windows. Instead of using all FC values in the typical way, networks identified through ICA were employed to extract only the weight values of these networks for each sliding window. In total, 1,144 consecutive weight values over time were calculated for each meaningful IC map by linearly projecting each windowed FC on IC basis. Furthermore, the temporal variability of each IC map was calculated to characterize the dynamic configuration of a specific IC map across all time windows. As a measure of variability, the variance of the FC values of each IC map was calculated for each person (Fig. 1C).
Clustering
Spectral clustering was used to find clusters of people through variability of temporal dynamic functional networks. Spectral clustering is capable of detecting clusters even when the k-means method fails [26]. Specifically, spectral clustering was performed on the variance of each person’s IC maps to group them together. Silhouette coefficient measures [27] and the Davies–Bouldin index [28] were used to measure clustering performance and determine the final number of clusters (Fig. 1D).
Statistical analysis
Baseline characteristics were presented as counts with proportions for categorical variables and as means with standard deviations for continuous variables. A chi-squared test was used to compare categorical variables between populations. Independent-sample t-test was used to compare continuous variables between populations. p-values < 0.05 were considered statistically significant in all analyses.
Mediation analyses were conducted using the lavaan package of R statistical software [29]. In this study, the association between clusters by temporal functional networks as a predictor variable and affective states were examined, including depression and anxiety, as an outcome variable through negative emotions as a mediator using mediation models, in which X, Y, and M each represent predictor, outcome, and mediator variables. As shown in Fig. 2, the mediation model separates the effects of various directed paths among the variables. Specifically, paths a, b, and c represent the association from X to M, association from M to Y, and direct effect of X on Y, respectively. The mediation effect from X to Y through M was estimated as the product of a and b. Moreover, we compared the differences between clusters for potential confounders such as socio-demographics (sex, family psychiatric history, employment status, alcohol use), as well as age and BMI. For any confounders that showed significant differences, we conducted mediation analyses adjusted for these confounders. The statistical significance of these paths was assessed using bootstrap tests with 10,000 samples, setting the significance level at a false discovery rate (FDR) of less than 0.15 [30]. All analyses except brain MRI scans were performed using R software version 4.1.0 (R Foundation for Statistical Computing, Vienna, Austria).
For brain MRI scans, including signal preprocessing and ICA, statistical analyses were performed with MATLAB (MathWorks, Sherborn, MA, USA) (“MATLAB - MathWorks - MATLAB &Simulink,” n.d.)-based custom software utilizing Fast-ICA and ICASSO toolbox (https://research.ics.aalto.fi/ica/fastica/ and https://research.ics.aalto.fi/ica/icasso/). The codes for this study have been released in an online repository for transparent disclosure of analysis (https://github.com/ABMI/TemporalClusteringEmotion).
Results
Demographic and clinical characteristics
Table 1 shows the demographic and clinical characteristics of the study population, with 54.2% women. The average age was 29.1 years old, with people in this dataset ranging from 22 to 37 years old. The participants’ data revealed that 19.7% and 14.3% had a paternal and maternal family history for mental illness, respectively. For the healthy participants, the psychiatric and negative affect scales averaged close to 50.
Functional networks by group independent component analysis
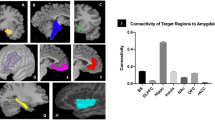
The optimal number of components determined through Laplace PCA for performing group ICA was 43. After group ICA analysis, we selected nine meaningful IC maps with a reliability of over 0.8, as calculated using ICASSO. Each IC map was named based on its corresponding brain functional connections: nucleus accumbens-related network [NAC-related network], nucleus accumbens-sensorimotor network [NAC-SM network], nucleus accumbens-cerebellum network [NAC-CRB network], nucleus accumbens-thalamus network [NAC-THL network], nucleus accumbens-basal ganglia network [NAC-BG network], amygdala-visual network [AMY-Visual network], amygdala-hippocampus network [AMY-HP network], amygdala-orbitofrontal cortex network [AMY-OFC network], and amygdala-inferior parietal lobe network [AMY-IPL network]. Figure 3 shows the specific brain regions of the nine IC maps.
Group clusters with similar temporal variability patterns
The temporal variability of the weight values across the nine IC maps was calculated for each person. Spectral clustering was performed to group similar variability patterns using this variability, which resulted in the classification of individuals into two clusters. The cluster count was automatically determined to optimize the Silhouette score and the Davies–Bouldin index.
All 1080 individuals in the HCP dataset were split into Clusters 1 and 2 with 700 and 380 persons, respectively (Supplementary Table 2). No statistical difference was observed in Cluster 1 and Cluster 2 with 55.7% and 51.3% women, respectively. Cluster 2 had an average age of 28.3 years and was significantly younger than Cluster 1 with an average age of 29.1 years. While most scores did not differ significantly between the clusters, Cluster 2 had significantly higher anger–physical aggression scores than Cluster 1. Although there were no overall differences in cognitive performance between the two clusters, Cluster 1 scored significantly higher than Cluster 2 on the Dimensional Change Card Sort Test and the Pattern Comparison Processing Speed Test. The distribution of subjects’ scores by cluster on the psychiatric scales is presented in Supplement Fig. 1.
Comparison of the weights for the IC maps showed that Cluster 2 had higher root mean squared (RMS) weight and weight variance values for all maps than Cluster 1 (Supplementary Table 3).
Mediating role of anger–physical aggression between clusters and affective states
The mediating roles of negative emotions between group clusters and affective symptoms (depressive and anxiety symptoms) were explored. The data were divided into two clusters, coding Cluster 1 as “0” and Cluster 2 as “1” for the mediation analysis. Table 2 shows the mediating role of negative emotions in the cluster–affective symptom relationship. Among negative emotions, the level of anger–physical aggression significantly mediated the positive relationships between the cluster and affective states (depressive symptoms: mediation coefficient [a × b] = 0.19, FDR = 0.07; anxiety symptoms: ab = 0.17, FDR = 0.05, respectively). Specifically, compared to Cluster 1, anger–physical aggression markedly mediated the positive relationships between Cluster 2 and symptoms of depression or anxiety. Although the indirect effect of anger-physical aggression was significant, both the direct and total effects of this emotion were not significant. Indirect, direct, and total effects were insignificant for other negative emotions. A significant difference in age was discovered between Cluster 1 and Cluster 2, so an additional mediation analysis we performed that was adjusted for age (Table 3). In this adjusted analysis, the results remained consistent with the main findings, which showed that the level of anger–physical aggression significantly mediated the relationship between cluster and depressive symptoms or anxiety symptoms (depressive symptoms: ab = 0.15, FDR = 0.14; anxiety symptoms: ab = 0.15, FDR = 0.13, respectively).
Discussion
In the present study, we utilized a temporal clustering approach to limbic activity to explore whether negative emotion mediates the relationship between temporal clusters and affective states in large HCP data. First, group-level spatial components and activation time courses were identified with respect to the amygdala and nucleus accumbens. Two distinct clusters were found in healthy adults according to the temporal variability of limbic networks. Second, anger–physical aggression was found to markedly mediate the positive relationship between temporal clusters and affective states, including depression and anxiety. Moreover, this association was independent of age. This suggests that a component of affective states to temporal variability in limbic networks is explained by the level of anger, with higher levels of anger suggesting worse affective states.
Interestingly, the nine meaningful brain maps, identified in the study, are categorized into two types of brain networks, based on the nucleus accumbens and amygdala. This raises the possibility that these two brain regions are independently involved in emotional processing, although the amygdala and nucleus accumbens markedly contribute to emotion processing in humans. Additionally, the networks involving the amygdala or nucleus accumbens can be delineated into those related to emotional information and high-order emotional processes. Four representative amygdala networks are linked to the visual brain regions, orbitofrontal cortex, hippocampus, and inferior parietal lobe. The connection between the amygdala and the visual brain areas, orbitofrontal cortex, and hippocampus, known to receive sensory input, is consistent with that the amygdala responds to emotional information such as the faces of fearful people [31]. The hippocampus and inferior parietal lobe, which are connected to the amygdala, would be involved in higher-order emotional processes, such as connecting memories to emotions and perceiving emotions in facial expressions. Meanwhile, the nucleus accumbens is linked to five brain regions. The brain networks between the nucleus accumbens and thalamus, sensorimotor, cerebellum would act in receiving sensory input and relaying sensory or emotional information. The nucleus accumbens-related, cerebellum, and basal ganglia networks would engage in higher-level emotional processing, including monitoring self-emotion and inducing emotion through rewarding process. Since the nucleus accumbens responds consistently to rewards such as money and happy facial expressions [32], it would tend to connect to brain regions that are mainly involved in advanced cognitive processes. These findings suggest that the representative brain maps, identified through ICA, capture the emotional processing within the brain. The amygdala and nucleus accumbens are particularly associated with the processing of negative emotions [33, 34]. Meanwhile, depressive and anxiety disorders are closely related to emotion regulation and are often referred to as disorders of emotion [7, 35]. Depressive and anxiety disorders are linked to the processing of negative emotions [36, 37]. Structural and functional changes in the amygdala and nucleus accumbens are linked to depressive and anxiety disorders in the emotional process [38,39,40,41,42]. Thus, given the complex and intimate relationships among limbic regions, negative emotion, depression, and anxiety disorders, this study investigated how the amygdala and nucleus accumbens contribute to affective state mediated by negative emotions in a general, non-clinical population.
A novel and key finding from our study is that the association of temporal clusters with worse affective states is partially mediated by anger levels. Notably, despite being in the healthy group, temporal brain connectivity and affective states in the participants were explained through the emotion of anger. One possible explanation is the potential for temporal variability in the limbic region to explain affective states. Temporal variability in brain regions can be used to capture emotional states [43,44,45]. For anxiety, changes in temporal variance and FC of the BOLD signal are linked to state anxiety during acute systemic inflammation [43]. For depression, differential patterns of dynamic FC variability in major depression were found across treatment course [44]. Increased variability in amygdala-medial prefrontal cortex connectivity is observed in other psychiatric disorders, such as schizophrenia [46]. Unlike previous fMRI studies, which assumed that FC remained fixed throughout the entire scan, utilization of temporal variability in this study has proven to be particularly effective in examination of depression and anxiety disorders [47, 48]. The direction of temporal variability, which was observed to worsen affective states in clusters with higher variability, corroborated previous studies [49,50,51]. Patients with depression showed higher temporal variability of fMRI than healthy controls [50]. Basolateral amygdala showed increased dynamic FC between the amygdala and the default mode network in individuals with high trait anxiety [49]. This increased variability in the amygdala occurs because individuals with anxiety might be overly sensitive and hyper-vigilant to emotion-associated stimuli [49]. The previous findings are consistent with our results, which show that increased variability is related to affective states. However, some researchers found that patients with depression disorder exhibited lower temporal variability compared to healthy individuals, and cognitive behavioral therapy (CBT) increased FC variability [44]. Demirtas et al. reported that depression patients displayed lower temporal variability than healthy controls [10]. These findings suggest potential explanations, such as the possibility that patients with certain symptoms are associated with lower FC variability. Considering that CBT is an intervention targeting distorted cognition in patients, the severity of cognitive symptoms would be related to lower FC variability in individuals with depression disorder. Another possible explanation relates to where the temporal variability is observed. Studies linking affective states to elevated temporal variability have focused on the brain FC involving limbic system, particularly the amygdala. In contrast, studies reporting decreased temporal variability in the patients investigated other brain regions within the default mode network.
Additionally, using networks identified by group ICA, rather than the anatomical locations of brain regions, provided an explanation to this. In particular, group ICA can efficiently characterize temporal changes, known as dynamic functional network connectivity [24]. Group ICA can parcellate the brain into networks, each of which has its own characteristic time course [52]. Another contributing factor is anger, which has a significant correlation with emotion dysregulation [53]. Healthy individuals experienced an increased state anxiety level due to maladaptive emotion dysregulation during the COVID-19 period [54]. Tore et al. reported that emotion regulation markedly mediates the impact of stressful life events on depressive symptoms [55]. There are several possibilities regarding whether the temporal dynamic functional connectivity (FC) of the limbic network is particularly related to the levels of anger-physical aggression. Deficient serotonin signaling has been implicated in impulsive aggression arising from weakened prefrontal inhibition of the amygdala [56]. For example, a negative association between 5-HT1A receptor binding and lifetime aggression score has been reported within the raphe, amygdala, cingulate, and prefrontal cortex [57]. Also, the amygdala and nucleus accumbens contribute to the anger and emotional regulation at the brain network level [58,59,60]. Siep et al. reported increased amygdala-paralimbic connectivity and decreased amygdala-medial prefrontal cortex connectivity in resting-state activity of violent offenders after anger provocation [58]. Denson et al. found increased connectivity between the amygdala and prefrontal cortex during efforts to control anger after an insult [59]. A significant correlation exists between the appetitive type of aggression and activation in the nucleus accumbens [60]. Studies have demonstrated association between anger and depressive disorder along with link between anger and anxiety disorder [61, 62]. Regarding the relationship between anger and depression, anger initiates depression through less adaptive coping strategies [61]. According to a self-regulatory executive function model, failure in the cognitive mechanisms of emotional self-regulation following anger leads to emotional maladjustment and the persistence of depressive symptoms. Regarding the relationship between anger and anxiety disorder, anger and anxiety disorder are functionally connected due to shared cognitive biases. For instance, high levels of anger involve similar transdiagnostic cognitive patterns, such as selective attention, which are also seen in anxiety disorders [63]. Thus, anger might have influenced affective states of healthy participants from the perspective of emotion regulation, related brain region, and psychiatric disorders.
In addition to providing the neurobiological perspective, these results offer several clinical implications. This study holds clinical significance in that it explores the brain functional networks associated with emotional processes. Emotional brain networks would be broadly categorized anatomically into the amygdala-related networks and nucleus accumbens-related networks, while functionally, they would consist of two types of emotional processes: processing emotional information and higher-order emotional processes. Furthermore, considering that cognitive symptoms in depression were linked to lower temporal variability, these findings would help build therapeutic strategies for individual with depression. For instance, psychological interventions such as relaxation or mindfulness-based therapies could be proposed for depressed patients exhibiting elevated temporal variability to promote calmness. In contrast, interventions aimed at improving cognitive symptoms might be more effective for those with decreased temporal variability. Lastly, as the associations among temporal variability, anger-physical aggression, and affective states were observed in the healthy group, this study highlights that anger could act as a trigger for depression or anxiety. Given that other emotional variables (i.e., sadness) do not significantly mediate the relationship between temporal variability and affective states, it suggests that managing anger adaptively may be critically important for maintaining mental health.
This study has certain limitations. First, our analysis was exploratory and hypothesis driven, which suggested that the actual mediating relationships could be more complex than those investigated in this study. In addition, the cross-sectional design of the analyses limits the interpretations of the causal relationships among temporal clusters, negative emotions, and affective states. Moreover, there are various possible approaches to clustering, such as normative modeling [64]. Future investigations should use a comprehensive set of variables and longitudinal design to robustly clarify the causal relationships among the variables. Second, the age range of study participants was limited to individuals in their 20 s and 30 s, all from a healthy group. This impeded the result generalization to other age ranges or different populations. Third, temporal variability might not fully reflect the nature of temporal FC. We believe that further attempts are warranted to reflect dynamic functional networks, which includes methods that learn the signal itself, such as autoencoders. Fourth, we were unable to include other regions associated with depression and anxiety, such as the hippocampus, as regions of interest. A limitation of the present study’s methodology is that increasing the number of regions leads to a larger number of networks, which complicates interpretation. Consequently, we were unable to include additional regions identified in various studies. In future research, it may be necessary to consider alternative methodological approaches to temporal variability and network analysis. Fifth, we were unable to differentiate between state and trait anger. Emotions can be classified into state and trait, and there are scales like the State-Trait Anxiety Inventory that enable such a distinction. However, our dataset did not include information for this purpose, making the analysis infeasible. Future research should address this limitation. Sixth, our FDR criterion was set at 0.15, which is less stringent than 0.05. A looser threshold for statistical significance can increase the risk of Type I errors. However, in neuroimaging research, a q-value in the range of 0.1–0.2 after false discovery rate control is generally considered acceptable for multiple comparisons [65]. Nevertheless, future studies should also incorporate analyses using more rigorous thresholds.
In conclusion, the study revealed that anger–physical aggression mediated the positive relationship between temporal clusters and affective states. The results indicate that anger may partially mediate and explain the relationships between the increased temporal variability of networks and depression and anxiety. These findings indicate that temporal variability in limbic regions might affect the emotion of anger, potentially influencing affective states. The study adds to the growing body of evidence demonstrating that the features of dynamic functional networks have clinical implications.
Data availability
All data used in our study were obtained from the HCP website (http://www.humanconnectomeproject.org/).
References
Lerner JS, Li Y, Valdesolo P, Kassam KS. Emotion and decision making. Annu Rev Psychol. 2015;66:799–823.
Schäfer JÖ, Naumann E, Holmes EA, Tuschen-Caffier B, Samson AC. Emotion regulation strategies in depressive and anxiety symptoms in youth: a meta-analytic review. J Youth Adolesc. 2017;46:261–76.
Morawetz C, Bode S, Derntl B, Heekeren HR. The effect of strategies, goals and stimulus material on the neural mechanisms of emotion regulation: A meta-analysis of fMRI studies. Neurosci Biobehav Rev. 2017;72:111–28.
Pessoa L, Adolphs R. Emotion processing and the amygdala: from a’low road’to’many roads’ of evaluating biological significance. Nat Rev Neurosci. 2010;11:773–82.
Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–87.
Floresco SB. The nucleus accumbens: an interface between cognition, emotion, and action. Annu Rev Psychol. 2015;66:25–52.
Monk CS, Klein RG, Telzer EH, Schroth EA, Mannuzza S, Moulton III, et al. Amygdala and nucleus accumbens activation to emotional facial expressions in children and adolescents at risk for major depression. Am J Psychiatry. 2008;165:90–8.
Duda M, Koutra D, Sripada C. Validating dynamicity in resting state fMRI with activation‐informed temporal segmentation. Hum Brain Mapp. 2021;42:5718–35.
Gaviria J, Rey G, Bolton T, Van De Ville D, Vuilleumier P. Dynamic functional brain networks underlying the temporal inertia of negative emotions. Neuroimage. 2021;240:118377.
Demirtaş M, Tornador C, Falcón C, López‐Solà M, Hernández‐Ribas R, Pujol J, et al. Dynamic functional connectivity reveals altered variability in functional connectivity among patients with major depressive disorder. Hum Brain Mapp. 2016;37:2918–30.
Kaiser RH, Whitfield-Gabrieli S, Dillon DG, Goer F, Beltzer M, Minkel J, et al. Dynamic resting-state functional connectivity in major depression. Neuropsychopharmacology. 2016;41:1822–30.
Van Essen DC, Smith SM, Barch DM, Behrens TE, Yacoub E, Ugurbil K, et al. The WU-Minn human connectome project: an overview. Neuroimage. 2013;80:62–79.
Achenbach TM, Rescorla L. Manual for the ASEBA adult forms & profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth …; 2003.
Salsman JM, Butt Z, Pilkonis PA, Cyranowski JM, Zill N, Hendrie HC, et al. Emotion assessment using the NIH Toolbox. Neurology. 2013;80:S76–S86.
Lin YC, Baete SH, Wang X, Boada FE. Mapping brain–behavior networks using functional and structural connectome fingerprinting in the HCP dataset. Brain Behav. 2020;10:e01647.
Marcus DS, Harms MP, Snyder AZ, Jenkinson M, Wilson JA, Glasser MF, et al. Human Connectome Project informatics: quality control, database services, and data visualization. Neuroimage. 2013;80:202–19.
Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD, Frackowiak RS. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp. 1994;2:189–210.
Glasser MF, Sotiropoulos SN, Wilson JA, Coalson TS, Fischl B, Andersson JL, et al. The minimal preprocessing pipelines for the Human Connectome Project. Neuroimage. 2013;80:105–24.
Smith SM, Beckmann CF, Andersson J, Auerbach EJ, Bijsterbosch J, Douaud G, et al. Resting-state fMRI in the human connectome project. Neuroimage. 2013;80:144–68.
Calhoun VD, Liu J, Adalı T. A review of group ICA for fMRI data and ICA for joint inference of imaging, genetic, and ERP data. Neuroimage. 2009;45:S163–S172.
Beckmann CF, Smith SM. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans Med Imaging. 2004;23:137–52.
Himberg J, Hyvärinen A, Esposito F. Validating the independent components of neuroimaging time series via clustering and visualization. Neuroimage. 2004;22:1214–22.
Murley AG, Coyle-Gilchrist I, Rouse MA, Jones PS, Li W, Wiggins J, et al. Redefining the multidimensional clinical phenotypes of frontotemporal lobar degeneration syndromes. Brain. 2020;143:1555–71.
Calhoun VD, Miller R, Pearlson G, Adalı T. The chronnectome: time-varying connectivity networks as the next frontier in fMRI data discovery. Neuron. 2014;84:262–74.
Zhuang X, Yang Z, Mishra V, Sreenivasan K, Bernick C, Cordes D. Single-scale time-dependent window-sizes in sliding-window dynamic functional connectivity analysis: a validation study. Neuroimage. 2020;220:117111.
Mirzal A. Statistical analysis of microarray data clustering using NMF, spectral clustering, Kmeans, and GMM. IEEE/ACM Trans Comput Biol Bioinform. 2020;19:1173–92.
Rousseeuw PJ. Silhouettes: a graphical aid to the interpretation and validation of cluster analysis. J Comput Appl Math. 1987;20:53–65.
Davies DL, Bouldin DW. A Cluster Separation Measure. IEEE Trans Pattern Anal Mach Intell. 1979;PAMI-1:224–7.
Rosseel Y. lavaan: an R package for structural equation modeling. J Stat Softw. 2012;48:1–36.
Lee JD, Park HJ, Park ES, Oh MK, Park B, Rha DW, et al. Motor pathway injury in patients with periventricular leucomalacia and spastic diplegia. Brain. 2011;134:1199–210.
Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. J Neurosci. 1998;18:411–8.
Reynolds SM, Berridge KC. Positive and negative motivation in nucleus accumbens shell: bivalent rostrocaudal gradients for GABA-elicited eating, taste “liking”/“disliking” reactions, place preference/avoidance, and fear. J Neurosci. 2002;22:7308–20.
Massaly N, Copits BA, Wilson-Poe AR, Hipólito L, Markovic T, Yoon HJ, et al. Pain-induced negative affect is mediated via recruitment of the nucleus accumbens kappa opioid system. Neuron. 2019;102:564–573.e566.
Dannlowski U, Kugel H, Huber F, Stuhrmann A, Redlich R, Grotegerd D, et al. Childhood maltreatment is associated with an automatic negative emotion processing bias in the amygdala. Hum Brain Mapp. 2013;34:2899–909.
Amstadter A. Emotion regulation and anxiety disorders. J Anxiety Disord. 2008;22:211–21.
Gray V, Douglas KM, Porter RJ. Emotion processing in depression and anxiety disorders in older adults: systematic review. BJPsych Open. 2021;7:e7.
Lemke H, Probst S, Warneke A, Waltemate L, Winter A, Thiel K, et al. The course of disease in major depressive disorder is associated with altered activity of the limbic system during negative emotion processing. Biol Psychiatry: Cogn Neurosci Neuroimaging. 2022;7:323–32.
Anand A, Shekhar A. Brain imaging studies in mood and anxiety disorders: special emphasis on the amygdala. Ann N Y Acad Sci. 2003;985:370–88.
Roy AK, Fudge JL, Kelly C, Perry JS, Daniele T, Carlisi C, et al. Intrinsic functional connectivity of amygdala-based networks in adolescent generalized anxiety disorder. J Am Acad Child Adolesc Psychiatry. 2013;52:290–299.e292.
Ho TC. Toward neurobiological-based treatments of depression and anxiety: A potential case for the nucleus accumbens. Elsevier; 2022;61:136-8.
Pizzagalli DA, Holmes AJ, Dillon DG, Goetz EL, Birk JL, Bogdan R, et al. Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am J Psychiatry. 2009;166:702–10.
Murray EA, Wise SP, Drevets WC. Localization of dysfunction in major depressive disorder: prefrontal cortex and amygdala. Biol Psychiatry. 2011;69:e43–e54.
Labrenz F, Ferri F, Wrede K, Forsting M, Schedlowski M, Engler H, et al. Altered temporal variance and functional connectivity of BOLD signal is associated with state anxiety during acute systemic inflammation. Neuroimage. 2019;184:916–24.
Zhou W, Yuan Z, Yingliang D, Chaoyong X, Ning Z, Chun W. Differential patterns of dynamic functional connectivity variability in major depressive disorder treated with cognitive behavioral therapy. J Affect Disord. 2021;291:322–8.
Luo Z, Chen G, Jia Y, Zhong S, Gong J, Chen F, et al. Shared and specific dynamics of brain segregation and integration in bipolar disorder and major depressive disorder: a resting-state functional magnetic resonance imaging study. J Affect Disord. 2021;280:279–86.
Yue JL, Li P, Shi L, Lin X, Sun HQ, Lu L. Enhanced temporal variability of amygdala-frontal functional connectivity in patients with schizophrenia. NeuroImage: Clin. 2018;18:527–32.
Sporns O. The human connectome: a complex network. Ann N Y Acad Sci. 2011;1224:109–25.
Horn A, Ostwald D, Reisert M, Blankenburg F. The structural–functional connectome and the default mode network of the human brain. Neuroimage. 2014;102:142–51.
Wang C, Wang Y, Lau WK, Wei X, Feng X, Zhang C, et al. Anomalous static and dynamic functional connectivity of amygdala subregions in individuals with high trait anxiety. Depress Anxiety. 2021;38:860–73.
Long Y, Cao H, Yan C, Chen X, Li L, Castellanos FX, et al. Altered resting-state dynamic functional brain networks in major depressive disorder: Findings from the REST-meta-MDD consortium. NeuroImage: Clin. 2020;26:102163.
Li W, Wang C, Lan X, Fu L, Zhang F, Ye Y, et al. Variability and concordance among indices of brain activity in major depressive disorder with suicidal ideation: A temporal dynamics resting-state fMRI analysis. J Affect Disord. 2022;319:70–78.
Calhoun VD, Sui J, Kiehl K, Turner J, Allen E, Pearlson G. Exploring the psychosis functional connectome: aberrant intrinsic networks in schizophrenia and bipolar disorder. Front Psychiatry. 2012;2:75.
McCloskey MS, New AS, Siever LJ, Goodman M, Koenigsberg HW, Flory JD, et al. Evaluation of behavioral impulsivity and aggression tasks as endophenotypes for borderline personality disorder. J Psychiatr Res. 2009;43:1036–48.
Brehl A-K, Schene A, Kohn N, Fernández G. Maladaptive emotion regulation strategies in a vulnerable population predict increased anxiety during the Covid-19 pandemic: a pseudo-prospective study. J Affect Disord Rep. 2021;4:100113.
Aune T, Hamiel D, Wolmer L. Does emotion regulation mediate the effect of stressful life events on depressive symptoms in adolescents? J Affect Disord. 2023;325:354–9.
da Cunha-Bang S, Knudsen GM. The modulatory role of serotonin on human impulsive aggression. Biol Psychiatry. 2021;90:447–57.
Parsey RV, Oquendo MA, Simpson NR, Ogden RT, Van Heertum R, Arango V, et al. Effects of sex, age, and aggressive traits in man on brain serotonin 5-HT1A receptor binding potential measured by PET using [C-11] WAY-100635. Brain Res. 2002;954:173–82.
Siep N, Tonnaer F, van de Ven V, Arntz A, Raine A, Cima M. Anger provocation increases limbic and decreases medial prefrontal cortex connectivity with the left amygdala in reactive aggressive violent offenders. Brain Imaging Behav. 2019;13:1311–23.
Denson TF, Ronay R, von Hippel W, Schira MM. Endogenous testosterone and cortisol modulate neural responses during induced anger control. Soc Neurosci. 2013;8:165–77.
Schöne M, Seidenbecher S, Kaufmann J, Antonucci LA, Frodl T, Koutsouleris N, et al. Appetitive aggression is associated with lateralized activation in nucleus accumbens. Psychiatry Res: Neuroimaging. 2022;319:111425.
Besharat MA, Nia ME, Farahani H. Anger and major depressive disorder: The mediating role of emotion regulation and anger rumination. Asian J Psychiatry. 2013;6:35–41.
Moscovitch DA, McCabe RE, Antony MM, Rocca L, Swinson RP. Anger experience and expression across the anxiety disorders. Depress Anxiety. 2008;25:107–13.
Deschênes SS, Dugas MJ, Fracalanza K, Koerner N. The role of anger in generalized anxiety disorder. Cogn Behav Ther. 2012;41:261–71.
Marquand AF, Rezek I, Buitelaar J, Beckmann CF. Understanding heterogeneity in clinical cohorts using normative models: beyond case-control studies. Biol Psychiatry. 2016;80:552–61.
Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–8.
Acknowledgements
This research was supported by a grant of the MD-Phd/Medical Scientist Training Program through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea. This work was supported by the Physician Scientist research fund of Ajou University School of Medicine. Also, this work was also supported and funded by the grant from National Research Foundation of Korea (NRF), funded by the Ministry of Science and ICT (NRF-2019R1A5A2026045).
Author information
Authors and Affiliations
Contributions
DYL: conceptualization, data curation, methodology, software, data analysis, visualization, writing-original draft, writing-review and editing, funding acquisition. SL: data curation, methodology, software, visualization, writing-original draft, writing-review and editing. SJS: data acquisition, supervision. RWP: supervision, funding support. BP: data acquisition, conceptualization, supervision, funding acquisition, writing and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval and consent informed
All participants provided their written informed consent to participate in the Human Connectome Project. And all methods used in this study were performed in accordance with the relevant guidelines and regulations. The utilization of behavioral and imaging data from the Human Connectome Project received approval from Ajou University Hospital’s Institutional Review Board (IRB) and Ethics Committee (AJIRB-BMR-EXP-21-122).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lee, D.Y., Lee, S., Son, S.J. et al. Identifying affective state via clustering of temporal variability in limbic activity and mediating role of negative emotion. Mol Psychiatry 31, 1546–1555 (2026). https://doi.org/10.1038/s41380-025-03282-9
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41380-025-03282-9