Abstract
The pituitary intermediate lobe (IL) is a surprisingly understudied pituitary region. Here, we find that chromatin-binding protein PHF6 is enriched in the pituitary IL and plays a crucial role in regulating stress susceptibility in mice. Conditional knockout of Phf6 in PHF6-positive IL cells (ILPHF6 cells) significantly reduces acute stress-induced anxiety-like behaviors and chronic stress-induced depression-like behaviors by impairing cellular activation and the release of stress-related hormones. Mechanistically, conditional knockout of Phf6 in the pituitary IL cells downregulates the calcium channel β3 subunit and suppresses transcription of stress-responsive genes. Chemogenetic inhibition of ILPHF6 cells reduces stress susceptibility, whereas activation of these cells increases anxiety-like behaviors. Circuit tracing demonstrates that ILPHF6 cells receive direct synaptic inputs from CRH-expressing neurons in the hypothalamus paraventricular nucleus (PVN), positioning ILPHF6 cells as a crucial interface between neural stress signals and endocrine output. These findings reveal a previously unknown mechanism in the regulation of stress susceptibility that operates through anxiogenic ILPHF6 cells, providing a perspective for understanding how stress susceptibility is molecularly tuned.
Similar content being viewed by others
Introduction
Stress susceptibility varies widely among individuals, shaping their resilience or vulnerability to environmental challenges [1]. The biological basis for individual differences in stress susceptibility is mediated by the interplay of neuroendocrine and neuronal activity, involving circuits such as the hypothalamic-pituitary-adrenal (HPA) axis, mesolimbic dopamine circuitry, prefrontal cortex (PFC), hippocampus, and locus coeruleus (LC), alongside cell-type-specific transcriptional and epigenetic regulation [1,2,3].
The pituitary gland plays a central role in stress regulation through hormone secretion. In contrast to the well-studied anterior and posterior lobes, the pituitary intermediate lobe (IL) is a surprisingly understudied pituitary region. The structures of the pituitary IL are highly variable across species and during different developmental stages [4]. The pituitary IL develops from the embryonic oral cavity (Rathke’s pouch) but differs from the anterior lobe in having rich innervation and poor vascularization [5,6,7]. The pituitary IL is composed of melanotrophic cells that secrete proopiomelanocortin (POMC)-derived peptides, such as α-melanocyte-stimulating hormone (α-MSH), β-endorphin and corticotropin-like intermediate lobe peptide (CLIP) [5,6,7,8,9,10]. The pituitary IL is well-developed in many mammals and in human fetuses and newborns [4, 11,12,13,14]. Evidence has shown that labor stress enhances the release of β-endorphin and α-MSH from the pituitary IL in the human fetus [15], and repeated predator threats or hypoglycemic stress increase IL secretory activity in rodents [16, 17], suggesting that the pituitary IL plays a role in regulating stress susceptibility, but this notion has never been tested.
Recent studies have highlighted genetic and epigenetic contributors to stress susceptibility [2]. Plant homeodomain finger protein 6 (PHF6) is a chromatin-binding protein that contains two plant homeodomain (PHD) fingers. Mutations in Phf6 cause Börjeson-Forssman-Lehmann (BFL) syndrome, an X-linked disorder characterized by intellectual disability, neurodevelopmental deficits, endocrine dysfunction, and mood disturbances [18,19,20]. PHF6 regulates transcription through interactions with multiple complexes, including PAF1 transcription elongation complex, the nucleosome remodeling and deacetylation (NuRD) complex, and the SWI/SNF complex [21,22,23]. Notably, BFL syndrome patients exhibit reduced anxiety scores [24, 25], and Phf6 R342X null-mutant mice display an anxiolytic phenotype [25].
In this study, we show that PHF6 is highly enriched in the pituitary IL of mice. Conditional knockout of Phf6 in pituitary IL cells significantly reduces both acute stress-induced anxiety-like behaviors and chronic stress-induced depression-like behaviors. These effects are mediated by impaired cellular activation and diminished release of stress-related hormones. PHF6-deficient IL cells exhibit decreased expression of the calcium channel β3 subunit and suppressed transcription of stress-responsive genes. Chemogenetic inhibition of ILPHF6 cells alleviates stress susceptibility, whereas activation induces anxiety-like behaviors. Circuit tracing reveals that ILPHF6 cells receive direct synaptic inputs from the paraventricular nucleus (PVN), supporting their role as an integrative node linking neural stress signals to endocrine output.
Results
PHF6 is enriched in the pituitary IL cells that respond to stress
PHF6 immunostaining revealed that PHF6 was highly enriched in the intermediate lobe (IL) of the pituitary, with no detectable expression in the anterior lobe (AL) or the posterior lobe (PL) (Fig. 1a, b). In adult C57BL/6 mice, 90.1% ± 1.7% of IL cells in males and 88.8% ± 1.9% of IL cells in females were positive for PHF6 (Fig. 1c). PHF6 expression remained stable across various developmental stages (P7, P14, P28, and P60) and did not differ between sexes (Fig. 1a). Co-immunostaining using PHF6 and POMC antibodies showed that PHF6 specifically marked pituitary IL cells, with 91.8% ± 1.9% POMC-expressing IL cells in males and 92.7% ± 1.3% POMC-expressing IL cells in females co-expressed PHF6 (Fig. 1d, e). These PHF6-expressing IL cells are hereafter referred to as ILPHF6 cells.
a Representative images of endogenous PHF6 (red) and DAPI (blue) in the pituitary of male and female wild type (WT) mice at postnatal day 7 (P7), P14, P28, and P60. AL, anterior lobe; IL, intermediate lobe; PL, posterior lobe. Scale bars, 100 μm. b Schematic diagram of the pituitary intermediate lobe (IL). c Quantification of PHF6+/DAPI+ cells in the IL of adult male and female mice (male, n = 9; female, n = 5). d Representative images showing colocalization of endogenous PHF6 (red) and endogenous POMC (green) in the IL of adult WT male mice. Scale bars, 100 μm (low magnification) and 20 μm (high magnification). e Quantification of PHF6+/POMC+ cells in the IL of adult male and female mice (male, n = 9; female, n = 5). f, i, l Experimental design for c-Fos staining following a 10-min stress exposure using one of the three paradigms: restraint stress (RS) (f), forced swim (FS) (i), or predator odor 2-methyl-2-thiazoline (2MT) (l). g, j, m Representative images of c-Fos (green) and DAPI (blue) staining in the pituitary under RS-stress (g), FS-stress (j), and 2MT-stress (m) conditions, along with their respective basal controls. Scale bars, 100 μm. h Quantification of the number of c-Fos-positive cells in the IL under basal and RS-stress conditions (Base: n = 4; RS-Stress: n = 5, **** p < 0.0001, unpaired Student’s t-test). k Quantification of the number of c-Fos-positive cells in the IL under basal and FS-stress conditions (Base: n = 3; FS-Stress: n = 4, *** p < 0.001, unpaired Student’s t-test). n Quantification of the number of c-Fos-positive cells in the IL under basal and 2MT-stress conditions (Base: n = 3; 2MT-Stress: n = 5, *** p < 0.001, unpaired Student’s t-test). Data are presented as mean ± SEM.
To investigate whether ILPHF6 cells respond to stress, we measured the induction of c-Fos expression (Δc-Fos), in the pituitary IL of adult C57BL/6 wild type male mice following various noxious stress stimuli, including restraint stress (RS), forced swim (FS), and predator odor 2-methyl-2-thiazoline (2MT) exposure (Fig. 1f, i, l). Significant increases in c-Fos-expressing cells in the pituitary IL were observed following all tested stressors compared to basal conditions (RS, p < 0.0001; FS, p < 0.001; 2MT, p < 0.001, Fig. 1f–n). Following RS stress, 88.7% ± 2.0% of ILPHF6 cells expressed c-Fos compared to 7.6% ± 2.2% at baseline (Supplementary Fig. 1a-c); Following FS stress, 90.9% ± 2.8% of ILPHF6 cells expressed c-Fos compared to 6.5% ± 0.8% at baseline (Supplementary Fig. 1d, e); Following 2MT stress, 85.8% ± 3.2% of ILPHF6 cells expressed c-Fos compared to 5.0% ± 0.8% at baseline (Supplementary Fig. 1f, g). These results demonstrate that ILPHF6 cells significantly respond to various stressors.
We next evaluated changes in Phf6 levels in response to stress. A time-course analysis of Phf6 mRNA levels by qPCR showed that Phf6 mRNA levels did not change at 15- or 30-min after FS stress compared to baseline (Supplementary Fig. 2a, b). At 60 min after FS stress, the Phf6 mRNA level decreased to 84.1% ± 3.0% of baseline, a finding that was also detected in an RNA sequencing analysis (Supplementary Fig. 2b, c). These findings suggest that Phf6 transcription show a moderate decrease following acute stress. However, no difference in Phf6 mRNA levels was observed after 14 days of chronic restraint stress, as compared to baseline (Supplementary Fig. 2d, e).
We then assessed the PHF6 protein distribution in ILPHF6 cells using immunostaining. Stress induced a redistribution of PHF6 within the nucleus of ILPHF6 cells, going from a concentrated signal in the nucleolus at baseline to a more dispersed distribution throughout the nucleus following stress (Supplementary Fig. 2f-h). To quantify this redistribution, we measured the proportion of IL cells with nucleolus-enriched PHF6 versus those with nucleus-dispersed PHF6. Following various stressors (RS, FS, and 2MT), the proportion of IL cells with nucleus-dispersed PHF6 significantly increased, while those with nucleolus-enriched PHF6 significantly decreased, as compared to basal conditions (Supplemental Fig. 2h). These findings reveal that stress triggers a dynamic PHF6 redistribution from nucleolar enrichment to a diffuse nuclear relocation in ILPHF6 cells, suggesting that PHF6 is involved in stress regulation through altered nuclear relocation rather than its expression level changes.
Conditional knockout of Phf6 in pituitary IL cells inhibits acute stress-induced anxiety-like behaviors
Given the robust activation of ILPHF6 cells by stressors, we hypothesized that PHF6 modulates stress response in these cells. To test this, we generated a conditional Phf6 knockout (Phf6 cKO) mouse line by crossing POMC-Cre mice [26] with mice harboring floxed Phf6 alleles (loxP sites flanking its exons 4 and 5 on the X chromosome, Phf6flox/flox; provided by Dr. Tim Thomas in WEHI) [27] (Fig. 2a). Immunostaining confirmed the complete knockout of PHF6 in the pituitary IL cells of Phf6 cKO mice (Fig. 2b, Supplementary Fig. 3a).
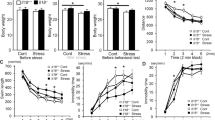
a Strategy for generating conditional Phf6 knockout (cKO) mice by crossing male POMC-Cre mice with female Phf6flox/flox mice. b Representative images of endogenous PHF6 (red) and endogenous POMC (green) in the pituitary of control and Phf6 cKO mice, confirming deletion of Phf6 in cKO mice. Scale bars, 100 μm. c Experimental design for assessing anxiety-like behaviors in control and Phf6 cKO mice under basal and stress conditions. d-f Under RS-stress conditions, Phf6 cKO mice displayed increased center area time in the OF test (d), increased open-arm time in the EPM test (e), and decreased latency to feed in the NSF test (f), compared to the control mice (Control, n = 16; Phf6 cKO, n = 14; * p < 0.05, ** p < 0.01, *** p < 0.001, two-way repeated-measures analysis of variance (ANOVA) with Bonferroni’s multiple comparisons test). Example locomotor trajectories of the Phf6 cKO and control mice in the OF test (d) and EPM test (e) under RS-stress conditions are shown, with the center area (d, dotted square) and open arms (e, dotted outline) highlighted. g-i Under FS-stress conditions, Phf6 cKO mice exhibited increased center area time in the OF (g), increased open-arm time in the EPM (h), and decreased latency to feed in the NSF test (i) relative to controls (Control, n = 9; Phf6 cKO, n = 9, * p < 0.05, ** p < 0.01, two-way repeated-measures ANOVA with Bonferroni’s multiple comparisons test). Example locomotor trajectories of the Phf6 cKO and control mice in the OF test (g) and EPM test (h) under FS-stress conditions are shown. j-l Under 2MT-stress conditions, Phf6 cKO mice demonstrated increased center area time in the OF test (j), increased open-arm time in the EPM test (k), and decreased latency to feed in the NSF test (l), compared to the control mice (Control, n = 8; Phf6 cKO, n = 11, * p < 0.05, ** p < 0.01, two-way repeated-measures ANOVA with Bonferroni’s multiple comparisons test). Example locomotor trajectories of the Phf6 cKO and control mice in the OF test (j) and EPM test (k) under 2MT-stress conditions are shown. m Experimental design of chronic restraint stress (CRS) induction and assessment of depression-like behaviors in control and Phf6 cKO mice. n Quantification of the immobility duration and latency to immobility in the FST. Compared to control mice, Phf6 cKO mice exhibited decreased immobile duration and increased latency to immobility in FST under CRS conditions (n = 11 per group; * p < 0.05, two-way repeated-measures ANOVA with Bonferroni’s multiple comparisons test). o Quantification of the sucrose preference in the SPT. Compared to control mice, Phf6 cKO mice showed increased sucrose preference in SPT under CRS conditions (n = 11 per group; * p < 0.05, two-way repeated-measures ANOVA with Bonferroni’s multiple comparisons test). Data are presented as mean ± SEM.
We evaluated the anxiety-like behaviors in adult male Phf6 cKO (POMC-Cre; Phf6flox/y) mice and littermate control mice using the open field (OF), elevated plus maze (EPM), and novelty suppressed feeding (NSF) tests under both basal (without stressors) and acute stress (RS, FS, 2MT) conditions (Fig. 2c). Under basal conditions, no differences were observed between Phf6 cKO mice and control mice (Fig. 2d–l). However, under stress conditions, Phf6 cKO mice exhibited a significant reduction in anxiety-like behaviors. Specifically, Phf6 cKO mice showed a significant increase in the center area time in the OF test (RS, p < 0.001; FS, p < 0.05; 2MT, p < 0.05, Fig. 2d, g, j), a significant increase in the open arms time in the EPM test (RS, p < 0.01; FS, p < 0.01; 2MT, p < 0.05, Fig. 2e, h, k), and a significant decrease in the latency to feed in the NSF test (RS, p < 0.05; FS, p < 0.05; 2MT, p < 0.01, Fig. 2f, i, l), compared to littermate control mice. No changes were detected in general development, locomotor activity, or the latency to feed (Supplementary Fig. 3b–k). These findings demonstrate that conditional knockout of Phf6 in pituitary IL cells reduces acute stress-induced increases in anxiety-like behaviors, indicating that PHF6 deficiency in pituitary IL cells reduces susceptibility to acute stress in rodents.
To exclude the possibility that phenotypes observed in Phf6 cKO mice resulted from deletion of Phf6 in POMC-lineage cells in the brain, we first examined whether PHF6 is expressed in any POMC-Cre-positive neurons in the brain by crossing the POMC-Cre mouse line with a reporter line expressing Cre-dependent histone-GFP (LSL-H2B-GFP). In POMC-Cre; LSL-H2B-GFP mice, we found that approximately 13.1% ± 0.3% of GFP-positive POMC-Cre neurons in the arcuate nucleus (ARC) express PHF6 (Supplementary Fig. 4a–c); none of the GFP-positive POMC-Cre neurons in other brain regions were positive for PHF6 (Supplementary Fig. 4a). In Phf6 cKO mice (POMC-Cre; Phf6flox/y), PHF6 expression remained unaffected in brain regions including the medial preoptic area (mPOA), the supraoptic nucleus (SON), the suprachiasmatic nucleus (SCN), and the ventral pallidum (VP); PHF6-expressing neurons were decreased by approximately 18% in the ARC (Supplementary Fig. 4d). To investigate whether this ~18% reduction in PHF6 expression in the ARC contributes to the anxiety-related phenotypes observed in Phf6 cKO mice, we specifically deleted Phf6 in the ARC by bilaterally injecting AAV-Cre (or AAV-GFP as control) into the ARC of Phf6flox/y mice (Supplementary Fig. 4e). We found that specific deletion of Phf6 in the ARC had no effect on anxiety-like behaviors as assessed by the open-field test and elevated plus maze test (Supplementary Fig. 4f, g). These findings exclude the possibility that the phenotypes observed in Phf6 cKO mice result from Phf6 deletion in POMC-lineage cells in the ARC.
Conditional knockout of Phf6 in pituitary IL cells reduces susceptibility to chronic stress
The persistence of long-term increase of stress-induced anxiety may lead to physiological dysfunction and maladaptive outcomes like depression [28, 29]. We further investigated whether conditional knockout of Phf6 in pituitary IL cells would affect susceptibility to chronic stress by measuring depression-like behaviors. We subjected mice to a chronic restraint stress (CRS) protocol for 14 days (2 h per day) (Fig. 2m) [30]. Depression-like behaviors were assessed using the forced swim test (FST) and the sucrose preference test (SPT), both of which evaluate the core depression symptom [31]. In basal condition, Phf6 cKO mice exhibited no difference in depression-like behaviors compared to littermate controls as measured by the FST and SPT paradigms (Fig. 2n, o). In contrast, upon CRS stress, Phf6 cKO mice exhibited a significant decrease in the immobile duration (p < 0.05) and a significant increase in the latency to immobility (p < 0.05) in the FST, as well as a significantly higher sucrose preference (p < 0.05) in the SPT, compared to the littermate controls (Fig. 2n, o). These results demonstrate that conditional knockout of Phf6 in pituitary IL cells attenuates CRS-induced depression-like behaviors, indicating PHF6 deficiency in pituitary IL cells reduces susceptibility to chronic stress in rodents.
PHF6-chromatin binding is increased in response to stress
Given that PHF6 is a chromatin-binding protein involved in gene transcription regulation [20], and that ILPHF6 cells respond to stress, we hypothesized that PHF6 may regulate stress-responsive gene transcription in these cells. We first investigated whether the recruitment of PHF6 to chromatin is triggered by stress stimulation. We performed PHF6 chromatin immunoprecipitation sequencing by the tagmentation-based epigenomic profiling method (CUT&Tag) using a validated PHF6 antibody [32] on enriched pituitary tissue from C57BL/6 wild type mice (Fig. 3a). Because PHF6 is exclusively expressed in the intermediate lobe of the pituitary, the CUT&Tag analysis reflects the genome-wide occupancy of PHF6 in ILPHF6 cells. Under both basal and RS-stress conditions, PHF6 occupancy predominantly surrounded the transcription start sites (TSSs) near the promoter regions of annotated genes (Fig. 3b, Supplementary Fig. 5a). Pairwise comparisons of PHF6 CUT&Tag analyses between basal and stress conditions revealed a distinct PHF6-chromtain binding profile in ILPHF6 cells under stress, with significantly increased PHF6 recruitment to TSSs after RS stress (Fig. 3b). We unveiled a combination of 16,203 stress-condition-specific binding sites in comparison with 329 basal-condition-specific binding sites (Fig. 3c, Supplementary Table 1), indicating that stress triggers increased genome-wide PHF6-chromatin binding near TSSs of downstream target genes in pituitary ILPHF6 cells. Particularly, following RS stress, PHF6 was recruited to the promotors of numerous activity-dependent immediate early genes (IEGs, e.g., Fos, Fosb, Fosl2, Jun, Junb, Nr4a1, Nr4a2, Egr1, and Myc) (Supplementary Fig. 5b), showing a stress-induced increase in PHF6-chromatin binding sites in activity-dependent genes.
a Schematic of CUT&Tag using an anti-PHF6 antibody on the enriched pituitary tissue dissected from 100 wild-type mice under basal conditions and 100 wild-type mice exposed to RS stress. b Heatmaps of genome-wide PHF6 binding in the pituitary IL, generated from normalized PHF6 CUT&Tag reads under basal and RS-stress conditions. PHF6 occupancy is shown within 3 kb upstream of transcription start sites (TSS) and 3 kb downstream of transcription end sites (TES). Right panels display the average PHF6 enrichment under basal and RS-stress conditions. c Pairwise comparison of PHF6 CUT&Tag under basal and RS-stress conditions revealed 2,121 overlapping, 329 basal-only, and 16,203 RS-stress-only annotated genes. d Schematic of SMART-RNA-seq performed on LCM-captured IL tissue from control and Phf6 cKO mice under basal and RS-stress conditions. Representative bright-field image of pituitary section after LCM is shown. LCM, Laser-capture Microdissection. e Volcano plot of the differentially expressed genes (DEGs) in ILPHF6 cells from Phf6 cKO mice and control mice under basal conditions (Ctrl-base, n = 6; Phf6 cKO-base, n = 6), with a pie chart indicating 21 stress-upregulated and 37 stress-downregulated genes. f GO analysis of functional categories enriched among DEGs in Phf6 cKO mice. g Genomic binding profile of PHF6 at the Cacnb3 locus under basal conditions. h Fold change in normalized SMART-RNA-seq counts of Cacnb3 under basal conditions in control (n = 6) and Phf6 cKO mice (n = 6). i Representative smFISH (RNAscope) images showing Cacnb3 mRNA (green) in the pituitary of control and Phf6 cKO mice. White dotted lines indicate the IL. Scale bars, 100 μm. j Left: Volcano plot of the DEGs in ILPHF6 cells from control mice after RS stress (Ctrl-base, n = 6; Ctrl-stress, n = 6), with a pie chart indicating 52 stress-upregulated and 14 stress-downregulated genes. Right: Volcano plot of the DEGs in ILPHF6 cells from Phf6 cKO mice after RS stress (Phf6 cKO-base, n = 6; Phf6 cKO-stress, n = 6), with a pie chart indicating 25 stress-upregulated and 26 stress-downregulated genes. k Heatmap of the quadrant-wise analysis of DEGs in control and Phf6 cKO mice under basal and RS-stress conditions. The heatmap illustrated 66 differentially expressed genes in control mice (RS-stress vs. basal), with cutoffs at FDR (adjusted p-value) < 0.05, and mean of normalized counts ≥ 50. l Fold changes in normalized counts of Fosb, Fosl2, and Nr4a2 from SMART-RNA-seq under RS-stress conditions versus basal conditions in control (n = 6) and Phf6 cKO mice (n = 6).
PHF6 regulates calcium channel β3 subunit expression in pituitary IL cells
To identify PHF6 target genes, we performed SMART-RNA sequencing (RNA-seq using Smartseq2 method) on enriched pituitary ILPHF6 cells dissected and captured by laser-capture microdissection (LCM) from Phf6 cKO and littermate control mice (Fig. 3d). Differentially expressed genes (DEGs) were identified based on a false discovery rate (FDR) adjusted p < 0.05 and mean normalized counts ≥50. Under basal conditions, we observed 21 upregulated DEGs and 37 downregulated DEGs in PHF6-deficient ILPHF6 cells (Fig. 3e, Supplementary Table 2). Gene ontology (GO) analysis of Phf6-cKO differentially expressed genes indicated enrichment in “calcium ion binding”, “gated channel activity”, etc. (Fig. 3f). We performed integrative analyses between PHF6 CUT&Tag dataset (from wild type mice) and SMART-RNA-seq dataset (from Phf6 cKO vs. control mice). We found that PHF6 directly regulates the expression of 4 Phf6 cKO-related DEGs in ILPHF6 cells because PHF6 directly bound to the promoter regions of these genes (Supplementary Table 2). Among them, Cacnb3, encoding the β3 subunit of voltage-gated calcium channels (VGCCs), was of particular interest due to its role in neuroendocrine calcium signaling, which regulates hormone secretion and cellular activity [33,34,35]. PHF6 was shown to directly bind to the Cacnb3 promoter, and Cacnb3 expression was significantly reduced in PHF6-deficient IL cells compared to the controls (p < 0.05, Fig. 3g, h). To confirm Cacnb3 downregulation at the cellular level, we performed single-molecule fluorescence in situ hybridization (smFISH) using a mouse Cacnb3 mRNA probe. We found that conditional knockout of Phf6 in pituitary IL cells significantly reduced Cacnb3 mRNA levels (Fig. 3i). These findings indicate that PHF6 regulates the expression of calcium channel β3 subunit in pituitary IL cells, suggesting that PHF6 may modulate cellular activation and hormone release via regulation of Cacnb3.
Conditional knockout of Phf6 in pituitary IL cells blunts the induction of stress-responsive genes
Given that PHF6 regulates Cacnb3 expression, which influences calcium-mediated cellular activity and activation, and that Cacnb3 has been previously implicated in anxiety-like behaviors [36], we investigated whether PHF6-deficiency-induced reduction of Cacnb3 expression accompanied a dysregulation of the downstream stress-responsive gene expression in the pituitary ILPHF6 cells. To address this, we performed gene expression profiling by SMART-RNA sequencing using enriched pituitary IL cells dissected and captured by LCM under both basal and RS-stress conditions from Phf6 cKO and littermate control mice (Fig. 3d). The pituitary IL tissue was harvested one hour after RS-stress treatment.
We conducted the quadrant-wise analyses of the DEGs among the control mice (RS-stress vs. base) and the Phf6 cKO mice (RS-stress vs. base) (Fig. 3j, k). In the littermate control mice, we identified 52 stress-upregulated genes and 14 stress-downregulated genes upon RS-stress treatment (Fig. 3j, Supplementary Table 3). In contrast, Phf6 cKO mice exhibited a marked decrease in the number of stress-responsive genes, with only 25 stress-upregulated genes identified (Fig. 3j, Supplementary Table 3). Most genes that were stress-induced in control mice displayed a diminished induction in Phf6 cKO mice (Fig. 3k). These blunted stress-induced genes in Phf6 cKO mice included several activity-dependent IEGs, such as Fosb, Fosl2, and Nr4a2, exhibiting diminished induction after stress in Phf6 cKO mice compared to controls (Fig. 3l). These results indicate that PHF6 deficiency impairs the induction of stress-responsive IEGs in ILPHF6 cells.
Conditional knockout of Phf6 in pituitary IL cells attenuates stress-induced cell activation and hormone release
Given the role of PHF6 in regulating calcium channel β3 subunit expression and stress-responsive IEGs induction, we next investigated whether PHF6 deficiency in ILPHF6 cells affects the activation of these cells in response to stress. Compared to the control mice, we found that conditional knockout of Phf6 in pituitary IL cells significantly inhibits the stress-induced expression of celluar avtivity markers c-Fos (RS, p < 0.05; FS, p < 0.01; 2MT, p < 0.01, Fig. 4a–d) and FosB (p < 0.001, Fig. 4e, f) in the pituitary IL, regardless of the stressor applied (RS, FS, or 2MT). These results indicate that PHF6 is required for full activation of pituitary IL cells under stress.
a Representative images of c-Fos (green) and DAPI (blue) staining in the pituitary under RS-, FS-, and 2MT-stress conditions, as well as basal conditions, in control and Phf6 cKO mice. White dotted lines indicate the IL. Scale bars, 100 μm. b-d Quantification of c-Fos-positive cells in the IL under RS (b), FS (c), and 2MT (d) stress conditions. Following stress exposure, Phf6 cKO mice showed a significant reduction in the number of c-Fos-positive cells in the IL compared to control mice (RS: n = 4 per group, FS: n = 3 per group under basal and n = 4 per group under stress; 2MT: n = 3 per group under basal and n = 4 per group under stress; * p < 0.05, ** p < 0.01, two-way ANOVA with Bonferroni’s multiple comparisons test). e Representative images of FosB (green) and DAPI (blue) staining in the pituitary under basal and RS-stress conditions in control and Phf6 cKO mice. White dotted lines indicate the IL. Scale bars, 100 μm. f Quantification of FosB-positive cells in the IL under RS-stress conditions. Following RS-stress exposure, Phf6 cKO mice showed a significant reduction in the number of FosB-positive cells in the IL compared to control mice (n = 4 per group, *** p < 0.001, two-way ANOVA with Bonferroni’s multiple comparisons test). g Experimental design for assessing stress-induced hormone release in control and Phf6 cKO mice. h Plasma concentrations of α-MSH, β-endorphin, and corticosterone were significantly reduced in Phf6 cKO mice compared to control mice following RS stress (Ctrl-base, n = 11; Ctrl-stress, n = 11; Phf6 cKO-base, n = 12; Phf6 cKO-stress, n = 12, * p < 0.05, two-way ANOVA with Bonferroni’s multiple comparisons test). The stress-induced increase in plasma ACTH levels was comparable in Phf6 cKO mice and control mice (Ctrl-base, n = 8; Ctrl-stress, n = 8; Phf6 cKO-base, n = 7; Phf6 cKO-stress, n = 8; p = 0.1238). Data are presented as mean ± SEM.
Given that ILPHF6 cells secrete stress-related POMC-derived IL hormones including α-MSH and β-endorphin, and that α-MSH has been shown to facilitate corticosterone release [37, 38], we investigated whether conditional knockout of Phf6 in pituitary IL cells affects stress-related hormone release. We measured the plasma levels of α-MSH, β-endorphin, ACTH and corticosterone in Phf6 cKO and littermate control mice under both basal and RS-stress conditions, using ELISA (Fig. 4g). Under basal condition, no differences in these hormone levels were observed between Phf6 cKO mice and control mice (Fig. 4h). However, following RS stress, Phf6 cKO mice exhibited a significant reduction in the extent of stress-induced increase in plasma α-MSH (p < 0.05), β-endorphin (p < 0.05), and corticosterone (p < 0.05) levels compared to the control mice (Fig. 4h). There was no difference in the extent of stress-induced increase in plasma ACTH levels between Phf6 cKO and control mice (p = 0.1238) (Fig. 4h), indicating that the anxiety-related phenotype in Phf6 cKO mice is independent of changes in ACTH levels. These results demonstrate that PHF6 deficiency in pituitary IL cells impairs the stress-induced secretion of POMC-derived IL hormones (α-MSH and β-endorphin) and corticosterone.
Inhibition of ILPHF6 cells decreases stress susceptibility
To investigate whether inhibiting ILPHF6 cell activity would decrease susceptibility to stress in mice, we generated a Phf6-CreER knock-in (KI) mouse line by inserting an IRES-CreERT2 cassette downstream of the TAG stop codon in exon 10 of the Phf6 gene (Fig. 5a). To evaluate the specificity of this Phf6-CreER driver line to label endogenous PHF6-expressing cells, we injected an adeno-associated virus (AAV) expressing Cre-dependent fluorescent protein (AAV2/9-Ef1a-DIO-mCherry) into the pituitary IL of adult Phf6-CreER mice (Fig. 5a, b). Upon tamoxifen-induced recombination, we found that 97.5% ± 0.3% of mCherry-positive cells co-expressed endogenous PHF6 (Fig. 5b, c), confirming CreER activity was restricted to PHF6-positive cells.
a Schematic illustration of the generation of Phf6-CreER knock-in (KI) mice and the experimental strategy for labeling ILPHF6 cells through stereotactic injection of AAV-DIO-mCherry into the pituitary intermediate lobe of adult male Phf6-CreER mice, followed by tamoxifen-induced recombination. b Representative images show colocalization of endogenous PHF6 (green) and mCherry (red) in the pituitary intermediate lobe. Scale bars, 100 μm. c Quantification revealed that 97.5% ± 0.3% of mCherry-positive cells co-expressed endogenous PHF6 (n = 3). d Experimental design for ILPHF6 cell inhibition and subsequent evaluation of stress-induced anxiety-like behaviors. e Left: Representative images showing expression of hM4D-mCherry (or mCherry) in the IL of Phf6-CreER mice, co-stained with c-Fos (green). Right: Quantification demonstrated significantly reduced c-Fos expression in hM4D-expressing IL cells compared to mCherry controls after RS-stress (mCherry, n = 3; hM4D, n = 3, *** p < 0.001, unpaired Student’s t-test). DAPI is shown in blue. Scale bars, 50 μm. f Representative locomotor trajectories and quantification of time spent in the center area of the OF test. Inhibition of ILPHF6 cells significantly increased the time spent in the center area of the OF test following RS stress (mCherry-saline, n = 9; hM4D-saline, n = 10; mCherry-CNO: n = 9; hM4D-CNO: n = 10; * p < 0.05, *** p < 0.001, two-way ANOVA with Bonferroni’s multiple comparisons test). g Representative locomotor trajectories and quantification of time spent in the open arms of the EPM test. Inhibition of ILPHF6 cells significantly increased the time spent in the open arms of the EPM test after RS stress (mCherry-saline, n = 9; hM4D-saline, n = 9; mCherry-CNO: n = 9; hM4D-CNO: n = 10; * p < 0.05, ** p < 0.01, two-way ANOVA with Bonferroni’s multiple comparisons test). h Quantification of the latency to feed in the NSF test. Inhibition of ILPHF6 cells significantly decreased the latency to feed in the NSF test following RS stress (mCherry-saline, n = 9; hM4D-saline, n = 10; mCherry-CNO: n = 9; hM4D-CNO: n = 10; * p < 0.05, ** p < 0.01, two-way ANOVA with Bonferroni’s multiple comparisons test. Data are presented as mean ± SEM.
We inhibited ILPHF6 cells by delivering the AAVs expressing Cre-dependent inhibitory Gi/o-coupled DREADD hM4D (AAV2/9-Ef1a-DIO-hM4D-mCherry), or AAV2/9-Ef1a-DIO-mCherry as control, into the pituitary IL of Phf6-CreER mice (Fig. 5d). Tamoxifen was administered seven days after viral injection, resulting in a specific expression of hM4D-mCherry in pituitary ILPHF6 cells (Fig. 5d, e). Administration of CNO (3 mg/kg, i.p.) 20 min before RS-stress treatment in hM4D-expressing mice effectively inhibited the stress-induced IL cell activation compared to the controls (p < 0.001, Fig. 5e). The anxiety-like behaviors were assessed using the OF, EPM, and NSF tests one hour following RS stress. Chemogenetic inhibition of hM4D-expressing ILPHF6 cells by CNO led to a significant increase in center time in the OF test compared to saline group (p < 0.001) or mCherry group (p < 0.05), a significant increase in open arm time in the EPM test compared to saline group (p < 0.01) or mCherry group (p < 0.05), and a significant decrease in latency to feed in the NSF test compared to saline group (p < 0.05) or mCherry group (p < 0.01) (Fig. 5f–h). Inhibition of ILPHF6 cells did not change the locomotor activity in either the OF test or the EPM test and had no effect on home cage food consumption after the NSF test (Supplementary Fig. 6a–c). These results demonstrate that inhibition of ILPHF6 cells significantly reduces susceptibility to stress.
Activation of ILPHF6 cells promotes anxiety-like behaviors and stress-related hormone release
To determine whether activation of ILPHF6 cells is sufficient to trigger stress-like responses, AAVs expressing Cre-dependent excitatory Gq-coupled DREADD hM3D (AAV2/9-Ef1a-DIO-hM3D-mCherry), or AAV2/9-Ef1a-DIO-mCherry as control, were injected into the pituitary IL of Phf6-CreER mice (Fig. 6a). Tamoxifen was administered seven days after viral injection, resulting in the specific expression of hM3D-mCherry in pituitary ILPHF6 cells (Fig. 6a, b). Administration of CNO (1 mg/kg, i.p.) effectively activated hM3D-expressing ILPHF6 cells, which led to increased c-Fos expression in these cells (p < 0.0001) (Fig. 6b). Chemogenetic activation of ILPHF6 cells did not alter PHF6 protein levels but increased the proportion of IL cells exhibiting nucleus-dispersed PHF6 distribution (Supplementary Fig. 6d–f), recapitulating the PHF6 redistribution pattern after stress. We found that chemogenetic activation of ILPHF6 cells, even in the absence of external stressors, resulted in increased anxiety-like behaviors, with a significant decrease in the center time in the OF test compared to saline group (p < 0.05) or mCherry group (p < 0.05), a significant decrease in the open arm time in the EPM test compared to saline group (p < 0.05) or mCherry group (p < 0.05), and a significant increase in the latency to feed in the NSF test compared to saline group (p < 0.05) or mCherry group (p < 0.05) (Fig. 6c–e). Chemogenetic activation of ILPHF6 cells did not change the locomotor activity in either the OF test or the EPM test and had no effect on home cage food consumption after the NSF test (Supplementary Fig. 6g–i). These results demonstrate that chemogenetic activation of ILPHF6 cells promotes anxiety-like behaviors.
a Experimental design for ILPHF6 cell activation and assessment of anxiety-like behaviors. b Left: Representative images showing hM3D-mChery (or mCherry) expression in the IL of Phf6-CreER mice, co-stained with c-Fos (green). Right: Quantification revealed significantly higher c-Fos expression in hM3D-expressing ILPHF6 cells compared to mCherry controls (mCherry, n = 3; hM3D, n = 3, **** p < 0.0001, unpaired Student’s t-test). DAPI is shown in blue. Scale bars, 50 μm. c Representative locomotor trajectories and quantification of time spent in the center area of the OF test. Activation of ILPHF6 cells significantly decreased the time spent in the center area of the OF test (mCherry-saline, n = 8; hM3D-saline, n = 8; mCherry-CNO, n = 10; hM3D-CNO, n = 8; * p < 0.05, two-way ANOVA with Bonferroni’s multiple comparisons test). d Representative locomotor trajectories and time spent in the open arms of the EPM test. Activation of ILPHF6 cells significantly decreased the time spent in the open arms of the EPM test (mCherry-saline, n = 8; hM3D-saline, n = 8; mCherry-CNO, n = 10; hM3D-CNO, n = 8; * p < 0.05, two-way ANOVA with Bonferroni’s multiple comparisons test). e Quantification of the latency to feed in the NSF test. Activation of ILPHF6 cells significantly increased the latency to feed in the NSF test (mCherry-saline, n = 8; hM3D-saline, n = 8; mCherry-CNO, n = 10; hM3D-CNO, n = 8; * p < 0.05, two-way ANOVA with Bonferroni’s multiple comparisons test). f Experimental design for assessing hormone release following ILPHF6 cell activation. g-i Plasma quantification showed significantly elevated α-MSH (g), β-endorphin (h), and corticosterone (i) levels in hM3D-expressing mice compared to controls. (mCherry, n = 10; hM3D, n = 8, * p < 0.05, unpaired Student’s t-test). j Schematic diagram of retrograde monosynaptic tracing using rabies virus in Phf6-CreER mice. Starter cells are labeled in yellow, as indicated by the white arrowheads. Scale bars, 100 μm. k Rabies-labeled upstream inputs to ILPHF6 cells. Scale bars, 100 μm. l Percentage of RV-traced tdTomato-positive neurons located in the paraventricular nucleus (PVN), the periventricular zone (Pe), and the arcuate nucleus (ARC) (n = 3). m Representative images of RV-traced tdTomato-positive neurons co-expressing CRH (green) in the PVN. Scale bars, 100 μm (low magnification) and 10 μm (high magnification). Data are presented as mean ± SEM.
To investigate whether activation of ILPHF6 cells would also increase secretion of stress-related hormones, we measured the plasma levels of α-MSH, β-endorphin, ACTH and corticosterone by ELISA one hour after CNO administration (Fig. 6f). Chemogenetic activation of ILPHF6 cells significantly increased plasma levels of α-MSH (p < 0.05), β-endorphin (p < 0.05), and corticosterone (p < 0.05) in hM3D-expressing mice one hour after CNO administration, compared to controls (Fig. 6g–i). Chemogenetic activation of ILPHF6 cells had no effect on plasma ACTH levels (Supplementary Fig. 6j). These findings demonstrate that activation of pituitary ILPHF6 cells, even in the absence of stressors, can mimic both behavioral and hormonal response to stress, indicating that ILPHF6 cells play an active role in shaping stress susceptibility.
ILPHF6 cells receive synaptic inputs from the PVNCRH neurons
Given the dense innervation and sparse vascularization of the pituitary IL [7, 39], we investigated whether ILPHF6 cells received direct neural inputs from stress-related brain regions. We performed monosynaptic retrograde tracing using pseudorabies virus (RV), a commonly strategy used in the central nerve system to retrogradely trace the upstream input neurons (Fig. 6j). The helper virus, AAVs expressing Cre-dependent TVA (AAV2/9-Ef1a-DIO-His-EGFP-2A-TVA) and RVG (AAV2/9-Ef1a-DIO-RVG) were first injected into the pituitary IL of Phf6-CreER mice, followed by the pseudorabies virus (RV-EnvA-ΔG-tdTomato) injected into the same site. The cells expressing both tdTomato and EGFP in the pituitary IL represented the “starter cells” in this RV-based retrograde tracing experiment (Fig. 6j). We traced a population of tdTomato-expressing neurons in the hypothalamus paraventricular nucleus (PVN) (Fig. 6k), a central regulator of stress responses [40]. We also traced tdTomato-expressing neurons in the periventricular zone (Pe) and the arcuate nucleus (ARC) (Fig. 6k, l). Using immunostaining, we found that a subset of RV-traced tdTomato-expressing neurons in the PVN were positive for corticotropin-releasing hormone (CRH) (Fig. 6m). Although tyrosine hydroxylase (TH)-expressing neurons in the hypothalamus are proposed as upstream inputs to IL cells [5, 41], we found very few RV-traced tdTomato-expressing neurons were positive for TH (Supplementary Fig. 7). These findings demonstrate that the pituitary ILPHF6 cells are embedded in the neuroendocrine stress circuitry and receive direct synaptic input from the hypothalamic PVNCRH neurons, implicating ILPHF6 cells as an important interface between neural stress signals and pituitary hormone responses.
Discussion
This study identified that PHF6 plays a crucial role in the gain control of stress susceptibility primarily through transcriptional regulation and controlling the cellular activity of ILPHF6 cells. Using Phf6 cKO (POMC-Cre; Phf6flox/y) male mice, we delete PHF6 in the pituitary IL, and we demonstrated that loss of PHF6 in ILPHF6 cells reduces acute stress-induced anxiety-like behaviors, as well as chronic stress-induced depression-like behaviors. Although a small proportion of POMC-Cre neurons in the arcuate nucleus (ARC) are positive for PHF6 and Phf6 cKO mice showed ~18% loss of PHF6 in the ARC, we have experimentally excluded the possibility that the phenotypes observed in the Phf6 cKO mice result from this partial PHF6 loss in the ARC. Using DREADD-based chemogenetic manipulation tools, we validated the positive correlation between the activity of ILPHF6 cells and the levels of stress response. Inhibition of ILPHF6 cells reduces stress susceptibility, whereas activation of these cells promotes anxiety-like behaviors. Mechanistically, using RNA-seq and CUT&Tag analyses, we demonstrated that loss of PHF6 in pituitary IL cells decreases the calcium channel β3 subunit and suppresses transcription of stress-responsive genes in the mouse pituitary IL. Using immunohistochemistry and ELISA, we demonstrated that PHF6 deficiency impairs IL cell activation and reduces the secretion of stress-related peptides in response to stress. Further, using RV-based circuit tracing, we demonstrated that ILPHF6 cells receive direct synaptic inputs from hypothalamic PVNCRH neurons, implicating ILPHF6 cells as an important interface between neural stress signals and pituitary hormone responses. Together, our study sheds light on the molecular and cellular mechanisms by which PHF6 regulates stress susceptibility in pituitary IL cells (Model shown in Supplementary Fig. 8).
The structure and physiological function of the pituitary IL have long been mysterious. It is present in some species but poorly defined in others. In humans, the IL is well-developed in newborns but becomes vestigial in adults [7, 9]. The evolutionary variance of the pituitary IL seems to be closely correlated with the animals’ demands for coping with stress. Notably, many newborn mammals exhibit hypersecretion of IL-derived hormones [4, 9, 15], suggesting a transient role in shaping early-life susceptibility to stress. The reduction or loss of the pituitary IL may be associated with changes in environmental responses or stress coping mechanisms during evolution or development. In this study, we demonstrated that the pituitary IL is significantly activated in response to stress, and this activation of IL cells is largely dependent on the transcriptional regulator PHF6. By employing DREADD-based chemogenetic tools, which are widely used in the central nervous system to manipulate neuronal activity, we selectively modulated the activity of ILPHF6 cells. We found that chemogenetic inhibition of ILPHF6 cells reduces stress susceptibility, whereas activation of these cells promotes anxiety-like behaviors. These findings demonstrate that ILPHF6 cell activity modulates stress response, supporting the notion that the pituitary IL contributes to the regulation of stress susceptibility.
PHF6 appears to have dual functions in the regulation of stress responses in the pituitary IL cells. Under basal conditions, PHF6 regulates the expression of ion channel genes such as calcium channel β3 subunit, which exerts functions in cell excitability and hormone secretion [33,34,35]. This indicates that PHF6 modulates the excitability of IL cells under non-stress conditions. Following stress exposure, although the Phf6 mRNA levels do not increase, we observed a stress-induced redistribution of PHF6 protein within the nucleus, characterized by a higher proportion of IL cells displaying a nucleus-dispersed PHF6 pattern and a lower proportion exhibiting a nucleolus-enriched PHF6 pattern. The stress-induced redistribution of the PHF6 protein from nucleolar enrichment under basal conditions to a more diffuse nuclear pattern after stress aligns with PHF6 CUT&Tag sequencing data, showing a marked increase in PHF6-chromatin binding sites across the genome upon stress. PHF6 was recruited to the promotors of numerous activity-dependent immediate early genes following stress, promoting ILPHF6 cell activation, revealing a molecular mechanism by which PHF6 modulates stress susceptibility through transcriptional and cellular activity regulation in the pituitary IL cells in response to stress. PHF6’s dual function under both basal conditions and stress conditions underscores its importance in both sustaining cellular readiness and orchestrating transcriptional responses to environmental stimuli.
Using RV-based monosynaptic retrograde tracing, we found that pituitary ILPHF6 cells receive direct synaptic inputs from the hypothalamic PVNCRH neurons, indicating ILPHF6 cells are embedded in the neuroendocrine stress circuitry and implicating ILPHF6 cells as an important interface between neural stress signals and pituitary hormone responses. In future studies, it would be interesting to know which extracellular signals from the brain and which signaling pathways within the IL cells following stress trigger the recruitment of PHF6 to chromatin to regulate stress-responsive gene transcription. It would also be interesting to determine which protein complexes PHF6 interacts with to regulate downstream gene transcription in pituitary ILPHF6 cells.
In conclusion, our findings uncover a novel role for PHF6 in regulating stress susceptibility through transcriptional regulation and controlling the cellular activity of pituitary ILPHF6 cells. This study provides new insights into the molecular and cellular mechanisms underlying BFL syndrome.
Materials and methods
Ethics statement
All experimental procedures were approved by the Institutional Animal Care and Use Committee of Shanghai Jiaotong University School of Medicine (JUMC2023-096-A). Written informed consent was not required, as no human participant was involved in this study. All methods were performed in accordance with the relevant guidelines and regulations.
Animals
The wild type C57BL/6J mice (SLAC Laboratory Animal, Shanghai) were used for immunostaining at different developmental stages and under various noxious stress conditions. To generate conditional knockout mice lacking Phf6 in pituitary IL cells, male POMC-IRES-Cre mice (C57BL/6J background; strain no. 005965, Jackson Laboratory) were crossed with female Phf6flox/flox mice (C57BL/6J background; a gift from Dr. Tim Thomas, The Walter and Eliza Hall Institute of Medical Research, Australia). The resulting male offspring included Phf6 cKO mice (POMC-Cre/+; Phf6flox/y) and littermate controls (Phf6flox/y), all on a C57BL/6J background. These mice were used for behavioral testing, immunostaining, ELISA, RNA-seq, and CUT&Tag experiments. For chemogenetic and circuit tracing experiments, Phf6-CreER mice (C57BL/6J background, generated by Cyagen Biosciences) were used. All mice were group-housed (4–5 per cage) under controlled conditions (25 °C; 12-h light/dark cycle, 7:00 a.m.−7:00 p.m.) with free access to chow and water. Adult males aged 3–4 months were used for behavioral testing.
Generation of Phf6-CreERT2 knock-in mice
Phf6-CreERT2 knock-in mice were generated in C57BL/6J mice using CRISPR/Cas9-mediated genome editing. The targeting strategy inserted an “IRES-CreERT2” cassette immediately downstream of the TAG stop codon in exon 10 of the Phf6 gene (NCBI RefSeq: NM_027642.2; Ensembl Transcript: ENSMUST00000078944) located on the X chromosome. Homology arms for the targeting vector were PCR-amplified from BAC clone RP23-133E22. The targeting vector containing the “IRES-CreERT2” cassette, along with Cas9 mRNA and gRNA targeting the mouse Phf6 gene (sequence: AGCTTAATGGAAACTAGGTATGG), were co-injected into fertilized C57BL/6J eggs. Founder pups were genotyped by PCR amplification of the targeted region, followed by Sanger sequencing to confirm correct insertion and absence of off-target mutations. Positive founders were subsequently backcrossed to the C57BL/6J strain for at least five generations before use in experiments.
Acute stress stimulation
For restraint stress (RS), mice were immobilized for 10 min in a small transparent tube (3 cm diameter × 10 cm length) equipped with air holes and a stopper to prevent turning. For the forced swim (FS) test, mice were placed individually in a cylinder (12 cm diameter × 30 cm height) filled with water (23–24 °C) and allowed to swim freely for 10 min. For predator odor exposure, mice were placed in a cage containing filter paper soaked with 20 μL of 2MT (Adamas Reagent Co., Ltd, Cat. No. 39657 A) for 10 min.
After stress exposure, mice were singly housed for one hour prior to anxiety-like behavioral testing or perfusion for immunostaining. Basal controls were undisturbed in their home cages. All stress protocols were administered between 8:00 a.m. and 10:00 a.m.
Chronic restraint stress (CRS)
Mice were placed in the same transparent tube (3 cm diameter × 10 cm length) for 2 h daily over 14 consecutive days. Following CRS, mice were subjected to the forced swim test (FST) and sucrose preference test (SPT).
Behavioral tests
Animals for behavioral tests were randomly assigned to experimental groups and data analyses were performed blinded to the genotype. Mice were habituated to testing conditions before behavioral experiments. A minimum 72-h interval was imposed between different behavioral assays in the same animal. To assess acute stress–induced anxiety-like behaviors, Phf6 cKO mice and littermate controls were randomly assigned to one of three acute stress groups (RS, FS, or 2MT). Behavioral testing—including open field (OF), elevated plus maze (EPM), and novelty suppressed feeding (NSF)—were performed under basal conditions and one hour after acute stress exposure. To evaluate chronic stress–induced depression-like behaviors, Phf6 cKO mice and littermate controls underwent CRS for 14 days, followed by the forced swim test (FST) and sucrose preference test (SPT).
Open field (OF) test
Mice were placed in one corner of an open-field arena (40 × 40 × 30 cm) and allowed to explore for 10 min. Movement trajectories were recorded and analyzed with EthoVision XT software (v3.0, Noldus). Total distance traveled was used to assess locomotor activity, and time spent in the center zone (20 × 20 cm) was used as an index of anxiety.
Elevated plus maze (EPM) test
The maze consisted of a central platform (7 × 7 cm), two open arms (30 × 7 cm, no walls), and two closed arms (30 × 7 cm with 15 cm walls). Mice were placed on the central platform, facing a closed arm and allowed to explore freely for 5 min. The apparatus was cleaned with 75% ethanol between trials. Time spent in the open arms and the total distance traveled were recorded. Time in open arms was used as an index of anxiety.
Novelty suppressed feeding (NSF) test
Mice were food-deprived for 24 h before testing. A single chow pellet was placed on filter paper (10 cm diameter) in the center of a novel open field arena (40 × 40 × 30 cm) with bedding. Each mouse was placed in a corner and allowed to explore for up to 10 min. The trial ended once the mouse first bit and consumed the pellet. Immediately afterward, the mouse was returned to its home cage, and food intake during a 5-min session was measured to control for feeding drive.
Forced swim test (FST)
Mice were placed individually in a cylinder (12 cm diameter × 30 cm height) of water (23–24 °C) for 6 min, with depth preventing tail or hindlimb support. Behavior was recorded from the side, and immobility during the last 4 min and latency to first immobility were quantified with EthoVision XT software (v3.0, Noldus). FST was performed between 8:00 a.m. and 10:00 a.m. on the day following CRS.
Sucrose preference test (SPT)
The sucrose preference index was measured as described previously [42]. Mice were individually housed and habituated to two bottles (one containing 1% sucrose solution and the other containing pure water) for two days, with the positions of the bottles switched after 24 h. After habituation, mice were water-deprived for 24 h and then exposed to one bottle of pure water and one bottle of 1% sucrose solution for 12 h during the dark phase. The positions of the bottles were switched every 6 h. Fluid consumption was determined by weighing bottles before and after testing. Sucrose preference was calculated as sucrose intake divided by total fluid intake.
Histology and immunostaining
Mice were anesthetized and perfused with saline, followed by 2% paraformaldehyde (PFA). The pituitary or brain were post-fixed in 2% PFA for 2 h at 4 °C, and then equilibrated in 30% sucrose overnight. Tissues were embedded at –20 °C, and coronal sections were cut using a cryostat microtome (CM1860, Leica): 20 μm for pituitary and 40 μm for brain. For staining, sections were rinsed three times in phosphate-buffered saline (PBS, 5 min each), then blocked for 2 h at room temperature in 10% goat serum, 0.4% Triton X-100, and 0.3% BSA. After blocking, sections were incubated with the primary antibody overnight at 4 °C. The following day, sections were washed three times with PBS (5 min each) and incubated with the secondary antibody and DAPI (1:10,000 dilution of 5 mg/mL) for 2 h at room temperature in the dark. Sections were washed three times and mounted with antifade mounting medium (SouthernBiotech, Cat. No. 0100-01). The primary antibodies used are as follows: rabbit anti-PHF6 antibody (NOVUS, NB100-68262, 1:500), mouse anti-POMC antibody (Santa Cruz, SC-373878; 1:500), rabbit anti-c-Fos antibody (Abcam, AB222699, 1:1000), rabbit anti-FosB antibody (Cell Signaling, 2251 T, 1:1000), rat anti-mCherry antibody (AB_2536611; 1:500), rabbit anti-CRH antibody (Abcam, ab272391, 1:100), mouse anti TH antibody (Millipore, MAB318, 1:500), and rat anti c-Fos antibody (Synaptic Systems, 226017, 1:1000). The secondary antibodies used are as follows: goat anti-rabbit 555 (A21428, 1:500), goat anti-mouse 488 (A11001, 1:500), goat anti-rabbit 488 (A11008, 1:500), goat anti-rat 555 (A21434, 1:500), goat anti-rat 488 (A11006, 1:500), or goat anti-mouse 633 (A21050, 1:500). Confocal images were acquired using an Olympus FV3000 and Leica SP8 X confocal system. For each staining (e.g., c-Fos, FosB, PHF6, POMC), immunopositive cells were manually counted using FIJI software. PHF6 fluorescence intensity in individual IL cells was quantified in Adobe Photoshop. Regions of interest (ROIs) corresponding to single cells were manually delineated, and the mean gray value of each ROI was obtained from the Histogram panel. Background intensity from adjacent signal-free regions was subtracted to yield net fluorescence.
PHF6 CUT&Tag and data analysis
CUT&Tag was performed on pituitaries from 100 wild-type mice under basal conditions and 100 subjected to restraint stress (RS) (6–7 weeks old). RS was induced by 10 min of restraint, followed by euthanasia 1 h later. Pituitaries were dissected in ice-cold solution (1× HBSS, 10 mM HEPES, 23 mM D-glucose), micro-dissected, and pooled. The CUT&Tag assay followed published protocols [43, 44], with modifications. Frozen tissue (~20 mg) was homogenized on ice in cold homogenization buffer using a Dounce homogenizer (10 strokes with pestle A, 20 with pestle B). The homogenate was filtered through an 80 µm mesh and centrifuged at 100 × g for 1 min. The cleared homogenate was layered on a 25/29/35% iodixanol gradient and centrifuged at 3000 × g for 20 min at 4 °C. Nuclei at the 29–35% interface were collected and washed twice in buffer (20 mM HEPES pH 7.5, 150 mM NaCl, 0.5 mM spermidine, protease inhibitor cocktail). Concanavalin A-coated magnetic beads (10 μL, Bangs Laboratories) were added and incubated for 10 min at room temperature. Bead-bound nuclei were resuspended in Dig-wash buffer (20 mM HEPES, pH 7.5, 150 mM NaCl, 0.5 mM spermidine, protease inhibitor cocktail, 0.05% Digitonin, 2 mM EDTA) and incubated overnight at 4 °C with PHF6 antibody (Sigma, HPA001023, 1:50) or IgG control antibody (Millipore, 12-370). After magnetic separation, the nuclei were incubated with goat anti-rabbit IgG (Millipore, AP132, 1:100) for 1 h at room temperature, followed by a 1-h incubation with the pA-Tn5 adapter complex. After washing with Dig-med buffer, samples were incubated in tagmentation buffer (10 mM MgCl₂ in Dig-med) at 37 °C for 1 h. Genomic DNA was purified using a phenol-chloroform-isoamyl alcohol extraction method and ethanol precipitation. Libraries were prepared following the manufacturer’s protocol, cleaned with XP beads (Beckman Counter), and sequenced on the Illumina Novaseq 6000 platform with 150 bp paired-end reads.
Adapter sequences were trimmed before mapping. Reads were aligned to the mouse reference genome (mm10) using Bowtie2. Spike-in DNA was quantified by alignment to the E. coli genome using Bowtie2 with parameters “--no-mixed --no-discordant --no-overlap --no-dovetail”. Peak calling was performed with SEACR2 in a stringent mode. BigWig files were generated from BAM files using deepTools (bamCoverage-scaleFactor), with scale factor calculated as S = 100 / (fragments mapped to the E. coli genome). Peaks were annotated with HOMER (annotatePeaks.pl). IGV (v2.14.1) was used for visualization, and transcription start site profiles were generated with deepTools (v1.5.3).
qPCR
Pituitaries were collected 15, 30, and 60 min after a 10-min forced swim (FS) stress, then snap-frozen on dry ice. For each time point, five pituitaries were pooled as one biological replicate. RNA was extracted using TRIzol (Invitrogen), followed by chloroform separation, and then purified with a RNeasy Micro Kit (Qiagen, 74104). RNA quality and concentration were assessed with Nanodrop (Thermo Fisher Scientific). For cDNA synthesis, 2 μg RNA was reverse transcribed for 1 h at 37 °C using random hexamers (5 pmol), M-MLV reverse transcriptase (200 U, Promega M1701), 2 mM dNTPs, and RNasin (20 U, Promega N2615). qPCR was performed with the FastStart Universal SYBR Green Master Mix (Roche, 4913850001). Primers: Phf6 (forward: CGAGACAGCGTAAATGTGGC; reverse: GAACACATCAGCTTTGTGCCT) and Gapdh (forward: GCCTTCCGTGTTCCTACC; reverse: CCTCAGTGTAGCCCAAGATG). Gene expression was analyzed with the 2(–ΔΔCT) method [45], normalized to Gapdh.
Laser capture microdissection (LCM)
The pituitary intermediate lobe (IL) tissue was isolated by LCM from frozen pituitary sections of Phf6 cKO mice (POMC-Cre/+; Phf6flox/y) and littermate controls (Phf6flox/y) (6–7 weeks old). For RS-stress samples, mice underwent 10 min of restraint and were euthanized 1 h later. Pituitaries were embedded in O.C.T. compound (Sakura) and sectioned at 10 μm onto the flat side of MMI MembraneSlides (Prod. No. 50102). Slides were air-dried at room temperature for 1–2 min. A sandwich was formed by placing a cover-glass slide under the stained membrane slide. LCM was performed using the CellCut laser microdissection system (CellCut System; MMI) under bright-field optics (10× objective). After laser dissection, tissue was collected with MMI IsolationCaps (Prod. No. 50208). Ten microliters of lysis buffer containing RNase inhibitor (SMART-Seq HT kit, Takara) were added to the bottoms of the IsolationCaps. The cap of the collection tube was gently closed, and the tube was inverted to ensure all the lysis buffer was in contact with the captured tissue. The tube was then incubated upside-down at room temperature for 5 min. Samples were stored at −80 °C until the preparation for SMART-RNA-seq.
SMART-RNA-seq and data analysis
IL tissues collected by LCM in 10 μL lysis buffer were subjected to RNA extraction using TRIzol reagent (Sigma). RNA quality was initially evaluated using a Nanodrop 2000 spectrophotometer (Thermo Fisher Scientific, USA) based on the A260/A280 ratio, and subsequently assessed with an Agilent 2100 Bioanalyzer (Agilent Technologies, USA). Only samples with RNA Integrity Number (RIN) >7 were selected for cDNA amplification using a SMART-Seq HT kit (Takara, USA). Libraries were subsequently prepared with a TruePrep RNA Library Prep Kit V2 for Illumina (Vazyme, China), and sequenced on an Illumina Novaseq 6000 platform, yielding an average of ~20 million 150-bp paired-end reads per sample.
Raw reads were aligned to the mouse reference genome (mm10) using Bowtie2. HTSeq (v2.0.2) was used to generate read counts of each gene. Normalization was performed with size factors using the median ratio method in DESeq2 (v1.36.0). Differentially expressed genes (DEGs) were identified with DESeq2 using a Benjamini–Hochberg-adjusted p-value (q-value) <0.05 as the cutoff. Count data were variance-stabilized using the regularized logarithm transformation and converted to z-scores for heatmap visualization. Gene ontology (GO) enrichment of DEGs was conducted using the over-representation test in clusterProfiler package (v3.10.1). Statistical significance was determined with cutoffs at FDR (adjusted p-value) <0.05, and a mean of normalized counts ≥50 for significantly expressed genes.
Single-molecule FISH (RNAscope)
Single-molecule fluorescence in situ hybridization (smFISH) was performed on fresh-frozen pituitary sections (20 μm) from 8-week-old Phf6 cKO and littermate controls using RNAScope Multiplex Reagent Kits (ACDBio, USA). Frozen sections of 20 μm thickness were cut and air-dried inside the cryostat for less than 20 min. Then, the frozen sections were fixed in ice-cold 4% paraformaldehyde (PFA) for 15 min, followed by dehydration in an ethanol series. The slices were treated with H2O2 for 10 min and washed in PBS. Protease Ⅲ was added and incubated for 20 min at room temperature. Hybridization with the Cacnb3 probe was performed in a HybEZ humidified incubator (40 °C, 2.5 h). After washing in ACD buffer, slices were sequentially incubated with AMP1-FL and AMP2-FL (30 min each), AMP3-FL (15 min), and counterstained with DAPI. Images were acquired using an Olympus FV3000 confocal microscope.
ELISA
Mice were euthanized by decapitation and blood was collected in EDTA-coated EP tubes. Samples were immediately centrifuged at 10,000 rpm for 10 min at 4 °C, and the resulting supernatants (plasma) were collected, aliquoted, and stored at −80 °C. ELISA kits were used to quantify α-MSH (EK-043-01, Phoenix Pharmaceuticals, USA), β-endorphin (EK-022-06, Phoenix Pharmaceuticals, USA), corticosterone (AR-E-8100, LDN, Germany), and ACTH (E-EL-M0079, Elabscience, China). Assays were performed according to the manufacturer’s protocols. Briefly, 50 μL of sample/standard, 25 μL of primary antibody, and 25 μL of biotinylated peptide were added per well and incubated at room temperature for 2 h. After washes, 100 μL of streptavidin–horseradish peroxidase was added and incubated for 1 h. Following additional washes, 100 μL of tetramethylbenzidine (TMB) substrate solution was added, and the plate was incubated in the dark for color development. The reaction was stopped with 100 μL 2 N HCl, and absorbance was measured at 450 nm using a BioTek Synergy HTX microplate reader (Agilent Technologies, USA). Hormone concentrations were determined using standard curves fitted with a four-parameter logistic regression model.
Stereotaxic viral injection
Mice were anesthetized with pentobarbital sodium (100 mg/kg, i.p.), and body temperature was maintained using a feedback-controlled heating system (RWD Life Science). After scalp incision, a small cranial window was drilled for viral delivery. Injections were performed at 25 nL/min with a quintessential stereotaxic injector (Stoelting Co.), and pipettes were left in place for 10 min before withdrawal. Postoperative analgesia was provided with ketoprofen (5 mg/kg, i.p.). Mice were allowed to recover for at least three weeks before behavioral assays to ensure full recovery and sufficient viral expression. The injection sites were carefully examined after behavioral tests, and only animals with correct injections were used for analysis. The following viral vectors and titers were used: AAV2/9-Ef1a-DIO-hM4D(Gi)-mCherry-WPREs-pA (5.18E + 12 v.g./mL); AAV2/9-Ef1a-DIO-mCherry-WPRE-pA (2.55E + 12 v.g./mL); AAV2/9-Ef1a-DIO-hM3D(Gq)-mCherry (6.91E + 12 v.g./mL); AAV2/9-Ef1a-DIO-RVG-WPRE-pA (2.00E + 12 v.g./mL); AAV2/9-Ef1a-DIO-His-EGFP-2A-TVA-WPRE-pA (2.00E + 12 v.g./mL); RV-EnvA-ΔG-tdTomato (7.00E + 08 IFU/mL); AAV-CMVbGl-eGFP (3.40E + 12 v.g./mL); AAV-CMVbGl-Cre-eGFP (4.15E + 12 v.g./mL). Viral delivery to the pituitary gland was performed using stereotaxic coordinates relative to bregma: anteroposterior (AP), −2.5 mm; mediolateral (ML), ± 0.15 to 0.25 mm; dorsoventral (DV), −6.15 to −6.25 mm. Virus bilaterally injections into the ARC used the following stereotaxic coordinates relative to bregma: AP, −1.5 mm; ML, ± 0.25 mm; DV, −5.85 mm.
Chemogenetic inhibition
To inhibit ILPHF6 cells, 200 nL of AAV2/9-Ef1a-DIO-hM4D(Gi)-mCherry-WPREs-pA (5.18E + 12 v.g./mL) or AAV2/9-Ef1a-DIO-mCherry-WPRE-pA (2.55E + 12 v.g./mL) was bilaterally injected into the pituitary IL of adult male Phf6-CreER mice (2–3 months old) at the following coordinates relative to bregma: AP, −2.5 mm; ML, ± 0.15 to 0.25 mm; DV, −6.15 to −6.25 mm. Postoperative analgesia (ketoprofen, 5 mg/kg, i.p.) was administered. On day 7, mice were intraperitoneally injected with tamoxifen (100 mg/kg, Sigma, Cat. NO. T5648) dissolved in corn oil (ABCONE) at a concentration of 20 mg/mL. After three weeks of recovery and AAV expression, mice were randomized into four groups: hM4D + saline, hM4D + clozapine N-oxide (CNO), mCherry + saline, and mCherry + CNO. CNO (3 mg/kg, Sigma, Cat. No. C0832) or saline was administered intraperitoneally 20 min before a 10-min body restraint stress. Behavioral assays were conducted one hour after acute restraint stress.
Chemogenetic activation
For activation of ILPHF6 cells, 200 nL of rAAV-Ef1a-DIO-hM3D(Gq)-mCherry-WPRE-pA (6.91E + 12 v.g./mL) or AAV2/9-Ef1a-DIO-mCherry-WPRE-pA (2.55E + 12 v.g./mL) was bilaterally injected into the pituitary IL of adult male Phf6-CreER mice (2–3 months old) at the same stereotaxic coordinates as above. Ketoprofen (5 mg/kg, i.p.) was given for postoperative analgesia. On day 7, mice received tamoxifen (100 mg/kg, i.p.) in corn oil (20 mg/mL). After three weeks of recovery and AAV expression, mice were randomly assigned to four groups: hM3D + saline, hM3D + CNO, mCherry + saline, and mCherry + CNO. CNO (1 mg/kg, Sigma, Cat. No. C0832) or saline was administered intraperitoneally 1 h before behavioral testing. For hormone measurements, both hM3D- and mCherry-expressing mice received CNO (1 mg/kg, i.p.) and were euthanized by decapitation. Blood was collected immediately for plasma preparation and ELISA.
Monosynaptic retrograde tracing
To map upstream inputs to pituitary ILPHF6 cells, we stereotactically co-injected AAV2/9-Ef1a-DIO-RVG-WPRE-pA (2.00E + 12 v.g./mL) and AAV2/9-Ef1a-DIO-His-EGFP-2A-TVA-WPRE-pA (2.00E + 12 v.g./mL) at a 1:1 volume ratio into the pituitary IL of adult Phf6-CreER mice (300 nL). Postoperative analgesia was provided with ketoprofen (5 mg/kg, i.p.). On day 7, tamoxifen was administered to induce Cre-dependent expression of TVA and RVG specifically in ILPHF6 cells. After three weeks of recovery and AAV expression, 200 nL of RV-EnvA-ΔG-tdTomato (7.00E + 08 IFU/mL) was injected into the pituitary IL under biosafety level 2 conditions. Mice were sacrificed one week later, and pituitary and brain tissues were fixed and sectioned. We obtained images of the pituitary and brain sections using an Olympus FV3000 confocal microscope.
AAV-mediated Phf6 deletion
To selectively delete Phf6 in the arcuate nucleus (ARC) of the hypothalamus, 400 nL of AAV-CMVbGl-Cre-eGFP or control AAV-CMVbGl-eGFP was bilaterally injected into the ARC of Phf6flox/y mice (coordinates: AP, −1.5 mm; ML, ± 0.25 mm; DV, −5.85 mm). Three weeks after virus injection, mice were subjected to behavioral experiments. Post hoc PHF6 immunostaining was performed to confirm and quantify AAV-Cre–mediated Phf6 deletion.
Statistical analysis
Statistical analyses were performed using GraphPad Prism 9, IBM SPSS Statistics (v26), or R (v4.2.1). Data are expressed as mean ± SEM unless otherwise indicated. The data were analyzed using unpaired Student’s t-test in Figs. 1h, k, n, 5e, 6b, g–i, and Supplementary Fig. 1c, 1e, 1g, 2e, 4d, 4f, 4g, 6e, 6f, 6j. Two-way repeated-measures ANOVA with the Bonferroni multiple comparisons test in Figs. 2d–l, n, o, and Supplementary Fig. 3c–k. Two-way ANOVA with the Bonferroni’s multiple comparisons test in Figs. 4b–d, f, h, 5f–h, 6c–e, and Supplementary Fig. 3b, 6a–c, 6g–i. One-way ANOVA with the Bonferroni’s multiple comparisons test in Supplementary Fig. 2b, 2g, 2h. SMART-RNA-seq cutoffs: FDR (adjusted p-value) < 0.05 and mean normalized counts ≥50. CUT&Tag cutoffs: q-value (BH-adjusted p-value) <0.05. Significance levels are indicated as * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001; n.s., not significant.
Data availability
The RNA-seq and CUT&Tag datasets have been deposited in NCBI’s Gene Expression Omnibus (GEO) under the accession codes GSE270456 and GSE270455 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE270456;https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE270455). All the original data for Figures in this paper are available at Mendeley Data (https://doi.org/10.17632/vrmzycxbvb.1). Additional data are available from the corresponding author on reasonable request.
References
Franklin TB, Saab BJ, Mansuy IM. Neural mechanisms of stress resilience and vulnerability. Neuron. 2012;75:747–61.
Nestler EJ, Russo SJ. Neurobiological basis of stress resilience. Neuron. 2024;112:1911–29.
Willmore L, Cameron C, Yang J, Witten IB, Falkner AL. Behavioural and dopaminergic signatures of resilience. Nature. 2022;611:124–32.
Murakami M, Yoshida T, Nakayama Y, Hashimoto J, Hirata S. The fine structure of the pars intermedia of the pituitary in human fetus. Arch Histol Jpn. 1968;30:61–73.
Goudreau JL, Lindley SE, Lookingland KJ, Moore KE. Evidence that hypothalamic periventricular dopamine neurons innervate the intermediate lobe of the rat pituitary. Neuroendocrinology. 1992;56:100–5.
Stoeckel ME, Schmitt G, Porte A. Fine structure and cytochemistry of the mammalian pars intermedia. Ciba Found Symp. 1981;81:101–27.
Saland LC. The mammalian pituitary intermediate lobe: an update on innervation and regulation. Brain Res Bull. 2001;54:587–93.
Low MJ. Neural Control of the Intermediate Lobe of the Pituitary Gland (Pars Intermedia) and Proopiomelanocortin, 2012.
Takeuchi M. The mammalian pars intermedia—structure and function. Zoological science. 2001;18:133–44.
Cawley NX, Li Z, Loh YP. 60 YEARS OF POMC: Biosynthesis, trafficking, and secretion of pro-opiomelanocortin-derived peptides. J Mol Endocrinol. 2016;56:T77–97.
Geiling EMK, Vos BJ Jr, Oldham FK. The pharmacology and anatomy of the hypophysis of the porpoise1¹. Endocrinology. 1940;27:309–16.
Wislocki GB. The topography of the hypophysis in the elephant, manatee and hyrax. The Anatomical Record. 1940;77:427–45.
Plaut A. Investigations on the pars intermedia of the hypophysis in anthropoid apes and man. J Anat. 1936;70:242–9.
Mauri A, Volpe A, Martellotta MC, Barra V, Piu U, Angioni G, et al. alpha-Melanocyte-stimulating hormone during human perinatal life. J Clin Endocrinol Metab. 1993;77:113–7.
Facchinetti F, Lanzani A, Genazzani AR. Fetal intermediate lobe is stimulated by parturition. Am J Obstet Gynecol. 1989;161:1267–70.
Blasco J, Martinez C, Gomez E, Sarrat R. Electron microscopic studies on the pars intermedia of the rat under stress. Histol Histopathol. 1987;2:383–9.
Takeuchi M, Takahashi S. Effect of hypoglycemic stress on the pars intermedia of the mouse pituitary gland: an ultrastructural analysis. Zoolog Sci. 1995;12:99–103.
Birrell G, Lampe A, Richmond S, Bruce SN, Gecz J, Lower K, et al. Borjeson-Forssman-Lehmann syndrome and multiple pituitary hormone deficiency. J Pediatr Endocrinol Metab. 2003;16:1295–300.
Voss AK, Gamble R, Collin C, Shoubridge C, Corbett M, Gecz J, et al. Protein and gene expression analysis of Phf6, the gene mutated in the Borjeson-Forssman-Lehmann Syndrome of intellectual disability and obesity. Gene Expr Patterns. 2007;7:858–71.
Jahani-Asl A, Cheng C, Zhang C, Bonni A. Pathogenesis of Borjeson-Forssman-Lehmann syndrome: Insights from PHF6 function. Neurobiol Dis. 2016;96:227–35.
Zhang C, Mejia LA, Huang J, Valnegri P, Bennett EJ, Anckar J, et al. The X-linked intellectual disability protein PHF6 associates with the PAF1 complex and regulates neuronal migration in the mammalian brain. Neuron. 2013;78:986–93.
Todd MA, Picketts DJ. PHF6 interacts with the nucleosome remodeling and deacetylation (NuRD) complex. J Proteome Res. 2012;11:4326–37.
Mittal P, Myers JA, Carter RD, Radko-Juettner S, Malone HA, Rosikiewicz W, et al. PHF6 cooperates with SWI/SNF complexes to facilitate transcriptional progression. Nat Commun. 2024;15:7303.
Visootsak J, Rosner B, Dykens E, Schwartz C, Hahn K, White SM, et al. Clinical and behavioral features of patients with Borjeson-Forssman-Lehmann syndrome with mutations in PHF6. J Pediatr. 2004;145:819–25.
Ahmed R, Sarwar S, Hu J, Cardin V, Qiu LR, Zapata G, et al. Transgenic mice with an R342X mutation in Phf6 display clinical features of Borjeson-Forssman-Lehmann Syndrome. Hum Mol Genet. 2021;30:575–94.
Balthasar N, Coppari R, McMinn J, Liu SM, Lee CE, Tang V, et al. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron. 2004;42:983–91.
McRae HM, Garnham AL, Hu Y, Witkowski MT, Corbett MA, Dixon MP, et al. PHF6 regulates hematopoietic stem and progenitor cells and its loss synergizes with expression of TLX3 to cause leukemia. Blood. 2019;133:1729–41.
Fenster RJ, Lebois LAM, Ressler KJ, Suh J. Brain circuit dysfunction in post-traumatic stress disorder: from mouse to man. Nat Rev Neurosci. 2018;19:535–51.
Merikangas KR, Zhang H, Avenevoli S, Acharyya S, Neuenschwander M, Angst J, et al. Longitudinal trajectories of depression and anxiety in a prospective community study: the Zurich Cohort Study. Arch Gen Psychiatry. 2003;60:993–1000.
Ma S, Chen M, Jiang Y, Xiang X, Wang S, Wu Z, et al. Sustained antidepressant effect of ketamine through NMDAR trapping in the LHb. Nature. 2023;622:802–9.
Yuan Z, Qi Z, Wang R, Cui Y, An S, Wu G, et al. A corticoamygdalar pathway controls reward devaluation and depression using dynamic inhibition code. Neuron. 2023;111:3837–53.e3835.
Gan L, Sun J, Yang S, Zhang X, Chen W, Sun Y, et al. Chromatin-Binding Protein PHF6 Regulates Activity-Dependent Transcriptional Networks to Promote Hunger Response. Cell Rep. 2020;30:3717–28.e3716.
Becker A, Wardas B, Salah H, Amini M, Fecher-Trost C, Sen Q, et al. Cavbeta3 Regulates Ca(2+) Signaling and Insulin Expression in Pancreatic beta-Cells in a Cell-Autonomous Manner. Diabetes. 2021;70:2532–44.
Li L, Cao XH, Chen SR, Han HD, Lopez-Berestein G, Sood AK, et al. Up-regulation of Cavbeta3 subunit in primary sensory neurons increases voltage-activated Ca2+ channel activity and nociceptive input in neuropathic pain. J Biol Chem. 2012;287:6002–13.
Lee K, Kim J, Kohler M, Yu J, Shi Y, Yang SN, et al. Blocking Ca(2+) channel beta(3) subunit reverses diabetes. Cell Rep. 2018;24:922–34.
Murakami M, Nakagawasai O, Yanai K, Nunoki K, Tan-No K, Tadano T, et al. Modified behavioral characteristics following ablation of the voltage-dependent calcium channel beta3 subunit. Brain Res. 2007;1160:102–12.
Vinson GP, Whitehouse BJ, Bateman A, Hruby VJ, Sawyer TK, Darman PS. alpha-MSH analogues and adrenal zona glomerulosa function. Life Sci. 1984;35:603–10.
Henville KL, Hinson JP, Vinson GP, Laird SM. Actions of desacetyl-alpha-melanocyte-stimulating hormone on human adrenocortical cells. J Endocrinol. 1989;121:579–83.
Lamacz M, Tonon MC, Louiset E, Cazin L, Vaudry H. Le lobe intermédiaire de l’hypophyse, modèle de communication neuroendocrinienne. Archives Internationales de Physiologie, de Biochimie et de Biophysique. 1991;99:205–19.
Rasiah NP, Loewen SP, Bains JS. Windows into stress: a glimpse at emerging roles for CRH(PVN) neurons. Physiol Rev. 2023;103:1667–91.
Luppi PH, Sakai K, Salvert D, Berod A, Jouvet M. Periventricular dopaminergic neurons terminating in the neuro-intermediate lobe of the cat hypophysis. J Comp Neurol. 1986;244:204–12.
Liu MY, Yin CY, Zhu LJ, Zhu XH, Xu C, Luo CX, et al. Sucrose preference test for measurement of stress-induced anhedonia in mice. Nat Protoc. 2018;13:1686–98.
Kaya-Okur HS, Wu SJ, Codomo CA, Pledger ES, Bryson TD, Henikoff JG, et al. CUT&Tag for efficient epigenomic profiling of small samples and single cells. Nat Commun. 2019;10:1930.
Corces MR, Trevino AE, Hamilton EG, Greenside PG, Sinnott-Armstrong NA, Vesuna S, et al. An improved ATAC-seq protocol reduces background and enables interrogation of frozen tissues. Nat Methods. 2017;14:959–62.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8.
Acknowledgements
We thank Dr. Tim Thomas in WEHI and Dr. Jozef Gécz in the University of Adelaide for providing the Phf6 floxed mice. This work was supported by grants from the National High-level Young Talents Program for J.H. (Q2023108); the program for Professor of Special Appointment-Eastern Scholar Tracking Plan for J.H. (GZ20220008); the Ministry of Science and Technology of the People’s Republic of China (2021ZD0202800/01); the Natural Science Foundation of China (32070957, 81771215, 32200820 and 32300838); and the innovative research teams of high-level local universities in Shanghai (SHSMU-ZDCX20211100).
Author information
Authors and Affiliations
Contributions
BL and JJW conducted most of the experiments and data analyses. LHG, XHW and JJS worked for immunostaining and behavioral tests. GZW and QL assisted in the data analyses. JH designed and supervised the project. JH and BL wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, B., Wang, J., Wu, X. et al. Regulation of stress susceptibility by chromatin-binding protein PHF6 in the pituitary intermediate lobe. Mol Psychiatry 31, 1665–1680 (2026). https://doi.org/10.1038/s41380-025-03300-w
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41380-025-03300-w