Abstract
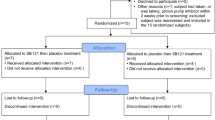
JNJ-42165279, a highly selective and orally bioavailable fatty acid amide (FAA) hydrolase inhibitor, was evaluated for efficacy and safety in adolescents and adults with autism spectrum disorder (ASD) in this phase 2, double-blind, placebo-controlled, multicenter study (NCT03664232). Participants aged 13–35 years, with a diagnosis of ASD (Diagnostic and Statistical Manual of Mental Disorders, 5th edition; Autism Diagnostic Observation Schedule, 2nd edition) were randomized (1:1) to 12 weeks of treatment with JNJ-42165279 (25 mg, twice-daily) or placebo. Primary endpoints were the change in the Autism Behavior Inventory (ABI) Core Domain (ABI-CD), ABI-Social Communication (ABI-SC), and ABI-Repetitive/Restrictive Behavior (ABI-RB) scores from baseline to day 85. Of the 61 participants (16 female, 45 male) included in the efficacy analyses, 53 (87%) completed the double-blind treatment. At day 85, the JNJ-42165279 group did not show a statistically significant reduction in ASD symptoms versus placebo, as assessed with ABI-CD (p = 0.284), ABI-SC (p = 0.290), and ABI-RB (p = 0.231). However, the following secondary outcomes exhibited small to moderate changes directionally favoring JNJ-42165279: Social Responsiveness Scale 2 (SRS, p = 0.064), Repetitive Behavior Scale-Revised (RBS-R, p = 0.006), Zarit Burden Interview short version (ZBI, p = 0.063), Child Adolescent Symptom Inventory-Anxiety (CASI-Anx, p = 0.048), and Caregiver Global Impression of Severity (p = 0.075). Notably, versus placebo, JNJ-42165279-treated participants showed increased concentrations of FAAs throughout the treatment period, with those achieving elevated concentrations experiencing the greatest reduction in the SRS total score at day 85. JNJ-42165279 demonstrated an acceptable safety profile. Although primary endpoints were not met, JNJ-42165279 may have a therapeutic effect on certain aspects of core ASD symptoms.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files. The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. As noted on this site, requests for access to the study data can be submitted through Yale Open Data Access [YODA] Project site at http://yoda.yale.edu.
Change history
31 January 2025
A Correction to this paper has been published: https://doi.org/10.1038/s41386-025-02056-9
References
American Psychiatric Association D, Association AP. Diagnostic and statistical manual of mental disorders: DSM-5. American Psychiatric Association Washington, DC; 2013.
Baxter AJ, Brugha TS, Erskine HE, Scheurer RW, Vos T, Scott JG. The epidemiology and global burden of autism spectrum disorders. Psychol Med. 2015;45:601–13.
Data & Statistics on Autism Spectrum Disorder. January 25, 2024. Avaialble at: https://www.cdc.gov/autism/data-research/index.html. Accessed 10 April, 2024.
Lamy M, Pedapati EV, Dominick KL, Wink LK, Erickson CA. Recent advances in the pharmacological management of behavioral disturbances associated with autism spectrum disorder in children and adolescents. Paediatr Drugs. 2020;22:473–83.d.
Lu H-C, Mackie K. An introduction to the endogenous cannabinoid system. Biol Psychiatry. 2016;79:516–25.
Devane WA, Hanuš L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–49.
Solinas M, Goldberg SR, Piomelli D. The endocannabinoid system in brain reward processes. Br J Pharmacol. 2008;154:369–83.
Fusar-Poli L, Cavone V, Tinacci S, Concas I, Petralia A, Signorelli MS, et al. Cannabinoids for people with ASD: a systematic review of published and ongoing studies. Brain Sci. 2020;10:572.
Pietropaolo S, Bellocchio L, Bouzon-Arnaiz I, Yee BK. The role of the endocannabinoid system in autism spectrum disorders: Evidence from mouse studies. Prog Mol Biol Transl Sci. 2020;173:183–208.
Aran A, Eylon M, Harel M, Polianski L, Nemirovski A, Tepper S, et al. Lower circulating endocannabinoid levels in children with autism spectrum disorder. Mol Autism. 2019;10:2.
Kerr DM, Downey L, Conboy M, Finn DP, Roche M. Alterations in the endocannabinoid system in the rat valproic acid model of autism. Behav Brain Res. 2013;249:124–32.
Trezza V, Vanderschuren LJ. Bidirectional cannabinoid modulation of social behavior in adolescent rats. Psychopharmacology (Berl). 2008;197:217–27.
Wei D, Dinh D, Lee D, Li D, Anguren A, Moreno-Sanz G, et al. Enhancement of anandamide-mediated endocannabinoid signaling corrects autism-related social impairment. Cannabis Cannabinoid Res. 2016;1:81–89.
Keith JM, Jones WM, Tichenor M, Liu J, Seierstad M, Palmer JA, et al. Preclinical characterization of the FAAH inhibitor JNJ-42165279. ACS Med Chem Lett. 2015;6:1204–8.
Schmidt ME, Liebowitz MR, Stein MB, Grunfeld J, Van Hove I, Simmons WK, et al. The effects of inhibition of fatty acid amide hydrolase (FAAH) by JNJ-42165279 in social anxiety disorder: a double-blind, randomized, placebo-controlled proof-of-concept study. Neuropsychopharmacology. 2021;46:1004–10.
Hus V, Gotham K, Lord C. Standardizing ADOS domain scores: separating severity of social affect and restricted and repetitive behaviors. J Autism Dev Disord. 2014;44:2400–12.
Gray SAO Kaufman Brief Intelligence Test. In: Volkmar FR, editor Encyclopedia of Autism Spectrum Disorders. New York, NY: Springer New York; 2013. p. 1673–75.
Pandina G, Ness S, Trudeau J, Stringer S, Knoble N, Lenderking WR, et al. Qualitative evaluation of the Autism Behavior Inventory: use of cognitive interviewing to establish validity of a caregiver report scale for autism spectrum disorder. Health Qual Life Outcomes. 2021;19:26.
Bangerter A, Ness S, Lewin D, Aman MG, Esbensen AJ, Goodwin MS, et al. Clinical validation of the autism behavior inventory: caregiver-rated assessment of core and associated symptoms of autism spectrum disorder. J Autism Dev Disord. 2020;50:2090–101.
Bangerter A, Ness S, Aman MG, Esbensen AJ, Goodwin MS, Dawson G, et al. Autism behavior inventory: a novel tool for assessing core and associated symptoms of autism spectrum disorder. J Child Adolesc Psychopharmacol. 2017;27:814–22.
Bangerter A, Manyakov NV, Lewin D, Boice M, Skalkin A, Jagannatha S, et al. Caregiver daily reporting of symptoms in autism spectrum disorder: observational study using web and mobile apps. JMIR Ment Health. 2019;6:e11365.
Busner J, Targum SD. The clinical global impressions scale: applying a research tool in clinical practice. Psychiatry. 2007;4:28–37.
Guy W. Clinical global impression. Assessment manual for Psychopharmacology. 1976:217–22.
Constantino JN, Gruber CP. Social responsiveness scale: SRS-2. 2012.
Kaat AJ, Lecavalier L, Aman MG. Validity of the aberrant behavior checklist in children with autism spectrum disorder. J Autism Dev Disord. 2014;44:1103–16.
Bodfish JW, Symons FJ, Parker DE, Lewis MH. Varieties of repetitive behavior in autism: comparisons to mental retardation. J Autism Dev Disord. 2000;30:237–43.
Scahill L, Aman MG, Lecavalier L, Halladay AK, Bishop SL, Bodfish JW, et al. Measuring repetitive behaviors as a treatment endpoint in youth with autism spectrum disorder. Autism. 2015;19:38–52.
Zarit SH, Reever KE, Bach-Peterson J. Relatives of the impaired elderly: correlates of feelings of burden. Gerontologist. 1980;20:649–55.
Lecavalier L, Wood JJ, Halladay AK, Jones NE, Aman MG, Cook EH, et al. Measuring anxiety as a treatment endpoint in youth with autism spectrum disorder. J Autism Dev Disord. 2014;44:1128–43.
Virues-Ortega J. Applied behavior analytic intervention for autism in early childhood: meta-analysis, meta-regression and dose-response meta-analysis of multiple outcomes. Clin Psychol Rev. 2010;30:387–99.
Brugha TS, Doos L, Tempier A, Einfeld S, Howlin P. Outcome measures in intervention trials for adults with autism spectrum disorders; a systematic review of assessments of core autism features and associated emotional and behavioural problems. Int J Methods Psychiatric Res. 2015;24:99–115.
Meyer, M (2022, May) Development of a Self-Report Tool for Measuring Change in Aspects of Autism. [Poster presentation].International Society for Autism Research, Austin Texas. Avaialble at: https://cdn.ymaws.com/www.autism-insar.org/resource/resmgr/files/insar_2022/2022_Program_Book.pdf. Accessed 20 August, 2024.
Parker KJ, Oztan O, Libove RA, Mohsin N, Karhson DS, Sumiyoshi RD, et al. A randomized placebo-controlled pilot trial shows that intranasal vasopressin improves social deficits in children with autism. Sci Transl Med. 2019;11:eaau7356.
Bolognani F, Del Valle Rubido M, Squassante L, Wandel C, Derks M, Murtagh L, et al. A phase 2 clinical trial of a vasopressin V1a receptor antagonist shows improved adaptive behaviors in men with autism spectrum disorder. Sci Transl Med. 2019;11:eaat7838.
Acknowledgements
The authors acknowledge Vaibhav Deshpande, PhD and Himabindu Gutha, PhD, CMPP (SIRO Clinpharm Pvt. Ltd.) for providing writing assistance, which was funded by Janssen Global Services, LLC. Ellen Baum, PhD (Janssen Global Services, LLC) for additional editorial support for this manuscript. We thank Seth Ness, MD, PhD for his commitment to this study including conception design, and implementation. We also want to express our gratitude and acknowledge the passing of Sandra Chaplan, MD (Study Physician) and Marlene Ball (Trial Manager), who played vital roles in this study.
Funding
The studies presented in this report were supported by Janssen Research & Development, LLC.
Author information
Authors and Affiliations
Contributions
MK: Study conduct, analysis, and interpretation. AB: Study design, conduct, analysis, and interpretation. KC: Formal Analysis. RH: Study design, conduct, analysis, and interpretation. ZA: Study design, conduct, analysis, and interpretation. CC: Study design, conduct, analysis, and interpretation. WD: Study design, conduct, analysis, and interpretation. MS: Safety monitoring, protocol review. GP: Study design, conduct, analysis, and interpretation. All authors participated in the writing and revising of this manuscript and approved the final manuscript content to be submitted to the journal.
Corresponding author
Ethics declarations
Competing interests
All authors are employees of Janssen Research & Development, LLC and may own stock or stock options.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Klein, M.E., Bangerter, A., Halter, R.J. et al. Efficacy and safety of JNJ-42165279, a fatty acid amide hydrolase inhibitor, in adolescents and adults with autism spectrum disorder: a randomized, phase 2, placebo-controlled study. Neuropsychopharmacol. 50, 480–487 (2025). https://doi.org/10.1038/s41386-024-02001-2
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41386-024-02001-2
This article is cited by
-
Deficiency of calretinin in prefrontal cortex causes behavioral deficits relevant to autism spectrum disorder in mice
Molecular Brain (2025)
-
Endocannabinoid contributions to the perception of socially relevant, affective touch in humans
Neuropsychopharmacology (2025)
-
Alterations of the endocannabinoid system in autism spectrum disorder: a systematic review and meta-analysis
European Archives of Psychiatry and Clinical Neuroscience (2025)
-
The efficacy of elevating anandamide via inhibition of fatty acid amide hydrolase (FAAH) combined with internet-delivered cognitive behavioral therapy in the treatment of post-traumatic stress disorder: a randomized, placebo-controlled clinical trial
Neuropsychopharmacology (2025)