Abstract
Psychotic disorders are complex and debilitating conditions that arise from the interplay of genetic and environmental factors. A wealth of research has identified various factors that confer risk for psychosis, while comparatively less work has focused on identifying protective or resilience-promoting factors that contribute to positive outcomes in the context of psychosis risk. Given the significant heterogeneity of outcomes for individuals at risk for psychosis, it is clear that both risk and resilience factors should be considered. In this narrative review, we synthesize current research on early adversity occurring across pre-/perinatal periods, childhood, and early adolescence, which confer risk for psychosis. We also discuss several protective factors and interventions that may buffer against the effects of early adversity, thus mitigating risk and promoting improved outcomes. By integrating findings across these domains, we underscore the importance of a developmental and multidimensional approach to understanding pathways to psychosis, which may inform future directions for prevention and intervention efforts.
Similar content being viewed by others
Introduction
Psychotic disorders, such as schizophrenia, are debilitating psychiatric conditions characterized by the presence of positive symptoms (e.g., delusions, hallucinations), negative symptoms (e.g., reductions in motivation, pleasure, and expressiveness), and/or disorganized symptoms (e.g., unusual or disorganized thoughts, speech, or motor behavior), and often include a range of cognitive impairments (e.g., reductions in attention, memory, and mental processing) [1, 2]. Despite advances in treatment, living with a psychotic disorder is associated with reduced life expectancy [3,4,5], greater incidence of homelessness and poverty [6,7,8], and poorer overall quality of life [9]. Therefore, significant efforts have been made towards identifying factors that increase risk for developing psychosis, with the hope that early intervention may prevent, postpone, or improve illness onset [10].
Although psychotic disorders typically emerge in late adolescence or early adulthood, subtle signs and symptoms often appear much earlier in development. Increasing evidence suggests that psychotic disorders have neurodevelopmental origins, beginning as early as the prenatal period, which are influenced by a dynamic interplay between genetic vulnerabilities and environmental exposures [11,12,13]. Thus, adopting a developmental framework is critical for understanding how and when adverse exposures exert their greatest influence. For example, the majority of human brain neurons are generated during the prenatal period [14], with the complexity of cortical neurons increasing rapidly during the first few years of life [15]. Adolescence represents another period of heightened neuroplasticity, in which the onset of puberty acts as a catalyst for key neurobiological changes, such as synaptic pruning and myelination [16, 17], as well as reorganization of neural circuits involved in executive functioning, reward processing, and social cognition [18,19,20,21]. These early years represent vulnerable periods in which neurodevelopment is heavily influenced by environmental inputs and individual experiences [22]. A prominent conceptual model that guides this literature in the context of psychosis is the diathesis-stress model [13, 23], which posits that individuals possess varying levels of biological vulnerability that interact with environmental stressors to determine the likelihood of developing a psychiatric disorder. Together, these frameworks highlight the importance of examining environmental influences during vulnerable periods of development to understand the trajectory of psychosis risk. Thus, in this review, we focus specifically on two categories of early life adversity: prenatal and obstetric complications and environmental exposures during childhood and adolescence. These forms of adversity were selected because they occur during vulnerable windows of brain development, are consistently linked to altered neurobiological pathways implicated in psychosis, and often precede the emergence of early signs of risk [12, 24,25,26,27].
While this review emphasizes prenatal and early life exposures, we do not discount the importance of genetic contributions to psychosis risk, which are well established [28]. Rather, we aim to highlight how early environmental factors contribute to neurodevelopmental vulnerability, both independently and as modifiers of genetic risk. Moreover, increasing evidence suggests that prenatal exposures themselves can shape neurobiological predispositions, influencing postnatal brain development and stress–response systems [29]. Nonetheless, where relevant, we highlight how these prenatal and early life exposures interact with underlying genetic vulnerabilities to provide a more integrated understanding of psychosis risk.
Critically, not all individuals who are exposed to risk factors during prenatal and early life periods go on to develop psychosis later in life. Even among individuals identified as at clinical high risk (CHR) for psychosis (i.e., individuals who experience attenuated psychotic symptoms, brief intermittent psychotic episodes, and/or a genetic risk for psychosis combined with functional decline; [30]), rates of transition to a threshold psychotic disorder range between 20 and 30% [31, 32], with some individuals experiencing full remission of psychotic symptoms [33,34,35]. A resilience-based framework, which complements traditional risk-focused models, emphasizes how some individuals resist illness, achieve recovery, or experience positive outcomes despite adversity [36]. Within this framework, resilience is a superordinate process across development that mitigates the impact of risk and improves prognosis. It arises through the interaction of protective factors (which are typically risk-dependent and protect against negative outcomes) and promotive factors (which more broadly encompass factors that enhance psychological well-being) across biological, psychological, and social domains [36]. Related terms include resilience factors and modifiable risk factors, as even established risks may be altered through intervention. Critically, these are not simply the absence of risk factors, but distinct influences that may modify psychosis risk independently or interactively. While a growing body of literature has begun to identify protective and promotive factors in the context of psychosis risk [26, 37,38,39], much remains to be discussed regarding the potential dynamic interactions between risk and protective factors during early life.
Thus, in this narrative review, we explore the complex interplay between various forms of early life adversity and potential protective factors in the development of psychosis. We focus on outcomes for both individuals with psychotic disorders and those identified as CHR, which represent a well-characterized population for the prospective study of risk and protective processes [40, 41]. Specifically, we discuss how various forms of early adversity and resilience-promoting factors contribute to a range of psychosis-related outcomes, including symptom severity, functional impairment, and transition to psychosis. We also explore the underlying mechanisms of these relationships, such as stress neurobiology and structural and functional brain changes. It is important to note that many of the risk and protective factors included in this chapter are transdiagnostic and are not exclusively related to psychosis, but are associated with vulnerability to other neurodevelopmental and psychiatric disorders (e.g., depression, anxiety, attention-deficit/hyperactivity disorder, and autism spectrum disorders), which are frequently comorbid with psychosis [42, 43]. Here, we review the literature linking various risk and protective factors to psychosis-related outcomes and, where indicated, provide evidence for specificity to psychosis. However, it is important to keep in mind that these factors may reflect broader disruptions in neurodevelopmental processes that can lead to a range of clinical outcomes. Finally, by adopting a developmental framework and examining the influences of early adversity and protective factors at vulnerable periods of development, from prenatal exposures through childhood and adolescence, we aim to provide an integrative overview of pathways implicated in psychosis, which highlights converging evidence and identifies further avenues for early intervention and prevention efforts.
Search strategy
To guide the scope of this narrative review, we focused on early life adversities and protective factors most consistently linked to psychosis risk in empirical research, particularly those supported by both human and preclinical evidence. These include pre- and perinatal exposures (e.g., maternal stress, inflammation, and obstetric complications) and early environmental stressors (e.g., trauma, peer victimization, neighborhood disadvantage, and substance use). This selection reflects a developmentally informed approach aimed at highlighting modifiable pathways to inform early intervention efforts. A search of peer-reviewed articles in English was conducted on PubMed and PsycINFO that examined each factor when experienced during early life, which we define as encompassing pre-/perinatal periods, childhood, and adolescence, in relation to risk for a psychotic disorder (e.g., schizophrenia, schizoaffective disorder, schizophreniform disorder). We included observational and intervention studies in CHR individuals to encompass research on both the modification of risk for transition to a psychotic disorder and the promotion of positive outcomes. Additionally, intervention studies conducted in individuals with psychotic disorders were also included as they related to improved outcomes (e.g., decreased symptom severity, improved functioning). Studies that examined subclinical psychotic-like experiences (PLEs) were excluded to limit the scope of the review. This is because PLEs are more common in the general population, particularly during childhood and adolescence, and because they are associated with increased risk for a range of psychopathology beyond psychotic disorders [44, 45]. In contrast, the CHR state represents an operationalized diagnostic construct with established utility for identifying risk of transition to psychosis.
Developmental risk factors for psychosis: influences of early adversity
Pre- and perinatal development: obstetric complications
As supported by Barker’s “Developmental Origins of Health and Disease” (DOHaD) [46], the pre- and perinatal periods are critical windows for fetal brain development, during which perturbations can have lasting effects on offspring psychopathology, also known as “fetal programming” [47,48,49,50]. Barker proposed that adverse conditions during critical periods of fetal development “program” long-term physiological changes that affect disease risk later in life. Since then, research has consistently shown that obstetric complications (OCs), broadly defined here as deviations from the typical course of pregnancy, labor, and delivery, and early neonatal development [51], are associated with an increased risk of psychotic disorders in offspring [24, 49, 52]. Importantly, OCs have also been associated with increased risk for a range of other cognitive, emotional, and behavioral difficulties, including anxiety [53], depression [54,55,56], ADHD [57], and autism spectrum disorder [58], indicating that their effects are not limited to pathways leading to psychosis. Although a series of OCs have been associated with schizophrenia [52], this section focuses on maternal stress, inflammation and infections, hypoxia-associated OCs and other associated pre-and perinatal factors, given that they occur at a high frequency in the population, have been found to influence each other, and have the most studies linking them to psychosis outcomes [24, 25, 49].
Prenatal maternal stress
Prenatal maternal stress (PNMS) is a well-documented risk factor for offspring psychopathology and disease [59,60,61], including psychotic disorders [25, 49, 62]. Ecological studies have consistently linked events presumed to be stressful during pregnancy to offspring schizophrenia, including famine [63, 64], warfare and genocide [65,66,67,68,69], natural disasters [70, 71], and loss of a spouse or close relative [72, 73]. However, ecologic studies suffer from the assumption that maternal stress occurred based on events that are believed to be stressful for an entire population, but often can co-occur with other potential teratogens (e.g., malnutrition) and/or did not induce stress [74]. Investigations on individual-level stressors in human pregnancy and offspring psychosis have ranged from stressful life events [75, 76], natural disasters [77, 78], the loss of a spouse [72, 73], and poverty [79], to measures of maternal daily life stress [62] and “unwanted” pregnancy [80]. A recent review of 33 studies on PNMS and offspring schizophrenia risk found predominantly positive associations in 26 studies [81].
Because psychosocial stress engages multiple bodily systems [82], PNMS may increase offspring psychosis risk through various pathways. The hypothalamic-pituitary-adrenal (HPA; i.e., the body’s primary stress–response system) axis is the most commonly proposed mechanism in non-pregnant samples [82], though it functions differently in pregnant populations. During pregnancy, a positive feedback loop between the maternal adrenal cortex and placenta increases cortisol and placental corticotropin-releasing hormone (CRH; derived entirely from the placenta in maternal blood sera during pregnancy). However, early in pregnancy, only a portion of cortisol reaches the fetus because the placental enzyme 11β-hydroxysteroid dehydrogenase-2 (11β-HSD-2) converts much of this cortisol to inactive cortisone, and its activity declines as pregnancy progresses [83, 84]. These hormonal changes support fetal organ development, parturition, and labor activation [85,86,87]. Pregnant women may also become increasingly desensitized to the effects of stress across pregnancy, reporting less stress in response to the same event during late pregnancy compared to those who experienced it in the first trimester [88]. Nevertheless, evidence suggests that both elevations in maternal cortisol and reported maternal stress are associated with adverse birth outcomes associated with schizophrenia [25, 62, 83, 89] as well as other psychopathologies, including depression [90], and neurodevelopmental conditions such as ADHD [91] and ASD [92].
Other hypothesized indirect pathways linking PNMS to offspring psychosis outcomes include interactions with OCs, such as fetal hypoxia (reviewed in later sections) [25]. Maternal and fetal genetic vulnerabilities have also been proposed as contributing factors, as certain genetic predispositions may interact with PNMS to amplify risk for psychosis [93]. PNMS has also been linked to premorbid cognitive changes, such as lower childhood IQ [94], executive functioning [95], and language abilities [96], as well as impairments in motor skills [97]; all of which have been identified prior to psychosis onset [49]. Similarly, PNMS has been linked to reduced gray matter and hippocampal and DA abnormalities seen in psychosis [98, 99]. Collectively, these findings suggest that PNMS contributes to neurodevelopmental outcomes, beginning at birth, that increase the likelihood of psychosis, as well as cognitive and neural alterations found in the course of psychosis.
Prenatal maternal infection and inflammation
Prenatal maternal infections and inflammation have become an important target of investigation for their role in offspring development of psychotic disorders [100]. Population-based ecological studies first provided evidence of these associations, finding increased risk of psychosis in offspring of those born during influenza epidemics compared to other periods [101,102,103,104]. Birth cohort studies further examined this association using prenatal medical records and/or analyses of prenatal blood sera that determined infections through antibodies [11, 100, 105, 106]. Based on these studies, findings indicated that multiple maternal infections during pregnancy were associated with an increased risk of offspring psychotic disorders, including respiratory infections, influenza, herpes simplex virus type 2, toxoplasma gondii, genital/reproductive infections, rubella, and bacterial infections [107,108,109,110,111,112,113], which have been extensively reviewed elsewhere [105, 114].
Many infections do not appear to cross the placenta [115]; therefore, one proposed mechanism linking maternal infections during pregnancy to offspring psychosis is maternal immune responses to infection, particularly elevations in proinflammatory proteins [115]. Increases in proinflammatory proteins during gestation (e.g., cytokines and chemokines), herein referred to as prenatal maternal inflammation (PNMI), have been repeatedly implicated in offspring development of psychosis [109, 116,117,118,119]. Specifically, interleukins (IL)-8, IL-6, IL-1β, TNF-α, and C-reactive protein (CRP) at various times of gestation have all been linked to an increased risk of psychotic disorders in offspring [109, 116, 118,119,120]. PNMI has been linked to increased inflammatory markers in the placenta and/or fetal circulation [121, 122], and there is also evidence that some maternal cytokines can cross the placenta [115, 121, 123].
The association between PNMI and offspring psychotic disorders may be explained, in part, by altered fetal neurodevelopment [117]. Preclinical research has found prenatal maternal immune activation (MIA) to be related to several neurological phenotypes found in schizophrenia (e.g., increased sensitivity to dopamine (DA) receptor agonists, decreased levels of serotonin, reductions in hippocampal and amygdala cortical volume, microgliosis, and increased microglial density in areas such as the hippocampus) [124, 125]. Similarly, rodent models of MIA have found cognitive deficits in offspring mirroring symptoms commonly seen in psychotic disorders (e.g., problems with executive functioning, cognitive flexibility, and sensory motor gating) [125]. In humans, PNMI has been associated with decreased left entorhinal cortex volumes, increased ventricular cerebrospinal fluid, and poorer performance on assessments of verbal memory and executive function, specifically in offspring with schizophrenia [115, 126,127,128]. Among offspring who go on to develop psychosis, there is evidence that cases with a history of prenatal infections have a more severe premorbid period, characterized by lower birth weight and poorer performance on cognitive tests in childhood, as well as a more severe course of the disorder, characterized by poorer cognitive performance and fine-motor coordination, and increased cavum septum pellucidum (CSP) length, a well-documented brain anomaly in psychosis [126, 127, 129]. Finally, PNMI may indirectly increase offspring psychosis risk through its interaction with other prenatal insults, such as PNMS and hypoxia-associated OCs, discussed elsewhere in this review [100, 117, 130, 131].
Hypoxia-associated obstetric complications
Hypoxia-associated OCs (herein referred to as fetal hypoxia) are defined by complications that involve oxygen deprivation to the fetus and serve as one of the primary OCs that have been linked to offspring risk for developing a psychotic disorder, with evidence suggesting that as many as 20–30% of cases of schizophrenia have a history of fetal hypoxia [38, 130]. Fetal hypoxia can occur at various stages of pregnancy and labor/delivery [132] and can include prenatal complications such as preeclampsia and/or complications occurring during birth (e.g., umbilical cord problems obstructing blood flow) [133,134,135]. Fetal hypoxia is associated with an earlier age of onset and a worsened premorbid period, characterized by childhood motor and cognitive difficulties [52, 136,137,138,139,140]. Importantly, many of these findings also differentiated unaffected siblings from cases who developed schizophrenia, suggesting that the interaction between fetal hypoxia and liability for schizophrenia portended a worsened course of the disorder [12, 138, 140]. Findings suggest that fetuses who later go on to develop schizophrenia, compared to control fetuses, exhibit a reduced neurotrophic response to hypoxia (assessed through brain-derived neurotrophic factor levels in cord blood) [141]. Although these results did not directly test genetic mechanisms, other studies have highlighted how genes implicated in schizophrenia (e.g., AKT1, BDNF) may render the fetus more vulnerable to hypoxic insult [142, 143]. For instance, genome-wide derived polygenic risk scores for schizophrenia were related to a higher prevalence of OCs [144]. The genes related to these OCs were also overexpressed in placentas from complicated pregnancies compared to control pregnancies, particularly when the fetuses were male [144]. Thus, genetic risk for psychosis may render the fetus more susceptible to OCs, increasing the probability of adverse neurodevelopmental outcomes.
There is also evidence that other OCs (like infection and PNMS) can increase the likelihood of fetal hypoxia. For instance, a review of the relationship between infection, proinflammatory cytokines, and hypoxia in the fetal brain indicated that infection exposure and proinflammatory cytokines may alter the threshold at which hypoxia becomes neurotoxic, increasing vulnerability to lesser hypoxic events [145]. Likewise, PNMS has been linked to increased rates of asphyxia during birth and decreased Apgar scores (rating newborn health after birth), both of which are used to assess the construct of hypoxia-associated OCs [146]. Taken together, evidence suggests that fetal hypoxia is a prominent OC linked to psychosis that has been found to interact with both risk genes and other OCs associated with psychotic disorders Nevertheless, challenges remain in understanding the hypoxia to psychosis pathway due to heterogeneous definitions of fetal hypoxia across existing studies, as well as methodological challenges in measuring hypoxia during pregnancy [12, 38, 89].
Maternal health behaviors during pregnancy
A range of prenatal substance exposures (e.g., nicotine, alcohol, and cannabis) and health behaviors (e.g., nutrition, sleep) have been explored in their link to offspring psychosis. Evidence has linked maternal smoking during pregnancy, measured via early-gestation serum cotinine levels, to increased schizophrenia risk, as well as greater severity of negative symptoms and reality distortion (e.g., hallucinations) in offspring who develop the disorder [147, 148]. Additionally, rodent models have linked Δ⁹-tetrahydrocannabinol (THC) exposure to epigenetic changes that mediate synaptic development [149]. This may offer insight into the mechanisms underlying human cohort findings linking prenatal cannabis use to increased risk of psychosis in offspring [150]. While evidence directly connecting maternal alcohol use to offspring schizophrenia is limited, high levels of maternal alcohol use during pregnancy are associated with OCs, such as low birth weight and umbilical cord vasoconstriction that elevate schizophrenia risk [151]. Animal studies further support that in utero exposures to nicotine, alcohol, and cannabis are associated with neural and behavioral outcomes found in schizophrenia [152].
Additionally, PNMS warrants consideration in relation to health behaviors during pregnancy due to evidence linking heightened PNMS with engagement in smoking, decreased exercise, unhealthy eating, and further adverse lifestyle behaviors [11, 153]. Adverse health behaviors are also associated with OCs (e.g., vitamin D deficiency and preeclampsia) that have been identified in association with offspring psychosis risk [11, 154]. The bidirectional nature of adverse health behaviors and PNMS suggests that either may serve as mediators in models of fetal programming following prenatal risk factors [155]. Recent reviews have highlighted associations between elevated maternal pre-pregnancy body mass index (BMI), obesity, and/or nutritional deficits (e.g., iron, vitamin D) with offspring psychosis-related outcomes [25, 38]. Nutritional intake’s relationship to fetal outcomes may also be understood through its role in inflammatory status and placental dysfunction, as nutrient deficiencies have been linked to immune system and CNS functioning in offspring [25, 156]. Similarly, maternal sleep disturbances have been linked to elevated levels of inflammatory markers, such as CRP and IL-6 [157], and may represent a potential mediator in the relationship between PNMS and fetal programming [155]. Consequently, modifiable risk factors, such as maternal health behaviors, may contribute to the observed associations between OCs and psychosis, potentially influencing fetal programming through interactions with inflammation, nutrition, sleep, and other lifestyle factors.
The role of offspring sex and timing of prenatal exposure
Research suggests that the effects of pre- and perinatal insults on offspring development likely vary based on both fetal sex and gestational timing. For example, male fetuses are thought to be at higher risk for mortality and morbidity in response to PNMS exposure, also known as the viability–vulnerability tradeoff hypothesis [87]. In line with this, exposure to prenatal maternal daily life stress has been shown to have a two-fold increase in the odds of psychotic disorders, primarily in male offspring [62]. Similarly, PNMI contributions to offspring outcomes are likely also dependent on fetal sex [54, 118, 158, 159]. Some studies report a greater risk for psychotic disorders in male offspring after PNMI or maternal infection, which may contribute to the male-biased prevalence of psychotic disorders [118, 160]. Similarly, animal studies have indicated greater reductions in learning task performance in male offspring following fetal hypoxia [161], which is bolstered by findings that schizophrenia risk genes in placentae may be associated with upregulation when the fetus is male. However, other findings suggest that chronic fetal hypoxia may be associated with cognitive difficulties (e.g., decreased verbal IQ, inhibition) in females [144, 162], suggesting that future research is warranted to better assess sex differences in offspring outcomes following fetal hypoxia.
Timing of prenatal exposures also may differentially contribute to psychosis risk. While PNMS exposure during the second trimester has been most consistently associated with offspring risk for psychosis, evidence is limited, and it still remains inconclusive regarding which trimester presents the highest vulnerability [25]. Offspring risk for psychotic disorders has been linked to infection or PNMI exposure at various times in gestation, suggesting inconsistencies in a gestational vulnerable period for psychosis risk [74, 106, 109, 118, 163, 164].
In summary, pre- and perinatal factors interact with each other, as well as with susceptibility genes [144, 165], to play a critical role in shaping offspring vulnerability to psychosis. These effects may be moderated by both the timing of exposure and fetal sex, though findings remain mixed. Importantly, not all individuals exposed to OCs go on to develop psychosis; rather, it has been proposed that such early insults may function as a “primer,” increasing the brain’s sensitivity to subsequent postnatal stressors [166]. In the following section, we examine early life environmental stressors that may build upon pre-existing vulnerabilities and further contribute to the development of psychosis.
Early life: environmental risk factors
Experiences during childhood and adolescence can exert profound, long-lasting effects on mental health trajectories, including risk for psychosis. A growing body of research highlights how adverse early life exposures interact with neurodevelopmental processes to shape brain circuits involved in systems associated with psychosis, including stress regulation, reward processing, and social cognition [167]. Here, we focus on specific forms of environmental exposures during childhood and adolescence that have been consistently associated with heightened psychosis risk: childhood trauma, peer victimization, and neighborhood disadvantage [24, 168,169,170,171]. In addition, adolescent substance use represents a particularly potent risk factor for psychosis during a time of heightened neuroplasticity, changes to reward circuitry, and increased susceptibility to social influences [18, 19, 172,173,174]. In this section, we synthesize evidence on these key psychosocial and environmental risk factors, emphasizing both epidemiological associations and emerging neurobiological mechanisms.
Childhood trauma
Childhood trauma, defined as experiences of abuse and or neglect which result in actual or potential harm to a child’s health, development, and/or dignity (WHO, 2024), has consistently been associated with increased risk for developing psychosis [169, 175, 176]. Meta-analytic findings suggest 80–90% of individuals at CHR have been exposed to at least one type of childhood trauma [170, 177]. Repeated evidence suggests that childhood trauma is associated with greater symptom severity for individuals at CHR [177,178,179] and those with psychotic disorders [175, 176, 180,181,182,183]. Moreover, among individuals with psychosis, histories of childhood trauma are associated with a range of negative outcomes, including poorer cognitive functioning [184, 185], poorer social and role functioning [186,187,188,189], poorer treatment response [190, 191], and lower symptom remission [192, 193].
Notably, recent models of childhood trauma have highlighted distinct dimensions of deprivation and threat, which differentially impact neural, cognitive, and emotional development [194,195,196,197]. As such, increasing attention has been focused on parsing the specific effects of different types of childhood trauma in the context of psychosis [198,199,200,201,202], though findings have been mixed. Although there is some evidence that threat-based traumas, such as physical and sexual abuse, may be associated with the largest increased odds of developing a psychotic disorder [200, 201], several studies have concluded that all types of childhood trauma are associated with increased risk, with exposure to multiple traumas increasing risk in a linear relationship [198, 201, 202]. Further, the specific effects of childhood trauma types may be difficult to differentiate as multiple types of trauma commonly co-occur [203, 204]. Moreover, recent evidence suggests that childhood neglect modulates neural profiles of abuse, such that individuals with high levels of both abuse and neglect show a qualitatively different pattern of neural activation, compared to those with high levels of either type of trauma alone [205].
Peer victimization
Peer victimization (e.g., bullying) has repeatedly been associated with a greater risk for developing a psychotic disorder [168, 206,207,208]. Critically, the relationship between peer victimization and psychotic symptoms remains after accounting for a range of other factors, including family adversity, comorbid psychopathology, and other negative life events [209]. A prospective cohort study provided evidence that victimization is associated with the development of a psychotic disorder in a dose-response fashion, with victims of sibling bullying being 2–3 times more likely to meet criteria for a psychotic disorder, and victims of both sibling and peer bullying having more than four times the odds of developing a psychotic disorder [168]. According to the social defeat hypothesis, repeated social exclusion has deleterious effects on DA functioning, heightening sensitivity of the mesolimbic DA pathway and increasing vulnerability to psychosis [210, 211]. It has also been suggested that certain pre-existing difficulties, such as cognitive impairments and non-specific effects of socioeconomic disadvantage, precede experiences of bullying and contribute to heightened risk of victimization [212], increasing risk for psychosis in a bidirectional and compounding manner [213]
Neighborhood disadvantage
Beyond individual-level adverse exposures, neighborhood-level factors may also contribute to risk for psychosis. Neighborhood disadvantage, which refers to growing up in impoverished conditions with limited access to social and economic resources, has been studied extensively in the context of psychosis, though findings are mixed. Some studies have suggested that individuals exposed to lower socioeconomic conditions at birth or during childhood and adolescence are at increased risk for the onset of a psychotic disorder in adulthood [214,215,216,217,218], while others found no or opposite associations [219]. However, the literature examining neighborhood disadvantage is complex, as this risk factor often encompasses other neighborhood-level factors, such as urbanicity, which has also been related to increased risk for psychotic disorders [214, 220, 221]. Other neighborhood conditions associated with increased risk for psychotic disorders include increased exposure to environmental toxins and lack of green space [24, 221, 222], and social disorganization, which itself encompasses a range of additional neighborhood conditions, including crime levels, single-person households, residential mobility, ethnic density, and voter turnout [223]. While it may be difficult to disentangle the precise risk conferred by each of these interrelated neighborhood-level factors, chronic exposure to multiple disadvantaged neighborhood conditions may be associated with greater cumulative stress [24, 224], likely converging on several neurobiological systems, discussed in later sections.
Adolescent substance use
As highlighted previously, adolescence is a vulnerable neurodevelopmental period characterized by heightened neuroplasticity and brain maturation [172,173,174], as well as identity formation, increased autonomy, and sensitivity to social influences [18, 225]. Thus, adolescents are more likely to engage in risk-taking behaviors, such as substance use [226], which has been identified as a primary predictor of transition to psychosis [227, 228]. During adolescence, the brain is particularly vulnerable to the effects of substance use, with cannabis frequently studied in relation to psychosis risk [228,229,230,231]. Moreover, individuals with a history of early life adversity are also at greater risk for initiation of substance use [232]. Earlier initiation and more chronic substance use during adolescence are also associated with a greater magnitude of risk for psychotic disorders [231, 233]. Some studies suggest that psychosis-spectrum symptoms may precede the initiation of substance use, with substances used to cope with distressing symptoms [234] or early life stressors [235]. However, evidence from prospective, longitudinal studies has found that the associations between adolescent substance use and risk for psychosis onset exist even after controlling for psychosis symptoms that preceded substance use [236, 237].
Biopsychosocial mechanisms: cascade of risk
As highlighted previously, the risk factors discussed here do not occur in isolation. While these exposures vary in content and timing, they likely converge through shared and overlapping mechanisms that unfold across development. Beginning in the prenatal period, exposure to stressors and environmental insults may initiate a cascade of interconnected neurobiological disruptions, cognitive changes, and behaviors that could set the stage for increased vulnerability to subsequent adversity during childhood and adolescence [11].
PNMI and other OCs may act as a neurodevelopmental ‘primer’, leading to subtle brain, immune, and hormonal alterations that can influence subsequent neurodevelopment [115, 238, 239]. OCs have been linked to a variety of cognitive difficulties [49, 240], which may in turn influence social functioning (e.g., interactions with peers, teachers, family members), potentially resulting in additional difficulties [55]. For example, evidence suggests that cognitive difficulties and other developmental risk factors increase the risk of being bullied and victimized by peers, which in turn increases the risk for developing psychosis [212, 213]. Additionally, stressors during prenatal and childhood periods have been found to prime the HPA-axis for exaggerated reactivity, increasing sensitivity to future stressful events, and increasing the likelihood that daily events will be perceived as stressful [241, 242]. In particular, elevated stress sensitivity may play a role in the early stages of psychosis development, as greater emotional reactivity to minor stressors has been associated with increases in subtle psychotic experiences [242]. Consistent with this, individuals at CHR tend to be more reactive to stressful life events, compared to controls [243, 244]. Moreover, compared to non-converters, CHR individuals who transitioned to psychosis exhibit a blunted cortisol awakening response, further indicating HPA-axis dysregulation [245]. Additionally, HPA-axis dysregulation is linked to disruptions in sleep patterns [246,247,248], which is another established risk factor for psychosis [249]. Sleep disturbances, in turn, further exacerbate cognitive dysfunction, increase inflammation, and are associated with increases in stress reactivity [157, 250, 251], creating a self-perpetuating cycle that contributes to vulnerability.
Over time, repeated stress exposure may initiate further neurobiological disruptions, such as altered DA signaling. For example, evidence suggests that dysregulated stress responses, likely in combination with genetic predispositions [252, 253], directly influence the DA system, contributing to hyperactivity of the mesolimbic DA pathway [254, 255]. DA dysregulation may be further compounded by substance use during adolescence, in part, through indirect effects of the endocannabinoid system on DA signaling [256]. DA dysregulation is a hallmark of psychosis [257,258,259], such that higher levels of DA in the striatum have been repeatedly observed and may amplify the salience of irrelevant stimuli, contributing to the development of delusions and hallucinations—a concept often referred to as “aberrant salience” [260]. Critically, these processes may unfold in a cascading and self-reinforcing manner. For example, early experiences of cognitive difficulties and peer victimization may further exacerbate stress sensitivity and increase risk for initiating substance use.
Additionally, it has been hypothesized that chronic HPA-axis activation may impair the regulation of glucocorticoids (GCs), leading to persistent elevations in GC levels [261,262,263]. Both chronic GC exposure and DA dysregulation may interact with and contribute to other biological processes, such as increased production of proinflammatory cytokines [264, 265] and excessive glutamatergic signaling, which has been associated with glutamate excitotoxicity [266, 267]. Together, these interactive processes have been proposed to contribute to progressive structural and functional brain alterations by disrupting normal developmental processes and contributing to decreased neurogenesis, impaired synaptic plasticity, and cell death [268,269,270]. Notably, many of the relationships described here are based on correlational findings or theoretical models. While these patterns offer insight into possible developmental mechanisms, additional longitudinal and mechanistic research is needed to delineate causality and interaction effects.
In line with this cascading model, it should be noted that early life adversity, including prenatal insults, may not exert immediate effects but instead alter the developmental trajectory of brain maturation in ways that only become apparent during later critical periods. For example, during adolescence, a time of significant neural and psychosocial changes [16,17,18, 225], previously latent vulnerabilities may be unmasked, leading to the emergence of psychosis symptoms [271, 272]. Specifically, evidence of reduced synaptic connectivity in schizophrenia derives from postmortem studies [273,274,275,276], and consistent findings of cortical gray matter reductions in individuals with psychosis [277,278,279] and CHR [280,281,282]. Accelerated gray matter loss during adolescence has also been linked to increased psychosis risk, suggesting that normative developmental processes may interact with prior insults to drive neurobiological change [271, 283]. Although genetic factors have been linked to increased pruning in schizophrenia [284], it has been proposed that early adversity could reduce baseline synaptic density or compromise neural organization, thereby lowering the threshold for pruning-related disruptions to precipitate psychotic symptoms [285]. Support for this model comes from preclinical studies indicating that a fetal hippocampal lesion is associated with schizophrenia-like brain pathology that emerges with increased pruning [286, 287], as well as other neurodevelopmental processes [288], which occur during adolescence. However, pre- and postnatal adversities are not focal events that result in lesions, but rather represent interacting exposures that likely impact multiple neurodevelopmental systems over time. Thus, pruning abnormalities are unlikely to represent the sole pathway through which early exposures confer risk for psychosis. For more comprehensive discussions of the various neurobiological processes linking prenatal and early life adversity with psychosis risk, we direct the readers to several excellent reviews [27, 115, 288,289,290]
Although OCs and early life stress increase risk for a range of psychopathologies [50, 54, 291], there is some evidence for specificity to psychosis. For example, our group has provided evidence that fetal hypoxia is associated with decreased birth weight among individuals with schizophrenia, but not affective psychosis (i.e., mood disorders with psychotic features) [292]. Additionally, trauma and stress in childhood are linked to subthreshold psychosis symptoms beyond their shared correlates, including comorbid psychopathology [293,294,295]. Further, common psychological sequelae of early adversity, such as anxiety and depression, may mediate this relationship, representing an affective pathway to psychosis [242, 296, 297]. Nevertheless, it is plausible that many of these early experiences increase risk for shared phenotypes across disorders (e.g., brain alterations, cognitive difficulties), but future studies are necessary to determine factors that contribute to the specificity of results. Importantly, these risk factors also offer opportunities for early identification and studies examining resilience in the face of early adversity, discussed further in our sections on protective factors.
Protective and promotive influences in psychosis: mitigating the effects of developmental risk factors
A resilience-based approach helps conceptualize how some individuals experience variable outcomes despite exposure to risk factors. This not only relates to rates of transition to psychosis, but also improved prognosis if psychosis occurs - manifesting in lower symptom severity, better functioning, and greater capacity for recovery [36, 298]. Central to this framework are protective and promotive factors, as well as protective and preventative interventions, which are not dependent on risk presence and typically introduced before possible risk exposure. Importantly, protective factors are not necessarily the absence or inverse of risk factors; though some, such as reduced maternal stress during pregnancy, are supported by intervention studies. Consequently, several studies have tested the efficacy of interventions based on these protective factors, which we review separately along with preventative interventions.
Pre-/perinatal protective and promotive factors
Serious infection reduction and responses to infection
Given that most pregnant individuals experience some type of infection during the course of their pregnancies, it is important to understand the differential impact of various infections during pregnancy [299]. The severity of infection, rather than exposure alone, might explain some of the variability in the impact of prenatal infections on offspring [74, 83]. However, there is evidence for individual genetic variation in maternal responses to infection [12, 106, 115]. For example, immune-related genetic polymorphisms in the IL-1 complex or TNF-alpha are associated with greater basal levels of proinflammatory cytokines and an increased inflammatory response to infection [12, 115]. It is possible that individuals with these and other genetic profiles are more vulnerable to the effects of prenatal infection, therefore increasing offspring risk for psychosis. Conversely, individuals with genetic profiles that promote more effective regulation of immune responses may be buffered against the adverse effects of infection, highlighting that stronger or more adaptive immune functioning could serve as a protective factor even prior to intervention. However, the relationship between specific genetic polymorphisms and prenatal immune response in relation to offspring risk for psychotic disorders has yet to be examined [12, 106, 115].
Maternal health behaviors during pregnancy
Several prenatal maternal health behaviors have received attention as possible protective factors with theoretical links to modified risk for psychosis. For example, prenatal nutritional deprivation has been linked to an increased risk of offspring psychotic disorders, suggesting enriched maternal nutrition may have the potential to reduce risk. Sufficient intake of folic acid and iron prenatally, crucial for preventing maternal anemia and fetal hypoxia, may be protective [300, 301]. In addition, prenatal plasma-free choline and a choline-rich diet during pregnancy have been linked to increased offspring cognitive functioning in infancy and childhood [302,303,304]. Higher fetal choline concentrations are also related to increases in alpha-7 nicotinic receptor activation, receptors that are typically highly expressed in the fetus and critical for development [303]. Further, genetic deficiencies in alpha-7 nicotinic receptors have been linked to the development of psychotic disorders [303]. Some evidence suggests that greater omega-3 fatty acid docasahexaenoic acid (DHA) levels in the offspring at birth are associated with better birth outcomes and improvements in cognitive, visual, and motor development in infancy [305, 306].
Additionally, some studies suggest that psychological factors like an optimistic disposition during pregnancy reduce the risk of preterm birth, which has independently been linked to changes in offspring development and an increased psychosis risk [307, 308]. Maternal optimism is also associated with the use of positive coping strategies during pregnancy [309, 310], with strategies like positive appraisal during pregnancy found to be related to lower levels of emotional distress [311]. These are relevant protective factors given the relationship between PNMS and psychosis risk discussed previously in this review.
Pre-/perinatal interventions
Intervention research conducted during pregnancy and early life has included both protective and preventative efforts. Based on research identifying maternal infection severity during pregnancy and offspring psychosis risk [312], offspring vaccination has been robustly tested and recommended as a preventative intervention. The Centers for Disease Control and Prevention (CDC) recommends that pregnant women receive the influenza vaccine, with research from large birth cohorts finding no adverse effects in offspring [312, 313], and studies have observed fewer instances of influenza illness in pregnant individuals who are vaccinated [312,313,314]. Although prenatal vaccination is also effective for promoting maternal and fetal health, longitudinal studies are needed to examine its potential in protecting against offspring development of psychiatric illnesses associated with prenatal infections, including psychotic disorders. Other preventative interventions that have been studied include dietary supplements during pregnancy. One randomized control trial demonstrated that infants born to a high prenatal choline diet had an inhibited P50 response (i.e., intact sensory gating) at 5 weeks old compared to placebo-treated infants [302,303,304], with P50 deficits consistently found in schizophrenia and, to an extent, in individuals at CHR [315]. Given mounting evidence in support of other nutrition and dietary supplements like those described above, studying maternal nutrition interventions during pregnancy as a preventative against psychosis represents a fruitful direction for further research. Additionally, poor sleep quality during pregnancy has been linked to differences in child development in areas of global cognition, brain structure, and behavioral problems, with sleep interventions (e.g., Cognitive Behavioral Therapy for Insomnia; CBT-I, mindfulness) found to improve sleep difficulties during pregnancy [316,317,318]. Although the efficacy of preventative prenatal sleep interventions has not yet been examined in the context of offspring development, it is another promising avenue of exploration for promoting resilience against developmental factors that may give rise to later psychopathology.
Numerous protective interventions during pregnancy have focused on targeting maternal mental health, specifically reducing maternal stress. Several randomized control trials (RCTs) have tested stress management interventions, cognitive behavioral therapy (CBT), and positive psychological interventions that are effective in decreasing maternal perceived stress and depression symptoms during pregnancy, with some studies also finding decreases in diurnal salivary cortisol [319,320,321,322]. Longitudinal follow-up studies with offspring are critical to explore any potential long-term benefits of protecting against the development of psychosis. Additionally, there is evidence to suggest that postnatal factors may promote offspring resilience after exposure to maternal prenatal stress, including parental sensitivity and environmental enrichment [323]. RCTs examining parental sensitivity and skin-to-skin contact interventions (e.g., Kangaroo Care) find widespread benefits to the infant and child (e.g., increased newborn brain structural connectivity, decreased cortisol and autonomic nervous system reactivity, and improved cognitive and language development), emphasizing the potential of resilience-promoting postnatal environments to protect against the effects of PNMS [323]. Lastly, increasing attention has been paid to understanding the importance of modifying exposures to adverse neighborhood and environmental factors during pregnancy. Research has linked exposure to adverse environmental conditions to elevations in inflammation during pregnancy, including higher levels of air pollution and other potential toxicants (e.g., plastics) [305, 324]. Therefore, potential protective interventions could include reducing exposure to ambient air pollution (e.g., not exercising outdoors during periods of high pollution, if living in urban areas), breathing through the nose when outside, having a high quality air purifier, and reducing exposure to plastics (e.g., frequently vacuuming and not drinking out of plastic bottles) (U.S. Environmental Protection Agency, 2019).
Childhood and adolescent protective and promotive factors
Psychological and emotional factors
Broader psychological processes—such as coping strategies, emotion regulation, and personality traits—may mitigate the effects of adverse experiences. Coping strategies have long been implicated in the development, maintenance, and outcomes of psychotic disorders [325,326,327,328,329]. An individual’s ability to adapt to change or cope with stress has been associated with decreased symptom severity in CHR individuals [330, 331], as well as lower rates of transition to psychosis [332, 333]. Studies find that more frequent use of certain coping strategies (e.g., avoidance, emotion-focused) by CHR individuals is associated with greater negative symptoms and lower psychosocial functioning [334]. In comparison, more frequent use of other coping strategies like problem-solving and seeking social support has been associated with less severe negative and psychotic symptoms, though it remains unclear whether specific coping strategies impact the likelihood of transition to psychosis [334]. Nevertheless, the use of adaptive coping strategies among individuals at CHR has been associated with better social and role functioning [335], symptom severity [335, 336], and treatment response [337]. In contrast, poor emotion regulation strategies, like emotional suppression, were shown to mediate the relationship between psychosis risk and mental health symptoms like emotion dysregulation and substance use [338]. Additionally, research suggests that certain traits like optimism, an internal locus of control, and positive self-appraisal may promote resilience as they are associated with decreased perceived stress and improved functioning among individuals with psychosis [339, 340]. Some of these resilience-promoting factors may also be modifiable; for example, implementation of mindfulness and self-affirmation strategies may improve emotion regulation, reduce stress, increase positive emotions, and increase optimism [341, 342].
Psychosocial factors
Beyond individual-level protective factors, social and community factors may further protect against psychosis risk. Affectionate familial relationships and effective extended family networks may be a protective factor among CHR individuals [343], with caregiver affection and positive engagement during the CHR period associated with symptom reduction [344]. Family relationships high in expressed emotion (e.g., the intensity and nature of hostile or critical emotional expressions) have been linked to heightened relapse rates and poorer functioning among individuals with psychosis [345, 346], while greater levels of warmth and optimal family involvement have been associated with improved functioning and reduced symptom severity in CHR individuals [346]. Further, evidence suggests that greater perceived social support is associated with reduced risk for psychosis and improved functioning [343, 347, 348], though social network research remains limited by a lack of standardization in conceptualizing social networks across studies [349, 350]. For individuals with marginalized social identities, other community-level factors such as social inclusion, education, job and food security, and access to healthcare may be particularly protective [24]. Increased ethnic group affiliation [351] and access to community individuals with similar ethnoracial backgrounds may potentially be protective against the effects of exposure to chronic social stressors like discrimination among children and adolescents [352, 353]. On a broader scale, systemic efforts—including changes to assessment and diagnosis training, multilevel interventions, and shifts in funding priorities—may be necessary to modify psychosis risk, particularly for marginalized racial/ethnic individuals [24, 354].
Childhood and adolescent interventions
Intervention studies conducted among children and adolescents have largely targeted lifestyle and psychosocial factors that may be protective or resilience-promoting in the context of psychosis risk. Modifying sleep habits has been one of the most studied and promising lifestyle targets. Sleep disturbances (i.e., sleep disorders, deviations in objective measures of sleep, subjective reports of poor sleep) are highly prevalent in psychosis [355,356,357], often precede psychosis onset, and have been found to be predictive of transition to psychosis [356, 357]; therefore, an intervention window prior to psychosis onset is emphasized. One study found CBT-I resulted in better sleep, improvements in negative affect, and reduced psychotic experiences among CHR individuals [358]. Additionally, stress and early adversity can disrupt sleep, and sleep disruptions, in turn, have been associated with cortisol reactivity and higher levels of inflammation [359,360,361]. Therefore, sleep improvements may buffer the effects of early adversity on mental health outcomes and represent a promising resilience-promoting factor [360].
Exercise and diet interventions have also been robustly tested as protective against psychosis. Physical activity has been linked to reduced symptom severity, improved mood, memory, psychosocial functioning, and quality of life across CHR and psychosis populations [362,363,364,365]. Further, longitudinal evidence suggests that greater physical activity levels are associated with a lower risk of transition to psychosis in those at CHR [366]. Importantly for those with a history of early adversity, exercise has been shown to buffer the effects of stress on mental health outcomes [367], likely through the modulation of multiple biological systems, such as lower overall cortisol levels [368, 369], reduced inflammation [370, 371], and increases in hippocampal neurogenesis [372, 373]. Additionally, exercise may be most beneficial in combination with a healthy diet and nutrition patterns that may reduce metabolic risk factors common among individuals with psychosis [374,375,376]. These combination exercise and diet interventions may further mitigate the effects of adverse exposures by reducing inflammation and regulating stress-sensitive hormonal systems [377, 378]. That said, many of the sleep, exercise, and diet studies just described were tested in late adolescence/early adult samples, suggesting a critical need for further studies that introduce these interventions earlier in development, which may help increase their efficacy in buffering against early life adversities and promoting positive outcomes.
Lastly, psychosocial interventions may help support individual-level resilience processes. An RCT revealed that a family psychoeducation approach, including training in communication and problem-solving skills, exhibits potential in improving attenuated positive symptoms in CHR individuals [379]. Similarly, another RCT suggests that reductions in perceived maternal criticism predict decreases in subthreshold positive symptoms [380]. Other clinical interventions have focused on social activation techniques, such as group sessions and constructive group activities, which have successfully decreased negative symptoms in individuals with recent-onset psychosis [381].
Mechanisms of resilience
Interactions between behavioral protective factors and various neurodevelopmental mechanisms may also support effective adaptation and recovery from stressors across early life. For example, neural plasticity, the brain’s ability to adaptively change synaptic connections over time [382], supports the strengthening of adaptive circuits and maintenance of various cognitive functions [383,384,385]. Therefore, it is possible that plasticity in response to stress is protective against poor mental health outcomes by promoting compensatory neural mechanisms and buffering the effects of environmental insults [386,387,388]. Critically, there is significant overlap in the neural circuits most affected by stress and those implicated in risk for psychosis [389]. For example, while hippocampal volume loss is characteristic of psychosis [390,391,392,393], longitudinal evidence suggests that CHR individuals who remitted did not exhibit reductions in hippocampal CA1 volume over time [394]. Similarly, among adults who have experienced childhood maltreatment, resilient individuals (i.e., those who did not develop psychopathology) exhibited greater hippocampal volume compared to adults with psychopathology [395]. Additionally, resilient individuals showed lower hippocampal activation to emotional faces and increased amygdala habituation to stress, suggesting a greater ability to regulate emotions, dampen threat processing, and modulate stress responses [395]. In line with this, CHR individuals who remitted showed greater accuracy, greater amygdala activation, and stronger negative amygdala-prefrontal functional connectivity during an emotional faces task [396]. This suggests that adaptive or compensatory mechanisms within these circuits—such as increased synaptic efficiency, strengthened top-down regulatory control, and more effective stress–response modulation—may protect against the development of psychosis, and could inform future neurobiological intervention research in psychosis.
Interplay between risk and resilience
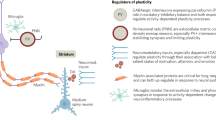
While several of the factors reviewed here have been studied in isolation, both risk and resilience factors should be conceptualized as dynamic and interacting factors across developmental periods and biopsychosocial levels [298]. Critically, individuals vary widely in their susceptibility to environmental inputs, including both positive and negative contexts [397,398,399,400]. Therefore, those with heightened susceptibility may be particularly vulnerable to negative contexts, but also more responsive to protective factors, potentially buffering against negative outcomes. As discussed, early life adversity, such as prenatal insults and/or childhood trauma, can disrupt neurodevelopment and increase risk for psychosis by altering stress–response systems, neural circuitry, and other biological systems (e.g., immune systems). However, resilience factors may buffer against these risks by promoting adaptive coping, support, and self-efficacy. For example, evidence suggests that the presence of supportive caregivers [401, 402] and other social support [403] buffers against the risk of psychopathology following trauma exposure in childhood, including for individuals with psychosis [404]. Risk and resilience can also interact across environmental and biological levels, such that social engagement may buffer against the deleterious effects of neighborhood poverty on hippocampal volume among CHR individuals [405]. Further, the use of effective coping mechanisms can reduce distress associated with psychotic symptoms [406], which likely has downstream effects on stress sensitivity and overall stress–response [407,408,409,410]. Expanding previously existing models of psychosis risk to incorporate resilience provides a more comprehensive understanding of the complex and dynamic processes that shape outcomes for individuals at risk for psychosis. Figure 1 provides an overview of the interacting and/or compounding effects of various risk factors, while highlighting promising protective factors and interventions that have the potential to buffer against risk. These interactions have critical implications for early intervention and prevention efforts, discussed below.
This model highlights the various mechanisms through which genetic risk interacts with a range of prenatal and environmental stressors to contribute to or compound risk for psychosis. Additionally, several promising protective and promotive factors, as well as preventive interventions, have been identified that may buffer or counteract the effects of exposures across developmental stages.
Clinical implications
Existing early intervention programs, like Coordinated Specialty Care (CSC), have demonstrated efficacy in the treatment of individuals experiencing a first-episode psychosis [411,412,413] through a combination of individual psychotherapy, medication management, family support, and psychoeducation (SAMHSA, 2023), and are being extended to individuals at CHR [414,415,416]. Meta-analytic findings also suggest that broader, non-psychosis-specific treatments, such as CBT [417] and antidepressant medications [418], may be effective in reducing transition rates and improving functional outcomes for CHR individuals, with the incorporation of psychoeducation about CHR status important to mitigate stigma-related stress [419,420,421,422]. However, these treatment models have not yet fully incorporated preventative and protective interventions into their approach. For example, CBT-I approaches could be incorporated for individuals with sleep difficulties [358], and Trauma-focused CBT (TF-CBT) strategies may also aid in decreasing both psychotic and post-traumatic stress symptoms for those with a history of trauma [423]. Mindfulness-based stress reduction (MBSR) techniques [424] could be more regularly offered to expecting mothers to mitigate exposure to maternal stress [425], as well as in children and adolescents to promote stress management. Additionally, families could engage in Family-Focused Therapy (FFT-CHR), which may improve psychotic symptoms among children and adolescents growing up in families with high emotional expression [380]. Beyond the individual-level, family-focused and culturally-informed treatment (CIT-S) has also been shown to significantly decrease psychotic symptoms [426, 427]. Effective integration of these approaches and other resilience-promoting factors discussed in this review requires individualized and targeted treatment plans for implementation at different stages of development. Additionally, understanding the systems and broader sociocultural conditions within which an individual grows up is necessary. Recent evidence finds significant socioeconomic disparities in treatment access and effectiveness of CSC programs [428], suggesting that addressing structural barriers, while challenging, will be critical to ensure early intervention efforts reach those most vulnerable. Finally, a prevention approach that aims to provide information at the population level irrespective of risk status holds the promise of improving large-scale access to education about psychosis risk and protective factors, and may be beneficial for implementation in maternal healthcare, school, and community settings [40, 429,430,431].
Conclusion and future directions
Expanding upon traditional risk-based models of psychosis to incorporate resilience-promoting factors will improve our ability to identify those who will go on to develop psychosis, with the aim of preventing or postponing illness onset and improving outcomes. Examining protective factors across biopsychosocial levels, particularly in early development, may help inform the development of novel interventions and prevention efforts. Given the nascent literature on protective factors in psychosis, further empirical testing of these factors through intervention studies, as well as identification of other resilience-promoting factors, should be a priority. Still, continued research on risk factors remains essential, particularly given the heterogeneous and developmentally dynamic pathways through which psychosis emerges. For example, a growing body of literature suggests that experiencing ethnoracial discrimination is a stressful and potentially traumatic event [432, 433] that may contribute to psychosis risk both independently and in interaction with other early adversities and neighborhood conditions shaped by structural racism [24, 354, 434]. These experiences may play a role in the well-documented racial disparities observed in schizophrenia-spectrum diagnoses [435, 436]. However, there remains a notable gap in research examining ethnoracial discrimination as a developmental risk factor for psychotic disorders, particularly when experienced in childhood or adolescence. Existing studies have more often focused on its relationship with subthreshold psychotic experiences rather than diagnosable psychotic disorders during these vulnerable periods [405, 437, 438]. This review also underscores the need for future research to differentiate more clearly between risk and resilience pathways for affective versus non-affective psychoses, as many existing studies do not distinguish or compare these diagnostic categories of psychosis in relation to specific risk and resilience factors. Adopting a developmental framework to extend current findings across both risk and protective domains, as well as addressing questions of diagnostic specificity, will be essential to advancing a more precise and equitable understanding of psychosis trajectories beginning in early life.
To move towards a resilience-based approach to psychosis, several recommendations are made for future directions. Importantly, protective factors should not be viewed merely as the absence or inverse of risk, but as distinct constructs that warrant dedicated investigation [36, 298]. Research should consider person-centered approaches, including computational or predictive modeling approaches, which characterize the interactions of an individual’s unique risk and resilience factors [224]. Secondly, given the complexity of protective and risk factors interacting across multiple biopsychosocial levels, research efforts should prioritize multi-disciplinary collaboration, including involving individuals with lived experience who may offer invaluable insights [439]. Prospective longitudinal studies tracking risk-resilience interactions over time are also needed to clarify their role across critical developmental periods. Ultimately, moving towards a resilience-based framework holds promise for reshaping how we understand, prevent, and intervene in psychosis. By integrating protective factors into developmental models, we can develop more nuanced, equitable, and effective approaches to supporting individuals at risk.
References
Kahn RS, Sommer IE, Murray RM, Meyer-Lindenberg A, Weinberger DR, Cannon TD, et al. Schizophrenia. Nat Rev Dis Prim. 2015;1:15067. https://doi.org/10.1038/nrdp.2015.67.
Tandon R, Nasrallah H, Akbarian S, Carpenter WT Jr, DeLisi LE, Gaebel W, et al. The schizophrenia syndrome, circa 2024: what we know and how that informs its nature. Schizophr Res. 2024;264:1–28. https://doi.org/10.1016/j.schres.2023.11.015.
Allebeck P. Schizophrenia: a life-shortening disease. Schizophr Bull. 1989;15:81–9. https://doi.org/10.1093/schbul/15.1.81.
Hjorthøj C, Stürup AE, McGrath JJ, Nordentoft M. Years of potential life lost and life expectancy in schizophrenia: a systematic review and meta-analysis. Lancet Psychiatry. 2017;4:295–301. https://doi.org/10.1016/S2215-0366(17)30078-0.
Laursen TM. Life expectancy among persons with schizophrenia or bipolar affective disorder. Schizophr Res. 2011;131:101–4. https://doi.org/10.1016/j.schres.2011.06.008.
Folsom DP, Hawthorne W, Lindamer L, Gilmer T, Bailey A, Golshan S, et al. Prevalence and risk factors for homelessness and utilization of mental health services among 10,340 patients with serious mental illness in a large public mental health system. Am J Psychiatry. 2005;162:370–6. https://doi.org/10.1176/appi.ajp.162.2.370.
Lévesque IS, Abdel-Baki A. Homeless youth with first-episode psychosis: a 2-year outcome study. Schizophr Res. 2020;216:460–9. https://doi.org/10.1016/j.schres.2019.10.031.
Lin D, Kim H, Wada K, Aboumrad M, Powell E, Zwain G, et al. Unemployment, homelessness, and other societal outcomes among US veterans with schizophrenia relapse: a retrospective cohort study. Prim Care Companion CNS Disord. 2022;24:21m03173. https://doi.org/10.4088/PCC.21m03173.
Attepe Özden S, Tekindal M, Tekindal MA. Quality of life of people with schizophrenia: a meta-analysis. Int J Soc Psychiatry. 2023;69:1444–52. https://doi.org/10.1177/00207640231164019.
Fusar-Poli P, Borgwardt S, Bechdolf A, Addington J, Riecher-Rössler A, Schultze-Lutter F, et al. The psychosis high-risk state. JAMA Psychiatry. 2013;70:107–20. https://doi.org/10.1001/jamapsychiatry.2013.269.
Ellman LM, Murphy SK, Maxwell SD. Pre- and perinatal risk factors for serious mental disorders: ethical considerations in prevention and prediction efforts. J Ethics Ment Health. 2018;10:5.
Mittal VA, Ellman LM, Cannon TD. Gene-environment interaction and covariation in schizophrenia: the role of obstetric complications. Schizophr Bull. 2008;34:1083–94. https://doi.org/10.1093/schbul/sbn080.
Pruessner M, Cullen AE, Aas M, Walker EF. The neural diathesis-stress model of schizophrenia revisited: an update on recent findings considering illness stage and neurobiological and methodological complexities. Neurosci Biobehav Rev. 2017;73:191–218. https://doi.org/10.1016/j.neubiorev.2016.12.013.
Bhardwaj RD, Curtis MA, Spalding KL, Buchholz BA, Fink D, Björk-Eriksson T, et al. Neocortical neurogenesis in humans is restricted to development. Proc Natl Acad Sci USA. 2006;103:12564–8. https://doi.org/10.1073/pnas.0605177103.
Gilmore JH, Santelli RK, Gao W. Imaging structural and functional brain development in early childhood. Nat Rev Neurosci. 2018;19:123–37. https://doi.org/10.1038/nrn.2018.1.
Juraska JM. The last stage of development: the restructuring and plasticity of the cortex during adolescence especially at puberty. Dev Psychobiol. 2024;66:e22468. https://doi.org/10.1002/dev.22468.
Pfeifer JH, Allen NB. Puberty initiates cascading relationships between neurodevelopmental, social, and internalizing processes across adolescence. Biol Psychiatry. 2021;89:99–108. https://doi.org/10.1016/j.biopsych.2020.09.002.
Blakemore S-J, Mills KL. Is adolescence a sensitive period for sociocultural processing? Annu Rev Psychol. 2014;65:187–207. https://doi.org/10.1146/annurev-psych-010213-115202.
Dahl RE. Adolescent brain development: a period of vulnerabilities and opportunities. Keynote address. Ann N Y Acad Sci. 2004;1021:1–22. https://doi.org/10.1196/annals.1308.001.
Reynolds LM, Flores C. Mesocorticolimbic dopamine pathways across adolescence: diversity in development. Front Neural Circuits. 2021;15:735625. https://doi.org/10.3389/fncir.2021.735625.
Vijayakumar N, Ball G, Seal ML, Mundy L, Whittle S, Silk T. The development of structural covariance networks during the transition from childhood to adolescence. Sci Rep. 2021;11:9451. https://doi.org/10.1038/s41598-021-88918-w.
Knudsen EI. Sensitive periods in the development of the brain and behavior. J Cogn Neurosci. 2004;16:1412–25. https://doi.org/10.1162/0898929042304796.
Walker EF, Diforio D. Schizophrenia: a neural diathesis-stress model. Psychol Rev. 1997;104:667–85. https://doi.org/10.1037/0033-295x.104.4.667.
Anglin DM, Ereshefsky S, Klaunig MJ, Bridgwater MA, Niendam TA, Ellman LM, et al. From womb to neighborhood: a racial analysis of social determinants of psychosis in the United States. Am J Psychiatry. 2021;178:599–610. https://doi.org/10.1176/appi.ajp.2020.20071091.
Lipner E, Murphy SK, Ellman LM. Prenatal maternal stress and the cascade of risk to schizophrenia spectrum disorders in offspring. Curr Psychiatry Rep. 2019;21:99. https://doi.org/10.1007/s11920-019-1085-1.
Radua J, Ramella-Cravaro V, Ioannidis J, Reichenberg A, Phiphopthatsanee N, Amir T, et al. What causes psychosis? An umbrella review of risk and protective factors. World Psychiatry. 2018;17:49–66. https://doi.org/10.1002/wps.20490.
Shah JL, Malla AK. Much ado about much: stress, dynamic biomarkers and HPA axis dysregulation along the trajectory to psychosis. Schizophr Res. 2015;162:253–60. https://doi.org/10.1016/j.schres.2015.01.010.
Owen MJ, Legge SE, Rees E, Walters JTR, O’Donovan MC. Genomic findings in schizophrenia and their implications. Mol Psychiatry. 2023;28:3638–47. https://doi.org/10.1038/s41380-023-02293-8.
Margolis ET, Gabard-Durnam LJ. Prenatal influences on postnatal neuroplasticity: integrating DOHaD and sensitive/critical period frameworks to understand biological embedding in early development. Infancy. 2025;30:e12588. https://doi.org/10.1111/infa.12588.
McGlashan TH, Walsh BC, Woods SW. The Psychosis-Risk Syndrome: Handbook for Diagnosis and Follow-up. Oxford University Press; New York: 2010.
Fusar-Poli P, Bonoldi I, Yung AR, Borgwardt S, Kempton MJ, Valmaggia L, et al. Predicting psychosis: meta-analysis of transition outcomes in individuals at high clinical risk. Arch Gen Psychiatry. 2012;69:220–9. https://doi.org/10.1001/archgenpsychiatry.2011.1472.
Salazar de Pablo G, Radua J, Pereira J, Bonoldi I, Arienti V, Besana F, et al. Probability of transition to psychosis in individuals at clinical high risk: an updated meta-analysis. JAMA Psychiatry. 2021;78:970–8. https://doi.org/10.1001/jamapsychiatry.2021.0830.
Addington J, Stowkowy J, Liu L, Cadenhead KS, Cannon TD, Cornblatt BA, et al. Clinical and functional characteristics of youth at clinical high-risk for psychosis who do not transition to psychosis. Psychol Med. 2019;49:1670–7. https://doi.org/10.1017/S0033291718002258.
Simon AE, Borgwardt S, Riecher-Rössler A, Velthorst E, de Haan L, Fusar-Poli P. Moving beyond transition outcomes: meta-analysis of remission rates in individuals at high clinical risk for psychosis. Psychiatry Res. 2013;209:266–72. https://doi.org/10.1016/j.psychres.2013.03.004.
Worthington MA, Addington J, Bearden CE, Cadenhead KS, Cornblatt BA, Keshavan M, et al. Individualized prediction of prodromal symptom remission for youth at clinical high risk for psychosis. Schizophr Bull. 2022;48:395–404. https://doi.org/10.1093/schbul/sbab115.
Thakkar KN, McCleery A, Minor KS, Lee J, Humpston CS, Chopik WJ, et al. Moving from risk to resilience in psychosis research. Nat Rev Psychol. 2023;2:537–55. https://doi.org/10.1038/s44159-023-00205-9.
Althwanay A, AlZamil NA, Almukhadhib OY, Alkhunaizi S, Althwanay R. Risks and protective factors of the prodromal stage of psychosis: a literature review. Cureus. 2020;12:e8639. https://doi.org/10.7759/cureus.8639.
Davies C, Segre G, Estradé A, Radua J, De Micheli A, Provenzani U, et al. Prenatal and perinatal risk and protective factors for psychosis: a systematic review and meta-analysis. Lancet Psychiatry. 2020;7:399–410. https://doi.org/10.1016/S2215-0366(20)30057-2.
Oliver D, Reilly TJ, Baccaredda Boy O, Petros N, Davies C, Borgwardt S, et al. What causes the onset of psychosis in individuals at clinical high risk? A Meta-analysis of Risk and Protective Factors. Schizophr Bull. 2020;46:110–20. https://doi.org/10.1093/schbul/sbz039.
Fusar-Poli P, McGorry PD, Kane JM. Improving outcomes of first-episode psychosis: an overview. World Psychiatry. 2017;16:251–65. https://doi.org/10.1002/wps.20446.
Poletti M, Pelizza L, Preti A, Raballo A. Clinical High-Risk for Psychosis (CHR-P) circa 2024: synoptic analysis and synthesis of contemporary treatment guidelines. Asian J Psychiatry. 2024;100:104142. https://doi.org/10.1016/j.ajp.2024.104142.
Buckley PF, Miller BJ, Lehrer DS, Castle DJ. Psychiatric comorbidities and schizophrenia. Schizophr Bull. 2009;35:383–402. https://doi.org/10.1093/schbul/sbn135.
Wilson RS, Yung AR, Morrison AP. Comorbidity rates of depression and anxiety in first episode psychosis: a systematic review and meta-analysis. Schizophr Res. 2020;216:322–9. https://doi.org/10.1016/j.schres.2019.11.035.
Remberk B. Clinical significance of psychotic-like experiences in children and adolescents. Psychiatr Pol. 2017;51:271–82. https://doi.org/10.12740/PP/63894.
Healy C, Brannigan R, Dooley N, Coughlan H, Clarke M, Kelleher I, et al. Childhood and adolescent psychotic experiences and risk of mental disorder: a systematic review and meta-analysis. Psychol Med. 2019;49:1589–99. https://doi.org/10.1017/S0033291719000485.
Barker DJP. Fetal origins of coronary heart disease. BMJ. 1995;311:171–4. https://doi.org/10.1136/bmj.311.6998.171.
Amgalan A, Andescavage N, Limperopoulos C. Prenatal origins of neuropsychiatric diseases. Acta Paediatr. 2021;110:1741–9. 10.1111/apa.15766.
Glynn LM, Sandman CA. Prenatal origins of neurological development: a critical period for fetus and mother. Curr Dir Psychol Sci. 2011;20:384–9. https://doi.org/10.1177/0963721411422056.
ELipner E, O’Brien KJ, Pike MR, Ered A, Ellman LM. Environmental risk factors and cognitive outcomes in psychosis: pre-, perinatal, and early life adversity. In: Current topics in behavioral neurosciences. Berlin, Heidelberg: Springer; 2022. pp. 1–36. Available from: https://link.springer.com/chapter/10.1007/7854_2022_378. Accessed 30 Oct 2022.
Monk C, Lugo-Candelas C, Trumpff C. Prenatal developmental origins of future psychopathology: mechanisms and pathways. Annu Rev Clin Psychol. 2019;15:317–44. https://doi.org/10.1146/annurev-clinpsy-050718-095539.
McNeil TF, Cantor-Graae E, Weinberger DR. Relationship of obstetric complications and differences in size of brain structures in monozygotic twin pairs discordant for schizophrenia. Am J Psychiatry. 2000;157:203–12. https://doi.org/10.1176/appi.ajp.157.2.203.
Cannon M, Jones PB, Murray RM. Obstetric complications and schizophrenia: historical and meta-analytic review. Am J Psychiatry. 2002;159:1080–92. https://doi.org/10.1176/appi.ajp.159.7.1080.
Gross C, Hen R. The developmental origins of anxiety. Nat Rev Neurosci. 2004;5:545–52. https://doi.org/10.1038/nrn1429.
Lipner E, Mac Giollabhui N, Breen EC, Cohn BA, Krigbaum NY, Cirillo PM, et al. Sex-specific pathways from prenatal maternal inflammation to adolescent depressive symptoms. JAMA Psychiatry. 2024;81:498–505. https://doi.org/10.1001/jamapsychiatry.2023.5458.
Pike MR, Lipner E, O'Brien KJ, Breen EC, Cohn BA, Cirillo PM, et al. Prenatal maternal Inflammation, childhood cognition and adolescent depressive symptoms. Brain Behav Immun. 2024;119:908–18. https://doi.org/10.1016/j.bbi.2024.05.012.
Serati M, Bertino V, Malerba MR, Mucci F, Barkin JL, Grassi S, et al. Obstetric complications and subsequent risk of mood disorders for offspring in adulthood: a comprehensive overview. Nord J Psychiatry. 2020;74:470–8. https://doi.org/10.1080/08039488.2020.1751878.
Serati M, Barkin JL, Orsenigo G, Altamura AC, Buoli M. Research review: the role of obstetric and neonatal complications in childhood attention deficit and hyperactivity disorder - a systematic review. J Child Psychol Psychiatry. 2017;58:1290–300. https://doi.org/10.1111/jcpp.12779.
Hisle-Gorman E, Susi A, Stokes T, Gorman G, Erdie-Lalena C, Nylund CM. Prenatal, perinatal, and neonatal risk factors of autism spectrum disorder. Pediatr Res. 2018;84:190–8. https://doi.org/10.1038/pr.2018.23.
Beydoun H, Saftlas AF. Physical and mental health outcomes of prenatal maternal stress in human and animal studies: a review of recent evidence. Paediatr Perinat Epidemiol. 2008;22:438–66. https://doi.org/10.1111/j.1365-3016.2008.00951.x.
Glover V, O’Donnell KJ, O’Connor TG, Fisher J. Prenatal maternal stress, fetal programming, and mechanisms underlying later psychopathology—a global perspective. Dev Psychopathol. 2018;30:843–54. https://doi.org/10.1017/S095457941800038X.
Manzari N, Matvienko-Sikar K, Baldoni F, O’Keeffe GW, Khashan AS. Prenatal maternal stress and risk of neurodevelopmental disorders in the offspring: a systematic review and meta-analysis. Soc Psychiatry Psychiatr Epidemiol. 2019;54:1299–309. https://doi.org/10.1007/s00127-019-01745-3.
Fineberg AM, Ellman LM, Schaefer CA, Maxwell SD, Shen L, Chaudhury NH, et al. Fetal exposure to maternal stress and risk for schizophrenia spectrum disorders among offspring: differential influences of fetal sex. Psychiatry Res. 2016;236:91–7. https://doi.org/10.1016/j.psychres.2015.12.026.
Roseboom TJ, Painter RC, van Abeelen AFM, Veenendaal MVE, de Rooij SR. Hungry in the womb: what are the consequences? Lessons from the Dutch famine. Maturitas. 2011;70:141–5. https://doi.org/10.1016/j.maturitas.2011.06.017.
Song S, Wang W, Hu P. Famine, death, and madness: schizophrenia in early adulthood after prenatal exposure to the Chinese Great Leap Forward Famine. Soc Sci Med. 2009;68:1315–21. https://doi.org/10.1016/j.socscimed.2009.01.027.
He P, Luo Y, Guo C, Chen G, Song X, Zheng X. Prenatal war exposure and schizophrenia in adulthood: evidence from the Sino-Japanese War of 1937-1945. Soc Psychiatry Psychiatr Epidemiol. 2019;54:313–20. https://doi.org/10.1007/s00127-018-1584-0.
Levine SZ, Levav I, Goldberg Y, Pugachova I, Becher Y, Yoffe R. Exposure to genocide and the risk of schizophrenia: a population-based study. Psychol Med. 2016;46:855–63. https://doi.org/10.1017/S0033291715002354.
Malaspina D, Corcoran C, Kleinhaus KR, Perrin MC, Fennig S, Nahon D, et al. Acute maternal stress in pregnancy and schizophrenia in offspring: a cohort prospective study. BMC Psychiatry. 2008;8:71. https://doi.org/10.1186/1471-244X-8-71.
Os JV, Selten J-P. Prenatal exposure to maternal stress and subsequent schizophrenia: the May 1940 invasion of the Netherlands. Br J Psychiatry. 1998;172:324–6. https://doi.org/10.1192/bjp.172.4.324.
Weinstein Y, Levav I, Gelkopf M, Roe D, Yoffe R, Pugachova I, et al. Association of maternal exposure to terror attacks during pregnancy and the risk of schizophrenia in the offspring: a population-based study. Schizophr Res. 2018;199:163–7. https://doi.org/10.1016/j.schres.2018.04.024.
Guo C, He P, Song X, Zheng X. Long-term effects of prenatal exposure to earthquake on adult schizophrenia. Br J Psychiatry. 2019;215:730–5. https://doi.org/10.1192/bjp.2019.114.
Selten J-P, van der Graaf Y, van Duursen R, Gispen-de Wied CC, Kahn RS. Psychotic illness after prenatal exposure to the 1953 Dutch Flood Disaster. Schizophr Res. 1999;35:243–5. https://doi.org/10.1016/S0920-9964(98)00143-1.
Abel KM, Heuvelman HP, Jörgensen L, Magnusson C, Wicks S, Susser E, et al. Severe bereavement stress during the prenatal and childhood periods and risk of psychosis in later life: population based cohort study. BMJ. 2014;348:f7679. https://doi.org/10.1136/bmj.f7679.
Huttunen MO, Niskanen P. Prenatal loss of father and psychiatric disorders. Arch Gen Psychiatry. 1978;35:429–31. https://doi.org/10.1001/archpsyc.1978.01770280039004.
Ellman LM, Yolken RH, Buka SL, Torrey EF, Cannon TD. Cognitive functioning prior to the onset of psychosis: the role of fetal exposure to serologically determined influenza infection. Biol Psychiatry. 2009;65:1040–7. https://doi.org/10.1016/j.biopsych.2008.12.015.
Betts KS, Williams GM, Najman JM, Scott J, Alati R. Exposure to stressful life events during pregnancy predicts psychotic experiences via behaviour problems in childhood. J Psychiatr Res. 2014;59:132–9. https://doi.org/10.1016/j.jpsychires.2014.08.001.
Pugliese V, Bruni A, Carbone EA, Calabrò G, Cerminara G, Sampogna G, et al. Maternal stress, prenatal medical illnesses and obstetric complications: risk factors for schizophrenia spectrum disorder, bipolar disorder and major depressive disorder. Psychiatry Res. 2019;271:23–30. https://doi.org/10.1016/j.psychres.2018.11.023.
King S, Laplante DP, Brunet A, Barr RG, Zelazo P, Saucier JF, et al. Project ice storm: a prospective study of the effects of prenatal maternal stress on other risk factors for schizophrenia. Schizophr Res. 2003;60:41–2. https://doi.org/10.1016/S0920-9964(03)80121-4.
King S, Laplante D, Joober R. Understanding putative risk factors for schizophrenia: retrospective and prospective studies. J Psychiatry Neurosci. 2005;30:342–8.
Lobel M. Conceptualizations, measurement, and effects of prenatal maternal stress on birth outcomes. J Behav Med. 1994;17:225–72.
McNeil TF, Schubert EW, Brossner M, Schubert P, Henriksson KM. Unwanted pregnancy as a risk factor for offspring schizophrenia-spectrum and affective disorders in adulthood: a prospective high-risk study. Psychol Med. 2008;39:957–65. https://doi.org/10.1017/S0033291708004479.
Mawson ER, Morris BJ. A consideration of the increased risk of schizophrenia due to prenatal maternal stress, and the possible role of microglia. Prog Neuropsychopharmacol Biol Psychiatry. 2023;125:110773. https://doi.org/10.1016/j.pnpbp.2023.110773.
Beijers R, Buitelaar JK, de Weerth C. Mechanisms underlying the effects of prenatal psychosocial stress on child outcomes: beyond the HPA axis. Eur Child Adolesc Psychiatry. 2014;23:943–56. https://doi.org/10.1007/s00787-014-0566-3.
Ellman LM, Schetter CD, Hobel CJ, Chicz-Demet A, Glynn LM, Sandman CA. Timing of fetal exposure to stress hormones: effects on newborn physical and neuromuscular maturation. Dev Psychobiol. 2008;50:232–41. https://doi.org/10.1002/dev.20293.
Sandman CA, Glynn L, Schetter CD, Wadhwa P, Garite T, Chicz-DeMet A, et al. Elevated maternal cortisol early in pregnancy predicts third trimester levels of placental corticotropin releasing hormone (CRH): priming the placental clock. Peptides. 2006;27:1457–63. https://doi.org/10.1016/j.peptides.2005.10.002.
Davis EP, Sandman CA. The timing of prenatal exposure to maternal cortisol and psychosocial stress is associated with human infant cognitive development. Child Dev. 2010;81:131–48. https://doi.org/10.1111/j.1467-8624.2009.01385.x.
Howland MA, Sandman CA, Glynn LM. Developmental origins of the human hypothalamic-pituitary-adrenal axis. Expert Rev Endocrinol Metab. 2017;12:321–39. https://doi.org/10.1080/17446651.2017.1356222.
Sandman CA. Prenatal CRH: An integrating signal of fetal distress. Dev Psychopathol. 2018;30:941–52. https://doi.org/10.1017/S0954579418000664.
Glynn LM, Wadhwa PD, Dunkel-Schetter C, Chicz-Demet A, Sandman CA. When stress happens matters: effects of earthquake timing on stress responsivity in pregnancy. Am J Obstet Gynecol. 2001;184:637–42. https://doi.org/10.1067/mob.2001.111066.
Ellman LM, Murphy SK, Maxwell SD, Calvo EM, Cooper T, Schaefer CA, et al. Maternal cortisol during pregnancy and offspring schizophrenia: influence of fetal sex and timing of exposure. Schizophr Res. 2019;213:15–22. https://doi.org/10.1016/j.schres.2019.07.002.
Liu Y, Heron J, Hickman M, Zammit S, Wolke D. Prenatal stress and offspring depression in adulthood: the mediating role of childhood trauma. J Affect Disord. 2022;297:45–52. https://doi.org/10.1016/j.jad.2021.10.019.
Grizenko N, Shayan YR, Polotskaia A, Ter-Stepanian M, Joober R. Relation of maternal stress during pregnancy to symptom severity and response to treatment in children with ADHD. J Psychiatry Neurosci. 2008;33:10–6.
Beversdorf DQ, Stevens HE, Jones KL. Prenatal stress, maternal immune dysregulation, and their association with autism spectrum disorders. Curr Psychiatry Rep. 2018;20:76. https://doi.org/10.1007/s11920-018-0945-4.
Abbott PW, Gumusoglu SB, Bittle J, Beversdorf DQ, Stevens HE. Prenatal stress and genetic risk: how prenatal stress interacts with genetics to alter risk for psychiatric illness. Psychoneuroendocrinology. 2018;90:9–21. https://doi.org/10.1016/j.psyneuen.2018.01.019.
Laplante DP, Barr RG, Brunet A, Galbaud du Fort G, Meaney ML, Saucier JF, et al. Stress during pregnancy affects general intellectual and language functioning in human toddlers. Pediatr Res. 2004;56:400–10. https://doi.org/10.1203/01.PDR.0000136281.34035.44.
Buss C, Davis EP, Hobel CJ, Sandman CA. Maternal pregnancy-specific anxiety is associated with child executive function at 6–9 years age. Stress. 2011;14:665–76. https://doi.org/10.3109/10253890.2011.623250.
Laplante DP, Brunet A, Schmitz N, Ciampi A, King S. Project Ice Storm: prenatal maternal stress affects cognitive and linguistic functioning in 5 1/2-year-old children. J Am Acad Child Adolesc Psychiatry. 2008;47:1063–72. https://doi.org/10.1097/CHI.0b013e31817eec80.
Grace T, Bulsara M, Robinson M, Hands B. The impact of maternal gestational stress on motor development in late childhood and adolescence: a longitudinal study. Child Dev. 2016;87:211–20. https://doi.org/10.1111/cdev.12449.
Buss C, Davis EP, Hobel CJ, Sandman CA. Maternal pregnancy-specific anxiety is associated with child executive function at 6-9 years age. Stress. 2011;14:665–76. https://doi.org/10.3109/10253890.2011.623250.
Straley ME, Van Oeffelen W, Theze S, Sullivan AM, O'Mahony SM, Cryan JF, et al. Distinct alterations in motor & reward seeking behavior are dependent on the gestational age of exposure to LPS-induced maternal immune activation. Brain Behav Immun. 2017;63:21–34. https://doi.org/10.1016/j.bbi.2016.06.002.
Kappelmann N, Perry BI, Khandaker GM. Prenatal and childhood immuno-metabolic risk factors for adult depression and psychosis. Harv Rev Psychiatry. 2022;30:8–23. https://doi.org/10.1097/HRP.0000000000000322.
Barr CE, Mednick SA, Munk-Jorgensen P. Exposure to influenza epidemics during gestation and adult schizophrenia. a 40-year stud. Arch Gen Psychiatry. 1990;47:869–74. https://doi.org/10.1001/archpsyc.1990.01810210077012.
Kendell RE, Kemp IW. Maternal influenza in the etiology of schizophrenia. Arch Gen Psychiatry. 1989;46:878–82. https://doi.org/10.1001/archpsyc.1989.01810100020004.
Mednick SA, Machon RA, Huttunen MO, Bonett D. Adult schizophrenia following prenatal exposure to an influenza epidemic. Arch Gen Psychiatry. 1988;45:189–92. https://doi.org/10.1001/archpsyc.1988.01800260109013.
Sham PC, O’Callaghan E, Takei N, Murray GK, Hare EH, Murray RM. Schizophrenia following pre-natal exposure to influenza epidemics between 1939 and 1960. Br J Psychiatry. 1992;160:461–6. https://doi.org/10.1192/bjp.160.4.461.
Brown AS, Derkits EJ. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry. 2010;167:261–80. https://doi.org/10.1176/appi.ajp.2009.09030361.
Ellman LM, Susser ES. The promise of epidemiologic studies: neuroimmune mechanisms in the etiologies of brain disorders. Neuron. 2009;64:25–7. https://doi.org/10.1016/j.neuron.2009.09.024.
Babulas V, Factor-Litvak P, Goetz R, Schaefer CA, Brown AS. Prenatal exposure to maternal genital and reproductive infections and adult schizophrenia. Am J Psychiatry. 2006;163:927–9. https://doi.org/10.1176/ajp.2006.163.5.927.
Brown AS, Cohen P, Greenwald S, Susser E. Nonaffective psychosis after prenatal exposure to rubella. Am J Psychiatry. 2000;157:438–43. https://doi.org/10.1176/appi.ajp.157.3.438.
Brown AS, Begg MD, Gravenstein S, Schaefer CA, Wyatt RJ, Bresnahan M, et al. Serologic evidence of prenatal influenza in the etiology of schizophrenia. Arch Gen Psychiatry. 2004;61:774–80. https://doi.org/10.1001/archpsyc.61.8.774.
Brown AS, Schaefer CA, Quesenberry CP, Liu L, Babulas VP, Susser ES. Maternal exposure to toxoplasmosis and risk of schizophrenia in adult offspring. Am J Psychiatry. 2005;162:767–73. https://doi.org/10.1176/appi.ajp.162.4.767.
Brown AS, Schaefer CA, Wyatt RJ, Goetz R, Begg MD, Gorman JM, et al. Maternal exposure to respiratory infections and adult schizophrenia spectrum disorders: a prospective birth cohort study. Schizophr Bull. 2000;26:287–95. https://doi.org/10.1093/oxfordjournals.schbul.a033453.
Buka SL, Cannon TD, Torrey EF, Yolken RH, Collaborative Study Group on the Perinatal Origins of Severe Psychiatric Disorders. Maternal exposure to herpes simplex virus and risk of psychosis among adult offspring. Biol Psychiatry. 2008;63:809–15. https://doi.org/10.1016/j.biopsych.2007.09.022.
Mortensen PB, Nørgaard-Pedersen B, Waltoft BL, Sørensen TL, Hougaard D, Torrey EF, et al. Toxoplasma gondii as a risk factor for early-onset schizophrenia: analysis of filter paper blood samples obtained at birth. Biol Psychiatry. 2007;61:688–93. https://doi.org/10.1016/j.biopsych.2006.05.024.
Zhou Y-Y, Zhang W-W, Chen F, Hu S-S, Jiang H-Y. Maternal infection exposure and the risk of psychosis in the offspring: a systematic review and meta-analysis. J Psychiatr Res. 2021;135:28–36. https://doi.org/10.1016/j.jpsychires.2020.12.065.
Fineberg AM, Ellman LM. Inflammatory cytokines and neurological and neurocognitive alterations in the course of schizophrenia. Biol Psychiatry. 2013;73:951–66. https://doi.org/10.1016/j.biopsych.2013.01.001.
Allswede DM, Yolken RH, Buka SL, Cannon TD. Cytokine concentrations throughout pregnancy and risk for psychosis in adult offspring: a longitudinal case-control study. Lancet Psychiatry. 2020;7:254–61. https://doi.org/10.1016/S2215-0366(20)30006-7.
Miller BJ, Culpepper N, Rapaport MH, Buckley P. Prenatal inflammation and neurodevelopment in schizophrenia: a review of human studies. Prog Neuropsychopharmacol Biol Psychiatry. 2013;42:92–100. https://doi.org/10.1016/j.pnpbp.2012.03.010.
Goldstein JM, Cherkerzian S, Seidman LJ, Donatelli JA, Remington AG, Tsuang MT, et al. Prenatal maternal immune disruption and sex-dependent risk for psychoses. Psychol Med. 2014;44:3249–61. https://doi.org/10.1017/S0033291714000683.
Canetta S, Sourander A, Surcel HM, Hinkka-Yli-Salomäki S, Leiviskä J, Kellendonk C, et al. Elevated maternal C-reactive protein and increased risk of schizophrenia in a national birth cohort. Am J Psychiatry. 2014;171:960–8. https://doi.org/10.1176/appi.ajp.2014.13121579.
Buka SL, Tsuang MT, Torrey EF, Klebanoff MA, Wagner RL, Yolken RH. Maternal cytokine levels during pregnancy and adult psychosis. Brain Behav Immun. 2001;15:411–20. https://doi.org/10.1006/brbi.2001.0644.
Dammann O, Leviton A. Maternal intrauterine infection, cytokines, and brain damage in the preterm newborn. Pediatr Res. 1997;42:1–8. https://doi.org/10.1203/00006450-199707000-00001.
Howerton CL, Bale TL. Prenatal programing: at the intersection of maternal stress and immune activation. Horm Behav. 2012;62:237–42. https://doi.org/10.1016/j.yhbeh.2012.03.007.
Zaretsky MV, Alexander JM, Byrd W, Bawdon RE. Transfer of inflammatory cytokines across the placenta. Obstet Gynecol. 2004;103:546–50. https://doi.org/10.1097/01.AOG.0000114980.40445.83.
Depino AM. Perinatal inflammation and adult psychopathology: From preclinical models to humans. Semin Cell Dev Biol. 2018;77:104–14. https://doi.org/10.1016/j.semcdb.2017.09.010.
Spencer SJ, Meyer U. Perinatal programming by inflammation. Brain Behav Immun. 2017;63:1–7. https://doi.org/10.1016/j.bbi.2017.02.007.
Brown AS, Vinogradov S, Kremen WS, Poole JH, Deicken RF, Penner JD, et al. Prenatal exposure to maternal infection and executive dysfunction in adult schizophrenia. Am J Psychiatry. 2009;166:683–90. https://doi.org/10.1176/appi.ajp.2008.08010089.
Brown AS, Vinogradov S, Kremen WS, Poole JH, Bao Y, Kern D, et al. Association of maternal genital and reproductive infections with verbal memory and motor deficits in adult schizophrenia. Psychiatry Res. 2011;188:179–86. https://doi.org/10.1016/j.psychres.2011.04.020.
Ellman LM, Deicken RF, Vinogradov S, Kremen WS, Poole JH, Kern DM, et al. Structural brain alterations in schizophrenia following fetal exposure to the inflammatory cytokine interleukin-8. Schizophr Res. 2010;121:46–54. https://doi.org/10.1016/j.schres.2010.05.014.
Brown AS, Deicken RF, Vinogradov S, Kremen WS, Poole JH, Penner JD, et al. Prenatal infection and cavum septum pellucidum in adult schizophrenia. Schizophr Res. 2009;108:285–7. https://doi.org/10.1016/j.schres.2008.11.018.
Cannon TD, van Erp TG, Rosso IM, Huttunen M, Lönnqvist J, Pirkola T, et al. Fetal hypoxia and structural brain abnormalities in schizophrenic patients, their siblings, and controls. Arch Gen Psychiatry. 2002;59:35–41. https://doi.org/10.1001/archpsyc.59.1.35.
Vilotić A, Nacka-Aleksić M, Pirković A, Bojić-Trbojević Ž, Dekanski D, Jovanović Krivokuća M. IL-6 and IL-8: an overview of their roles in healthy and pathological pregnancies. Int J Mol Sci. 2022;23:14574. https://doi.org/10.3390/ijms232314574.
Piešová M, Mach M. Impact of perinatal hypoxia on the developing brain. Physiol Res. 2020;69:199–213. https://doi.org/10.33549/physiolres.934198.
Fox R, Kitt J, Leeson P, Aye CYL, Lewandowski AJ. Preeclampsia: risk factors, diagnosis, management, and the cardiovascular impact on the offspring. J Clin Med. 2019;8:1625 https://doi.org/10.3390/jcm8101625.
Giussani DA. Breath of life: heart disease link to developmental hypoxia. Circulation. 2021;144:1429–43. https://doi.org/10.1161/CIRCULATIONAHA.121.054689.
Sharma S, Norris WE, Kalkunte S. Beyond the threshold: an etiological bridge between hypoxia and immunity in preeclampsia. J Reprod Immunol. 2010;85:112–6. https://doi.org/10.1016/j.jri.2010.01.002.
Cannon TD, Mednick SA, Parnas J. Genetic and perinatal determinants of structural brain deficits in schizophrenia. Arch Gen Psychiatry. 1989;46:883–9. https://doi.org/10.1001/archpsyc.1989.01810100025005.
Cannon TD, Rosso IM, Hollister JM, Bearden CE, Sanchez LE, Hadley T. A prospective cohort study of genetic and perinatal influences in the etiology of schizophrenia. Schizophr Bull. 2000;26:351–66. https://doi.org/10.1093/oxfordjournals.schbul.a033458.
Rosso I, Cannon T, Huttunen T, Huttunen M, Lonnqvist J, Gasperoni T. Obstetric risk factors for early-onset schizophrenia in a finnish birth cohort. Am J psychiatry. 2000;157:801–7. https://doi.org/10.1176/appi.ajp.157.5.801.
Seidman LJ, Buka SL, Goldstein JM, Horton NJ, Rieder RO, Tsuang MT. The relationship of prenatal and perinatal complications to cognitive functioning at age 7 in the New England Cohorts of the National Collaborative Perinatal Project. Schizophr Bull. 2000;26:309–21. https://doi.org/10.1093/oxfordjournals.schbul.a033455.
van Erp TG, Saleh PA, Rosso IM, Huttunen M, Lönnqvist J, Pirkola T, et al. Contributions of genetic risk and fetal hypoxia to hippocampal volume in patients with schizophrenia or schizoaffective disorder, their unaffected siblings, and healthy unrelated volunteers. Am J Psychiatry. 2002;159:1514–20. https://doi.org/10.1176/appi.ajp.159.9.1514.
Cannon TD, Yolken R, Buka S, Torrey EF, Collaborative Study Group on the Perinatal Origins of Severe Psychiatric Disorders. Decreased neurotrophic response to birth hypoxia in the etiology of schizophrenia. Biol Psychiatry. 2008;64:797–802. https://doi.org/10.1016/j.biopsych.2008.04.012.
Nicodemus KK, Marenco S, Batten AJ, Vakkalanka R, Egan MF, Straub RE, et al. Serious obstetric complications interact with hypoxia-regulated/vascular-expression genes to influence schizophrenia risk. Mol Psychiatry. 2008;13:873–7. https://doi.org/10.1038/sj.mp.4002153.
Schmidt-Kastner R, van Os J, Steinbusch HWM, Schmitz C. Gene regulation by hypoxia and the neurodevelopmental origin of schizophrenia. Schizophr Res. 2006;84:253–71. https://doi.org/10.1016/j.schres.2006.02.022.
Ursini G, Punzi G, Chen Q, Marenco S, Robinson JF, Porcelli A, et al. Convergence of placenta biology and genetic risk for schizophrenia. Nat Med. 2018;24:792–801. https://doi.org/10.1038/s41591-018-0021-y.
Kendall G, Peebles D. Acute fetal hypoxia: the modulating effect of infection. Early Hum Dev. 2005;81:27–34.
Aldinger JK, Abele H, Kranz A. Prenatal Maternal Psychological Stress (PMPS) and its effect on the maternal and neonatal outcome: a retrospective cohort study. Healthcare. 2024;12:2431 https://doi.org/10.3390/healthcare12232431.
Niemelä S, Sourander A, Surcel HM, Hinkka-Yli-Salomäki S, McKeague IW, Cheslack-Postava K, et al. Prenatal nicotine exposure and risk of schizophrenia among offspring in a national birth cohort. Am J Psychiatry. 2016;173:799–806. https://doi.org/10.1176/appi.ajp.2016.15060800.
Verdolini N, Mezquida G, Valli I, Garcia-Rizo C, Cuesta M, Vieta E, et al. Obstetric complications and clinical presentation in first episode of psychosis. Acta Neuropsychiatr. 2023;35:156–64. https://doi.org/10.1017/neu.2023.9.
Ellis RJ, Bara A, Vargas CA, Frick AL, Loh E, Landry J, et al. Prenatal Δ9-tetrahydrocannabinol exposure in males leads to motivational disturbances related to striatal epigenetic dysregulation. Biol Psychiatry. 2022;92:127–38. https://doi.org/10.1016/j.biopsych.2021.09.017.
Fine JD, Moreau AL, Karcher NR, Agrawal A, Rogers CE, Barch DM, et al. Association of prenatal cannabis exposure with psychosis proneness among children in the Adolescent Brain Cognitive Development (ABCD) study. JAMA Psychiatry. 2019;76:762–4. https://doi.org/10.1001/jamapsychiatry.2019.0076.
Burd L, Roberts D, Olson M, Odendaal H. Ethanol and the placenta: a review. J Matern Fetal Neonatal Med. 2007;20:361–75. https://doi.org/10.1080/14767050701298365.
Zammit S, Odd D, Horwood J, Thompson A, Thomas K, Menezes P, et al. Investigating whether adverse prenatal and perinatal events are associated with non-clinical psychotic symptoms at age 12 years in the ALSPAC birth cohort. Psychol Med. 2009;39:1457–67. https://doi.org/10.1017/S0033291708005126.
Lobel M, Cannella DL, Graham JE, DeVincent C, Schneider J, Meyer BA. Pregnancy-specific stress, prenatal health behaviors, and birth outcomes. Health Psychol. 2008;27:604–15. https://doi.org/10.1037/a0013242.
Zeng S, Cheng X, Chen R, Wu J, Zhou J. Low level of vitamin D is a risk factor for the occurrence of early and late onset pre-eclampsia in pregnant women. Clin Lab. 2020. https://doi.org/10.7754/Clin.Lab.2019.191022.
De Weerth C. Prenatal stress and the development of psychopathology: Lifestyle behaviors as a fundamental part of the puzzle. Dev Psychopathol. 2018;30:1129–44. https://doi.org/10.1017/S0954579418000494.
Marques AH, Bjørke-Monsen A-L, Teixeira AL, Silverman MN. Maternal stress, nutrition and physical activity: impact on immune function, CNS development and psychopathology. Brain Res. 2015;1617:28–46. https://doi.org/10.1016/j.brainres.2014.10.051.
Irwin MR, Olmstead R, Carroll JE. Sleep disturbance, sleep duration, and inflammation: a systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biol Psychiatry. 2016;80:40–52. https://doi.org/10.1016/j.biopsych.2015.05.014.
Gabory A, Roseboom TJ, Moore T, Moore LG, Junien C. Placental contribution to the origins of sexual dimorphism in health and diseases: sex chromosomes and epigenetics. Biol Sex Differ. 2013;4:5. https://doi.org/10.1186/2042-6410-4-5.
Mac Giollabhui N, Breen EC, Murphy SK, Maxwell SD, Cohn BA, Krigbaum NY, et al. Maternal inflammation during pregnancy and offspring psychiatric symptoms in childhood: timing and sex matter. J Psychiatr Res. 2019;111:96–103. https://doi.org/10.1016/j.jpsychires.2019.01.009.
Dalman C, Allebeck P, Cullberg J, Grunewald C, Köster M. Obstetric complications and the risk of schizophrenia: a longitudinal study of a national birth cohort. Arch Gen Psychiatry. 1999;56:234–40. https://doi.org/10.1001/archpsyc.56.3.234.
Grimm VE, Frieder B. Differential vulnerability of male and female rats to the timing of various perinatal insults. Int J Neurosci. 1985;27:155–64. https://doi.org/10.3109/00207458509149763.
Anastario M, Salafia CM, Fitzmaurice G, Goldstein JM. Impact of fetal versus perinatal hypoxia on sex differences in childhood outcomes: developmental timing matters. Soc Psychiatry Psychiatr Epidemiol. 2012;47:455–64. https://doi.org/10.1007/s00127-011-0353-0.
Brown AS. Prenatal infection as a risk factor for schizophrenia. Schizophr Bull. 2006;32:200–2. https://doi.org/10.1093/schbul/sbj052.
Meyer U, Yee BK, Feldon J. The neurodevelopmental impact of prenatal infections at different times of pregnancy: the earlier the worse. Neuroscientist. 2007;13:241–56. https://doi.org/10.1177/1073858406296401.
Zornberg GL, Buka SL, Tsuang MT. Hypoxic-ischemia-related fetal/neonatal complications and risk of schizophrenia and other nonaffective psychoses: a 19-year longitudinal study. Am J Psychiatry. 2000;157:196–202. https://doi.org/10.1176/appi.ajp.157.2.196.
Estes ML, McAllister AK. Maternal immune activation: implications for neuropsychiatric disorders. Science. 2016;353:772–7. https://doi.org/10.1126/science.aag3194.
Bick J, Nelson CA. Early adverse experiences and the developing brain. Neuropsychopharmacol. 2016;41:177–96. https://doi.org/10.1038/npp.2015.252.
Dantchev S, Zammit S, Wolke D. Sibling bullying in middle childhood and psychotic disorder at 18 years: a prospective cohort study. Psychol Med. 2018;48:2321–8. https://doi.org/10.1017/S0033291717003841.
Inyang B, Gondal FJ, Abah GA, Minnal Dhandapani M, Manne M, Khanna M, et al. The role of childhood trauma in psychosis and schizophrenia: a systematic review. Cureus. 2022;14:e21466. https://doi.org/10.7759/cureus.21466.
Mayo D, Corey S, Kelly LH, Yohannes S, Youngquist AL, Stuart BK, et al. The role of trauma and stressful life events among individuals at clinical high risk for psychosis: a review. Front Psychiatry. 2017;8:55. https://doi.org/10.3389/fpsyt.2017.00055.
Varese F, Smeets F, Drukker M, Lieverse R, Lataster T, Viechtbauer W, et al. Childhood adversities increase the risk of psychosis: a meta-analysis of patient-control, prospective- and cross-sectional cohort studies. Schizophr Bull. 2012;38:661–71. https://doi.org/10.1093/schbul/sbs050.
Galván A. Adolescent brain development and contextual influences: a decade in review. J Res Adolesc. 2021;31:843–69. https://doi.org/10.1111/jora.12687.
Green R, Meredith LR, Mewton L, Squeglia LM. Adolescent neurodevelopment within the context of impulsivity and substance use. Curr Addict Rep. 2023;10:166–77. https://doi.org/10.1007/s40429-023-00485-4.
Thorpe HHA, Hamidullah S, Jenkins BW, Khokhar JY. Adolescent neurodevelopment and substance use: receptor expression and behavioral consequences. Pharmacol Ther. 2020;206:107431. https://doi.org/10.1016/j.pharmthera.2019.107431.
Read J, van Os J, Morrison AP, Ross CA. Childhood trauma, psychosis and schizophrenia: a literature review with theoretical and clinical implications. Acta Psychiatr Scand. 2005;112:330–50. https://doi.org/10.1111/j.1600-0447.2005.00634.x.
Stanton KJ, Denietolis B, Goodwin BJ, Dvir Y. Childhood trauma and psychosis: an updated review. Child Adolesc Psychiatr Clin N Am. 2020;29:115–29. https://doi.org/10.1016/j.chc.2019.08.004.
Kraan T, Velthorst E, Smit F, de Haan L, van der Gaag M. Trauma and recent life events in individuals at ultra high risk for psychosis: review and meta-analysis. Schizophr Res. 2015;161:143–9. https://doi.org/10.1016/j.schres.2014.11.026.
Fusar-Poli P, Tantardini M, De Simone S, Ramella-Cravaro V, Oliver D, Kingdon J, et al. Deconstructing vulnerability for psychosis: meta-analysis of environmental risk factors for psychosis in subjects at ultra high-risk. Eur Psychiatry. 2017;40:65–75. https://doi.org/10.1016/j.eurpsy.2016.09.003.
Loewy RL, Corey S, Amirfathi F, Dabit S, Fulford D, Pearson R, et al. Childhood trauma and clinical high risk for psychosis. Schizophr Res. 2019;205:10–4. https://doi.org/10.1016/j.schres.2018.05.003.
Beards S, Gayer-Anderson C, Borges S, Dewey ME, Fisher HL, Morgan C. Life events and psychosis: a review and meta-analysis. Schizophr Bull. 2013;39:740–7. https://doi.org/10.1093/schbul/sbt065.
Bendall S, Jackson HJ, Hulbert CA, McGorry PD. Childhood trauma and psychotic disorders: a systematic, critical review of the evidence. Schizophr Bull. 2008;34:568–79. https://doi.org/10.1093/schbul/sbm121.
Dvir Y, Denietolis B, Frazier JA. Childhood trauma and psychosis. Child Adolesc Psychiatr Clin N Am. 2013;22:629–41. https://doi.org/10.1016/j.chc.2013.04.006.
Giannopoulou I, Georgiades S, Stefanou M-I, Spandidos DA, Rizos E. Links between trauma and psychosis (Review). Exp Ther Med. 2023;26:386. https://doi.org/10.3892/etm.2023.12085.
Kilian S, Asmal L, Chiliza B, Olivier MR, Phahladira L, Scheffler F, et al. Childhood adversity and cognitive function in schizophrenia spectrum disorders and healthy controls: evidence for an association between neglect and social cognition. Psychol Med. 2018;48:2186–93. https://doi.org/10.1017/S0033291717003671.
Vargas T, Lam PH, Azis M, Osborne KJ, Lieberman A, Mittal VA. Childhood trauma and neurocognition in adults with psychotic disorders: a systematic review and meta-analysis. Schizophr Bull. 2019;45:1195–208. https://doi.org/10.1093/schbul/sby150.
Alameda L, Golay P, Baumann PS, Progin P, Mebdouhi N, Elowe J, et al. Mild depressive symptoms mediate the impact of childhood trauma on long-term functional outcome in early psychosis patients. Schizophr Bull. 2017;43:1027–35. https://doi.org/10.1093/schbul/sbw163.
Christy A, Cavero D, Navajeeva S, Murray-O'Shea R, Rodriguez V, Aas M, et al. Association between childhood adversity and functional outcomes in people with psychosis: a meta-analysis. Schizophr Bull. 2023;49:285–96. https://doi.org/10.1093/schbul/sbac105.
Hjelseng IV, Vaskinn A, Ueland T, Lunding SH, Reponen EJ, Steen NE, et al. Childhood trauma is associated with poorer social functioning in severe mental disorders both during an active illness phase and in remission. Schizophr Res. 2022;243:241–6. https://doi.org/10.1016/j.schres.2020.03.015.
Turner S, Harvey C, Hayes L, Castle D, Galletly C, Sweeney S, et al. Childhood adversity and clinical and psychosocial outcomes in psychosis. Epidemiol Psychiatr Sci. 2020;29:e78. https://doi.org/10.1017/S2045796019000684.
Hassan AN, De Luca V. The effect of lifetime adversities on resistance to antipsychotic treatment in schizophrenia patients. Schizophr Res. 2015;161:496–500. https://doi.org/10.1016/j.schres.2014.10.048.
Misiak B, Frydecka D. A history of childhood trauma and response to treatment with antipsychotics in first-episode schizophrenia patients: preliminary results. J Nerv Ment Dis. 2016;204:787–92. https://doi.org/10.1097/NMD.0000000000000567.
Hegelstad W, Berg AO, Bjornestad J, Gismervik K, Johannessen JO, Melle I, et al. Childhood interpersonal trauma and premorbid social adjustment as predictors of symptom remission in first episode psychosis. Schizophr Res. 2021;232:87–94. https://doi.org/10.1016/j.schres.2021.05.015.
Pruessner M, King S, Veru F, Schalinski I, Vracotas N, Abadi S, et al. Impact of childhood trauma on positive and negative symptom remission in first episode psychosis. Schizophr Res. 2021;231:82–9. https://doi.org/10.1016/j.schres.2021.02.023.
McLaughlin KA, Sheridan MA, Lambert HK. Childhood adversity and neural development: deprivation and threat as distinct dimensions of early experience. Neurosci Biobehav Rev. 2014;47:578–91. https://doi.org/10.1016/j.neubiorev.2014.10.012.
McLaughlin KA, Weissman D, Bitrán D. Childhood adversity and neural development: a systematic review. Annu Rev Dev Psychol. 2019;1:277–312. https://doi.org/10.1146/annurev-devpsych-121318-084950.
McLaughlin KA, Sheridan MA. Beyond cumulative risk: a dimensional approach to childhood adversity. Curr Dir Psychol Sci. 2016;25:239–45. https://doi.org/10.1177/0963721416655883.
Sheridan MA, McLaughlin KA. Dimensions of early experience and neural development: deprivation and threat. Trends Cogn Sci. 2014;18:580–5. https://doi.org/10.1016/j.tics.2014.09.001.
Croft J, Heron J, Teufel C, Cannon M, Wolke D, Thompson A, et al. Association of trauma type, age of exposure, and frequency in childhood and adolescence with psychotic experiences in early adulthood. JAMA Psychiatry. 2019;76:79–86. https://doi.org/10.1001/jamapsychiatry.2018.3155.
Ered A, Ellman LM. Specificity of childhood trauma type and attenuated positive symptoms in a non-clinical sample. J Clin Med. 2019;8:1537.
Fisher HL, Jones PB, Fearon P, Craig TK, Dazzan P, Morgan K, et al. The varying impact of type, timing and frequency of exposure to childhood adversity on its association with adult psychotic disorder. Psychol Med. 2010;40:1967–78. https://doi.org/10.1017/S0033291710000231.
Morgan C, Gayer-Anderson C, Beards S, Hubbard K, Mondelli V, Di Forti M, et al. Threat, hostility and violence in childhood and later psychotic disorder: population-based case-control study. Br J Psychiatry. 2020;217:575–82. https://doi.org/10.1192/bjp.2020.133.
Trauelsen AM, Bendall S, Jansen JE, Nielsen HG, Pedersen MB, Trier CH, et al. Childhood adversity specificity and dose-response effect in non-affective first-episode psychosis. Schizophr Res. 2015;165:52–9. https://doi.org/10.1016/j.schres.2015.03.014.
Finkelhor D, Ormrod RK, Turner HA. Poly-victimization: a neglected component in child victimization. Child Abus Negl. 2007;31:7–26. https://doi.org/10.1016/j.chiabu.2006.06.008.
Ford JD. Polyvictimization and developmental trauma in childhood. Eur J Psychotraumatol. 2021;12:1866394. https://doi.org/10.1080/20008198.2020.1866394.
Yang R, Yu Q, Owen CE, Ibarra Aspe G, Wiggins JL. Contributions of childhood abuse and neglect to reward neural substrates in adolescence. Neuroimage Clin. 2021;32:102832. https://doi.org/10.1016/j.nicl.2021.102832.
Bebbington PE, Bhugra D, Brugha T, Singleton N, Farrell M, Jenkins R, et al. Psychosis, victimisation and childhood disadvantage: evidence from the second British National Survey of Psychiatric Morbidity. Br J Psychiatry. 2004;185:220–6. https://doi.org/10.1192/bjp.185.3.220.
Trotta A, Di Forti M, Mondelli V, Dazzan P, Pariante C, David A, et al. Prevalence of bullying victimisation amongst first-episode psychosis patients and unaffected controls. Schizophr Res. 2013;150:169–75. https://doi.org/10.1016/j.schres.2013.07.001.
Trotta A, Murray RM, Fisher HL. The impact of childhood adversity on the persistence of psychotic symptoms: a systematic review and meta-analysis. Psychol Med. 2015;45:2481–98. https://doi.org/10.1017/S0033291715000574.
Schreier A, Wolke D, Thomas K, Horwood J, Hollis C, Gunnell D, et al. Prospective study of peer victimization in childhood and psychotic symptoms in a nonclinical population at age 12 years. Arch Gen Psychiatry. 2009;66:527–36. https://doi.org/10.1001/archgenpsychiatry.2009.23.
Selten JP, Ormel J. Low status, humiliation, dopamine and risk of schizophrenia. Psychol Med. 2023;53:609–13. https://doi.org/10.1017/S0033291722003816.
Selten J-P, van der Ven E, Rutten BPF, Cantor-Graae E. The social defeat hypothesis of schizophrenia: an update. Schizophr Bull. 2013;39:1180–6. https://doi.org/10.1093/schbul/sbt134.
Danese A, Moffitt TE, Arseneault L, Bleiberg BA, Dinardo PB, Gandelman SB, et al. The origins of cognitive deficits in victimized children: implications for neuroscientists and clinicians. Am J Psychiatry. 2017;174:349–61. https://doi.org/10.1176/appi.ajp.2016.16030333.
Liu Y, Mendonça M, Cannon M, Jones PB, Lewis G, Thompson A, et al. Testing the independent and joint contribution of exposure to neurodevelopmental adversity and childhood trauma to risk of psychotic experiences in adulthood. Schizophr Bull. 2020;47:776–84. https://doi.org/10.1093/schbul/sbaa174.
Castillejos MC, Martín-Pérez C, Moreno-Küstner B. A systematic review and meta-analysis of the incidence of psychotic disorders: the distribution of rates and the influence of gender, urbanicity, immigration and socio-economic level. Psychol Med. 2018;48:2101–15. https://doi.org/10.1017/S0033291718000235.
Hastings PD, Serbin LA, Bukowski W, Helm JL, Stack DM, Dickson DJ, et al. Predicting psychosis-spectrum diagnoses in adulthood from social behaviors and neighborhood contexts in childhood. Dev Psychopathol. 2020;32:465–79. https://doi.org/10.1017/S095457941900021X.
O’Donoghue B, Roche E, Lane A. Neighbourhood level social deprivation and the risk of psychotic disorders: a systematic review. Soc Psychiatry Psychiatr Epidemiol. 2016;51:941–50. https://doi.org/10.1007/s00127-016-1233-4.
Schneider M, Müller CP, Knies AK. Low income and schizophrenia risk: a narrative review. Behav Brain Res. 2022;435:1–16. https://doi.org/10.1016/j.bbr.2022.114047.
Wicks S, Hjern A, Gunnell D, Lewis G, Dalman C. Social adversity in childhood and the risk of developing psychosis: a national cohort study. Am J Psychiatry. 2005;162:1652–7. https://doi.org/10.1176/appi.ajp.162.9.1652.
Kwok W. Is there evidence that social class at birth increases risk of psychosis? A systematic review. Int J Soc Psychiatry. 2014;60:801–8. https://doi.org/10.1177/0020764014524737.
Fett A-KJ, Lemmers-Jansen ILJ, Krabbendam L. Psychosis and urbanicity: a review of the recent literature from epidemiology to neurourbanism. Curr Opin Psychiatry. 2019;32:232–41. https://doi.org/10.1097/YCO.0000000000000486.
Pignon B, Szöke A, Ku B, Melchior M, Schürhoff F. Urbanicity and psychotic disorders: Facts and hypotheses. Dialogues Clin Neurosci. 2023;25:122–38. https://doi.org/10.1080/19585969.2023.2272824.
Murawiec S. The incidence of schizophrenia in the context of growing up in urbanised vs. green areas – a narrative review. Psychiatr Psychol Klin. 2023;23:18–26. https://doi.org/10.15557/PiPK.2023.0003.
Veling W, Susser E, Selten J-P, Hoek HW. Social disorganization of neighborhoods and incidence of psychotic disorders: a 7-year first-contact incidence study. Psychol Med. 2015;45:1789–98. https://doi.org/10.1017/S0033291714002682.
Barzilay R, Pries LK, Moore TM, Gur RE, van Os J, Rutten B, et al. Exposome and trans-syndromal developmental trajectories toward psychosis. Biol Psychiatry Glob Open Sci. 2022;2:197–205. https://doi.org/10.1016/j.bpsgos.2022.05.001.
Andrews JL, Ahmed SP, Blakemore S-J. Navigating the social environment in adolescence: the role of social brain development. Biol Psychiatry. 2021;89:109–18. https://doi.org/10.1016/j.biopsych.2020.09.012.
Gray KM, Squeglia LM. Research review: what have we learned about adolescent substance use? J Child Psychol Psychiatry. 2018;59:618–27. https://doi.org/10.1111/jcpp.12783.
Cannon TD, Cadenhead K, Cornblatt B, Woods SW, Addington J, Walker E, et al. Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Arch Gen Psychiatry. 2008;65:28–37. https://doi.org/10.1001/archgenpsychiatry.2007.3.
Semple DM, McIntosh AM, Lawrie SM. Cannabis as a risk factor for psychosis: systematic review. J Psychopharmacol. 2005;19:187–94. https://doi.org/10.1177/0269881105049040.
Addington J, Case N, Saleem MM, Auther AM, Cornblatt BA, Cadenhead KS. Substance use in clinical high risk for psychosis: a review of the literature. Early Interv Psychiatry. 2014;8:104–12. https://doi.org/10.1111/eip.12100.
Barkus E, Murray RM. Substance use in adolescence and psychosis: clarifying the relationship. Annu Rev Clin Psychol. 2010;6:365–89. https://doi.org/10.1146/annurev.clinpsy.121208.131220.
Wilkinson ST, Radhakrishnan R, D’Souza DC. Impact of cannabis use on the development of psychotic disorders. Curr Addict Rep. 2014;1:115–28. https://doi.org/10.1007/s40429-014-0018-7.
María-Ríos CE, Morrow JD. Mechanisms of shared vulnerability to post-traumatic stress disorder and substance use disorders. Front Behav Neurosci. 2020;14:6. https://doi.org/10.3389/fnbeh.2020.00006.
Matheson SL, Laurie M, Laurens KR. Substance use and psychotic-like experiences in young people: a systematic review and meta-analysis. Psychol Med. 2023;53:305–19. https://doi.org/10.1017/S0033291722003440.
Osborne KJ, Barch DM, Jackson JJ, Karcher NR. Psychosis spectrum symptoms before and after adolescent cannabis use initiation. JAMA Psychiatry. 2025;82:181–90. https://doi.org/10.1001/jamapsychiatry.2024.3525.
Gibson LE, Alloy LB, Ellman LM. Trauma and the psychosis spectrum: a review of symptom specificity and explanatory mechanisms. Clin Psychol Rev. 2016;49:92–105. https://doi.org/10.1016/j.cpr.2016.08.003.
Arseneault L, Cannon M, Poulton R, Murray R, Caspi A, Moffitt TE. Cannabis use in adolescence and risk for adult psychosis: longitudinal prospective study. BMJ. 2002;325:1212–3.
Zammit S, Allebeck P, Andreasson S, Lundberg I, Lewis G. Self reported cannabis use as a risk factor for schizophrenia in Swedish conscripts of 1969: historical cohort study. BMJ. 2002;325:1199. https://doi.org/10.1136/bmj.325.7374.1199.
Meyer U. Developmental neuroinflammation and schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2013;42:20–34. https://doi.org/10.1016/j.pnpbp.2011.11.003.
Murphy SK, Pike MR, Lipner E, Maxwell SD, Cohn BA, Cirillo P, et al. Contributions of maternal prenatal infection and antibiotic exposure to offspring infection and risk for allergic respiratory conditions through age 5. Brain Behav Immun Health. 2024;42:100892. https://doi.org/10.1016/j.bbih.2024.100892.
Wortinger LA, Engen K, Barth C, Lonning V, Jørgensen KN, Andreassen OA, et al. Obstetric complications and intelligence in patients on the schizophrenia-bipolar spectrum and healthy participants. Psychol Med. 2020;50:1914–22. https://doi.org/10.1017/S0033291719002046.
Collip D, Myin-Germeys I, Van Os J. Does the concept of ‘sensitization’ provide a plausible mechanism for the putative link between the environment and schizophrenia? Schizophr Bull. 2008;34:220–5. https://doi.org/10.1093/schbul/sbm163.
Myin-Germeys I, van Os J. Stress-reactivity in psychosis: evidence for an affective pathway to psychosis. Clin Psychol Rev. 2007;27:409–24. https://doi.org/10.1016/j.cpr.2006.09.005.
Muñoz-Samons D, Tor J, Rodríguez-Pascual M, Álvarez-Subiela X, Sugranyes G, de la Serna E, et al. Recent stressful life events and stress sensitivity in children and adolescents at clinical risk for psychosis. Psychiatry Res. 2021;303:114017. https://doi.org/10.1016/j.psychres.2021.114017.
van der Steen Y, Gimpel-Drees J, Lataster T, Viechtbauer W, Simons C, Lardinois M, et al. Clinical high risk for psychosis: the association between momentary stress, affective and psychotic symptoms. Acta Psychiatr Scand. 2017;136:63–73. https://doi.org/10.1111/acps.12714.
Valli I, Crossley NA, Day F, Stone J, Tognin S, Mondelli V, et al. HPA-axis function and grey matter volume reductions: imaging the diathesis-stress model in individuals at ultra-high risk of psychosis. Transl Psychiatry. 2016;6:e797. https://doi.org/10.1038/tp.2016.68.
Buckley TM, Schatzberg AF. On the Interactions of the hypothalamic-pituitary-adrenal (HPA) axis and sleep: normal HPA axis activity and circadian rhythm, exemplary sleep disorders. J Clin Endocrinol Metab. 2005;90:3106–14. https://doi.org/10.1210/jc.2004-1056.
Nicolaides NC, Vgontzas AN, Kritikou I, Chrousos G. HPA axis and sleep. In: Feingold KR, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, de Herder WW, Dhatariya K, Dungan K, Hofland J, Kalra S, Kaltsas G, Kapoor N, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrère B, Levy M, McGee EA, McLachlan R, Muzumdar R, Purnell J, Sahay R, Shah AS, Singer F, Sperling MA, Stratakis CA, Trence DL, Wilson DP, editors. Endotext. South Dartmouth, MA: MDText.com, Inc.; 2000. Available from: http://www.ncbi.nlm.nih.gov/books/NBK279071/. Accessed 14 Mar 2025.
van Dalfsen JH, Markus CR. The influence of sleep on human hypothalamic-pituitary-adrenal (HPA) axis reactivity: a systematic review. Sleep Med Rev. 2018;39:187–94. https://doi.org/10.1016/j.smrv.2017.10.002.
Kim J, Prasad S, Roshan NS, Hasan BF, Gill G, Gunturu S. Sleep disruptions and the pathway to psychosis: an in-depth case and literature review. Clin Case Rep. 2024;12:e9108. https://doi.org/10.1002/ccr3.9108.
Balbo M, Leproult R, Van Cauter E. Impact of sleep and its disturbances on hypothalamo-pituitary-adrenal axis activity. Int J Endocrinol. 2010;2010:759234. https://doi.org/10.1155/2010/759234.
Yap Y, Slavish DC, Taylor DJ, Bei B, Wiley JF. Bi-directional relations between stress and self-reported and actigraphy-assessed sleep: a daily intensive longitudinal study. Sleep. 2020;43:zsz250. https://doi.org/10.1093/sleep/zsz250.
Howes OD, McCutcheon R, Owen MJ, Murray RM. The Role of Genes, Stress, and Dopamine in the Development of Schizophrenia. Biol Psychiatry. 2017;81:9–20. https://doi.org/10.1016/j.biopsych.2016.07.014.
Howes OD, Shatalina E. Integrating the neurodevelopmental and dopamine hypotheses of schizophrenia and the role of cortical excitation-inhibition balance. Biol Psychiatry. 2022;92:501–13. https://doi.org/10.1016/j.biopsych.2022.06.017.
Baik J-H. Stress and the dopaminergic reward system. Exp Mol Med. 2020;52:1879–90. https://doi.org/10.1038/s12276-020-00532-4.
Belujon P, Grace AA. Regulation of dopamine system responsivity and its adaptive and pathological response to stress. Proc Biol Sci. 2015;282:20142516. https://doi.org/10.1098/rspb.2014.2516.
Covey DP, Mateo Y, Sulzer D, Cheer JF, Lovinger DM. Endocannabinoid modulation of dopamine neurotransmission. Neuropharmacology. 2017;124:52–61. https://doi.org/10.1016/j.neuropharm.2017.04.033.
Brunelin J, Fecteau S, Suaud-Chagny M-F. Abnormal striatal dopamine transmission in schizophrenia. Curr Med Chem. 2013;20:397–404. https://doi.org/10.2174/0929867311320030011.
Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III-the final common pathway. Schizophr Bull. 2009;35:549–62. https://doi.org/10.1093/schbul/sbp006.
Millard SJ, Bearden CE, Karlsgodt KH, Sharpe MJ. The prediction-error hypothesis of schizophrenia: new data point to circuit-specific changes in dopamine activity. Neuropsychopharmacology. 2022;47:628–40. https://doi.org/10.1038/s41386-021-01188-y.
Howes OD, Nour MM. Dopamine and the aberrant salience hypothesis of schizophrenia. World Psychiatry. 2016;15:3–4. https://doi.org/10.1002/wps.20276.
McEwen BS, Sapolsky RM. Stress and cognitive function. Curr Opin Neurobiol. 1995;5:205–16. https://doi.org/10.1016/0959-4388(95)80028-x.
Sapolsky RM, Krey LC, McEwen BS. Prolonged glucocorticoid exposure reduces hippocampal neuron number: implications for aging. J Neurosci. 1985;5:1222–7. https://doi.org/10.1523/JNEUROSCI.05-05-01222.1985.
Sapolsky RM, Krey LC, McEwen BS. The neuroendocrinology of stress and aging: the glucocorticoid cascade hypothesis. Endocr Rev. 1986;7:284–301. https://doi.org/10.1210/edrv-7-3-284.
Brenhouse HC, Danese A, Grassi-Oliveira R. Neuroimmune impacts of early-life stress on development and psychopathology. In: Coolen LM, Grattan DR, editors. Neuroendocrine regulation of behavior. Cham: Springer International Publishing; 2019. pp. 423–47. https://doi.org/10.1007/7854_2018_53.
Rivest S. Regulation of innate immune responses in the brain. Nat Rev Immunol. 2009;9:429–39. https://doi.org/10.1038/nri2565.
Armada-Moreira A, Gomes JI, Pina CC, Savchak OK, Gonçalves-Ribeiro J, Rei N, et al. Going the extra (synaptic) mile: excitotoxicity as the road toward neurodegenerative diseases. Front Cell Neurosci. 2020;14:90. https://doi.org/10.3389/fncel.2020.00090.
Osborne DM, Pearson-Leary J, McNay EC. The neuroenergetics of stress hormones in the hippocampus and implications for memory. Front Neurosci. 2015;9:164. https://doi.org/10.3389/fnins.2015.00164.
Goosens KA, Sapolsky RM. Stress and glucocorticoid contributions to normal and pathological aging. In: Riddle DR, editor. Brain aging: models, methods, and mechanisms. Boca Raton, FL: CRC Press/Taylor & Francis; 2007. Available from: http://www.ncbi.nlm.nih.gov/books/NBK3870/. Accessed 8 Feb 2024.
Miguel PM, Pereira LO, Silveira PP, Meaney MJ. Early environmental influences on the development of children’s brain structure and function. Dev Med Child Neurol. 2019;61:1127–33. https://doi.org/10.1111/dmcn.14182.
Podgorny OV, Gulyaeva NV. Glucocorticoid-mediated mechanisms of hippocampal damage: contribution of subgranular neurogenesis. J Neurochem. 2021;157:370–92. https://doi.org/10.1111/jnc.15265.
Cannon TD, Chung Y, He G, Sun D, Jacobson A, van Erp TG, et al. Progressive reduction in cortical thickness as psychosis develops: a multisite longitudinal neuroimaging study of youth at elevated clinical risk. Biol Psychiatry. 2015;77:147–57. https://doi.org/10.1016/j.biopsych.2014.05.023.
Feinberg I. Schizophrenia: caused by a fault in programmed synaptic elimination during adolescence? J Psychiatr Res. 1982;17:319–34. https://doi.org/10.1016/0022-3956(82)90038-3.
Black JE, Kodish IM, Grossman AW, Klintsova AY, Orlovskaya D, Vostrikov V, et al. Pathology of layer V pyramidal neurons in the prefrontal cortex of patients with schizophrenia. Am J Psychiatry. 2004;161:742–4. https://doi.org/10.1176/appi.ajp.161.4.742.
Garey LJ, Ong WY, Patel TS, Kanani M, Davis A, Mortimer AM, et al. Reduced dendritic spine density on cerebral cortical pyramidal neurons in schizophrenia. J Neurol Neurosurg Psychiatry. 1998;65:446–53. https://doi.org/10.1136/jnnp.65.4.446.
Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000;57:65–73. https://doi.org/10.1001/archpsyc.57.1.65.
Lewis DA, Pierri JN, Volk DW, Melchitzky DS, Woo TU. Altered GABA neurotransmission and prefrontal cortical dysfunction in schizophrenia. Biol Psychiatry. 1999;46:616–26. https://doi.org/10.1016/s0006-3223(99)00061-x.
Howes OD, Cummings C, Chapman GE, Shatalina E. Neuroimaging in schizophrenia: an overview of findings and their implications for synaptic changes. Neuropsychopharmacology. 2023;48:151–67. https://doi.org/10.1038/s41386-022-01426-x.
Kraguljac NV, McDonald WM, Widge AS, Rodriguez CI, Tohen M, Nemeroff CB. Neuroimaging biomarkers in schizophrenia. Am J Psychiatry. 2021;178:509–21. https://doi.org/10.1176/appi.ajp.2020.20030340.
Vita A, De Peri L, Deste G, Sacchetti E. Progressive loss of cortical gray matter in schizophrenia: a meta-analysis and meta-regression of longitudinal MRI studies. Transl Psychiatry. 2012;2:e190. https://doi.org/10.1038/tp.2012.116.
Chung Y, Allswede D, Addington J, Bearden CE, Cadenhead K, Cornblatt B, et al. Cortical abnormalities in youth at clinical high-risk for psychosis: findings from the NAPLS2 cohort. Neuroimage Clin. 2019;23:101862. https://doi.org/10.1016/j.nicl.2019.101862.
ENIGMA Clinical High Risk for Psychosis Working G, Jalbrzikowski M, Hayes RA, Wood SJ, Nordholm D, Zhou JH, et al. Association of structural magnetic resonance imaging measures with psychosis onset in individuals at clinical high risk for developing psychosis: an ENIGMA Working Group Mega-analysis. JAMA Psychiatry. 2021;78:753–66. https://doi.org/10.1001/jamapsychiatry.2021.0638.
Hua J, Loewy RL, Stuart B, Fryer SL, Niendam TA, Carter CS, et al. Cortical and subcortical brain morphometry abnormalities in youth at clinical high-risk for psychosis and individuals with early illness schizophrenia. Psychiatry Res Neuroimaging. 2023;332:111653. https://doi.org/10.1016/j.pscychresns.2023.111653.
Collins MA, Ji JL, Chung Y, Lympus CA, Afriyie-Agyemang Y, Addington JM, et al. Accelerated cortical thinning precedes and predicts conversion to psychosis: the NAPLS3 longitudinal study of youth at clinical high-risk. Mol Psychiatry. 2023;28:1182–9. https://doi.org/10.1038/s41380-022-01870-7.
Sekar A, Bialas AR, de Rivera H, Davis A, Hammond TR, Kamitaki N, et al. Schizophrenia risk from complex variation of complement component 4. Nature. 2016;530:177–83. https://doi.org/10.1038/nature16549.
McGlashan TH, Hoffman RE. Schizophrenia as a disorder of developmentally reduced synaptic connectivity. Arch Gen Psychiatry. 2000;57:637–48. https://doi.org/10.1001/archpsyc.57.7.637.
Lavin A, Moore HM, Grace AA. Prenatal disruption of neocortical development alters prefrontal cortical neuron responses to dopamine in adult rats. Neuropsychopharmacology. 2005;30:1426–35. https://doi.org/10.1038/sj.npp.1300696.
Moore H, Jentsch JD, Ghajarnia M, Geyer MA, Grace AA. A neurobehavioral systems analysis of adult rats exposed to methylazoxymethanol acetate on E17: implications for the neuropathology of schizophrenia. Biol Psychiatry. 2006;60:253–64. https://doi.org/10.1016/j.biopsych.2006.01.003.
Gomes FV, Zhu X, Grace AA. Stress during critical periods of development and risk for schizophrenia. Schizophr Res. 2019;213:107–13. https://doi.org/10.1016/j.schres.2019.01.030.
Aguilar-Valles A, Rodrigue B, Matta-Camacho E. Maternal immune activation and the development of dopaminergic neurotransmission of the offspring: relevance for schizophrenia and other psychoses. Front Psychiatry. 2020;11:852. https://doi.org/10.3389/fpsyt.2020.00852.
Holtzman CW, Trotman HD, Goulding SM, Ryan AT, Macdonald AN, Shapiro DI, et al. Stress and neurodevelopmental processes in the emergence of psychosis. Neuroscience. 2013;249:172–91. https://doi.org/10.1016/j.neuroscience.2012.12.017.
Giannopoulou I, Pagida MA, Briana DD, Panayotacopoulou MT. Perinatal hypoxia as a risk factor for psychopathology later in life: the role of dopamine and neurotrophins. Hormones. 2018;17:25–32. https://doi.org/10.1007/s42000-018-0007-7.
Fineberg AM, Ellman LM, Buka S, Yolken R, Cannon TD. Decreased birth weight in psychosis: influence of prenatal exposure to serologically determined influenza and hypoxia. Schizophr Bull. 2013;39:1037–44. https://doi.org/10.1093/schbul/sbs084.
Barzilay R, Calkins ME, Moore TM, Wolf DH, Satterthwaite TD, Cobb Scott J, et al. Association between traumatic stress load, psychopathology, and cognition in the Philadelphia Neurodevelopmental Cohort. Psychol Med. 2019;49:325–34. https://doi.org/10.1017/S0033291718000880.
Gibson LE, Cooper S, Reeves LE, Anglin DM, Ellman LM. The association between traumatic life events and psychological symptoms from a conservative, transdiagnostic perspective. Psychiatry Res. 2017;252:70–4. https://doi.org/10.1016/j.psychres.2017.02.047.
Karcher NR, Niendam TA, Barch DM. Adverse childhood experiences and psychotic-like experiences are associated above and beyond shared correlates: findings from the adolescent brain cognitive development study. Schizophr Res. 2020;222:235–42. https://doi.org/10.1016/j.schres.2020.05.045.
Isvoranu A-M, van Borkulo CD, Boyette LL, Wigman JT, Vinkers CH, Borsboom D, et al. A network approach to psychosis: pathways between childhood trauma and psychotic symptoms. Schizophr Bull. 2017;43:187–96. https://doi.org/10.1093/schbul/sbw055.
Sideli L, Murray RM, Schimmenti A, Corso M, La Barbera D, Trotta A, et al. Childhood adversity and psychosis: a systematic review of bio-psycho-social mediators and moderators. Psychol Med. 2020;50:1761–82. https://doi.org/10.1017/S0033291720002172.
Ungar M, Theron L. Resilience and mental health: how multisystemic processes contribute to positive outcomes. Lancet Psychiatry. 2020;7:441–8. https://doi.org/10.1016/S2215-0366(19)30434-1.
Ellman LM, Cannon TD. Environmental pre- and perinatal influences. In: Mueser KT, Jeste DV, editors. The clinical handbook of schizophrenia. New York: Guilford Press; 2008. pp. 65–73.
Brown AS, Susser ES. Prenatal nutritional deficiency and risk of adult schizophrenia. Schizophr Bull. 2008;34:1054–63. https://doi.org/10.1093/schbul/sbn096.
McGrath J, Brown A, St Clair D. Prevention and schizophrenia-the role of dietary factors. Schizophr Bull. 2011;37:272–83. https://doi.org/10.1093/schbul/sbq121. Art. no. 2.
Boeke CE, Gillman MW, Hughes MD, Rifas-Shiman SL, Villamor E, Oken E. Choline intake during pregnancy and child cognition at age 7 years. Am J Epidemiol. 2013;177:1338–77. https://doi.org/10.1093/aje/kws395.
Freedman R, Ross RG. Prenatal choline and the development of schizopvhrenia. Shanghai Arch Psychiatry. 2015;27:90–102. https://doi.org/10.11919/j.issn.1002-0829.215006.
Wu BTF, Dyer RA, King DJ, Richardson KJ, Innis SM. Early second trimester maternal plasma choline and betaine are related to measures of early cognitive development in term infants. PLoS ONE. 2012;7:e43448 https://doi.org/10.1371/journal.pone.0043448.
Jordan RG. Prenatal omega-3 fatty acids: review and recommendations. J Midwifery Womens Health. 2010;55:520–8. https://doi.org/10.1016/j.jmwh.2010.02.018.
Larqué E, Gil-Sánchez A, Prieto-Sánchez MT, Koletzko B. Omega 3 fatty acids, gestation and pregnancy outcomes. Br J Nutr. 2012;107:S77–84. https://doi.org/10.1017/S0007114512001481.
Boog G. Obstetrical complications and subsequent schizophrenia in adolescent and young adult offsprings: is there a relationship? Eur J Obstet Gynecol Reprod Biol. 2004;114:130–6. https://doi.org/10.1016/j.ejogrb.2003.09.041.
Giangiordano I, Sahani H, Di Mascio D, Saccone G, Bellussi F, Berghella A, et al. Optimism during pregnancy and obstetrical outcomes: a systematic review. Eur J Obstet Gynecol Reprod Biol. 2020;248:95–101. https://doi.org/10.1016/j.ejogrb.2020.03.029.
Auerbach MV, Lobel M, Cannella DT. Psychosocial correlates of health-promoting and health-impairing behaviors in pregnancy. J Psychosom Obstet Gynaecol. 2014;35:76–83. https://doi.org/10.3109/0167482X.2014.943179.
Lobel M, DeVincent CJ, Kaminer A, Meyer BA. The impact of prenatal maternal stress and optimistic disposition on birth outcomes in medically high-risk women. Health Psychol. 2000;19:544–53. 10.1037//0278-6133.19.6.544.
Ibrahim SM, Nicoloro-SantaBarbara J, Auerbach MV, Rosenthal L, Kocis C, Busso CE, et al. Pregnancy-specific coping and changes in emotional distress from mid- to late pregnancy. J Reprod Infant Psychol. 2019;37:397–412. https://doi.org/10.1080/02646838.2019.1578871.
Brown AS, Patterson PH. Maternal infection and schizophrenia: implications for prevention. Schizophr Bull. 2011;37:284–90. https://doi.org/10.1093/schbul/sbq146.
Black SB, Shinefield HR, France EK, Fireman BH, Platt ST, Shay D, et al. Effectiveness of influenza vaccine during pregnancy in preventing hospitalizations and outpatient visits for respiratory illness in pregnant women and their infants. Am J Perinatol. 2004;21:333–9. https://doi.org/10.1055/s-2004-831888.
Munoz FM, Greisinger AJ, Wehmanen OA, Mouzoon ME, Hoyle JC, Smith FA, et al. Safety of influenza vaccination during pregnancy. Am J Obstet Gynecol. 2005;192:1098–106. https://doi.org/10.1016/j.ajog.2004.12.019.
Lepock JR, Mizrahi R, Korostil M, Bagby RM, Pang EW, Kiang M. Event-related potentials in the clinical high-risk (CHR) state for psychosis: a systematic review. Clin EEG Neurosci. 2018;49:215–25. https://doi.org/10.1177/1550059418755212.
Bacaro V, Benz F, Pappaccogli A, De Bartolo P, Johann AF, Palagini L, et al. Interventions for sleep problems during pregnancy: a systematic review. Sleep Med Rev. 2020;50:101234. https://doi.org/10.1016/j.smrv.2019.101234.
Nevarez-Brewster M, Han D, Todd EL, Keim P, Doom JR, Davis EP. Sleep during pregnancy and offspring outcomes from infancy to childhood: a systematic review. Biopsychosoc Sci Med. 2025;87:7–32. https://doi.org/10.1097/PSY.0000000000001352.
Pires GN, Benedetto L, Cortese R, Gozal D, Gulia KK, Kumar VM, et al. Effects of sleep modulation during pregnancy in the mother and offspring: evidences from preclinical research. J Sleep Res. 2021;30:e13135. https://doi.org/10.1111/jsr.13135.
Ertekin Pinar S, Duran Aksoy O, Daglar G, Yurtsal ZB, Cesur B. Effect of stress management training on depression, stress and coping strategies in pregnant women: a randomised controlled trial. J Psychosom Obstet Gynaecol. 2018;39:203–10. https://doi.org/10.1080/0167482X.2017.1321632.
Glover V. Maternal depression, anxiety and stress during pregnancy and child outcome; what needs to be done. Best Pract Res Clin Obstet Gynaecol. 2014;28:25–35. https://doi.org/10.1016/j.bpobgyn.2013.08.017.
Matvienko-Sikar K, Dockray S. Effects of a novel positive psychological intervention on prenatal stress and well-being: A pilot randomised controlled trial. Women Birth. 2017;30:e111–8. https://doi.org/10.1016/j.wombi.2016.10.003.
Richter J, Bittner A, Petrowski K, Junge-Hoffmeister J, Bergmann S, Joraschky P, et al. Effects of an early intervention on perceived stress and diurnal cortisol in pregnant women with elevated stress, anxiety, and depressive symptomatology. J Psychosom Obstet Gynaecol. 2012;33:162–70. https://doi.org/10.3109/0167482X.2012.729111.
Nolvi S, Merz EC, Kataja E-L, Parsons CE. Prenatal stress and the developing brain: postnatal environments promoting resilience. Biol Psychiatry. 2023;93:942–52. https://doi.org/10.1016/j.biopsych.2022.11.023.
Margolis AE, Liu R, Conceição VA, Ramphal B, Pagliaccio D, DeSerisy ML, et al. Convergent neural correlates of prenatal exposure to air pollution and behavioral phenotypes of risk for internalizing and externalizing problems: potential biological and cognitive pathways. Neurosci Biobehav Rev. 2022;137:104645. https://doi.org/10.1016/j.neubiorev.2022.104645.
Godoy Izquierdo D, Vázquez Pérez ML, Lara Moreno R, Godoy García JF. Training coping skills and coping with stress self-efficacy for successful daily functioning and improved clinical status in patients with psychosis: a randomized controlled pilot study. Sci Prog. 2021;104:00368504211056818. https://doi.org/10.1177/00368504211056818.
Phillips LJ, Francey SM, Edwards J, McMurray N. Strategies used by psychotic individuals to cope with life stress and symptoms of illness: a systematic review. Anxiety Stress Coping. 2009;22:371–410. https://doi.org/10.1080/10615800902811065.
Piotrowski P, Rymaszewska J, Stańczykiewicz B, Małecka M, Kotowicz K, Samochowiec J, et al. Stress coping strategies and their clinical correlates in patients with psychosis at various stages of illness: a case-control study. Early Interv Psychiatry. 2020;14:559–67. https://doi.org/10.1111/eip.12880.
Stramecki F, Kotowicz K, Piotrowski P, Beszłej JA, Rymaszewska J, Samochowiec J, et al. Coping styles and symptomatic manifestation of first-episode psychosis: Focus on cognitive performance. Psychiatry Res. 2019;272:246–51. https://doi.org/10.1016/j.psychres.2018.12.083.
Yanos PT, Moos RH. Determinants of functioning and well-being among individuals with schizophrenia: an integrated model. Clin Psychol Rev. 2007;27:58–77. https://doi.org/10.1016/j.cpr.2005.12.008.
DeLuca JS, Rakhshan Rouhakhtar P, Klaunig MJ, Akouri-Shan L, Jay SY, Todd TL, et al. Psychosis-like experiences and resilience: a systematic and critical review of the literature. Psychol Serv. 2022;19:120–38. https://doi.org/10.1037/ser0000585.
Marulanda S, Addington J. Resilience in individuals at clinical high risk for psychosis. Early Interv Psychiatry. 2016;10:212–9. https://doi.org/10.1111/eip.12174.
Kim KR, Song YY, Park JY, Lee EH, Lee M, Lee SY, et al. The relationship between psychosocial functioning and resilience and negative symptoms in individuals at ultra-high risk for psychosis. Aust N Z J Psychiatry. 2013;47:762–71. https://doi.org/10.1177/0004867413488218.
Shi J, Wang L, Yao Y, Chen F, Su N, Zhao X, et al. Protective factors in Chinese university students at clinical high risk for psychosis. Psychiatry Res. 2016;239:239–44. https://doi.org/10.1016/j.psychres.2016.03.036.
Mian L, Lattanzi GM, Tognin S. Coping strategies in individuals at ultra-high risk of psychosis: a systematic review. Early Interv Psychiatry. 2018;12:525–34. https://doi.org/10.1111/eip.12492.
Jalbrzikowski M, Sugar CA, Zinberg J, Bachman P, Cannon TD, Bearden CE. Coping styles of individuals at clinical high risk for developing psychosis. Early Interv Psychiatry. 2014;8:68–76. https://doi.org/10.1111/eip.12005.
Schmidt SJ, Schultze-Lutter F, Bendall S, Groth N, Michel C, Inderbitzin N, et al. Mediators linking childhood adversities and trauma to suicidality in individuals at risk for psychosis. Front Psychiatry. 2017;8:242. https://doi.org/10.3389/fpsyt.2017.00242.
Kommescher M, Wagner M, Pützfeld V, Berning J, Janssen B, Decker P, et al. Coping as a predictor of treatment outcome in people at clinical high risk of psychosis. Early Interv Psychiatry. 2016;10:17–27. https://doi.org/10.1111/eip.12130.
Fernández I, Vallina-Fernández Ó, Alonso-Bada S, Rus-Calafell M, Paino M. Emotional regulation as a mediating variable between risk of psychosis and common mental health problems in adolescents. J Psychiatr Res. 2025;181:273–81. https://doi.org/10.1016/j.jpsychires.2024.11.058.
Bozikas V, Parlapani E. Resilience in patients with psychotic disorder. Psychiatriki. 2016;27:13–6.
Johnson J, Gooding PA, Wood AM, Taylor PJ, Pratt D, Tarrier N. Resilience to suicidal ideation in psychosis: positive self-appraisals buffer the impact of hopelessness. Behav Res Ther. 2010;48:883–9. https://doi.org/10.1016/j.brat.2010.05.013.
Dutcher JM, Eisenberger NI, Woo H, Klein W, Harris PR, Levine JM, et al. Neural mechanisms of self-affirmation’s stress buffering effects. Soc Cogn Affect Neurosci. 2020;15:1086–96. https://doi.org/10.1093/scan/nsaa042.
Lindsay EK, Chin B, Greco CM, Young S, Brown KW, Wright A, et al. How mindfulness training promotes positive emotions: dismantling acceptance skills training in two randomized controlled trials. J Pers Soc Psychol. 2018;115:944–73. https://doi.org/10.1037/pspa0000134.
Brasso C, Giordano B, Badino C, Bellino S, Bozzatello P, Montemagni C, et al. Primary psychosis: risk and protective factors and early detection of the onset. Diagnostics. 2021;11:2146. https://doi.org/10.3390/diagnostics11112146.
Cojocaru A, Braha A, Anastasescu CM, Folescu R, Bugi MA, Puiu M, et al. A systematic review of resilience in at-risk youth for psychotic disorders: an analysis of protective and risk factors from recent literature. Behav Sci. 2024;14:898 https://doi.org/10.3390/bs14100898.
Butzlaff RL, Hooley JM. Expressed emotion and psychiatric relapse: a meta-analysis. Arch Gen Psychiatry. 1998;55:547–52. https://doi.org/10.1001/archpsyc.55.6.547.
Izon E, Berry K, Law H, French P. Expressed emotion (EE) in families of individuals at-risk of developing psychosis: a systematic review. Psychiatry Res. 2018;270:661–72. https://doi.org/10.1016/j.psychres.2018.10.065.
Riches S, Arseneault L, Bagher-Niakan R, Alsultan M, Crush E, Fisher HL. Protective factors for early psychotic phenomena among children of mothers with psychosis. Front Psychiatry. 2019;9:750. https://doi.org/10.3389/fpsyt.2018.00750.
Serra-Arumí C, Vila-Badia R, Del Cacho N, Butjosa A, Abella M, Colomer-Salvans A, et al. Association of perceived social support with sociodemographic, clinical, and psychosocial variables in patients with first-episode psychosis. J Psychiatr Res. 2023;162:30–6. https://doi.org/10.1016/j.jpsychires.2023.04.008.
Gayer-Anderson C, Morgan C. Social networks, support and early psychosis: a systematic review. Epidemiol Psychiatr Sci. 2013;22:131–46. https://doi.org/10.1017/S2045796012000406.
Palumbo C, Volpe U, Matanov A, Priebe S, Giacco D. Social networks of patients with psychosis: a systematic review. BMC Res Notes. 2015;8:560 https://doi.org/10.1186/s13104-015-1528-7.
Anglin DM, Lui F, Espinosa A, Tikhonov A, Ellman L. Ethnic identity, racial discrimination and attenuated psychotic symptoms in an urban population of emerging adults. Early Interv Psychiatry. 2018;12:380–90. https://doi.org/10.1111/eip.12314.
Baker SJ, Jackson M, Jongsma H, Saville CWN. The ethnic density effect in psychosis: a systematic review and multilevel meta-analysis. Br J Psychiatry. 2021;219:632–43. https://doi.org/10.1192/bjp.2021.96.
Bosqui TJ, Hoy K, Shannon C. A systematic review and meta-analysis of the ethnic density effect in psychotic disorders. Soc Psychiatry Psychiatr Epidemiol. 2014;49:519–29. https://doi.org/10.1007/s00127-013-0773-0.
Anglin DM. Racism and social determinants of psychosis. Annu Rev Clin Psychol. 2023;19:277–302. https://doi.org/10.1146/annurev-clinpsy-080921-074730.
Barrett EA, Aminoff SR, Simonsen C, Romm KL. Opening the curtains for better sleep in psychotic disorders - considerations for improving sleep treatment. Compr Psychiatry. 2020;103:152207. https://doi.org/10.1016/j.comppsych.2020.152207.
Clarke L, Chisholm K, Cappuccio FP, Tang N, Miller MA, Elahi F, et al. Sleep disturbances and the at risk mental state: a systematic review and meta-analysis. Schizophr Res. 2021;227:81–91. https://doi.org/10.1016/j.schres.2020.06.027.
Poe S-L, Brucato G, Bruno N, Arndt LY, Ben-David S, Gill KE, et al. Sleep disturbances in individuals at clinical high risk for psychosis. Psychiatry Res. 2017;249:240–3. https://doi.org/10.1016/j.psychres.2016.12.029.
Bradley J, Freeman D, Chadwick E, Harvey AG, Mullins B, Johns L, et al. Treating sleep problems in young people at ultra-high risk of psychosis: a feasibility case series. Behav Cogn Psychother. 2018;46:276–91. https://doi.org/10.1017/S1352465817000601.
Capaldi Ii VF, Handwerger K, Richardson E, Stroud LR. Associations between sleep and cortisol responses to stress in children and adolescents: a pilot study. Behav Sleep Med. 2005;3:177–92. https://doi.org/10.1207/s15402010bsm0304_1.
Fuligni AJ, Chiang JJ, Tottenham N. Sleep disturbance and the long-term impact of early adversity. Neurosci Biobehav Rev. 2021;126:304–13. https://doi.org/10.1016/j.neubiorev.2021.03.021.
Irwin M. Effects of sleep and sleep loss on immunity and cytokines. Brain, Behav, Immun. 2002;16:503–12. https://doi.org/10.1016/S0889-1591(02)00003-X.
Bredin S, Kaufman KL, Chow MI, Lang DJ, Wu N, Kim DD, et al. Effects of aerobic, resistance, and combined exercise training on psychiatric symptom severity and related health measures in adults living with schizophrenia: a systematic review and meta-analysis. Front Cardiovasc Med. 2021;8:753117. https://doi.org/10.3389/fcvm.2021.753117.
Firth J, Carney R, Jerome L, Elliott R, French P, Yung AR. The effects and determinants of exercise participation in first-episode psychosis: a qualitative study. BMC Psychiatry. 2016;16:36. https://doi.org/10.1186/s12888-016-0751-7.
Lederman O, Ward PB, Rosenbaum S, Maloney C, Watkins A, Teasdale S, et al. Stepping up early treatment for help-seeking youth with at-risk mental states: feasibility and acceptability of a real-world exercise program. Early Interv Psychiatry. 2020;14:450–62. https://doi.org/10.1111/eip.12871.
Mittal V, Dean D, Gupta T, Bryan A. SU20. Aerobic exercise intervention for clinical high-risk youth improves cognitive and hippocampal abnormalities. Schizophr Bull. 2017;43:S168. https://doi.org/10.1093/schbul/sbx024.019.
De Micheli A, Provenzani U, Krakowski K, Oliver D, Damiani S, Brondino N, et al. Physical health and transition to psychosis in people at clinical high risk. Biomedicines. 2024;12:523 https://doi.org/10.3390/biomedicines12030523.
Liu P. Stress buffering effects of physical activity in adolescents: the moderating role of physical activity attitudes. BMC Public Health. 2025;25:463. https://doi.org/10.1186/s12889-025-21674-y.
Beserra AHN, Kameda P, Deslandes AC, Schuch FB, Laks J, Moraes HSde. Can physical exercise modulate cortisol level in subjects with depression? A systematic review and meta-analysis. Trends Psychiatry Psychother. 2018;40:360–8. https://doi.org/10.1590/2237-6089-2017-0155.
De Nys L, Anderson K, Ofosu EF, Ryde GC, Connelly J, Whittaker AC. The effects of physical activity on cortisol and sleep: a systematic review and meta-analysis. Psychoneuroendocrinology. 2022;143:105843. https://doi.org/10.1016/j.psyneuen.2022.105843.
Mahalakshmi B, Maurya N, Lee S-D, Bharath Kumar V. Possible neuroprotective mechanisms of physical exercise in neurodegeneration. Int J Mol Sci. 2020;21:5895. https://doi.org/10.3390/ijms21165895.
Silverman MN, Deuster PA. Biological mechanisms underlying the role of physical fitness in health and resilience. Interface Focus. 2014;4:20140040. https://doi.org/10.1098/rsfs.2014.0040.
Liu PZ, Nusslock R. Exercise and hippocampal neurogenesis: a dogma re-examined and lessons learned. Neural Regen Res. 2018;13:1354–5. https://doi.org/10.4103/1673-5374.235225.
Yau S, Gil-Mohapel J, Christie BR, So K. Physical exercise-induced adult neurogenesis: a good strategy to prevent cognitive decline in neurodegenerative diseases? Biomed Res Int. 2014;2014:403120. https://doi.org/10.1155/2014/403120.
Aucoin M, LaChance L, Cooley K, Kidd S. Diet and psychosis: a scoping review. Neuropsychobiology. 2018;79:20–42. https://doi.org/10.1159/000493399.
Bonfioli E, Berti L, Goss C, Muraro F, Burti L. Health promotion lifestyle interventions for weight management in psychosis: a systematic review and meta-analysis of randomised controlled trials. BMC Psychiatry. 2012;12:78. https://doi.org/10.1186/1471-244X-12-78.
Mucheru D, Hanlon M-C, McEvoy M, Thakkinstian A, MacDonald-Wicks L. Comparative efficacy of lifestyle intervention strategies targeting weight outcomes in people with psychosis: a systematic review and network meta-analysis. JBI Database Syst Rev Implement Rep. 2019;17:1770–825. https://doi.org/10.11124/JBISRIR-2017-003943.
Bottaccioli F, Bottaccioli A, Marzola E, Longo P, Minelli A, Abbate-Daga G. Nutrition, exercise, and stress management for treatment and prevention of psychiatric disorders. A narrative review psychoneuroendocrineimmunology-based. Endocrines. 2021. Available from: https://www.semanticscholar.org/paper/Nutrition%2C-Exercise%2C-and-Stress-Management-for-and-Bottaccioli-Bottaccioli/00ef3ed6675099b2f7c03dcd62e2a2f10bdbbe85. Accessed 13 Mar 2025
Cha HY, Yang SJ. Anti-inflammatory diets and schizophrenia. Clin Nutr Res. 2020;9:241–57. https://doi.org/10.7762/cnr.2020.9.4.241.
Miklowitz DJ, O'Brien MP, Schlosser DA, Addington J, Candan KA, Marshall C, et al. Family-focused treatment for adolescents and young adults at high risk for psychosis: results of a randomized trial. J Am Acad Child Adolesc Psychiatry. 2014;53:848–58. https://doi.org/10.1016/j.jaac.2014.04.020.
O’Brien MP, Miklowitz DJ, Cannon TD. Decreases in perceived maternal criticism predict improvement in subthreshold psychotic symptoms in a randomized trial of family-focused therapy for individuals at clinical high risk for psychosis. J Fam Psychol. 2015;29:945–51. https://doi.org/10.1037/fam0000123.
Pos K, Franke N, Smit F, Wijnen B, Staring A, Van der Gaag M, et al. Cognitive behavioral therapy for social activation in recent-onset psychosis: Randomized controlled trial. J Consul Clin Psychol. 2019;87:151–60. https://doi.org/10.1037/ccp0000362.
Citri A, Malenka RC. Synaptic plasticity: multiple forms, functions, and mechanisms. Neuropsychopharmacology. 2008;33:18–41. https://doi.org/10.1038/sj.npp.1301559.
Caroni P, Donato F, Muller D. Structural plasticity upon learning: regulation and functions. Nat Rev Neurosci. 2012;13:478–90. https://doi.org/10.1038/nrn3258.
Johansen JP, Cain CK, Ostroff LE, LeDoux JE. Molecular mechanisms of fear learning and memory. Cell. 2011;147:509–24. https://doi.org/10.1016/j.cell.2011.10.009.
Yin HH, Mulcare SP, Hilário MR, Clouse E, Holloway T, Davis MI, et al. Dynamic reorganization of striatal circuits during the acquisition and consolidation of a skill. Nat Neurosci. 2009;12:333–41. https://doi.org/10.1038/nn.2261.
Hasler G, Inta D. Emerging perspectives on neuroprotection. Psychother Psychosom. 2024;93:285–91. https://doi.org/10.1159/000540032.
McEwen BS. Plasticity of the hippocampus: adaptation to chronic stress and allostatic load. Ann N Y Acad Sci. 2001;933:265–77. https://doi.org/10.1111/j.1749-6632.2001.tb05830.x.
McIlvried LA, Del Rosario JS, Pullen MY, Wangzhou A, Sheahan TD, Shepherd AJ, et al. Intrinsic adaptive plasticity in mouse and human sensory neurons. J Gen Physiol. 2025;157:e202313488. https://doi.org/10.1085/jgp.202313488.
DeRosse P, Barber AD. Overlapping neurobiological substrates for early-life stress and resilience to psychosis. Biol Psychiatry Cogn Neurosci Neuroimaging. 2021;6:144–53. https://doi.org/10.1016/j.bpsc.2020.09.003.
Bois C, Levita L, Ripp I, Owens DC, Johnstone EC, Whalley HC, et al. Hippocampal, amygdala and nucleus accumbens volume in first-episode schizophrenia patients and individuals at high familial risk: a cross-sectional comparison. Schizophr Res. 2015;165:45–51. https://doi.org/10.1016/j.schres.2015.03.024.
Ebdrup BH, Glenthøj B, Rasmussen H, Aggernaes B, Langkilde AR, Paulson OB, et al. Hippocampal and caudate volume reductions in antipsychotic-naive first-episode schizophrenia. J Psychiatry Neurosci. 2010;35:95–104. https://doi.org/10.1503/jpn.090049.
Nakahara S, Matsumoto M, van Erp TGM. Hippocampal subregion abnormalities in schizophrenia: a systematic review of structural and physiological imaging studies. Neuropsychopharmacol Rep. 2018;38:156–66. https://doi.org/10.1002/npr2.12031.
Steen RG, Mull C, McClure R, Hamer RM, Lieberman JA. Brain volume in first-episode schizophrenia: systematic review and meta-analysis of magnetic resonance imaging studies. Br J Psychiatry. 2006;188:510–8. https://doi.org/10.1192/bjp.188.6.510.
Ho NF, Holt DJ, Cheung M, Iglesias JE, Goh A, Wang M, et al. Progressive decline in hippocampal CA1 volume in individuals at ultra-high-risk for psychosis who do not remit: findings from the longitudinal youth at risk study. Neuropsychopharmacol. 2017;42:1361–70. https://doi.org/10.1038/npp.2017.5.
Moreno-López L, Ioannidis K, Askelund AD, Smith AJ, Schueler K, van Harmelen A-L. The resilient emotional brain: a scoping review of the medial prefrontal cortex and limbic structure and function in resilient adults with a history of childhood maltreatment. Biol Psychiatry Cogn Neurosci Neuroimaging. 2020;5:392–402. https://doi.org/10.1016/j.bpsc.2019.12.008.
Gee D. Amygdala-prefrontal function and clinical course among adolescents and young adults at clinical high risk for psychosis. UCLA, 2015. Available from: https://escholarship.org/uc/item/41n0x4rm. Accessed 3 Feb 2025.
Belsky J, Pluess M. Beyond diathesis stress: differential susceptibility to environmental influences. Psychol Bull. 2009;135:885–908. https://doi.org/10.1037/a0017376.
Boyce WT. Differential susceptibility of the developing brain to contextual adversity and stress. Neuropsychopharmacology. 2016;41:142–62. https://doi.org/10.1038/npp.2015.294.
Boyce WT, Ellis BJ. Biological sensitivity to context: I. An evolutionary-developmental theory of the origins and functions of stress reactivity. Dev Psychopathol. 2005;17:271–301. https://doi.org/10.1017/s0954579405050145.
Obradović J, Boyce WT. Individual differences in behavioral, physiological, and genetic sensitivities to contexts: implications for development and adaptation. Dev Neurosci. 2009;31:300–8. https://doi.org/10.1159/000216541.
McLaughlin KA, Lambert HK. Child trauma exposure and psychopathology: mechanisms of risk and resilience. Curr Opin Psychol. 2017;14:29–34. https://doi.org/10.1016/j.copsyc.2016.10.004.
Trickey D, Siddaway AP, Meiser-Stedman R, Serpell L, Field AP. A meta-analysis of risk factors for post-traumatic stress disorder in children and adolescents. Clin Psychol Rev. 2012;32:122–38. https://doi.org/10.1016/j.cpr.2011.12.001.
Pinto R, De Castro MV, Silva L, Jongenelen I, Maia A, Levendosky AA. The impact of psychopathology associated with childhood trauma on quality of life in portuguese adolescents: a two-wave longitudinal study. Front Psychiatry. 2021;12:650700. https://doi.org/10.3389/fpsyt.2021.650700.
Wambua GN, Kilian S, Chiliza B. A qualitative study of coping strategies and resilience in the aftermath of childhood adversity in first-episode psychosis. Early Interv Psychiatry. 2025;19:e13551. https://doi.org/10.1111/eip.13551.
Ku BS, Aberizk K, Addington J, Bearden CE, Cadenhead KS, Cannon TD, et al. The association between neighborhood poverty and hippocampal volume among individuals at clinical high-risk for psychosis: the moderating role of social engagement. Schizophr Bull. 2022;48:1032–42. https://doi.org/10.1093/schbul/sbac055.
Osborne KJ, Willroth EC, DeVylder JE, Mittal VA, Hilimire MR. Investigating the association between emotion regulation and distress in adults with psychotic-like experiences. Psychiatry Res. 2017;256:66–70. https://doi.org/10.1016/j.psychres.2017.06.011.
Höhne N, Poidinger M, Merz F, Pfister H, Brückl T, Zimmermann P, et al. Increased HPA axis response to psychosocial stress in remitted depression: the influence of coping style. Biol Psychol. 2014;103:267–75. https://doi.org/10.1016/j.biopsycho.2014.09.008.
Perez VM, Gonzales NA, Tein J-Y, Ibrahim MH, Luecken LJ, Losoya S. Dispositional active coping predicts patterns of adolescents’ cortisol responsivity in the context of school-related stressors. J Clin Child Adolesc Psychol. 2023;52:604–15. https://doi.org/10.1080/15374416.2021.1969651.
Seitz R, Vracotas N, Bechard-Evans L, King S, Abadi S, Joober R, et al. The Trier Social Stress Test in first episode psychosis patients: impact of perceived stress, protective factors and childhood trauma. Psychoneuroendocrinology. 2019;105:155–63. https://doi.org/10.1016/j.psyneuen.2019.01.010.
Sladek MR, Doane LD, Stroud CB. Individual and day-to-day differences in active coping predict diurnal cortisol patterns among early adolescent girls. J Youth Adolesc. 2017;46:121–35. https://doi.org/10.1007/s10964-016-0591-2.
Kane JM, Robinson DG, Schooler NR, Mueser KT, Penn DL, Rosenheck RA, et al. Comprehensive versus usual community care for first-episode psychosis: 2-year outcomes from the NIMH RAISE Early Treatment Program. Am J Psychiatry. 2016;173:362–72. https://doi.org/10.1176/appi.ajp.2015.15050632.
Moe AM, Kilicoglu MFV, Angers K, Huang K, Breitborde NJK. Coordinated specialty care for first-episode psychosis: effects on social motivation and social pleasure. Schizophr Res. 2023;262:130–1. https://doi.org/10.1016/j.schres.2023.10.039.
Westfall M, Kohler CG, Hurford I, Abegunde C, Agosti D, Brinen A, et al. Pennsylvania coordinated specialty care programs for first-episode psychosis: 6- and 12-month outcomes. Early Interv Psychiatry. 2021;15:1395–408. https://doi.org/10.1111/eip.13084.
Bosnjak Kuharic D, Kekin I, Hew J, Rojnic Kuzman M, Puljak L. Interventions for prodromal stage of psychosis. Cochrane Database Syst Rev. 2019;2019:CD012236. https://doi.org/10.1002/14651858.CD012236.pub2.
Lieberman JA, Small SA, Girgis RR. Early detection and preventive intervention in schizophrenia: from fantasy to reality. Am J Psychiatry. 2019;176:794–810. https://doi.org/10.1176/appi.ajp.2019.19080865.
Zheng Y, Xu T, Zhu Y, Li C, Wang J, Livingstone S, et al. Cognitive behavioral therapy for prodromal stage of psychosis-outcomes for transition, functioning, distress, and quality of life: a systematic review and meta-analysis. Schizophr Bull. 2022;48:8–19. https://doi.org/10.1093/schbul/sbab044.
van der Gaag M, van den Berg D, Ising H. CBT in the prevention of psychosis and other severe mental disorders in patients with an at risk mental state: a review and proposed next steps. Schizophr Res. 2019;203:88–93. https://doi.org/10.1016/j.schres.2017.08.018.
Raballo A, Poletti M, Preti A. Do antidepressants prevent transition to psychosis in individuals at clinical high-risk (CHR-P)? Systematic review and meta-analysis. Psychol Med. 2023;53:4550–60. https://doi.org/10.1017/S0033291722001428.
Parrish EM, Kim NS, Woodberry KA, Friedman-Yakoobian M. Clinical high risk for psychosis: the effects of labelling on public stigma in a undergraduate population. Early Interv Psychiatry. 2019;13:874–81. https://doi.org/10.1111/eip.12691.
Schiffman J, Horton LE, Landa Y, Woods SW. Considerations for providing feedback to patients and families regarding clinical high-risk for psychosis status. Schizophr Res. 2022;244:55–7. https://doi.org/10.1016/j.schres.2022.01.059.
Uttinger M, Koranyi S, Papmeyer M, Fend F, Ittig S, Studerus E, et al. Early detection of psychosis: helpful or stigmatizing experience? A qualitative study. Early Interv Psychiatry. 2018;12:66–73. https://doi.org/10.1111/eip.12273.
Woodberry KA, Powers KS, Bryant C, Downing D, Verdi MB, Elacqua KM, et al. Emotional and stigma-related experiences relative to being told one is at risk for psychosis. Schizophr Res. 2021;238:44–51. https://doi.org/10.1016/j.schres.2021.09.017.
Hellen I, Døsvig SG, Liseth BE, Mørkved N, Johannessen JO, Løberg E-M. Trauma-focused treatment in psychosis: a systematic review. Early Interv Psychiatry. 2025;19:e70064. https://doi.org/10.1111/eip.70064.
Kabat-Zinn J. Mindfulness-based interventions in context: past, present, and future. Clin Psychol Sci Pract. 2003;10:144–56. https://doi.org/10.1093/clipsy.bpg016.
Zhang J-Y, Cui Y-X, Zhou Y-Q, Li Y-L. Effects of mindfulness-based stress reduction on prenatal stress, anxiety and depression. Psychol Health Med. 2019;24:51–8. https://doi.org/10.1080/13548506.2018.1468028.
Weisman de Mamani A, Weintraub MJ, Gurak K, Maura J. A randomized clinical trial to test the efficacy of a family-focused, culturally informed therapy for schizophrenia. J Fam Psychol. 2014;28:800–10. https://doi.org/10.1037/fam0000021.
Weisman de Mamani A, McLaughlin M, Altamirano O, Lopez D, Ahmad SS. Culturally informed therapy for schizophrenia: a family-focused cognitive behavioral approach, clinician guide. Oxford University Press; 2021. https://doi.org/10.1093/med-psych/9780197500644.001.0001.
Bennett D, Rosenheck R. Socioeconomic status and the effectiveness of treatment for first-episode psychosis. Health Serv Res. 2021;56:409–17. https://doi.org/10.1111/1475-6773.13606.
McGrath J. Universal Interventions For The Primary Prevention Of Schizophrenia. Aust N Z J Psychiatry. 2000;34:A58–A64. https://doi.org/10.1177/000486740003401S10.
Murray RM, David AS, Ajnakina O. Prevention of psychosis: moving on from the at-risk mental state to universal primary prevention. Psychol Med. 2021;51:223–7. https://doi.org/10.1017/S003329172000313X.
Woods SW, Bearden CE, Sabb FW, Stone WS, Torous J, Cornblatt BA, et al. Counterpoint. Early intervention for psychosis risk syndromes: minimizing risk and maximizing benefit. Schizophr Res. 2021;227:10–7. https://doi.org/10.1016/j.schres.2020.04.020.
Berger M, Sarnyai Z. “More than skin deep”: stress neurobiology and mental health consequences of racial discrimination. Stress. 2015;18:1–10. https://doi.org/10.3109/10253890.2014.989204.
Carter RT. Racism and psychological and emotional injury: recognizing and assessing race-based traumatic stress. Couns Psychol. 2007;35:13–105. https://doi.org/10.1177/0011000006292033.
Kirkbride JB, Jones PB, Ullrich S, Coid JW. Social deprivation, inequality, and the neighborhood-level incidence of psychotic syndromes in east london. Schizophr Bull. 2014;40:169–80. https://doi.org/10.1093/schbul/sbs151.
Bresnahan M, Begg MD, Brown A, Schaefer C, Sohler N, Insel B, et al. Race and risk of schizophrenia in a US birth cohort: another example of health disparity? Int J Epidemiol. 2007;36:751–8. https://doi.org/10.1093/ije/dym041.
van der Ven E, Olino TM, Diehl K, Nuñez SM, Thayer G, Bridgwater MA, et al. Ethnoracial risk variation across the psychosis continuum in the us: a systematic review and meta-analysis. JAMA Psychiatry. 2024;81:447–55. https://doi.org/10.1001/jamapsychiatry.2023.5497.
Karcher NR, Klaunig MJ, Elsayed NM, Taylor RL, Jay SY, Schiffman J. Understanding associations between race/ethnicity, experiences of discrimination, and psychotic-like experiences in middle childhood. J Am Acad Child Adolesc Psychiatry. 2022;61:1262–72. https://doi.org/10.1016/j.jaac.2022.03.025.
Petti E, Schiffman J, Oh H, Karcher NR. Evidence for environmental risk factors and cumulative stress linking racial/ethnic identity and psychotic-like experiences in ABCD study data. J Am Acad Child Adolesc Psychiatry. 2025;64:386–97. https://doi.org/10.1016/j.jaac.2024.04.017.
Jones N, Atterbury K, Byrne L, Carras M, Brown M, Phalen P. Lived experience, research leadership, and the transformation of mental health services: building a researcher pipeline. Psychiatr Serv. 2021;72:591–3. https://doi.org/10.1176/appi.ps.202000468.
Funding
This work was supported by the National Institute of Mental Health (Grants R01MH120091, R01 MH112613, and R01MH118545 to LE, F31MH135667 to KO) and the National Science Foundation Graduate Research Fellowship Program (2038235 to ZH).
Author information
Authors and Affiliations
Contributions
KO led the development, writing, and editing of the manuscript, as well as conducting literature reviews. ZH, MP, ES, and ND also conducted relevant literature reviews and contributed to the writing and editing of the manuscript. LE provided critical revisions, additional insights, and final approval. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflicts of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
O’Brien, K.J., Huque, Z.M., Pike, M.R. et al. Synergistic pathways to psychosis: understanding developmental risk and resilience factors. Neuropsychopharmacol. 51, 273–292 (2026). https://doi.org/10.1038/s41386-025-02261-6
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41386-025-02261-6