Abstract
RNase H2 is a heterotrimeric endoribonuclease that resolves RNA:DNA hybrids and genome-embedded ribonucleotides, which are implicated in DNA replication stress and cancer development. Protein and/or mRNA levels of individual RNase H2 subunits are elevated in some cancers, but little is known about the mechanisms or consequences of RNase H2 upregulation. We report that RNase H2 subunits are upregulated at the protein level in response to replication stress induced by oncogenes and chemotherapy drugs in human cancer and non-cancer cell lines. We show that inducible overexpression of the RNASEH2B subunit increases levels of the active RNase H2 heterotrimer. While causing only subtle changes to gene expression, RNASEH2B overexpression is unexpectedly associated with increased RNA:DNA hybrid levels. RNASEH2B overexpression prevents further increases in RNA:DNA hybrid levels by camptothecin or hydroxyurea and reduces replication fork stalling in presence of these drugs. Surprisingly, RNase H2 levels do not strongly impact survival of chemotherapy treatments but appear to have more subtle effects on genome instability and innate immune signalling. In contrast, increased RNase H2 levels in presence of oncogenic HRAS limit not only RAS-induced replication fork stalling but also cell death. Our findings shed new light on the functions of RNase H2 and suggest that upregulation of RNase H2 may be an important aspect of replication stress responses in cancer.
Similar content being viewed by others
Introduction
Replication stress can contribute to tumorigenesis via genome instability [1] but is also a therapeutic target and a central mechanism of action for many cytotoxic cancer therapies [2]. Nuclear processes such as transcription can contribute to replication stress by generating barriers to replication fork progression. Transcription can interfere with replication fork progression through topological stress, collisions of the RNA and DNA polymerase machineries or co-transcriptional RNA:DNA hybrids called R-loops [3].
Oncogenes such as HRAS, SS18-SSX1 and EWS–FLI1 increase RNA:DNA hybrid levels and transcription-replication conflicts (TRCs) [4,5,6]. Chemotherapies can also induce RNA:DNA hybrids. For instance, camptothecins (CPT) inhibit DNA topoisomerase I (TOP1), which alleviates supercoiling, increasing the likelihood of R-loop formation [7]. Gemcitabine and hydroxyurea deplete deoxyribonucleotides which can lead to increased ribonucleotide incorporation [8, 9] and stalled replication forks, which can in turn promote R-loop formation [10]. RNA:DNA hybrids can thereby contribute to DNA damage, genome instability and cell death caused by chemotherapies. They may also contribute to the toxicity of targeted cancer therapies such as ATR inhibitors [11] and to innate immune activation [12].
R-loop homoeostasis is a fine balance between R-loop formation and removal. In eukaryotes, RNase H1 and RNase H2 can digest the RNA component of RNA:DNA hybrids [13]. RNase H2 is a heterotrimer where RNASEH2A contains the catalytic domain, whereas RNASEH2B and RNASEH2C are essential accessory subunits [14, 15]. RNASEH2B is required for the nuclear localisation of the RNase H2 complex and interacts with the DNA sliding clamp, PCNA [16, 17]. RNase H2 initiates ribonucleotide excision repair (RER), the removal of mis-incorporated single ribonucleotides from duplex DNA [18] and can remove R-loops (Fig. 1A). RNase H2 is the predominant RNase H activity in yeast and mammals [19, 20]. In S. cerevisiae RNase H2 protein levels are cell-cycle regulated [21, 22] and R-loops at replication forks are thought to be processed specifically by RNase H2 [23]. However, in mammals RNase H2 levels appear to be constant throughout the cell cycle [8, 9] and the contribution of this enzyme to R-loop removal is less well studied. RNase H2 deficiency can cause neuroinflammation [24] or contribute to cancer development [25, 26], whereas RNase H2 upregulation is associated with cancer progression [27,28,29]. R-loop interacting proteins such as RNase H2 are potential therapeutic targets in cancer [29].
A RNase H2 complex subunits and functions. B Protein levels of HRAS, RNASEH2B (RH2B), RNASEH2C (RH2C) and Vinculin (loading control) in BJ-hTERT HRASV12ERTAM cells ± 72 h HRASG12V induction with 4-hydroxytamoxifen (4-OHT). C Relative protein levels of RH2B and RH2C after 24, 72 or 96 h HRASG12V induction normalised to ethanol (con) for each protein. N = 5 (24 and 96 h), N = 13 (RH2B 72 h), N = 6 (RH2C 72 h). D RT-qPCR analysis of RNASEH2A, RNASEH2B and RNASEH2C expression after HRASG12V induction. Asterisks compare to con. N = 4. E Substrates used for the RNase H2 activity assay with forward strand covalently coupled to 3′-fluorescein (green) and reverse strand linked to 5′-DABCYL quencher (orange). F Representative time course of DRD:DNA substrate conversion during incubation with whole cell extract ±72 h HRASG12V induction (control). N = 2 measured on the same plate. G Relative RNase H2 activities in cell extracts ±72 h HRASG12V induction. N = 4. H Protein levels of CYCLIN E, RH2A, RH2B, RH2C and Tubulin (loading control) in U2OS-CYCLIN E cells ± CYCLIN E induction with tetracycline removal (-tet) for the times indicated. I Protein levels of RH2A, RH2B, RH2C and Tubulin (loading control) in BJ-hTERT cells after 4 h treatment with 200 μM hydroxyurea (HU), 25 nM gemcitabine (GEM), 10 μM camptothecin (CPT) or DMSO (con). Means and SEM (bars) of independent experiments are shown. Asterisks indicate p values (ANOVA or mixed-effects analysis, *p < 0.05, **p < 0.01).
RNase H2 deficiency has long been studied due to its association with the neuroinflammatory disorder Aicardi-Goutières Syndrome [15, 17]. RNase H2 loss causes spontaneous replication stress [15], and interferes with the proper processing and progression of replication forks in presence of exogenous replication stress-inducing agents [30, 31]. However, RNase H2 protein levels are often increased in cancers such as colon cancer [29, 32] where they can contribute to aggressive phenotypes such as cell migration and growth [33, 34]. The roles of increased RNase H2 activity during replication stress responses are not well understood.
Here we investigate roles of RNase H2 in response to replication stress induced by oncogene activation and chemotherapy drugs, both in cancer and non-cancer cells. We show that RNase H2 protein levels and activity are up-regulated in response to replication stress. Using inducible non-cancer and cancer cell models, we show that overexpression of the RNASEH2B subunit can increase protein levels and activity of the entire RNase H2 complex. RNASEH2B overexpression unexpectedly increases global RNA:DNA hybrid levels, but prevents further RNA:DNA hybrid increases after treatment with chemotherapy drugs. RNASEH2B overexpression counteracts drug- and oncogene-induced replication fork stalling, with potential implications for genome instability, cytosolic nucleic acid sensing and cell survival.
Materials and methods
Cell lines and reagents
U2OS cells were obtained from ATCC. HCT116, HT55, NCIH747 and LS174T cells were from the Charles Swanton lab [1]. Human BJ-hTERT HRASV12ER-TAM were from the Agami and de Vita labs [35]. U2OS-Cyclin E cells were from the Jiri Lukas lab [36]. Cell lines were authenticated using 17- locus STR profiling (LGC Standards) and verified to be Mycoplasma-free by PCR. All cell lines were grown in Dulbecco’s modified Eagle’s Medium (DMEM), supplemented with 10% foetal bovine serum and 1% L-glutamine, in a humidified atmosphere containing 5% CO2. U2OS-Cyclin E cells were also grown in presence of G418 (400 µg/ml), puromycin (1 µg/ml) and tetracycline (2 µg/ml). HRASV12 was induced with 500 nM 4-hydroxytamoxifen (4OHT, Sigma). RNASEH2A, RNASEH2B or RNASEH2C were induced with 1 µg/ml doxycycline (Sigma). Cyclin E was induced by washing cells three times in PBS and transferring into medium containing 10% tetracycline-free foetal calf serum (PAA-GE Healthcare), G418 and puromycin. Camptothecin, hydroxyurea and 5,6-dichloro-1-β-D-ribofuranosylbenzimidazole (DRB) were from Sigma. Gemcitabine and triptolide were from Tocris Bioscience.
Lentiviral transfections
Lentiviral vectors were generated by Genscript Biotech, using pLIX403-ccdB-Blast, a gift from Alejandro Chavez (Addgene plasmid # 158560; http://n2t.net/addgene:158560; RRID:Addgene 158560), to generate pLIX403-ccdB-Blast-RNASEH2B. 10 µg purified vector DNA, lentiviral packaging plasmids (RRE, REV and VSV) and lipofectamine 2000 (Invitrogen) were incubated for 20 min at room temperature in OptiMEM (Gibco) before being added dropwise to HEK293FT cells in antibiotic-free DMEM and incubated for 48 h. Collected medium was passed through a 0.45 µm filter and supplemented with 8 µg/ml polybrene (Sigma), then mixed with cells followed by centrifugation for 90 min at 4000 rpm (3739 rcf), before replacing with fresh medium and repeating the polybrene infection 24 h later. Cells were washed with PBS and selected with 10 µg/ml blasticidin (Sigma) for 7 days. Clones were isolated by plating selected cells at 1000 cells per 10 cm dish.
siRNA and plasmid transfections
For siRNA transfection, cells were seeded in 6-well plates and incubated with DharmaFECT 1 reagent (Horizon Discovery) and 50 nM siRNA per well. ‘All stars negative control siRNA’ was purchased from Qiagen. ON-TARGETplus SMARTpool siRNAs against RNASEH2A (L-003535-01-0005), RNASEH2B (L-014369-01-0005) and RNASEH2C (L-014801-00-0005) were purchased from Horizon Discovery. For plasmid transfection, cells were seeded in 6-well plates and incubated with TransIT-2020 reagent (Mirus Bio) and 2.5 µg plasmid DNA per well. Control plasmid pEGFP-C2 was from Clontech and pCMV6-AC-RNASEH1-GFP was from Origene. pcDNA3.1(+)-RNASEH2A was generated by Genscript Biotech.
Western blot
For RNASEH2B detection, cells should not be freeze-thawed prior to extraction to avoid loss of protein. Cells were extracted in UTB buffer (50 mM Tris-HCl pH 7.5, 150 mM β-mercaptoethanol and 8 M urea) unless indicated otherwise, sonicated and protein concentration determined by Bradford assay. Primary antibodies were: Rabbit anti-RNASEH2B (Invitrogen PA5-59095, 1:250; Abcam ab122619, 1:250; Invitrogen PA5-83560, 1:250), anti-RNASEH2C (Proteintech 16518-1-AP, 1:500), rabbit anti-RNASEH2A (Proteintech 16132-1-AP, 1:2000), mouse anti-HRAS (Santa Cruz sc-29, 1:1000), rabbit anti-MYC-tag (Cell Signaling 2272, 1:1000), mouse anti-αtubulin (B512 Sigma T6074, 1:40000), rabbit anti-vinculin (Abcam ABCAAB1 29002-100, 1:10,000), rabbit anti-TBK1 (Cell Signaling 3504S, 1:5,000), rabbit anti-phospho(S172) TBK1 (Cell Signaling 5483S, 1:5000), rabbit anti-RNASEH1 (Proteintech 15606-1-AP, 1:1000), mouse anti-GFP (Biolegend 902601, 1:5000). Secondary antibodies were goat anti-rabbit-HRP (Cell Signaling, 7074, 1:5000), goat anti-mouse-HRP (Cell Signaling 7076, 1:5000). Protein quantification of scanned blots was performed using ImageJ (http://rsbweb.nih.gov/ij/) [37].
RNase H2 activity assay
Desalted oligonucleotides were from Eurogentec (Liege, Belgium) and were reconstituted at 100 µM in sterile nuclease-free water. Forward strands: DRD, 5′-GATCTGAGCCTGGGaGCT-3′, DNA, 5′-GATCTGAGCCTGGGAGCT-3′, RNA, 5′-gaucugagccugggagcu-3′. Reverse strand: DNA, 5′-AGCTCCCAGGCTCAGATC-3′. Lower case denotes RNA. Forward strands have a 3′-fluorescein residue and reverse strands have a 5′ DABCYL quencher residue. Oligonucleotides were annealed by denaturing 10 µM forward strand and 10 µM reverse strand at 95 °C for 5 min in 1X oligo buffer (60 mM KCl, 50 mM Tris-HCl pH 8) then gradually cooling them to room temperature. Standard curves comprise forwards strands being annealed to reverse strands that lack the 5′-DABCYL quencher. Cells were washed twice with 1X PBS, trypsinized and pelleted at 845 x g for 1 min at 4 °C. Cell pellets were snap frozen in dry ice and stored at −80 °C. Cell extracts were prepared using whole cell extract buffer (50 mM Tris pH8, 280 mM NaCl, 0.5% NP40, 0.2 mM EDTA, 0.2 mM EGTA and 10% glycerol) for 10 min on ice followed by adding an equal volume of CB buffer (20 mM HEPES pH7.9, 10 mM KCl, 1 mM EDTA and 10% glycerol) for 10 min on ice. Both lysis buffers were supplemented with 1 mM phenylmethylsulfonyl fluoride, 1 mM dithiothreitol, 1X protease inhibitor cocktail (Roche) and 1X phosSTOP (Roche) prior to use. Cell lysates were centrifuged for 10 min at 21,130 × g and 4 °C and protein concentration measured by Bradford assay. Lysates were diluted in RNase H2 assay (RA) buffer (50 mM Tris pH8, 60 mM KCl, 10 mM MgCl2, 0.01% bovine serum albumin (BSA) and 0.01% Triton X-100) to a final protein concentration of 100 ng/µl and incubated with 250 nM substrate in black 96-well flat-bottomed plates (Costar) in technical triplicates. Fluorescence intensity was measured (10 flashes per well), using 485 nm excitation and 520 nm emission filters, every 5 min for 90 min at 24 °C on a BMG Labtech PHERAstar FS multimode plate reader or a Perkin-Elmer EnSpire multimode plate reader. Standard curves were generated to convert relative fluorescence units (RFU) to pmol of substrate converted by linear regression. For absolute values, correction was performed by subtracting the negative control wells, containing substrate only. For the DRD:DNA substrate, values from DNA:DNA substrate wells were subtracted for additional correction.
Co-immunoprecipitation
Immunoprecipitation was carried out using the ChromoTek Myc-trap® Magnetic Agarose Kit (Proteintech) according to the manufacturer’s instructions with some modifications. Buffers provided in the kit were supplemented with DNase I, 2.5 mM MgCl2, 1X EDTA-free protease inhibitor cocktail (Roche), 1X phosSTOP (Roche) and 1 mM phenylmethylsulfonyl fluoride (ThermoFisher). A total of 1 mg protein per condition was incubated with the anti-Myc-tag nanobody for 1 h. Supernatants were eluted in 5X Laemmli buffer and denatured at 95 °C for 5 min. Samples were analysed with SDS-PAGE and Western blot.
Slot blot
Genomic DNA was extracted using the DNeasy Blood & Tissue kit (Qiagen) according to the manufacturer’s instructions. 10 µg genomic DNA per sample was mock treated or treated with 2 U/µg DNA of RNaseH (NEB, M0297) for 2 h at 37 °C. 250 ng DNA per well was loaded onto the slot blot apparatus, and transferred onto a pre-wetted nylon membrane (Amersham Hybond N+) by vacuum suction. The membrane was UV-crosslinked at 1200 µJ and blocked in sterile 5% milk/TBST for 1 h at room temperature. The membrane was incubated overnight at 4 °C in mouse anti-DNA-RNA hybrid antibody (S9.6 hybridoma growth medium, ATCC HB-8730, 1:1000; MABE1095, 1:1000) or mouse anti-dsDNA antibody (Abcam ab27156, 1:100000) in sterile 5% BSA/TBST, before being washed in TBST and incubated in goat anti-mouse HRP (Cell Signaling 7074, 1:5000) 5% milk/TBST for 1 h at room temperature. Images were taken using a ChemiDoc™ MP Imaging System (BioRad) and quantifications performed using ImageJ.
DNA fibre assay
Cells were pulse-labelled with 25 µM CldU followed by 250 µM IdU as indicated. DNA fibres were spread by spotting 2 µl of cells at 5 × 105 cells/ml in PBS onto microscope slides (Menzel Gläser) and allowed to settle for 5 min. Cells were mixed with 7 µl of spreading buffer (0.5% SDS, 200 mM Tris-HCl pH 7.4 and 50 mM EDTA) for 2 min. DNA fibres were spread by tilting the slides for 3 min and fixed in methanol/ glacial acetic acid (3:1). Rehydrated fibre spreads were denatured with 2.5 M HCl for 75 min at room temperature. Slides were blocked with 1% BSA and 0.1% Tween-20 in PBS before incubation with rat anti-BrdU (BU1/75, Abcam ab6326, 1:700) and mouse anti-BrdU (B44, Becton Dickinson 347580, 1:500) for 1 h followed by fixation with 4% PFA for 10 min. Slides were incubated with secondary antibodies anti-mouse IgG AlexaFluor 488 (ThermoFisher, 1:500) and anti-rat IgG AlexaFluor 594 (Invitrogen, 1:500). Slides were mounted (Fluoroshield, Sigma) and imaged using a Nikon E600 microscope with a Nikon Plan Apo 60x (1.3NA) oil lens, an Andor Zyla sCMOS digital camera and the Nikon NIS elements BR 5.41.01 software. The lengths of the CldU and IdU tracts were measured using the ImageJ software and lengths converted into μm using a micrometre slide. For replication structures, stalled forks were quantified by scoring the percentages of CldU-only labelled fibres of other quantifiable structures. Numbers of fibres analysed per experiment are listed in Table S1.
Immunofluorescence
Cells were washed and fixed to coverslips with 4% paraformaldehyde for 10 min at room temperature, then permeabilized with 0.25% Triton X-100 in PBS for 5 min at room temperature before blocking with 1% BSA and 0.1% Tween-20 in PBS. For ssDNA and dsDNA staining, coverslips were fixed and permeabilized, then treated with 0.1 mg/ml RNase A (ThermoFisher, EN0531) for 30 min at 37 °C and 5% CO2. Primary antibodies were mouse anti-ssDNA (Merck MAB3868, 1:1000), mouse anti-dsDNA (Merck MAB1293, 1:100), rabbit anti-53BP1 (Bethyl A300-272A, 1:3000), mouse anti-phospho-Histone H2AX (Merck JBW301, 1:1000), rabbit anti-RAD51 (Abcam ab63801, 1:800). Secondary antibodies were anti-mouse IgG AlexaFluor 488 (ThermoFisher, 1:500) and anti-rabbit IgG AlexaFluor 594 (ThermoFisher, 1:500). Coverslips were mounted in Fluoreshield with 4.6-diamidino-2-phenylindole (DAPI) (Sigma). For quantification of γH2AX nuclear intensity, nuclear masks were created using ImageJ software based on DAPI-staining and mean fluorescence intensities per pixel were quantified per nucleus.
RNA extraction
Cells were harvested in TRI Reagent® (Zymo Research) and RNA extraction was performed using the Direct-zol RNA Miniprep Kit (Zymo Research), including DNase I treatment, according to the manufacturer’s instructions. RNA quality and concentration was determined either using the 4200 tapestation system or a NanoDrop Lite spectrophotometer (ThermoFisher).
Reverse transcription quantitative real-time PCR (RT-qPCR)
Total RNA (1 μg) was reverse-transcribed using qScript cDNA synthesis kit (Quantabio), following the manufacturer’s instructions. RT-qPCR primers for amplification were obtained from Merck and their sequences are as follows: ACTB Forward TTGCGTTACACCCTTTCTTG, ACTB Reverse CACCTTCACCGTTCCAGTTT;
RNASEH2A Forward AATGGAGGACACGGACTTTG, RNASEH2A Reverse ATGTCTCTGGCATCCCTACG;
RNASEH2B Forward CTGGTGACCAAGCTTCCACT, RNASEH2B Reverse ATGGAGGATTTGGCAATGAG;
RNASEH2C Forward CTTAACTTGGCCCAGCCTTG, RNASEH2C Reverse TCCTTTATTGGGGTGATGGA.
ISG15 Forward GCGAACTCATCTTTGCCAGTA, ISG15 Reverse CCAGCATCTTCACCGTCAG
IL6 Forward CAGCCCTGAGAAAGGAGACAT, IL6 Reverse GGTTCAGGTTGTTTTCTGCC
RPLP0 Forward CAGATTGGCTACCCAACTGTT, RPLP0 Reverse GGAAGGTGTAATCCGTCTCCAC
2 μl of cDNA was incubated with primers and SYBR™ Green PCR Master Mix (ThermoFisher) and analysed using the Real Time PCR QuantStudio 5 system (ThermoFisher). Cycling parameters were 50 °C for 2 min and 95 °C for 5 min followed by 40 cycles of 95 °C for 30 s, 60 °C for 30 s and 72 °C for 30 s. Results were normalised to ACTB (Fig. 1B) or RPLP0 (all others).
Steady-state eukaryotic directional mRNA-sequencing
Library preparation and sequencing on the Illumina platform NovaSeq 6000 S4 (PE150) were performed by Novogene. Sequences were first quality checked using FastQC v0.11.9 (https://www.bioinformatics.babraham.ac.uk/projects/fastqc/), then quality filtered and trimmed using TrimGalore v0.6.6 (https://www.bioinformatics.babraham.ac.uk/projects/trim_galore/). Genome mapping was performed with STAR [38] (v2.7.10b) against the human reference genome (hg38 assembly). Reads were counted using FeatureCounts [39] (subread v2.0.3) and differential analysis was performed using DESeq2 [40], with p value calculated using the Wald test. Differential genes were identified by P value < 0.01 and LogFC>2. Volcano plots were generated using EnhancedVolcano.
Normalised read counts were converted into GCT files compatible with GSEA 4.3.3 (Broad Institute) [41, 42]. GSEA was run with the following parameters: number of permutations (1000), permutation type (gene_set), ChIP platform (Human_Ensembl_Gene_ID_MSigDB.v2024.1.Hs.chip). All other parameters were left as the default setting. The gene sets databases used were from the Molecular Signatures Database (MSigDB) [43, 44].
Colony survival assay
For siRNA transfections, HCT116 cells at 70% confluency were transfected for 24 h prior to being plated. Cells were allowed 24 h to adhere before doxycycline or drug treatments. Doxycycline was replenished every 2 days. To stain colonies, cells were washed twice with 1X PBS and fixed in 50% ethanol, 2% methylene blue for 20 min at room temperature. Colonies of >50 cells were counted. For cell proliferation assays, 20,000 cells were seeded per well of a 6-well plate. After treatments, cells were trypsinized, resuspended in 1 ml of PBS and counted using cell counting chambers.
Flow cytometry
Cells were fixed in 70% ice-cold ethanol and centrifuged at 250 × g for 5 min. For inclusion of the sub-G1 population, culture medium and pre-trypsinisation washes were also collected. Cells were washed 3 times with PBS prior to incubating with 10 µg/ml propidium iodide and 25 µg/ml ribonuclease A from bovine pancreas (Merck) in PBS. Cell cycle profiles were generated using the Fortessa X-20 flow cytometer and analysed with the BD FacsDiva software.
TCGA data analysis
Cancer Genome Atlas datasets were accessed using cBioPortal. Colorectal Adenocarcinoma (COAD, TCGA, PanCancer Atlas), all complete samples, was interrogated for RNASEH2A, RNASEH2B and RNASEH2C mRNA expression z-scores relative to diploid samples (RNA Seq V2 RSEM). The correlation with aneuploidy scores was obtained from the built-in cBioPortal Comparison/Survival tab.
Statistical analysis
Experimenters were blinded to the sample allocation for DNA fibre image acquisition and DNA foci analysis. We aimed to perform at least three independent biological repeats of each experiment for adequate statistical power. Numbers of independent biological repeats (N) are indicated in the figure legends. Unless specified, all values for bar graphs are the mean ± s.e.m of results from biological replicates. Scatter plots represent individual data points and medians from independent biological repeats. Statistical tests were performed using the GraphPad Prism 10 software, version 10.2.0 (392). Test were chosen depending on the structure of the datasets. Gaussian distribution was determined using GraphPad Prism. Paired or unpaired student’s t-test was used for two comparisons with Gaussian distribution or a Mann-Whitney test for two comparisons with non-Gaussian distribution. For multiple comparisons, one-or two-way ANOVA or mixed-effects analysis with Dunnett’s, Tukey’s or Sidak’s test for datasets with Gaussian distribution and Kruskal-Wallis test with Dunn’s test for datasets with non-Gaussian distribution. We did not determine whether variance was similar between the groups that were statistically compared. For analysis of TCGA datasets, the cBioPortal in-built Wilcoxon Test was used.
Results
Induction of RNase H2 protein levels and activity in response to oncogene-induced replication stress
We had previously shown that the expression of oncogenic HRASG12V in BJ-hTERT fibroblasts increased RNA:DNA hybrid levels, caused TRCs and increased mRNA and protein levels of RNase H1 [6]. We therefore decided to further investigate how HRASG12V induction influences the protein levels of RNA:DNA hybrid-resolving enzymes. We found that HRASG12V induction for 3 or 4 days, but not for 24 h, increased protein levels of RNASEH2B and RNASEH2C (Fig. 1B, C; Fig. S1A–C). HRASG12V induction was accompanied by increased mRNA expression of genes encoding RNase H2 subunits, especially RNASEH2A (Fig. 1D).
To measure the impact of oncogenic HRASG12V on cellular RNase H2 activity, we employed a fluorescence resonance energy transfer-based activity assay [14] to measure nuclease activity against RNA:DNA hybrid substrate or double stranded DNA with a single ribonucleotide (DRD:DNA) (Fig. 1E). As RNase H2 is the only known enzyme to specifically cleave DNA-embedded ribonucleotides, the DRD:DNA substrate is considered a specific readout of RNase H2 activity [8]. Both RNase H2-specific and RNA:DNA hybrid resolution activities were increased after HRASG12V induction (Fig. 1F, G).
HRASG12V will accelerate S phase entry, which may increase RNase H2 protein levels due to cell cycle regulation. However, the biggest increase in S/G2 phase percentage occurred 24 h after HRASG12V induction, when RNASEH2B and RNASEH2C protein levels were not elevated (Fig. S1D, E). We also previously showed that MAP kinase signalling and replication stress are more strongly elevated after 72 h compared to 24 h HRASG12V induction [6]. Therefore, RNase H2 protein levels increase in response to RAS signalling and/or replication stress rather than S phase entry.
To further investigate whether the increase in RNase H2 protein and activity was specific to RAS signalling or more generally observed during oncogene-induced replication stress, we used U2OS cells inducibly overexpressing the oncogene Cyclin E [45], which also increased RNase H2 protein levels (Fig. 1H; Fig. S1F). We next used short 4 h treatments with replication stress-inducing drugs hydroxyurea (HU), gemcitabine (GEM), or CPT in BJ-hTERT cells without HRASG12V induction. This also increased RNase H2 protein levels (Fig. 1I, Fig. S1G). Taken together, these data suggest that RNase H2 subunits are up-regulated at the protein level in response to replication stress.
Induction of RNase H2 protein and activity in response to drug-induced replication stress
We further investigated the role of RNase H2 in response to replication stress-inducing drugs using colon cancer (CRC) cell lines relevant to chemotherapy drug responses and cancers with elevated RNase H2 subunit levels [29, 32]. mRNA expression of at least one RNase H2 subunit, most commonly RNASEH2B, is increased in over 25% of CRCs which correlates with higher aneuploidy, and therefore likely with higher chromosomal instability and possibly higher replication stress [1] (Fig. 2A, B). We used four CRC cell lines. HT55 and NCIH747 are characterised by high chromosomal instability and replication stress, while LS174T and HCT116 cells are characterised by microsatellite instability and low replication stress [1]. We then investigated protein levels of RNASEH2B and RNASEH2C after 2 h treatment with HU, GEM or CPT (Fig. 2C–J; Fig. S2A–H). Despite relatively high variability between experiments, there was an overall trend of drug-induced increases of RNASEH2B and RNASEH2C levels across most cell lines. Although 2 h CPT treatment increased RNASEH2B protein levels in HCT116 cells (Fig. 2I), in contrast to HRASG12V activation this was not accompanied by a trend towards increased RNASEH2A mRNA expression (Fig. 2K). RNase H2 activity increased in response to replication stress inducing agents in selected cell lines, HT55 and HCT116, in line with the increases in protein levels (Fig. 2L, M).
A RNASEH2A, -B, and -C mRNA levels are increased in colorectal adenocarcinoma (TCGA Pan-Cancer Atlas [74, 75], RNA Seq V2 RSEM, z-score threshold ± 2.0). B Increased RNASEH2A, -B, and -C mRNA levels are correlated with higher aneuploidy in colorectal adenocarcinoma (TCGA Pan-Cancer Atlas). Medians with 25% and 75% quartiles are shown. Wilcoxon Test, **** p < 0.0001. C Relative protein levels of RH2B and RH2C in HT55 cells after 2 h treatment with 200 μM HU, 10 μM CPT or DMSO. N = 3–6. D Relative protein levels of RH2B and RH2C in HT55 cells after 2 h treatment with 25 nM GEM or DMSO. N = 7. E Relative protein levels of RH2B and RH2C in NCIH747 cells after treatment as in (C). N = 3–4. F Relative protein levels of RH2B and RH2C in NCIH747 cells after treatment as in (D). N = 5. G Relative protein levels of RH2B and RH2C in LS174T cells after treatment as in (C). N = 3–5. H Relative protein levels of RH2B and RH2C in LS174T cells after treatment as in (D). N = 5. I Relative protein levels of RH2B and RH2C in HCT116 cells after treatment as in (C). N = 4–7. J Relative protein levels of RH2B and RH2C in HCT116 cells after treatment as in (D). N = 5. K RT-qPCR analysis of RH2A, RH2B, RH2C expression in HCT116 cells after 2 h treatment with 10 μM CPT. N = 4. L RNase H2 activity (DRD:DNA substrate) in HT55 cells after 2 h treatment with 200 μM HU or CPT. N = 5 (1 μM CPT and 200 μM HU), 3 (10 μM CPT). M RNase H2 activity (DRD:DNA substrate) in HCT116 cells after 2 h treatment with CPT. N = 3–4. Bar graphs: Means and SEM (bars) of independent experiments are shown. Asterisks indicate p values (ANOVA, ns: not significant, *p < 0.05, **p < 0.01).
Overexpression of RNASEH2B increases ribonucleotide and R-loop resolving activity
To develop tools for studying the role of increased RNase H2 activity in the response to replication stress, we generated HCT116 and BJ-hTERT cell lines for inducible overexpression of MYC-tagged RNASEH2B. When RNASEH2B was overexpressed in these cell lines, RNASEH2A and RNASEH2C protein levels also increased (Fig. 3A, B; Fig. S3A, B) without a corresponding increase in RNASEH2A or RNASEH2C mRNA expression (Fig. 3C; Fig. S3C). Co-immunoprecipitation showed that ectopic MYC-RNASEH2B interacts with endogenous RNASEH2A and RNASEH2C forming the RNase H2 complex (Fig. 3D). Ectopic RNASEH2B overexpression further increased RNase H2-specific and RNA:DNA hybrid resolution activity (Fig. 3E; Fig. S3D–G).
A Protein levels of RH2A, RH2B, RH2C and Tubulin (loading control) in HCT116-RH2B cells ±48 h treatment with doxycyline (Dox). B Protein levels of RH2A, RH2B, RH2C and Tubulin in BJ-hTERT-RH2B cells ±48 h Dox. C RT-qPCR analysis of RNASEH2A (RH2A), RNASEH2B (RH2B) and RNASEH2C (RH2C) expression in HCT116-RH2B cells after 48 h Dox normalised to no Dox control. N = 3. D Co-immunoprecipitation using MYC-trap to precipitate ectopic RH2B 48 h after RH2B induction, followed by immunoblotting for RH2A, RH2B, RH2C and Tubulin (loading control). Non-spec: non-specific band. E RNase H2 activity (DRD:DNA substrate) in HCT116-RH2B and BJ-hTET-RH2B cells ±48 h Dox. N = 5 (HCT116), N = 3 (BJ-hTERT). F Slot blot analysis of RNA:DNA hybrid levels in genomic DNA after 50 min treatment with 200 μM HU, 10 μM CPT or DMSO. S9.6: RNA:DNA hybrids; dsDNA: double-stranded DNA (loading control). G Quantification of slot blot analysis in HCT116-RH2B cells. S9.6 intensities were normalised to dsDNA and to DMSO control. N = 6. H Quantification of slot blot analysis in BJ-hTERT-RH2B cells as in (G). N = 4. I Volcano plot of differentially expressed genes in parental HCT116 cells treated with doxycycline (control for doxycycline effects) and HCT116-RH2B cells treated with doxycycline (RH2B overexpression). Red dots indicate differentially expressed genes with a P < 0.01 and LogFC > 2. Data from N = 3. The means and SEM (bars) of independent experiments are shown. Asterisks indicate p values (ANOVA, ns: not significant, *p < 0.05, **p < 0.01).
To test whether increased RNase H2 activity would impact on global RNA:DNA hybrid levels, RNASEH2B overexpression was combined with a 50 min CPT or HU treatment to induce RNA:DNA hybrids. Slot blot analysis of isolated genomic DNA using the S9.6 antibody that detects RNA/DNA hybrids [46] revealed that RNASEH2B overexpression alone increased RNA:DNA hybrid signal to some extent in untreated cells (Fig. 3F–H). CPT or HU treatment both induced around 2-fold increase in global RNA:DNA hybrid signal, but there was less or no additional increase in RNA:DNA hybrids when the drug treatments were combined with RNASEH2B overexpression (Fig. 3G, H, Fig. S4A, B). These effects were significant in BJ-hTERT fibroblasts (Fig. 3H, Fig. S4B), but not in HCT116 cells (Fig. 3G, Fig. S4A) where the RNA:DNA hybrid signal was more variable.
The unchanged or increased RNA:DNA hybrid signal after RNASEH2B overexpression could suggest that overexpressed RNASEH2B has a dominant negative effect outside of the RNase H2 complex, although this seemed unlikely because protein levels of endogenous RNASEH2A and RNASEH2C increased with RNASEH2B overexpression (Fig. S3A, B). Alternatively, RNASEH2B overexpression might increase RNA:DNA hybrids that are normally degraded by RNase H1, either because these are different classes of hybrids or because RNASEH2B overexpression inhibits RNase H1. To investigate this, we combined RNASEH2B overexpression with transient overexpression of either RNase H1 or RNASEH2A in HCT116 cells followed by S9.6 slot blot analysis (Fig. S4C–E). This revealed a trend towards decreased RNA:DNA hybrid signal specifically after combined RNASEH2B and RNase H1 overexpression, suggesting that while RNASEH2B overexpression may reduce hybrid levels, this could be masked by a concomitant increase in hybrids that require RNase H1 for degradation. RNASEH2B depletion using siRNA did not alter RNA:DNA hybrid levels in HCT116 cells, either alone or in combination with CPT or HU treatment (Fig. S4F–H).
RNase H2 has been shown to promote efficient transcription by interacting with RNA polymerase II [47]. Therefore, to investigate whether RNASEH2B overexpression alters the expression of cell cycle or DNA damage response genes with impact on replication stress phenotypes, we performed steady-state RNA sequencing after 48 h RNASEH2B induction in HCT116-RH2B cells. 48 h doxycycline treatment of parental HCT116 cells was used as a control (Fig. 3I). RNASEH2B overexpression changed the expression of few genes (13 genes up and 4 genes down, Fig. 3I, Table S2). Three transcripts, EPCAS, CBX5 and RN7SL390P (the latter an snRNA pseudogene embedded within CBX5) were significantly increased after RNASEH2B induction, but this was not reproducible by RT-qPCR (Fig. S5A, B). Gene set enrichment analysis suggested increased expression of genes involved in inflammatory responses and epithelial-mesenchymal transition, which are also responses to DNA damage or stress [48,49,50,51,52,53], and a down-regulation of proliferation pathways (Fig. S5C, D). Taken together, while overexpression of RNASEH2B in HCT116 cells for 48 h has very little effect on expression of individual genes, subtle changes in gene expression patterns are consistent with some cellular stress in line with the increased RNA:DNA hybrid levels.
RNASEH2B overexpression counteracts chemotherapy-induced replication fork stalling
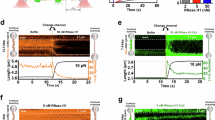
To characterise the impact of RNASEH2B overexpression on DNA replication and replication stress, we used DNA fibre approaches. RNASEH2B overexpressing BJ-hTERT fibroblasts displayed no overt growth defects for up to 7–10 days after RNASEH2B induction (Fig. S6A–C). DNA fibre analysis using thymidine analogues chlorodeoxyuridine (CldU) and iododeoxyuridine (IdU) for 20 min each (Fig. 4A), showed that 48 h RNASEH2B overexpression caused no significant change in average fork speed (Fig. 4B) or CldU/IdU length ratios, with ratios above 1 indicating increased replication fork stalling [54] (Fig. 4C).
A DNA fibre labelling protocols after 48 h RH2B overexpression. B Replication fork speeds in BJ-hTERT-RH2B cells ±48 h RH2B induction. N = 3. C CldU/IdU ratios in BJ-hTERT-RH2B cells ±48 h RH2B induction. Data from 6 repeats. D DNA fibre labelling protocols after 48 h RH2B overexpression with drug treatments. E Replication fork speeds (IdU label) in BJ-hTERT-RH2B cells ±48 h RH2B induction ±10 μM CPT. N = 3 F Percentages of stalled forks (CldU labelled only) in BJ-hTERT-RH2B cells ±48 h RH2B induction ±10 μM CPT. N = 4. G Percentages of stalled forks in HCT116-RH2B cells ±48 h RH2B induction ±100 nM CPT for during the IdU label. CldU and IdU treatment were 30 min each. N = 3. H Percentages of stalled forks in HCT116-RH2B cells ±48 h RH2B induction ±200 μM HU. N = 3. I Percentages of BJ-hTERT-RH2B cells with >8 53BP1 foci ±48 h RH2B induction ±10 μM CPT. N = 3. J Percentages of stalled forks in BJ-hTERT-RH2B cells without RH2B induction and treated with 10 μM CPT ± 100 μM DRB. N = 2–3. K CldU/IdU ratios in HCT116-RH2B cells treated with 200 μM HU ± 48 h RH2B induction and ±1 μM Triptolide (TRIP). Data from 3 repeats. L DNA fibre labelling protocol for fork restart assay. M Percentages of stalled forks (CldU-labelled only) in HCT116-RH2B cells ±48 h RH2B induction after release from 2 mM HU. N = 3. N Speeds of restarting replication forks (IdU label) in HCT116-RH2B cells treated as in (L). N = 3. O Track lengths of stalled replication forks (CldU label) in HCT116-RH2B cells treated as in (L). Data from 3 repeats. Bar graphs show the means and SEM (bars) of independent experiments. Scatter plots show aggregate data with medians (lines), and medians (white dots) of independent experiments. Asterisks indicate p values (ANOVA, Kruskal-Wallis or Mann-Whitney test, ns: not significant, *p < 0.05, **p < 0.01, ****p < 0.0001). Numbers of fibres analysed per experiment are listed in Table S1.
We then investigated the impact of RNASEH2B overexpression on chemotherapy-induced replication fork slowing and stalling, by adding CPT with the second label (IdU) for 50 min (Fig. 4D). CPT reduced IdU fork speeds by half, indicative of replication fork slowing. CPT-induced replication fork slowing was not significantly affected by RNASEH2B overexpression (Fig. 4E). However, when quantifying stalled replication forks (fibres containing only the first or CldU label), RNASEH2B overexpression significantly reduced CPT-induced fork stalling (Fig. 4F). Similar results were obtained when using HCT116 cells instead of BJ-hTERT fibroblasts (Fig. 4G) and when treating HCT116 cells with HU instead of CPT (Fig. 4H). Therefore, RNase H2 activity counteracts CPT and HU-induced fork stalling. While fork stalling can promote fork collapse into double-strand breaks (DSBs), RNASEH2B overexpression did not affect CPT-induced DSB levels as measured by 53BP1 foci formation, with a slight but non-significant increase in 53BP1 foci after RNASEH2B overexpression alone (Fig. 4I).
It has been reported that RNase H2 depletion decreases the degradation of HU-stalled forks and that this could be rescued with the transcription inhibitor triptolide, but not with another transcription inhibitor, DRB [31]. In our hands both DRB and triptolide treatments rescued CPT- or HU-induced fork stalling to a significant extent (Fig. 4J, K). This supports that RNase H2 activity reduces transcription-dependent fork stalling regardless of whether the stalling is induced by CPT or HU. To investigate whether RNASEH2B overexpression promotes restart or degradation of stalled forks, we used a DNA fibre labelling protocol in HCT116 cells where ongoing forks were pre-labelled with CldU for 20 min, then stalled with 2 mM HU for 2 h and released into fresh IdU for 1 h to measure fork restart [55] (Fig. 4L). RNASEH2B overexpression only had a minor effect on the percentage of stalled forks or the speed of restarting forks (Fig. 4M, N). We then reasoned that if RNASEH2B overexpression increases stalled fork degradation during the HU block, this would manifest in reduced length of the CldU-labelled tracks. However, RNASEH2B overexpression did not decrease the length of CldU-labelled tracks during the HU block (Fig. 4O), suggesting there was no increase in fork degradation.
Impact of RNASEH2B overexpression on cell survival and genome stability
To investigate the impact of RNASEH2B overexpression on cell survival, we used colony formation assays. Surprisingly, RNASEH2B overexpression did not substantially affect the survival of HCT116 cells in response to CPT, HU or GEM. This held true whether the treatment was administered for 24 h followed by washout (Fig. 5A–C) or continuously (Fig. S7A, B). We also investigated the effect of siRNA depletion of RNASEH2A, RNASEH2B or RNASEH2C on survival. RNASEH2B depletion reduced RNase H2-specific activity to less than 20% of control, and to the same extent as co-depletion of all subunits (Fig. 5D, E). However, depletion of any of the RNase H2 subunits did not affect the survival of HCT116 cells in response to CPT or GEM (Fig. 5F, G), with only a small reduction in survival at lower HU concentrations when RNASEH2A was depleted (Fig. 5H).
A Colony survival of HCT116-RH2B cells after release from 24 h CPT. N = 3. B Colony survival of HCT116-RH2B cells after release from 24 h HU. N = 6 (5 mM: N = 5). C Colony survival of HCT116-RH2B cells after release from 24 h GEM. N = 2. D Protein levels of RH2B, RH2C and tubulin (loading control) in HCT116 cells after 72 h transfection with non-targeting control siRNA (siNTC) or siRNA against RH2A (siRH2A), RH2B (siRH2B) and/or RH2C (siRH2C). E Relative RNase H2 and RNA:DNA hybrid resolving activity in HCT116 cell extracts after 72 h transfection with non-targeting control siRNA (siNTC) or siRNA against RH2A (siRH2A), RH2B (siRH2B) and/or RH2C (siRH2C). N = 3. F Colony survival of HCT116 cells after 72 h transfection with siNTC, siRH2B or siRH2C followed by 24 h treatment with CPT and release into fresh medium. N = 6. G Colony survival of HCT116 cells after 72 h transfection with siNTC, siRH2B or siRH2C followed by 24 h treatment with GEM and release into fresh medium. N = 5 (5 nM, 10 nM), N = 6 (DMSO, 20 nM, 40 nM, 60 nM). H Colony survival of HCT116 cells after 72 h transfection with siNTC, siRH2B or siRH2C followed by 24 h treatment with HU and release into fresh medium. N = 5. The means and SEM (bars) of independent experiments are shown. Asterisks indicate p values (ANOVA, ns: not significant, *p < 0.05, **p < 0.01, ***p < 0.001).
Rather than promoting cell survival, RNASEH2B overexpression may protect against sub-lethal genome instability and/or prevent innate immune activation. To investigate the impact of RNASEH2B overexpression on genome instability, we quantified micronucleus formation as a marker of genome instability in response to short-term treatment with CPT, GEM or HU in HCT116-RH2B cells (Fig. 6A). RNASEH2B overexpression prevented HU-induced micronucleus formation (Fig. 6B; Fig. S7C), had a small effect on reducing micronuclei in response to GEM (Fig. 6C; Fig. S7D), but no effect on the induction of micronuclei in response to CPT (Fig. 6D; Fig. S7E). In BJ-hTERT-RH2B cells, we observed micronucleus induction only after 24 h continuous drug treatment and under these conditions the effect of RNASEH2B overexpression was less pronounced (Fig. S7F–H).
A Treatment schematic for micronucleus quantification in HCT116 cells. B Fold increase in HCT116-RH2B cells with micronuclei ±RH2B induction ±2 mM HU. N = 4. C Fold increase in HCT116-RH2B cells with micronuclei ±RH2B induction ±1 μM GEM. N = 3. D Fold increase in HCT116-RH2B cells with micronuclei ± RH2B induction ±10 μM CPT. N = 4. E Treatment schematic and representative images for cytosolic DNA staining after drug treatments. F Fold increase in percentages of BJ-hTERT-RH2B cells with cytoplasmic ssDNA or dsDNA staining ±48 h RH2B induction and 24 h treatment with 1 μM CPT, 25 nM GEM, 200 μM HU or DMSO. Circles: dsDNA antibody, squares: ssDNA antibody. N = 4 (CPT and GEM), N = 3 (HU). G Relative intensity of cytoplasmic ssDNA and dsDNA staining, normalised to image background, in BJ-hTERT-RH2B cells ±48 h RH2B induction and 24 h treatment with 1 μM CPT, 25 nM GEM, 200 μM HU or DMSO. Circles: dsDNA antibody, squares: ssDNA antibody. N = 4 (CPT and GEM), N = 3 (HU). H Protein levels of phospho-S172 TBK1, TBK1 and Tubulin (loading control) in BJ-RH2B cells ±48 or 72 h RH2B induction and 48 h treatment with drugs or DMSO as indicated. I Relative protein levels of phospho-TBK1 normalised to TBK1 in BJ-RH2B cells after treatment as in (H). N = 3, N = 2 (10 μM CPT). J Relative protein levels of phospho-TBK1 as in (I), normalised to DMSO also for samples with RH2B induction. K RT-qPCR analysis of IL6 (Interleukin 6) expression after in BJ-RH2B cells ±72 h RH2B induction and 48 h treatment with 1 μM CPT, 25 nM GEM, 200 μM HU or DMSO. Values were normalised to DMSO for each condition. N = 3. The means and SEM (bars) of independent experiments are shown. Asterisks indicate p values (ANOVA or Kruskal-Wallis test, ns: not significant, *p < 0.05, **p < 0.01, ***p < 0.001, **** p < 0.0001).
Replication stress can lead to accumulation of nucleic acids in the cytoplasm, including RNA:DNA hybrids, that can potentially activate innate immune signalling pathways [12]. DNA damage could similarly activate this pathway through accumulation of micronuclear DNA [48,49,50] or other sources of double-stranded DNA (dsDNA) [52, 56] or single-stranded DNA (ssDNA) [51]. Because HCT116 cells are cGAS-STING signalling-deficient [57], we also used BJ-hTERT-RH2B cells to investigate the impact of RNASEH2B overexpression on cytoplasmic nucleic acid signalling pathways. We first stained for cytosolic DNA using antibodies raised against ssDNA or dsDNA. 24 h treatment with CPT or GEM resulted in higher percentages of cells with large cytoplasmic DNA foci as measured by single- or double-stranded DNA antibodies (Fig. 6E, F; Fig. S8A–C), but the percentages of cells with foci were unaffected by RNASEH2B overexpression (Fig. 6F; Fig. S8A–C). We quantified the fluorescence intensity of cytoplasmic DNA staining in BJ-hTERT-RH2B cells; this revealed a slight increase in global cytoplasmic DNA intensity only in response to CPT treatment. RNASEH2B overexpression slightly reduced global cytoplasmic DNA intensity in untreated and CPT-treated cells, but not in response to GEM or HU (Fig. 6G). We then quantified autophosphorylation of tank binding kinase 1 (TBK1), a serine/threonine kinase directly activated by several nucleic acid sensing pathways including cGAS-STING and RIG-I, which regulates innate immunity responses including activation of type I interferon and interferon-stimulated genes (ISGs) [58]. Activating TBK1 autophosphorylation on serine 172 was increased both after 72 h RNASEH2B overexpression and by 48 h drug treatments in uninduced BJ-hTERT-RH2B cells and there was no further increase when drugs were combined with RNASEH2B overexpression (Fig. 6H–J). We then performed expression analysis of pro-inflammatory genes that are downstream of STING and/or TBK1 such as interleukin 6 (IL6) and interferon-stimulated gene 15 (ISG15) [59, 60]. 48 h drug treatments induced expression of both genes, while RNASEH2B overexpression caused a minor increase in IL6 expression and a slight attenuation of IL6 induction in response CPT (Fig. 6K; Fig. S8D–F). These results support that RNASEH2B overexpression can activate some pro-inflammatory signalling and attenuate the drug-induced formation of cytoplasmic nucleic acids, but that increased RNase H2 levels have limited effect on drug-induced downstream pro-inflammatory signalling.
RNASEH2B counteracts HRAS-induced replication fork stalling and cell death
To investigate the role of RNase H2 upregulation in response to RAS activation, we combined HRASG12V induction with siRNA depletion of RNASEH2B to counteract the RAS-induced increase (Fig. 7A, B). Depleting RNASEH2B together with HRASG12V induction further increased RNA:DNA hybrid signal (Fig. 7C). As reported previously, HRASG12V strongly decreased replication fork speeds [6], however, RNASEH2B depletion did not decrease fork speeds further (Fig. 7D). Instead, RNASEH2B depletion increased CldU/IdU length ratios specifically when combined with HRASG12V induction, suggesting increased replication fork stalling when preventing HRASG12V-driven RNase H2 upregulation (Fig. 7E). Combining RNASEH2B depletion with HRASG12V induction led to no additional increase in γH2AX intensity or 53BP1 foci formation compared to HRASG12V induction alone (Fig. 7F, G). Oncogenic HRASG12V is known to initially increase proliferation, but then cause growth arrest due to apoptosis and senescence [61]. After HRASG12V induction, cell numbers increase for 3 days, while growth stops between 6 and 8 days [4,5,6]. To investigate the impact of RNase H2 on cell growth in presence of HRASG12V, we measured proliferation by cell counting over a 7-day period after HRASG12V induction with or without depleting RNASEH2B using siRNA. RNASEH2B siRNA transfection was maintained for 8 days for all samples, while the length of HRASG12V induction was varied (Fig. 7H). While there was no difference in HRASG12V-induced proliferation up to day 3 when HRASG12V was combined with RNASEH2B depletion, at day 7 this led to an additional decrease in cell numbers (Fig. 7I, Fig. S9A). Flow cytometry analysis revealed increased percentages of cells with sub-G1 DNA content when HRASG12V was combined with RNASEH2B depletion, indicating increased cell death (Fig. 7J, Fig. S9B). Staining for β-galactosidase-positive cells, a measure of HRASG12V-induced senescence, showed no difference when HRASG12V was combined with RNASEH2B depletion (Fig. 7K). This suggests that RNase H2 activity is required to reduce replication stress for longer-term cell proliferation and survival, but does not to prevent senescence, in the presence of oncogenic RAS.
A BJ-hTERT HRASV12ERTAM cells were transfected for 96 h with non-targeting control siRNA (siNTC) or RH2B siRNA (siRH2B) and HRASG12V was induced after 24 h for 72 h. B Protein levels of RH2A, RH2B, RH2C, HRAS and Tubulin (loading control) after treatment as in (A). C Quantification of slot blot analysis of RNA:DNA hybrid levels in genomic DNA after treatment as in (A). S9.6 intensities were normalised to dsDNA and to siNTC control. N = 5. D Replication fork speeds ±72 h HRASG12V induction in presence of siNTC or siRH2B. N = 3. E CldU/IdU ratios ±96 h siNTC or siRH2B and ±72 h HRASG12V induction. CldU and IdU treatment were 20 min each (con) and 30 min each (HRASG12V). Data from 3 repeats. F Mean intensities of γH2AX staining ±72 h HRASG12V induction in presence of siNTC or siRH2B. Data from N = 3. G Percentages of cells with >8 53BP1 foci ±72 h HRASG12V induction in presence of siNTC or siRH2B. N = 3. H Strategy for long-term HRASG12V induction with RNASEH2B depletion. I Relative proliferation after 8 days transfection with non-targeting control (siNTC) or RH2B siRNA (siRH2B) and HRASG12V induction for the times indicated. Cell counts for siNTC or siRH2B were each normalised to no HRASG12V induction (0). N = 3. J Percentages of sub-G1 (dead) versus alive cells after 8 days transfection with siNTC or RH2B siRNA and HRASG12V induction for the times indicated, measured by flow cytometry with propidium iodide. N = 4 (siNTC 7 days N = 3). K Percentages of cells positive for β-galactosidase staining (senescence) after 8 days transfection with non-targeting control or RH2B siRNA and HRASG12V induction for the times indicated. N = 3. L Model: Oncogenic HRASG12V, CPT and HU cause RNA:DNA hybrids (red) ahead of or behind the fork. RNA:DNA hybrids stall forks unless RNase H2 levels are increased. RNase H2 overexpression potentially comes at the expense of increased RNA:DNA hybrids elsewhere in the genome. Bar graphs: Means and SEM (bars) of independent experiments are shown. Scatter plots show aggregate data with medians (lines), and medians (white dots) of independent experiments. Asterisks indicate p values (ANOVA or Kruskal-Wallis-test, ns: not significant, *p < 0.05, **p < 0.01, ****p < 0.0001).
Discussion
We have shown that RNase H2 protein levels increase in response to replication stress from different sources including oncogenes and cancer chemotherapy drugs. RNase H2 levels may be regulated transcriptionally, but possibly also post-transcriptionally in response to replication stress. RNASEH2B overexpression increases RNase H2 activity and prevents chemotherapy- and oncogene-induced replication fork stalling, with the potential to impact on innate immune sensing of nucleic acids or cell survival.
Co-regulation of RNASEH2B and other RNase H2 subunits
To model increased RNase H2 protein levels, we chose to focus on RNASEH2B. Although not the catalytic subunit, RNASEH2B is required for the nuclear localisation of the RNase H2 complex [17] and interacts with the DNA sliding clamp, PCNA, which could localise it to replication forks and sites of DNA repair [16]. We occasionally observed that RNASEH2B was up-regulated while RNASEH2C remained constant, such as in response to Cyclin E overexpression. RNASEH2B appears to have a crucial role in regulating localisation and function of the RNase H2 complex and RNASEH2B depletion or knockout has been repeatedly used to reduce RNase H2 activity [25, 26, 31], which was also the most effective strategy in our hands.
Our data agree with observations from knockout models showing that the three RNase H2 subunits are co-regulated at the protein level. RNASEH2B knockout or mutation can destabilise the complex via loss or reduction of both RNASEH2A and RNASEH2C, respectively [14, 15]. Similarly, RNASEH2B overexpression can increase RNASEH2A and RNASEH2C. RNASEH2B overexpression is sufficient to increase cellular RNase H2 activity (Fig. 3E). Therefore, importantly, phenotypic changes resulting from RNASEH2B overexpression currently do not imply specific non-RNase H2-related functions of this subunit.
Impact on RNA:DNA hybrids and transcription
Surprisingly, RNASEH2B overexpression appeared to slightly increase rather than decrease global RNA:DNA hybrid levels, and RNASEH2B depletion alone had no effect on global RNA:DNA hybrid levels. It was previously reported that RNASEH2A depletion did not increase global RNA:DNA hybrid levels [31]. The impact of altering RNase H2 activity on global hybrid levels is therefore not straightforward. Overexpressing RNASEH2B may disrupt hybrid homoeostasis in otherwise unstressed cells, increasing as well as decreasing subsets of RNA:DNA hybrids. RNASEH2B overexpression might increase hybrids that are resolved by RNase H1, while still mitigating drug- or replication stress-induced increases in hybrids. In response to HU treatment, RNase H2 is recruited to transcription termination sites by BRCA2 to resolve RNA:DNA hybrids [62]. This suggests that RNase H2 could be responsive to specific hybrids induced by replication stress.
Excessive RNase H2 activity may increase hybrid levels by disrupting RNA:DNA hybrid homoeostasis through RNase H2 functions from regulating transcription to DSB repair [63, 64]. We observed a small increase in levels of 53BP1 foci, a marker of DSB formation, in cells overexpressing RNASEH2B (Fig. 4I). RNASEH2B overexpression could also increase binding to interaction partners such as PCNA [14], which might disrupt DNA replication and DNA repair processes that are regulated by PCNA. Other putative RNase H2 interactors are involved in transcriptional regulation [65]. While there was no overt impact of RNASEH2B overexpression on global nascent RNA synthesis or gene expression, there could be subtler changes at RNA:DNA hybrid hotspots such as promoter and terminator regions of genes [3].
RNASEH2B overexpression rescues drug-induced replication fork stalling
RNASEH2B overexpression rescued a subset of CPT- and HU-induced replication stress, specifically replication fork stalling, potentially via reducing TRCs or RNA:DNA hybrids at replication forks. This supports that in addition to nucleotide depletion, transcription is involved during fork stalling induced by HU. In line with this, HU also increases reactive oxygen species and causes fork stalling that can be alleviated by removing RNA:DNA hybrids [66]. Our data complement previous reports in yeast and human cells using RNase H2 loss or depletion to show that RNase H2 promotes nascent strand degradation in presence of HU or CPT, and replication fork progression in presence of HU, by removing RNA:DNA hybrids derived either from ongoing transcription or from Okazaki primers [30, 31]. Our data expand on these findings by directly showing that RNase H2 counteracts replication fork stalling in human cells. Our data are consistent with the idea that RNase H2 helps to counteract adverse effects of ongoing transcription at perturbed replication forks, as acute inhibition of transcription both promoted nascent strand degradation [31] and prevented fork stalling (this study).
The DNA fibre labelling protocol used in this study (Fig. 4D) could theoretically lead to forks resected during drug treatments appearing as stalled forks. However, in that case, promotion of resection by RNase H2 would lead to increased, not decreased, fork stalling when RNASEH2B is overexpressed. RNASEH2B overexpression had minor effects on fork restart after release from HU block, suggesting that in our system, increased RNase H2 activity prevents replication fork stalling more than promoting restart. RNASEH2B overexpression did not promote shortening of the first (CldU) label during an HU block (Fig. 4O). If there is no sign of increased fork degradation in presence of RNASEH2B overexpression during 2 h of 2 mM HU treatment, then it seems unlikely that fork degradation would play a role in the rescue of fork stalling by RNASEH2B overexpression during 50 min of 200 μM HU treatment (Fig. 4H).
There are still open questions regarding the role of RNase H2 at stalled replication forks. Increased RNase H2 activity has been proposed to interfere with fork restart after HU or aphidicolin if RNase H2 localisation to replication forks is not properly controlled by factors such as the proteasome shuttle protein DDI1/2 [67]. We also observed that RNASEH2B overexpression resulted in some signs of stress, especially increased RNA:DNA hybrid levels. The molecular mechanisms underlying both HU- or CPT-induced replication fork stalling and their relationship to RNA:DNA hybrids and nascent strand degradation require further investigation. It would be interesting to overexpress wild type RNASEH2A and the RNASEH2A separation-of-function RED mutant that can resolve long hybrids but not genome-embedded ribonucleotides [68] to investigate whether increased RER activity can contribute to reducing fork stalling.
Effects of RNASEH2B overexpression on genome stability and cell survival
Even though RNase H2 activity is implicated not only in replication stress responses but also in DSB repair [63, 64], RNASEH2B overexpression had little effect on CPT-induced DSB levels (Fig. 4I) or colony survival in response to CPT, GEM or HU in HCT116 cells. It was previously reported that constitutive RNASEH2A overexpression protected LNCaP prostate cancer cells against CPT or etoposide [34] and that RNase H2 depletion increased HU sensitivity in HeLa cells [15]. In contrast, RNASEH2A depletion was shown to decrease HU sensitivity in U2OS cells [67]. Finally, RNase H2 deficient budding yeast are more sensitive to replication stress-inducing drugs [69]. Altogether, more remains to be discovered about the precise impact of RNase H2 levels on drug sensitivity in human cells.
Even if replication stress response factors do not promote survival, they can be important to safeguard genome stability in surviving cells. RNASEH2B overexpression reduced micronuclei formation in response to HU treatment. It is possible that the extent of micronucleus induction by CPT and GEM matched the extent of DSB formation [70] more than levels of fork stalling.
We observed phosphorylation of TBK1 in BJ-hTERT cells in response to drug treatments, indicating activation of cytoplasmic nucleic acid sensing pathways. RNASEH2B overexpression attenuated drug-induced TBK1 phosphorylation compared to RNASEH2B overexpression alone, which could be linked to decreased formation of micronuclei in response to HU and GEM and reduced formation of other cytosolic DNA species in response to CPT. Unexpectedly however, even though RNASEH2B overexpression did not increase levels of micronuclei or cytoplasmic DNA, it did increase TBK1 phosphorylation (Fig. 6H, I) and interferon gene expression (Fig. S5C; Fig. S8D). Cytoplasmic DNA quantification was performed after 48 h overexpression, and it is possible that longer overexpression would increase cytoplasmic DNA signal. It has been reported that micronuclei formation does not always translate into cGAS-STING pathway activation because micronuclei-bound cGAS is inhibited by the presence of nucleosomes [71]. TBK1 activation could therefore correlate with nucleic acid species other than micronuclei. As RNASEH2B overexpression increases genomic RNA:DNA hybrid signal in BJ-hTERT cells (Fig. 3H), changes in cytosolic RNA:DNA hybrids levels might also contribute to the observed effects on TBK1 phosphorylation and interferon activation, both in absence and presence of drugs [12]. Nevertheless, RNASEH2B overexpression had no strong effect on downstream pro-inflammatory signalling. Overall, our data suggest that the strongest downstream impact of RNASEH2B overexpression is on drug-induced genomic instability through micronucleus formation.
RNase H2 prevents RAS-induced replication fork stalling
As with the response to replication stress-inducing drugs, RNase H2 specifically alleviates fork stalling rather than fork slowing induced by HRASG12V. This suggests that HRASG12V-induced transcription and/or RNA:DNA hybrids can cause fork stalling through a mechanism that resembles the fork stalling induced by HU or CPT. In contrast to HU or CPT, HRASG12V only causes fork stalling when RNase H2 is also depleted. This suggests that increased RNase H2 levels after HRASG12V induction have a similar rescuing effect as ectopic RNase H2 overexpression (Fig. 7L), or fork stalling may require accumulation of higher levels of RNA:DNA hybrids, if there is no additional nucleotide depletion [6] or DNA damage. Again, the relationship of fork stalling to nascent strand degradation requires further investigation. RNase H2 depletion accelerated growth arrest and cell death in response to oncogenic HRAS. This complements previous reports that down-regulation of other DNA repair and -replication proteins, such as BRIP1 (involved in BRCA1-mediated DSB repair) and RRM2 (a ribonucleotide reductase subunit) accelerates HRAS-induced senescence or growth arrest [72, 73], presumably by further exacerbating HRAS-induced replication stress. Our data suggest that oncogenic HRAS-expressing cells rely on RNase H2 to keep replication stress at tolerable levels for proliferation and survival.
In conclusion, increased levels of RNase H2 subunits may be indicators of the response to chemotherapy or oncogene-induced replication stress, warranting further investigation. RNase H2 mRNA levels are increased in some CIN+ colon cancers, and it is possible that protein levels are also be up-regulated post-transcriptionally. Deciphering the mechanisms that regulate RNase H2 protein levels, activity and localisation in response to cellular stress will contribute to our understanding of replication stress responses in cancer.
Data availability
The authors declare that all the data supporting the findings of this study are available within the article and its supplementary information files and from the corresponding authors upon reasonable request. The datasets generated during and analysed during the current study are available in the GEO repository, https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE276623.
References
Burrell RA, McClelland SE, Endesfelder D, Groth P, Weller MC, Shaikh N, et al. Replication stress links structural and numerical cancer chromosomal instability. Nature. 2013;494:492–6.
Cybulla E, Vindigni A. Leveraging the replication stress response to optimize cancer therapy. Nat Rev Cancer. 2023;23:6–24.
Petermann E, Lan L, Zou L. Sources, resolution and physiological relevance of R-loops and RNA-DNA hybrids. Nat Rev Mol Cell Biol. 2022;23:521–40.
Gorthi A, Romero JC, Loranc E, Cao L, Lawrence LA, Goodale E, et al. EWS-FLI1 increases transcription to cause R-loops and block BRCA1 repair in Ewing sarcoma. Nature. 2018;555:387–91.
Jones SE, Fleuren EDG, Frankum J, Konde A, Williamson CT, Krastev DB, et al. ATR is a therapeutic target in synovial sarcoma. Cancer Res. 2017;77:7014–26.
Kotsantis P, Silva LM, Irmscher S, Jones RM, Folkes L, Gromak N, et al. Increased global transcription activity as a mechanism of replication stress in cancer. Nat Commun. 2016;7:13087.
Manzo SG, Hartono SR, Sanz LA, Marinello J, De Biasi S, Cossarizza A, et al. DNA Topoisomerase I differentially modulates R-loops across the human genome. Genome Biol. 2018;19:100.
Reijns MA, Rabe B, Rigby RE, Mill P, Astell KR, Lettice LA, et al. Enzymatic removal of ribonucleotides from DNA is essential for mammalian genome integrity and development. Cell. 2012;149:1008–22.
Ewald B, Sampath D, Plunkett W. Nucleoside analogs: molecular mechanisms signaling cell death. Oncogene. 2008;27:6522–37.
Hamperl S, Bocek MJ, Saldivar JC, Swigut T, Cimprich KA. Transcription-replication conflict orientation modulates R-loop levels and activates distinct DNA damage responses. Cell. 2017;170:774–786.e19.
Lloyd RL, Urban V, Muñoz-Martínez F, Ayestaran I, Thomas JC, de Renty C, et al. Loss of cyclin C or CDK8 provides ATR inhibitor resistance by suppressing transcription-associated replication stress. Nucleic Acids Res. 2021;49:8665–83.
Crossley MP, Song C, Bocek MJ, Choi JH, Kousorous J, Sathirachinda A, et al. R-loop-derived cytoplasmic RNA-DNA hybrids activate an immune response. Nature. 2023;613:187–94.
Cerritelli SM, Crouch RJ. Ribonuclease H: the enzymes in eukaryotes. FEBS J. 2009;276:1494–505.
Reijns MAM, Bubeck D, Gibson LCD, Graham SC, Baillie GS, Jones EY, et al. The structure of the human RNase H2 complex defines key interaction interfaces relevant to enzyme function and human disease. J Biol Chem. 2011;286:10530–9.
Pizzi S, Sertic S, Orcesi S, Cereda C, Bianchi M, Jackson AP, et al. Reduction of hRNase H2 activity in Aicardi-Goutières syndrome cells leads to replication stress and genome instability. Hum Mol Genet. 2015;24:649–58.
Bubeck D, Reijns MAM, Graham SC, Astell KR, Jones EY, Jackson AP. PCNA directs type 2 RNase H activity on DNA replication and repair substrates. Nucleic Acids Res. 2011;39:3652–66.
Kind B, Muster B, Staroske W, Herce HD, Sachse R, Rapp A, et al. Altered spatio-temporal dynamics of RNase H2 complex assembly at replication and repair sites in Aicardi-Goutieres syndrome. Hum Mol Genet. 2014;23:5950–60.
Williams JS, Lujan SA, Kunkel TA. Processing ribonucleotides incorporated during eukaryotic DNA replication. Nat Rev Mol Cell Biol. 2016;17:350–63.
O’Connell K, Jinks-Robertson S, Petes TD. Elevated Genome-wide instability in yeast mutants lacking RNase H activity. Genetics. 2015;201:963–75.
Frank P, Albert S, Cazenave C, Toulmé JJ. Purification and characterization of human ribonuclease HII. Nucleic Acids Res. 1994;22:5247–54.
Shiromoto Y, Sakurai M, Minakuchi M, Ariyoshi K, Nishikura K. ADAR1 RNA editing enzyme regulates R-loop formation and genome stability at telomeres in cancer cells. Nat Commun. 2021;12:1654.
Lockhart A, Pires VB, Bento F, Kellner V, Luke-Glaser S, Yakoub G, et al. RNase H1 and H2 are differentially regulated to process RNA-DNA hybrids. Cell Rep. 2019;29:2890–2900.
Chon H, Sparks JL, Rychlik M, Nowotny M, Burgers PM, Crouch RJ, et al. RNase H2 roles in genome integrity revealed by unlinking its activities. Nucleic Acids Res. 2013;41:3130–43.
Crow YJ, Leitch A, Hayward BE, Garner A, Parmar R, Griffith E, et al. Mutations in genes encoding ribonuclease H2 subunits cause Aicardi-Goutières syndrome and mimic congenital viral brain infection. Nat Genet. 2006;38:910–6.
Aden K, Bartsch K, Dahl J, Reijns MAM, Esser D, Sheibani-Tezerji R, et al. Epithelial RNase H2 maintains genome integrity and prevents intestinal tumorigenesis in mice. Gastroenterology. 2019;156:145–59.
Hiller B, Hoppe A, Haase C, Hiller C, Schubert N, Muller W, et al. Ribonucleotide excision repair is essential to prevent squamous cell carcinoma of the skin. Cancer Res. 2018;78:5917–26.
Deasy SK, Uehara R, Vodnala SK, Yang HH, Dass RA, Hu Y, et al. Aicardi-Goutières syndrome gene Rnaseh2c is a metastasis susceptibility gene in breast cancer. PLoS Genet. 2019;15:e1008020.
Shen J, Lin S, Liu L, Wang C. Ribonuclease H2 Subunit A impacts invasiveness and chemoresistance resulting in poor survivability of breast cancer in ER dependent manner. Am J Transl Res. 2020;12:2281–94.
Boros-Oláh B, Dobos N, Hornyák L, Szabó Z, Karányi Z, Halmos G, et al. Drugging the R-loop interactome: RNA-DNA hybrid binding proteins as targets for cancer therapy. DNA Repair (Amst). 2019;84:102642.
Audoynaud C, Schirmeisen K, Ait Saada A, Gesnik A, Fernandez-Varela P, Boucherit V, et al. RNA:DNA hybrids from Okazaki fragments contribute to establish the Ku-mediated barrier to replication-fork degradation. Mol Cell. 2023;83:1061–74.
Heuzé J, Kemiha S, Barthe A, Vilarrubias AT, Aouadi E, Aiello U, et al. RNase H2 degrades toxic RNA: DNA hybrids behind stalled forks to promote replication restart. EMBO J. 2023;42:e113104.
Zhang L, Yang Y, Cheng L, Cheng Y, Zhou HH, Tan ZR. Identification of common genes refers to colorectal carcinogenesis with paired cancer and noncancer samples. Dis Markers. 2018;2018:3452739.
Yang CA, Huang HY, Chang YS, Lin CL, Lai IL, Chang JG. DNA-sensing and nuclease gene expressions as markers for colorectal cancer progression. Oncology. 2017;92:115–24.
Kimura N, Takayama K-I, Yamada Y, Kume H, Fujimura T, Inoue S. Ribonuclease H2 subunit A preserves genomic integrity and promotes prostate cancer progression. Cancer Res Commun. 2022;2:870–83.
De Vita G, Bauer L, da Costa VM, De Felice M, Baratta MG, De Menna M, et al. Dose-dependent inhibition of thyroid differentiation by RAS oncogenes. Mol Endocrinol. 2005;19:76–89.
Santoni-Rugiu E, Falck J, Mailand N, Bartek J, Lukas J. Involvement of Myc activity in a G(1)/S-promoting mechanism parallel to the pRb/E2F pathway. Mol Cell Biol. 2000;20:3497–509.
Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–5.
Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21.
Liao Y, Smyth GK, Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014;30:923–30.
Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550.
Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci. 2005;102:15545–50.
Mootha VK, Lindgren CM, Eriksson K-F, Subramanian A, Sihag S, Lehar J, et al. PGC-1α-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34:267–73.
Liberzon A, Subramanian A, Pinchback R, Thorvaldsdóttir H, Tamayo P, Mesirov JP. Molecular signatures database (MSigDB) 3.0. Bioinformatics. 2011;27:1739–40.
Liberzon A, Birger C, Thorvaldsdóttir H, Ghandi M, Mesirov JP, Tamayo P. The molecular signatures database hallmark gene set collection. Cell Syst. 2015;1:417–25.
Jones RM, Mortusewicz O, Afzal I, Lorvellec M, García P, Helleday T, et al. Increased replication initiation and conflicts with transcription underlie Cyclin E-induced replication stress. Oncogene. 2013;32:3744–53.
Boguslawski SJ, Smith DE, Michalak MA, Mickelson KE, Yehle CO, Patterson WL, et al. Characterization of monoclonal antibody to DNA.RNA and its application to immunodetection of hybrids. J Immunol Methods. 1986;89:123–30.
Cristini A, Tellier M, Constantinescu F, Accalai C, Albulescu LO, Heiringhoff R, et al. RNase H2, mutated in Aicardi-Goutières syndrome, resolves co-transcriptional R-loops to prevent DNA breaks and inflammation. Nat Commun. 2022;13:2961.
MacKenzie KJ, Carroll P, Martin CA, Murina O, Fluteau A, Simpson DJ, et al. CGAS surveillance of micronuclei links genome instability to innate immunity. Nature. 2017;548:461–5.
Bartsch K, Knittler K, Borowski C, Rudnik S, Damme M, Aden K, et al. Absence of RNase H2 triggers generation of immunogenic micronuclei removed by autophagy. Hum Mol Genet. 2017;26:3960–72.
Harding SM, Benci JL, Irianto J, Discher DE, Minn AJ, Greenberg RA. Mitotic progression following DNA damage enables pattern recognition within micronuclei. Nature. 2017;548:466–70.
Coquel F, Silva MJ, Techer H, Zadorozhny K, Sharma S, Nieminuszczy J, et al. SAMHD1 acts at stalled replication forks to prevent interferon induction. Nature. 2018;557:57–61.
Vanpouille-Box C, Alard A, Aryankalayil MJ, Sarfraz Y, Diamond JM, Schneider RJ, et al. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat Commun. 2017;8:15618.
Debaugnies M, Rodríguez-Acebes S, Blondeau J, Parent M-A, Zocco M, Song Y, et al. RHOJ controls EMT-associated resistance to chemotherapy. Nature. 2023;616:168–5.
Petermann E, Maya-Mendoza A, Zachos G, Gillespie DAF, Jackson DA, Caldecott KW. Chk1 requirement for high global rates of replication fork progression during normal vertebrate S phase. Mol Cell Biol. 2006;26:3319–26.
Petermann E, Orta ML, Issaeva N, Schultz N, Helleday T. Hydroxyurea-stalled replication forks become progressively inactivated and require two different RAD51-mediated pathways for restart and repair. Mol Cell. 2010;37:492–502.
West AP, Khoury-Hanold W, Staron M, Tal MC, Pineda CM, Lang SM, et al. Mitochondrial DNA stress primes the antiviral innate immune response. Nature. 2015;520:553–7.
Suter MA, Tan NY, Thiam CH, Khatoo M, MacAry PA, Angeli V, et al. cGAS–STING cytosolic DNA sensing pathway is suppressed by JAK2-STAT3 in tumor cells. Sci Rep. 2021;11:7243.
Zhou R, Zhang Q, Xu P. TBK1, a central kinase in innate immune sensing of nucleic acids and beyond. Acta Biochim Biophys Sin (Shanghai). 2020;52:757–67.
Wardlaw CP, Petrini JHJ. ISG15 conjugation to proteins on nascent DNA mitigates DNA replication stress. Nat Commun. 2022;13:5971.
Hong C, Schubert M, Tijhuis AE, Requesens M, Roorda M, van den Brink A, et al. cGAS–STING drives the IL-6-dependent survival of chromosomally instable cancers. Nature. 2022;607:366–73.
Di Micco R, Fumagalli M, Cicalese A, Piccinin S, Gasparini P, Luise C, et al. Oncogene-induced senescence is a DNA damage response triggered by DNA hyper-replication. Nature. 2006;444:638–42.
Goehring L, Keegan S, Lahiri S, Xia W, Kong M, Jimenez-Sainz J, et al. Dormant origin firing promotes head-on transcription-replication conflicts at transcription termination sites in response to BRCA2 deficiency. Nat Commun. 2024;15:4716.
Pryor JM, Conlin MP, Carvajal-Garcia J, Luedeman ME, Luthman AJ, Small GW, et al. Ribonucleotide incorporation enables repair of chromosome breaks by nonhomologous end joining. Science. 2018;361:1126–9.
D’Alessandro G, Whelan DR, Howard SM, Vitelli V, Renaudin X, Adamowicz M, et al. BRCA2 controls DNA:RNA hybrid level at DSBs by mediating RNase H2 recruitment. Nat Commun. 2018;9:5376.
Shapson-Coe A, Valeiras B, Wall C, Rada C. Aicardi–Goutières Syndrome associated mutations of RNase H2B impair its interaction with ZMYM3 and the CoREST histone-modifying complex. PLoS ONE. 2019;14. https://doi.org/10.1371/journal.pone.0213553.
Andrs M, Stoy H, Boleslavska B, Chappidi N, Kanagaraj R, Nascakova Z, et al. Excessive reactive oxygen species induce transcription-dependent replication stress. Nat Commun. 2023;14:1791.
Conti BA, Ruiz PD, Broton C, Blobel NJ, Kottemann MC, Sridhar S, et al. RTF2 controls replication repriming and ribonucleotide excision at the replisome. Nat Commun. 2024;15:1943.
Uehara R, Cerritelli SM, Hasin N, Sakhuja K, London M, Iranzo J, et al. Two RNase H2 mutants with differential rNMP processing activity reveal a threshold of ribonucleotide tolerance for embryonic development. Cell Rep. 2018;25:1135–45.
Arudchandran A, Cerritelli SM, Narimatsu SK, Itaya M, Shin DY, Shimada Y, et al. The absence of ribonuclease H1 or H2 alters the sensitivity of Saccharomyces cerevisiae to hydroxyurea, caffeine and ethyl methanesulphonate: Implications for roles of RNases H in DNA replication and repair. Genes Cells. 2000;5:789–802.
Jones RM, Kotsantis P, Stewart GS, Groth P, Petermann E. BRCA2 and RAD51 promote double-strand break formation and cell death in response to gemcitabine. Mol Cancer Ther. 2014;13:2412–21.
Takaki T, Millar R, Hiley CT, Boulton SJ. Micronuclei induced by radiation, replication stress, or chromosome segregation errors do not activate cGAS-STING. Mol Cell. 2024;84:2203–13.
Tu Z, Aird KM, Bitler BG, Nicodemus JP, Beeharry N, Xia B, et al. Oncogenic RAS regulates BRIP1 expression to induce dissociation of BRCA1 from chromatin, inhibit DNA repair, and promote senescence. Dev Cell. 2011;21:1077–91.
Aird KM, Zhang G, Li H, Tu Z, Bitler BG, Garipov A, et al. Suppression of nucleotide metabolism underlies the establishment and maintenance of oncogene-induced senescence. Cell Rep. 2013;3:1252–65.
Hoadley KA, Yau C, Hinoue T, Wolf DM, Lazar AJ, Drill E, et al. Cell-of-origin patterns dominate the molecular classification of 10,000 tumors from 33 types of cancer. Cell. 2018;173:291–304 e6.
Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:pl1.
Acknowledgements
RJW, CW and RDWK were supported by a Cancer Research UK Programme Foundation award to EP and AdK (C25526/A28275). PK was supported by a Worldwide Cancer Research grant (13-1048) to EP. MAMR is supported by an UK Medical Research Council (MRC) Human Genetics Unit grant (MC_UU_00035/10). We thank Dr Angelo Agathanggelou for technical help.
Author information
Authors and Affiliations
Contributions
EP, CHMT and PK conceived the study; RJW, AbK, SAP and RDWK performed experiments; RJW, AbK, PK, MAMR and EP designed experiments; CW and AdK performed and supervised RNA-seq data analysis; RJW and EP wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This work relied exclusively immortalised human cell lines and therefore did not require ethics approval or consent to participate. No live vertebrates, human patients or patient-derived samples were used. All experiments conducted in this study have been performed in accordance with relevant guidelines and regulations.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wilkins, R.J., Kannan, A., Plass, S.A. et al. Human RNase H2 upregulation counteracts oncogene- and chemotherapy-induced replication stress. Oncogene 44, 3255–3271 (2025). https://doi.org/10.1038/s41388-025-03489-8
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41388-025-03489-8