Abstract
Background
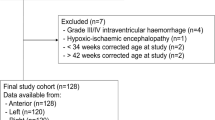
Extremely low birth weight (ELBW) infants are at risk for end-organ hypoxia and ischemia. Regional tissue oxygenation of the brain and gut as monitored with near-infrared spectroscopy (NIRS) may change with postnatal age, but normal ranges are not well defined.
Methods
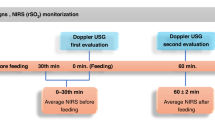
A prospective study of ELBW preterm infants utilized NIRS monitoring to assess changes in cerebral and mesenteric saturation (Csat and Msat) over the first week after birth. This secondary study of a multicenter trial comparing hemoglobin transfusion thresholds assessed cerebral and mesenteric fractional tissue oxygen extraction (cFTOE and mFTOE) and relationships with perinatal variables.
Results
In 124 infants, both Csat and Msat declined over the first week, with a corresponding increase in oxygen extraction. With lower gestational age, lower birth weight, and 5-min Apgar score ≤5, there was a greater increase in oxygen extraction in the brain compared to the gut. Infants managed with a lower hemoglobin transfusion threshold receiving ≥2 transfusions in the first week had the lowest Csat and highest cFTOE (p < 0.001).
Conclusion
Brain oxygen extraction preferentially increased in more immature and anemic preterm infants. NIRS monitoring may enhance understanding of cerebral and mesenteric oxygenation patterns and inform future protective strategies in the preterm ELBW population.
Impact
-
Simultaneous monitoring of cerebral and mesenteric tissue saturation demonstrates the balance of oxygenation between preterm brain and gut and may inform protective strategies.
-
Over the first week, oxygen saturation of the brain and gut declines as oxygen extraction increases.
-
A low hemoglobin transfusion threshold is associated with lower cerebral saturation and higher cerebral oxygen extraction compared to a high hemoglobin transfusion threshold, although this did not translate into clinically relevant differences in the TOP trial primary outcome.
-
Greater oxygen extraction by the brain compared to the gut occurs with lower gestational age, lower birth weight, and 5-min Apgar score ≤5.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Bruckner, M. et al. Normal regional tissue oxygen saturation in neonates: a systematic qualitative review. Pediatr. Res. https://doi.org/10.1038/s41390-021-01786-y (2021).
Alderliesten, T. et al. Reference values of regional cerebral oxygen saturation during the first 3 days of life in preterm neonates. Pediatr. Res. 79, 55–64 (2016).
McNeill, S., Gatenby, J. C., McElroy, S. & Engelhardt, B. Normal cerebral, renal and abdominal regional oxygen saturations using near-infrared spectroscopy in preterm infants. J. Perinatol. 31, 51–57 (2011).
Hyttel-Sorensen, S. et al. Cerebral near infrared spectroscopy oximetry in extremely preterm infants: phase II randomised clinical trial. BMJ 350, g7635 (2015).
Hoeller, N. et al. Cerebral and peripheral muscle oxygenation and perfusion: course in moderate and late preterm neonates during the first day after birth. Physiol. Int. https://doi.org/10.1556/2060.2020.00028 (2020).
Cohen, E. et al. Growth restriction and gender influence cerebral oxygenation in preterm neonates. Arch. Dis. Child Fetal Neonatal Ed. 101, F156–F161 (2016).
Cortez, J. et al. Noninvasive evaluation of splanchnic tissue oxygenation using near-infrared spectroscopy in preterm neonates. J. Matern. Fetal Neonatal Med. 24, 574–582 (2011).
Kuik, S. J. et al. The effect of enteral bolus feeding on regional intestinal oxygen saturation in preterm infants is age-dependent: a longitudinal observational study. BMC Pediatr. 19, 404 (2019).
van der Heide, M. et al. Regional splanchnic oxygen saturation for preterm infants in the first week after birth: reference values. Pediatr. Res. https://doi.org/10.1038/s41390-020-01323-3 (2021).
Kirpalani, H. et al. Higher or lower hemoglobin transfusion thresholds for preterm infants. N. Engl. J. Med. 383, 2639–2651 (2020).
Metcalfe, K. H. M., Stienstra, R. & McHoney, M. NIRS as a biomarker of bowel ischaemia & surgical pathology: a meta-analysis of studies in newborns. Early Hum. Dev. 161, 105437 (2021).
Gillam-Krakauer, M. et al. Correlation of abdominal rSO2 with superior mesenteric artery velocities in preterm infants. J. Perinatol. 33, 609–612 (2013).
Cerbo, R. M. et al. Global perfusion assessment and tissue oxygen saturation in preterm infants: where are we? Early Hum. Dev. 89(Suppl 1), S44–S46 (2013).
Banerjee, J., Leung, T. S. & Aladangady, N. Cerebral blood flow and oximetry response to blood transfusion in relation to chronological age in preterm infants. Early Hum. Dev. 97, 1–8 (2016).
Mohamed, M. A. et al. Changes in cerebral tissue oxygenation and fractional oxygen extraction with gestational age and postnatal maturation in preterm infants. J. Perinatol. https://doi.org/10.1038/s41372-020-00794-w (2020).
Chock, V. Y. et al. Cerebral oxygenation and autoregulation in preterm infants (Early NIRS Study). J. Pediatr. 227, 94–100.e1 (2020).
Patel, A. K. et al. Abdominal near-infrared spectroscopy measurements are lower in preterm infants at risk for necrotizing enterocolitis. Pediatr. Crit. Care Med. 15, 735–741 (2014).
Havranek, T., Miladinovic, B., Wadhawan, R. & Carver, J. D. Factors that affect the postnatal increase in superior mesenteric artery blood flow velocity in very low birth weight preterm infants. J. Perinat. Med. 40, 565–570 (2012).
Maruyama, K., Koizumi, T., Tomomasa, T. & Morikawa, A. Intestinal blood-flow velocity in uncomplicated preterm infants during the early neonatal period. Pediatr. Radiol. 29, 472–477 (1999).
Martinussen, M., Brubakk, A. M., Vik, T. & Yao, A. C. Mesenteric blood flow velocity and its relation to transitional circulatory adaptation in appropriate for gestational age preterm infants. Pediatr. Res. 39, 275–280 (1996).
Thompson, A., Silva, C. T., Gork, A. S., Wang, D. & Ehrenkranz, R. A. Intestinal blood flow by Doppler ultrasound: the impact of gestational age and time from first enteral feeding in preterm neonates. Am. J. Perinatol. 31, 261–268 (2014).
Dani, C., Pratesi, S., Fontanelli, G., Barp, J. & Bertini, G. Blood transfusions increase cerebral, splanchnic, and renal oxygenation in anemic preterm infants. Transfusion 50, 1220–1226 (2010).
Bennet, L., Rossenrode, S., Gunning, M. I., Gluckman, P. D. & Gunn, A. J. The cardiovascular and cerebrovascular responses of the immature fetal sheep to acute umbilical cord occlusion. J. Physiol. 517(Pt 1), 247–257 (1999).
Andersen, C. C. et al. The cerebral critical oxygen threshold of ventilated preterm lambs and the influence of antenatal inflammation. J. Appl. Physiol. (1985) 111, 775–781 (2011).
Bozzetti, V. et al. Cerebral and somatic NIRS-determined oxygenation in IUGR preterm infants during transition. J. Matern. Fetal Neonatal Med. 29, 443–446 (2016).
Howarth, C. et al. Cerebral oxygenation in preterm infants with necrotizing enterocolitis. Pediatrics 146, e20200337 (2020).
Hintz, S. R. et al. Neurodevelopmental and growth outcomes of extremely low birth weight infants after necrotizing enterocolitis. Pediatrics 115, 696–703 (2005).
Bailey, S. M., Hendricks-Muñoz, K. D., Wells, J. T. & Mally, P. Packed red blood cell transfusion increases regional cerebral and splanchnic tissue oxygen saturation in anemic symptomatic preterm infants. Am. J. Perinatol. 27, 445–453 (2010).
Mintzer, J. P., Parvez, B. & La Gamma, E. F. Regional tissue oxygen extraction and severity of anemia in very low birth weight neonates: a pilot NIRS analysis. Am. J. Perinatol. 35, 1411–1418 (2018).
Whitehead, H. V., Vesoulis, Z. A., Maheshwari, A., Rao, R. & Mathur, A. M. Anemia of prematurity and cerebral near-infrared spectroscopy: should transfusion thresholds in preterm infants be revised? J. Perinatol. 38, 1022–1029 (2018).
Whitehead, H. V., Vesoulis, Z. A., Maheshwari, A., Rambhia, A. & Mathur, A. M. Progressive anemia of prematurity is associated with a critical increase in cerebral oxygen extraction. Early Hum. Dev. 140, 104891 (2019).
van Hoften, J. C. R., Verhagen, E. A., Keating, P., ter Horst, H. J. & Bos, A. F. Cerebral tissue oxygen saturation and extraction in preterm infants before and after blood transfusion. Arch. Dis. Child. Fetal Neonatal Ed. 95, F352–F358 (2010).
Sandal, G. et al. Assessment of red blood cell transfusion and transfusion duration on cerebral and mesenteric oxygenation using near-infrared spectroscopy in preterm infants with symptomatic anemia. Transfusion 54, 1100–1105 (2014).
Jain, D., D’Ugard, C., Bancalari, E. & Claure, N. Cerebral oxygenation in preterm infants receiving transfusion. Pediatr. Res. 85, 786–789 (2019).
Jani, P. et al. Liberal hemoglobin threshold affects cerebral arterial pulsed Doppler and cardiac output, not cerebral tissue oxygenation: a prospective cohort study in anemic preterm infants. Transfusion 59, 3093–3101 (2019).
Akotia, D. H., Durham, J. T., Arnell, K. M., Petruzzelli, D. L. & Katheria, A. C. Relationship between near-infrared spectroscopy and transabdominal ultrasonography: noninvasive monitoring of intestinal function in neonates. Med. Sci. Monit. 22, 61–68 (2016).
Thompson, A., Benni, P., Seyhan, S. & Ehrenkranz, R. Meconium and transitional stools may cause interference with near-infrared spectroscopy measurements of intestinal oxygen saturation in preterm infants. Adv. Exp. Med. Biol. 765, 287–292 (2013).
Acknowledgements
The National Institutes of Health, the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the National Heart, Lung, and Blood Institute (NHLBI), the National Center for Research Resources (NCRR), and the National Center for Advancing Translational Sciences (NCATS) provided grant support for the Neonatal Research Network’s Transfusion of Preemies (TOP) trial through cooperative agreements. While NICHD and NHLBI staff had input into the trial design, conduct, analysis, and manuscript drafting, the comments and views of the authors do not necessarily represent the views of NICHD, the National Institutes of Health, the Department of Health and Human Services, or the U.S. Government. Data collected at participating sites of the NICHD Neonatal Research Network were transmitted to RTI International, the data coordinating center (DCC) for the network, which stored, managed, and analyzed the data included in this trial. On behalf of the NRN, RTI International had full access to all the data in the trial and take responsibility for the integrity of the data and accuracy of the data analysis. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We are indebted to our medical and nursing colleagues and the infants and their parents who agreed to take part in this trial. The investigators listed in Supplementary Table S3, in addition to those listed as authors, participated in this trial.
Funding
The National Institutes of Health, National Heart, Lung, and Blood Institute (R01HL12216701A1, U01 HL112776, U01 HL112748), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) (U10 HD21373, UG1 HD21364, UG1 HD21385, UG1 HD27851, UG1 HD27853, UG1 HD27856, UG1 HD27880, UG1 HD27904, UG1 HD34216, UG1 HD36790, UG1 HD40492, UG1 HD40689, UG1 HD53089, UG1 HD53109, UG1 HD68244, UG1 HD68270, UG1 HD68278, UG1 HD68263, UG1 HD68284; UG1 HD87226, UG1 HD87229) and the National Center for Advancing Translational Sciences (NCATS) (UL1 TR6, UL1 TR41, UL1 TR42, UL1 TR77, UL1 TR93, UL1 TR105, UL1 TR442, UL1 TR454, UL1 TR1117) provided grant support for the Neonatal Research Network.
Author information
Authors and Affiliations
Consortia
Contributions
Substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data: VYC, ES, ST, MBB, AD, SRH, HK, EFB, and KPVM. Drafting the article or revising it critically for important intellectual content: VYC, ES, ST, AD, SRH, HK, EFB, RMP, and KPVM. Final approval of the version to be published: VYC, ES, ST, MBB, AD, SRH, HK, EFB, LFC, WAC, CMC, JAW, KAK, RKO, RBS, RMP, ARL, TM, GMS, MCW, BAY, BBP, SC, CTD, RDH, and KPVM.
Corresponding author
Ethics declarations
Competing interests
This study was supported by loan of equipment from Medtronic (Minneapolis, MN). The authors have no additional conflicts of interest to disclose.
Consent to participate
Informed parental consent was obtained for study participation.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Chock, V.Y., Smith, E., Tan, S. et al. Early brain and abdominal oxygenation in extremely low birth weight infants. Pediatr Res 92, 1034–1041 (2022). https://doi.org/10.1038/s41390-022-02082-z
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41390-022-02082-z
This article is cited by
-
Neonatal somatic oxygenation and perfusion assessment using near-infrared spectroscopy
Pediatric Research (2024)
-
Imperative to accelerate research aligning real-time clinical demand with mental health supply
Pediatric Research (2022)