Abstract
Background and objectives
Cardiac rhabdomyoma (CR) is the principal cardiac tumor diagnosed in pediatric age and is commonly associated with tuberous sclerosis complex. In some patients, these masses can cause heart failure and difficult-to-control arrhythmias. There are multiple case reports on use of mammalian target of rapamycin (mTOR) inhibitors, everolimus or sirolimus, in treatment of CRs. We reviewed the current data regarding effectiveness of everolimus and sirolimus in treating of CRs in newborns with hemodynamic repercussions.
Methods
This systematic review was reported according to the PRISMA guidelines. The EBSCO, PubMed, EMBASE, and Lilacs databases were searched for full-text articles reporting the use of everolimus or sirolimus in the treatment of CRs in neonates and infants.
Results
Thirty-one articles met inclusion criteria, totaling 48 patients. Hemodynamic instability prompted treatment in 89.5% of cases. Everolimus was used in 83.3% of cases and sirolimus in 16.6%. The median treatment duration was 67 days, with a 57 ± 23% average CR size reduction. Common adverse events included hypertriglyceridemia, infections, and hematological abnormalities.
Conclusions
mTOR inhibitors appear effective and safe for treating CRs in neonates and infants. The average daily doses were 1.03 mg/m²/day for everolimus and 1.37 mg/m²/day for sirolimus. Randomized controlled clinical trials are necessary to confirm these findings and establish optimal treatment protocols.
Impact
-
Currently, there are no results from randomized clinical trials evaluating the efficacy of mammalian target of rapamycin inhibitors in patients with symptomatic cardiac rhabdomyomas.
-
This is the first systematic review that evaluates the efficacy and safety of the use of everolimus and sirolimus in the non-surgical treatment of cardiac rhabdomyomas with hemodynamic repercussions in neonates.
-
Everolimus and sirolimus may be particularly useful in the neonatal period when the hemodynamic complications caused by cardiac rhabdomyomas are more severe.
Similar content being viewed by others
Introduction
Cardiac rhabdomyoma (CR) is the principal cardiac tumor diagnosed in the pediatric age.1 Seventy-five percent of affected patients are under one year old, with an approximate incidence in newborns of 0.02 to 0.08%.2 Sixty to eighty percent of CR cases are associated with tuberous sclerosis complex (TSC), an autosomal dominant disorder that causes the growth of hamartomas in multiple organs, including the heart.3 Mutations present in TSC deactivate the genes encoding the hamartin (TSC1) or tuberin (TSC2) proteins, which are responsible for inhibiting the mammalian target of rapamycin (mTOR), a family of serine-threonine kinases involved in the regulation of cell growth and proliferation.4
Most CRs present as multiple masses located mainly in the ventricles.1,2 A significant number of CRs undergo spontaneous regression in early childhood. Although most patients may remain asymptomatic, in some cases, these masses can obstruct blood flow, cause heart failure, and difficult-to-control arrhythmias.1,5,6,7,8,9 Death in the fetal and early neonatal period can occur due to the obstructive effect of large masses or incessant arrhythmias leading to cardiogenic shock.10,11,12 Surgery is reserved for cases with severe hemodynamic compromise5; however, it is not without complications and risk of death.13,14,15,16,17
Although mTOR inhibitors (mTORi) such as everolimus and sirolimus have demonstrated their effectiveness in treating TSC-associated tumors such as subependymal giant cell astrocytoma (SEGA) and renal angiomyolipomas,18,19 there are currently no results from randomized clinical trials evaluating the efficacy of these drugs in patients with symptomatic CRs.13,16,17
There are multiple case reports in the literature on the use of everolimus or sirolimus as a therapeutic option (off-label) in the management of RC,5,13,16,20 with no consensus on essential aspects such as dose, duration of treatment, and adverse effects.16 This study aimed to review and synthesize the effectiveness of mTORi (everolimus and sirolimus) in treating of CRs in newborns with hemodynamic repercussions.
Methods
This review was reported according to the guidelines of the PRISMA statement.21 This review was not registered in any publicly accessible database for systematic reviews.
Search strategy and data sources
A literature search was conducted for articles published up to July 16, 2023, in four electronic databases (EBSCO, PubMed, EMBASE, and Lilacs) with no language restrictions. Initially, terms related to the PICO elements were used. Population and intervention-related terms were combined: Infant [MeSH], Newborn [MeSH] OR Neonate [MeSH], MTOR inhibitor [Free], OR Everolimus [Free] OR Sirolimus [Free], Rhabdomyoma [Free]. The search algorithms were: (((((Infant, Newborn[Title/Abstract]) OR (Neonate[Title/Abstract])) AND (MTOR inhibitor[Title/Abstract])) OR (Everolimus[Title/Abstract])) OR (Sirolimus[Title/Abstract])) AND (Rhabdomyoma[Title/Abstract]) and (newborn:ti,ab OR neonate:ti,ab) AND (everolimus:ti,ab OR sirolimus:ti,ab OR ‘mtor inhibitor’:ti,ab) AND rhabdomyoma:ti,ab and TX Infant, Newborn AND TX Everolimus OR TX Sirolimus TX Rhabdomyoma and Infant, Newborn OR Neonate AND MTOR inhibitor OR Everolimus OR Sirolimus AND Rhabdomyoma. The final searches were merged and managed for screening in the rayyan.ai web application.
Eligibility criteria and study selection
Studies were eligible if: (1) the study population consisted of neonates and infants; (2) they used everolimus or sirolimus for the treatment of CR; (3) the outcome of interest was the size of the CR causing intractable cardiac arrhythmias or hemodynamic instability, and (4) any epidemiological design was included. Abstracts from scientific events and studies conducted in animals were excluded. Initially, four reviewers (two pairs) independently examined the titles and abstracts of all identified studies according to the selection criteria. Subsequently, full-text articles that met the selection criteria were retrieved and read entirely, and the eligibility criteria were reapplied. Any disagreements were resolved through consensus or consultation with the research team’s pediatric cardiologist and echocardiographer (DHS).
Data extraction
A data extraction form was created to collect relevant information from the studies included in the Microsoft Excel program. Three reviewers independently extracted the following data from each study: first author’s name, year of publication, country, continent, study design, gestational age of the newborn, sex, weight, height, single or multiple CR, CR location, initial CR size (cm2 or mm), the reason for starting treatment (hemodynamic/arrhythmias), age at the start of treatment, mTORi used (everolimus/sirolimus), initial mTORi dose, cumulative mTORi dose, target serum level of mTORi, achieved serum mTORi level, changes made to the initial dose, reduction in CR size (mm, cm2, or %), hemodynamic improvement or resolution of arrhythmia, total treatment duration, adverse pharmacological effects, treatment suspension or dose reduction due to adverse effects, rebound after treatment suspension (mm, cm2, or %), treatment restart in case of rebound, duration of treatment in case of a second cycle, and total follow-up time (years). Any disagreements were resolved through consensus or consultation with a fourth independent reviewer. Finally, to clarify or complete relevant information, some of the authors of the publications were contacted via email.
Quality assessment of studies
The methodological quality of the included studies was evaluated using the Joanna Briggs Institute (JBI) critical appraisal checklist for case reports.22 This evaluation was conducted independently by two reviewers, and in case of disagreement, it was discussed until a consensus was reached; otherwise, a third reviewer intervened. The checklist evaluated eight essential criteria and was standardized for this review as follows: (1) sociodemographic characteristics of the patient (report of gestational age at birth, sex, and weight of the newborn); (2) detailed timeline of the patient’s clinical history (report of CR dimensions and clinical condition at least two points in time); (3) current clinical condition (description of hemodynamic compromise and/or arrhythmia); (4) diagnostic methods and results (report of serum levels of mTORi and CR dimensions assessed by echocardiography); (5) description of the therapeutic procedure (detail of the dose and duration of mTORi treatment); (6) patient’s post-intervention status (report of CR size and evolution of the hemodynamic condition and/or arrhythmia); (7) identification of adverse events (specification of the occurrence or absence of side events) and 8) lessons learned (recommendations or implications for clinical practice).
Statistical analysis
The data obtained were synthesized narratively, and descriptive statistics and frequency measurements were carried out (tables and supplementary material 1). The heterogeneity of the results did not allow meta-analysis.
Results
Selection of studies
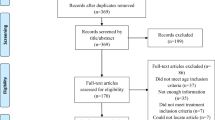
The initial search identified 407 studies, of which 31 met the inclusion criteria, totaling 48 cases. Figure 1 shows the flow diagram of study selection and exclusion.
Characteristics of the studies
The studies were published from 2012 to 2023, primarily reported from Asia (n = 26; 54.17%), followed by North America (n = 10; 20.83%), Europe (n = 6; 12.50%), Latin America (n = 5; 10.42%), and Oceania (n = 1; 2.08%). Case reports constituted 58.33% (n = 28) of the studies, followed by case series (n = 15; 31.25%) and case-control studies (n = 5; 10.42%).
Among the patients, 53.8% were full-term newborns, and 70.4% were male, with a median weight of 2927 grams (Q1 = 2150; Q3 = 3240). In 72.9% of cases, CR was multiple (more than one), primarily located in the left ventricular outflow tract (36.1%), followed by the right ventricular cavity (27.6%). Hemodynamic instability was the main reason for initiating treatment with a mTORi (89.5%). Everolimus was administered in 83.3% of cases (n = 40) and sirolimus in 16.6% (n = 8), with a median age of drug initiation of 6 days (Q1 = 3; Q3 = 18). Other study and population characteristics can be found in Tables 1 and 2.
Doses of mTOR inhibitors and duration of treatment
Considering the heterogeneity in dose reporting, the information was consolidated into cumulative weekly dose in mg/m2 and mg/kg. The cumulative dose for everolimus in mg/m2 was calculated in 25 cases, with a median of 4.5 mg/m2/week (Q1 = 3.9; Q3 = 5), a minimum of 0.6, and a maximum of 31.5 mg/m2/week. The cumulative dose in mg/kg was estimated in 28 cases, with a median of 0.33 mg/kg/week (Q1 = 0.23; Q3 = 0.64), a minimum of 0.12, and a maximum of 1.47 mg/kg/week. The cumulative dose for sirolimus in mg/m2 was calculated in 5 cases, with a median of 10.5 mg/m2/week (Q1 = 8; Q3 = 10.9), a minimum of 4.2, and a maximum of 15.9 mg/m2/week. The cumulative dose in mg/kg was estimated in 6 cases, with a median of 0.74 mg/kg/week (Q1 = 0.70; Q3 = 1.4), a minimum of 0.42, and a maximum of 1.46 mg/kg/week (Table 3).
A high percentage of studies (68.4%) used the target serum level of mTORi reported in the EXIST studies of 5–15 ng/mL.18,19 The maximum serum level of everolimus was reported in 28 cases, with a median of 11.5 ng/mL (Q1 = 8.7; Q3 = 18), and for sirolimus, it was reported in 6 cases, with a median of 26.5 ng/mL (Q1 = 24.3; Q3 = 42.1). In more than 50% of the cases, the maximum serum level of mTORi was reached within 7 days of treatment. The median duration of treatment was 67 days (Q1 = 36; Q3 = 112). In 41.67% of cases, changes in the initial dose were reported, the main cause being serum levels of mTORi outside the established range (68.4%) and, to a lesser extent, adverse events (26.3%) (Table 4).
Effect of mTORi on CR size and long-term follow-up
In all cases, an improvement or resolution of the symptoms for which the treatment was initiated was reported. The average total reduction in CR size was 57 ± 23%. In 5 patients, an average CR reduction of 48.5 ± 33.8% was achieved between days 16 and 30 of treatment, and in 11 patients, the reduction was 50.3 ± 12.8% between days 31 and 60. The median total follow-up time was 10 months (Q1 = 6; Q3 = 21). In 34 cases, the evolution of CR after mTORi discontinuation was reported, finding an increase in the mass size (rebound) in 58.82% (20 patients). Treatment was restarted in 50% (10 out of 20 patients).
The association between the total percentage reduction of the mass and the maximum serum level of the mTORi was explored through linear regression, finding a significant relationship. For each unit ng/mL of mTORi, a reduction of 0.41% in the mass was observed (β = −0.41; 95% CI −0.75 to -0.08; p = 0.018). This association remained significant after adjusting for the medication and the newborn’s gestational age (β = −0.43; 95% CI −0.78 to −0.07; p = 0.020).
Adverse events related to mTORi
Adverse events were reported in 41.6% of the cases (n = 20); however, only 6 patients (12.5%) required permanent treatment discontinuation. For the remaining cases, the dose was reduced, or the mTORi was temporarily suspended. The most common adverse events were hypertriglyceridemia, infections, and hematological abnormalities (Table 5).
Evaluation of study quality
The detailed description of each article’s JBI Critical Appraisal Checklist can be reviewed in the Supplemental Table S1. Overall, the studies assessed mostly met the criteria outlined in the checklist. The criterion that showed the lowest compliance was demographic characteristics, with no data in 14 articles. Additionally, 9 articles did not meet diagnostic methods and outcomes criteria due to inaccuracies in CR dimensions or reported mTORi serum levels. With the collaboration of the articles’ authors, missing data were clarified for only two patients. Figure 2 provides a summary of the compliance percentage for each evaluated criterion.
Discussion
There are randomized controlled clinical trials demonstrating the efficacy and safety of mTORi in the treatment of certain manifestations associated with TSC, such as subependymal giant cell astrocytoma, drug-resistant focal seizures, and renal angiomyolipomas in children.18,19,23 To date, there is one phase II clinical trial evaluating the efficacy of mTOR inhibitors in the treatment of symptomatic cardiac rhabdomyomas in children, but the results are not yet available.13
Our review revealed a significant increase in case reports and case series documenting the off-label use of mTORi in treating hemodynamically significant CRs. This increase has been particularly notable in the last five years, with more than 60% of case reports made in this period. The proven efficacy of mTORi in treating other TSC manifestations has led to the increasingly frequent use of these medications in symptomatic CRs.16
Although spontaneous regression of CRs is common and most patients remain asymptomatic, neonates are particularly vulnerable to hemodynamic complications or arrhythmias caused by these masses, which can lead to death in the early neonatal period.1,5,9,10,11 In our review, initiating treatment with mTORi was mainly a life-saving measure, such as in patients with severe ventricular outflow tract obstruction, interfering with ventricular contraction by giant masses, or incessant arrhythmias that were difficult to control.
There is significant variability in the age at which mTORi treatment is initiated, largely determined by the severity of hemodynamic compromise. In most cases reviewed, treatment was initiated within the first 7 days of life. Currently, no studies standardize the age at which mTORi treatment should commence in neonates with symptomatic CRs.24
There is no consensus on the doses of mTORi used for treating hemodynamically significant CRs. Fixed doses were used in some cases, while others calculated doses per kilogram or body surface area. We calculated the cumulative weekly dose based on information obtained from each report, observing wide variability for both everolimus and sirolimus. The accumulated dose of everolimus ranged widely from 0.6 to 31.5 mg/m2/week, primarily administered as a daily dose over 7 days. The average daily dose of everolimus was 1.03 mg/m2/day, significantly lower than the FDA-approved dose for treating SEGA and pharmacoresistant focal onset seizures associated with TSC (4.5 mg/m2/day).18,23 The accumulated dose of sirolimus ranged of 4.2 to 15.9 mg/m2/week, mainly administered daily over 7 days. The average daily dose of sirolimus was 1.37 mg/m2/day. Like us, Sugalska et al. could not determine a standardized dose of mTORi due to marked variability in the reported data.16
In 2017, Mizuno et al. reported the results of a pharmacokinetic study for precise dosing of sirolimus in pediatric patients with vascular anomalies, concluding that younger children, such as infants and neonates, require lower doses of sirolimus to achieve the target serum level, as well as having lower drug clearance compared to children older than 2 years.25 In this study, the average dose of sirolimus needed to achieve the target serum level close to 10 ng/ml was 1.8 mg/m2/day for children older than 2 years and ranged from 0.7 to 1.6 mg/m2/day for patients aged 3 weeks to 2 years. Based on available evidence and data from our review, we suggest that it is reasonable to use lower doses of mTORi in newborns and infants than those approved for other TSC-associated manifestations, changing doses according to the target serum level.
Recently, Ng et al. proposed a dosing and monitoring strategy for sirolimus in neonates and infants.26 For premature newborns, the proposed initial dose is 0.25 mg/m2/day; for term newborns - 0.5 mg/m2/day; for infants aged 2 to 6 months - 0.5 to 1 mg/m2/day; and for infants aged 6 months to 12 months - 1 to 1.5 mg/m2/day, with a 10 to 15% dose increase in all cases based on sirolimus serum level.26
Neonates and infants are predisposed to achieve high serum levels of mTORi due to the low activity of enzymes that metabolize these drugs.26 Everolimus and sirolimus are mainly metabolized through the CYP3A enzyme family, which is expressed at very low levels during gestation and remains functionally immature after birth, reaching only 30-40% of adult values at 4 weeks and full levels around 3 years.27,28,29
Although we found a significant relationship between the total percentage reduction in mass and mTORi serum level, the goal is to maintain the drug’s serum level within the reference range. In our review, the median maximum serum level for everolimus was within the reference range; however, some patients reported values as high as 108 ng/mL or 83.5 ng/mL. We also observed that the maximum mTORi level was reached early in the first week of treatment. Considering these data and the neonatal predisposition to achieve high mTORi serum levels, the first serum measurement of the drug should be performed in the first week after starting treatment. Thus, Ng et al. propose monitoring mTORi serum levels on days 3 and 7 after the first dose, continuing with weekly measurements until a stable state is achieved.26
Sirolimus and everolimus have been used interchangeably in preclinical studies; however, few studies have directly compared the two drugs.30 In the absence of comparative clinical trials, the specific selection of everolimus or sirolimus in treating manifestations associated with TSC will be guided by the best evidence published to date.30 For example, there is greater availability of controlled randomized clinical trials on the use of everolimus in treating SEGA, renal angiomyolipomas, and refractory epilepsy,8,19,23 or more controlled randomized clinical trials on treating lymphangioleiomyomatosis with sirolimus.31 For treating CRs with mTORi, only one double-blind, multicenter, placebo-controlled randomized clinical trial of everolimus in patients with TSC and symptomatic CRs (ORACLE) is currently underway.13
In a small case-control study, Aw et al. reported a reduction in CR size of at least 50% in the group of patients treated with everolimus over a period of 1.13 ± 0.33 months (median 29.5 days), compared to the 72.9 ± 53.03 months it took for the control group. The CR reduction rate for the treatment group was 11.8 times faster than for the control group.12 In our review, all included cases reported a significant reduction in CR size and hemodynamic improvement and/or resolution of arrhythmia that prompted the initiation of treatment. Some patients experienced a reduced CR size of nearly 50% when nearing completion of the first or second month of treatment. While the types of studies included in this review do not allow for generalization of CR response to mTORi treatment, it is evident that these medications are particularly useful in the neonatal period when hemodynamic complications from these masses are more critical.
The optimal duration of mTORi treatment for hemodynamically significant CRs is not established. Cleary and McMahon reported significant variation in treatment duration among different centers, ranging from approximately 1 to 3 months.26 Our review reported treatment duration in 87.5% of cases, with a median of 2.2 months. In most patients, mTORi was discontinued once a significant reduction in CR size was achieved and the initial hemodynamic condition that prompted medication initiation was surpassed. In a smaller proportion, mTORi was discontinued due to adverse events. Sugalska et al. could not correlate treatment duration and CR reduction due to divergence found in reported data.16
Although not all authors in our review reported the evolution of CRs once treatment was discontinued, rebound growth of the mass occurred in 20 cases, becoming significant enough in some of them to necessitate restarting mTORi. In Sugalska et al.‘s review, the post-treatment evolution of CRs was reported in very few cases, with rebound growth present in 7 out of 12 patients (58.3%); however, the size reached by the mass was smaller than the initial size, and patients remained hemodynamically stable without requiring a new cycle of mTORi.16 Ng et al. propose extending treatment until achieving a 50 to 70% reduction in CR size or improvement in obstructive gradient.26 Considering the variability in reported data, treatment duration should be individualized and guided by clinical manifestations, hemodynamic alterations, CR size during echocardiographic follow-up, and the potential for rebound growth upon discontinuing mTORi.
Like findings from other studies, most adverse events found in our review were mild and dose-dependent.18,23,32,33 Although standardized scales for classifying adverse events are infrequently used in case reports, the majority of events in our review would be categorized as mild (grade 1) or moderate (grade 2), with very few cases classified as severe (grade 3) or potentially life-threatening (grade 4), according to the Common Terminology Criteria for Adverse Events scale.34 Dyslipidemia (hypercholesterolemia or hypertriglyceridemia) represents by far the primary reported adverse event, not only by us but also by Sugalska et al. and Cleary et al.16,24
Stomatitis has been reported by other authors as the most frequent adverse event in young children receiving mTORi treatment.32,35,36 Other commonly reported adverse events include transient neutropenia or lymphopenia, anemia, upper respiratory tract infections, gastrointestinal symptoms (diarrhea or vomiting), elevated liver enzymes, and infantile acne, mostly classified as grade 1 or 2.16,24,33,36,37 Non-infectious pneumonitis, although rare in patients under 3 years old,34 can be a severe adverse event (grade 3 or 4).35 Most of these adverse events can be managed by reducing the dose of mTORi and less frequently by temporary or permanent discontinuation of treatment.32,36
This systematic review stands out for being the most recent and complete review related to the effectiveness and safety of mTORi in the treatment of CRs in the neonatal period; however, publication bias cannot be ruled out considering that most of the information came from observational studies. In some cases, it was not possible to recover the missing information, or it was of low quality.
Recommendations
Neonates and infants are predisposed to achieving high serum levels of mTORi, so the first serum level measurement of the medication should be done within the first week after initiation.
It is reasonable in newborns and infants to use lower doses of mTORi than those approved for other manifestations associated with TSC, adjusting the dose according to the target serum level. Based on our study, an average daily dose of 1.03 mg/m²/day for everolimus and 1.37 mg/m²/day for sirolimus appears to be effective and safe. The most frequently used target serum level of mTORi was 5-15 ng/mL. These doses should be used carefully, considering that the dose of mTORi in neonates is not yet standardized.
More specific information on the pharmacokinetics of mTORi in neonates is needed to ensure optimal dose selection in future clinical trials.
The duration of treatment should be individualized and guided by clinical manifestations, hemodynamic alterations, and the size of cardiac rhabdomyoma during echocardiographic follow-up, as well as the potential for rebound growth upon discontinuing mTORi.
Conclusion
The use of mTORi in the treatment of symptomatic CRs in neonates at a lower dose than those approved for other manifestations associated with TSC appears to be effective and safe. The average day dose of mTOR inhibitors found in this review was 1.03 mg/m²/day for everolimus and 1.37 mg/m²/day for sirolimus. The most frequently used target serum level of mTORi was 5-15 ng/mL. In our review, all included cases reported a significant reduction in CR size, hemodynamic improvement, and/or resolution of arrhythmia. The speed of mass reduction is significant within the first month of treatment. Rebound growth is an expected effect and may sometimes require restarting treatment. Most adverse events found in our review were mild and dose-dependent. Randomized controlled clinical trials are required.
Data availability
All data generated or analysed during this study are included in this published article.
References
Uzun, O., Wilson, D. G., Vujanic, G. M., Parsons, J. M. & De Giovanni, J. V. Cardiac tumours in children. Orphanet J. Rare Dis. 2, 11 (2007).
Isaacs, H. Fetal and neonatal cardiac tumors. Pediatr. Cardiol. 25, 252–273 (2004).
Northrup, H. et al. Updated International Tuberous Sclerosis Complex Diagnostic Criteria and Surveillance and Management Recommendations. Pediatr. Neurol. 123, 50–66 (2021).
Huang, J. & Manning, B. D. The TSC1-TSC2 complex: a molecular switchboard controlling cell growth. Biochem J. 412, 179–190 (2008).
Hinton, R. B. et al. Cardiovascular manifestations of tuberous sclerosis complex and summary of the revised diagnostic criteria and surveillance and management recommendations from the International Tuberous Sclerosis Consensus Group. J. Am. Heart Assoc. 3, e001493 (2014).
Bornaun, H. et al. Regression of cardiac rhabdomyomas in a neonate after Everolimus treatment. Case Rep. Pediatr. 2016, 8712962 (2016).
Nespoli, L. F. et al. Efficacy of Everolimus low-dose treatment for cardiac Rhabdomyomas in neonatal tuberous sclerosis: case report and literature review. Pediatr. Rep. 13, 104–112 (2021).
Dahdah, N. Everolimus for the Treatment of Tuberous Sclerosis Complex-Related Cardiac Rhabdomyomas in Pediatric Patients. J. Pediatr. 190, 21–6.e7 (2017).
Fesslova, V., Villa, L., Rizzuti, T., Mastrangelo, M. & Mosca, F. Natural history and long-term outcome of cardiac rhabdomyomas detected prenatally. Prenat. Diagn. 24, 241–248 (2004).
Jóźwiak, S. et al. Clinical and genotype studies of cardiac tumors in 154 patients with tuberous sclerosis complex. Pediatrics 118, e1146–e1151 (2006).
Chao, A. S. et al. Outcome of antenatally diagnosed cardiac rhabdomyoma: case series and a meta-analysis. Ultrasound Obstet. Gynecol. 31, 289–295 (2008).
Aw, F. et al. Accelerated cardiac Rhabdomyoma regression with Everolimus in infants with tuberous Sclerosis complex. Pediatr. Cardiol. 38, 394–400 (2017).
Stelmaszewski, E. V. et al. Everolimus for cardiac rhabdomyomas in children with tuberous sclerosis. The ORACLE study protocol (everOlimus for caRdiac rhAbdomyomas in tuberous sCLErosis): a randomised, multicentre, placebo-controlled, double-blind phase II trial. Cardiol. Young-.-. 30, 337–345 (2020).
Garg, A., Gorla, S. R., Kardon, R. E. & Swaminathan, S. Rapid Involution of Large Cardiac Rhabdomyomas With Everolimus Therapy. World J. Pediatr. Congenit. Heart Surg. 12, 426–429 (2021).
Hoshal, S. G., Samuel, B. P., Schneider, J. R., Mammen, L. & Vettukattil, J. J. Regression of massive cardiac rhabdomyoma on everolimus therapy. Pediatr. Int. 58, 397–399 (2016).
Sugalska, M., Tomik, A., Jóźwiak, S. & Werner, B. Treatment of cardiac Rhabdomyomas with mTOR inhibitors in children with tuberous Sclerosis complex-a systematic review. Int J. Environ. Res Public Health 18, 4907 (2021).
Liu, X. et al. Treatment strategies for primary tumors of the heart in children: a 10-year experience. Ann. Thorac. Surg. 100, 1744–1749 (2015).
Franz, D. N. et al. Efficacy and safety of everolimus for subependymal giant cell astrocytomas associated with tuberous sclerosis complex (EXIST-1): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet 381, 125–132 (2013).
Bissler, J. J. et al. Everolimus for angiomyolipoma associated with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis (EXIST-2): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet 381, 817–824 (2013).
Bevacqua, M. et al. Off-Label use of Sirolimus and Everolimus in a pediatric center: a case series and review of the literature. Paediatr. Drugs 21, 185–193 (2019).
Page, M. J. et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372, n71 (2021).
Moola, S. et al. Systematic reviews of etiology and risk (2020). In JBI Manual for Evidence Synthesis (Aromataris, E., Lockwood, C., Porritt, K., Pilla, B. & Jordan, Z., eds) https://synthesismanual.jbi.global (JBI, 2024).
French, J. A. et al. Adjunctive everolimus therapy for treatment-resistant focal-onset seizures associated with tuberous sclerosis (EXIST-3): a phase 3, randomised, double-blind, placebo-controlled study. Lancet 388, 2153–2163 (2016).
Cleary, A. & McMahon, C. J. Literature review of international mammalian target of rapamycin inhibitor use in the non-surgical management of haemodynamically significant cardiac rhabdomyomas. Cardiol. Young-.-. 30, 923–933 (2020).
Mizuno, T. et al. Model-based precision dosing of sirolimus in pediatric patients with vascular anomalies. Eur. J. Pharm. Sci. 109S, S124–S131 (2017).
Ng, L. Y. et al. Cardiac Rhabdomyomas presenting with critical cardiac obstruction in neonates and infants: treatment strategies and outcome, a single-center experience. Pediatr. Cardiol. 45, 1132–1141 (2024).
Zanger, U. M. & Schwab, M. Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharm. Ther. 138, 103–141 (2013).
Hines, R. N. Ontogeny of human hepatic cytochromes P450. J. Biochem Mol. Toxicol. 21, 169–175 (2007).
Emoto, C. et al. Characterizing the developmental trajectory of sirolimus clearance in neonates and infants. CPT Pharmacomet. Syst. Pharm. 5, 411–417 (2016).
MacKeigan, J. P. & Krueger, D. A. Differentiating the mTOR inhibitors everolimus and sirolimus in the treatment of tuberous sclerosis complex. Neuro Oncol. 17, 1550–1559 (2015).
Takada, T. et al. Efficacy and safety of long-term Sirolimus Therapy for Asian patients with Lymphangioleiomyomatosis. Ann. Am. Thorac. Soc. 13, 1912–1922 (2016).
Jóźwiak, S., Kotulska, K., Berkowitz, N., Brechenmacher, T. & Franz, D. N. Safety of Everolimus in Patients Younger than 3 Years of Age: Results from EXIST-1, a Randomized, Controlled Clinical Trial. J. Pediatr. 172, 151–155.e1 (2016).
Saffari, A. et al. Safety and efficacy of mTOR inhibitor treatment in patients with tuberous sclerosis complex under 2 years of age - a multicenter retrospective study. Orphanet J. Rare Dis. 14, 96 (2019).
Freites-Martinez, A., Santana, N., Arias-Santiago, S. & Viera, A. Using the Common Terminology Criteria for Adverse Events (CTCAE - Version 5.0) to evaluate the severity of adverse events of anticancer therapies. Actas Dermosifiliogr. (Engl. Ed.). 112, 90–92 (2021).
Zhang, Z. et al. Safety Evaluation of Oral Sirolimus in the treatment of childhood diseases: a systematic review. Children 9, 1295 (2022).
Krueger, D. A. et al. Short-term safety of mTOR inhibitors in infants and very young children with tuberous sclerosis complex (TSC): Multicentre clinical experience. Eur. J. Paediatr. Neurol. 22, 1066–1073 (2018).
Sadowski, K., Kotulska, K. & Jóźwiak, S. Management of side effects of mTOR inhibitors in tuberous sclerosis patients. Pharm. Rep. 68, 536–542 (2016).
Demir, H. A., Ekici, F., Yazal Erdem, A., Emir, S. & Tunç, B. Everolimus: a challenging drug in the treatment of multifocal inoperable cardiac rhabdomyoma. Pediatrics 130, e243–e247 (2012).
Breathnach, C., Pears, J., Franklin, O., Webb, D. & McMahon, C. J. Rapid regression of left ventricular outflow tract rhabdomyoma after sirolimus therapy. Pediatrics 134, e1199–e1202 (2014).
Goyer, I., Dahdah, N. & Major, P. Use of mTOR inhibitor everolimus in three neonates for treatment of tumors associated with tuberous sclerosis complex. Pediatr. Neurol. 52, 450–453 (2015).
Wagner, R. et al. Oral everolimus for treatment of a giant left ventricular rhabdomyoma in a neonate-rapid tumor regression documented by real time 3D Echocardiography. Echocardiography 32, 1876–1879 (2015).
Colaneri, M., Quarti, A. & Pozzi, M. Everolimus-induced near-resolution of giant cardiac rhabdomyomas and large renal angiomyolipoma in a newborn with tuberous sclerosis complex. Cardiol. Young. 26, 1025–1028 (2016).
Chang, J. S., Chiou, P. Y., Yao, S. H., Chou, I. C. & Lin, C. Y. Regression of neonatal cardiac Rhabdomyoma in two months through low-dose Everolimus therapy: a report of three cases. Pediatr. Cardiol. 38, 1478–1484 (2017).
Lee, S. J. et al. Rapid regression of obstructive cardiac Rhabdomyoma in a preterm neonate after Sirolimus Therapy. Biomed. Hub. 2, 1–6 (2017).
Schmidt-Fittschen, M., Spahn, S., Al Naimi, A., Schranz, D. & Bahlmann, F. Everolimus treatment of a fetal intracardiac rhabdomyoma not associated with the tuberous sclerosis complex: a case report. Case Rep. Perinat. Med. 6, 20160067 (2017).
Martínez-García, A. et al. Giant left ventricular rhabdomyoma treated successfully with everolimus: case report and review of literature. Cardiol. Young. 28, 903–909 (2018).
Weiland, M. D., Bonello, K. & Hill, K. D. Rapid regression of large cardiac rhabdomyomas in neonates after sirolimus therapy. Cardiol. Young. 28, 485–489 (2018).
Dhulipudi, B., Bhakru, S., Rajan, S., Doraiswamy, V. & Koneti, N. R. Symptomatic improvement using everolimus in infants with cardiac rhabdomyoma. Ann. Pediatr. Cardiol. 12, 45–48 (2019).
Lawley, C., Popat, H., Wong, M., Badawi, N. & Ayer, J. A dramatic response to Sirolimus Therapy in a premature infant with massive cardiac Rhabdomyoma. JACC Case Rep. 1, 327–331 (2019).
Shibata, Y. et al. Effect and complications of Everolimus use for giant cardiac Rhabdomyomas with neonatal tuberous sclerosis. AJP Rep. 9, e213–e217 (2019).
Esmer-Sánchez, M. et al. Everolimus response in a newborn with cardiac rhabdomyoma associated to tuberous sclerosis complex: Case report. Acta Pediatr. Mex. 41, 208–214 (2020).
Prasad, K. et al. Accelerated regression of cardiac rhabdomyoma by mTOR inhibitors in a neonate with heart failure: A case report. IHJ Cardiovasc. Case Rep. (CVCR) 4, 142–145 (2020).
Çetin, M., Aydın, A. A. & Karaman, K. Everolimus treatment in a 3-month-old infant with tuberous sclerosis complex cardiac rhabdomyoma, severe left ventricular outflow tract obstruction, and hearing loss. Cardiol. Young. 31, 1359–1362 (2021).
Nir-David, Y., Brosilow, S. & Khoury, A. Rapid response of a cardiac rhabdomyoma causing severe right ventricular outflow obstruction to Sirolimus in an infant with negative genetics for Tuberous sclerosis. Cardiol. Young. 31, 312–314 (2021).
Relan, J. et al. Prenatal Pericardiocentesis and Postnatal Sirolimus for a Giant Inoperable Cardiac Rhabdomyoma. JACC Case Rep. 3, 1473–1479 (2021).
Silva-Sánchez, M. P. et al. Everolimus for severe arrhythmias in tuberous sclerosis complex related cardiac rhabdomyomas. Am. J. Med. Genet. A 185, 1525–1531 (2021).
Tsuchihashi, T., Tsuno, Y., Kakimoto, N., Riko, M. & Kumagai, T. Use of everolimus for cardiac rhabdomyomas in a very-low-birthweight infant. Pediatr. Int. 63, 726–727 (2021).
Beyazal, M., Özyazıcı, A. & Yeşil, Ş Multiple intracardiac benign tumors treated with low-dose everolimus. Anatol. J. Cardiol. 26, 141–142 (2022).
Çetiner, N., Kavas, N. & Küçükosmanoğlu, O. Rapid regression everolimus therapy in a neonate with cardiac rhabdomyoma. Pediatr. Int. 64, e15188 (2022).
Inoue, S., Inuzuka, R., Kakiuchi, S., Sato, A. & Matsui, H. Successful treatment with everolimus for severe heart failure with large cardiac rhabdomyomas. Pediatr. Int. 64, e15219 (2022).
Sagiv, E., Chikkabyrappa, S. M., Conwell, J., Lewin, M. & Chun, T. U. H. Use of Everolimus to treat cardiac rhabdomyomas and incessant arrhythmias in a newborn: Benefits and complications. Ann. Pediatr. Cardiol. 15, 58–60 (2022).
Winkie, C., Gelman, J., Verhoeven, P. & Chaudhuri, N. R. Sirolimus-induced regression of Tuberous Sclerosis-associated cardiac Rhabdomyoma causing left ventricular outflow tract obstruction. CASE 6, 361–365 (2022).
Babaoğlu, K., Başar, E. Z., Usta, E., Yılmaz, E. H. & Günlemez, A. Effect of different dose regimens of everolimus in a series of neonates with giant cardiac rhabdomyomas. Cardiol. Young. 33, 2291–2296 (2023).
Hurtado-Sierra, D., Ramos Garzón, J. X., Rojas, L. Z., Fernández-Gómez, O. & Manrique-Rincón, F. Case report: Accelerated regression of giant cardiac rhabdomyomas in neonates with low dose everolimus. Front. Pediatr. 11, 1109646 (2023).
Montaguti, E. et al. A case of massive fetal cardiac rhabdomyoma: ultrasound features and management. J. Matern. Fetal Neonatal Med. 36, 2197099 (2023).
Acknowledgements
No financial assistance was received in support of this study.
Funding
Open Access funding provided by Colombia Consortium.
Author information
Authors and Affiliations
Contributions
D. H-S., J. R., S. R-G., A. S-G., and L. R. conceptualized and designed this project and contributed to acquisition of data. L. R. participated in data analysis and interpretation. All authors contributed to drafting, critically appraising, and/or revising the manuscript. All authors approved the final version of the manuscript and are accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hurtado-Sierra, D., Ramos Garzón, J.X., Romero-Guevara, S.L. et al. Everolimus and sirolimus in the treatment of cardiac rhabdomyomas in neonates. Pediatr Res (2025). https://doi.org/10.1038/s41390-025-04043-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41390-025-04043-8