Abstract
Human milk is far more than a source of infant nutrition. It is a dynamic, living fluid packed with cells, bioactive molecules, and a complex microbiome that shapes neonatal development and lifelong health. Recent advances have illuminated the remarkable cellular diversity of human milk, including epithelial, immune, microbial and stem cells, each contributing essential biological functions. Milk contains distinct membrane-bound structures in the form of milk fat globules and extracellular vesicles that package a diverse cargo of lipids, proteins and nucleic acids for neonate nutrition, development and immune regulation. This review explores the composition of human milk, highlighting its nutrient and bioactive components and discussing growing concerns of xenobiotic and viral burden. We describe how milk-derived cells offer non-invasive windows into lactation biology and how emerging 3D mammary organoid models, particularly those generated from human milk cells, provide unprecedented tools to study breast development, lactation disorders, and regenerative therapies. We outline the potential of milk cells and extracellular vesicles in neonatal care, personalized medicine, and biobanking, while addressing current technical challenges and future research opportunities. By harnessing the unique properties of human milk, we stand at the threshold of transformative insights into maternal-infant health and novel biomedical applications.
Impact
-
Up to date summary of bioactives, living cells and membrane bound compartments found in human milk.
-
Primer on human mammary organoid technology, including advantages, recent advances and step by step methods.
-
Highlights the unrealized potential of human milk in organoid technology, therapeutics, and regenerative medicine.
Similar content being viewed by others
Introduction
Human milk has evolved to be the optimum infant food.1,2 Produced by the mammary glands, milk provides nutrition, immune protection, and developmental support to the neonate. Milk is a complex fluid that consists of myriad components including nutrients (lipids, proteins, carbohydrates), bioactive molecules (vitamins and immunomodulatory factors), various cells (lactocytes, macrophages, stem cells and bacteria) and cell-like components (milk fat globules and extracellular vesicles) with important biological functions.3 Together, the nutrient and non-nutrient components of human milk are highly effective for the survival and healthy development of human infants. However, nutritional deficiencies in human milk (e.g., pre-term milk4), coupled with the increasing burden of xenobiotics,5 pose significant and long-term health risks to the infant. A contemporary understanding of human milk composition and the state-of-the-art tools and technologies applicable to milk research is prerequisite to improving the quality and safety of human milk.
Breastfeeding is a key public health strategy with major health organizations recommending exclusive breastfeeding in the first six months of life.6,7 However, infants are frequently weaned before this period due to insufficiencies in lactation, as reported by 40–50% of breast feeding persons in the US and 60–90% world-wide.7 Examination of gene and protein expression in lactating breast tissue could serve to gauge the differentiation and functionality of breast tissue during lactation and to evaluate the potential for successful lactation. This could be especially beneficial for addressing lactation challenges, including issues with milk supply and delayed secretory activation in lactating persons with premature infants. However, studies on lactation historically rely heavily on animal models which fail to fully recapitulate human lactation physiology, or on healthy breast tissue that is difficult to obtain, especially during lactation, and has limited scalability. Differences between human and animal models include the number and location of mammary glands, the timing and trajectory of mammary gland development, and the composition of milk.8,9
In contrast, as we describe in this review, human milk is a readily available and non-invasive source of milk fat globules, extracellular vesicles, microbiota and living cells from the lactating breast that could offer valuable insight into the natural function of this organ and its associated disorders. Recent advancements in stem cell technology and 3D cell cultures may be applied to human milk cells, opening new avenues for studying lactation biology. We describe how organoids could lead to a deeper understanding of the human mammary gland, and we speculate on the potential use of milk-derived stem cells in regenerative medicine. Finally, we explore the potential biotechnological and clinical applications of human milk.
Cell-derived components of human milk
Human milk is a complex fluid composed of diverse solutes, membrane bound structures and living cells3,10,11 (Fig. 1). The composition of milk varies with the stages of lactation, extent of nursing, maternal genetics and diet, health of the breast feeding dyad, whether the infant is born full-term or preterm, and many other factors.4,12 Establishing a thorough and reliable reference that encompasses all essential nutrients and bioactive factors in human milk will require standardized population studies in the future. A comprehensive understanding of the components of human milk could inform on lifestyle and diet choices that impact milk production and composition, and better formulation of supplements for the changing needs of infant nutrition in the first few years of life.
This figure depicts the multifaceted nature of human milk as both a nutritional and immunological source for infant development. Left panel: biochemical components include macronutrients (carbohydrates, proteins, and lipids), micronutrients (essential minerals like calcium, iron, sodium, and potassium), bioactive components (such as immunoglobulins, hormones, and growth factors), and xenobiotics (external compounds like medications and environmental contaminants). Right panel: cellular fraction features milk fat globules for lipid transport, extracellular vesicles carrying proteins and RNA, and various cell types, including luminal epithelial cells, stem cells, immune cells (B-cells, T-cells, macrophages, and monocytes), as well as microbes and viruses that contribute to gut colonization and immune modulation in infants.
Macromolecules and micronutrients
The solutes in milk include macronutrients, micronutrients, bioactive components including immunomodulatory and growth factors, and a variable composition of xenobiotics. On average, milk comprises ~87% water, 7% lactose, 3.8% fat, and 1% protein, with lactose being a predominant carbohydrate averaging 62 g/L.11 The fat content is primarily composed of triglycerides, polyunsaturated fatty acids, cholesterol, and phospholipids that are critical for cognitive and brain development. Whey proteins (e.g., lactoferrin, lysozyme, secretory IgA) and casein support growth, intestinal health, and immunity.3,13 Human milk oligosaccharides constitute the third most abundant class of solutes, with more than 150 distinct structures influenced by maternal genetics.14 They serve as food substrate for gut bacteria and when fermented, release beneficial compounds that improve intestinal barrier integrity, and modulate immune function.15 Other bioactive components like beta-casomorphins enhance gut function and regulate satiety.16,17 Examples of micronutrients are vitamins and minerals, including ionic and complexed calcium, magnesium, iron, zinc, and copper, that are critical to meet infant needs.18 Human milk contains numerous immunomodulatory factors and growth-promoting elements that extend its benefits beyond nutrition. Immunoglobulins, predominantly secretory IgA, provide passive immunity by safeguarding mucosal surfaces and protecting against infections during the early months when the infant immune system is still developing.10 Growth factors such as Epidermal Growth Factor (EGF), Insulin-like Growth Factor-1 (IGF-I), and Vascular Endothelial Growth Factor (VEGF) are critical for intestinal development, vascularization, and tissue repair, especially in preterm infants.19 Adiponectin, a hormone found in human milk, regulates infant metabolism and is inversely correlated with infant Body Mass Index (BMI), suggesting a potential role in preventing childhood obesity.20 Collectively, these milk components support physical growth, cognitive development, and immune health of the neonate, underscoring their value during infancy.
Milk fat globule
When observed under a microscope, the visual characteristics of human milk are remarkable. Despite being a fluid, human milk possesses significant structure in the form of compartments that sequester nutrients and bioactive compounds. An exemplary illustration of this arrangement is the milk-fat globule, a natural colloidal assembly ranging in diameter from 0.1 to 20 μm composed of a triglyceride core encapsulated within a complex membrane derived from the secretory mammary epithelial cell.21 The lipid core is surrounded by a tripartite membrane: an inner monolayer of proteins and polar lipids with the fatty acid tails facing the globule core that is derived from the cytoplasmic leaflet of the endoplasmic reticulum and a bilayer of polar lipids, proteins, glycoproteins and cholesterol derived from the plasma membrane during the budding process. In addition, there is an entrained layer of cytoplasm about 10–20 nm wide between the inner single membrane and outer bilayer.22,23
The milk-fat globule sequesters specific proteins, growth hormones, and vitamins, which may be incorporated into the limiting membrane.21 The membrane functions as a stabilizing barrier between the aqueous milk components and segregated fat globule and facilitates the regulated liberation of lipolysis products and the transfer of polar substances into the aqueous phase of milk serum. In addition to it nutritional value, the human milk fat globule plays a major role in protecting the infant against infection through the lipase dependent production of free fatty acids and monoglycerides that exert potent antimicrobial and antiviral properties in the infant gut, reviewed in ref. 24 Mucins, lactadherins and other glycoproteins in the milk fat globule membrane bind to pathogens, preventing their attachment to the gastric mucosa.24
Extracellular vesicles
Exosomes and extracellular vesicles (EV) constitute another important cell-derived component of milk.25 They vary widely in size depending on their mode of production.26,27 Fusion of multivesicular endosomes with the plasma membrane in mammary epithelial cells gives rise to 30–100 nm exosomes. Direct budding of vesicles from the plasma membrane in a process called ectocytosis leads to the formation of microvesicles, with a wider diameter range of 100–1000 nm. In addition, extracellular compartments can arise from apoptotic bodies and have annexin V as a marker. These nanoscale vesicles encapsulate different bioactive molecules, including microRNA (miRNA), protein, and lipid, that survive the acidic milieu of the neonate intestine and may be endocytosed by intestinal cells to exert immunomodulatory and regenerative effects.28 The cargo content of EVs in transitional milk was found to depend on gestational age, with differences observed between preterm and term milk.29 As discussed ahead, the important role of extracellular vesicles in signal transduction, delivery of bioactive cargo, and maternal-infant communication is increasingly recognized.30,31
Viruses
Human milk can be a reservoir for certain viruses and serve as a transmissible route for infant infection. For this to happen, the virus must infect and replicate in mammary epithelia and be secreted into milk. Furthermore, for infection to be successful, the virus must survive contact with the oral and digestive tract of the infant, proliferate and exit from enterocytes, and enter the bloodstream for systemic infection.32 Studies in the 1980’s established that human milk contains both cell-free and cell-associated particles of human immunodeficiency virus type 1 (HIV-1) that can be transmitted to breast fed infants. Paradoxically, milk from HIV-positive people has potent HIV-inhibitory activity and breast-fed infants have a lower rate of acquiring AIDS.33 During the COVID pandemic, concerns that lactating persons could transmit SARS-CoV-2 through milk combined with shortage of formula-based alternatives led to extensive testing and evaluation, with the conclusion that SARS-CoV-2 infection through human milk has limited transmission risk, and benefits for infants far outweigh the risk.32 Since 2024, surprisingly high titers of the highly pathogenic avian influenza virus HPAI H5N1 have been found in raw dairy milk, underscoring the importance of pasteurization and potential for human transmission, including to lactating persons.34 Although influenza is considered a respiratory virus, HPAI H5N1 virus shows a marked tropism to the mammary gland,35 which is the primary replication site in dairy cows leading to mastitis and high viral loads in milk.36 The unexpected ability of HPAI to replicate efficiently in the mammary gland has been linked to sialic acid receptors: recent immunohistochemistry reports showing expression of specific sialic acid receptors essential for HPAI binding in human mammary tissues37,38 highlights the risk of human transmission which could be a rising concern for lactating persons.
Xenobiotics
Although human milk is the gold standard for infant health and nutrition, it may contain varying levels of xenobiotics, including pesticides and industrial contaminants, mycotoxins, heavy metals and pharmaceuticals. These foreign compounds occur as environmental contaminants, medications, or are found in cosmetics and food, entering the maternal bloodstream through absorption, inhalation or ingestion.39 By partitioning into milk fat by maternal transfer, or transported across the mammary epithelium by efflux transporters such as the breast cancer resistance protein BCRP,40 xenobiotics are passed on to the infant where they exert harmful effects on infant development, act as endocrine disrupters, neurotoxins or allergens with long lasting health repercussions. Most biomonitoring studies use targeted analysis and can miss emerging or unknown xenobiotics, whereas non-targeted methodologies and multi-omics approaches, using gas chromatography and mass spectrometry for example, can identify novel compounds in human milk.41 Other challenges include developing guidelines for acceptable daily intake and evaluating aggregated and cumulative exposure from multiple sources.
Cellular diversity of human milk
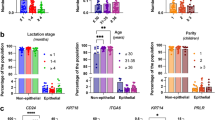
Human milk includes diverse populations of maternal-derived epithelial, immunological, microbial and stem cells. The number of cells varies with lactation stage, with peak levels reported in colostrum (146,000 cells per milliliter), and decreasing postpartum.42 As discussed below, bulk and single cell RNA sequencing studies have demonstrated that the cellular composition of milk changes throughout lactation. Gene expression cluster analysis on longitudinal samples obtained from human donors revealed up to ten broad cell types, including seven types of immune cells and three epithelial cell compartments.43 These studies reveal the existence of distinct mammary cells and their patterns of gene expression in human milk, emphasizing the intricate and biologically diverse cellular characteristics of human milk.
Epithelial cells
The presence of epithelial cells in human milk was initially believed to be a byproduct of apoptosis. However, the discovery that many of these cells are viable and able to form mammospheres in primary cultures suggests that these are cells that detach from the ducts and alveoli during milk synthesis and secretion. There is evidence that milk cells delivered to the infant subsequently infiltrate into different organs for the benefit of the offspring.44,45
Epithelial cells, identified by expression of the mature mammary epithelial cell markers cytokeratin 18 (CK18) and CK19,46 are the most abundant cells of the mammary gland and critical for maintaining the structure and function of the organ.47 There are two categories of epithelial cells: ductal epithelial cells, which line the mammary gland ducts and alveolar or luminal epithelial cells, which differentiate into lactocytes during in milk production.48 One study showed that lactocyte epithelial cells accounted for an average of 81.7+/− 24% of all cells per milk sample, across both donor and lactation stage.43 It is unclear if basal/myoepithelial cells are in human milk: CK14 was detected in milk cells, but it is a marker common to both mammary progenitor cells and myoepithelial cells.49
All epithelial cells found in milk express genes involved in milk synthesis. However, subsets of these cells are differentially enriched for secretory gene expression (“secretory lactocytes”), tight junction function, cell cycle regulators or cytoskeleton functions pointing to their origin within discrete subdomains of structure or function within the lactating mammary gland.43,50 Whereas all lactocyte epithelial cells expressed lactalbumin (LALBA), Claudin 4 (CLDN4) and Krueppel-like factor 6 (KLF6)) highlighted the so-called LC1 cluster whereas xanthine dehydrogenase (XDH), casein alpha S1 (CSN1S1) identified a larger group of LC2 cells.51 These studies and many others reveal that the expression of different markers in human milk cells tracks with their stage of differentiation. A complex transcriptional program controls biosynthesis of milk and response to signals throughout lactation. Both the diversity and abundance of epithelial cells increased over the course of lactation, although cycling and secretory lactocytes decreased in proportion, possibly as an outcome of increased specialization over time.
Immune cells
Macrophages and lymphocytes found in human milk migrate from the blood stream and maternal mucosal immune system, particularly the gut and respiratory tract, and are drawn to the mammary gland by specific signaling molecules and adhesion molecules.52,53 The presence of immune cells in human milk varies depending on the stage of lactation and the health of both the birthing parent and infant.54 During the initial stages of lactation, as in colostrum, a considerable proportion of human milk cells is made up of leukocytes, including macrophages, neutrophils, and lymphocytes. Macrophages comprise the most abundant immune cell type, at 50.5+/− 34% per sample.52 These cells travel through the intestinal mucosa and enter the infant’s bloodstream to strengthen immune defense and development.55,56 Flow cytometry analysis of human milk cells revealed an infiltration of CD45+ cells in response to mastitis and other infections. The correlation between milk immune cell counts and mastitis could be harnessed to develop diagnostic test for the disease which is a major cause of unrecoverable milk production.57 Intriguingly, there are hints of shifting macrophage composition in transcriptional metadata encompassing infant health, feed supplementation and daycare attendance, although larger and more robust datasets will be needed to tease out correlations.43
Stem cells
Early reports on the identification of a population of nestin and p63 positive cells in human milk,58,59 together with demonstrations that cells obtained from human milk could expand, differentiate and propagate in culture pointed to the presence of a resident population of stem cells.60,61 Stem cells in human milk express pluripotency markers including OCT4, SOX2, and NANOG which comprise the critical transcription factor circuitry found in human embryonic stem cells.62 These cells are scarce in the non-lactating breast but are activated during pregnancy to direct the development of mammary tissue to a lactating organ. Milk stem cells can develop into a wide range of cell types, encompassing those from all three germ layers (endoderm, mesoderm, and ectoderm), a hallmark of pluripotency.63 In contrast to embryonic stem cells, milk stem cells did not form tumors when injected subcutaneously into immunocompromised mice in the teratoma assay.62 While the relative abundance of stem cells in human milk is unclear, they are ingested in significant quantities by the nursing infant.
Similar to milk leukocytes, stem cells ingested through milk may diapedese through the intestinal epithelium and enter the blood circulation of the infant to be distributed to various niches in the body. This could result in tissue microchimerism, continuing a process that has been observed in utero.64 Strikingly, studies in mouse models have revealed the integration of milk-derived stem cells into brains of suckling pups.65 The observation that maternal transplants are better tolerated in individuals who have been breast fed suggests that infiltrating stem cells from human milk may confer immune tolerance to maternal antigens in the infant, strengthening the case for human milk over formula milk.66
Microorganisms
Human milk contains over 200 microbial phylotypes. In terms of abundance, about half can be assigned to a common milk microbiome and the remaining show individual variation.67 Initially, microbial presence in milk was attributed to contamination from the infant’s oral cavity or maternal skin, or from maternal infection. These mechanisms, however, do not explain the presence of microorganisms in precolostrum secreted prior to parturition,68 or the detection of anaerobic species found in the gut.69 There is emerging evidence for an entero-mammary pathway by which bacteria found in maternal gut are sampled by dendritic cells that can reach through tight junctions in the epithelial gastrointestinal lining.57 These bacteria travel through the lymphatic system to reach the lactating mammary gland. In human trials of women who received oral administration of lactobacilli strains, the presence of these bacteria in the milk was subsequently detected in >50% of participants.70,71
Infants can greatly benefit from the wide variety of probiotics found in human milk. Among the microbial variety, Bifidobacterium and Lactobacillus stand out as particularly noteworthy. Bifidobacterium is essential for maintaining a well-balanced gut microbiota, boosting the immune system, and safeguarding against infections.71 These bacteria play a crucial role in the digestion of human milk oligosaccharides, supporting the growth of other beneficial microbes and preventing proliferation of harmful pathogens.72 Lactobacillus, a crucial microbial species, plays a vital role in preserving infant gut health. It achieves this by producing lactic acid, which effectively reduces the pH levels in the intestines and inhibits the growth of detrimental bacteria.73,74,75 There are several other noteworthy microorganisms worth mentioning. One of them is Akkermansia muciniphila, which plays a crucial role in fortifying the intestinal barrier and mitigating inflammation.76 Additionally, human milk contains Staphylococcus and Streptococcus, which have significant impact on the infant’s developing immune system, bolstering its effectiveness and safeguarding against disease.77
Metabolites generated by milk microorganisms, including short-chain fatty acids such as acetate, butyrate and propionate, have crucial functions in maintaining gut health, regulating the immune system, and supporting energy metabolism. These metabolites play an important role in promoting infant immune tolerance, mitigating inflammation, and maintaining gut health.78,79 Indeed, human milk is the exclusive source of short-chain fatty acids in the first year of life before the maturation of the infant microbiome. The protective role of maternal milk is especially critical in preterm infants whose reduced gut microbial diversity increases their susceptibility to pathogenic bacteria.80 Overall, microorganisms and their byproducts in human milk have a probiotic effect, promoting the growth and well-being of babies by strengthening the immune system, promoting a healthy gut, and safeguarding against various infections.81
Emerging applications of milk
Bioactive compounds and cells isolated from milk are useful in both basic and disease-oriented applications. Milk-derived cells may be used in vitro studies to model mammary gland biology and lactation, and in understanding breast cancer, mastitis, and other lactation-related conditions. Potential therapeutic applications include the use of milk-derived stem cells for tissue engineering and regenerative therapies. The immunological properties of milk cells could be harnessed for developing new immunotherapeutic strategies. Here, we summarize the tools and techniques in applications of milk cells.
Therapeutics
Drug delivery
Milk exosomes have many features conducive to their application in drug delivery, including stability, protection of cargo, oral bioavailability, scalability, low immunotoxicity, and ability to cross the intestinal barrier.82 Milk extracellular vesicles may be loaded with drugs, vaccines or nucleic acids for therapeutic and gene editing applications. Studies indicate that human and bovine milk vesicles can traverse the whole gut, remaining intact against enzymatic degradation in the acidic environment of the stomach, thereby, improving the delivery of their cargo to target cells.30,83 These advantages suggest that milk-derived exosomes could be modified to deliver therapeutic agents to diseased tissues, although the full potential of this technology remains to be realized. Developing robust and scalable procedures for the production, and refining strategies for precision and safety will be key to successful use of this platform.
Regenerative medicine
Stem cells obtained from human milk are ethically acceptable, pluripotent and non-teratogenic.60,62 In this respect, milk stem cells resemble umbilical cord and bone marrow-derived pluripotent cells that also display lower tumorigenic potential in immunodeficient mice where they can integrate into damaged tissue. Markers of neurons (β-tubulin, nestin and MAP2) as well as glia (s100b) have been detected in milk stem cell-derived lineage, making them potential candidates for cell replacement therapy in neurodegenerative disorders.84 Human milk derived spheroids cultured in appropriate differentiation media have the capacity to secrete milk protein (mammospheres), insulin (pancreatic beta-cell organoids) or express troponin T (cardiospheres).62 Interestingly, the tolerance of human milk-fed infants to maternal cells could be considered a form of “natural stem cell therapy” and bodes well for their application in regenerative medicine.60
Immunomodulation
Human milk has antibacterial and immunomodulating properties, and has traditionally been used in many cultures for the treatment of skin problems like dermatitis and eczema.85 More specifically, extracellular vesicles derived from human milk are increasingly recognized as important mediators for intestinal health by protecting against inflammation, promoting epithelial regeneration and modulating the composition of gut microbiota. Exosomes purified from both raw and pasteurized human milk, as well as from colostrum or mature milk, were effective in protecting against necrotizing enterocolitis in mouse models.86,87,88 MicroRNA cargo in EVs were found to shield neonates against inflammatory diseases, including necrotizing enterocolitis, through their regulation of immune signaling.29 Notably, human milk EV-associated miR-29 induced activation of intestinal stem cells to promote epithelial proliferation and repair damaged mucosa.89,90 The ability of milk EVs to modulate various inflammatory pathways such as the TLR4-NF-κB signaling cascade and NLRP3 inflammasome activation demonstrates their potential as therapeutics for inflammatory bowel disease (IBD) and necrotizing enterocolitis (NEC).91 At physiological concentrations, EVs were found to increase the barrier function of infant oral mucosa by targeting multiple signaling pathways.92 In the same way, EVs seemingly bolster the neonatal intestinal barrier, affecting mucus layer formation and modulation of tight junction proteins, with enormous implications for conditions associated with increased intestinal permeability, such as allergies and autoimmune disease.93,94
Diagnostic medicine
Diagnostic applications
Beyond its nutritional value, human milk offers enormous diagnostic potential for insights into maternal and infant health, from the detection of infectious agents to early warning signs for infants at risk for neurodevelopmental disorders and microbial analysis for childhood asthma and atopy.95 Using milk as a tissue-specific biospecimen, one study identified significant differences in DNA methylation in subjects with subsequent diagnosis of breast cancer.96 Studies on human milk antibodies to respiratory pathogens have evaluated the effect of infection and maternal vaccination.97
Pharmacogenetics
Human milk is a source of DNA and can be analyzed in pharmacogenetic studies, helping to understand how individuals respond to medications. Maternal genetic factors can significantly affect milk pharmacokinetics and infant exposure to medication. Thus, pharmacogenetic differences in membrane transporter expression and/or activity impact the concentrations of key nutrients and drugs in human milk.40 For example, a single polymorphism in the ABCG2 gene encoding the BCRP transporter alters milk levels of nifedipine in hypertensive lactating persons treated with this drug.98
Biomanufacturing
Bioactives
Beyond infant nutrition, bioactives in milk have a range of potential applications in food industry, pharmaceuticals and nutraceuticals. These include the biomanufacturing of human milk oligosaccharides as probiotics, by large scale production using either cell- based approached, transgenic plants or microbial fermentation.99,100
Artificial or lab-grown milk
There is public interest in harvesting milk-like fluids produced from cultured lactogenic human mammary cells for a variety of ethical and practical reasons.101,102 Lab grown milk could be more sustainable than traditional dairy, reduce greenhouse gas emissions and use less land and water resources. Unlike lab-grown meat, milk-producing cells themselves are not harvested which means that they may be re-used to continuously generate milk and may be genetically engineered for quantity and quality without the milk itself being genetically modified. To produce raw milk from bioreactors, mammary epithelial cells are cultured on straw-like capillary devices either in the inside-out or physiologically correct topology to secrete their product into the surrounding medium or into the capillary lumen, respectively.103 Although promising, these efforts are still in early stages and face uphill challenges in economics of scale and limitations in reproducing many critical components of human milk such as immune cells.
Research and development
Biobanking
Traditionally, milk banks have collected, screened, stored and distributed milk to infants needing access to the nutritional and immunological benefits of human milk. Milk biobanks also serve as valuable storage facilities for research and clinical purposes.104 Donor milk offers an abundant source of milk components including lactocytes, milk fat globules and extracellular vesicles that could provide insight on transcriptome and proteome changes through the course of lactation. For example, donor milk obtained from milk banks was an effective source of extracellular vesicles used to demonstrate protection against inflammation in enteroids and necrotizing enterocolitis in mouse models.86 Cell biobanking specifically refers to the methodical gathering, handling, and preservation of human milk cells from nursing women, typically using non-invasive techniques. The process entails the isolation of the cells, evaluation of their quality, and the subsequent preparation for cryopreservation.105 Optimal treatment and storage conditions are essential to prevent cellular deterioration and guarantee their viability for future scientific investigation. Pasteurization should be avoided to preserve cell components. These cells can be utilized by researchers to examine the impact of milk on newborn growth, immunological function, and disease prevention.106 Compared to the banking of umbilical cord derived stem cells, human milk derived stem cells have the potential to be collected in greater numbers and banked for future applications.107 Thus, from a clinical perspective, milk cells possess significant potential for the advancement of infant care and individualized therapy.
Generation of mammary organoids
Mammary organoids are three dimensional structures that recapitulate the complexity of the mammary gland, offering insights into lactation, mammary development and disease states including breast cancer.108 Mammary organoids are typically obtained from breast tissue. However, there is increasing interest in deriving mammary organoids from viable epithelial cells isolated from human milk, although published studies are scarce. We consider the advantages, applications and limitations of mammary organoids in greater detail below.
Mammary organoids
Since 1987, 3D culture systems have been established to generate organoids that replicate a wide range of organs. These organoids comprise diverse cell types and exhibit self-organization akin to their in vivo behavior through processes of proliferation and differentiation. Consequently, they establish structures that preserve the original organ identity in vivo.109
Advantages of mammary organoids
The use of in vitro organoid models for lactation and mammary research avoids the ethical, practical and cost issues associated with animal and human models. Furthermore, organoids generated from human cells more accurately replicate human physiology in comparison to animal models. Organoids can also be cultivated in a controlled biomimetic environment, allowing for systematic investigation of experimental and biological variables. The use of genetic tools coupled with real time imaging allows lineage tracing during organoid development. Organoid models are increasingly used for investigating diseases associated with lactation, including breast cancer, and for evaluating personalized therapeutic interventions within a system that more closely resembles individual human physiology.110
Advances in mammary organoid research
In recent years, lab-grown organoids have replicated the intricate structures of mammary organoids in a controlled environment, offering valuable perspectives on biological processes and disease mechanisms. Primary mammary organoids derived from mouse tissue have significantly contributed to our understanding of the mechanisms behind mammary branching morphogenesis, particularly regarding the roles of the extracellular matrix and stromal cells within 3D culture models.111,112 Tumor organoids have been used for drug screening113 and a biobank with over 100 primary and metastatic breast cancer has been established114 with significant potential to advance personalized medicine, enabling dosage determination tailored to the specific needs of each patient. The incorporation of accessible and numerically controlled bioprinting systems has facilitated accurate spatial organization and scalable generation of consistent human mammary organoids, addressing the constraints of arbitrary cell distribution observed in conventional 3D culture techniques.115 These advancements enhance experimental consistency and throughput while facilitating the creation of customizable organoid arrays to examine intricate cell-cell and cell-matrix interactions pertinent to mammary gland biology and diseases.116
Sources for generation of lactating mammary organoids
Human-specific organoids have been generated from breast tissue obtained from reduction mammoplasty, prophylactic mastectomy, breast biopsy, or resected cancerous tissues from donors or patients. Rosenbluth et al. successfully produced mammary organoids that exhibited the expression of both luminal and basal cell markers (CK8 and CK14, respectively).117 These organoids exhibited ductal and alveolar morphologies that closely resembled those present in the native mammary gland. Key breast tissue markers118 including estrogen receptor, progesterone receptor, and HER2neu, were preserved within the developed organoid model.
Induced pluripotent stem cells (iPSCs) are somatic cells that have undergone reprogramming, enabling them to differentiate into various cell types, encompassing all endodermal, mesodermal, and ectodermal lineages. Mammary-like organoids derived from human iPSC (hiPSC) display appropriate gene and protein expression and could be induced with lactogenic hormones to express milk protein.119 Human iPSCs can be derived from cells specific to individual patients, offering enhanced opportunities for personalized disease modeling and therapeutic approaches. This method mitigates many ethical issues typically linked to alternative sources such as tissue or embryonic stem cells.
Stem cells from human milk have self-renewal and pluripotency characteristics, which make them a promising source for generating mammary organoids. Although there have been reports of culturing human milk cells as spheroids,62 there are few published protocols showing differentiated mammary organoids derived from milk, likely due to the small population of viable stem cells in human milk. The yield of viable epithelial cells per mL of human milk varies widely with biology (lactation stage, time since expression, parity, donor health), sample features (foremilk vs hindmilk, fat content, transport/holding conditions), isolation settings, and downstream factors that together determine organoid-forming efficiency. As such, generalizable volume to yield conversions are not yet available. Nevertheless, we believe human milk has the potential to be a useful and physiologically relevant source for lactating mammary organoids in the future. Future studies, with larger sample sets and harmonized protocols, should focus on systematically reporting milk volume processed, cell yield per mL, viability, epithelial enrichment, and organoid-forming efficiency, which will ultimately allow the field to establish practical volume guidelines.
As enumerated in Table 1, there are distinct advantages and disadvantages associated with each of these sources for mammary organoid generation. Breast tissue is a source of differentiated mammary cells that readily assemble to natural tissue architectures. However, tissue collection requires invasive procedures, with significant limitations in scalability and issues of donor variability. These disadvantages are obviated by iPSC-derived organoids, but they are expensive and time consuming to generate. Milk-derived cells offer an excellent compromise as a non-invasive and readily available source of lactating cells and, as such, hold great promise for mammary organoid formation.
Generation of lactating mammary organoids
The main steps in mammary organoid generation have been summarized from published protocols114,119,120,121,122,123 and enumerated below and schematically, in Fig. 2.
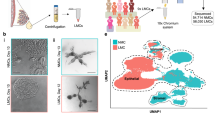
Step-by-step process for generating mammary organoids from three different sources: induced pluripotent stem cells (iPSCs), breast tissue, and milk-derived cells. Each approach follows a distinct isolation and differentiation protocol using key growth factors and signaling molecules to promote lineage specification. Cocktail 1 contains parathyroid hormone for mammary lineage differentiation; hydrocortisone, insulin, FGF10, hepatocyte growth factor for branch and alveolar formation, and hydrocortisone, insulin, FBS for lactation. Cocktail 2 contains R-spondin, neuregulin, FGF7, FGF10, EGF, noggin, A83-01, Y27632, SB202190, B27, NAC, nicotinamide, and Cocktail 3 contains R-spondin, neuregulin, FGF7, FGF10, EGF, noggin, A83-01, Y27632, SB202190, B27, NAC, nicotinamide. Milk-derived organoids do not need prolactin as they are already lactogenic. The final organoids, cultured in a supportive extracellular matrix, exhibit heterogeneous cellular composition, including luminal, basal, and adipocyte populations, mimicking the mammary gland microenvironment.
Step 1: Cell isolation and preparation
Mammary epithelial cells are isolated from breast tissue via enzymatic digestion or mechanical mincing, followed by filtration and centrifugation to obtain a clean epithelial cell population. Cells from human milk are enriched using centrifugation and carefully plated. Low cell numbers make this step challenging but manageable with optimized protocols. Reprogrammed somatic cells (iPSC) require lineage-specific differentiation into mammary epithelial cells. This involves a series of growth factors and cytokines, making the process labor-intensive and time-consuming.
Step 2: Embedding in extracellular matrix
All cell types are embedded in a basement membrane matrix rich in key extracellular matrix proteins like laminin, collage IV, entactin and heparin sulfate proteoglycan, which provide a biomimetic 3-D microenvironment. These components are able to form a gel at 37 °C, to support cell attachment and promoting cell polarization, and enhancing cell-cell interactions.
Step 3: Organoid culture and differentiation
The embedded cells are cultured in organoid-specific media supplemented with essential growth factors. For iPSCs, differentiation protocols involve multiple media changes with precise combinations of epidermal growth factor (EGF), fibroblast growth factor (FGF), and Wnt activators to induce mammary epithelial lineage commitment.
Step 4: Induction of lactation
Prolactin supplementation is a pivotal step for inducing lactation-specific functionality in the organoids. Prolactin, in combination with hydrocortisone and insulin, activates the lactation machinery by upregulating milk protein genes such as casein and alpha-lactalbumin. This step is essential for mimicking lactating mammary physiology in cells derived under non-lactogenic conditions, including mammary tissue and iPSC.
Step 5: Maturation and analysis
After 10–14 days of culture, organoids begin to exhibit alveolar-like structures and milk protein expression. Organoids derived from tissue and milk cells achieve this stage more rapidly due to their pre-differentiated state, whereas iPSC-derived organoids take significantly longer to mature.
The use of basement membrane extracts such as Cultrex® or Matrigel® ensures robust growth and differentiation, while prolactin supplementation is indispensable for functional maturation. These protocols underscore the relative ease of using tissue and milk-derived cells compared to the labor-intensive process required for iPSC differentiation, highlighting the trade-offs between source availability, time, and reproducibility.
Benefits and limitations of using human milk models
Human milk has great potential for the improvement of infant nutrition and development through a better understanding of breast biology, and the development of new therapeutic solutions. Figure 3 summarizes the advantages and disadvantages associated with human milk research. Despite many advantages, we also note the challenges and limitations in working with human milk arising from the inherent heterogeneity and variability of milk cells depending on donor, lactation stage and environmental factors. The composition of human milk can be influenced by maternal variables such as nutrition, stress, and infections. For instance, mastitis infection can modify gene expression patterns in milk cells,46 potentially impacting function. Table 2 specifically focuses on the technical aspects and experimental considerations of utilizing milk-derived cells. For example, it lists the capability for single-cell analysis, allowing researchers to distinguish between different epithelial subpopulations. However, it notes important limitations, such as variable cell viability between donors, the potential need for tailored culture conditions, and the presence of immune cells that might complicate epithelial specific studies. The accessibility of milk makes repeat and non-invasive sampling possible, though factors like donor physiology and sample handling can lead to inconsistencies. Analyzing the influence of these maternal variables on milk cells will be essential for interpreting research findings and designing treatments to improve lactation.124,125
The schematic presents the pros and cons of harnessing human milk cells for functional and mechanistic organoid studies, weighing the ease of collection and relative affordability against challenges like propensity for microbial contamination, donor and batch variability, and labor-intensive processing and separation of lactocytes from immune cells.
Conclusion and prospective future directions
Human milk offers a non-invasive and readily available window into the physiology of lactation through the culturing of mammary organoids, harvesting of milk fat globules and extracellular vesicles and isolation of bioactive components, immune and stem cells. This review demonstrates the diverse and most promising applications of human milk, from the immunotherapeutic effects of extracellular vesicles to the diagnostic use of epigenetic and genetic markers in milk. Some of these technologies, such as the manufacturing of artificial or lab-grown milk, while intriguing and potentially impactful from a sustainable and ethical viewpoint, are still in early stages of development and face significant challenges of economic scale. Still others are largely unrealized, including the harvesting of stem cells in milk for applications in regenerative medicine, such as cell replacement therapy in neurodegenerative disorders. Future collaborations between academics, clinicians and milk banks, especially in low-income countries, should be fostered for greater access to the potential applications of human milk.126 The prospective utilization of mammary organoids in conjunction with bioprinting technology presents considerable potential for advancing tissue engineering, disease modeling, and precision medicine.
We highlight safety concerns from the increasing burden of xenobiotics from environmental pollutants and medications that impact lactating persons, and the potential for viral spillover into milk in the face of new pandemic threats. Milk-derived extracellular vesicles have exhibited the capacity to influence gut microbiota, facilitate tissue healing, and convey regulatory signals to developing tissues, thereby emulating certain natural advantages of human milk. Integrating EVs into milk formula could enhance individualized newborn nutrition and help reconcile the differences between formula and human milk in promoting early-life gut and immunological health. We foresee a growing role for milk biobanks in supporting longitudinal research on the composition of human milk using state of the art proteomic, metabolomic and transcriptomic approaches.
In light of recent shifts in funding priorities in the United States which discourage projects relying solely on animal-based studies, there has been a growing emphasis on developing alternative model systems. Among these, human organoids have emerged as one of the most promising platforms, offering physiologically relevant, scalable, and ethically favorable models that bridge the gap between traditional cell culture and in vivo systems. While mammary organoid research is well established, organoids generated from human milk cells are underreported, pointing to promising opportunities for productive research in the future.
References
Hinde, K. & German, J. B. Food in an evolutionary context: insights from mother’s milk. J. Sci. Food Agric 92, 2219–2223 (2012).
Oftedal, O. T. The evolution of milk secretion and its ancient origins. Animal 6, 355–368 (2012).
Ballard, O. & Morrow, A. L. Human milk composition: nutrients and bioactive factors. Pediatr. Clin. North Am. 60, 49–74 (2013).
Gates, A., Marin, T., Leo, G. & Stansfield, B. K. Review of Preterm Human-Milk Nutrient Composition. Nutr. Clin. Pr. 36, 1163–1172 (2021).
Anadón, A., Martínez-Larrañaga, M. R., Ares, I. & Martínez, M. A. in Reproductive and Developmental Toxicology, 3rd ed. (ed R. C. Gupta) 1019–1052 (Academic Press, 2022).
Gartner, L. M. et al. Breastfeeding and the use of human milk. Pediatrics 115, 496–506 (2005).
Pérez-Escamilla, R., Buccini, G. S., Segura-Pérez, S. & Piwoz, E. Perspective: should exclusive breastfeeding still be recommended for 6 months?. Adv. Nutr. 10, 931–943 (2019).
Dontu, G. & Ince, T. A. Of mice and women: a comparative tissue biology perspective of breast stem cells and differentiation. J. Mammary Gland Biol. Neoplasia 20, 51–62 (2015).
McNally, S. & Stein, T. Overview of mammary gland development: a comparison of mouse and human. Methods Mol. Biol. 1501, 1–17 (2017).
Cacho, N. T. & Lawrence, R. M. Innate immunity and breast milk. Front. Immunol. 8, https://doi.org/10.3389/fimmu.2017.00584 (2017).
Martin, C. R., Ling, P. R. & Blackburn, G. L. Review of infant feeding: key features of breast milk and infant formula. Nutrients 8 https://doi.org/10.3390/nu8050279 (2016).
Nutrition during lactation (National Academics Press, 1991).
Bakshi, S. et al. A comprehensive review on infant formula: nutritional and functional constituents, recent trends in processing and its impact on infants’ gut microbiota. Front. Nutr. 10, https://doi.org/10.3389/fnut.2023.1194679 (2023).
Bode, L. & Donovan, S. M. Fructooligosaccharides are not the same as Fucosylated Human Milk Oligosaccharides. Adv. Nutr. 13, 972–973 (2022).
Dinleyici, M., Barbieur, J., Dinleyici, E. C. & Vandenplas, Y. Functional effects of human milk oligosaccharides (HMOs). Gut Microbes 15, 2186115 (2023).
Osborne, S. et al. In vitro transport and satiety of a beta-lactoglobulin dipeptide and beta-casomorphin-7 and its metabolites. Food Funct. 5, 2706–2718 (2014).
Enjapoori, A. K., Kukuljan, S., Dwyer, K. M. & Sharp, J. A. In vivo endogenous proteolysis yielding beta-casein derived bioactive beta-casomorphin peptides in human breast milk for infant nutrition. Nutrition 57, 259–267 (2019).
Reyes, S. M. et al. Human milk micronutrients and child growth and body composition in the first 2 years: a systematic review. Adv. Nutr. 15, 100082 (2024).
York, D. J., Smazal, A. L., Robinson, D. T. & De Plaen, I. G. Human milk growth factors and their role in NEC prevention: a narrative review. Nutrients 13, 3751 (2021).
Newburg, D. S., Woo, J. G. & Morrow, A. L. Characteristics and potential functions of human milk adiponectin. J. Pediatr. 156, S41–S46 (2010).
Garcia, C. & Innis, S. Structure of the human milk fat globule. Lipid Technol. 25, 223–226 (2013).
Heid, H. W. & Keenan, T. W. Intracellular origin and secretion of milk fat globules. Eur. J. Cell Biol. 84, 245–258 (2005).
Huston, G. E. & Patton, S. Factors related to the formation of cytoplasmic crescents on milk fat globules. J. Dairy Sci. 73, 2061–2066 (1990).
Hamosh, M. et al. Protective function of human milk: the milk fat globule. Semin. Perinatol. 23, 242–249 (1999).
Di, S. et al. Advances in the isolation and characterization of milk-derived extracellular vesicles and their functions. Front. Nutr. 11, 1512939 (2024).
Bongiovanni, L. et al. Extracellular vesicles: novel opportunities to understand and detect neoplastic diseases. Vet. Pathol. 58, https://doi.org/10.1177/0300985821999328 (2021).
Akers, J. C., Gonda, D., Kim, R., Carter, B. S. & Chen, C. C. Biogenesis of extracellular vesicles (EV): exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J. Neuro Oncol. 113, 1–11 (2013).
Yung, C. et al. Neonatal enteroids absorb extracellular vesicles from human milk-fed infant digestive fluid. J. Extracell. Vesicles 13, e12422 (2024).
Vahkal, B. et al. Human milk extracellular vesicles modulate inflammation and cell survival in intestinal and immune cells. Pediatric Res. https://doi.org/10.1038/s41390-024-03757-5 (2024).
Jiang, X. et al. Biological properties of milk-derived extracellular vesicles and their physiological functions in infant. Front. Cell Dev. Biol. 9, 693534 (2021).
Kumar, M. A. et al. Extracellular vesicles as tools and targets in therapy for diseases. Sig. Transduct Target Ther. 9, https://doi.org/10.1038/s41392-024-01735-1 (2024).
Le, N. P., Cravo, E., Burke, T., Brooks, B. & Tucker, A. Perspective on the potential vertical transmission of SARS-CoV-2 through breast milk. J. Paediatr. Child Health 61, 148–152 (2025).
Wahl, A. et al. Breast milk of HIV-positive mothers has potent and species-specific in vivo HIV-inhibitory activity. J. Virol. 89, 10868–10878 (2015).
Schafers, J. et al. Pasteurisation temperatures effectively inactivate influenza A viruses in milk. Nat. Commun. 16, 1173 (2025).
Kelvin Alyson, A., Baker Pari, H., Ghosh, S., Schultz-Cherry, S. & Langel Stephanie, N. Influenza infection of the mammary gland. J. Virol. 0, e01940–01924 (2025).
Butt, S. L., Nooruzzaman, M., Covaleda, L. M. & Diel, D. G. Hot topic: Influenza A H5N1 virus exhibits a broad host range, including dairy cows. JDS Commun. 5, S13–S19 (2024).
Song, H. et al. Receptor binding, structure, and tissue tropism of cattle-infecting H5N1 avian influenza virus hemagglutinin. Cell 188, 919–929.e919 (2025).
Nelli, R. K. et al. Exploring influenza A virus receptor distribution in the lactating mammary gland of domesticated livestock and in human breast tissue. bioRxiv, https://doi.org/10.1101/2025.04.16.649193 (2025).
Chi, Z. H., Goodyer, C. G., Hales, B. F. & Bayen, S. Characterization of different contaminants and current knowledge for defining chemical mixtures in human milk: a review. Environ. Int. 171, 107717 (2023).
Beers, J. L., Hebert, M. F. & Wang, J. Transporters and drug secretion into human breast milk. Expert Opin. Drug Metab. Toxicol. 21, 409–428 (2025).
Tran, C. D. et al. Organic contaminants in human breast milk identified by non-targeted analysis. Chemosphere 238, 124677 (2020).
Witkowska-Zimny, M. & Kaminska-El-Hassan, E. Cells of human breast milk. Cell Mol. Biol. Lett. 22, 11 (2017).
Nyquist, S. K. et al. Cellular and transcriptional diversity over the course of human lactation. Proc. Natl. Acad. Sci. USA 119, e2121720119 (2022).
Cabinian, A. et al. Transfer of maternal immune cells by breastfeeding: maternal cytotoxic T lymphocytes present in breast milk localize in the Peyer’s patches of the nursed infant. PLoS One 11, e0156762 (2016).
Zhou, L. et al. Two independent pathways of maternal cell transmission to offspring: through placenta during pregnancy and by breast-feeding after birth. Immunology 101, 570–580 (2000).
Sharp, J. A., Lefevre, C., Watt, A. & Nicholas, K. R. Analysis of human breast milk cells: gene expression profiles during pregnancy, lactation, involution, and mastitic infection. Funct. Integr. Genomics 16, 297–321 (2016).
Hassiotou, F. & Geddes, D. Anatomy of the human mammary gland: current status of knowledge. Clin. Anat. 26, 29–48 (2013).
Gaffney, E. V., Polanowski, F. P., Blackburn, S. E. & Lambiase, J. P. Origin, concentration and structural features of human mammary gland cells cultured from breast secretions. Cell Tissue Res. 172, 269–279 (1976).
Fan, Y., Chong, Y. S., Choolani, M. A., Cregan, M. D. & Chan, J. K. Unravelling the mystery of stem/progenitor cells in human breast milk. PLoS One 5, e14421 (2010).
Twigger, A. J. et al. Transcriptional changes in the mammary gland during lactation revealed by single cell sequencing of cells from human milk. Nat. Commun. 13, 562 (2022).
LeMaster, C. et al. The cellular and immunological dynamics of early and transitional human milk. Commun. Biol. 6, 539 (2023).
Goldman, A. S. The immune system of human milk: antimicrobial, antiinflammatory and immunomodulating properties. Pediatr. Infect. Dis. J. 12, 664–671 (1993).
Michie, C. A., Tantscher, E. & Rot, A. The long term effects of breastfeeding: a role for the cells in breast milk?. J. Tropical Pediatrics 44, 2–3 (1998).
Hosea Blewett, H. J. Cicalo, M. C., Holland, C. D. & Field, C. J. in Advances in Food and Nutrition Research, Vol. 54 (ed. Steve L. Taylor) 45–80 (Academic Press, 2008).
Hassiotou, F. & Geddes, D. T. Immune cell-mediated protection of the mammary gland and the infant during breastfeeding. Adv. Nutr. 6, 267–275 (2015).
Lokossou, G. A. G., Kouakanou, L., Schumacher, A. & Zenclussen, A. C. Human breast milk: from food to active immune response with disease protection in infants and mothers. Front Immunol. 13, 849012 (2022).
Bode, L. et al. It’s alive: Microbes and cells in human milk and their potential benefits to mother and infant. Adv. Nutr. 5, 571–573 (2014).
Cregan, M. D. et al. Identification of nestin-positive putative mammary stem cells in human breastmilk. Cell Tissue Res 329, 129–136 (2007).
Thomas, E., Zeps, N., Cregan, M., Hartmann, P. & Martin, T. 14-3-3sigma (sigma) regulates proliferation and differentiation of multipotent p63-positive cells isolated from human breastmilk. Cell Cycle 10, 278–284 (2011).
Hassiotou, F., Geddes, D. T., Blancafort, P., Filgueira, L. & Hartmann, P. E. Breastmilk stem cells: Recent advances and future prospects, 185–195 (Springer, 2015).
Hassiotou, F., Geddes, D. T. & Hartmann, P. E. Cells in human milk: state of the science. J. Hum. Lact 29, 171–182 (2013).
Hassiotou, F. et al. Breastmilk is a novel source of stem cells with multilineage differentiation potential. Stem Cells 30, 2164–2174 (2012).
Hassiotou, F. & Hartmann, P. E. At the dawn of a new discovery: the potential of breast milk stem cells. Adv. Nutr. 5, 770–778 (2014).
Barinaga, M. Cells exchanged during pregnancy live on. Science 296, 2169–2172 (2002).
Aydin, M. S., Yigit, E. N., Vatandaslar, E., Erdogan, E. & Ozturk, G. Transfer and Integration of Breast Milk Stem Cells to the Brain of Suckling Pups. Sci. Rep. 8, 14289 (2018).
Hanson, L. A. The mother-offspring dyad and the immune system. Acta Paediatr. 89, 252–258 (2000).
Hunt, K. M. et al. Characterization of the diversity and temporal stability of bacterial communities in human milk. PLoS One 6, e21313 (2011).
Ruiz, L. et al. Microbiota of human precolostrum and its potential role as a source of bacteria to the infant mouth. Sci. Rep. 9, 8435 (2019).
Jost, T., Lacroix, C., Braegger, C. P., Rochat, F. & Chassard, C. Vertical mother-neonate transfer of maternal gut bacteria via breastfeeding. Environ. Microbiol. 16, 2891–2904 (2014).
Rodríguez, J. M. The origin of human milk bacteria: is there a bacterial entero-mammary pathway during late pregnancy and lactation?. Adv. Nutr. 5, 779–784 (2014).
Repa, A. et al. Probiotics (Lactobacillus acidophilus and Bifidobacterium infantis) prevent NEC in VLBW infants fed breast milk but not formula [corrected]. Pediatr. Res. 77, 381–388 (2015).
Thongaram, T., Hoeflinger, J. L., Chow, J. & Miller, M. J. Human milk oligosaccharide consumption by probiotic and human-associated bifidobacteria and lactobacilli. J. Dairy Sci. 100, 7825–7833 (2017).
Gyllenberg, H. & Carlberg, G. The dominance of a specific nutritional type of Lactobacillus bifidus in breast-fed infants. Acta Pathol. Microbiol. Scand. 42, 380–384 (1958).
Martín, R. et al. Isolation of bifidobacteria from breast milk and assessment of the bifidobacterial population by PCR-denaturing gradient gel electrophoresis and quantitative real-time PCR. Appl. Environ. Microbiol. 75, 965–969 (2009).
Singh, P. et al. Unveiling the dynamics of the breast milk microbiome: impact of lactation stage and gestational age. J. Transl. Med. 21, 784 (2023).
Hou, F. et al. Safety evaluation and probiotic potency screening of Akkermansia muciniphila strains isolated from human feces and breast milk. Microbiol. Spectr. 11, e0336122 (2023).
Xi, X. et al. Assessment of microbial community structure in human colostrum and mature milk based on geographical location and delivery mode. Sci. Bull. 62, 745–747 (2017).
Kumari, M. & Kozyrskyj, A. L. Gut microbial metabolism defines host metabolism: an emerging perspective in obesity and allergic inflammation. Obes. Rev. 18, 18–31 (2017).
Stinson, L. F. & Geddes, D. T. Microbial metabolites: the next frontier in human milk. Trends Microbiol. 30, 408–410 (2022).
Natal, A. C. D. C., de Paula Menezes, R. & de Brito Röder, D. V. D. Role of maternal milk in providing a healthy intestinal microbiome for the preterm neonate. Pediatric Res. https://doi.org/10.1038/s41390-024-03751-x (2024).
Zhang, M. et al. Human breast milk: the role of its microbiota and metabolites in infant health. J. Agric. Food Chem. 72, 10665–10678 (2024).
Barathan, M., Ng, S. L., Lokanathan, Y., Ng, M. H. & Law, J. X. Milk-derived extracellular vesicles: a novel perspective on comparative therapeutics and targeted nanocarrier application. Vaccines 12, https://doi.org/10.3390/vaccines12111282 (2024).
Zhong, Y. et al. Multifunctional milk-derived small extracellular vesicles and their biomedical applications. Pharmaceutics 15, https://doi.org/10.3390/pharmaceutics15051418 (2023).
Twigger, A. J., Hodgetts, S., Filgueira, L., Hartmann, P. E. & Hassiotou, F. From breast milk to brains: the potential of stem cells in human milk. J. Hum. Lact 29, 136–139 (2013).
Witkowska-Zimny, M., Kamińska-El-Hassan, E. & Wróbel, E. Milk therapy: unexpected uses for human breast milk. Nutrients 11, 944 (2019).
Miyake, H. et al. Human breast milk exosomes attenuate intestinal damage. Pediatr. Surg. Int 36, 155–163 (2020).
Gao, R. et al. Exosomes derived from colostrum and mature human breast milk protect against experimental necrotizing enterocolitis. Pediatr. Surg. Int. 41, 218 (2025).
Pisano, C. et al. Human breast milk-derived extracellular vesicles in the protection against experimental necrotizing enterocolitis. J. Pediatr. Surg. 55, 54–58 (2020).
Lin, Y. et al. The regeneration of intestinal stem cells is driven by miR-29-induced metabolic reprogramming. Engineering 42, 39–58 (2024).
Wu, Q. et al. Insights into the unique roles of extracellular vesicles for gut health modulation: Mechanisms, challenges, and perspectives. Curr. Res. Microb. Sci. 7, 100301 (2024).
Muttiah, B. & Law, J. X. Milk-derived extracellular vesicles and gut health. npj Sci. Food 9, 12 (2025).
Zonneveld, M. I. et al. Human milk extracellular vesicles target nodes in interconnected signalling pathways that enhance oral epithelial barrier function and dampen immune responses. J. Extracell. Vesicles 10, e12071 (2021).
Aarts, J. et al. Flood control: how milk-derived extracellular vesicles can help to improve the intestinal barrier function and break the gut-joint axis in rheumatoid arthritis. Front. Immunol. 12, 703277 (2021).
Tong, L. et al. Milk-derived extracellular vesicles protect intestinal barrier integrity in the gut-liver axis. Sci. Adv. 9, eade5041 (2023).
Li, K. et al. Metabolomic and exposomic biomarkers of risk of future neurodevelopmental delay in human milk. Pediatr. Res. 93, 1710–1720 (2023).
Salas, L. A. et al. Prediagnostic breast milk DNA methylation alterations in women who develop breast cancer. Hum. Mol. Genet 29, 662–673 (2020).
Young, B. E. et al. Association of human milk antibody induction, persistence, and neutralizing capacity with SARS-CoV-2 infection vs mRNA vaccination. JAMA Pediatrics 176, 159–168 (2022).
Malfará, B. N. et al. ABCG2 c.421C>A polymorphism alters nifedipine transport to breast milk in hypertensive breastfeeding women. Reprod. Toxicol. 85, 1–5 (2019).
Palur, D. S. K., Pressley, S. R. & Atsumi, S. Microbial production of human milk oligosaccharides. Molecules 28, 1491 (2023).
Barnum, C. R. et al. Engineered plants provide a photosynthetic platform for the production of diverse human milk oligosaccharides. Nat. Food 5, 480–490 (2024).
Kwon, H. C., Jung, H. S., Kothuri, V. & Han, S. G. Current status and challenges for cell-cultured milk technology: a systematic review. J. Anim. Sci. Biotechnol. 15, 81 (2024).
Lelis, C. A., Alvares, T. D. S. & Conte Junior, C. A. Can lab-grown milk be a novel trend in the dairy industry? Crit. Rev. Food Sci. Nutr. https://doi.org/10.1080/10408398.2025.2471013 (2025).
Eisner, M. D. Milk without animals – a dairy science perspective. Int. Dairy J. 156, 105978 (2024).
Malsagova, K. et al. Biobanks-A platform for scientific and biomedical research. Diagnostics 10, 485 (2020).
Whaley, D. et al. Cryopreservation: an overview of principles and cell-specific considerations. Cell Transpl. 30, 963689721999617 (2021).
Murtagh, M. J., Demir, I., Harris, J. R. & Burton, P. R. Realizing the promise of population biobanks: a new model for translation. Hum. Genet. 130, 333–345 (2011).
Kaingade, P. et al. Breast milk cell components and its beneficial effects on neonates: need for breast milk cell banking. J. Pediatr. Neonatal Ind. Med. 6, e060115 (2017).
Srivastava, V., Huycke, T. R., Phong, K. T. & Gartner, Z. J. Organoid models for mammary gland dynamics and breast cancer. Curr. Opin. Cell Biol. 66, 51–58 (2020).
Corrò, C., Novellasdemunt, L. & Li, V. S. W. A brief history of organoids. Am. J. Physiol. Cell Physiol. 319, C151–c165 (2020).
Niazi, V. & Parseh, B. Organoid models of breast cancer in precision medicine and translational research. Mol. Biol. Rep. 52, 2 (2024).
Caruso, M., Huang, S., Mourao, L. & Scheele, C. A mammary organoid model to study branching morphogenesis. Front. Physiol. 13, 826107 (2022).
Sahu, S. et al. Spatiotemporal modulation of growth factors directs the generation of multilineage mouse embryonic stem cell-derived mammary organoids. Dev. Cell 59, 175–186.e178 (2024).
Tzeng, Y.-D. T., Hsiao, J.-H., Tseng, L.-M., Hou, M.-F. & Li, C.-J. Breast cancer organoids derived from patients: A platform for tailored drug screening. Biochem. Pharmacol. 217, 115803 (2023).
Sachs, N. et al. A living biobank of breast cancer organoids captures disease heterogeneity. Cell 172, 373–386.e310 (2018).
Reid, J. A., Mollica, P. A., Bruno, R. D. & Sachs, P. C. Consistent and reproducible cultures of large-scale 3D mammary epithelial structures using an accessible bioprinting platform. Breast Cancer Res. 20, 122 (2018).
Bruno, R. D., Reid, J. & Sachs, P. C. The revolution will be open-source: how 3D bioprinting can change 3D cell culture. Oncotarget 10, 4724–4726 (2019).
Rosenbluth, J. M. et al. Organoid cultures from normal and cancer-prone human breast tissues preserve complex epithelial lineages. Nat. Commun. 11, 1711 (2020).
Slepicka, P. F., Somasundara, A. V. H. & Dos Santos, C. O. The molecular basis of mammary gland development and epithelial differentiation. Semin Cell Dev. Biol. 114, 93–112 (2021).
Qu, Y. et al. Differentiation of human induced pluripotent stem cells to mammary-like organoids. Stem Cell Rep. 8, 205–215 (2017).
Carter, M. E. et al. A three-dimensional organoid model of primary breast cancer to investigate the effects of oncolytic virotherapy. Front. Mol. Biosci. 9, 826302 (2022).
Charifou, E., Sumbal, J., Koledova, Z., Li, H. & Chiche, A. A robust mammary organoid system to model lactation and involution-like processes. Bio Protoc. 11, e3996 (2021).
Dekkers, J. F. et al. High-resolution 3D imaging of fixed and cleared organoids. Nat. Protoc. 14, 1756–1771 (2019).
Dekkers, J. F. et al. Long-term culture, genetic manipulation and xenotransplantation of human normal and breast cancer organoids. Nat. Protoc. 16, 1936–1965 (2021).
Johnson, K. E. et al. Human milk variation is shaped by maternal genetics and impacts the infant gut microbiome. Cell Genomics 4, 100638 (2024).
Urrutia-Baca, V. H. et al. Exploring the impact of maternal factors and dietary habits on human milk oligosaccharide composition in early breastfeeding among Mexican women. Sci. Rep. 14, 14685 (2024).
Siziba, L. P. et al. Diversity and trends of human milk banking: a scoping review from 1946 to 2021. Arch. Dis. Child. Fetal Neonatal Ed. 108, 210 (2023).
Aboul-Soud, M. A. M., Alzahrani, A. J. & Mahmoud, A. Induced pluripotent stem cells (iPSCs)-roles in regenerative therapies, disease modelling and drug screening. Cells 10, https://doi.org/10.3390/cells10092319 (2021).
Baranek, M. et al. Effect of small molecules on cell reprogramming. Mol. Biosyst. 13, 277–313 (2017).
Biswas, S. K., Banerjee, S., Baker, G. W., Kuo, C. Y. & Chowdhury, I. The mammary gland: basic structure and molecular signaling during development. Int. J. Mol. Sci. 23 https://doi.org/10.3390/ijms23073883 (2022)
Pang, W. W. & Hartmann, P. E. Initiation of human lactation: secretory differentiation and secretory activation. J. Mammary Gland Biol. Neoplasia 12, 211–221 (2007).
Lee, J., Liu, Y., Ray, E., Giuliano, A. E. & Cui, X. Human breast organoid models for lactation research. Reprod. Breed. 3, 125–130 (2023).
Funding
This manuscript is the result of funding in whole or in part by the National Institutes of Health National Institute of Child Health and Development (NIH/NICHD), grant UC2 HD113036 to R.R. It is subject to the NIH Public Access Policy. Through acceptance of this federal funding, NIH has been given a right to make this manuscript publicly available in PubMed Central upon the Official Date of Publication, as defined by NIH.
Author information
Authors and Affiliations
Contributions
M.M. and R.R. were responsible for drafting the review, contributing intellectual content, generating figures and tables and final approval of the version to be published.
Corresponding author
Ethics declarations
Competing interests
R.R. receives compensation as a consultant for Formation Venture Engineering.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Majood, M., Rao, R. Human milk: insights on cell composition, organoids and emerging applications. Pediatr Res (2025). https://doi.org/10.1038/s41390-025-04458-3
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41390-025-04458-3
This article is cited by
-
Impact of lactation stage, maternal age, and parity on mammary epithelial cell populations in fresh milk
Scientific Reports (2025)