Abstract
Background
Despite the evidence supporting the use of focal therapy (FT) in patients with localized prostate cancer (PCa), considerable variability exists in the patient selection criteria across current studies. This study aims to review the most recent evidence concerning the optimal approach to patient selection for FT in PCa.
Methods
PubMed database was systematically queried for studies reporting patient selection criteria in FT for PCa before December 31, 2023. After excluding non-relevant articles and a quality assessment, data were extracted, and results were described qualitatively.
Results
There is no level I evidence regarding the best patient selection approach for FT in patients with PCa. Current international multidisciplinary consensus statements recommend multiparametric magnetic resonance imaging (mpMRI) followed by MRI-targeted and systematic biopsy for all candidates. FT may be considered in clinically localized, intermediate risk (Gleason 3 + 4 and 4 + 3), and preferably unifocal disease. Patients should have an acceptable life expectancy. Those with prostate volume >50 ml and erectile dysfunction should not be excluded from FT. Prostate-specific antigen (PSA) level of < 20 (ideally < 10) ng/mL is recommended. However, the utility of other molecular and genomic biomarkers in patient selection for FT remains unknown.
Conclusions
FT may be considered in well-selected patients with localized PCa. This review provides a comprehensive insight regarding the optimal approach for patient selection in FT.

Similar content being viewed by others
Background
Focal therapy (FT) has emerged as a viable treatment option in the management of patients with localized prostate cancer (PCa). The goal of FT is to mitigate the side effects commonly associated with more aggressive treatments without compromising cancer control [1,2,3]. Numerous FT modalities are currently available, including high intensity focused ultrasound (HIFU), cryotherapy, irreversible electroporation (IRE), laser, photodynamic therapy (PDT), and brachytherapy (BT). Several single-center and multi-institutional studies have reported the outcomes of these novel therapies [4]. Currently, the American Urological Association (AUA) guidelines consider FT in select, appropriately informed patients with intermediate-risk PCa, with an emphasis on prioritizing enrollment in clinical trials [5]. However, patients should be informed that high-quality data comparing the outcomes of FT to other PCa management options, including radiation therapy, surgery, and active surveillance, are currently lacking.
Despite the evidence supporting the use of FT in patients with localized PCa, reported studies exhibit considerable variability in terms of patient selection criteria and treatment planning approaches [4]. Precise selection of patients is a crucial step in achieving the optimal outcomes following FT. The identification of the best candidate has evolved dynamically in the past two decades alongside the increasing comprehension and constraints of FT (Fig. 1). Nevertheless, this process is still dependent on the current available evidence, expert opinions, and international multidisciplinary consensus statements [6,7,8,9,10,11,12,13]. These statements incorporate several criteria, including the type of imaging, prostate biopsy techniques, pathological features and anatomical location of the lesion(s), and a comprehensive assessment of the patient’s overall health and life expectancy. In recent years, advancements in diagnostic modalities, such as multiparametric magnetic resonance imaging (mpMRI) have reshaped the paradigm for patient selection [14]. In addition, the emergence of novel genomic markers has engendered optimism regarding the prospect of refining risk stratification for patients with PCa [15].
The objective of this study is to review the most recent evidence concerning the optimal approach to patient selection for FT in PCa.
Methods
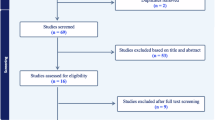
Our search was performed using PubMed database for articles published before December 31, 2023. A systematic review was conducted according to the Preferred Reporting Items for Systematic Review and Meta-analyses (PRISMA) statement to assess patient selection criteria in FT for PCa. Unrelated articles, letters, editorial comments, replies from authors, non-human and non-English language articles were excluded. The detailed search terms, filters, and exclusions are presented in Fig. 2.
After critical and quality assessments, data were extracted, and results were described qualitatively. Two investigators (A.G. and A.H.L.) were independently involved in the data assessment and extraction. The following data were extracted: first-author, year of publication, consensus methos, consensus threshold, number of expert participants, response rates, characteristics of participants, summary of recommendations for imaging and biopsy in FT, and summary of consensus statements in patient selection for FT.
Results
Several single-center and multicenter reports on FT with different sources of energy have been reported during the past two decades, with the majority being small-sample size single-arm phase I or II studies [16,17,18,19,20,21,22,23]. With increasing experience in this field, multicenter reports and larger sample size studies have been recently published [24,25,26,27,28]. The only randomized trial reported to date in the field of FT included patients with low-risk PCa who underwent focal PDT [29]. Hence, due to the lack of level I evidence, patient selection for FT is currently based on several international multidisciplinary consensus statements [6,7,8,9,10,11,12,13]. These statements define several aspects of the ideal patient selection for FT in PCa, including diagnostic modalities (imaging and biopsy) to characterize the lesion(s), as well as patient-related and disease-related factors contributing to the optimal decision making.
In this systematic review, a total of 11 articles were included in the qualitative synthesis. Characteristics of these studies are presented in Table 1. Additionally, a summary of recommendations for imaging and biopsy, along with patient selection criteria outlined in these reports, is provided in Tables 2 and 3.
Lesion characterization
The first step in assessing the eligibility of a patient who might be eligible for FT is to characterize the PCa lesion(s). FT initially relied on the cancer characteristics of transrectal ultrasound (TRUS)-guided biopsies [30]. The introduction of mpMRI has revolutionized cancer detection and consequently improved the process of patient selection for FT. The use of mpMRI in FT candidates was first presented in a consensus statement by the experts in this field in 2010 [7]. Subsequently, panels of experts re-emphasized the importance of mpMRI as the preferred approach for accomplishing the necessary objectives in FT [31, 32]. Recent consensus panels on patient selection for FT have consensually concurred that mpMRI stands as the preferred imaging modality for preoperative evaluation [7,8,9,10,11,12,13]. mpMRI has a high sensitivity in detecting clinically significant PCa. In a Cochrane meta-analysis comparing MRI to template biopsies in biopsy-naïve and repeat-biopsy settings, MRI had a pooled sensitivity and specificity of 0.91 and 0.37 for the International Society of Urological Pathology (ISUP) grade >2 cancers, respectively. For grade >3 cancers, the pooled sensitivity of MRI was 0.95, and the specificity was 0.35 [33].
MRI-TRUS fusion is the recommended technique to perform biopsies following mpMRI. The findings of PRECISION trial (PRostate Evaluation for Clinically Important Disease: Sampling Using Image-guidance Or Not?) demonstrated that an mpMRI-guided biopsy leads to higher detection of clinically significant PCa while avoiding detection of insignificant disease [34]. According to an International Delphi Consensus, in the presence of an mpMRI-suspicious lesion (i.e., Prostate Imaging Reporting & Data System: PIRADS 4 or 5), histological confirmation using MRI-TRUS-fusion biopsy is necessary prior to treatment with FT [10]. Systematic biopsy is still required in this setting to assess mpMRI-negative areas prior to treating a histologically confirmed mpMRI lesion. However, minimum standard for the extent of systematic biopsy outside of the mpMRI lesion (i.e., number of cores/approach) remains indeterminate. When mpMRI is unavailable, three-dimensional (3D) mapping biopsies are recommended [13]. Prostate-specific membrane antigen (PSMA) positron emission tomography (PET) scan, an effective imaging modality in patients with PCa, may be used more frequently in the future as experience increases with its use in the field of FT [35].
Prostate biopsy can be performed using the transrectal (TR) or transperineal (TP) approach. The TR approach has been traditionally favored due to its less invasive nature and feasibility under local anesthesia [36]. However, a significant drawback of this approach is its relatively high rate of infectious complications. In a systematic review of 165 articles, TR, compared to TP approach, was associated with a significantly higher incidence of sepsis (0.8% vs 0.1%) and hospitalization (1.1% vs. 0.9%) [37]. Recent prospective studies have demonstrated that the TP approach can safely omit antibiotics without increasing the risk of infection, while maintaining comparable detection rates of PCa to the TR approach [38,39,40,41]. Consequently, the TP approach is now preferred for patients undergoing prostate biopsy [36].
Patient features
Overall health and clinical features of the patients are important points that should be considered during appropriate candidate selection for FT. According to an the Delphi Consensus by Tay et al., life expectancy considerations are similar to those stated in major guidelines, with no upper or lower boundary beyond which FT is contraindicated [10]. According to the current guidelines, minimum estimated life expectancy of 8–10 years is required in order for treatment to result in a reduction in the risk of death [5]. Nevertheless, Tan et al. considered the age range of 60–80 when considering FT for patients who are discontinuing active surveillance [13]. Additionally, similar to other surgical procedures, those with less comorbidities are more appropriate candidates for FT compared to sicker patients.
Genitourinary symptoms are also important when considering a patient for FT. Although preservation of erectile function is an important reason for choosing FT over radical treatments, the lack of erectile function at baseline should not exclude a patient from FT [10]. In addition, the presence of mild to moderate lower urinary tract symptoms are not contraindications for FT. Men with prostate volumes of less than 50 ml are more suitable for FT compared to those with prostate volumes >50 ml. Patients with a larger prostate should not be excluded from FT but they need to be counseled with caution. FT in these patients depends on the location of index lesion, amount of tissue requiring ablation, and type of ablative energy [10]. For instance, in the case of HIFU treatment for a large prostate, ultrasound waves may dissipate over longer focal points, resulting in prostatic edema. This could potentially displace the treatment target from the firing zone, especially in lesions located in the anterior zone [42]. On the contrary, FT of posterior lesions is not affected by the prostate size.
Initial consensus statements for FT patient selection have excluded salvage cases, including those with previous treatment of the primary cancer within the prostate, recent hormone treatment for PCa, and previous radiation to the pelvis [8]. In recent years, more experience has been gained with salvage FT. The recent AUA guidelines recommend offering cryoablation and HIFU to patients with biopsy-documented PCa recurrence after primary radiation as part of a shared decision-making approach [43]. Nevertheless, data regarding salvage FT following primary PCa ablation is limited. Salvage FT should preferably be performed in experienced centers as part of a clinical trial or well-designed prospective cohort study. In addition, patients should be made aware of the potential complications and functional outcomes associated with this procedure [44,45,46].
Finally, patients should understand the lack of randomized clinical trial and long-term outcomes following FT. In addition, due to the slight risk of infield recurrence resulting from incomplete ablation or outfield recurrence caused by small, overlooked satellite lesions, or the de novo occurrence of PCa in the untreated gland, the patient must be compliant for close surveillance after treatment [47]. Patients may require re-treatments (e.g., repeat FT, radiation, or radical prostatectomy), which could lead to suboptimal outcomes when compared to primary treatments [44,45,46].
Disease features
Pathological characteristics
Gleason grade is an important factor to be considered in the evaluation of a patient with PCa for FT. In the past decade, there has been a shift from low-grade cancers toward a higher grade. In the earlier days, FT was only considered for low-risk patients, and the presence of Gleason 4 in the biopsy was among the exclusion criteria. In 2010, the consensus statement by de la Rosette et al. was first to include patients with Gleason pattern 4 PCa for FT [7]. The advancement of imaging and biopsy techniques has led to wide acceptance of these new criteria among focal therapists. Currently, major guidelines recommend active surveillance as the preferred management option for patients with low-risk (i.e., Gleason 3 + 3) PCa [5, 48]. This recommendation is based on the high-level evidence from the ProtecT trial, which demonstrated no significant differences in long-term all-cause mortality among patients with localized PCa who underwent radical prostatectomy, radiation therapy, or active monitoring [49]. FT is currently accepted for patients with intermediate-risk PCa, and those having Gleason 3 + 4 cancer representing the ideal candidates [5, 10]. Given the limited evidence supporting the use of FT in patients with Gleason grade >7, this treatment should be offered with caution and only to those in whom additional diagnostic evaluations have confirmed the absence of extraprostatic disease (Expert Opinion). In cases where a patient presents with a single core of Gleason 8, accompanied by multiple cores of Gleason 6 and 7 in surrounding areas, the single Gleason 8 core may disproportionately represent the disease burden. In such cases, whole mount pathology may reveal the predominant presence of Gleason ≤ 7 following gland extirpation. It is important to note that various consensus statements have employed different criteria for calculating PCa risk groups (e.g., D’Amico vs. National Comprehensive Cancer Network: NCCN). However, this discrepancy has minimal impact on the selection criteria for FT.
Anatomical characteristics
Patients with PCa who are candidates for FT should have a clinically localized (clinical stage ≤ T2c) disease [10, 13]. While select patients with extra-prostatic extension (T3a) may be considered for FT, those with seminal vesicle invasion (T3b) and bladder neck invasion (T3b) should be counseled with caution [24, 25]. FT in such a high-risk group of patients should only be performed by highly experienced urologists. It is worth noting that FT in patients with T3 disease may be associated with a higher failure rate. In a prospective study of 625 consecutive patients with non-metastatic clinically significant PCa undergoing focal HIFU stage T3 was a significant predictor of failure, with multivariable hazard ratio of 3.06 (95%CI 1.11–8.44; p = 0.03) [24].
The tumor volume in both imaging and needle biopsies was a limitation to FT during the period when random biopsies were used for the detection of PCa. In the initial consensus statement for FT by Eggener et al., the inclusion criteria consisted of a single lesion with a maximum size of 12 mm in the imaging, as well as maximal cancer percentage in core < 20%, maximal cancer length in each core < 7 mm, and maximal cores with cancer < 33% [6]. Nowadays, with the availability of mpMRI, evaluation of cancer size is calculated more accurately. According to recent consensus statements, visible cancer foci < 1.5 ml on mpMRI are suitable for FT. Furthermore, foci < 3 ml but localized to one hemi-gland are also be considered for FT if an appropriate ablation with a good margin (5–10 mm) can be achieved. It is important to highlight that optimizing the treatment margin in FT is crucial. An excessive treatment margin might harm critical structures, while insufficient margins could compromise the treatment outcomes. The type of energy source plays an important role when making decisions for FT of lesions of >1.5 mL. Considering the prostate volume, cancer foci occupying 20% of the prostate on mpMRI are deemed suitable for FT. Additionally, foci occupying up to 25%, yet confined to one hemi-gland, may also be considered for FT [10].
The location of PCa lesion is not a limitation for FT but may impact the choice of energy source used for ablation. Prostatic edema during HIFU may push away the target area and decrease the efficacy of treatment, particularly for anterior lesions [42]. Consideration should also be given to apical lesions. Due to the proximity of these lesions to the sphincter, thermal-based FT modalities, such as cryotherapy and HIFU can cause some degree of sphincteric dysfunction, which may result in a higher rates of urinary incontinence [42].
Patients with multi-focal lesions are not ideal candidates for FT [13]. Most of the experience on FT has specifically included unilateral and unifocal lesions. Although feasible, ablation of multifocal tumors may attenuate the advantages of FT and compromised the oncological control associated with whole-gland treatments. However, select cases with multifocal disease may be considered for FT in experienced hands.
Molecular biomarkers
Prostate-specific antigen (PSA) is a known biomarker used in patient selection for FT. Most consensus statements agree on the PSA level of < 10 ng/mL when considering a patient for FT [6, 10, 12, 13]. However, some experts suggest that a PSA level ranging between 10 and 20 ng/mL should also be considered acceptable [11]. It should be noted that decision about performing FT should not be based solely on PSA levels, particularly when those levels exhibit volatility and may be influenced by other factors, such as infections or urinary obstruction. Therefore, even those with elevated PSA levels above 20, may be considered for FT if additional diagnostic evaluations, such as PSMA PET scan, rule out the presence of extraprostatic disease (Expert Opinion). The consensus statements regarding the use of PSA velocity and density are variable. These markers were incorporated into the initial consensus statements for FT [6]. Additionally, the potential role of PSA density in this context has been recently endorsed by Marra. et al. [15]. However, Tay et al. did not reach a consensus on this matter [10]. Other PSA-related markers, such as prostate health index (PHI) and 4k score, have demonstrated a higher accuracy in identifying clinically significant disease compared to PSA alone [50, 51]. However, their role in patient selection for FT remains unknown. In a recent Delphi consensus by Marra et al. 80% of participants agreed that that evidence for molecular biomarkers in FT is absent or low. Hence, the panel did not endorse their utilization in routine clinical decision-making [15]. Nevertheless, some experts believe that these biomarkers may play a role in the process of patient selection for FT with localizing occult clinically significant PCa and quantifying the potential risk of cancer progression.
Future directions
There is increasing data on the use of genetic testing in the management of all stages of PCa; however, there have been no definitive studies on the utility of germ line or somatic genetic testing in evaluating patients for FT. The earliest data that is similar to men when considering FT concerns genetic testing and long-term outcomes in PCa and in when deciding on active surveillance.
Men who have a BRCA 1/2 or ATM mutation with newly diagnosed PCa are more likely to have aggressive disease and die from PCa [52]. In studies of genetic mutation on men considering active surveillance these same mutations are associated with upgrading and progression in men on active surveillance [53]. While not directly addressing decision making in FT, men with these germ line mutations do not appear to be ideal candidates for FT due to the high likelihood of progression and death from the disease.
In recent years, several serum, urine, and tissue-based genetic and epigenetic biomarkers have emerged as novel risk-stratification tools for PCa. Some of the tests using these biomarkers include:
-
Prolaris: It is a tissue-based quantitative reverse transcription polymerase chain reaction (RT-PCR) test that measures mRNA levels of 31 cell cycle progression and 15 housekeeping genes. This test provides an independent prediction of PCa-specific mortality and is available for use in men diagnosed with very low and low-risk PCa [54, 55].
-
OncotypeDX: It is a tissue-based quantitative RT-PCR test that measures the expression 12 cancer-specific and 5 housekeeping genes. It predicts favorable vs. adverse pathology (defined as Gleason ≥ 4 + 3 or ≥ pT3). The results are presented as genomic prostate score (GPS). For every 20-point increase in the GPS, there is a twofold elevation in the risk of adverse pathology observed at radical prostatectomy. This test is currently validated for men with low- to low-intermediate risk disease considering active surveillance [56,57,58].
-
Decipher: It is a tissue-based gene expression classifier which is designed based on the microarray expression of 22 genomic marker signatures. This broadly validated test provides several prognostic variables, including the risk of adverse pathology, percentage risk of distant metastasis in 5- and 10-years, and 15-year disease specific mortality [59,60,61,62].
Despite the promising role of these biomarkers in selecting patients for biopsy, active surveillance, and definitive therapy, which is endorsed by current guidelines [5, 48], their utility and efficacy in the field of FT has yet to be determined.
Conclusion
During the past two decades, there has been a gradual shift in FT towards targeting larger volumes and higher grades of PCa moving away from primarily addressing low-volume and low-grade tumors. Currently, FT is considered in well-selected patients with intermediate-risk PCa. Precise patient selection is a fundamental step in achieving the optimal functional and oncological outcomes in these patients. Current selection criteria are based on the international multidisciplinary consensus statements. The ongoing trials and studies investigating novel genomic and molecular biomarkers will provide a higher level of evidence in this setting and shed light on the optimal patient selection criteria for FT.
Data availability
Data supporting the findings of this study are available within the article.
References
Ashrafi AN, Tafuri A, Cacciamani GE, Park D, de Castro Abreu AL, Gill IS. Focal therapy for prostate cancer: concepts and future directions. Curr Opin Urol. 2018;28:536–43.
Ghoreifi A, Kaneko M, Peretsman S, Iwata A, Brooks J, Shakir A, et al. Patient-reported Satisfaction and Regret Following Focal Therapy for Prostate Cancer: A Prospective Multicenter Evaluation. Eur Urol Open Sci. 2023;50:10–6.
Lebastchi AH, Gill IS, Abreu AL. A Focus on Focal Therapy for Prostate Cancer. JAMA Surg. 2021;156:881–2.
Hopstaken JS, Bomers JGR, Sedelaar MJP, Valerio M, Fütterer JJ, Rovers MM. An Updated Systematic Review on Focal Therapy in Localized Prostate Cancer: What Has Changed over the Past 5 Years? Eur Urol. 2022;81:5–33.
Eastham JA, Auffenberg GB, Barocas DA, Chou R, Crispino T, Davis JW, et al. Clinically Localized Prostate Cancer: AUA/ASTRO Guideline, Part II: Principles of Active Surveillance, Principles of Surgery, and Follow-Up. J Urol. 2022;208:19–25.
Eggener SE, Scardino PT, Carroll PR, Zelefsky MJ, Sartor O, Hricak H, et al. Focal therapy for localized prostate cancer: a critical appraisal of rationale and modalities. J Urol. 2007;178:2260–7.
de la Rosette J, Ahmed H, Barentsz J, Johansen TB, Brausi M, Emberton M, et al. Focal therapy in prostate cancer-report from a consensus panel. J Endourol. 2010;24:775–80.
van den Bos W, Muller BG, Ahmed H, Bangma CH, Barret E, Crouzet S, et al. Focal therapy in prostate cancer: international multidisciplinary consensus on trial design. Eur Urol. 2014;65:1078–83.
Donaldson IA, Alonzi R, Barratt D, Barret E, Berge V, Bott S, et al. Focal therapy: patients, interventions, and outcomes–a report from a consensus meeting. Eur Urol. 2015;67:771–7.
Tay KJ, Scheltema MJ, Ahmed HU, Barret E, Coleman JA, Dominguez-Escrig J, et al. Patient selection for prostate focal therapy in the era of active surveillance: an International Delphi Consensus Project. Prostate Cancer Prost Dis. 2017;20:294–9.
van Luijtelaar A, Greenwood BM, Ahmed HU, Barqawi AB, Barret E, Bomers JGR, et al. Focal laser ablation as clinical treatment of prostate cancer: report from a Delphi consensus project. World J Urol. 2019;37:2147–53.
Borkowetz A, Blana A, Böhmer D, Cash H, Ehrmann U, Franiel T, et al. German S3 Evidence-Based Guidelines on Focal Therapy in Localized Prostate Cancer: The First Evidence-Based Guidelines on Focal Therapy. Urol Int. 2022;106:431–9.
Tan WP, Rastinehad AR, Klotz L, Carroll PR, Emberton M, Feller JF, et al. Utilization of focal therapy for patients discontinuing active surveillance of prostate cancer: Recommendations of an international Delphi consensus. Urol Oncol. 2021;39:781.e17–781.
Scheltema MJ, Tay KJ, Postema AW, de Bruin DM, Feller J, Futterer JJ, et al. Utilization of multiparametric prostate magnetic resonance imaging in clinical practice and focal therapy: report from a Delphi consensus project. World J Urol. 2017;35:695–701.
Marra G, Laguna MP, Walz J, Pavlovich CP, Bianco F, Gregg J, et al. Molecular biomarkers in the context of focal therapy for prostate cancer: recommendations of a Delphi Consensus from the Focal Therapy Society. Minerva Urol Nephrol. 2022;74:581–9.
Oishi M, Gill IS, Tafuri A, Shakir A, Cacciamani GE, Iwata T, et al. Hemigland Cryoablation of Localized Low, Intermediate and High Risk Prostate Cancer: Oncologic and Functional Outcomes at 5 Years. J Urol. 2019;202:1188–98.
Abreu AL, Peretsman S, Iwata A, Shakir A, Iwata T, Brooks J, et al. High Intensity Focused Ultrasound Hemigland Ablation for Prostate Cancer: Initial Outcomes of a United States Series. J Urol. 2020;204:741–7.
Eggener SE, Yousuf A, Watson S, Wang S, Oto A. Phase II Evaluation of Magnetic Resonance Imaging Guided Focal Laser Ablation of Prostate Cancer. J Urol. 2016;196:1670–5.
Lepor H, Llukani E, Sperling D, Fütterer JJ. Complications, Recovery, and Early Functional Outcomes and Oncologic Control Following In-bore Focal Laser Ablation of Prostate Cancer. Eur Urol. 2015;68:924–6.
Chao B, Llukani E, Lepor H. Two-year Outcomes Following Focal Laser Ablation of Localized Prostate Cancer. Eur Urol Oncol. 2018;1:129–33.
Chuang R, Kinnaird A, Kwan L, Sisk A, Barsa D, Felker E, et al. Hemigland Cryoablation of Clinically Significant Prostate Cancer: Intermediate-Term Followup via Magnetic Resonance Imaging Guided Biopsy. J Urol. 2020;204:941–9.
Wysock JS, Becher E, Gogaj R, Velazquez N, Lepor H. Early oncological control following partial gland cryo-ablation: a prospective experience specifying reflex MRI guided biopsy of the ablation zone. Prostate Cancer Prost Dis. 2021;24:114–9.
Taneja SS, Bennett J, Coleman J, Grubb R, Andriole G, Reiter RE, et al. Final Results of a Phase I/II Multicenter Trial of WST11 Vascular Targeted Photodynamic Therapy for Hemi-Ablation of the Prostate in Men with Unilateral Low Risk Prostate Cancer Performed in the United States. J Urol. 2016;196:1096–104.
Guillaumier S, Peters M, Arya M, Afzal N, Charman S, Dudderidge T, et al. A Multicentre Study of 5-year Outcomes Following Focal Therapy in Treating Clinically Significant Nonmetastatic Prostate Cancer. Eur Urol. 2018;74:422–9.
Shah TT, Peters M, Eldred-Evans D, Miah S, Yap T, Faure-Walker NA, et al. Early-Medium-Term Outcomes of Primary Focal Cryotherapy to Treat Nonmetastatic Clinically Significant Prostate Cancer from a Prospective Multicentre Registry. Eur Urol. 2019;76:98–105.
Ganzer R, Hadaschik B, Pahernik S, Koch D, Baumunk D, Kuru T, et al. Prospective Multicenter Phase II Study on Focal Therapy (Hemiablation) of the Prostate with High Intensity Focused Ultrasound. J Urol. 2018;199:983–9.
Habashy D, Reddy D, Peters M, Shah TT, van Son M, van Rossum PSN, et al. Evaluation of Outcomes Following Focal Ablative Therapy for Treatment of Localized Clinically Significant Prostate Cancer in Patients >70 Years: A Multi-institute, Multi-energy 15-Year Experience. J Urol. 2023;210:108–16.
de la Rosette J, Dominguez-Escrig J, Zhang K, Teoh J, Barret E, Ramon-Borja JC, et al. A Multicenter, Randomized, Single-blind, 2-Arm Intervention Study Evaluating the Adverse Events and Quality of Life After Irreversible Electroporation for the Ablation of Localized Low-intermediate Risk Prostate Cancer. J Urol. 2023;209:347–53.
Gill IS, Azzouzi AR, Emberton M, Coleman JA, Coeytaux E, Scherz A, et al. Randomized Trial of Partial Gland Ablation with Vascular Targeted Phototherapy versus Active Surveillance for Low Risk Prostate Cancer: Extended Followup and Analyses of Effectiveness. J Urol. 2018;200:786–93.
Gravas S, Tzortzis V, de la Riva SIM, Laguna P, de la Rosette J. Focal therapy for prostate cancer: patient selection and evaluation. Expert Rev Anticancer Ther. 2012;12:77–86.
Muller BG, Fütterer JJ, Gupta RT, Katz A, Kirkham A, Kurhanewicz J, et al. The role of magnetic resonance imaging (MRI) in focal therapy for prostate cancer: recommendations from a consensus panel. BJU Int. 2014;113:218–27.
Ahmed HU, Akin O, Coleman JA, Crane S, Emberton M, Goldenberg L, et al. Transatlantic Consensus Group on active surveillance and focal therapy for prostate cancer. BJU Int. 2012;109:1636–47.
Drost FJH, Osses DF, Nieboer D, Steyerberg EW, Bangma CH, Roobol MJ, et al. Prostate MRI, with or without MRI-targeted biopsy, and systematic biopsy for detecting prostate cancer. Cochrane Database Syst Rev. 2019;4:CD012663.
Kasivisvanathan V, Rannikko AS, Borghi M, Panebianco V, Mynderse LA, Vaarala MH, et al. MRI-Targeted or Standard Biopsy for Prostate-Cancer Diagnosis. N. Engl J Med. 2018;378:1767–77.
Manfredi C, Fernández-Pascual E, Arcaniolo D, Emberton M, Sanchez-Salas R, Artigas Guix C, et al. The Role of Prostate-specific Membrane Antigen Positron Emission Tomography/Magnetic Resonance Imaging in Primary and Recurrent Prostate Cancer: A Systematic Review of the Literature. Eur Urol Focus. 2022;8:942–57.
Zattoni F, Rajwa P, Miszczyk M, Fazekas T, Carletti F, Carrozza S, et al. Transperineal Versus Transrectal Magnetic Resonance Imaging-targeted Prostate Biopsy: A Systematic Review and Meta-analysis of Prospective Studies. Eur Urol Oncol. 2024;1;S2588-9311(24)00182-2.
Bennett HY, Roberts MJ, Doi SAR, Gardiner RA. The global burden of major infectious complications following prostate biopsy. Epidemiol Infect. 2016;144:1784–91.
Hu JC, Assel M, Allaf ME, Ehdaie B, Vickers AJ, Cohen AJ, et al. Transperineal Versus Transrectal Magnetic Resonance Imaging-targeted and Systematic Prostate Biopsy to Prevent Infectious Complications: The PREVENT Randomized Trial. Eur Urol. 2024;86:61–8.
Mian BM, Feustel PJ, Aziz A, Kaufman RP, Bernstein A, Avulova S, et al. Complications Following Transrectal and Transperineal Prostate Biopsy: Results of the ProBE-PC Randomized Clinical Trial. J Urol. 2024;211:205–13.
Mian BM, Feustel PJ, Aziz A, Kaufman RP, Bernstein A, Fisher HAG. Clinically Significant Prostate Cancer Detection Following Transrectal and Transperineal Biopsy: Results of the Prostate Biopsy Efficacy and Complications Randomized Clinical Trial. J Urol. 2024;212:21–31.
Ploussard G, Barret E, Fiard G, Lenfant L, Malavaud B, Giannarini G, et al. Transperineal Versus Transrectal Magnetic Resonance Imaging-targeted Biopsies for Prostate Cancer Diagnosis: Final Results of the Randomized PERFECT trial (CCAFU-PR1). Eur Urol Oncol. 2024 Feb 24;S2588-9311(24)00049-X.
Huber PM, Afzal N, Arya M, Boxler S, Dudderidge T, Emberton M, et al. Focal HIFU therapy for anterior compared to posterior prostate cancer lesions. World J Urol. 2021;39:1115–9.
Morgan TM, Boorjian SA, Buyyounouski MK, Chapin BF, Chen DYT, Cheng HH, et al. Salvage Therapy for Prostate Cancer: AUA/ASTRO/SUO Guideline Part III: Salvage Therapy After Radiotherapy or Focal Therapy, Pelvic Nodal Recurrence and Oligometastasis, and Future Directions. J Urol. 2024;211:526–32.
Crouzet S, Blana A, Murat FJ, Pasticier G, Brown SCW, Conti GN, et al. Salvage high-intensity focused ultrasound (HIFU) for locally recurrent prostate cancer after failed radiation therapy: Multi-institutional analysis of 418 patients. BJU Int. 2017;119:896–904.
Hostiou T, Gelet A, Chapelon JY, Rouvière O, Mège-Lechevalier F, Lafon C, et al. Salvage high-intensity focused ultrasound for locally recurrent prostate cancer after low-dose-rate brachytherapy: oncological and functional outcomes. BJU Int. 2019;124:746–57.
Li YH, Elshafei A, Agarwal G, Ruckle H, Powsang J, Jones JS. Salvage focal prostate cryoablation for locally recurrent prostate cancer after radiotherapy: initial results from the cryo on-line data registry. Prostate. 2015;75:1–7.
Lebastchi AH, George AK, Polascik TJ, Coleman J, de la Rosette J, Turkbey B, et al. Standardized Nomenclature and Surveillance Methodologies After Focal Therapy and Partial Gland Ablation for Localized Prostate Cancer: An International Multidisciplinary Consensus. Eur Urol. 2020;78:371–8.
Schaeffer EM, Srinivas S, Adra N, An Y, Barocas D, Bitting R, et al. NCCN Guidelines® Insights: Prostate Cancer, Version 1.2023. J Natl Compr Cancer Netw JNCCN. 2022;20:1288–98.
Hamdy FC, Donovan JL, Lane JA, Mason M, Metcalfe C, Holding P, et al. 10-Year Outcomes after Monitoring, Surgery, or Radiotherapy for Localized Prostate Cancer. N. Engl J Med. 2016;375:1415–24.
Bryant RJ, Sjoberg DD, Vickers AJ, Robinson MC, Kumar R, Marsden L, et al. Predicting high-grade cancer at ten-core prostate biopsy using four kallikrein markers measured in blood in the ProtecT study. J Natl Cancer Inst. 2015;107:djv095.
Kawada T, Shim SR, Quhal F, Rajwa P, Pradere B, Yanagisawa T, et al. Diagnostic Accuracy of Liquid Biomarkers for Clinically Significant Prostate Cancer Detection: A Systematic Review and Diagnostic Meta-analysis of Multiple Thresholds. Eur Urol Oncol. 2023;17:S2588-9311(23)00248-1.
Na R, Zheng SL, Han M, Yu H, Jiang D, Shah S, et al. Germline Mutations in ATM and BRCA1/2 Distinguish Risk for Lethal and Indolent Prostate Cancer and are Associated with Early Age at Death. Eur Urol. 2017;71:740–7.
Carter HB, Helfand B, Mamawala M, Wu Y, Landis P, Yu H, et al. Germline Mutations in ATM and BRCA1/2 Are Associated with Grade Reclassification in Men on Active Surveillance for Prostate Cancer. Eur Urol. 2019;75:743–9.
Cooperberg MR, Simko JP, Cowan JE, Reid JE, Djalilvand A, Bhatnagar S, et al. Validation of a cell-cycle progression gene panel to improve risk stratification in a contemporary prostatectomy cohort. J Clin Oncol J Am Soc Clin Oncol. 2013;31:1428–34.
Shore ND, Kella N, Moran B, Boczko J, Bianco FJ, Crawford ED, et al. Impact of the Cell Cycle Progression Test on Physician and Patient Treatment Selection for Localized Prostate Cancer. J Urol. 2016;195:612–8.
Klein EA, Cooperberg MR, Magi-Galluzzi C, Simko JP, Falzarano SM, Maddala T, et al. A 17-gene assay to predict prostate cancer aggressiveness in the context of Gleason grade heterogeneity, tumor multifocality, and biopsy undersampling. Eur Urol. 2014;66:550–60.
Van Den Eeden SK, Lu R, Zhang N, Quesenberry CP, Shan J, Han JS, et al. A Biopsy-based 17-gene Genomic Prostate Score as a Predictor of Metastases and Prostate Cancer Death in Surgically Treated Men with Clinically Localized Disease. Eur Urol. 2018;73:129–38.
Cullen J, Rosner IL, Brand TC, Zhang N, Tsiatis AC, Moncur J, et al. A Biopsy-based 17-gene Genomic Prostate Score Predicts Recurrence After Radical Prostatectomy and Adverse Surgical Pathology in a Racially Diverse Population of Men with Clinically Low- and Intermediate-risk Prostate Cancer. Eur Urol. 2015;68:123–31.
Kim HL, Li P, Huang HC, Deheshi S, Marti T, Knudsen B, et al. Validation of the Decipher Test for predicting adverse pathology in candidates for prostate cancer active surveillance. Prostate Cancer Prost Dis. 2019;22:399–405.
Herlemann A, Huang HC, Alam R, Tosoian JJ, Kim HL, Klein EA, et al. Decipher identifies men with otherwise clinically favorable-intermediate risk disease who may not be good candidates for active surveillance. Prostate Cancer Prostatic Dis. 2020;23:136–43.
Karnes RJ, Choeurng V, Ross AE, Schaeffer EM, Klein EA, Freedland SJ, et al. Validation of a Genomic Risk Classifier to Predict Prostate Cancer-specific Mortality in Men with Adverse Pathologic Features. Eur Urol. 2018;73:168–75.
Spratt DE, Zhang J, Santiago-Jiménez M, Dess RT, Davis JW, Den RB, et al. Development and Validation of a Novel Integrated Clinical-Genomic Risk Group Classification for Localized Prostate Cancer. J Clin Oncol J Am Soc Clin Oncol. 2018;36:581–90.
Acknowledgements
We extend our sincere gratitude to the Czyzyk family for their generous donation in support of prostate cancer research.
Funding
Open access funding provided by SCELC, Statewide California Electronic Library Consortium.
Author information
Authors and Affiliations
Contributions
Conceptualization, A.H.L.; methodology, A.H.L., and A.G.; investigation, A.G.; writing—original draft preparation, A.G., and A.H.L.; writing—review and editing, L.G., J.C.H., B.K., L.L., A.R.R., G.S., S.T., R.T-B.; visualization, A.G.; supervision, A.H.L.
Corresponding author
Ethics declarations
Competing interests
A.H.L. is a consultant for Koelis and EDAP TMS.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ghoreifi, A., Gomella, L., Hu, J.C. et al. Identifying the best candidate for focal therapy: a comprehensive review. Prostate Cancer Prostatic Dis 28, 684–692 (2025). https://doi.org/10.1038/s41391-024-00907-y
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41391-024-00907-y
This article is cited by
-
Postoperative functional complications and quality of life following robot-assisted prostatectomy and radiotherapy in localized prostate cancer: evidence from a systematic review and meta-analysis
Journal of Robotic Surgery (2025)
-
The current landscape of single-port robotic surgery in urology
Nature Reviews Urology (2025)