Abstract
High-risk biochemical recurrence (BCR) definition varies across clinical studies/practice guidelines. We evaluated metastasis-free survival (MFS) by blinded, independent, central review and safety in EMBARK (NCT02319837) patients defined as high-risk BCR per European Association of Urology (EAU) criteria. Patients post-radical prostatectomy (prostatic-specific antigen doubling time ≤9 months) or post-radiation therapy with a Gleason score > 7, were considered EAU high-risk. MFS improved with enzalutamide + leuprolide (HR 0.37, 95% CI 0.25‒0.57) and enzalutamide monotherapy (HR 0.57, 95% CI 0.39‒0.83) versus leuprolide alone. MFS and safety for enzalutamide ± leuprolide versus leuprolide alone were similar in EMBARK patients with EAU-consistent or protocol-defined high-risk BCR. Clinical trial registration number: NCT02319837.
Similar content being viewed by others
Introduction
In patients with prostate cancer and biochemical recurrence (BCR) after primary radical prostatectomy (RP) or primary radiation therapy (RT), those with unfavorable clinicopathological characteristics have an increased risk of metastases and/or mortality [1]. There is inconsistency across clinical studies and no consensus among medical societies/guidelines regarding the definition of high-risk BCR [2]. According to US guidelines, high-risk features include: short prostate-specific antigen doubling time (PSADT) < 12 months, short interval to biochemical failure (IBF) < 18 months, advanced tumor stage, Gleason scores (GS) of 8–10, International Society of Urological Pathologists (ISUP) grade of 4–5, node-positive disease, high genomic classifier (GC) risk, and positive molecular-targeted imaging [2,3,4]. The European Association of Urology (EAU) defines high-risk BCR as PSADT ≤ 12 months or pathological ISUP grade 4–5 (equivalent to a GS > 7) after RP, and IBF ≤ 18 months or biopsy ISUP grade 4‒5 (equivalent to a GS > 7) after RT [5].
Recent data show that patients with high-risk BCR can benefit from androgen receptor (AR) suppression with AR pathway inhibitors [4, 5]. The US Food and Drug Administration and European Commission approved enzalutamide with or without androgen deprivation therapy for treatment of nonmetastatic castration-sensitive prostate cancer with BCR at high risk for metastasis, based on the primary results from the global, phase 3 EMBARK trial. EMBARK showed clinically meaningful metastasis-free survival (MFS) improvements in patients with protocol-defined high-risk BCR who received enzalutamide plus leuprolide (combination) or enzalutamide monotherapy (monotherapy) versus leuprolide plus placebo (leuprolide alone), with preserved quality of life and no new safety signals [6, 7]. This post hoc analysis compares baseline characteristics, MFS, and safety across treatment groups in EMBARK patients who met the EAU high-risk BCR definition.
Methods
As described previously [6], EMBARK (NCT02319837) enrolled patients with high-risk BCR, defined as a PSADT ≤ 9 months and a screening PSA > 1 ng/mL post RP (± postoperative/salvage RT) or ≥ 2 ng/mL above the nadir post RT only, and no evidence of distant metastasis on conventional imaging. Patients were randomized (1:1:1) to receive combination (double blind), leuprolide alone (double blind), and monotherapy (open label).
The primary endpoint was MFS for combination versus leuprolide alone. A key secondary endpoint was MFS for monotherapy versus leuprolide alone. Imaging-based bone and/or soft tissue progression was assessed by blinded, independent, central review using conventional imaging.
This post hoc analysis of EMBARK excluded RT-only patients with a GS ≤ 7. Hence, the EAU high-risk subpopulation included all post-RP patients (all had a PSADT ≤ 9 months per EMBARK protocol) and all post-RT-only patients with a GS > 7 (ISUP grade 4–5). This post hoc subgroup analysis provided descriptive results.
Pfizer’s generative artificial intelligence-assisted technology, MAIA (Medical Artificial Intelligence Assistant), was used to develop an initial draft. The authors reviewed and edited the content as needed, and take full responsibility for the final communication.
Results
Of the total EMBARK population (N = 1068), 853 patients (80%) met the EAU high-risk BCR definition (post RP: all patients [PSADT < 12 months]; post RT: patients with a GS > 7); 290 received combination, 283 received monotherapy, and 280 received leuprolide alone. Baseline characteristics were generally balanced across treatment groups, with some differences in the distribution of PSADT categories and pelvic soft tissue lesions that followed the observed trends in the overall EMBARK population (Supplementary Table S1) [6].
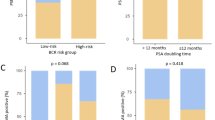
In this subpopulation, the 5-year MFS rate (95% confidence interval [CI]) was 88.8% (84.1–92.1) for combination, 81.3% (75.8–85.6) for monotherapy, and 72.9% (66.6–78.3) for leuprolide alone. MFS was improved with both combination (hazard ratio [HR] 0.37, 95% CI 0.25–0.57; P < 0.0001) and monotherapy (HR 0.57, 95% CI 0.39–0.83; P = 0.003) versus leuprolide alone. Median MFS was not reached in any treatment group. Improved MFS outcomes with combination (Fig. 1A) and monotherapy (Fig. 1B) versus leuprolide alone in the EAU-defined high-risk BCR subpopulation were similar to the primary results in the protocol-defined EMBARK population [6].
A Enzalutamide combination versus leuprolide alone in the EAU-defined subpopulationa and B Enzalutamide combination versus leuprolide alone in the protocol-defined EMBARK population; C Enzalutamide monotherapy versus leuprolide alone in the EAU-defined subpopulationa; and D Enzalutamide monotherapy versus leuprolide alone in the protocol-defined EMBARK population. Data cutoff date: January 31, 2023. P-value was based on a stratified log-rank test and hazard ratio (with associated 95% CIs) was calculated using a stratified Cox regression model, with Kaplan–Meier method to summarize time-to-event endpoints. Stratification factors included PSA level at screening (≤ 10 ng/mL vs > 10 ng/mL), PSADT (≤ 3 mo vs > 3 mo to ≤ 9 mo), and prior hormonal therapy (yes or no) as reported in the interactive web-response system. P-values for post hoc MFS comparisons in the EAU high-risk subpopulation are nominal without adjustment for multiplicity. In the primary analysis of EMBARK, MFS (primary endpoint) improvements with enzalutamide combination versus leuprolide alone were consistent across multiple prespecified baseline characteristic subgroups, including the short-PSADT subgroups (≤ 3 mo and > 3 to ≤ 6 mo) [6]. Thus, the results in the EAU-consistent subpopulation were not adjusted for the observed imbalances in baseline characteristics, i.e., PSADT categories and pelvic soft tissue lesions, and were presented descriptively. aThe EAU high-risk BCR subpopulation excluded EMBARK patients with prior RT only and a GS ≤ 7. Per EMBARK protocol, all patients with prior RP had a PSADT ≤ 9 mo, regardless of their GS, and therefore met EAU criteria for post-RP high-risk BCR; patients with prior RT only and a GS > 7 (equivalent to ISUP grade 4‒5) were also considered high-risk, consistent with the EAU definition of post-RT high-risk BCR. bFrom The New England Journal of Medicine, Freedland SJ, de Almeida Luz M, De Giorgi U, Gleave M, Gotto GT, Pieczonka CM, Haas GP, Kim CS, Ramirez-Backhaus M, Rannikko A, Tarazi J, Sridharan S, Sugg J, Tang Y, Tutrone RF Jr, Venugopal B, Villers A, Woo HH, Zohren F, Shore ND, Improved Outcomes with Enzalutamide in Biochemically Recurrent Prostate Cancer, 389 (16), 1453–65. Copyright © (2023) Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society. BCR biochemical recurrence, CI confidence interval, combination enzalutamide plus leuprolide, EAU European Association of Urology, GS Gleason score, ISUP International Society of Urological Pathologists, leuprolide alone leuprolide plus placebo, MFS metastasis-free survival, mo month, monotherapy enzalutamide monotherapy, no number, NR not reached, PSA prostate-specific antigen, PSADT PSA doubling time, RP radical prostatectomy, RT radiation therapy.
The safety profiles for all treatment groups in the EAU-defined high-risk BCR subpopulation (Supplementary Table S2) were generally consistent with the overall EMBARK population [6]. Most patients in each treatment group (97.2–97.8%) experienced treatment-emergent adverse events (AEs) during the on-treatment period, with similar trends for grade ≥ 3 AEs (41.2–47.8%), serious AEs, (29.6–35.3%), and treatment-related AEs (80.1–88.0%).
Discussion
This post hoc analysis demonstrated that in the patient subpopulation who met EAU-defined high-risk BCR, both combination and monotherapy improved MFS versus leuprolide alone, consistent with the primary results of EMBARK [6]. However, this EMBARK subpopulation does not represent all patients who meet EAU criteria [5], as patients with post-RP PSADT 9–12 months or > 12 months but a GS > 7 (ISUP grade 4‒5) were not enrolled, and post-RT patients with a GS ≤ 7 but IBF ≤ 18 months were not included due to unavailable IBF data. Nevertheless, the similar results in the EAU-defined and protocol-defined EMBARK high-risk BCR populations indicate that the observed MFS benefits in EMBARK are likely applicable to the broader EAU high-risk BCR population.
Patients with PSA values below the EMBARK protocol-defined threshold, who would otherwise meet the high-risk BCR definition, were not included although they may have been at increased risk of disease progression and mortality [8, 9]. Other BCR-related prognostic factors, including tumor stage, surgical margin, GC score, and findings on molecular-targeted imaging [1,2,3], are also not incorporated in the EAU guidelines or this post hoc analysis. Notably, detection of metastatic disease at baseline and radiographic progression during the EMBARK study were based on conventional imaging rather than molecular-targeted imaging, which was not validated in the BCR setting at trial initiation. Similar to the overall EMBARK population [6], the EAU-consistent subpopulation underrepresented Black patients (< 5%). Given the differences between Black and non-Black patients in terms of social determinants of health and genomic variations that may both correlate with disease outcomes and treatment response, more studies are urgently needed in Black patients [10, 11].
In conclusion, our findings suggest that the EAU criteria identify patients who would benefit clinically from enzalutamide ± leuprolide. Nevertheless, shared decision-making remains crucial for risk stratification and treatment individualization in this heterogenous patient population.
Data availability
Upon request and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.
References
Van den Broeck T, van den Bergh RCN, Arfi N, Gross T, Moris L, Briers E, et al. Prognostic Value of Biochemical Recurrence Following Treatment with Curative Intent for Prostate Cancer: A Systematic Review. Eur Urol. 2019;75:967–87.
Efstathiou JA, Morgans AK, Bland CS, Shore ND. Novel Hormone Therapy and Coordination of Care in High-risk Biochemically Recurrent Prostate Cancer. Cancer Treat Rev. 2024;122:102630.
Morgan TM, Boorjian SA, Buyyounouski MK, Chapin BF, Chen DYT, Cheng HH, et al. Salvage Therapy for Prostate Cancer: AUA/ASTRO/SUO Guideline Part I: Introduction and Treatment Decision-Making at the Time of Suspected Biochemical Recurrence after Radical Prostatectomy. J Urol. 2024;211:509–17.
Morgan TM, Boorjian SA, Buyyounouski MK, Chapin BF, Chen DYT, Cheng HH, et al. Salvage Therapy for Prostate Cancer: AUA/ASTRO/SUO Guideline Part II: Treatment Delivery for Non-metastatic Biochemical Recurrence After Primary Radical Prostatectomy. J Urol. 2024;211:518–25.
Cornford P, Tilki D, van den Bergh RCN, Briers E, Patient Advocate (European Prostate Cancer Coalition/Europa UOMO), Eberli D, et al. EAU - EANM - ESTRO - ESUR - ISUP - SIOG Guidelines on Prostate Cancer. 2024. https://d56bochluxqnz.cloudfront.net/documents/full-guideline/EAU-EANM-ESTRO-ESUR-ISUP-SIOG-Guidelines-on-Prostate-Cancer-2024_2024-04-09-132035_ypmy.pdf.
Freedland SJ, de Almeida Luz M, De Giorgi U, Gleave M, Gotto GT, Pieczonka CM, et al. Improved Outcomes with Enzalutamide in Biochemically Recurrent Prostate Cancer. N Engl J Med. 2023;389:1453–65.
Freedland SJ, Gleave M, De Giorgi U, Rannikko A, Pieczonka CM, Tutrone RF, et al. Enzalutamide and Quality of Life in Biochemically Recurrent Prostate Cancer. NEJM Evid. 2023;2:EVIDoa2300251.
Tilki D, Preisser F, Graefen M, Huland H, Pompe RS. External Validation of the European Association of Urology Biochemical Recurrence Risk Groups to Predict Metastasis and Mortality After Radical Prostatectomy in a European Cohort. Eur Urol. 2019;75:896–900.
Stish BJ, Pisansky TM, Harmsen WS, Davis BJ, Tzou KS, Choo R, et al. Improved Metastasis-Free and Survival Outcomes With Early Salvage Radiotherapy in Men With Detectable Prostate-Specific Antigen After Prostatectomy for Prostate Cancer. J Clin Oncol. 2016;34:3864–71.
Gong J, Kim DM, De Hoedt AM, Bhowmick N, Figlin R, Kim HL, et al. Disparities With Systemic Therapies for Black Men Having Advanced Prostate Cancer: Where Do We Stand? J Clin Oncol. 2024;42:228–36.
Gong J, Kim DM, Freeman MR, Kim H, Ellis L, Smith B, et al. Genetic and biological drivers of prostate cancer disparities in Black men. Nat Rev Urol. 2024;21:274–89.
Acknowledgements
The authors thank the patients, their families, all other investigators, and all investigational site members involved in this study. Medical writing and editorial support were provided by Roham Sadeghimakki, MD, PhD, Valerie Moss, PhD, CMPP™, and Rosie Henderson, MSc, all of Onyx (a division of Prime, London, UK), funded by the sponsors. The authors were involved in the collection and interpretation of the information provided in the manuscript and ultimate responsibility for opinions and conclusions lies with the authors.
Funding
This study was sponsored by Pfizer Inc. and Astellas Pharma Inc., the co-developers of enzalutamide.
Author information
Authors and Affiliations
Contributions
Design and conduct of the study: UDG, SJF, MR-B, GPH, NDS. Collection, management, analysis, and interpretation of the data: UDG, SJF, MR-B, JT, YT, GPH, MR, NDS. Preparation of the manuscript: All authors. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: YT, MR. Supervision: SJF, NDS. Other: None.
Corresponding author
Ethics declarations
Competing interests
UDG reports consulting for Amgen, Astellas Pharma Inc., AstraZeneca, Bayer, Bristol Myers Squibb, Esai, Ipsen, Johnson & Johnson Innovative Medicine (formerly Janssen), Merck KGaA, Merck Sharp & Dohme, Novartis, Pfizer Inc., and Roche; receiving travel expenses from AstraZeneca, Ipsen, Pfizer Inc., Roche, and Sanofi; and having other financial or non-financial interests in AstraZeneca, Roche, and Sanofi. SJF reports consulting for Astellas Pharma Inc., AstraZeneca, Bayer, Eli Lilly, Exact Sciences, Johnson & Johnson Innovative Medicine (formerly Janssen), Merck, Novartis, Pfizer Inc., Sanofi, and Sumitomo Pharma America, Inc. (formerly Myovant Sciences). AR reports being an advisory board member for Bayer, Johnson & Johnson Innovative Medicine (formerly Janssen), and Orion Pharma; holding a leadership role in the Orion Research Foundation (board chair); and being a member of the board for the Ida Montin Research Foundation. MR-B reports consulting and receiving honoraria from Astellas Pharma Inc., Bayer, and Johnson & Johnson Innovative Medicine (formerly Janssen); receiving payment for expert testimony from Johnson & Johnson Innovative Medicine (formerly Janssen); and receiving travel, accommodation, and expenses from Astellas Pharma Inc. and Johnson & Johnson Innovative Medicine (formerly Janssen). AV reports consulting for Astellas Pharma Inc., AstraZeneca, Ipsen, and Johnson & Johnson Innovative Medicine (formerly Janssen). JT reports being a former employee of and shareholder in Pfizer Inc. YT reports being an employee of and shareholder in Pfizer Inc. GPH reports being an employee of Astellas Pharma Inc.; and stock ownership in Sumitomo Pharma America, Inc. (formerly Myovant Sciences). MR reports being an employee of and shareholder in Astellas Pharma Inc. NDS reports consulting for or receiving research funding from Accord, Alessa Therapeutics, Amgen, Antev, Arquer, Asieris, Astellas Pharma Inc., AstraZeneca, Aura, Bayer, Bristol Myers Squibb, Boston Scientific, CG Oncology (formerly Cold Genesys), Clarity, Clovis Oncology, Dendreon, Eli Lilly, Exact Imaging, Ferring, Fize Medical, Foundation Medicine, GenesisCare US, Guardant Health, Immunitybio, Incyte, InVitae, Johnson & Johnson Innovative Medicine (formerly Janssen), Lantheus, MDxHealth, Merck, Minomic, Myriad, NGM, Nonagen, Novartis, Pfizer Inc., Photocure, PlatformQ, Point Biopharma, Promaxo, Propella, Protara, Sanofi, Specialty Networks, Steba, Sumitomo, Telix, Theralase, Tolmar, Urogen, and Zenflow.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
De Giorgi, U., Freedland, S.J., Rannikko, A. et al. Enzalutamide in patients with high-risk biochemically recurrent prostate cancer according to the European Association of Urology definition: a post hoc analysis of EMBARK. Prostate Cancer Prostatic Dis (2025). https://doi.org/10.1038/s41391-025-00959-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41391-025-00959-8