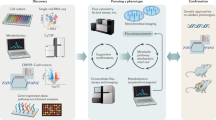
Abstract
Metabolism, including glycolysis, oxidative phosphorylation, fatty acid oxidation, and other metabolic pathways, impacts the phenotypes and functions of immune cells. The metabolic regulation of the immune system is important in the pathogenesis and progression of numerous diseases, such as cancers, autoimmune diseases and metabolic diseases. The concept of immunometabolism was introduced over a decade ago to elucidate the intricate interplay between metabolism and immunity. The definition of immunometabolism has expanded from chronic low-grade inflammation in metabolic diseases to metabolic reprogramming of immune cells in various diseases. With immunometabolism being proposed and developed, the metabolic regulation of the immune system can be gradually summarized and becomes more and more clearer. In the context of many diseases including cancer, autoimmune diseases, metabolic diseases, and many other disease, metabolic reprogramming occurs in immune cells inducing proinflammatory or anti-inflammatory effects. The phenotypic and functional changes of immune cells caused by metabolic regulation further affect and development of diseases. Based on experimental results, targeting cellular metabolism of immune cells becomes a promising therapy. In this review, we focus on immune cells to introduce their metabolic pathways and metabolic reprogramming, and summarize how these metabolic pathways affect immune effects in the context of diseases. We thoroughly explore targets and treatments based on immunometabolism in existing studies. The challenges of translating experimental results into clinical applications in the field of immunometabolism are also summarized. We believe that a better understanding of immune regulation in health and diseases will improve the management of most diseases.
Similar content being viewed by others
Introduction
The immune system removes pathogens and maintains balance in the body, with metabolism playing a crucial role in supporting immune functions.1 The interactions between metabolism and immunity have attracted many researchers. The relationship between metabolism and immunity have been considered bidirectional because of the prominent inflammatory responses in metabolic diseases and the variable metabolic pathways of immune cells.2 However, studies on the crosstalk between metabolic regulation and immune system is inseparable from the regulation of immune functions. This can be observed through changes in the phenotypes of immune cells, alterations in metabolic pathways and metabolic levels of immune cells, and changed cytokine levels. Therefore, we believe that the bidirectional relationship is essentially metabolic regulation of the immune system.
More than a decade ago, the concept of immunometabolism was proposed to summarize the interaction between metabolism and immunity (Fig. 1).3 In the beginning, researchers noted only inflammatory responses in metabolic disorders, including obesity, insulin resistance, and type 2 diabetes mellitus (T2DM), to define immunometabolism.3,4,5,6 After summarizing the differences of metabolic pathways in activated and quiescent immune cells, the definition of immunometabolism has been greatly expanded.7,8 Soon afterward, metabolic pathways of T cells have been discussed as a promising entry point for cancer immunotherapy.9 Then, benefiting from accessibility of measuring cellular metabolism of immune cells, immunometabolism has been introduced in studies on many other diseases, becoming an emerging and booming field. Metabolic regulation of the immune system becomes more and more clearer after the concept of immunometabolism being proposed.
Timeline for metabolic regulation of the immune system. Events mainly involving new findings or important reviews on metabolic pathways are in green boxes. Events mainly focusing on macrophages and T cells are are in bule and purple boxes. Light green boxes show events involving important molecules in immunometabolism. A light red box shows a special event that the concept of immunometabolism has been introduced and discussed in metabolic diseases in 2011. Before 2011, the studies on Warburg effect in cancer and metabolic characteristics of macrophages both contributes to the development of the immunometabolism field. In the last more than twenty years, the concept immunometabolism has been generally accepted and studied. Abbreviation: OXPHOS oxidative phosphorylation, mTOR mechanistic target of rapamycin, HIF-1α hypoxia-inducible factor 1α, PI3K phosphatidyl-inositol 3 kinase
Here, we are going to summarize metabolic regulation of the immune system in health and diseases by focusing on cellular metabolism and metabolic pathways in immune cells. First, metabolic pathways of immune cells, primarily including glycometabolism and lipid metabolism pathways, should be elaborated according to the phenotypes of immune cells. Second, we will discuss the changes in metabolic pathways of immune cells in different diseases. Although metabolic patterns of immune cells with proinflammatory or anti-inflammatory phenotypes can be highly generalized, studies in the context of different diseases can provide validation and identify specific characteristics or targets related to those diseases. Finally, our summary of studies on immunometabolism aims to further summarize existing and potential therapies targeting immunometabolism, ultimately improving the management of diseases in clinical practice.
Metabolic pathways and reprogramming in immune cells
The metabolism in immune cells is characterized by intricate links between metabolic reprogramming and immune cell activation.10 Metabolism contributes to immune cell activation and immune function, with immune cells adopting specific metabolic programs.11 Metabolic patterns of immune cells can be changed according to their different phenotypes, a process known as metabolic reprogramming.12 We are going to summarize the key metabolic pathways and metabolic reprogramming in immune cells (Table 1, Fig. 2). For the close association with energy production, the reprogramming of glycometabolism and lipid metabolism has gained much attention in immunometabolism. Amino acid signals have also shown their important roles in regulating immune responses.
Metabolic pathways in immune cells. The most reported metabolic pathways in immune cells are glycolysis, especially aerobic glycolysis, fatty acid oxidation (FAO), fatty acid synthesis (FAS), and oxidative phosphorylation (OXPHOS). Aerobic glycolysis and FAS are always active in immune cells with proinflammatory phenotypes including M1 macrophages and effector T cells, while FAO and OXPHOS are always active in immune cells with anti-inflammatory phenotypes including M2 macrophages and regulatory T (Treg) cells. Pentose phosphate pathway (PPP) is a branch of glycometabolism but studies on immune cells are not enough. Glutamine is the most important amino acid in immunometabolism, of which the metabolism is associated with other pathways by α-ketoglutarate
Metabolic pathways and reprogramming in macrophages
Macrophages are a heterogeneous population of immune cells with different functions. Diverse macrophages have been simply categorized into two polarizations: M1 and M2 subsets, simplifying the complexity in related studies.13 M1 macrophages are proinflammatory and are activated through a classical activation by interferon-γ (IFN-γ), interleukin (IL)-1, and LPS.14 M2 macrophages are anti-inflammatory and are activated through an alternative pathway by IL-4 and IL-1.14 M2 macrophages can be further subdivided into M2a, M2b, M2c, and M2d macrophages according to the different stimuli.15,16,17,18 How to polarize macrophages through adding certain stimuli in vitro has been described.19 In specific diseases, certain macrophage phenotypes have been identified.20 The metabolic reprogramming that occurs during the phenotypic and functional changes of macrophages is a highly concerned and somewhat controversial topic in immunometabolism.
Glycometabolism in macrophages
Proinflammatory M1 macrophages have higher glucose consumption and lactate release than M2 macrophages, mainly relying on aerobic glycolysis, while anti-inflammatory M2 macrophages mainly rely on oxidative phosphorylation (OXPHOS).14,21 With the immunometabolism rapidly developing, the mechanisms of macrophage activation and phenotypic changes are becoming clearer. However, contradictory results are also emerging. Directly relating glycolysis to proinflammatory effects or oxidative metabolism to anti-inflammatory effects is not able to summarize the full picture of immunometabolism.22
Aerobic glycolysis, also known as the Warburg effect, is a process in which pyruvate is converted into lactate instead of being oxidized in the mitochondria to produce 2 molecules of ATP under aerobic conditions.23 The Warburg effect was first reported in cancer by Warburg et al. one hundred years ago and has since received increasing attention in the field of cancer metabolism.24,25,26,27 Then, the Warburg effect has gradually been shown to play roles in other diseases.28,29 Nearly a decade ago, the Warburg effect has been linked to immunometabolism in discussions on how aerobic glycolysis conducts post-transcriptional control in T cell function.30,31 After observing significantly increased glycolysis in multiple activated immune cells under aerobic or anaerobic conditions, aerobic glycolysis has been recognized as an important metabolic pathway in the field of immunometabolism.32,33
In M1 macrophages, glycolysis is a crucial metabolic event with many factors involved.21 Downregulation of glycolysis in macrophages is always accompanied by reduced secretion of inflammatory cytokines.34 Hypoxia-inducible factor (HIF) 1α is a well-studied molecule that induces glycolysis and M1 polarization. The overexpression of HIF-1α can upregulate glycolysis and pentose phosphate pathway (PPP) of macrophages.35 Accumulated succinate and citrate from the altered tricarboxylic acid (TCA) cycle, mechanistic target of rapamycin (mTOR) complex 1 (mTORC1), and pyruvate kinase muscle isozyme M2 (PKM2) have been identified as upstream regulators of HIF-1α during glycolysis regulation and macrophage polarization.36,37,38,39,40,41 However, binding of succinate to activate succinate receptor on macrophages can sometimes promote immunosuppressive macrophage polarization through the HIF-1α signaling pathway.42 Citrate can increase proinflammatory factors and is accumulated at high concentrations outside M1 macrophages but not M2 macrophages.43,44 Although mTORC1 has been considered a key regulator of glycolysis, its role in regulating macrophage functions remains unclear.45 After knocking down mTORC1 in macrophages, an unexpected enhancement in the function of M1 macrophages has been observed, along with impaired glycolysis.45 Enhanced PKM2-dependent glycolysis has been observed simultaneously with upregulated M1 polarization.46,47 The upregulated PPP of M1 macrophages has received less attention compared to glycolysis, although some previous reviews have discussed the PPP of macrophages.48,49 Upregulation of the PPP in macrophages can also enhance inflammatory responses and contribute to the development of inflammatory diseases.50,51
Glycometabolism in M2 macrophages is partly controversial, due to the role of glycolysis.21 As mentioned before, it has been widely accepted that OXPHOS is associated anti-inflammatory macrophages, while glycolysis is associated with proinflammatory macrophages. However, the metabolic pathways that can distinguish macrophages with different phenotypes are likely to be OXPHOS instead of glycolysis.52 For M2 polarization, OXPHOS, but not glycolysis, is necessary.52 Some researchers have reported that inhibiting glycolysis in unpolarized macrophages cannot significantly affect M2 differentiation.53 In addition, inhibiting glycolysis in M2 macrophages can prevent M1 polarization.54 Thus, the question of whether glycolysis is active or inactive in M2 macrophages remains unclear.
Lipid metabolism in macrophages
In addition to OXPHOS, active fatty acid oxidation (FAO) is another metabolic characteristic of M2 macrophages.55 Fatty acids serve as precursors for producing inflammatory mediators, so catabolism including FAO is considered anti-inflammation by some researchers.56 Peroxisome proliferator-activated receptor (PPAR) is an important promotion factor for FAO of macrophages, downstream of receptor-interacting protein kinase 3 (RIPK3).57 Activation of PPAR induces M2 polarization.57 However, some researchers have found that inhibiting FAO does not disrupt M2 polarization, indicating the complex roles of FAO in regulating macrophage functions.58 FAO can also induce inflammasome activation in M1 macrophages, further indicating that associating FAO with anti-inflammation cannot summarize the entire story.22,59
Amino acid metabolism in macrophages
Amino acid metabolism in macrophages needs to be studied. Glutamine catabolism of macrophages can induce M2 polarization, and glutamine is essential for M2 polarization.60,61,62,63 Through glutaminolysis, α-ketoglutarate can be produced, inducing FAO and epigenetic reprogramming to promote M2 macrophage polarization.64 In environments with low levels of glutamine, macrophages can upregulate glutamine synthetase activity to secrete glutamine, but how the glutamine synthesis capability affects the functions of macrophages is still unclear.65 Serine synthesis in M1 macrophages supports IL-1β production through phosphoglycerate dehydrogenase signaling, and also activates NACHT, LRR, and PYD domains-containing protein 3 (NLRP3) inflammasome.66 Serine metabolism has been also reported to regulate macrophage polarization. Inhibiting serine synthesis can induce M1 polarization and reduce M2 polarization by activating the JAK/STAT signaling pathway.67 However, when serine synthesis induces M1 polarization, the inflammasome is not activated by serine metabolism.68 Upregulating serine synthesis can induce M2 polarization.69
Lipid synthesis might be the other side of lipid catabolism, which is positively associated with inflammation. Inhibiting lipid synthesis and enhancing lipid catabolism can synergistically promote M2 polarization and alleviate inflammation.70 Inhibiting lipid synthesis of macrophages can reduce the levels of proinflammatory cytokines.71 Lipid synthesis also impacts phagocytosis of macrophages.72 The sterol responsive element binding protein (SREBP)-1a-dependent lipid synthesis regulated by mTORC1 is essential for phagocytosis in macrophages.72
Metabolic pathways and reprogramming in T cells
The metabolic activity of T cells depends on their phenotypes and subtypes. Naive T cells remain quiescent with limited requirements for inducing metabolic pathways.73 As naive T cells differentiate into effector T cells, there is an increased requirement for biomass and the rapid proliferation, leading to active metabolism.74 The metabolic changes in T cells to meet the bioenergetic demand for rapid proliferation are also defined as metabolic reprogramming.75 As an immunosuppressive subset, regulatory T (Treg) cells exhibit distinct metabolic features compared to naive T cells and effector T cells.76 The metabolic features of memory T cells differ from those of other T cells.73 We will attempt to summarize the metabolic features of T cells through different metabolic pathways.
Glycometabolism in T cells
Naive T cells are regarded as a quiescent cell population with low metabolic demands.77 For only requiring energy for survival and migration, naive T cells utilize OXPHOS as the main metabolic pathway to metabolize glucose.78,79 Through OXPHOS in the mitochondria, naive T cells metabolize pyruvate to access an optimal yield of adenosine triphosphate (ATP) per glucose molecule.74
During and after T cell activation, the glucose uptake significantly increases and aerobic glycolysis occurs in T cells.74 To increase the glucose uptake, the expression of glucose transporter (GLUT) proteins is upregulated in T cells.80 Overexpression of GLUT3 can enhance glucose uptake in CD8+ T cells.81 GLUT1 can be upregulated by the phosphatidylinositol 3-kinase (PI3K)/ protein kinase B (Akt) pathway, which can be activated by CD28 co-stimulation with T-cell receptor (TCR) signals, and then participates in activating CD4+ T cells.80,82,83 The reason effector T cells significantly upregulate aerobic glycolysis might be to provide glycolytic precursors for biosynthetic reactions.73 An important factor for aerobic glycolysis, HIF-1α, is highly expressed in effector T cells under aerobic conditions.80 HIF-1α can enhance aerobic glycolysis by inducing the transcription of the enzymes pyruvate dehydrogenase kinase 1 (PDK1) and lactate dehydrogenase A (LDHA). Although OXPHOS is also increased during T cell activation, the extent is lower compared to aerobic glycolysis.80
Memory T cells and Treg cells also exhibit distinct metabolic characteristics. Memory T cells and Treg cells mainly rely on OXPHOS for more efficient energy sources like naive T cells.76 The metabolic pathways regulating glycolytic rate in memory T cells have been more fully studied than naive T cells. Memory T cells with high metabolic fitness are prepared to engage in active metabolism on demand, aiming for a rapid immune response.73 In the rapid recall response of CD8+ memory T cells, glycolysis plays a promoting role with intracellular glycogen serving as the major carbon source instead of extracellular glucose.84 After antigenic stimulation, TCR signaling phosphorylates glycogen phosphorylase together with LCK and ZAP70, then inducing glycogenolysis and increasing glucose-6-phosphate (G6P). Glycolysis is the main downstream metabolic pathway for G6P, and PPP also metabolizes G6P. Thus, memory CD8+ T cells rely specifically on glycogen for activation. However, studies on metabolic characteristics of memory CD4+ T cells are still lacking. The role of glucose metabolism in Treg cells is a controversial issue, with significantly varying results across different studies.84 In conjunction with the interaction between Treg cells and other T cell subsets, the metabolic network in Treg cells becomes increasingly complex. Treg cells and IL-17-producing T helper (Th17) cells cells are closely connected in existing studies and mutually antagonistic both in differentiation and function.85 Th17 cells have proinflammatory effects, while Treg cells have immunosuppressive functions. As a subset of effector CD4+ T cells, Th17 cells exhibit active glycolysis and utilize the pentose phosphate pathway. Treg cells depend more on FAO and OXPHOS for energy.86 Decreasing glucose levels results in reduced differentiation of Th17 cells but enhances the differentiation of Treg cells, thereby enhancing their immunosuppressive activity.85,87
Lipid metabolism in T cells
In addition to metabolizing glucose-derived pyruvate through OXPHOS, naive T cells carry out FAO as the default metabolic program, which is also an efficient way to generate energy.74,88
Increased anabolism is a characteristic of the metabolic reprogramming during and after activation of T cells.74 FAS is induced as a key cellular lipid biosynthetic pathway for T cell activation, accompanied by the downregulation of FAO. mTOR is a serine/threonine kinase regulating cellular metabolism, including T cell metabolism.89 T cells depend on mTOR to meet the demands for nutrient uptake during activation and differentiation.90 The upstream molecules of mTOR in immune signal pathways are numerous. TCR, co-stimulatory receptors, and cytokines can activate mTOR.90 Upon activation of mTORC1, both glycolysis and de novo FAS are enhanced in T cells.91 SREBPs are important downstream molecules of mTORC1 that regulate the metabolism of fatty acids and cholesterol.80 In effector CD8+ T cells, SREBPs induce the expression of the rate limiting enzymes in FAS and cholesterol synthesis, such as acetyl-CoA carboxylase (ACC).74,91 Additionally, PPARγ serves as a critical downstream molecule of mTORC1 in regulating fatty acid uptake in effector CD4+ T cells.92 PPARγ can directly bind to genes involved in fatty acid metabolism, including ACC1, thereby promoting fatty acid uptake in CD4+ T cells.75 The de novo lipid synthesis mediated by ACC1 plays a critical role in the differentiation of Th17 cells from CD4+ T cells.88 The upstream or downstream relationships between PPARγ and SREBPs in T cell lipid metabolism have not been reported.93,94
Like naive T cells, memory T cells and Treg cells maintain FAO and OXPHOS as the default metabolic program.80,88,91,95 Fatty acids, rather than glucose, are the preferent metabolites for memory T cells and Treg cells due to the survival environment with limited glucose.95 The survival of certain subsets of memory CD8+ T cells is dependent on the uptake and metabolism of exogenous lipids.96 Different from effector CD8+ T cells, the development of memory CD8+ T cells does not depend on SREBP activity and can be inhibited by mTOR activation.97 The features of lipid metabolism in memory CD4+ T cells remain unclear. Treg cells also prefer to uptake extracellular fatty acids rather than undergo de novo FAS.88 Forkhead/winged helix transcriptional factor P3 (FoxP3) is a nuclear-specific transcription factor expressed in Treg cells, important for maintaining immunological self-tolerance.98 Foxp3 enables Treg cells to tolerate high fatty acid concentrations by inducing FAO and triglyceride synthesis.76 Cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) and programmed death 1 (PD-1) can act as upstream molecules to upregulate Foxp3 expression.99
Amino acid metabolism in T cells
Amino acid metabolism in T cells is complex, with studies focusing on amino acid metabolism during T cell activation. Multiple amino acids are necessary for the activation, proliferation and function of T cells.100 Alanine is involved in protein synthesis rather than catabolism during T cell activation. While alanine can be synthesized from pyruvate through transamination, extracellular alanine is still required for naive T cell activation and memory T cell restimulation.101 Arginine concentration decreases in activated T cells, while the levels of other amino acids remain stable or increase.102 Increasing L-arginine levels in the experiment, can lead to a shift from glycolysis to OXPHOS, promoting the formation of memory T cell.102 Also, the uptake of cysteine and cystine is necessary for T cell activation, but it is noteworthy that cysteine can modify electrophilic compounds to impair T cell activation.100,103 Glutamine has been recognized as an immunomodulatory nutrient for a long time. The activation of naive T cells is accompanied by rapid glutamine uptake, depending on the amino acid transporter ASCT2, which could induce Th1 and Th17 cells activation in immunity and autoimmunity.104 Leucine is considered as an important nutrient signal for activating mTORC1.105 Methionine has been identified as a key nutrient signal in epigenetic reprogramming in CD4+ Th cells, including Th17.106 Serine controls proliferative capacity of effector T cells by supplying glycine and one-carbon units for de novo nucleotide biosynthesis.107 In summary, although studies on the synthesis and catabolism of amino acid in T cells are lacking, the results on how amino acid uptake regulates T cells already indicate the important role of amino acid in connecting metabolism and T cells.
Metabolic pathways and reprogramming in B cells
B cells are lymphocytes that develop from hematopoietic precursor cells and maturate in an ordered and selective process.108 Several B cell subsets have been identified. B1 cells, including B1a and B1b, are mainly generated in the foetal liver and play important roles in innate immunity.109 B2 cells, consisting of follicular B and marginal zone B cells, are derived from the bone marrow and play conventional roles in adaptive immunity.109 Regulatory B (Breg) cells are immunosuppressive cells that support immunological tolerance and can differentiate from immature B cells, mature B cells, and plasmablasts.110
B cells at different stages of maturity vary in metabolic activity, and the metabolic pathways in different B cell subsets are also different.111 Like T cells, resting B cells have a limited requirement for metabolic pathway activation, while activated B cells undergo metabolic reprogramming to meet the bioenergetic demands for rapid proliferation and antibody production.112,113 However, studies on metabolic pathways in B cells are significantly less than that in T cells.
Glycometabolism in B cells
After B cell activation, glucose uptake increases dramatically.114 The expression of GLUT1 and mitochondrial mass in B cells are upregulated following stimulation with lipopolysaccharide (LPS) or B cell receptor to enhance glucose uptake.115 Unlike the metabolic reprogramming in T cells, subsequently increased glycolysis and OXPHOS in B cells depends on tolerance.115 Anergic B cells, which do not respond to antigen, remain metabolically quiescent, while B cells stimulated by chronic B cell-activating factor rapidly increase glycolysis.115,116 In addition, aerobic glycolysis is not highlighted in nonneoplastic B cells, leading researchers to consider glycometabolic patterns in B cells as conventional.117,118
Lipid metabolism in B cells
Although B cells have metabolic requirements for lipids, studies on lipid metabolism in B cells are limited. As mentioned before, FAS is an important type of metabolism for T cell activation, with corresponding downregulation of FAO. However, germinal center B cells, critical cells for long-term humoral immunity, conduct active FAO and minimal glycolysis to achieve rapid proliferation, indicating different metabolic patterns from T cells.119 The important roles of short-chain fatty acids in regulating immune functions in B cells have been reported, also indicating that lipid metabolism in B cells requires more attention.120,121
Amino acid metabolism in B cells
Due to the ability of B cells to secrete large amounts of antibodies, amino acid metabolism and related signaling are particularly important. Glutamine is the most frequently reported amino acid for B cells. The uptake and utilization of extracellular glutamine are important for B cell activation.122 Glutamine can be catabolized to generate energy for B cells through a glucose-independent TCA cycle, which promotes cell proliferation under hypoxia and glucose deficiency.123 Glutamine metabolism can also mediate mitochondrial function enhancement.124 Furthermore, glutamine can promote the generation of Breg cells in different subsets of B cells through the mTOR/glycogen synthase kinase 3 pathway.125 The number of IgA+ plasma cells in the ileum can be increased by glutamine supplementation.126 Other amino acids are also important for B cells. Arginine undergoes increased methylation after B-cell activation, which is essential for the proliferation, differentiation, and survival of B cells.127,128 Tryptophan can be catabolized through a pathway mediated by the indoleamine 2,3-dioxygenases, whose members negatively regulate B cell proliferation.129 Leucine nutrient preferring B cells induce immune escape from tumors.130
Metabolic pathways and reprogramming in neutrophils
Neutrophils are short-lived innate immune cells that regulate acute injury and repair, cancer, autoimmunity, and inflammatory processes.131,132 Neutrophils can act as the first responders to acute inflammation, contributing to anti-inflammatory effects, and also play important roles in chronic inflammation.133 The existence of different neutrophil subsets has been proven, but studies often do not focus on specific subsets.134 Neutrophils mainly rely on glycolysis for ATP production with very limited OXPHOS because of their low mitochondrial density.135 In environments where glucose availability is limited, FAO is an alternative pathway for obtaining energy.134
Metabolic pathways and reprogramming in dendritic cells
Dendritic cells are innate immune and antigen-presenting cells that communicate environmental signals with T cells to bridge innate and adaptive immunity.136 Dendritic cells have complex subsets and participate in protective proinflammatory responses and tolerogenic immune responses.137,138 Dendritic cells undergo rapid metabolic reprogramming during the generation of specific immune responses, but the cellular metabolism of dendritic cells is still unclear.139 During pathogen infection and migration toward lymph nodes, metabolic reprogramming toward glycolysis in dendritic cells, which can be induced by the chemokine-mediated HIF-1α activation.140,141 In some other cases, upregulated FAO and OXPHOS have also been observed in activated dendritic cells, and differences in metabolic reprogramming can be caused by different Toll-like receptors.141 FAS is indispensable for the development and activation of dendritic cells.142,143 Lipid accumulation in dendritic cells can impair their capacity to process antigens.144
Metabolic pathways and reprogramming in other innate immune cells
Innate lymphoid cells (ILCs), including natural killer (NK) cells, non-cytotoxic ILC1s, ILC2s and ILC3s, are emerging innate immune cells that participate in immune responses to pathogens.145,146,147 NK cells are important cells with antiviral and anti-tumor effects.148 OXPHOS can cover the metabolic demand of NK cells at a quiescent state.149 To maintain their functions including anti-tumor effect, glycolysis has been proven to be indispensable.150,151 FAO can enhance the responses of NK cells against infection and cancer.152 FAS has also been found to be required for proinflammatory effect of NK cells.153 Because their function of exerting direct cytotoxic responses is similar to CD8+ T cells, studies on the metabolic patterns of NK cells may be inspired by those on CD8+ T cells.154 Studies on the cellular metabolism of non-cytotoxic ILC1s, ILC2s and ILC3s are more limited than that on NK cells.155 The transcriptional and epigenetic identity of ILCs in the small intestinal has been reported, which may be helpful to study their cellular metabolism.156 It has been noted that FAO predominantly supports the function of ILC2s during helminth infection.149,157 In activated ILC2s, glycolysis is upregulated and OXPHOS is at steady state.158 In activated ILC3s, both glycolysis and OXPHOS are upregulated.159,160
Metabolic regulation of the immune system in diseases
Immunometabolism in cancers
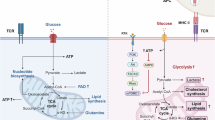
During the development and progression of cancer, dramatic metabolic reprogramming occurs in many cells, which has been fully discussed in studies focused on cancer metabolism.161,162,163 Immune cells also change their metabolic patterns to adapt to the stressful microenvironment of hypoxia and nutrient deprivation, simultaneously causing changes in immune function (Fig. 3).164 In addition to metabolic pathways, metabolites in the tumor microenvironment (TME) have also helped researchers to understand metabolic regulation of immune cells in cancer.165
Metabolic regulation of immune cells in cancer. During the development and progression of cancer, dramatic metabolic reprogramming happens in tumor cells and immune cells. Tumor cells have high metabolic demand and metabolic competition with immune cells. The tumor microenvironment is an immunosuppressive environment with the proportion of proinflammatory immune cells decreasing and anti-inflammatory cells increasing. Tumor derived-lactate is an important metabolite to regulate the phenotypes of immune cells. M2 macrophages have an abnormally high capacity to take up glucose, while glucose metabolism is reduced in effector T cells and glycolysis. Nuclear factor-kappaB (NF-κB), Toll-like receptor-2 (TLR2) and forkhead/winged helix transcriptional factor P3 (FoxP3) can regulate glucose metabolism. Lipid accumulation is upregulated in M2 macrophages and effector T cells, and lipid synthesis is upregulated in regulatory T (Treg) cells mediated by sterol regulatory-element binding proteins (SREBPs). Fatty acid oxidation (FAO) is active in M2 macrophages, with peroxisome proliferator-activated receptor γ (PPARγ) and CD36 involved in the regulation. Glutamine metabolism and serine synthesis is increased in M2 macrophages, and protein kinase RNA-like ER kinase can upregulate the serine synthesis. In effector T cells, glutamine uptake is reduced
Glycometabolism of immune cells in cancer
The most recognized metabolic phenotype of cancer cells is the altered glucose metabolism, which is known as Warburg effect or aerobic glycolysis.166,167 Similar to some immune cells, including effector T cells, tumor cells, tumor cells prefer to catalyze glucose into lactate instead of carbon dioxide, even in an oxygen-sufficient environment.168 Which should be noted is that in a variety of cancers, immune cells are a component of the TME, and their metabolic pattern is a topic different from that of tumor cells.169 The TME is an immunosuppressive environment in which glucose metabolism is altered in immune cells, which participate in building the environment.79
The metabolism of macrophages has been shown to be altered in the TME, and then change the polarization and anti-tumor response of macrophages.170 Tumor-associated macrophages cannot be completely classified into the M1 and M2 subtypes because of their general M2 phenotype, which promotes tumor growth and invasion.171 Polarization to the immunosuppressive phenotype has been proven to be caused by tumor-derived exosomes, which induce glycolytic-dominant metabolic reprogramming.172 Through Toll-like receptor-2 and nuclear factor-kappa B (NF-κB), tumor-derived exosomes can increase glucose uptake of macrophages. M2-like tumor-associated macrophages have an abnormally high capacity to take up intratumoral glucose, which has been proven to induce the hexosamine biosynthetic pathway and cardiac O-GlcNAcylation, subsequently promoting metastasis and chemoresistance.173
In a TME with low glucose and high lactate levels, the metabolic phenotypes of T cells change to promote immune tolerance. Foxp3, a Treg transcription factor, has been considered as a potential factor that regulates the metabolism of T cells in the TME. Foxp3 can suppress myelocytomatosis oncogene (Myc) and glycolysis, enhance OXPHOS, and increase nicotinamide adenine dinucleotide oxidation, possibly resulting in a metabolic advantage of Treg cells in the TME.79 In colorectal cancer, glucose intake and glycolysis are highly activated in Treg cells. Treg-specific MondoA knockout can induce Th17-like Treg cells to promote the initiation of cancer, indicating the important role of the MondoA-TXNIP axis in regulating the metabolism of Treg cells.174 The glucose metabolism of effector T cells in cancer is always reduced. In advanced non-small-cell lung cancer, upregulation of Sirtuin 2, which can suppress glycolysis in T cells, is associated with negative response to immunotherapy.175 In sarcoma, glycolysis of T cells is suppressed by the low glucose in the TME, and then mTOR activity and IFN-γ production are decreased, promoting tumor progression.163 In gastric cancer, glucose intake of CD8+ T cells is suppressed by cancer cells through CD155T/TIGIT signaling and the effector function is impaired.176
In contrast to studies on macrophages and T cells, studies on immunometabolism have focused on the malignant phenotype of B cells instead of considering them to be components of the TME. B-cell malignancies, including various leukemias and lymphomas, are prevalent worldwide.177,178 The metabolism of malignant B cells is complex and has been fully discussed in previous reviews.179,180,181 As cells with a low mitochondrial number and a small cytoplasmic volume, B cells are limited in obtaining glucose.182 However, oncogenes can drive B cells to obtain additional glucose, even resulting in permanently increased metabolic demands. Compared with that in healthy cells, the importance of pyruvate, the key product of glycolysis, is lowered for mitochondrial metabolism in malignant B cells, while glutaminolysis becomes more important.179,183 Flux of the PPP has been observed in chronic lymphocytic leukemia, where it protects malignant B cells from oxidative stress.183
Lipid metabolism of immune cells in cancer
Lipid metabolism of immune cells has been reported to be changed in cancer, participating in building an immunosuppressive microenvironment and tumor progression.
The intracellular metabolic lipid profiles of macrophages undergo significant changes in the TME, partly contributing to their pro-tumor effects.184 Generally, lipid accumulation and metabolism and M2 polarization are enhanced in macrophages when they participate in tumorigenesis or tumor progression.185 In lymphoma and myeloma mice, highly expressed CD36 induces macrophages to accumulate lipids and upregulate FAO to obtain energy.185 In breast cancer, M2 macrophages is associated with poor survival.186 Researchers have defined specific macrophage subsets with highly expressed genes related to lipid metabolism including fatty acid binding protein (FABP) 3, FABP4, FABP5, and myeloid cells 2, to provide a novel direction for combating breast cancer and anti-lung metastasis.187 PPARγ is considered as an important factor for FAO upregulation to induce M2 polarization in a breast cancer model.188 PPARγ can be induced by S100A4, which is highly abundant in macrophages and can be induced by IL-4. Some researchers even believe that long-chain fatty acid metabolism can control the M2 phenotype of macrophages in cancer, and lipid droplets are essential.189 However, we believe that although the polarization is important for elaborating the roles of macrophages in disease states, how lipid metabolism in macrophages promotes cancer progression cannot be completely generalized by abnormal polarization due to the complexity of the underlying mechanisms in addition to polarization. In prostate cancer, a special group of macrophages has shown a high lipid accumulation depending on the scavenger receptor Marco which can be activated by IL-1β.190 Macrophages with a high lipid accumulation can release CCL6 to promote cancer cell migration.
In the TME with a relative absence of glucose, T cells tend to accumulate lipids for obtaining energy.88 However, abnormal lipid accumulation in the TME can change the normal lipid accumulation and metabolism of T cells, causing dysfunction of effector T cells.191 Increased concentrations of lipids have been observed in CD8+ T cells of murine colon cancer and melanoma, inducing lipid peroxidation and activating p38 kinase, promoting CD8+ T cell dysfunction in the tumors.192 During the metabolic dysregulation, the expression of the scavenger receptor CD36 is increased its expression on CD8+ T cells, suggesting that this receptor is a potential immunometabolic target. Activated p38 mitogen-activated protein kinases (MAPKs) and lipid oxidation pathways have also been reported in the T cells from male breast cancer patients.193 However, as the central to Treg cell activation, the upregulation of lipid metabolism in Treg cells can drive Treg cells to enhance immunosuppression in the TME.194 Intratumoral Treg cells exhibit increased SREBP activity, indicating the upregulation of fatty acid synthase.195 Inhibiting lipid synthesis and metabolic signaling via SREBPs can enhance anti-tumour immune responses, which are related to PD-1.195
There are only a few published studies on lipid metabolism in B cells in cancer, and studies on lipid metabolism of healthy B cells have been summarized in a previous review.196 Although the evidence is limited, lipid metabolism of B cells in cancer has been considered to be altered. In chronic lymphocytic leukemia, changes of lipid metabolism in malignant B cells have been reported.197,198 Lipases and phospholipases are significantly overexpressed in chronic lymphocytic leukemia cells, and a lipase inhibitor can induce apoptosis of B-cells.198 PPARδ can change cholesterol metabolism and related signaling in malignant B cells.197 More than ten years ago, PPARα has been discovered to be an effective therapeutic target for murine B-cell lymphoma through the regulation of lipid metabolism.199
In other immune cells, abnormal lipid accumulation has also been reported in cancer. Lipid accumulation in dendritic cells is associated with the decreased antigen processing ability, which has been proven in ovarian cancer and hepatocellular carcinoma (HCC).200 Although NK cells are important in tumor immunotherapy, how their metabolism changes in cancer is unclear. In murine colorectal carcinoma and melanoma, surgery can increase the lipid accumulation in NK cells, by upregulating MSR1, CD36 and CD68 and decreasing their ability to lyse tumor cells.201 In HCC, hepatocytes can produce excessive cholesterol and enhance lipid accumulation in NK cells, resulting in deficient NK cell cytotoxicity.202 In aggressive B-cell lymphoma, the high levels of fatty acids in the environment can impair the function of NK cells.203
Amino acid metabolism of immune cells in cancer
Amino acids are metabolic regulators that support cancer cell proliferation, and also essential regulators of immune cells.204,205 However, existing studies have considered amino acids to be extrinsic signaling molecules through which immune cells to regulate immune responses in cancer. The amino acid anabolism and catabolism of immune cells in cancer are still difficult to summarize, but we believe that amino acid metabolism of immune cells in cancer has certain unique characteristics based on limited evidence.
The expression of EPHB2, which is an important gene of glutamine metabolism, is high in macrophages, especially M2 macrophages, from lung adenocarcinoma patients.206 Although the glutamine metabolism pattern of macrophages in cancer has still not been fully characterized, inhibiting glutamine metabolism of macrophages has shown the ability to convert M2 macrophages into M1 macrophages.207 The serine synthesis of macrophages has been reported to be upregulated in the TME through a protein kinase RNA-like ER kinase-signaling cascade, enhancing mitochondrial function and M2 macrophage activation.208
In the TME of triple-negative breast cancer, effector T cells competitively consume glutamine with cancer cells, and the high glutamine metabolism level in tumors is associated with decreased cytotoxicity of T cells.209 In lung cancer, blocking glutamine metabolism can improve anticancer immunity by increasing the infiltration of CD8+ T cells and CD4+ Th1 cells.210 Serine and glycine are elevated in mice with T-cell acute lymphoblastic leukemia, which is related to the upregulation of phosphoserine phosphatase.211
Studies on amino acid metabolism of other immune cells in cancer are still limited. In colorectal cancer, a subset of immunoregulatory B cells with leucine nutrient preference is correlated with poor survival.130 Otherwise, there are more studies on amino acid related signaling and treatments targeting amino acid metabolism to treat cancer, which is going to be discussed in the following sections.
Effects of metabolites on immune cells in cancer
Although it is important for understanding immunometabolism in cancer, studies on the changed metabolic pathways of immune cells in cancer are still limited. Some studies have shown that metabolites from the TME can influence the functions of immune cells, which can also enhance our understanding of immunometabolism in cancer. The metabolic state of the TME is disordered. The levels of many metabolites increase in the TME, contributing to the immunosuppression.
Lactate is an important immune inhibitory metabolite generated by tumor cells.212,213,214 Polarization of macrophages in the TME can be induced by lactate, the transport of which depends on mitochondrial pyruvate carrier.215 Researchers believe that lactate, rather than its downstream metabolites results in the M2 polarization.215 In pituitary adenoma, lactate induces M2 polarization through the mTORC2 and ERK pathways, after which the M2 macrophages secrete CCL17 to promote invasion.216 In colorectal cancer, methylation of MCT1, a monocarboxylate transporter protein, can promote M2 polarization and lactate shuttling.217 Odorant receptors also participate in M2 polarization in cancer.218 After polarization, the functions of macrophages can be influenced by lactate. The expression of the macrophage-specific vacuolar ATPase subunit ATP6V0d2 can be downregulated by lactate, enhancing the production of VEGF mediated by HIF-2α and promoting cancer growth.219 In glioma, GPR65 is highly expressed on macrophages, which can sense the stimulation of lactate in the TME and then secrete HMGB1 to promote glioma progression.220 Lactate generated from tumor cells also regulates the activation and function of T cells, weakening the immune surveillance.221,222,223,224 Lactate can enhance the function of Treg cells. Lactate-enhanced MOESIN lactylation and TGF-β signaling in Treg cells can promote tumorigenesis.225 Lactate can also regulate Foxp3-dependent RNA splicing in Treg cells in the TME via CTLA-4.226 Moreover, the cytotoxicity of CD8+ T cells can be inhibited by tumor-derived lactate, which is considered as a potential therapeutic target.227,228
Amino acids are other important metabolites in the TME.229 Glutamine is the most studied amino acid in immunometabolism. Glutamine metabolism is upregulated in trastuzumab-resistant gastric cancer, and tumor-derived glutaminase microvesicles can induce M2 polarization of macrophages.61 In clear cell renal cell carcinoma, tumor cells consume glutamine, promoting macrophages to secrete IL-23 through activating HIF-1α and ultimately suppressing tumor-cell killing.230 The competition for glutamine uptake with tumor cells inhibits dendritic cells from priming T cells, and the transporter solute carrier (SLC) family 38 member 2 (SLC38A2) is important for this competition.231 In patients with natural-killer T-cell lymphoma, tumor cells increase the glutamine uptake through the ectopic expression of SLC1A1, resulting in the reduced activity of CD3+ and CD8+ T cells.232 In HCC, decreased glutamine uptake can impair the function of CD8+ T cells by inducing mitochondrial damage and apoptosis.233 Other amino acids also regulate the function of immune cells in cancer. In bladder cancer, increased serine synthesis in cancer cells can induce the M2 macrophage polarization by activating the PI3K/Akt pathway in macrophages.69 In gastric cancer, the serine protease PRSS23 can promote macrophage infiltration and result in a poor prognosis through FGF2.234 Enriched serine in the TME can also promote the accumulation of Treg cells by regulating sphinganine-mediated c-Fos.235
Immunometabolism in autoimmune diseases
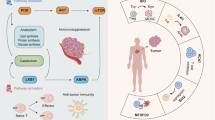
Inflammation is involved in multiple autoimmune diseases. Figure 4 summarizes the metabolic regulation of immune cells in autoimmune disorders.
Metabolic regulation of immune cells in autoimmune diseases. Systemic lupus erythematosus (SLE), inflammatory bowel diseases (IBDs) and rheumatoid arthritis (RA) are typical representatives of autoimmune diseases. Generally, affected sites of autoimmune diseases are dominated by immune cells with proinflammatory phenotypes. The metabolism of immune cells with proinflammatory phenotypes are active in the context of autoimmune diseases. Mechanistic target of rapamycin complex 1 (mTORC1)/hypoxia-inducible factor 1α (HIF-1α) signaling, mechanistic target of rapamycin complex 2 (mTORC2)/peroxisome proliferator-activated receptor γ (PPARγ) signaling, Zip8, glucose transporter type 1 (GLUT1), cellular myelocytomatosis oncogene (c-Myc), lactate dehydrogenase A (LDHA), and interleukin-27 (IL-27) have been proved to participate in the regulation of glycometabolism. Nuclear factor-kappaB (NF-κB) and CD36 can regulate fatty acid oxidation (FAO). In RA, lactate from synovial tissues is involved in regulating the metabolism of immune cells
Immunometabolism in systemic lupus erythematosus
Systemic lupus erythematosus (SLE) is a prototypical autoimmune disease characterized by the dysregulation of many immune cells including autoreactive B cells, macrophages, CD4+ T cells, dendritic cells, and neutrophils.236 The altered metabolic patterns of immune cells in SLE have been reported and are considered as potential therapeutic targets.237,238
In the context of SLE, glycometabolism is upregulated in the proinflammatory immune cells.85 Glycolysis of human and mouse macrophages can be induced by IgG immune complex and depends on mTOR and HIF-1α, resulting in the upregulation of IL-1β.239 Activated lymphocyte-derived DNA, which can induce M2b polarization of macrophages and has been reported to be an important factor in the development of SLE, can also enhance glycolysis and glycogenesis and downregulate the PPP.240 Notably, M2b macrophages are considered to be anti-inflammatory, but additional studies are needed to validate these conflicting results.241 The cellular metabolism of CD4+ T cells, including glycolysis, in SLE patients and mouse is overactivated, and CD4+ T cells are considered key immune cells for treating SLE through the regulation of cellular metabolism.242,243,244 Inhibitors of glycolysis can block glucose uptake of CD4+ T cells to attenuate autoimmune activation.242,245 In Treg cells, the impaired immunosuppressive functions in SLE can be enhanced by exogenous phosphofructokinase P through phosphorylating the glycolysis rate-limiting enzyme 6-phosphofructokinase and upregulating aerobic glycolysis.246 The differentiation of follicular helper T cells in SLE mice has been proven to be related to glycolysis and the PKM2 may be a key factor in SLE pathogenesis.247,248 B cells have also been considered to play a role in the glucose metabolic dysfunction in SLE mice.249 Breg cells, which secrete anti-inflammatory IL-10, can be changed the phenotype into aggressive inflammatory phenotype by upregulated cellular Myc (c-Myc) and enhanced glycolysis through the MAPK signaling in SLE.250
Lipid metabolism in immune cells from SLE patients or mice is changed, and lipid metabolism is considered as a potential therapeutic target, but research on lipid metabolism is insufficient.251 FcgRIIB dysfunction is common in SLE patients. In vitro, FcgRIIB-/- macrophages exhibit greater lipid accumulation, indicating that lipid metabolism dysregulation in macrophages may be related to the pathogenic mechanisms of SLE.252 Two decades ago, researchers have reported that the composition of lipid rafts on T cells from SLE patients is changed, causing aberrant autoimmune responses.253,254 However, besides lipid signaling, studies on lipid metabolism in T cells are still limited. Generally, the lipid synthesis in T cells from SLE patients is enhanced, favoring the function of proinflammatory Th17 cells.85 As the essential cells for the development of SLE, autoreactive B cells might have enhanced lipid uptake mediated by CD36 and upregulated FAO, and during this process, acetylcholine from spleen fibroblastic reticular cells is important.255
Amino acid metabolism of immune cells in SLE is also important, but related studies in the context of SLE are limited.256 Limited evidences have shown that glutaminolysis of T cells in SLE is enhanced, helping Th17 cells to differentiate.85 Glutaminase 1 inhibition can inhibit CD4+ T cells from SLE patients from differentiating into Th17 cells, and simultaneously inhibit the expression of HIF-1α and downregulate glycolysis.257,258 The absence of glutamine can inhibit OXPHOS and plasmablast differentiation in peripheral B cells from SLE patients.124 Although summarizing the metabolic patterns of amino acids in immune cells from SLE patients is difficult, the importance of related signaling molecules in SLE is undoubted. The expression of serine/threonine protein phosphatase 2 A (PP2A) is increased its expression in SLE patients, resulting in decreased IL-2 and increased IL-17 in T cells, which induces the development of glomerulonephritis.259 The regulatory subunit PPP2R2A is upregulated in T cells from SLE patients and can enhance Th1 and Th17 differentiation by activating the GEF-H1/RhoA/ROCK pathway.260 However, the production of PPP2R2B is reduced in SLE patients, helping T cells become resistant to apoptosis.261 Increased activity of PP2A has also been observed in B cells from SLE mice and patients, and is related to the expression of purine nucleoside phosphorylases.262 Another important factor, serine/arginine-rich splicing factor 1 (SRSF1), is downregulated in T cells from SLE patients, and the ubiquitination of SRSF1 is increased, which is also related to decreased IL-2.263,264 In SRSF1-deficient mice, the activity of mechanistic targets of mTORC1 is upregulated, indicating a potential therapeutic target for reducing the activity of T cells in SLE.265 Decreased SRSF1 levels are also correlated with lymphopenia in SLE patients, which is associated with decreased expression of the anti-apoptotic protein Bcl-xL.266
Immunometabolism in inflammatory bowel disease
Inflammatory bowel diseases (IBDs), including ulcerative colitis and Crohn’s disease, has spread globally. The spread of Western diet patterns contributes to the prevalence of IBDs, leading researchers to consider the roles of metabolism and immunometabolism in IBDs.267
Like in SLE patients, glycometabolism in immune cells from IBD patients is dominated by the cells with a proinflammatory phenotype. M1 macrophage polarization is upregulated in IBD. mTORC1/HIF-1α and mTORC2/PPAR-γ signaling have been shown to be associated with aerobic glycolysis of macrophages from mice with ulcerative colitis.267,268 T cells are excessively activated in IBDs.269 Impaired mitochondrial respiration has been shown in colitogenic T cells.270 In ulcerative colitis, HIF-1α also mediates glycolysis in Th17 cells, and glycolysis in Treg cells is associated with the aryl hydrocarbon receptor.271,272 In patients with acute severe ulcerative colitis, HIF-1α expression and glycolysis are also increased in neutrophils.273
Lipid metabolism contributes to in the development of therapies for IBD.274 However, in the context of IBD, changes in lipid metabolism of immune cells have not been fully described, with only a few studies reporting possible factors or pathways regulating lipid metabolism in immune cells. Fatty acid binding protein 5 can regulate lipid metabolism, which is upregulated in the mucosa of IBD patients.275 Inhibiting fatty acid binding protein 5 can promote the M2 polarization of macrophages and plays a protective role against colitis.275 Increased FAO has been found in tissue-resident memory CD4+ T cells from patients with Crohn’s disease, which induces a proinflammatory phenotype, and the activated NF-κB signaling participates in the upregulated lipid metabolism.276
The roles of signaling molecules related to amino acids in the development of IBDs have been reported. Members of the receptor-interacting serine/threonine kinase (RIPK) family are typical representatives. Loss-of-function mutations in RIPK1 are related to immunodeficiency and IBDs, which are also related to reduced NF-κB activity and abnormal differentiation of T and B cells.277 The expression of RIPK2 is upregulated in the colonic mucosa of IBD patients, and is important for NOD2 signaling.278,279 In intestinal biopsy specimens from IBD patients, inhibiting RIPK2 can decrease proinflammatory cytokine release.280 The inhibition of RIPK3 can downregulate proinflammatory cytokine expression in mononuclear cells from ulcerative colitis patients.281 Other molecules have also been reported. The upregulation of leucine-rich repeats containing X1 can protect mice from IBDs, and in vitro, leucine-rich repeats containing X1 can decrease the differentiation of CD4+ T cells into Th1 and Th17 cells and increase OXPHOS.282 The deficiency of leucine-rich repeat kinase 2 can enhance susceptibility to colitis in mice.283
The gastrointestinal tract is both an immune organ and a digestive organ. Different from other diseases, in the context of IBDs, researchers have focused on changes in metabolism patterns in a specific organ, the gastrointestinal tract. Glycolysis in intestinal samples from IBD patients has been observed to be significantly upregulated compared with that in intestinal samples from healthy people, which is associated with the upregulated expression of 6-phosphofructo-2-kinase/fructose-2, 6-bisphosphatase 3.284 Inhibiting 6-phosphofructo-2-kinase/fructose-2, 6-bisphosphatase 3 can reduce the infiltration of immune cells in the intestine.284 In intestinal epithelial cells from ulcerative colitis patients, glucose metabolism is increased, but how impaired glucose metabolism homeostasis in intestinal epithelial cells is associated with immune cells is still unclear.285 With the increasing recognition that diet is a pivotal factor for IBD development and progression, dietary lipids have been explored for the treatment of IBDs. Generally, short-chain fatty acids are protective factors with anti-inflammatory properties, but some long-chain fatty acids, including saturated fatty acids and trans fatty acids, are proinflammatory.286 How dietary lipids are associated with lipid metabolism and immune responses is a valuable topic for IBDs. Commonly increased serum lipids in IBD patients also indicate the considerable roles for lipid metabolism in managing IBD patients, but how the altered lipid metabolism causes an intestinal inflammatory state and IBD progression is unclear.287
Immunometabolism in rheumatoid arthritis
Rheumatoid arthritis (RA) is also a typical autoimmune disease characterized by immune cell dysfunction. The metabolic patterns of immune cells in RA have several distinct characteristics. Generally, at the site of RA involvement, immune cells are proinflammatory and under metabolic stress, building a glucose-deficient microenvironment that is somewhat similar to the TME.288,289,290
In rheumatoid synovial tissue and peripheral blood from RA patients, some immune cells with high metabolic demands undergo a metabolic shift from OXPHOS to glycolysis.291 The lactate level increases in synovial samples from RA patients, with increased glucose uptake and upregulated GLUT1.292 In macrophages from RA patients, the expression of Zip8, a zinc-specific importer, is upregulated, which is related to the activation of glycolysis induced by mTORC1, resulting in IL-1β upregulation.293 Inhibiting glycolysis in macrophages from RA rats and patients can promote their transition from the M1 phenotype into the M2 phenotype.294,295 Changes in the transcriptional regulation of glycolysis in peripheral T cells from RA patients have been shown.296 Increased LDHA activity and aerobic glycolysis have been reported in peripheral CD8+ T cells from RA patients.297 The glycolysis of T cells in RA is associated with ICOSL, which is regulated by B cells and participates in the polarization of Th cells.298 Then activated Th cells can upregulate aerobic glycolysis and drive the inflammatory phenotype transition of synovial fibroblasts, resulting in the progression of RA.299 Dendritic cells in synovial tissue are activated in RA, with enhanced glycolysis and anabolism, and their ability to activate T cells is enhanced.300 In contrast to CD8+ T cells, CD4+ T cells from RA patients exhibit decreased glycolysis and upregulated PPP.289 Glycolysis of peripheral B cells from RA patients is also increased, and IL-27 is a possible upstream activating factor.301 Glucose-6-phosphate isomerase, which can catalyze the interconversion between D-glucose-6-phosphate and D-fructose-6-phosphate, can induce chronic arthritis via B cells in mice to establish a type of RA model.302
Lipid metabolism in immune cells from RA patients is associated with the severity of RA. Data from the Gene Expression Omnibus database have shown that the expression profiles of fatty acid metabolism-related genes in peripheral CD8+ T cells are different between RA patients and healthy people, and the expression of fatty acid metabolism-related genes including FABP4 and GPR84 are upregulated the expression in RA patients who respond well to methotrexate.303 In mice with collagen-induced arthritis, the level of α2-glycoprotein 1, a protein that stimulates lipolysis, is increased, increasing the Th17 population of splenocytes and exacerbating RA.304
Lactate has been considered as a junction of metabolism, inflammation, and autoimmunity.305 Dysregulated lactate metabolism and lactate accumulation in the synovial tissue are important characteristics of RA. Local hypoxia at the involved sites is a main cause of lactate accumulation.306 The functions of both synovial fibroblasts and immune cells can be affected by lactate in RA. Genes regulating lactate intake and secretion can be upregulated upon inflammation, and the members SLC16A1 and SLC16A3 are expressed in synovial fibroblasts and macrophages.307 Lactate can upregulate glycolysis in fibroblasts and downregulate glycolysis of macrophages in the synovial tissue of individuals with RA.307 The lactate transporter SLC5A12 in CD4+ T cells can be upregulated by lactate, increasing IL-17 production and fatty acid synthesis and decreasing glycolysis in CD4+ T cells, thus promoting the development of RA.308,309 The Warburg effect is upregulated in CD8+ T cells from RA patients, and LDHA is overexpressed, participating in the induction of the proinflammatory phenotype of B cells.297
Immunometabolism in multiple sclerosis
Multiple sclerosis (MS) is characterized by the chronic inflammation of the central nervous system, which causes neurological disability in young adults.310,311 Multiple immune cells, especially T cells, participate in the development and progression of MS.312
Changes in glucose metabolism in immune cells contribute to inflammatory responses in MS. Glycolysis in CD4+ T cells can be upregulated by the CD28-mediated c-Myc and GLUT1 upregulation in relapsing-remitting MS patients, which is important for the production of inflammatory cytokines by Th17 cells.313 However, some researchers believe that glycolysis and OXPHOS of T cells in relapsing-remitting MS patients are impaired, as determined by measuring the extracellular acidification rate and oxygen consumption rate, but they also reported the important role of GLUT1.314
Lipid metabolism of immune cells has long been considered as a promising therapeutic target for MS, but related studies are still limited.315 RNA-sequencing analysis has revealed the dysregulation of lipid metabolism genes in CD4+ T cells from relapsing-remitting MS patients, with liver X receptors mediating lipid metabolism pathways.316 As the most important cells for the clearance of myelin, the lipid metabolism of macrophages might change in MS, which also deserves attention.317
Amino acid homeostasis is also impaired in MS. An animal model of MS has shown that glutaminase is upregulated in macrophages near dystrophic axons.318 Transglutaminase has been found to be expressed in macrophages but not in T cells or B cells, indicating its possible role in macrophage infiltration into the central nervous system.319,320 The peripheral blood mononuclear cells of MS patients have exhibited decreased activity of enzymes involved in tryptophan and arginine catabolism, resulting in a decrease in the number of Treg cells.321
Immunometabolism in metabolic diseases
Chronic low-grade inflammation in patients with metabolic diseases has been considered as an important trait. In Fig. 5, we have summarized the changes in phenotypes and metabolism of immune cells, and cytokines affecting disease progression in metabolic diseases.
Metabolic regulation of immune cells in metabolic diseases. Chronic low-grade inflammation has been considered as an important trait of metabolic diseases. Obesity, type 2 diabetes mellitus (T2DM) and non-alcoholic fatty liver disease (NAFLD) are typical representatives of metabolic diseases. Tumor necrosis factor (TNF)-α from adipocytes can induce M1 macrophage polarization, and insulin supports interleukin (IL)-1-producing T helper (Th1) cells cell differentiation. Generally, in immune cells with proinflammatory phenotype, the metabolic pathways are upregulated. Glucose transporter type 1 (GLUT1), Hypoxia-inducible factor-1α (HIF-1α) and pyruvate kinase muscle isozyme M2 (PKM2) are involved in regulating the metabolism of immune cells. After being activated, M1 macrophages can infiltrate adipose tissue, cause inflammation in islets and induce inflammation in the liver. Pro-inflammatory cytokines and chemokines produced by immune cells participate in disease progression
Immunometabolism in obesity
Obesity, always accompanied by the accumulation of body fat, increases the risk of diabetes, non-alcoholic fatty liver disease (NAFLD), and many other diseases.322 Chronic low-grade inflammation in the adipose tissue is an important characteristic of obesity, motivating researchers to explore the causal relationship between immune responses and obesity.323 The functions of immune cells can be regulated by cellular metabolic reprogramming.324
Many immune cells exhibit a proinflammatory phenotype in individuals with obesity. Macrophages are abundant immune cells in adipose tissue.325 In obese individuals, macrophages in adipose tissue switch from the M2 phenotype to the M1 phenotype, and M1 macrophages infiltrate adipose tissue.326,327,328 As the proinflammatory phenotype, M1 macrophages release cytokines and chemokines to initiate inflammatory responses in obese individuals.329 Many studies have reported changes in the activation and function of T cells in obesity and obesity-related pathological conditions. The anti-inflammatory environment predominates in healthy adipose tissues.330 In obesity, the levels of anti-inflammatory T cell subsets are decreased, including Th2 and Treg cells, while the numbers and proportions of proinflammatory effector T cells, including Th1 and Th17 cells, in adipose tissue and in the circulation are increased.331
Accompanied by changes in the proinflammatory phenotype, immune cells also change their intracellular metabolic patterns. First, we discuss the glycometabolism of immune cells in obesity. Increased glycolysis and OXPHOS of adipose tissue macrophages from obese mice are important metabolic characteristics of macrophages under conditions of obesity.324 The increased glucose uptake and metabolism of macrophages from obese individuals is related to GLUT1 and HIF-1α, which promote the production of IL-1β, leading to oxidative stress and the proinflammatory response.324,332,333 In obese individuals, glycolysis of T cells is upregulated and CD4+ T cells are overactivated.334 Insulin increases the glucose uptake into the cells to upregulate glycolysis of T cells, supporting Th1 cell differentiation.330 Obesity increases B cell recruitment to adipose tissue, indicating the importance of B cells in obesity, but knowledge of glycometabolic patterns of B cells in obesity is still lacking.335
In obesity, lipid metabolism of immune cells is modified. Obesity promotes macrophages to induce intracellular lipid catabolism, which depends on lysosomes.336 The FAO of CD8+ T cells is upregulated in obese mice with breast cancer through the activation of STAT3, which is also related to decreased glycolysis of CD8+ T cells.337 The FAS of Th17 cells from obese mice is regulated by acetyl-CoA carboxylase 1, then regulating the function of RORγt.338 The FAO of dendritic cells from obese mice is upregulated, promoting the intracellular ROS accumulation and impairing antigen presentation.339 In addition to the changes in lipid metabolism of immune cells, changes in lipid metabolism of the obese individual are also important for regulating immune cell activities. In obese individuals, adipocytes release large amounts of fatty acids which can increase tumor necrosis factor (TNF)-α, which can induce M1 macrophage polarization.340
Changes in the levels of cytokines and chemokines in obesity have received some attention. Because metabolic diseases are known as disorders of metabolism, immunometabolism in metabolic diseases sometimes does not mention metabolic pathways in specific cells. Considering their important functions in regulating immune cell function, cytokines are also involved in immunometabolism in obesity. For chronic inflammation in obesity, IL-6 is one of the most discussed mediators, and whether IL-6 is protective or nonprotective to obese individuals is still controversial.341 The level of IL-6 increases in obese patients.341 IL-6 can increase islet GLP-1 production to attenuate obesity and exogenous IL-6 reduces diet-induced obesity in mice, but IL-6 also worsens insulin resistance in the liver and adipose tissue.342,343 Other cytokines, including IFN-γ and TNFs, are upregulated in obese individuals, promoting chronic inflammation and worsening the prognosis.344,345,346 The expression of CC chemokines and CXC chemokines in visceral adipose tissue is upregulated in obese patients and is associated with increased inflammation.347,348,349,350
Immunometabolism in T2DM
Chronic and low-grade inflammatory disease with long-term immune system imbalance is also a characteristic of T2DM.351
The proinflammatory phenotype is the dominant phenotype of immune cells in T2DM. In T2DM, M1 macrophages are the dominant immune cells that cause inflammation in islets.352,353,354 The expression levels of PPARγ in macrophages from T2DM patients are decreased, and macrophage-specific PPARγ controls M2 macrophage activation.355,356 The proportion of CD4+ T cells increases and the proportion of CD8+ T cells decreases after glucose loading in people with or without diabetes, indicating a more active state for CD4+ T cells in T2DM.357 Th1 and Th17 cells are proinflammatory subsets of CD4+ T cells, while Th2 and Treg cells are anti-inflammatory subsets of CD4+ T cells.358 The numbers of Th1 and Th17 cells increase in T2DM patients.359,360 IL-17 released by Th17 cells plays a key role in inflammation, insulin resistance, and T2DM, with harmful effects on T2DM patients.361 The percentage of circulating Th2 cells is inversely correlated with insulin resistance.362 The percentage of peripheral Treg cells decreases in T2DM patients.363 CD8+ T cells can also secrete chemokines to induce macrophage activation, but the proinflammatory mechanisms involved in T2DM or insulin resistance are still unclear.351
Related studies on obesity can focus on immune cells in adipose tissues, but studies on T2DM always focus on the general symptoms, making it difficult to focus on a specific organ or tissue. In the context of T2DM, studies on the metabolic pattern of immune cells are very limited. Macrophages extracted from the peritoneum of T2DM mice exhibit decreased glucose uptake and glycolysis with downregulated GLUT1.364 Moreover, peripheral T cells from T2DM patients prefer glycolysis instead of FAO to obtain energy.365
As T2DM is a metabolic disease, changes in cytokines levels might be a simpler topic for researchers to associate metabolism with immunity in T2DM. IL-6 is also one of the most discussed mediators of T2DM. The role of IL-6 in diabetes has been discussed in a previous review.366,367 Recent studies have shown that IL-6 can suppress the expression of suppressor of cytokine signaling-3 to impair the phosphorylation of insulin receptors, leading to insulin resistance.368 The level of plasma IL-6 is positively correlated with the risk of the cardiovascular and kidney outcomes in T2DM patients.369,370 A small number of ILs have been proven to be protective against T2DM. As a prominent anti-inflammatory cytokine, IL-10 reduces neurogenic inflammation in a T2DM mouse model.371 More studies have reported the nonprotective effects of ILs in T2DM. IL-1, a cytokine family with proinflammatory functions, its signals can promote the progression of insulin resistance and diabetes, which has been summarized in a previous review.372,373 IL-2 and IL-18 play proinflammatory roles and have been shown increased levels in T2DM.374,375 The levels of serum IL-19, IL-38, and IL-39 are positively correlated with the risk of diabetic complications.376,377,378 IFN-γ plays non-protective roles in T2DM patients, but the underlying mechanisms are still unclear. A decrease in the level of IFN-γ decreases in the serum of T2DM patients is associated with increased oral candidiasis incidence, increased risk of tuberculosis and infection of diabetic ulcers.379,380,381,382 The serum TNF-α concentration is increased in T2DM patients.383 In T2DM patients, urinary TNF-α levels, but not serum TNF-α levels, are associated with the progression of nephropathy.384,385 Upregulated chemokine levels are nonprotective to T2DM patients.386,387 The increased chemokine levels are possibly caused by LPS stimulation in T2DM patients, which can predict hepatic steatosis.388,389
Immunometabolism in NAFLD
With the increasing prevalence of obesity and diabetes, the prevalence of NAFLD is increasing.390 During the variable course of NAFLD, ignorable hepatic lipid accumulation and liver inflammation are developed.
Immune cells play important roles in the pathological processes of NAFLD. In NAFLD, macrophages are activated and changed to the proinflammatory phenotype in NAFLD. Macrophages play important roles in the pathological processes of NAFLD. In a healthy liver, Kupffer cells secrete the anti-inflammatory cytokine IL-10 to provide an anti-inflammatory microenvironment.391 In NAFLD, macrophages can be activated by endotoxins, fatty acids, cholesterol and any other stimuli.392 Then, macrophages, including Kupffer cells, participate in inducing inflammation in NAFLD by releasing cytokines such as IL-1β and TNF-α.393,394,395 The expression of TLR4 is also upregulated in NAFLD patients.396 The TLR4-MyD88-mediated NF-κB and MAPK pathways can induce excessive immune responses in NAFLD.397 The activation of macrophages, which is a key event in fibrogenesis, can promote liver fibrosis and HCC in NAFLD through complex mechanisms that have been fully discussed in some reviews.396,398 Generally, macrophages are an integral part of the hepatocyte-macrophage-hepatic stellate cell network in NASH and are involved in signal transduction.398 The mechanism by which T cells are activated by metabolic dysregulation in NAFLD has not been fully elucidated. Lipid accumulation in hepatocytes initiates the pathogenesis of NAFLD and subsequently increases the release of reactive oxygen species (ROS) and free lipids.399 It can be noted that ROS have been considered as important signaling messengers to regulate the activation of T cells.400,401 The liver has been considered as an immunological organ for a long time, with B cells being the most abundant intrahepatic lymphocytes.402,403 Although the subsets of B cells in the liver tissue of NAFLD patients are unclear, B2 cells are the main B cell populations in the liver tissue of NASH mice.404 With the progression of NAFLD, serum B cell-activating factor levels increase.405,406 Nevertheless, whether the accumulation and activation of B cells and B2 subsets are causal or consequential to NASH is still unclear.407 It is relatively clear that B cells promote the progression of NAFLD. In the early stages of NAFLD, B2 cells are activated.408 During NAFLD, intrahepatic B cells express proinflammatory genes, secrete IL‐6 and TNF-α and subsequently promote a chronic inflammation and fibrogenesis.404,409
Studies on the metabolic patterns of immune cells in NAFLD are limited, but can indicate changes in metabolic patterns. In high-fat-diet induced mice, glycolysis and OXPHOS of macrophages are upregulated, with caspase-11 and PKM2 are involved in the upregulation of glucose metabolism.410,411,412,413 The lipid uptake and accumulation of Kupffer cells are increased in NAFLD, which can be mediated by macrophage scavenger receptor 1, leading to liver inflammation.393
Cytokines are also the entry point for immunometabolism in NAFLD. The levels of IL-6, IL-12, IL-32 and IL-38 in the blood are upregulated in NAFLD patients, with positive correlations to severity of NAFLD.414,415,416,417 In NASH mice, inhibiting IL-11 signaling can reduce inflammation and liver fibrosis, possibly due to the ability of IL-11 to activate myofibroblasts, indicating the nonprotective role of IL-11.418,419 Blocking the IL-6 receptor can increase the risk of NAFLD, indicating the protective role of IL-6 in NAFLD.420 IL-17 can promote microbiota-related intestinal barrier restoration to alleviate NASH in high-fat-diet induced NASH mice, but increased IL-17A correlates positively with steatosis in humans with NASH.421,422 IL-22 alleviates NASH by blocking hepatic oxidative stress through the induction of metallothionein.423 The role of IFN-γ in NAFLD are controversial. Type I IFN responses can drive T cells in the liver to promote metabolic syndrome.424 Treating NAFLD mice with IFN-α showing aggravated liver fibrosis.425 However, adipose-specific deletion of IFN-α and IFN-β receptors promotes metabolic dysregulation in NAFLD mice, which indicates possible protective roles of IFN in NAFLD.426 The level of TNF-α also increases in NAFLD individuals.427 TNF-α is considered as a central player in liver inflammation with multiple functions, and overall, it is a harmful molecule to the liver.428 Inhibiting the receptor of TNF-α can reduce liver steatosis, hepatocellular injury and fibrosis in NAFLD mice.429 The levels of chemokines are upregulated in NAFLD patients, with unclear mechanisms but clear results indicate the harmful roles of chemokines in NAFLD.430,431 Chemokines promote the development of steatosis, NASH, and fibrosis, as discussed in previous reviews.432,433,434,435 Recent studies have shown that CCL3 can promote liver macrophage infiltration and M1 polarization during the progression of NAFLD.436 The expression of CCL20 increases with the NAFLD stage, and can be regulated by microRNA-590-5p.437 Inhibiting CCL11 in NAFLD mice can reduce immune cell infiltration and liver fibrosis, with IFN regulatory factor 1 serving as a mediator.438 Inhibiting CCR9 also reduces fibrosis progression in NASH mice.430 The highly upregulated expression of CXCL1 in the liver can drive steatosis to NASH through neutrophil-derived ROS.423 CXCL5 can promote lipotoxicity in hepatocytes in mice with NASH, potentially through the regulation of signaling related to IL-1 in Kupffer cells.439 Inhibiting CXCL6 can upregulate PPARα in cell models of NAFLD.440 In NAFLD mice, CXCL10 can mediate the polarization of M1 macrophages and macrophage infiltration in the liver.441 In summary, although the mechanisms are still unclear, especially in humans, the results of recent studies support the supposition that chemokines play harmful roles in NAFLD and can be potential therapeutic targets.
Immunometabolism in infection
During infection, immune cell activity substantially changes. Metabolic reprogramming of immune cells also occurs upon infection, determining the pathogen growth or containment.442
In most cases, glycometabolism of immune cells is positively correlated with anti-infectious immunity.443 Upon Brucella infection, glycolysis of macrophages is upregulated by MyD88 to control Brucella infection.444 Reduced glycolytic activity is an important characteristic of exhausted CD8+ T cells in HIV-infected individuals, with dysregulated mTOR signaling.445 Glycolysis of CD4+ T cells is increased upon HIV-1 infection, with upregulated expression of GLUT and hexokinase 1.446 Blocking the glycolysis can increase HIV-1 reverse transcription in CD4+ T cells.447 On NK cells from HIV-infected individuals, the expression of CD160, which can upregulate glucose uptake through the PI3K/Akt pathway to enhance NK cell function, was reduced.448 Upon H. pylori infection, glycolysis of macrophages can be upregulated by HIF-1α, which then stabilizes Nos2 messenger RNA to produce nitric oxide and control infection.449 However, the upregulated glycometabolism of immune cells can also promote the progression of infection. Upon SARS-CoV-2 infection, glycolysis of monocytes and macrophages is upregulated by ROS and HIF-1α, directly inhibiting the T-cell response.450
Lipid metabolism of immune cells is complex and important to immune responses, existing evidence has revealed the roles of FAS. Upon Mycobacterium tuberculosis infection, the FAS of macrophages and dendritic cells is induced to mediate the innate response against mycobacteria.451 Upon Plasmodium chabaudi infection, inhibiting FAS during T-cell priming rather than the survival phase can reduce the number of memory T cells.452 Related studies on FAO are more limited than those on FAS. Lipid peroxidation caused by selenoenzyme glutathione (GSH) peroxidase 4 knockout suppresses the expansion of effector T cells.453
In the context of infection, studies have reported that the metabolism of glutamine in immune cells can influence immune functions.454 Glutamine is an important carbon and nitrogen source for the metabolic reprogramming of Mycobacterium tuberculosis induced M1 macrophage polarization, which is a complementary metabolic program of M1 macrophages in addition to HIF-1α mediated glycolysis upregulation.455 In vitro, glutamine deficiency and glutamine pathway inhibition can reduce the cytokine responses of mononuclear cells and T cells stimulated with Mycobacterium tuberculosis, including IFN-γ, IL-1β, IL-17 and IL-22.456 At the early stage of HIV-1 infection, the TCA cycle and OXPHOS of naive T cells and memory CD4+ T cells are majorly fuelled by glutaminolysis, which favors HIV-1 retrotranscription.447
Immunometabolism in diseases of specific systems and organs
Immunometabolism has inspired novel therapies for many diseases, beyond cancer, autoimmune diseases, metabolic diseases, and infection. Here, we summarize some other representative diseases according to the involved and lesion sites.
Immunometabolism in cardiovascular diseases
Immune cells carry out functions in the myocardium, that can be regulated by intracellular metabolism and metabolites of cardiac parenchymal cells, motivating researchers to focus on immunometabolism in cardiovascular diseases.457
Normal glucose metabolism of immune cells is impaired in cardiovascular diseases. The glycolysis of naive CD4+ T cells from mice with atherosclerosis is downregulated, impairing the proliferation and activation of CD4+ T cells.458 Upon atherosclerosis and myocardial ischemia-reperfusion, glycolysis of macrophages is upregulated, promoting M1 polarization and increasing the levels of inflammatory cytokines.459,460 At the early stage of myocardial infarction, glycolysis of macrophages is upregulated and macrophages are mainly in the M1 phenotype, while at the late stage, glycolysis decreases to basal levels, and macrophages are mainly in the M2 phenotype.461 Additionally, at the late stage of myocardial infarction, the activity of the PPP in macrophages is upregulated.461
The lipid metabolism of macrophages is critical in atherogenesis. Macrophages engulf lipid droplets to form foam cells, populating atherosclerotic plaques, which indicates that lipid uptake by macrophages in atherosclerosis is significantly upregulated.462,463,464 However, the activity of lipid metabolism including FAO in macrophages is insufficient, resulting in lipid accumulation and the development of atherosclerosis.465,466 Excessive lipid accumulation has also been observed in the dendritic cells of atherosclerotic lesions.467 The lipid metabolism patterns of other immune cells in cardiovascular diseases have not been reported.
Although studying amino acid metabolism seems to be more difficult than studying glucose and lipid metabolism, some studies have reported amino acid metabolism or related signals. Leucine is a key activator of mTOR in macrophages and can drive atherosclerosis in mice fed a high-protein diet.468,469
Immunometabolism in neurological disorders
Microglia are a specialized macrophages in the central nervous system.470 Due to the important roles of microglia in neurological disorders, immunometabolism in nervous system diseases has been noted by researchers, with Alzheimer’s disease as a representative disease.471
In multiple neurological disorders, immune cells are changed into a proinflammatory phenotype, with metabolic reprogramming occurring. In Alzheimer’s disease, a decrease in M2b macrophages has been considered an indicator of cognitive function.472 Microglia are activated into the proinflammatory phenotype, with a switch from OXPHOS to glycolysis.473 The glucose uptake of microglia is positively correlated with their activity.474 Nevertheless, in neurodegenerative disorders, the glucose uptake of microglia is upregulated, but glucose hypometabolism occurs in the affected region of the brain.475
Lipid metabolism of microglia differs between individuals with and without Alzheimer’s disease.476,477 Abnormal accumulation of lipid droplets has been observed in microglia from individuals with Alzheimer’s disease, with downregulated expression of BHLHE40/41.478,479 A recent study believes that the APOE4 genotype causes the abnormal lipid metabolism of microglia in Alzheimer’s disease, impairing the phagocytic capabilities of microglia and interactions with neurons.480
We believe that amino acid metabolism of immune cells may change in neurological disorders based on limited evidence. The release of D-serine can be increased by the inflammatory activation of microglia, which affects synaptogenesis and enhances NMDAR-dependent excitotoxicity, resulting in neurodegeneration.481 Two decades ago, a study reported that amyloid β-peptide can induce the release of D-serine in microglia.482 When the nervous system is in an inflammatory state, microglia may metabolize glutamate into glutamine instead of requiring the retention of glutamate.483
Immunometabolism in respiratory disorders
In addition to pulmonary neoplasms and pulmonary infections, immunometabolism also participates in other respiratory disorders. Related studies are limited, and glycometabolism is relatively well-studied.
Upregulated glycolysis and downregulated OXPHOS of M1 macrophages in acute lung injury have been observed, activating NLRP3 inflammasome, and triggering receptor expressed on myeloid cells-1 can mediate this metabolic reprogramming.484,485 In acute rejection after lung transplantation, the glucose uptake of immune cells is increased, with CD8+ T cells being the largest glucose utilizers.486 In pulmonary ischemia-reperfusion injury, glycolysis of activated neutrophils is upregulated, promoting the progression of the disease.487
The changes of amino acid metabolism in many respiratory disorders are still unclear, and the results from existing studies prefer to consider amino acids as signal molecules. In lung injuries caused by hyperoxia, the inhibition of glutamine metabolism and decreased GSH are protective to the diseased individuals, but how this regulation affects the activity of immune cells is unclear.488,489 Glycine can attenuate acute lung injury by decreasing the upregulation of NLRP3 and increasing the downregulation of NRF2.490
Targets and treatments based on immunometabolism
As previously summarized, immune cells with different phenotypes have different metabolic characteristics, and metabolic pathways can impact the functions of immune cells. The phenotypes and metabolic pathways of immune cells undergo changes when diseases occur and develop, prompting researchers to explore potential therapeutic targets by regulating metabolic pathways (Table 2).
Targeting glycolysis of immune cells to treat diseases
Glycolysis is one of the most studied metabolic pathways in immune cells, with the high metabolic flux representing the activated status of immune cells that is typically seen in proinflammatory immune cells.
To reduce inflammation, downregulating glycolysis of immune cells has been studied, which can be suitable for treating autoimmune diseases, metabolic diseases and other inflammatory diseases. HIF-1α is the factor received most attention, and its activation can upregulate glycolysis in immune cells.491 HIF-1α is one of the two subunits of HIF-1, activated in oxygen-free environments, and plays important roles in oxidative stress, cancer progression, and many other diseases.492,493,494 In vitro and in vivo experiments, researchers have tried to suppress HIF-1α to reduce the glycolysis of immune cells, including macrophages and T cells.268,271,493,495,496,497,498 Inhibitors of HIF-1α are numerous, most of which have been used for macrophages in the previous studies, with dioscin being a typical example.495 Dioscin is a natural compound with multiple functions including anti-inflammation and immunoregulation, which also means that dioscin is not specific for targeting HIF-1α expression in macrophages.499 In ulcerative colitis mice, dioscin can reduce M1 polarization and induce M2 polarization. Through the in vitro experiments, the inhibition of mTORC1/HIF-1α pathway is responsible for the reduced glycolysis observed under dioscin treatment.495 The PI3K/Akt pathway is a classic upstream pathway of mTORC1 and HIF-1α signaling.500,501 In T2DM mice treated with autologous blood transfusion, the upregulation of PI3K/Akt pathway in macrophages has been observed simultaneously with reduced levels of HIF-1α and glycolysis.497 In addition to macrophages, the glycolysis of effector T cells can also be reduced by inhibiting HIF-1α.271 An in vitro experiment has shown that costunolide treatment can induce HIF-1α degradation, leading to reduced glycolysis in Th17 cells and decreased Th17 cell differentiation.271 PKM2, a rate-limiting enzyme in glycolysis, is also an important target to control glycolysis in many diseases.411,502,503 The PKM2 inhibitor can alleviate microglial dysfunction of mice with Alzheimer’s disease by disrupting the upregulation of glycolysis.473 PKM2 is downstream of the PI3K/Akt pathway, indicating that inhibiting PKM2 may be one mechanism leading to reduced glycolysis through suppression of HIF-1α.497 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 3 (PFKFB3) is another downstream target of the PI3K/Akt pathway that induces glycolysis. Specific inhibitors of PFKFB3 have been used to reduce glycolysis and activity of inflammatory cells.284,504,505 As we mentioned before, GLUT1 also mediate glycolysis and can be regulated by the PI3K/Akt pathway. When exploring treatment options for blocking the glycolysis to inhibit M1 polarization in cardiac ischemia and reperfusion injury, researchers have found that sDR5-Fc can reduce GLUT1.506 In acute lung injury, phloretin has similar effects.507 With the downregulation of glycolysis in immune cells, the upregulation of AMP-activated protein kinase (AMPK) has been observed.294,508,509 AMPK is a guardian of mitochondrial homeostasis, and also controls lipid and glucose metabolism.510 Metformin, the first-line drug treatment for T2DM, has been found the capability to activate AMPK.511 Researchers have initiated an observational study to validate the capability of metformin in reversing the phenotypes of CD4+ T cells in obese children (NCT03960333). They believe that CD4+ T cells in obese children skew towards effector T cells rather than Treg cells due to upregulated mTOR-driven glycolysis and downregulated AMPK-driven OXPHOS. Unfortunately, their recently published results have not reported the role of metformin which was mentioned in their registration.512 Adiponectin can also inhibit glycolysis and the differentiation of Th17 cells by activating AMPK in obese mice.509 2-deoxyglucose (2-DG) is a well-known glycolytic inhibitor.513,514 In RA rats, 2-DG can promote M2 macrophage polarization through activating AMPK, exerting anti-arthritic effects.294 In treating acute lung injury, 2-DG has shown the ability to reduce NLRP3 inflammasome.487,515 Adiponectin can inhibit the glycolysis of CD4+ T cells through activating AMPK, thereby reducing Th17 cell differentiation.509 The upregulated glycolysis of CD4+ T cells upon obesity can be induced by the increased FAO, promoting the activation of T cells and resulting in proinflammation, which also provides a viewpoint to target glycolysis.334 Carnitine palmitoyltransferase 1 (CPT-1) is a rate-limiting enzyme in FAO. CPT-1a is an important subtype of CPT-1 that stabilizes Goliath, a mitochondrial E3 ubiquitin ligase.334 Researchers have used a specific Goliath inhibitor, DC-Gonib32, to destroy the upregulated glycolysis of CD4+ T cells in obese mice.334
To treat cancer, inhibiting glycolysis of immune cells have also been studied. However, because cancer is dominated by an immunosuppressive environment, inhibiting glycolysis of immune cells in cancer besides immune cell-derived tumors should be discussed separately. JHU083, a glutamine antagonist, can simultaneously inhibit glycolysis in tumor cells and effector CD8+ T cells.516 However, when glycolysis is reduced, OXPHOS is upregulated in the CD8+ T cells, resulting in activating anti-tumor immune responses. This mechanism is related to TCA intermediate replenishment, which has been fully introduced.516 The function of L-arginine is similar to glutamine antagonists. L-arginine can induce a shift from glycolysis to OXPHOS in effector T cells, enhancing their anti-tumor immune effects.102 The researchers have conducted a randomized controlled trial to evaluate the efficacy of oral arginine in immune function improvement in patients with glioblastoma multiforme (NCT02017249), but the results have not been published. Hexokinase (HK) 2 is an important rate-limiting enzyme in glycolysis and has been identified as a potential therapeutic target for cancer.517 HK inhibitor can suppress glycolysis to block the PD-L1 expression, resulting in CD8+ T cell activation.518 In summary, contrary to suppressing inflammation, glycolysis inhibitors have shown the anti-tumor immune effects by enhancing OXPHOS, possibly because existing glycolysis inhibitors are unable to specifically target the glycometabolism of immune cells.
Upregulating glycolysis can enhance anti-tumor immune effects of immune cells including macrophages, effector T cells and dendritic cells.175,519,520,521,522 Vismodegib, a small molecule that can inhibit the hedgehog pathway, has been approved for the treatment of advanced basal-cell carcinoma.523 Later, in a breast cancer mouse model, vismodegib has been found to have the capability to shift the metabolism of M2 macrophages from FAO to glycolysis, thus alleviating immunosuppression.520 However, this effect has not been validated in human studies.520 The hedgehog pathway may participate in changing the metabolic patterns of immune cells.
Targeting OXPHOS of immune cells to treat diseases
Also due to the non-specific targeting, to treat cancer, both downregulation and upregulation of OXPHOS have been taken into consideration, with downregulating OXPHOS being the less studied one.
Promising targets for cancer have been provided by existing studies fousing on OXPHOS downregulation of immune cells. FABP5 is a member of FABPs, a protein family known by its ability to promote cellular lipid uptake and intracellular transport, is expressed in T cells.96 Inhibiting FABP5 can cause mitochondrial changes and reduce OXPHOS in Treg cells, thereby impairing the suppressive activity of Treg cells due to the release of IL-10 induced by FABP5.194 IACS-010759 is a OXPHOS inhibitor developed for treating leukemia.524 IACS-010759 prefers to kill cells with Myc activity in the context of lymphoma.525 Myc can regulate metabolic reprogramming in cancer by upregulating glycolysis, and is also involved in mitochondrial diseases.526 However, why the OXPHOS inhibitor can specifically target cells with overexpressed Myc is unclear.526 In addition, in cancers with neoplastic lymphocytes, researchers also inhibit glycolysis as a treatment approach, similar to the downregulation of glycolysis by suppressing Myc function.527,528 It is important to note that regulating the metabolism of neoplastic lymphocytes aims to kill immune cells rather than regulate immune functions.
Anti-inflammatory and anti-tumor effects can be provided by upregulating OXPHOS to treat inflammatory diseases and cancer. The upregulation of OXPHOS is always accompanied by the downregulation of glycolysis, as discussed in the previous sections.529 Upregulating OXPHOS also has anti-inflammatory effects. NX-13, a new gut-restricted drug targeting leucine rich repeat containing X1, can upregulate OXPHOS and reduce NF-κB activation of CD4+ T cells in vitro, resulting in the inhibition of differentiation.282 A phase 1b study has reported that NX-13 is generally safe in patients with ulcerative colitis.530 The study has also showed that rectal bleeding and stool frequency can be improved at week 2, and endoscopic response can be observed at week 4.530 OXPHOS of CD8+ T cells can be significantly upregulated by including 4-1BB domain in the chimeric antigen receptor architecture, helping researchers to improve cancer immunotherapy.531 Linoleic acid, a commonly consumed polyunsaturated fatty acid, exhibits anti-inflammatory and anti-tumor effects.532,533 Although excessive intake of linoleic acid can lead to the formation of oxidized linoleic acid metabolites, increasing the risks of many diseases, linoleic acid also helps us to understand immunometabolism in cancer.534,535 Linoleic acid is a positive regulator of CD8+ T cells, upregulating calcium signaling and mitochondrial energy metabolism in CD8+ T cells.536 In HCC, T cells lose in the competition for linoleic acid with tumor cells, leading to T cell dysfunction and HCC progression.537 This suggests a protective role of linoleic acid in promoting anti-tumor immune responses.537
Targeting lipid metabolism of immune cells to treat diseases
Lipid metabolism of activated or proinflammatory immune cells is predominantly dominated by lipid synthesis rather than lipid catabolism. Researchers have attempted to modulate the lipid metabolism of immune cells to alter the phenotype and function of immune cells for the treatment of diseases.
FAO is the most investigated lipid metabolic pathway in immunometabolism. Regulating FAO in immune cells has been explored to treat multiple disease including cancer, autoimmune diseases, metabolic disease, atherosclerosis and lung inflammation.57,315,334,465,466,495,519,520,538,539 FAO of macrophages is the most studied. Downregulation of FAO can be accompanied by upregulation of glycolysis, resulting in alleviating immunosuppression caused by M2 macrophages, which is one of the effects of the anticancer drug vismodegib.520 RIPK3 is a newly identified lipid metabolism regulator, contributing to the development of fatty liver, liver fibrosis, and HCC, with the signaling not fully explored.540,541 Although the upregulation of RIPK3 appears to be non‐protective in patients, the downregulation of RIPK3 in M2 macrophages during HCC also contributes to tumorigenesis.57 The downregulation of RIPK3 in macrophages can activate PPAR, leading to the upregulation of FAO and M2 polarization. Therefore, researchers have used a RIPK3 activator to downregulate FAO and suppress the immunosuppressive activity of M2 macrophages.57 The inconsistent effects of RIPK3 indicate that specific targeting of metabolism in immune cells is essential. Downregulating FAO in T cells has also been studied. CPT-1, the key rate-limiting enzyme in FAO, can promote cells to adapt to the environment when energy demand changes.542 In mice with MS, CPT-1 inhibitor treatment can alleviate neurological inflammation by reducing overall FAO, including in T cells.315
In most cases, upregulation of FAO in macrophages is usually anti-inflammatory. Upregulated FAO induced by dioscin is accompanied by decreased glycolysis, which collectively enhances M2 macrophage polarization and reduces inflammation in ulcerative colitis mice.495 The mTORC2/PPARγ signaling pathway may be a potential mechanism for dioscin to promote FAO in macrophages.495 The upregulation of PPARα has been repeatedly observed when upregulating FAO in macrophages.465,539 PPAR is a nuclear receptor with capacities of anti-inflammation and metabolic regulation.543 PPARα activators, including the fibrates, are commonly used for lowering lipid levels.544 The anti-inflammatory effects of PPARα activators support the importance of studying immunometabolism. Angiotensin-converting enzyme of macrophages can increase cellular lipid uptake and FAO by upregulating PPARα in the context of atherosclerosis.465 In respiratory syncytial virus induced lung inflammation, Qingfei oral liquid can upregulate PPARα to increase FAO, leading to M2 polarization.539 The Akt activator SC-79 can reduce the anti-inflammatory effects of Qingfei oral liquid by increasing FAS, validating the possible association between PPARα and Akt signaling in immunometabolism.539 However, the upregulated FAO of immune cells in cancer sometimes exhibits proinflammatory effects.538 CD40, a member of TNF receptor superfamily, can be activated to promote T cell activation and tumor infiltration.545 CD40 activation can induce FAO and glutamine metabolism in macrophages, promoting M1 polarization to enhance anti-tumor activity, which provides a side effect of anti-CD40 treatments in cancer.538 CD40 activators provide an anti-tumor effect of macrophages by upregulating FAO, indicating that FAO is not completely negatively associated with pro-inflammation. This could be attributed to its potential crosstalk with other metabolic pathways, which needs further discussion in the future.
The inhibition of lipid synthesis has been used in vitro to treat cancer and rheumatic diseases. Balancing normal immune tolerance and abnormal immunosuppression in the TME caused by Treg cells is a challenging issue. Researchers have found that inhibiting SREBP signaling can inhibit lipid synthesis and the activity of PI3K in Treg cells to suppress immunosuppression, resulting in inhibiting tumor growth without causing autoimmune toxicity.195 In CD8+ T cells from RA patients, LDHA is overexpressed, and researchers have used the LDHA inhibitor FX11 to reduce lipogenesis of CD8+ T cells, leading CD8+ T cells to lose their proinflammatory capacities.297 The results from the two studies have shown that inhibiting lipid synthesis is possibly able to downregulate anti-inflammatory effects of Treg cells and proinflammatory effects of CD8+ T cells, which should be validated in further studies.
Studies have explored the ability of approved drugs in inflammatory diseases and cancer to regulate lipid metabolism in immune cells. Tofacitinib, a Janus kinase inhibitor, is used to treat RA, ulcerative colitis, psoriatic arthritis, and other diseases.546,547,548 In rabbits with chronic arthritis, tofacitinib can reduce lipid accumulation and increase lipid release of macrophages by inhibiting JAK/STAT signaling pathway, thereby reducing systemic and synovial inflammation.549 The mechanism by which orlistat causes apoptosis in B-cell chronic lymphocytic leukemia cells may involve the inhibition of lipidolysis in B cells.198 In summary, studies on immunometabolism can help researchers to understand the mechanisms of current drugs.
Lipid peroxidation is a special lipid metabolism occurring in pathological and physiological conditions, and can regulate several cellular processes through its metabolites.550 In acute myeloid leukemia, an in vitro experiment has shown that the chenodeoxycholic acid treatment can inhibit M2 macrophage polarization by inducing lipid peroxidation through the activation of the p38 MAPK signaling pathway, resulting in the inhibition of tumor cell proliferation.551 However, lipid peroxidation and p38 activation related to CD36 can impair the function of CD8+ T cells in the TME. The use of a p38 inhibitor can restore CD8+ T cell function by upregulating GSH peroxidase 4 and inhibiting lipid peroxidation.192 N-acetylcysteine, a GSH precursor, has been shown to reduce the level of lipid peroxidation biomarker 8-iso-prostaglandin F2α in SLE mice, and activated FoxP3 expression in CD4+ T cells has also been observed.552,553
Other treatments targeting metabolism of immune cells to treat diseases
Although glycometabolism and lipid metabolism of immune cells are still not thoroughly studied, other metabolic pathways have also shown their possible roles in immunometabolism.
Possibly benefiting from numerous studies on cancer metabolism, glutamine is almost the most studied amino acid in the field of immunometabolism. Downregulating glutamine metabolism has been considered as a promising treatment for cancer for a long time, given the addiction of cancer cells to nutrients and hypoxic environments.554,555,556,557 The heterogeneity of glutamine metabolism in cancer promotes researchers to seek more evidence.555 The inhibitor of glutamine metabolism can reduce immunosuppressive cells and increase proinflammatory macrophages.207 Along with the enhanced anti-tumor immune effects of macrophages, the blocked glutamine metabolism also occurs in tumor cells, inhibiting tumor growth.207,558 In triple-negative breast cancer, inhibiting glutamine metabolism can increase the presence of activated CD8+ T cells within the tumor.559 Later, other researchers have used JPH203, a SLC7A5 inhibitor, to target glutamine metabolism, resulting in increased CD8+ T cell infiltration.560 In lung cancer, the SLC7A5 inhibitor DRP-104 can inhibit glutamine-dependent nucleotide synthesis, enhancing the functions of CD4+ and CD8+ T cells while reducing Treg cells.561 In lymphoma, asparaginase can target tumor cells with high demand for glutamine.562 Subsequently, SLC1A1 has been identified as the key regulator that upregulates cellular glutamine metabolism.232 Asparaginase treatment can inhibit glutamine metabolism upregulated by SLC1A1 and restore impaired T-cell immunity in lymphoma.232 In summary, inhibiting glutamine metabolism can both suppress tumor cell growth and enhance anti-tumor immunity, providing potential strategies for other cells with reduced immune activity, such as those affected by infection. However, downregulating glutamine metabolism in cancer may actually upregulate glutamine metabolism of T cells. A metabolic competition exists between tumor cells and T cells. The glutamine transporter inhibitor V-9302 can selectively block glutamine uptake in tumor cells and improve the anti-tumor effects of CD8+ T cells.209 In addition, upregulating glutamine metabolism through the CD40 activator can enhance the proinflammatory and anti-tumor effects of macrophages, indicating that upregulating glutamine metabolism of immune cells is more beneficial for anti-tumor effects rather than immunosuppressive effects.538
Other metabolites or metabolic pathways have been studied in the field of immunometabolism. The serine metabolism of immune cells is also associated with cancer treatment. The serine synthesis inhibitor treatment can enhance NK cell activation and improve the efficacy of anti-PD1 immunotherapy.563 The serine/glycine metabolites can be reduced by lycorine to eliminate B-cell acute lymphoblastic leukemia cells, with phosphoserine aminotransferase 1 as a possible target.564 Lactic acid is a natural energy metabolite which can suppress anti-tumor immunity.565 Lithium carbonate, a mood stabilizer, can promote the transport of lactic acid into mitochondria of CD8+ T cells to obtain energy, resulting in the inhibition of immunosuppression.566
Potential clinical applications related to immunometabolism
Although few drugs which are developed based on immunometabolism have been approved for clinical use (Table 2), existing studies have identified potential clinical applications associated with immunometabolism.
Measuring the metabolic activity of immune cells provides a possible method to perform early diagnosis and prognosis prediction. There are many indicators of cellular metabolism, such as oxygen consumption rate, extracellular acidification rate, mitochondrial membrane potential and enzymatic activity.567,568 CD14+ monocytes isolated from the blood of RA patients and individuals at risk have shown higher levels of glycolytic enzymes, including HIF-1α, HK2, and PFKFB3, than those from healthy individuals.569 The proinflammatory and hypermetabolic phenotype of CD14+ monocytes occur before RA onset, providing a potential method for timely diagnosis and treatment of RA.569 In glomerulonephritis, including nephrotic syndrome, antineutrophil cytoplasmic antibody-associated vasculitis, and SLE nephritis, renal biopsies of patients have shown that enzymes of the PPP are associated with macrophage markers.570 Due to the association between activation of the PPP and reduced kidney function, directly measuring related indicators of peripheral blood monocytes might be a rapid diagnostic method before renal biopsy.570 Isolating peripheral blood monocytes has been widely used in experiments, and the diagnostic and prognostic value of peripheral blood monocytes has been discussed in many diseases.571,572,573,574 In immunometabolism, there is significant interest in exploring the diagnostic and prognostic value of peripheral blood monocytes. Single-cell applications are also helpful for studies on immunometabolism.575 Additionally, some researchers have combined metabolic indicators, including body mass index with inflammatory indicators, such as C-reactive protein levels, to predict antidepressant treatment outcomes. This approach has been also defined as immunometabolism in their study.576
Metabolic regulation can be taken into consideration in cellular therapies based on immune cells. Chimeric antigen receptor (CAR)-T cell therapy is a new and rapidly developing immunotherapy against cancer.577 The interactions between the TME and CAR-T cells critically influence CAR-T cell function.577 Improving anti-tumor activity of CAR-T cells is important. The respiratory capacity and FAO of CD8+ central memory T cells can be significantly enhanced by including the 4-1BB signaling domain in the CAR architecture.531 Adding domains that can enhance the metabolism of immune cells provides a promising method for designing future CAR-T cell therapies. Exogenous immune cells with anti-inflammatory phenotype have been considered as a possible immunotherapy for autoimmune diseases.578 The researchers have infused Treg cells intravenously to four patients with autoimmune hepatitis and measured the metabolism of the exogenous Treg cells.578 Treg infusion has not cause high-grade adverse effects and further research is needed for efficacy.578
The immune checkpoint inhibitor, which targets immune checkpoints suppressing the activity of immune cells, is an important breakthrough in cancer.579 However, resistance to immune checkpoint inhibitors remains common, and metabolic intervention has been considered a promising therapy to enhance anti-tumor immune responses triggered by immune checkpoint inhibitors.580 The association between immune checkpoint inhibitors and the metabolism of immune cells is an important topic in the field of immunometabolism. Before the emergence of immunometabolism, cancer metabolism mainly focused on the abnormal metabolism of tumor cells. The metabolic reprogramming of immune cells in cancer is also important and has been discussed in the previous sections.
Conclusions and perspectives
In this review, we have summarized how metabolism regulates immune effects in health and diseases, a concept known as immunometabolism. Immune cells exhibit abilities to metabolize various nutrients with complex metabolic reprogramming during activation. The multiple metabolic pathways affect the differentiation, phenotypes, and functions of immune cells. Among immune cells, macrophages and T cells are the most studied in the field of immunometabolism. Glycolysis, OXPHOS, FAO are the most studied metabolic pathways in immunometabolism. Immune cells with proinflammatory and anti-inflammatory phenotypes exhibit different metabolic patterns. Upregulation or downregulation of metabolic pathways can change the phenotypes of immune cells, resulting in regulation of immune effects. In the context of diseases, with cancer as the most studied one, metabolic reprogramming occurs in immune cells, promoting proinflammation or immunosuppression. Targeting metabolic pathways provides an attractive for the treatment of multiple diseases.
To translate experimental results into clinical applications, challenges remain in confirming the essential role of immunometabolism in disease treatment. A variety of factors, including different metabolic pathways, different cells and different diseases collectively influence the role of immunometabolism in treating diseases, which can be summarized as heterogeneity.555
First, heterogeneity exists in metabolic pathways and the effects of regulating metabolic pathways. The same regulation of metabolic pathways can lead to different immune effects. Upregulation of OXPHOS can induce anti-inflammatory M2 macrophage polarization, but can also activate T cells with proinflammatory effects.52,80 Upregulation of FAO can induce both anti-inflammatory polarization and inflammasome activation of macrophages.57,58 Active glutamine metabolism is associated with both M2 polarization and proinflammatory T cell differentiation.60,61,62,63,104 How generic metabolic pathways regulate immune effects is not sufficiently understood. It is more difficult to elaborate on the heterogeneity of metabolic pathways between immune cells and other cells, and further among different immune cells. Targeting metabolism of immune cells specifically is still difficult. A limitation of our study is that we have not discussed the crosstalk of different metabolic pathways in immune cells due to the limited evidence. A classic study in immunometabolism has discussed the intersection of glutamine metabolism and the TCA cycle in macrophages, but just one study may not be sufficient.60 In addition, available studies have paid much attention on glycolysis, but OXPHOS might have better targetability.52 Furthermore, existing studies on immunometabolism still focused on cellular metabolism of immune cells. How to expand cellular metabolic processes to metabolic processes without confusion is a big challenge for future studies on immunometabolism.
Second, heterogeneity exists in different cells. While the immunometabolism of macrophages and T cells are the most studied, it is important to recognize that other immune cells also play significant roles in the field of immunometabolism. We have summarized some metabolic pathways of B cells, but how metabolism affects the functions of B cells in diseases is not fully described. The number of subtypes of T cells are significantly more than that of macrophages. Further subdividing subtypes of immune cells may pose difficulties in research, but it can also obtain more reliable results. We guess that the contradictory results regarding the metabolism of macrophages may be resulted from the oversimplified classification of subtypes. Using quantitative indicators to replace M1 and M2 subtypes might be feasible. Additionally, Immunometabolism of other immune cells, including NK cells and ILCs, remains unclear. Besides immune cells, other cells relevant to various diseases should also be taken into consideration, including tumor cells in cancer, synovial fibroblasts in RA, adipocytes in obesity, and hepatocytes in NAFLD. If targeting conserved metabolic pathways, upregulating metabolism of immune cells can be accompanied by activating the cells that can promote disease development. The association between immune cells and other cells in the field of immunometabolism deserves further study. While the precise isolation of immune cells and specific cell types may be somewhat challenging, it is important.
Third, heterogeneity exists in different diseases. In many fields, cancer is always the most extensively studied disease, but studies on other diseases in immunometabolism are also important. Immune cells in cancer are dominated by an immunosuppressive phenotype, different from many inflammatory diseases such as autoimmune diseases and metabolic diseases. The prominent metabolic reprogramming of tumor cells may confuse the studies on metabolic pathways in immune cells. In cancer, new drugs are rapidly developed, so preclinical studies can be appropriate at present. However, in other diseases, exploring how widely used drugs control disease progression by regulating the metabolism of immune cells may be more feasible. Data from humans is precious. Randomized controlled trials are difficult to conduct, and observational studies are also valuable for obtaining data from humans. Distinguishing altered metabolism in tissues and immune cells can also be challenging, but important for exploring specific targets in immune cells, especially in diseases characterized by significant metabolism changes including obesity and T2DM.
In conclusion, metabolic pathways and metabolic reprogramming influence the phenotypes and functions of immune cells, thereby promoting disease progression. Based on experimental results, targeting the metabolic regulation of immune cells is a promising therapy for multiple diseases. To translate these experimental results into clinical applications, obtaining data from humans and elucidating mechanisms of immunometabolism in various metabolic pathways, different cells, and different diseases is necessary.
References
Ganeshan, K. & Chawla, A. Metabolic regulation of immune responses. Annu. Rev. Immunol. 32, 609–634 (2014).
Ning, L., Shishi, Z., Bo, W. & Huiqing, L. Targeting immunometabolism against acute lung injury. Clin. Immunol. 249, 109289 (2023).
Mathis, D. & Shoelson, S. E. Immunometabolism: an emerging frontier. Nat. Rev. Immunol. 11, 81 (2011).
Fullerton, M. D., Steinberg, G. R. & Schertzer, J. D. Immunometabolism of AMPK in insulin resistance and atherosclerosis. Mol. Cell Endocrinol. 366, 224–234 (2013).
Schipper, H. S., Prakken, B., Kalkhoven, E. & Boes, M. Adipose tissue-resident immune cells: key players in immunometabolism. Trends Endocrinol. Metab. 23, 407–415 (2012).
Martinez-Santibañez, G. & Lumeng, C. N. Macrophages and the regulation of adipose tissue remodeling. Annu. Rev. Nutr. 34, 57–76 (2014).
Rathmell, J. C. Metabolism and autophagy in the immune system: immunometabolism comes of age. Immunol. Rev. 249, 5–13 (2012).
Pearce, E. L. & Pearce, E. J. Metabolic pathways in immune cell activation and quiescence. Immunity 38, 633–643 (2013).
Mockler, M. B., Conroy, M. J. & Lysaght, J. Targeting T cell immunometabolism for cancer immunotherapy; understanding the impact of the tumor microenvironment. Front. Oncol. 4, 107 (2014).
Pearce, E. L., Poffenberger, M. C., Chang, C. H. & Jones, R. G. Fueling immunity: insights into metabolism and lymphocyte function. Science 342, 1242454 (2013).
Silveira Rossi, J. L. et al. Metabolic syndrome and cardiovascular diseases: going beyond traditional risk factors. Diab. Metab. Res. Rev. 38, e3502 (2022).
Kelly, B. & O’Neill, L. A. Metabolic reprogramming in macrophages and dendritic cells in innate immunity. Cell Res. 25, 771–784 (2015).
Mantovani, A. et al. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 23, 549–555 (2002).
Zhu, L. et al. Cellular metabolism and macrophage functional polarization. Int Rev. Immunol. 34, 82–100 (2015).
Mantovani, A. et al. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 25, 677–686 (2004).
Zhang, Q. & Sioud, M. Tumor-associated macrophage subsets: shaping polarization and targeting. Int. J. Mol. Sci. 24, 7493 (2023).
Nakai, K. Multiple roles of macrophage in skin. J. Dermatol Sci. 104, 2–10 (2021).
Arora, S. et al. Macrophages: their role, activation and polarization in pulmonary diseases. Immunobiology 223, 383–396 (2018).
Huang, X., Li, Y., Fu, M. & Xin, H. B. Polarizing macrophages in vitro. Methods Mol. Biol. 1784, 119–126 (2018).
Colin, S., Chinetti-Gbaguidi, G. & Staels, B. Macrophage phenotypes in atherosclerosis. Immunol. Rev. 262, 153–166 (2014).
Viola, A. et al. The metabolic signature of macrophage responses. Front. Immunol. 10, 1462 (2019).
Van den Bossche, J., O’Neill, L. A. & Menon, D. Macrophage immunometabolism: where are we (going)? Trends Immunol. 38, 395–406 (2017).
Warburg, O., Gawehn, K. & Geissler, A. W. Metabolism of leukocytes. Z. Naturforsch. B 13b, 515–516 (1958).
Warburg, O., Negelein, E. & Posener, K. Versuche an überlebendem Ca. Gewebe. Klin. Wochenschr (1923).
Warburg, O., Posener, K. & Negelein, E. Über den stoffwechsel der carcinomzelle. Naturwissenschaften 12, 1131–1137 (1924).
Vaupel, P. & Multhoff, G. Revisiting the Warburg effect: historical dogma versus current understanding. J. Physiol. 599, 1745–1757 (2021).
Wang, N. et al. PIWIL1 governs the crosstalk of cancer cell metabolism and immunosuppressive microenvironment in hepatocellular carcinoma. Signal Transduct. Target. Ther. 6, 86 (2021).
Barros, L. F. et al. Aerobic glycolysis in the brain: warburg and crabtree contra pasteur. Neurochem. Res. 46, 15–22 (2021).
Vallée, A., Lecarpentier, Y., Guillevin, R. & Vallée, J. N. Aerobic glycolysis in amyotrophic lateral sclerosis and Huntington’s disease. Rev. Neurosci. 29, 547–555 (2018).
Chang, C. H. et al. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell 153, 1239–1251 (2013).
Minton, K. Immunometabolism: what is the point of warburg? Nat. Rev. Immunol. 13, 472 (2013).
Poznanski, S. M., Barra, N. G., Ashkar, A. A. & Schertzer, J. D. Immunometabolism of T cells and NK cells: metabolic control of effector and regulatory function. Inflamm. Res. 67, 813–828 (2018).
Krejčová, G. et al. Drosophila macrophages switch to aerobic glycolysis to mount effective antibacterial defense. Elife 8, e50414 (2019).
Yuan, Y. et al. The transcription factor KLF14 regulates macrophage glycolysis and immune function by inhibiting HK2 in sepsis. Cell Mol. Immunol. 19, 504–515 (2022).
Wang, T. et al. HIF1α-induced glycolysis metabolism is essential to the activation of inflammatory macrophages. Mediat. Inflamm. 2017, 9029327 (2017).
Tannahill, G. M. et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature 496, 238–242 (2013).
Kim, Y. J. et al. Cassiaside C inhibits M1 polarization of macrophages by downregulating glycolysis. Int J. Mol. Sci. 23, 1696 (2022).
Palsson-McDermott, E. M. et al. Pyruvate kinase M2 regulates Hif-1α activity and IL-1β induction and is a critical determinant of the warburg effect in LPS-activated macrophages. Cell Metab. 21, 65–80 (2015).
Wang, J. et al. Lactylation of PKM2 suppresses inflammatory metabolic adaptation in pro-inflammatory macrophages. Int. J. Biol. Sci. 18, 6210–6225 (2022).
O’Neill, L. A. & Pearce, E. J. Immunometabolism governs dendritic cell and macrophage function. J. Exp. Med. 213, 15–23 (2016).
Williams, N. C. & O’Neill, L. A. J. A role for the krebs cycle intermediate citrate in metabolic reprogramming in innate immunity and inflammation. Front. Immunol. 9, 141 (2018).
Wu, J. Y. et al. Cancer-derived succinate promotes macrophage polarization and cancer metastasis via succinate receptor. Mol. Cell 77, 213–227.e215 (2020).
Duan, J. X. et al. Extracellular citrate serves as a DAMP to activate macrophages and promote LPS-induced lung injury in mice. Int. Immunopharmacol. 101, 108372 (2021).
Wu, X. et al. Metabolic control during macrophage polarization by a citrate-functionalized scaffold for maintaining bone homeostasis. Adv. Healthc. Mater. e2400770 (2024). https://doi.org/10.1002/adhm.202400770.
Collins, S. L. et al. mTORC1 signaling regulates proinflammatory macrophage function and metabolism. J. Immunol. 207, 913–922 (2021).
Rao, J. et al. FSTL1 promotes liver fibrosis by reprogramming macrophage function through modulating the intracellular function of PKM2. Gut 71, 2539–2550 (2022).
Zhang, Y. et al. M2 macrophage exosome-derived lncRNA AK083884 protects mice from CVB3-induced viral myocarditis through regulating PKM2/HIF-1α axis mediated metabolic reprogramming of macrophages. Redox Biol. 69, 103016 (2024).
Nagy, C. & Haschemi, A. Time and demand are two critical dimensions of immunometabolism: the process of macrophage activation and the pentose phosphate pathway. Front. Immunol. 6, 164 (2015).
Dionísio, F., Tomas, L. & Schulz, C. Glycolytic side pathways regulating macrophage inflammatory phenotypes and functions. Am. J. Physiol. Cell Physiol. 324, C558–c564 (2023).
He, D. et al. Pentose phosphate pathway regulates tolerogenic apoptotic cell clearance and immune tolerance. Front. Immunol. 12, 797091 (2021).
Nakamizo, S. et al. Activation of the pentose phosphate pathway in macrophages is crucial for granuloma formation in sarcoidosis. J. Clin. Investig. 133, e171088 (2023).
Wculek, S. K. et al. Oxidative phosphorylation selectively orchestrates tissue macrophage homeostasis. Immunity 56, 516–530.e519 (2023).
Wang, F. et al. Glycolytic stimulation is not a requirement for m2 macrophage differentiation. Cell Metab. 28, 463–475.e464 (2018).
Dang, C. P. & Leelahavanichkul, A. Over-expression of miR-223 induces M2 macrophage through glycolysis alteration and attenuates LPS-induced sepsis mouse model, the cell-based therapy in sepsis. PLoS One 15, e0236038 (2020).
Yan, J. & Horng, T. Lipid metabolism in regulation of macrophage functions. Trends Cell Biol. 30, 979–989 (2020).
Batista-Gonzalez, A., Vidal, R., Criollo, A. & Carreño, L. J. New insights on the role of lipid metabolism in the metabolic reprogramming of macrophages. Front. Immunol. 10, 2993 (2019).
Wu, L. et al. RIPK3 orchestrates fatty acid metabolism in tumor-associated macrophages and hepatocarcinogenesis. Cancer Immunol. Res. 8, 710–721 (2020).
Nomura, M. et al. Fatty acid oxidation in macrophage polarization. Nat. Immunol. 17, 216–217 (2016).
Li, X. et al. Macrophage HIF-2α suppresses NLRP3 inflammasome activation and alleviates insulin resistance. Cell Rep. 36, 109607 (2021).
Jha, A. K. et al. Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Immunity 42, 419–430 (2015).
Hu, X. et al. Glutamine metabolic microenvironment drives M2 macrophage polarization to mediate trastuzumab resistance in HER2-positive gastric cancer. Cancer Commun.43, 909–937 (2023).
Zhu, Y. et al. Cigarette smoke promotes oral leukoplakia via regulating glutamine metabolism and M2 polarization of macrophage. Int. J. Oral. Sci. 13, 25 (2021).
Zhu, Y. et al. Glutamine mitigates murine burn sepsis by supporting macrophage M2 polarization through repressing the SIRT5-mediated desuccinylation of pyruvate dehydrogenase. Burns Trauma 10, tkac041 (2022).
Liu, P. S. et al. α-ketoglutarate orchestrates macrophage activation through metabolic and epigenetic reprogramming. Nat. Immunol. 18, 985–994 (2017).
Shang, M. et al. Macrophage-derived glutamine boosts satellite cells and muscle regeneration. Nature 587, 626–631 (2020).
Wang, C. et al. Serine synthesis sustains macrophage IL-1β production via NAD(+)-dependent protein acetylation. Mol. Cell 84, 744–759.e746 (2024).
Shan, X. et al. Serine metabolism orchestrates macrophage polarization by regulating the IGF1-p38 axis. Cell Mol. Immunol. 19, 1263–1278 (2022).
Rodriguez, A. E. et al. Serine metabolism supports macrophage IL-1β production. Cell Metab. 29, 1003–1011.e1004 (2019).
Ouyang, Y. et al. FGFR3 alterations in bladder cancer stimulate serine synthesis to induce immune-inert macrophages that suppress t-cell recruitment and activation. Cancer Res. 83, 4030–4046 (2023).
Li, J. et al. Crocin alleviates coronary atherosclerosis via inhibiting lipid synthesis and inducing M2 macrophage polarization. Int. Immunopharmacol. 55, 120–127 (2018).
Meana, C. et al. Lipin-1 integrates lipid synthesis with proinflammatory responses during TLR activation in macrophages. J. Immunol. 193, 4614–4622 (2014).
Lee, J. H. et al. SREBP-1a-stimulated lipid synthesis is required for macrophage phagocytosis downstream of TLR4-directed mTORC1. Proc. Natl Acad. Sci. USA 115, E12228–e12234 (2018).
Almeida, L., Lochner, M., Berod, L. & Sparwasser, T. Metabolic pathways in T cell activation and lineage differentiation. Semin. Immunol. 28, 514–524 (2016).
Kidani, Y. et al. Sterol regulatory element-binding proteins are essential for the metabolic programming of effector T cells and adaptive immunity. Nat. Immunol. 14, 489–499 (2013).
Xu, X. et al. Madecassic acid, the contributor to the anti-colitis effect of madecassoside, enhances the shift of Th17 toward Treg cells via the PPARγ/AMPK/ACC1 pathway. Cell Death Dis. 8, e2723 (2017).
Pinzon Grimaldos, A. et al. The role of lipid metabolism in shaping the expansion and the function of regulatory T cells. Clin. Exp. Immunol. 208, 181–192 (2022).
van den Broek, T., Borghans, J. A. M. & van Wijk, F. The full spectrum of human naive T cells. Nat. Rev. Immunol. 18, 363–373 (2018).
Chen, H., Yang, T., Zhu, L. & Zhao, Y. Cellular metabolism on T-cell development and function. Int. Rev. Immunol. 34, 19–33 (2015).
Angelin, A. et al. Foxp3 reprograms T Cell metabolism to function in low-glucose, high-lactate environments. Cell Metab. 25, 1282–1293.e1287 (2017).
Raud, B. et al. Fatty acid metabolism in CD8(+) T cell memory: challenging current concepts. Immunol. Rev. 283, 213–231 (2018).
Cribioli, E. et al. Enforcing GLUT3 expression in CD8(+) T cells improves fitness and tumor control by promoting glucose uptake and energy storage. Front. Immunol. 13, 976628 (2022).
Frauwirth, K. A. et al. The CD28 signaling pathway regulates glucose metabolism. Immunity 16, 769–777 (2002).
Macintyre, A. N. et al. The glucose transporter Glut1 is selectively essential for CD4 T cell activation and effector function. Cell Metab. 20, 61–72 (2014).
Zhang, H. et al. TCR activation directly stimulates PYGB-dependent glycogenolysis to fuel the early recall response in CD8(+) memory T cells. Mol. Cell 82, 3077–3088.e3076 (2022).
Shan, J., Jin, H. & Xu, Y. T cell metabolism: a new perspective on Th17/Treg cell imbalance in systemic lupus erythematosus. Front. Immunol. 11, 1027 (2020).
Gerriets, V. A. et al. Metabolic programming and PDHK1 control CD4+ T cell subsets and inflammation. J. Clin. Investig. 125, 194–207 (2015).
Zhang, S. et al. The alterations in and the role of the Th17/treg balance in metabolic diseases. Front Immunol. 12, 678355 (2021).
Lou, W. et al. Lipid metabolic features of T cells in the tumor microenvironment. Lipids Health Dis. 21, 94 (2022).
Kim, Y. C. & Guan, K. L. mTOR: a pharmacologic target for autophagy regulation. J. Clin. Investig. 125, 25–32 (2015).
Huang, H. et al. mTOR signaling at the crossroads of environmental signals and T-cell fate decisions. Immunol. Rev. 295, 15–38 (2020).
Lochner, M., Berod, L. & Sparwasser, T. Fatty acid metabolism in the regulation of T cell function. Trends Immunol. 36, 81–91 (2015).
Angela, M. et al. Fatty acid metabolic reprogramming via mTOR-mediated inductions of PPARγ directs early activation of T cells. Nat. Commun. 7, 13683 (2016).
Chyau, C. C. et al. Antrodan alleviates high-fat and high-fructose diet-induced fatty liver disease in C57BL/6 mice model via AMPK/Sirt1/SREBP-1c/PPARγ pathway. Int J. Mol. Sci. 21, 360 (2020).
Teresi, R. E., Planchon, S. M., Waite, K. A. & Eng, C. Regulation of the PTEN promoter by statins and SREBP. Hum. Mol. Genet. 17, 919–928 (2008).
Maciolek, J. A., Pasternak, J. A. & Wilson, H. L. Metabolism of activated T lymphocytes. Curr. Opin. Immunol. 27, 60–74 (2014).
Pan, Y. et al. Survival of tissue-resident memory T cells requires exogenous lipid uptake and metabolism. Nature 543, 252–256 (2017).
Endo, Y., Kanno, T. & Nakajima, T. Fatty acid metabolism in T-cell function and differentiation. Int. Immunol. 34, 579–587 (2022).
Barbi, J., Pardoll, D. & Pan, F. Treg functional stability and its responsiveness to the microenvironment. Immunol. Rev. 259, 115–139 (2014).
Pereira, J. A. et al. PD-1 and CTLA-4 exert additive control of effector regulatory T cells at homeostasis. Front. Immunol. 14, 997376 (2023).
Wang, W. & Zou, W. Amino acids and their transporters in T cell immunity and cancer therapy. Mol. Cell 80, 384–395 (2020).
Ron-Harel, N. et al. T cell activation depends on extracellular alanine. Cell Rep. 28, 3011–3021.e3014 (2019).
Geiger, R. et al. L-arginine modulates T cell metabolism and enhances survival and anti-tumor activity. Cell 167, 829–842.e813 (2016).
Vinogradova, E. V. et al. An activity-guided map of electrophile-cysteine interactions in primary human T cells. Cell 182, 1009–1026.e1029 (2020).
Nakaya, M. et al. Inflammatory T cell responses rely on amino acid transporter ASCT2 facilitation of glutamine uptake and mTORC1 kinase activation. Immunity 40, 692–705 (2014).
Ananieva, E. A., Powell, J. D. & Hutson, S. M. Leucine metabolism in T cell activation: mTOR signaling and beyond. Adv. Nutr. 7, 798s–805s (2016).
Roy, D. G. et al. Methionine metabolism shapes t helper cell responses through regulation of epigenetic reprogramming. Cell Metab. 31, 250–266.e259 (2020).
Ma, E. H. et al. Serine is an essential metabolite for effector t cell expansion. Cell Metab. 25, 345–357 (2017).
Pieper, K., Grimbacher, B. & Eibel, H. B-cell biology and development. J. Allergy Clin. Immunol. 131, 959–971 (2013).
Prieto, J. M. B. & Felippe, M. J. B. Development, phenotype, and function of non-conventional B cells. Comp. Immunol. Microbiol. Infect. Dis. 54, 38–44 (2017).
Rosser, E. C. & Mauri, C. Regulatory B cells: origin, phenotype, and function. Immunity 42, 607–612 (2015).
Raza, I. G. A. & Clarke, A. J. B. Cell metabolism and autophagy in autoimmunity. Front. Immunol. 12, 681105 (2021).
Frasca, D. & Blomberg, B. B. Obesity accelerates age defects in mouse and human B cells. Front. Immunol. 11, 2060 (2020).
Chapman, N. M. & Chi, H. Metabolic adaptation of lymphocytes in immunity and disease. Immunity 55, 14–30 (2022).
Boothby, M. & Rickert, R. C. Metabolic regulation of the immune humoral response. Immunity 46, 743–755 (2017).
Caro-Maldonado, A. et al. Metabolic reprogramming is required for antibody production that is suppressed in anergic but exaggerated in chronically BAFF-exposed B cells. J. Immunol. 192, 3626–3636 (2014).
Cambier, J. C., Gauld, S. B., Merrell, K. T. & Vilen, B. J. B-cell anergy: from transgenic models to naturally occurring anergic B cells? Nat. Rev. Immunol. 7, 633–643 (2007).
Galicia-Vázquez, G. & Aloyz, R. Metabolic rewiring beyond Warburg in chronic lymphocytic leukemia: how much do we actually know? Crit. Rev. Oncol. Hematol. 134, 65–70 (2019).
Noble, R. A. et al. Simultaneous targeting of glycolysis and oxidative phosphorylation as a therapeutic strategy to treat diffuse large B-cell lymphoma. Br. J. Cancer 127, 937–947 (2022).
Weisel, F. J. et al. Germinal center B cells selectively oxidize fatty acids for energy while conducting minimal glycolysis. Nat. Immunol. 21, 331–342 (2020).
Zhou, C. J. et al. Short-chain fatty acids promote immunotherapy by modulating immune regulatory property in B cells. J. Immunol. Res. 2021, 2684361 (2021).
Tian, G. X. et al. Propionic acid regulates immune tolerant properties in B Cells. J. Cell Mol. Med. 26, 2766–2776 (2022).
Jiang, S., Yan, W., Wang, S. E. & Baltimore, D. Let-7 suppresses B cell activation through restricting the availability of necessary nutrients. Cell Metab. 27, 393–403.e394 (2018).
Le, A. et al. Glucose-independent glutamine metabolism via TCA cycling for proliferation and survival in B cells. Cell Metab. 15, 110–121 (2012).
Sumikawa, M. H. et al. An enhanced mitochondrial function through glutamine metabolism in plasmablast differentiation in systemic lupus erythematosus. Rheumatol. Oxf. 61, 3049–3059 (2022).
Mielle, J. et al. Glutamine promotes the generation of B10(+) cells via the mTOR/GSK3 pathway. Eur. J. Immunol. 52, 418–430 (2022).
Wu, M. et al. Glutamine promotes intestinal SIgA secretion through intestinal microbiota and IL-13. Mol. Nutr. Food Res. 60, 1637–1648 (2016).
Infantino, S. et al. Arginine methylation catalyzed by PRMT1 is required for B cell activation and differentiation. Nat. Commun. 8, 891 (2017).
Hata, K. & Mizuguchi, J. Arginine methylation regulates antibody responses through modulating cell division and isotype switching in B cells. Microbiol. Immunol. 57, 185–192 (2013).
Dagenais-Lussier, X. et al. Latest developments in tryptophan metabolism: understanding its role in B cell immunity. Cytokine Growth Factor Rev. 59, 111–117 (2021).
Wang, Z. et al. Leucine-tRNA-synthase-2-expressing B cells contribute to colorectal cancer immunoevasion. Immunity 55, 1067–1081.e1068 (2022).
Liew, P. X. & Kubes, P. The neutrophil’s role during health and disease. Physiol. Rev. 99, 1223–1248 (2019).
Kolaczkowska, E. & Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 13, 159–175 (2013).
Herrero-Cervera, A., Soehnlein, O. & Kenne, E. Neutrophils in chronic inflammatory diseases. Cell Mol. Immunol. 19, 177–191 (2022).
Curi, R. et al. The critical role of cell metabolism for essential neutrophil functions. Cell Physiol. Biochem 54, 629–647 (2020).
Willson, J. A. et al. Neutrophil HIF-1α stabilization is augmented by mitochondrial ROS produced via the glycerol 3-phosphate shuttle. Blood 139, 281–286 (2022).
You, Z. & Chi, H. Lipid metabolism in dendritic cell biology. Immunol. Rev. 317, 137–151 (2023).
Macri, C., Pang, E. S., Patton, T. & O’Keeffe, M. Dendritic cell subsets. Semin Cell Dev. Biol. 84, 11–21 (2018).
Worbs, T., Hammerschmidt, S. I. & Förster, R. Dendritic cell migration in health and disease. Nat. Rev. Immunol. 17, 30–48 (2017).
Sen, K. et al. NCoR1 controls immune tolerance in conventional dendritic cells by fine-tuning glycolysis and fatty acid oxidation. Redox Biol. 59, 102575 (2023).
Liu, J. et al. CCR7 chemokine receptor-inducible lnc-Dpf3 restrains dendritic cell migration by inhibiting HIF-1α-mediated glycolysis. Immunity 50, 600–615.e615 (2019).
Basit, F. & de Vries, I. J. M. Dendritic cells require PINK1-mediated phosphorylation of BCKDE1α to promote fatty acid oxidation for immune function. Front. Immunol. 10, 2386 (2019).
Rehman, A. et al. Role of fatty-acid synthesis in dendritic cell generation and function. J. Immunol. 190, 4640–4649 (2013).
Goretzki, A. et al. Role of glycolysis and fatty acid synthesis in the activation and T cell-modulating potential of dendritic cells stimulated with a TLR5-ligand allergen fusion protein. Int. J. Mol. Sci. 23, 12695 (2022).
Herber, D. L. et al. Lipid accumulation and dendritic cell dysfunction in cancer. Nat. Med. 16, 880–886 (2010).
Spits, H. et al. Innate lymphoid cells–a proposal for uniform nomenclature. Nat. Rev. Immunol. 13, 145–149 (2013).
Jacquelot, N., Seillet, C., Vivier, E. & Belz, G. T. Innate lymphoid cells and cancer. Nat. Immunol. 23, 371–379 (2022).
Vivier, E. et al. Innate lymphoid cells: 10 Years On. Cell 174, 1054–1066 (2018).
Gardiner, C. M. NK cell metabolism. J. Leukoc. Biol. 105, 1235–1242 (2019).
Pelletier, A. & Stockmann, C. The metabolic basis of ILC plasticity. Front. Immunol. 13, 858051 (2022).
Cong, J. et al. Dysfunction of natural killer cells by FBP1-induced inhibition of glycolysis during lung cancer progression. Cell Metab. 28, 243–255.e245 (2018).
Hu, X. et al. Downregulation of NK cell activities in Apolipoprotein C-III-induced hyperlipidemia resulting from lipid-induced metabolic reprogramming and crosstalk with lipid-laden dendritic cells. Metabolism 120, 154800 (2021).
Sheppard, S. et al. Fatty acid oxidation fuels natural killer cell responses against infection and cancer. Proc. Natl Acad. Sci. USA 121, e2319254121 (2024).
Koh, J. et al. De novo fatty-acid synthesis protects invariant NKT cells from cell death, thereby promoting their homeostasis and pathogenic roles in airway hyperresponsiveness. Elife 12, RP87536 (2023).
Kobayashi, T. & Mattarollo, S. R. Natural killer cell metabolism. Mol. Immunol. 115, 3–11 (2019).
Son, S. E., Koh, J. M. & Im, D. S. Free fatty acid receptor 4 (FFA4) activation attenuates obese asthma by suppressing adiposity and resolving metaflammation. Biomed. Pharmacother. 174, 116509 (2024).
Gury-BenAri, M. et al. The spectrum and regulatory landscape of intestinal innate lymphoid cells are shaped by the microbiome. Cell 166, 1231–1246.e1213 (2016).
Wilhelm, C. et al. Critical role of fatty acid metabolism in ILC2-mediated barrier protection during malnutrition and helminth infection. J. Exp. Med. 213, 1409–1418 (2016).
Surace, L. et al. Dichotomous metabolic networks govern human ILC2 proliferation and function. Nat. Immunol. 22, 1367–1374 (2021).
Di Luccia, B. et al. ILC3s integrate glycolysis and mitochondrial production of reactive oxygen species to fulfill activation demands. J. Exp. Med. 216, 2231–2241 (2019).
Fachi, J. L. et al. Hypoxia enhances ILC3 responses through HIF-1α-dependent mechanism. Mucosal Immunol. 14, 828–841 (2021).
Golonka, R. M. & Vijay-Kumar, M. Atypical immunometabolism and metabolic reprogramming in liver cancer: Deciphering the role of gut microbiome. Adv. Cancer Res. 149, 171–255 (2021).
Hay, N. Reprogramming glucose metabolism in cancer: can it be exploited for cancer therapy? Nat. Rev. Cancer 16, 635–649 (2016).
Chang, C. H. et al. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell 162, 1229–1241 (2015).
Dang, Q. et al. Cancer immunometabolism: advent, challenges, and perspective. Mol. Cancer 23, 72 (2024).
Singer, K. et al. Immunometabolism in cancer at a glance. Dis. Model Mech. 11, dmm034272 (2018).
Kamarajugadda, S. et al. Glucose oxidation modulates anoikis and tumor metastasis. Mol. Cell Biol. 32, 1893–1907 (2012).
Lian, X. et al. Immunometabolic rewiring in tumorigenesis and anti-tumor immunotherapy. Mol. Cancer 21, 27 (2022).
Xu, K. et al. Glycolytic ATP fuels phosphoinositide 3-kinase signaling to support effector T helper 17 cell responses. Immunity 54, 976–987.e977 (2021).
Stine, Z. E., Schug, Z. T., Salvino, J. M. & Dang, C. V. Targeting cancer metabolism in the era of precision oncology. Nat. Rev. Drug Discov. 21, 141–162 (2022).
Mehla, K. & Singh, P. K. Metabolic regulation of macrophage polarization in cancer. Trends Cancer 5, 822–834 (2019).
Xia, Y. et al. Engineering macrophages for cancer immunotherapy and drug delivery. Adv. Mater. 32, e2002054 (2020).
Morrissey, S. M. et al. Tumor-derived exosomes drive immunosuppressive macrophages in a pre-metastatic niche through glycolytic dominant metabolic reprogramming. Cell Metab. 33, 2040–2058.e2010 (2021).
Shi, Q. et al. Increased glucose metabolism in TAMs fuels O-GlcNAcylation of lysosomal Cathepsin B to promote cancer metastasis and chemoresistance. Cancer Cell 40, 1207–1222.e1210 (2022).
Lu, Y. et al. MondoA-thioredoxin-interacting protein axis maintains regulatory t-cell identity and function in colorectal cancer microenvironment. Gastroenterology 161, 575–591.e516 (2021).
Hamaidi, I. et al. Sirt2 inhibition enhances metabolic fitness and effector functions of tumor-reactive T cells. Cell Metab. 32, 420–436.e412 (2020).
He, W. et al. CD155T/TIGIT signaling regulates CD8(+) T-cell metabolism and promotes tumor progression in human gastric cancer. Cancer Res. 77, 6375–6388 (2017).
Kochenderfer, J. N. & Rosenberg, S. A. Treating B-cell cancer with T cells expressing anti-CD19 chimeric antigen receptors. Nat. Rev. Clin. Oncol. 10, 267–276 (2013).
Chu, T. W., Kosak, K. M., Shami, P. J. & Kopeček, J. Drug-free macromolecular therapeutics induce apoptosis of patient chronic lymphocytic leukemia cells. Drug Deliv. Transl. Res. 4, 389–394 (2014).
Simon-Molas, H., Del Prete, R. & Kabanova, A. Glucose metabolism in B cell malignancies: a focus on glycolysis branching pathways. Mol. Oncol. 18, 1777–1794 (2023).
Pi, M. et al. Targeting metabolism to overcome cancer drug resistance: a promising therapeutic strategy for diffuse large B cell lymphoma. Drug. Resist. Updat. 61, 100822 (2022).
Poulaki, A. & Giannouli, S. Metabolic swifts govern normal and malignant B cell lymphopoiesis. Int. J. Mol. Sci. 22, 8269 (2021).
Müschen, M. Metabolic gatekeepers to safeguard against autoimmunity and oncogenic B cell transformation. Nat. Rev. Immunol. 19, 337–348 (2019).
Chen, Z. et al. Characterization of metabolic alterations of chronic lymphocytic leukemia in the lymph node microenvironment. Blood 140, 630–643 (2022).
Guo, C. et al. Immunometabolism: a new target for improving cancer immunotherapy. Adv. Cancer Res. 143, 195–253 (2019).
Su, P. et al. Enhanced lipid accumulation and metabolism are required for the differentiation and activation of tumor-associated macrophages. Cancer Res. 80, 1438–1450 (2020).
Liu, Z. et al. Lipid-associated macrophages in the tumor-adipose microenvironment facilitate breast cancer progression. Oncoimmunology 11, 2085432 (2022).
Huggins, D. N. et al. Characterizing macrophage diversity in metastasis-bearing lungs reveals a lipid-associated macrophage subset. Cancer Res. 81, 5284–5295 (2021).
Liu, S. et al. S100A4 enhances protumor macrophage polarization by control of PPAR-γ-dependent induction of fatty acid oxidation. J. Immunother. Cancer 9, e002548 (2021).
Wu, H. et al. Lipid droplet-dependent fatty acid metabolism controls the immune suppressive phenotype of tumor-associated macrophages. EMBO Mol. Med. 11, e10698 (2019).
Masetti, M. et al. Lipid-loaded tumor-associated macrophages sustain tumor growth and invasiveness in prostate cancer. J. Exp. Med. 219, e20210564 (2022).
Wang, R., Liu, Z., Fan, Z. & Zhan, H. Lipid metabolism reprogramming of CD8(+) T cell and therapeutic implications in cancer. Cancer Lett. 567, 216267 (2023).
Xu, S. et al. Uptake of oxidized lipids by the scavenger receptor CD36 promotes lipid peroxidation and dysfunction in CD8(+) T cells in tumors. Immunity 54, 1561–1577.e1567 (2021).
Sun, H. et al. Single-cell transcriptome analysis indicates fatty acid metabolism-mediated metastasis and immunosuppression in male breast cancer. Nat. Commun. 14, 5590 (2023).
Field, C. S. et al. Mitochondrial integrity regulated by lipid metabolism is a cell-intrinsic checkpoint for treg suppressive function. Cell Metab. 31, 422–437.e425 (2020).
Lim, S. A. et al. Lipid signalling enforces functional specialization of T(reg) cells in tumours. Nature 591, 306–311 (2021).
Yang, F. & Wan, F. Lipid metabolism in tumor-associated B cells. Adv. Exp. Med. Biol. 1316, 133–147 (2021).
Sun, L. et al. PPAR-delta modulates membrane cholesterol and cytokine signaling in malignant B cells. Leukemia 32, 184–193 (2018).
Pallasch, C. P. et al. Targeting lipid metabolism by the lipoprotein lipase inhibitor orlistat results in apoptosis of B-cell chronic lymphocytic leukemia cells. Leukemia 22, 585–592 (2008).
Huang, J. et al. The PPARα agonist fenofibrate suppresses B-cell lymphoma in mice by modulating lipid metabolism. Biochim. Biophys. Acta 1831, 1555–1565 (2013).
Sun, Z., Zhang, L. & Liu, L. Reprogramming the lipid metabolism of dendritic cells in tumor immunomodulation and immunotherapy. Biomed. Pharmacother. 167, 115574 (2023).
Niavarani, S. R. et al. Lipid accumulation impairs natural killer cell cytotoxicity and tumor control in the postoperative period. BMC Cancer 19, 823 (2019).
Tang, W. et al. Aberrant cholesterol metabolic signaling impairs antitumor immunosurveillance through natural killer T cell dysfunction in obese liver. Cell Mol. Immunol. 19, 834–847 (2022).
Kobayashi, T. et al. Increased lipid metabolism impairs NK cell function and mediates adaptation to the lymphoma environment. Blood 136, 3004–3017 (2020).
Li, Z. & Zhang, H. Reprogramming of glucose, fatty acid and amino acid metabolism for cancer progression. Cell Mol. Life Sci. 73, 377–392 (2016).
Yang, L. et al. Amino acid metabolism in immune cells: essential regulators of the effector functions, and promising opportunities to enhance cancer immunotherapy. J. Hematol. Oncol. 16, 59 (2023).
Liu, J. et al. Prediction of prognosis, immunogenicity and efficacy of immunotherapy based on glutamine metabolism in lung adenocarcinoma. Front. Immunol. 13, 960738 (2022).
Oh, M. H. et al. Targeting glutamine metabolism enhances tumor-specific immunity by modulating suppressive myeloid cells. J. Clin. Investig. 130, 3865–3884 (2020).
Raines, L. N. et al. PERK is a critical metabolic hub for immunosuppressive function in macrophages. Nat. Immunol. 23, 431–445 (2022).
Edwards, D. N. et al. Selective glutamine metabolism inhibition in tumor cells improves antitumor T lymphocyte activity in triple-negative breast cancer. J. Clin. Investig. 131, e140100 (2021).
Huang, M. et al. Targeting glutamine metabolism to enhance immunoprevention of EGFR-Driven lung cancer. Adv. Sci. 9, e2105885 (2022).
Kampen, K. R. et al. Translatome analysis reveals altered serine and glycine metabolism in T-cell acute lymphoblastic leukemia cells. Nat. Commun. 10, 2542 (2019).
Colegio, O. R. et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature 513, 559–563 (2014).
Wang, Z. H. et al. Lactate in the tumour microenvironment: from immune modulation to therapy. EBioMedicine 73, 103627 (2021).
Jin, M. et al. Tumor-derived lactate creates a favorable niche for tumor via supplying energy source for tumor and modulating the tumor microenvironment. Front. Cell Dev. Biol. 10, 808859 (2022).
Fang, X. et al. Lactate induces tumor-associated macrophage polarization independent of mitochondrial pyruvate carrier-mediated metabolism. Int. J. Biol. Macromol. 237, 123810 (2023).
Zhang, A. et al. Lactate-induced M2 polarization of tumor-associated macrophages promotes the invasion of pituitary adenoma by secreting CCL17. Theranostics 11, 3839–3852 (2021).
She, X. et al. SETDB1 Methylates MCT1 promoting tumor progression by enhancing the lactate shuttle. Adv. Sci. 10, e2301871 (2023).
Vadevoo, S. M. P. et al. The macrophage odorant receptor Olfr78 mediates the lactate-induced M2 phenotype of tumor-associated macrophages. Proc. Natl. Acad. Sci. USA 118, e2102434118 (2021).
Liu, N. et al. Lactate inhibits ATP6V0d2 expression in tumor-associated macrophages to promote HIF-2α-mediated tumor progression. J. Clin. Investig. 129, 631–646 (2019).
Yan, C. et al. GPR65 sensing tumor-derived lactate induces HMGB1 release from TAM via the cAMP/PKA/CREB pathway to promote glioma progression. J. Exp. Clin. Cancer Res. 43, 105 (2024).
Oberholtzer, N., Quinn, K. M., Chakraborty, P. & Mehrotra, S. New developments in T Cell immunometabolism and implications for cancer immunotherapy. Cells 11, 708 (2022).
Brand, A. et al. LDHA-associated lactic acid production blunts tumor immunosurveillance by T and NK cells. Cell Metab. 24, 657–671 (2016).
Watson, M. J. et al. Metabolic support of tumour-infiltrating regulatory T cells by lactic acid. Nature 591, 645–651 (2021).
Anderson, K. G., Stromnes, I. M. & Greenberg, P. D. Obstacles posed by the tumor microenvironment to T cell activity: a case for synergistic therapies. Cancer Cell 31, 311–325 (2017).
Gu, J. et al. Tumor metabolite lactate promotes tumorigenesis by modulating MOESIN lactylation and enhancing TGF-β signaling in regulatory T cells. Cell Rep. 39, 110986 (2022).
Ding, R. et al. Lactate modulates RNA splicing to promote CTLA-4 expression in tumor-infiltrating regulatory T cells. Immunity 57, 528–540.e526 (2024).
Wang, K., Zhang, Y. & Chen, Z. N. Metabolic interaction: tumor-derived lactate inhibiting CD8(+) T cell cytotoxicity in a novel route. Signal Transduct. Target. Ther. 8, 52 (2023).
Wang, Z. et al. Tumor-secreted lactate contributes to an immunosuppressive microenvironment and affects CD8 T-cell infiltration in glioblastoma. Front. Immunol. 14, 894853 (2023).
Yu, M. & Zhang, S. Influenced tumor microenvironment and tumor immunity by amino acids. Front. Immunol. 14, 1118448 (2023).
Fu, Q. et al. Tumor-associated macrophage-derived interleukin-23 interlinks kidney cancer glutamine addiction with immune evasion. Eur. Urol. 75, 752–763 (2019).
Guo, C. et al. SLC38A2 and glutamine signalling in cDC1s dictate anti-tumour immunity. Nature 620, 200–208 (2023).
Xiong, J. et al. SLC1A1 mediated glutamine addiction and contributed to natural killer T-cell lymphoma progression with immunotherapeutic potential. EBioMedicine 72, 103614 (2021).
Zhang, Y. et al. Targeted inhibition of the immunoproteasome blocks endothelial MHC class II antigen presentation to CD4(+) T cells in chronic liver injury. Int. Immunopharmacol. 107, 108639 (2022).
Qin, S. et al. Serine protease PRSS23 drives gastric cancer by enhancing tumor associated macrophage infiltration via FGF2. Front. Immunol. 13, 955841 (2022).
Ma, S. et al. Serine enrichment in tumors promotes regulatory T cell accumulation through sphinganine-mediated regulation of c-Fos. Sci. Immunol. 9, eadg8817 (2024).
Morel, L. Immunometabolism in systemic lupus erythematosus. Nat. Rev. Rheumatol. 13, 280–290 (2017).
Choi, S. C. et al. Immune cell metabolism in systemic lupus erythematosus. Curr. Rheumatol. Rep. 18, 66 (2016).
Takeshima, Y., Iwasaki, Y., Fujio, K. & Yamamoto, K. Metabolism as a key regulator in the pathogenesis of systemic lupus erythematosus. Semin. Arthritis Rheum. 48, 1142–1145 (2019).
Jing, C. et al. Macrophage metabolic reprogramming presents a therapeutic target in lupus nephritis. Proc. Natl Acad. Sci. USA 117, 15160–15171 (2020).
Zhao, H., Wen, Z. & Xiong, S. Activated lymphocyte-derived DNA drives glucose metabolic adaptation for inducing macrophage inflammatory response in systemic lupus erythematosus. Cells 12, 2093 (2023).
Wang, L. X. et al. M2b macrophage polarization and its roles in diseases. J. Leukoc. Biol. 106, 345–358 (2019).
Li, W. et al. Targeting T cell activation and lupus autoimmune phenotypes by inhibiting glucose transporters. Front. Immunol. 10, 833 (2019).
Yin, Y. et al. Glucose oxidation is critical for CD4+ T cell activation in a mouse model of systemic lupus erythematosus. J. Immunol. 196, 80–90 (2016).
Yin, Y. et al. Normalization of CD4+ T cell metabolism reverses lupus. Sci. Transl. Med. 7, 274ra218 (2015).
Abboud, G. et al. Glucose requirement of antigen-specific autoreactive B cells and CD4+ T cells. J. Immunol. 210, 377–388 (2023).
Scherlinger, M. et al. Phosphofructokinase P fine-tunes T regulatory cell metabolism, function, and stability in systemic autoimmunity. Sci. Adv. 8, eadc9657 (2022).
Zou, X. et al. Metabolic regulation of follicular helper T cell differentiation in a mouse model of lupus. Immunol. Lett. 247, 13–21 (2022).
Lin, M. et al. Modulation of PKM2 inhibits follicular helper T cell differentiation and ameliorates inflammation in lupus-prone mice. J. Autoimmun. 145, 103198 (2024).
Gabriel, C. L. et al. Autoimmune-mediated glucose intolerance in a mouse model of systemic lupus erythematosus. Am. J. Physiol. Endocrinol. Metab. 303, E1313–E1324 (2012).
Wang, X. Y. et al. c-Myc-driven glycolysis polarizes functional regulatory B cells that trigger pathogenic inflammatory responses. Signal Transduct. Target Ther. 7, 105 (2022).
Sun, W. et al. Lipid Metabolism: immune regulation and therapeutic prospectives in systemic lupus erythematosus. Front. Immunol. 13, 860586 (2022).
Jaroonwitchawan, T. et al. Dysregulation of lipid metabolism in macrophages is responsible for severe endotoxin tolerance in FcgRIIB-deficient lupus mice. Front. Immunol. 11, 959 (2020).
Krishnan, S. et al. Alterations in lipid raft composition and dynamics contribute to abnormal T cell responses in systemic lupus erythematosus. J. Immunol. 172, 7821–7831 (2004).
Jury, E. C. et al. Altered lipid raft-associated signaling and ganglioside expression in T lymphocytes from patients with systemic lupus erythematosus. J. Clin. Investig. 113, 1176–1187 (2004).
Zeng, Q. et al. Spleen fibroblastic reticular cell-derived acetylcholine promotes lipid metabolism to drive autoreactive B cell responses. Cell Metab. 35, 837–854.e838 (2023).
Kono, M., Yoshida, N. & Tsokos, G. C. Amino acid metabolism in lupus. Front. Immunol. 12, 623844 (2021).
Kono, M. et al. Glutaminase 1 inhibition reduces glycolysis and ameliorates lupus-like disease in mrl/lpr mice and experimental autoimmune encephalomyelitis. Arthritis Rheumatol. 71, 1869–1878 (2019).
Zhang, X. et al. Inhibition of glutaminolysis ameliorates lupus by regulating T and B cell subsets and downregulating the mTOR/P70S6K/4EBP1 and NLRP3/caspase-1/IL-1β pathways in MRL/lpr mice. Int. Immunopharmacol. 112, 109133 (2022).
Sharabi, A., Kasper, I. R. & Tsokos, G. C. The serine/threonine protein phosphatase 2A controls autoimmunity. Clin. Immunol. 186, 38–42 (2018).
Pan, W. et al. The regulatory subunit PPP2R2A of PP2A Enhances Th1 and Th17 differentiation through activation of the GEF-H1/RhoA/ROCK signaling pathway. J. Immunol. 206, 1719–1728 (2021).
Crispín, J. C., Apostolidis, S. A., Finnell, M. I. & Tsokos, G. C. Induction of PP2A Bβ, a regulator of IL-2 deprivation-induced T-cell apoptosis, is deficient in systemic lupus erythematosus. Proc. Natl. Acad. Sci. USA 108, 12443–12448 (2011).
Meidan, E. et al. Serine/threonine phosphatase PP2A is essential for optimal B cell function. JCI Insight 5, e130655 (2020).
Kono, M. et al. Decreased expression of serine/arginine-rich splicing factor 1 in T cells from patients with active systemic lupus erythematosus accounts for reduced expression of RasGRP1 and DNA methyltransferase 1. Arthritis Rheumatol. 70, 2046–2056 (2018).
Moulton, V. R., Gillooly, A. R. & Tsokos, G. C. Ubiquitination regulates expression of the serine/arginine-rich splicing factor 1 (SRSF1) in normal and systemic lupus erythematosus (SLE) T cells. J. Biol. Chem. 289, 4126–4134 (2014).
Katsuyama, T. et al. Splicing factor SRSF1 controls T cell hyperactivity and systemic autoimmunity. J. Clin. Investig. 129, 5411–5423 (2019).
Katsuyama, T. et al. Splicing factor SRSF1 controls T cell homeostasis and its decreased levels are linked to lymphopenia in systemic lupus erythematosus. Rheumatology 59, 2146–2155 (2020).
Adolph, T. E. et al. The metabolic nature of inflammatory bowel diseases. Nat. Rev. Gastroenterol. Hepatol. 19, 753–767 (2022).
Zhuang, H. et al. Tiliroside ameliorates ulcerative colitis by restoring the M1/M2 macrophage balance via the HIF-1α/glycolysis pathway. Front. Immunol. 12, 649463 (2021).
Komuro, M. et al. Glucosylceramide in T cells regulates the pathology of inflammatory bowel disease. Biochem. Biophys. Res. Commun. 599, 24–30 (2022).
Lee, H., Jeon, J. H. & Kim, E. S. Mitochondrial dysfunctions in T cells: focus on inflammatory bowel disease. Front. Immunol. 14, 1219422 (2023).
Lv, Q. et al. Costunolide ameliorates colitis via specific inhibition of HIF1α/glycolysis-mediated Th17 differentiation. Int. Immunopharmacol. 97, 107688 (2021).
Lv, Q. et al. Norisoboldine, a natural AhR agonist, promotes Treg differentiation and attenuates colitis via targeting glycolysis and subsequent NAD(+)/SIRT1/SUV39H1/H3K9me3 signaling pathway. Cell Death Dis. 9, 258 (2018).
Lu, H. et al. Cyclosporine modulates neutrophil functions via the SIRT6-HIF-1α-glycolysis axis to alleviate severe ulcerative colitis. Clin. Transl. Med. 11, e334 (2021).
Karaskova, E. et al. Role of adipose tissue in inflammatory bowel disease. Int. J. Mol. Sci. 22, 4226 (2021).
Xu, J. et al. Inhibition of FABP5 attenuates inflammatory bowel disease by modulating macrophage alternative activation. Biochem. Pharm. 219, 115974 (2024).
Liang, G. et al. Fatty acid oxidation promotes apoptotic resistance and proinflammatory phenotype of CD4(+) tissue-resident memory t cells in crohn’s disease. Cell Mol. Gastroenterol. Hepatol. 17, 939–964 (2024).
Li, Y. et al. Human RIPK1 deficiency causes combined immunodeficiency and inflammatory bowel diseases. Proc. Natl. Acad. Sci. USA 116, 970–975 (2019).
Honjo, H. et al. RIPK2 as a new therapeutic target in inflammatory bowel diseases. Front. Pharm. 12, 650403 (2021).
Parkhouse, R. & Monie, T. P. Dysfunctional crohn’s disease-associated NOD2 polymorphisms Cannot be reliably predicted on the basis of RIPK2 binding or membrane association. Front. Immunol. 6, 521 (2015).
Lai, Y. et al. Discovery of a novel RIPK2 inhibitor for the treatment of inflammatory bowel disease. Biochem. Pharm. 214, 115647 (2023).
Lee, S. H. et al. Inhibition of RIPK3 pathway attenuates intestinal inflammation and cell death of inflammatory bowel disease and suppresses necroptosis in peripheral mononuclear cells of ulcerative colitis patients. Immune Netw. 20, e16 (2020).
Leber, A. et al. Activation of NLRX1 by NX-13 alleviates inflammatory bowel disease through immunometabolic mechanisms in CD4(+) T cells. J. Immunol. 203, 3407–3415 (2019).
Liu, Z. et al. The kinase LRRK2 is a regulator of the transcription factor NFAT that modulates the severity of inflammatory bowel disease. Nat. Immunol. 12, 1063–1070 (2011).
Zhou, Z. et al. Increased stromal PFKFB3-mediated glycolysis in inflammatory bowel disease contributes to intestinal inflammation. Front. Immunol. 13, 966067 (2022).
Ni, S., Liu, Y., Zhong, J. & Shen, Y. Inhibition of LncRNA-NEAT1 alleviates intestinal epithelial cells (IECs) dysfunction in ulcerative colitis by maintaining the homeostasis of the glucose metabolism through the miR-410-3p-LDHA axis. Bioengineered 13, 8961–8971 (2022).
Yan, D. et al. Fatty acids and lipid mediators in inflammatory bowel disease: from mechanism to treatment. Front. Immunol. 14, 1286667 (2023).
Sands, B. E. et al. Lipid profiles in patients with ulcerative colitis receiving tofacitinib-implications for cardiovascular risk and patient management. Inflamm. Bowel Dis. 27, 797–808 (2021).
Qiu, J. et al. Metabolic control of autoimmunity and tissue inflammation in rheumatoid arthritis. Front. Immunol. 12, 652771 (2021).
Weyand, C. M. & Goronzy, J. J. Immunometabolism in early and late stages of rheumatoid arthritis. Nat. Rev. Rheumatol. 13, 291–301 (2017).
Pucino, V. et al. Metabolic checkpoints in rheumatoid arthritis. Front. Physiol. 11, 347 (2020).
Gan, P. R. et al. Glycolysis, a driving force of rheumatoid arthritis. Int. Immunopharmacol. 132, 111913 (2024).
Zezina, E., Sercan-Alp, O., Herrmann, M. & Biesemann, N. Glucose transporter 1 in rheumatoid arthritis and autoimmunity. Wiley Interdiscip. Rev. Syst. Biol. Med. 12, e1483 (2020).
Kim, B. et al. Cytoplasmic zinc promotes IL-1β production by monocytes and macrophages through mTORC1-induced glycolysis in rheumatoid arthritis. Sci. Signal 15, eabi7400 (2022).
Cai, W. et al. The glycolysis inhibitor 2-deoxyglucose ameliorates adjuvant-induced arthritis by regulating macrophage polarization in an AMPK-dependent manner. Mol. Immunol. 140, 186–195 (2021).
Umar, S. et al. Metabolic regulation of RA macrophages is distinct from RA fibroblasts and blockade of glycolysis alleviates inflammatory phenotype in both cell types. Cell Mol. Life Sci. 78, 7693–7707 (2021).
Harshan, S., Dey, P. & Raghunathan, S. Altered transcriptional regulation of glycolysis in circulating cd8(+) t cells of rheumatoid arthritis patients. Genes 13, 1216 (2022).
Souto-Carneiro, M. M. et al. Effect of increased lactate dehydrogenase a activity and aerobic glycolysis on the proinflammatory profile of autoimmune CD8+ T cells in rheumatoid arthritis. Arthritis Rheumatol. 72, 2050–2064 (2020).
Zeng, Q. H. et al. B cells polarize pathogenic inflammatory T helper subsets through ICOSL-dependent glycolysis. Sci. Adv. 6, eabb6296 (2020).
Kvacskay, P. et al. Increase of aerobic glycolysis mediated by activated T helper cells drives synovial fibroblasts towards an inflammatory phenotype: new targets for therapy? Arthritis Res. Ther. 23, 56 (2021).
Suwa, Y., Nagafuchi, Y., Yamada, S. & Fujio, K. The role of dendritic cells and their immunometabolism in rheumatoid arthritis. Front. Immunol. 14, 1161148 (2023).
Qi, J. et al. IL-27 enhances peripheral B cell glycolysis of rheumatoid arthritis patients via activating mTOR signaling. Int. Immunopharmacol. 121, 110532 (2023).
Bockermann, R., Schubert, D., Kamradt, T. & Holmdahl, R. Induction of a B-cell-dependent chronic arthritis with glucose-6-phosphate isomerase. Arthritis Res. Ther. 7, R1316–R1324 (2005).
Kraus, F. V. et al. Reduction of proinflammatory effector functions through remodeling of fatty acid metabolism in CD8+ T cells from rheumatoid arthritis patients. Arthritis Rheumatol. 75, 1098–1109 (2023).
Na, H. S. et al. Th17 and IL-17 cause acceleration of inflammation and fat loss by inducing α(2)-glycoprotein 1 (AZGP1) in rheumatoid arthritis with high-fat diet. Am. J. Pathol. 187, 1049–1058 (2017).
Pucino, V., Bombardieri, M., Pitzalis, C. & Mauro, C. Lactate at the crossroads of metabolism, inflammation, and autoimmunity. Eur. J. Immunol. 47, 14–21 (2017).
Yi, O. et al. Lactate metabolism in rheumatoid arthritis: Pathogenic mechanisms and therapeutic intervention with natural compounds. Phytomedicine 100, 154048 (2022).
Pucino, V. et al. Differential effect of lactate on synovial fibroblast and macrophage effector functions. Front. Immunol. 14, 1183825 (2023).
Pucino, V. et al. Lactate buildup at the site of chronic inflammation promotes disease by inducing Cd4(+) T Cell metabolic rewiring. Cell Metab. 30, 1055–1074.e1058 (2019).
Haas, R. et al. Lactate regulates metabolic and pro-inflammatory circuits in control of T cell migration and effector functions. PLoS Biol. 13, e1002202 (2015).
Marcus, R. What is multiple sclerosis? JAMA 328, 2078 (2022).
Oh, J., Vidal-Jordana, A. & Montalban, X. Multiple sclerosis: clinical aspects. Curr. Opin. Neurol. 31, 752–759 (2018).
Yamout, B. I. & Alroughani, R. Multiple sclerosis. Semin Neurol. 38, 212–225 (2018).
Kunkl, M. et al. CD28 autonomous signaling up-regulates C-Myc expression and promotes glycolysis enabling inflammatory T cell responses in multiple sclerosis. Cells 8, 575 (2019).
La Rocca, C. et al. Immunometabolic profiling of T cells from patients with relapsing-remitting multiple sclerosis reveals an impairment in glycolysis and mitochondrial respiration. Metabolism 77, 39–46 (2017).
Shriver, L. P. & Manchester, M. Inhibition of fatty acid metabolism ameliorates disease activity in an animal model of multiple sclerosis. Sci. Rep. 1, 79 (2011).
Martin-Gutierrez, L. et al. Dysregulated lipid metabolism networks modulate T-cell function in people with relapsing remitting multiple sclerosis. Clin. Exp. Immunol. 217, 204–218 (2024).
Kamermans, A. et al. Reduced angiopoietin-like 4 expression in multiple sclerosis lesions facilitates lipid uptake by phagocytes via modulation of lipoprotein-lipase activity. Front. Immunol. 10, 950 (2019).
Werner, P., Pitt, D. & Raine, C. S. Multiple sclerosis: altered glutamate homeostasis in lesions correlates with oligodendrocyte and axonal damage. Ann. Neurol. 50, 169–180 (2001).
Chrobok, N. L. et al. Tissue transglutaminase appears in monocytes and macrophages but not in lymphocytes in white matter multiple sclerosis lesions. J. Neuropathol. Exp. Neurol. 78, 492–500 (2019).
van Strien, M. E. et al. Tissue transglutaminase contributes to experimental multiple sclerosis pathogenesis and clinical outcome by promoting macrophage migration. Brain. Behav. Immun. 50, 141–154 (2015).
Negrotto, L. & Correale, J. Amino acid catabolism in multiple sclerosis affects immune homeostasis. J. Immunol. 198, 1900–1909 (2017).
Engin, A. The definition and prevalence of obesity and metabolic syndrome. Adv. Exp. Med. Biol. 960, 1–17 (2017).
Qi, X. et al. Multifaceted roles of T cells in obesity and obesity-related complications: a narrative review. Obes. Rev. e13621, (2023).
Sharma, M. et al. Enhanced glycolysis and HIF-1α activation in adipose tissue macrophages sustains local and systemic interleukin-1β production in obesity. Sci. Rep. 10, 5555 (2020).
Weisberg, S. P. et al. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 112, 1796–1808 (2003).
Kawanishi, N., Yano, H., Yokogawa, Y. & Suzuki, K. Exercise training inhibits inflammation in adipose tissue via both suppression of macrophage infiltration and acceleration of phenotypic switching from M1 to M2 macrophages in high-fat-diet-induced obese mice. Exerc. Immunol. Rev. 16, 105–118 (2010).
Murray, P. J. & Wynn, T. A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 11, 723–737 (2011).
Lumeng, C. N., Bodzin, J. L. & Saltiel, A. R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Investig. 117, 175–184 (2007).
Thomas, D. & Apovian, C. Macrophage functions in lean and obese adipose tissue. Metabolism 72, 120–143 (2017).
Schmidt, V., Hogan, A. E., Fallon, P. G. & Schwartz, C. Obesity-mediated immune modulation: one step forward, (Th)2 steps back. Front. Immunol. 13, 932893 (2022).
SantaCruz-Calvo, S. et al. Adaptive immune cells shape obesity-associated type 2 diabetes mellitus and less prominent comorbidities. Nat. Rev. Endocrinol. 18, 23–42 (2022).
Freemerman, A. J. et al. Metabolic reprogramming of macrophages: glucose transporter 1 (GLUT1)-mediated glucose metabolism drives a proinflammatory phenotype. J. Biol. Chem. 289, 7884–7896 (2014).
Dror, E. et al. Postprandial macrophage-derived IL-1β stimulates insulin, and both synergistically promote glucose disposal and inflammation. Nat. Immunol. 18, 283–292 (2017).
Hao, S. et al. Goliath induces inflammation in obese mice by linking fatty acid β-oxidation to glycolysis. EMBO Rep. 24, e56932 (2023).
Ying, W. et al. Adipose tissue B2 cells promote insulin resistance through leukotriene LTB4/LTB4R1 signaling. J. Clin. Investig. 127, 1019–1030 (2017).
Xu, X. et al. Obesity activates a program of lysosomal-dependent lipid metabolism in adipose tissue macrophages independently of classic activation. Cell Metab. 18, 816–830 (2013).
Zhang, C. et al. STAT3 activation-induced fatty acid oxidation in CD8(+) T effector cells is critical for obesity-promoted breast tumor growth. Cell Metab. 31, 148–161.e145 (2020).
Endo, Y. et al. Obesity drives Th17 cell differentiation by inducing the lipid metabolic kinase, ACC1. Cell Rep. 12, 1042–1055 (2015).
Chen, I. C. et al. High-fat diet-induced obesity alters dendritic cell homeostasis by enhancing mitochondrial fatty acid oxidation. J. Immunol. 209, 69–76 (2022).
Engin, A. B. Adipocyte-macrophage cross-talk in obesity. Adv. Exp. Med. Biol. 960, 327–343 (2017).
Eder, K., Baffy, N., Falus, A. & Fulop, A. K. The major inflammatory mediator interleukin-6 and obesity. Inflamm. Res. 58, 727–736 (2009).
El-Kadre, L. J. & Tinoco, A. C. Interleukin-6 and obesity: the crosstalk between intestine, pancreas and liver. Curr. Opin. Clin. Nutr. Metab. Care 16, 564–568 (2013).
Bobbo, V. C. et al. Interleukin-6 actions in the hypothalamus protects against obesity and is involved in the regulation of neurogenesis. J. Neuroinflamm. 18, 192 (2021).
Pacifico, L. et al. Increased T-helper interferon-gamma-secreting cells in obese children. Eur. J. Endocrinol. 154, 691–697 (2006).
Yarla, N. S., Polito, A. & Peluso, I. Effects of Olive Oil on TNF-α and IL-6 in humans: implication in obesity and frailty. Endocr. Metab. Immune. Disord. Drug Targets 18, 63–74 (2018).
Wu, P. et al. Serum TNF-α, GTH and MDA of high-fat diet-induced obesity and obesity resistant rats. Saudi Pharm. J. 24, 333–336 (2016).
Huber, J. et al. CC chemokine and CC chemokine receptor profiles in visceral and subcutaneous adipose tissue are altered in human obesity. J. Clin. Endocrinol. Metab. 93, 3215–3221 (2008).
Kopasov, A. E., Blokhin, S. N., Volkova, E. N. & Morozov, S. G. Chemokine expression in neutrophils and subcutaneous adipose tissue cells obtained during abdominoplasty from patients with obesity and normal body weight. Bull. Exp. Biol. Med. 167, 728–731 (2019).
Dommel, S. & Blüher, M. Does C-C motif chemokine ligand 2 (CCL2) link obesity to a pro-inflammatory state? Int. J. Mol. Sci. 22, 1500 (2021).
Ullah, A. et al. A narrative review: CXC chemokines influence immune surveillance in obesity and obesity-related diseases: Type 2 diabetes and nonalcoholic fatty liver disease. Rev. Endocr. Metab. Disord. 24, 611–631 (2023).
Xia, C., Rao, X. & Zhong, J. Role of T lymphocytes in type 2 diabetes and diabetes-associated inflammation. J. Diab. Res. 2017, 6494795 (2017).
Ehses, J. A. et al. Increased number of islet-associated macrophages in type 2 diabetes. Diabetes 56, 2356–2370 (2007).
Eguchi, K. & Manabe, I. Macrophages and islet inflammation in type 2 diabetes. Diab. Obes. Metab. 15, 152–158 (2013).
Kraakman, M. J., Murphy, A. J., Jandeleit-Dahm, K. & Kammoun, H. L. Macrophage polarization in obesity and type 2 diabetes: weighing down our understanding of macrophage function? Front. Immunol. 5, 470 (2014).
Ni, H. X., Yu, N. J. & Yang, X. H. The study of ginsenoside on PPARgamma expression of mononuclear macrophage in type 2 diabetes. Mol. Biol. Rep. 37, 2975–2979 (2010).
Odegaard, J. I. et al. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature 447, 1116–1120 (2007).
Miya, A. et al. Impact of glucose loading on variations in CD4(+) and CD8(+) T cells in japanese participants with or without type 2 diabetes. Front. Endocrinol. 9, 81 (2018).
Raphael, I., Nalawade, S., Eagar, T. N. & Forsthuber, T. G. T cell subsets and their signature cytokines in autoimmune and inflammatory diseases. Cytokine 74, 5–17 (2015).
Li, J. et al. Effect of type 2 diabetes mellitus and periodontitis on the Th1/Th2 and Th17/Treg paradigm. Am. J. Dent. 35, 55–60 (2022).
Touch, S., Clément, K. & André, S. T cell populations and functions are altered in human obesity and Type 2 diabetes. Curr. Diab Rep. 17, 81 (2017).
Abdel-Moneim, A., Bakery, H. H. & Allam, G. The potential pathogenic role of IL-17/Th17 cells in both type 1 and type 2 diabetes mellitus. Biomed. Pharmacother. 101, 287–292 (2018).
McLaughlin, T. et al. T-cell profile in adipose tissue is associated with insulin resistance and systemic inflammation in humans. Arterioscler. Thromb. Vasc. Biol. 34, 2637–2643 (2014).
Qiao, Y. C. et al. Changes of regulatory T cells and of proinflammatory and immunosuppressive cytokines in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. J. Diab. Res. 2016, 3694957 (2016).
Matsuura, Y. et al. Diabetes suppresses glucose uptake and glycolysis in macrophages. Circ. Res. 130, 779–781 (2022).
Nicholas, D. A. et al. Fatty acid metabolites combine with reduced β oxidation to activate Th17 inflammation in human type 2 diabetes. Cell Metab. 30, 447–461.e445 (2019).
Kristiansen, O. P. & Mandrup-Poulsen, T. Interleukin-6 and diabetes: the good, the bad, or the indifferent? Diabetes 54, S114–S124 (2005).
Akbari, M. & Hassan-Zadeh, V. IL-6 signalling pathways and the development of type 2 diabetes. Inflammopharmacology 26, 685–698 (2018).
Rehman, K. et al. Role of interleukin-6 in development of insulin resistance and type 2 diabetes mellitus. Crit. Rev. Eukaryot. Gene Expr. 27, 229–236 (2017).
Koshino, A. et al. Interleukin-6 and cardiovascular and kidney outcomes in patients with type 2 diabetes: new insights from CANVAS. Diab. Care 45, 2644–2652 (2022).
Alharby, H. et al. Association of lipid peroxidation and interleukin-6 with carotid atherosclerosis in type 2 diabetes. Cardiovasc. Endocrinol. Metab. 8, 73–76 (2019).
Yanik, B. M., Dauch, J. R. & Cheng, H. T. Interleukin-10 reduces neurogenic inflammation and pain behavior in a mouse model of type 2 diabetes. J. Pain. Res. 13, 3499–3512 (2020).
Banerjee, M. & Saxena, M. Interleukin-1 (IL-1) family of cytokines: role in type 2 diabetes. Clin. Chim. Acta 413, 1163–1170 (2012).
Maedler, K., Dharmadhikari, G., Schumann, D. M. & Størling, J. Interleukin-1 beta targeted therapy for type 2 diabetes. Expert Opin. Biol. Ther. 9, 1177–1188 (2009).
Suri, S. et al. Role of interleukin-2 and interleukin-18 in newly diagnosed type 2 diabetes mellitus. J. Basic Clin. Physiol. Pharm. 33, 185–190 (2021).
Zaharieva, E. et al. Interleukin-18 serum level is elevated in type 2 diabetes and latent autoimmune diabetes. Endocr. Connect. 7, 179–185 (2018).
Li, L. et al. Association between interleukin-19 and angiopoietin-2 with vascular complications in type 2 diabetes. J. Diab. Investig. 7, 895–900 (2016).
Gurău, F. et al. Plasma levels of interleukin-38 in healthy aging and in type 2 diabetes. Diab. Res. Clin. Pract. 171, 108585 (2021).
Nussrat, S. W. & Ad’hiah, A. H. Interleukin-39 is a novel cytokine associated with type 2 diabetes mellitus and positively correlated with body mass index. Endocrinol. Diab. Metab. 6, e409 (2023).
Halimi, A. et al. The relation between serum levels of interleukin 10 and interferon-gamma with oral candidiasis in type 2 diabetes mellitus patients. BMC Endocr. Disord. 22, 296 (2022).
Hammad, R. et al. T-Natural killers and interferon gamma/interleukin 4 in augmentation of infection in foot ulcer in type 2 diabetes. Diab. Metab. Syndr. Obes. 14, 1897–1908 (2021).
Stalenhoef, J. E. et al. The role of interferon-gamma in the increased tuberculosis risk in type 2 diabetes mellitus. Eur. J. Clin. Microbiol. Infect. Dis. 27, 97–103 (2008).
Syal, K., Srinivasan, A. & Banerjee, D. VDR, RXR, coronin-1 and interferonγ levels in PBMCs of type-2 diabetes patients: molecular link between diabetes and tuberculosis. Indian J. Clin. Biochem. 30, 323–328 (2015).
Chen, Y. L. et al. Serum TNF-α concentrations in type 2 diabetes mellitus patients and diabetic nephropathy patients: a systematic review and meta-analysis. Immunol. Lett. 186, 52–58 (2017).
Lampropoulou, I. T. et al. TNF-α and microalbuminuria in patients with type 2 diabetes mellitus. J. Diab. Res. 2014, 394206 (2014).
Wu, J. et al. Urinary TNF-α and NGAL are correlated with the progression of nephropathy in patients with type 2 diabetes. Exp. Ther. Med. 6, 1482–1488 (2013).
Herder, C. et al. Chemokines as risk factors for type 2 diabetes: results from the MONICA/KORA Augsburg study, 1984-2002. Diabetologia 49, 921–929 (2006).
Pan, X., Kaminga, A. C., Wen, S. W. & Liu, A. Chemokines in prediabetes and type 2 diabetes: a meta-analysis. Front. Immunol. 12, 622438 (2021).
Bala, M. et al. Type 2 diabetes and lipoprotein metabolism affect LPS-induced cytokine and chemokine release in primary human monocytes. Exp. Clin. Endocrinol. Diab. 119, 370–376 (2011).
Matsushita, Y. et al. Serum C-X-C motif chemokine ligand 14 levels are associated with serum C-peptide and fatty liver index in type 2 diabetes mellitus patients. J. Diab. Investig. 12, 1042–1049 (2021).
Friedman, S. L., Neuschwander-Tetri, B. A., Rinella, M. & Sanyal, A. J. Mechanisms of NAFLD development and therapeutic strategies. Nat. Med. 24, 908–922 (2018).
Barreby, E., Chen, P. & Aouadi, M. Macrophage functional diversity in NAFLD—more than inflammation. Nat. Rev. Endocrinol. 18, 461–472 (2022).
Ioannou, G. N. The role of cholesterol in the pathogenesis of NASH. Trends Endocrinol. Metab. 27, 84–95 (2016).
Govaere, O. et al. Macrophage scavenger receptor 1 mediates lipid-induced inflammation in non-alcoholic fatty liver disease. J. Hepatol. 76, 1001–1012 (2022).
Mridha, A. R. et al. NLRP3 inflammasome blockade reduces liver inflammation and fibrosis in experimental NASH in mice. J. Hepatol. 66, 1037–1046 (2017).
Chatterjee, S. et al. Leptin is key to peroxynitrite-mediated oxidative stress and Kupffer cell activation in experimental non-alcoholic steatohepatitis. J. Hepatol. 58, 778–784 (2013).
Kazankov, K. et al. The role of macrophages in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Nat. Rev. Gastroenterol. Hepatol. 16, 145–159 (2019).
Li, J. et al. Resolvin D1 mitigates non-alcoholic steatohepatitis by suppressing the TLR4-MyD88-mediated NF-κB and MAPK pathways and activating the Nrf2 pathway in mice. Int. Immunopharmacol. 88, 106961 (2020).
Schwabe, R. F., Tabas, I. & Pajvani, U. B. Mechanisms of fibrosis development in nonalcoholic steatohepatitis. Gastroenterology 158, 1913–1928 (2020).
Mao, T., Yang, R., Luo, Y. & He, K. Crucial role of T cells in NAFLD-related disease: a review and prospect. Front Endocrinol. 13, 1051076 (2022).
Belikov, A. V., Schraven, B. & Simeoni, L. T cells and reactive oxygen species. J. Biomed. Sci. 22, 85 (2015).
Chávez, M. D. & Tse, H. M. Targeting mitochondrial-derived reactive oxygen species in T cell-mediated autoimmune diseases. Front. Immunol. 12, 703972 (2021).
Racanelli, V. & Rehermann, B. The liver as an immunological organ. Hepatology 43, S54–S62 (2006).
Deng, C. J. et al. Role of B lymphocytes in the pathogenesis of NAFLD: a 2022 update. Int. J. Mol. Sci. 23, (2022).
Barrow, F. et al. Microbiota-driven activation of intrahepatic B cells aggravates NASH through innate and adaptive signaling. Hepatology 74, 704–722 (2021).
Miyake, T. et al. B cell-activating factor is associated with the histological severity of nonalcoholic fatty liver disease. Hepatol. Int 7, 539–547 (2013).
Kanemitsu-Okada, K. et al. Role of B cell-activating factor in fibrosis progression in a murine model of non-alcoholic steatohepatitis. Int. J. Mol. Sci. 24, 2509 (2023).
Barrow, F., Khan, S., Wang, H. & Revelo, X. S. The emerging role of B cells in the pathogenesis of NAFLD. Hepatology 74, 2277–2286 (2021).
Bruzzì, S. et al. B2-Lymphocyte responses to oxidative stress-derived antigens contribute to the evolution of nonalcoholic fatty liver disease (NAFLD). Free Radic. Biol. Med. 124, 249–259 (2018).
Schmidt-Arras, D. & Rose-John, S. IL-6 pathway in the liver: from physiopathology to therapy. J. Hepatol. 64, 1403–1415 (2016).
Drummer, C. 4th et al. Caspase-11 promotes high-fat diet-induced NAFLD by increasing glycolysis, OXPHOS, and pyroptosis in macrophages. Front. Immunol. 14, 1113883 (2023).
Inomata, Y. et al. Downregulation of miR-122-5p activates glycolysis via PKM2 in Kupffer cells of rat and mouse models of non-alcoholic steatohepatitis. Int. J. Mol. Sci. 23, 5230 (2022).
Dong, T. et al. Activation of GPR3-β-arrestin2-PKM2 pathway in Kupffer cells stimulates glycolysis and inhibits obesity and liver pathogenesis. Nat. Commun. 15, 807 (2024).
Fan, N. et al. Covalent Inhibition of pyruvate kinase M2 reprograms metabolic and inflammatory pathways in hepatic macrophages against non-alcoholic fatty liver disease. Int. J. Biol. Sci. 18, 5260–5275 (2022).
Darmadi, D. & Ruslie, R. H. Association between serum interleukin (IL)-12 level and severity of non-alcoholic fatty liver disease (NAFLD). Rom. J. Intern. Med. 59, 66–72 (2021).
Baselli, G. A. et al. Liver transcriptomics highlights interleukin-32 as novel NAFLD-related cytokine and candidate biomarker. Gut 69, 1855–1866 (2020).
Cao, J. et al. Serum interleukin-38 levels correlated with insulin resistance, liver injury and lipids in non-alcoholic fatty liver disease. Lipids Health Dis. 21, 70 (2022).
Wieckowska, A. et al. Increased hepatic and circulating interleukin-6 levels in human nonalcoholic steatohepatitis. Am. J. Gastroenterol. 103, 1372–1379 (2008).
Widjaja, A. A. et al. Inhibiting Interleukin 11 signaling reduces hepatocyte death and liver fibrosis, inflammation, and steatosis in mouse models of nonalcoholic steatohepatitis. Gastroenterology 157, 777–792.e714 (2019).
Cook, S. A. & Schafer, S. Hiding in plain sight: interleukin-11 emerges as a master regulator of fibrosis, tissue integrity, and stromal inflammation. Annu. Rev. Med. 71, 263–276 (2020).
Li, S., Chen, L. & Lv, G. Interleukin-6 receptor blockade can increase the risk of nonalcoholic fatty liver disease: indications from mendelian randomization. Front. Pharm. 13, 905936 (2022).
He, S. et al. Interleukin-17 weakens the NAFLD/NASH process by facilitating intestinal barrier restoration depending on the gut microbiota. mBio 13, e0368821 (2022).
Gomes, A. L. et al. Metabolic inflammation-associated IL-17A causes non-alcoholic steatohepatitis and hepatocellular carcinoma. Cancer Cell 30, 161–175 (2016).
Hwang, S. et al. Interleukin-22 ameliorates neutrophil-driven nonalcoholic steatohepatitis through multiple targets. Hepatology 72, 412–429 (2020).
Ghazarian, M. et al. Type I interferon responses drive intrahepatic t cells to promote metabolic syndrome. Sci. Immunol. 2, eaai7616 (2017).
Møhlenberg, M. et al. The presence of interferon affects the progression of non-alcoholic fatty liver disease. Genes Immun. 23, 157–165 (2022).
Wieser, V. et al. Adipose type I interferon signalling protects against metabolic dysfunction. Gut 67, 157–165 (2018).
Kakino, S. et al. Pivotal role of TNF-α in the development and progression of nonalcoholic fatty liver disease in a murine model. Horm. Metab. Res. 50, 80–87 (2018).
Tiegs, G. & Horst, A. K. TNF in the liver: targeting a central player in inflammation. Semin. Immunopathol. 44, 445–459 (2022).
Wandrer, F. et al. TNF-Receptor-1 inhibition reduces liver steatosis, hepatocellular injury and fibrosis in NAFLD mice. Cell Death Dis. 11, 212 (2020).
Morikawa, R. et al. Role of CC chemokine receptor 9 in the progression of murine and human non-alcoholic steatohepatitis. J. Hepatol. 74, 511–521 (2021).
Kriss, M. et al. Increased hepatic and circulating chemokine and osteopontin expression occurs early in human NAFLD development. PLoS One 15, e0236353 (2020).
Xu, L., Kitade, H., Ni, Y. & Ota, T. Roles of chemokines and chemokine receptors in obesity-associated insulin resistance and nonalcoholic fatty liver disease. Biomolecules 5, 1563–1579 (2015).
Roh, Y. S. & Seki, E. Chemokines and chemokine receptors in the development of NAFLD. Adv. Exp. Med. Biol. 1061, 45–53 (2018).
Chen, W., Zhang, J., Fan, H. N. & Zhu, J. S. Function and therapeutic advances of chemokine and its receptor in nonalcoholic fatty liver disease. Ther. Adv. Gastroenterol. 11, 1756284818815184 (2018).
Braunersreuther, V., Viviani, G. L., Mach, F. & Montecucco, F. Role of cytokines and chemokines in non-alcoholic fatty liver disease. World J. Gastroenterol. 18, 727–735 (2012).
Xu, L. et al. CC chemokine ligand 3 deficiency ameliorates diet-induced steatohepatitis by regulating liver macrophage recruitment and M1/M2 status in mice. Metabolism 125, 154914 (2021).
Hanson, A. et al. Chemokine ligand 20 (CCL20) expression increases with NAFLD stage and hepatic stellate cell activation and is regulated by miR-590-5p. Cytokine 123, 154789 (2019).
Fan, Z. et al. C-C motif chemokine CCL11 is a novel regulator and a potential therapeutic target in non-alcoholic fatty liver disease. JHEP Rep. 5, 100805 (2023).
Qi, J. et al. CXCL5 promotes lipotoxicity of hepatocytes through upregulating NLRP3/Caspase-1/IL-1β signaling in Kupffer cells and exacerbates nonalcoholic steatohepatitis in mice. Int. Immunopharmacol. 123, 110752 (2023).
Zou, Y. et al. CXCL6 promotes the progression of NAFLD through regulation of PPARα. Cytokine 174, 156459 (2024).
Zhou, C. et al. FABP4 in LSECs promotes CXCL10-mediated macrophage recruitment and M1 polarization during NAFLD progression. Biochim. Biophys. Acta Mol. Basis Dis. 1869, 166810 (2023).
Russell, D. G., Huang, L. & VanderVen, B. C. Immunometabolism at the interface between macrophages and pathogens. Nat. Rev. Immunol. 19, 291–304 (2019).
Li, C. et al. HIF1α-dependent glycolysis promotes macrophage functional activities in protecting against bacterial and fungal infection. Sci. Rep. 8, 3603 (2018).
Lacey, C. A. et al. MyD88-dependent glucose restriction and itaconate production control brucella infection. Infect. Immun. 89, e0015621 (2021).
Rahman, A. N. et al. Elevated glycolysis imparts functional ability to CD8(+) T cells in HIV infection. Life Sci. Alliance 4, e202101081 (2021).
Kavanagh Williamson, M. et al. Upregulation of glucose uptake and hexokinase activity of primary human CD4+ T cells in response to infection with HIV-1. Viruses 10, 114 (2018).
Clerc, I. et al. Entry of glucose- and glutamine-derived carbons into the citric acid cycle supports early steps of HIV-1 infection in CD4 T cells. Nat. Metab. 1, 717–730 (2019).
Sun, Z. et al. CD160 Promotes NK cell functions by upregulating glucose metabolism and negatively correlates With HIV disease progression. Front. Immunol. 13, 854432 (2022).
Modi, N. et al. BRD4 regulates glycolysis-dependent Nos2 expression in macrophages upon H pylori infection. Cell Mol. Gastroenterol. Hepatol. 17, 292–308.e291 (2024).
Codo, A. C. et al. Elevated glucose levels favor SARS-CoV-2 infection and monocyte response through a hif-1α/glycolysis-dependent axis. Cell Metab. 32, 437–446.e435 (2020).
Stüve, P. et al. De novo fatty acid synthesis during mycobacterial infection is a prerequisite for the function of highly proliferative T cells, but not for dendritic cells or macrophages. Front. Immunol. 9, 495 (2018).
Ibitokou, S. A. et al. Early inhibition of fatty acid synthesis reduces generation of memory precursor effector T cells in chronic infection. J. Immunol. 200, 643–656 (2018).
Matsushita, M. et al. T cell lipid peroxidation induces ferroptosis and prevents immunity to infection. J. Exp. Med. 212, 555–568 (2015).
Karinch, A. M. et al. Glutamine metabolism in sepsis and infection. J. Nutr. 131, 2535S–2538S (2001). discussion 2550S-2531S.
Jiang, Q. et al. Glutamine is required for M1-like polarization of macrophages in response to Mycobacterium tuberculosis infection. mBio 13, e0127422 (2022).
Koeken, V. et al. Role of glutamine metabolism in host defense against mycobacterium tuberculosis infection. J. Infect. Dis. 219, 1662–1670 (2019).
DeBerge, M., Chaudhary, R., Schroth, S. & Thorp, E. B. Immunometabolism at the heart of cardiovascular disease. JACC Basic Transl. Sci. 8, 884–904 (2023).
Gaddis, D. E. et al. Atherosclerosis impairs naive CD4 T-cell responses via disruption of glycolysis. Arterioscler Thromb. Vasc. Biol. 41, 2387–2398 (2021).
Li, L. et al. Calenduloside e modulates macrophage polarization via KLF2-regulated glycolysis, contributing to attenuates atherosclerosis. Int. Immunopharmacol. 117, 109730 (2023).
Zhao, M. et al. Salvianolic acid B regulates macrophage polarization in ischemic/reperfused hearts by inhibiting mTORC1-induced glycolysis. Eur. J. Pharm. 871, 172916 (2020).
Mouton, A. J. et al. Temporal changes in glucose metabolism reflect polarization in resident and monocyte-derived macrophages after myocardial infarction. Front. Cardiovasc. Med. 10, 1136252 (2023).
Yuan, Y., Li, P. & Ye, J. Lipid homeostasis and the formation of macrophage-derived foam cells in atherosclerosis. Protein Cell 3, 173–181 (2012).
Sukhorukov, V. N. et al. Lipid metabolism in macrophages: focus on atherosclerosis. Biomedicines. 8, 262 (2020).
Huangfu, N. et al. TDP43 exacerbates atherosclerosis progression by promoting inflammation and lipid uptake of macrophages. Front. Cell Dev. Biol. 9, 687169 (2021).
Cao, D. et al. Macrophage angiotensin-converting enzyme reduces atherosclerosis by increasing peroxisome proliferator-activated receptor α and fundamentally changing lipid metabolism. Cardiovasc. Res. 119, 1825–1841 (2023).
Nomura, M. et al. Macrophage fatty acid oxidation inhibits atherosclerosis progression. J. Mol. Cell Cardiol. 127, 270–276 (2019).
Paulson, K. E. et al. Resident intimal dendritic cells accumulate lipid and contribute to the initiation of atherosclerosis. Circ. Res. 106, 383–390 (2010).
Zhang, X. et al. Identification of a leucine-mediated threshold effect governing macrophage mTOR signalling and cardiovascular risk. Nat. Metab. 6, 359–377 (2024).
Rose, A. J. & Rusu, P. M. A leucine-macrophage mTORC1 connection drives increased risk of atherosclerosis with high-protein diets. Nat. Metab. 6, 203–204 (2024).
Nayak, D., Roth, T. L. & McGavern, D. B. Microglia development and function. Annu. Rev. Immunol. 32, 367–402 (2014).
Shippy, D. C. & Ulland, T. K. Microglial Immunometabolism in Alzheimer’s disease. Front. Cell Neurosci. 14, 563446 (2020).
Hsieh, S. W. et al. M2b macrophage subset decrement as an indicator of cognitive function in Alzheimer’s disease. Psychiatry Clin. Neurosci. 74, 383–391 (2020).
Pan, R. Y. et al. Positive feedback regulation of microglial glucose metabolism by histone H4 lysine 12 lactylation in Alzheimer’s disease. Cell Metab. 34, 634–648.e636 (2022).
Xiang, X. et al. Microglial activation states drive glucose uptake and FDG-PET alterations in neurodegenerative diseases. Sci. Transl. Med. 13, eabe5640 (2021).
Tondo, G. et al. The combined effects of microglia activation and brain glucose hypometabolism in early-onset Alzheimer’s disease. Alzheimers Res. Ther. 12, 50 (2020).
Shippy, D. C. & Ulland, T. K. Lipid metabolism transcriptomics of murine microglia in Alzheimer’s disease and neuroinflammation. Sci. Rep. 13, 14800 (2023).
Xu, J. et al. Integrated lipidomics and proteomics network analysis highlights lipid and immunity pathways associated with Alzheimer’s disease. Transl. Neurodegener. 9, 36 (2020).
Claes, C. et al. Plaque-associated human microglia accumulate lipid droplets in a chimeric model of Alzheimer’s disease. Mol. Neurodegener. 16, 50 (2021).
Podleśny-Drabiniok, A. et al. BHLHE40/41 regulate microglia and peripheral macrophage responses associated with Alzheimer’s disease and other disorders of lipid-rich tissues. Nat. Commun. 15, 2058 (2024).
Hu, X., Ma, Y. N. & Xia, Y. Association between abnormal lipid metabolism and Alzheimer’s disease: new research has revealed significant findings on the APOE4 genotype in microglia. Biosci. Trends 18,195–197 (2024).
Beltrán-Castillo, S., Eugenín, J. & von Bernhardi, R. Impact of aging in microglia-mediated D-serine balance in the CNS. Mediators Inflamm. 2018, 7219732 (2018).
Wu, S. Z. et al. Induction of serine racemase expression and D-serine release from microglia by amyloid beta-peptide. J. Neuroinflamm. 1, 2 (2004).
Nakajima, K., Kanamatsu, T., Takezawa, Y. & Kohsaka, S. Up-regulation of glutamine synthesis in microglia activated with endotoxin. Neurosci. Lett. 591, 99–104 (2015).
Zhong, W. J. et al. TREM-1 governs NLRP3 inflammasome activation of macrophages by firing up glycolysis in acute lung injury. Int. J. Biol. Sci. 19, 242–257 (2023).
Zhang, Y. et al. Paraquat promotes acute lung injury in rats by regulating alveolar macrophage polarization through glycolysis. Ecotoxicol. Environ. Saf. 223, 112571 (2021).
Chen, D. L. et al. Increased T cell glucose uptake reflects acute rejection in lung grafts. Am. J. Transpl. 13, 2540–2549 (2013).
Liao, F. et al. Nanoparticle targeting of neutrophil glycolysis prevents lung ischemia-reperfusion injury. Am. J. Transplant. 24,1382–1394 (2024).
Qin, H. et al. Targeting CXCR1 alleviates hyperoxia-induced lung injury through promoting glutamine metabolism. Cell Rep. 42, 112745 (2023).
Vigeland, C. L. et al. Inhibition of glutamine metabolism accelerates resolution of acute lung injury. Physiol. Rep. 7, e14019 (2019).
Zhang, Y. et al. Glycine attenuates lipopolysaccharide-induced acute lung injury by regulating NLRP3 inflammasome and NRF2 signaling. Nutrients. 12, 611 (2020).
Cheng, S. C. et al. mTOR- and HIF-1α-mediated aerobic glycolysis as metabolic basis for trained immunity. Science 345, 1250684 (2014).
Rashid, M. et al. Up-down regulation of HIF-1α in cancer progression. Gene 798, 145796 (2021).
Meng, Y. et al. Histone methyltransferase SETD2 inhibits M1 macrophage polarization and glycolysis by suppressing HIF-1α in sepsis-induced acute lung injury. Med. Microbiol. Immunol. 212, 369–379 (2023).
Hao, H. et al. HIF-1α promotes astrocytic production of macrophage migration inhibitory factor following spinal cord injury. CNS Neurosci. Ther. 29, 3802–3814 (2023).
Wu, M. M. et al. Dioscin ameliorates murine ulcerative colitis by regulating macrophage polarization. Pharm. Res. 172, 105796 (2021).
Yuan, Y. et al. Adipose-derived mesenchymal stem cells reprogram M1 macrophage metabolism via PHD2/HIF-1α pathway in colitis mice. Front. Immunol. 13, 859806 (2022).
Cheng, Y. et al. Autologous blood transfusion impedes glycolysis in macrophages to inhibit red blood cell injury in type 2 diabetes through PI3K/Akt/PKM2 signaling axis. Acta Diabetol. 60, 481–492 (2023).
Du, N. et al. N-phenethyl-5-phenylpicolinamide alleviates inflammation in acute lung injury by inhibiting HIF-1α/glycolysis/ASIC1a pathway. Life Sci. 309, 120987 (2022).
Tao, X., Yin, L., Xu, L. & Peng, J. Dioscin: a diverse acting natural compound with therapeutic potential in metabolic diseases, cancer, inflammation and infections. Pharm. Res. 137, 259–269 (2018).
Zhang, Z. et al. PI3K/Akt and HIF‑1 signaling pathway in hypoxia‑ischemia (Review). Mol. Med. Rep. 18, 3547–3554 (2018).
Jang, H. J. et al. Nectandrin B-mediated activation of the AMPK pathway prevents cellular senescence in human diploid fibroblasts by reducing intracellular ROS levels. Aging 11, 3731–3749 (2019).
Wang, J. Z. et al. The role of the HIF-1α/ALYREF/PKM2 axis in glycolysis and tumorigenesis of bladder cancer. Cancer Commun. 41, 560–575 (2021).
Dayton, T. L., Jacks, T. & Vander Heiden, M. G. PKM2, cancer metabolism, and the road ahead. EMBO Rep. 17, 1721–1730 (2016).
Hu, X. et al. PI3K-Akt-mTOR/PFKFB3 pathway mediated lung fibroblast aerobic glycolysis and collagen synthesis in lipopolysaccharide-induced pulmonary fibrosis. Lab. Investig. 100, 801–811 (2020).
He, Q., Yin, J., Zou, B. & Guo, H. WIN55212-2 alleviates acute lung injury by inhibiting macrophage glycolysis through the miR-29b-3p/FOXO3/PFKFB3 axis. Mol. Immunol. 149, 119–128 (2022).
Zhai, G. Y. et al. sDR5-Fc inhibits macrophage M1 polarization by blocking the glycolysis. J. Geriatr. Cardiol. 18, 271–280 (2021).
Songyang, Y. et al. The inhibition of GLUT1-induced glycolysis in macrophage by phloretin participates in the protection during acute lung injury. Int. Immunopharmacol. 110, 109049 (2022).
Abboud, G. et al. Inhibition of glycolysis reduces disease severity in an autoimmune model of rheumatoid arthritis. Front. Immunol. 9, 1973 (2018).
Surendar, J. et al. Adiponectin limits IFN-γ and IL-17 producing CD4 T cells in obesity by restraining cell intrinsic glycolysis. Front. Immunol. 10, 2555 (2019).
Herzig, S. & Shaw, R. J. AMPK: guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 19, 121–135 (2018).
Foretz, M. et al. Metformin: from mechanisms of action to therapies. Cell Metab. 20, 953–966 (2014).
Rose, S. et al. Regulatory T cells and bioenergetics of peripheral blood mononuclear cells linked to pediatric obesity. Immunometabolism 6, e00040 (2024).
Laussel, C. & Léon, S. Cellular toxicity of the metabolic inhibitor 2-deoxyglucose and associated resistance mechanisms. Biochem. Pharm. 182, 114213 (2020).
Kang, H. T. & Hwang, E. S. 2-Deoxyglucose: an anticancer and antiviral therapeutic, but not any more a low glucose mimetic. Life Sci. 78, 1392–1399 (2006).
Zhong, W. J. et al. Inhibition of glycolysis alleviates lipopolysaccharide-induced acute lung injury in a mouse model. J. Cell Physiol. 234, 4641–4654 (2019).
Leone, R. D. et al. Glutamine blockade induces divergent metabolic programs to overcome tumor immune evasion. Science 366, 1013–1021 (2019).
Wiel, C. et al. BACH1 stabilization by antioxidants stimulates lung cancer metastasis. Cell 178, 330–345.e322 (2019).
Guo, D. et al. Aerobic glycolysis promotes tumor immune evasion by hexokinase2-mediated phosphorylation of IκBα. Cell Metab. 34, 1312–1324.e1316 (2022).
Kawalekar, O. U. et al. Distinct signaling of coreceptors regulates specific metabolism pathways and impacts memory development in CAR T Cells. Immunity 44, 712 (2016).
Hinshaw, D. C. et al. Hedgehog signaling regulates metabolism and polarization of mammary tumor-associated macrophages. Cancer Res. 81, 5425–5437 (2021).
Hermans, D. et al. Lactate dehydrogenase inhibition synergizes with IL-21 to promote CD8(+) T cell stemness and antitumor immunity. Proc. Natl Acad. Sci. USA 117, 6047–6055 (2020).
Inamdar, S. et al. Rescue of dendritic cells from glycolysis inhibition improves cancer immunotherapy in mice. Nat. Commun. 14, 5333 (2023).
Sekulic, A. et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl. J. Med. 366, 2171–2179 (2012).
Kalyanaraman, B., Cheng, G., Hardy, M. & You, M. OXPHOS-targeting drugs in oncology: new perspectives. Expert Opin. Ther. Targets 27, 939–952 (2023).
Donati, G. et al. Oxidative stress enhances the therapeutic action of a respiratory inhibitor in MYC-driven lymphoma. EMBO Mol. Med. 15, e16910 (2023).
Purhonen, J., Klefström, J. & Kallijärvi, J. MYC-an emerging player in mitochondrial diseases. Front. Cell Dev. Biol. 11, 1257651 (2023).
Xiao, G. et al. B-Cell-specific diversion of glucose carbon utilization reveals a unique vulnerability in B cell malignancies. Cell 173, 470–484.e418 (2018).
Hu, J. et al. WEE1 inhibition induces glutamine addiction in T-cell acute lymphoblastic leukemia. Haematologica 106, 1816–1827 (2021).
Yang, Y. et al. Mitochondrial UQCC3 modulates hypoxia adaptation by orchestrating OXPHOS and glycolysis in hepatocellular carcinoma. Cell Rep. 33, 108340 (2020).
Verstockt, B. et al. The safety, tolerability, pharmacokinetics and clinical efficacy of the NLRX1 agonist NX-13 in active ulcerative colitis: results of a phase 1b study. J. Crohns Colitis, 18, 762–772 (2023).
Kawalekar, O. U. et al. Distinct signaling of coreceptors regulates specific metabolism pathways and impacts memory development in CAR T Cells. Immunity 44, 380–390 (2016).
Whelan, J. & Fritsche, K. Linoleic acid. Adv. Nutr. 4, 311–312 (2013).
Alarcon-Gil, J. et al. Neuroprotective and anti-inflammatory effects of linoleic acid in models of parkinson’s disease: the implication of lipid droplets and lipophagy. Cells. 11, 229 (2022).
Mercola, J. & D’Adamo, C. R. Linoleic acid: a narrative review of the effects of increased intake in the standard american diet and associations with chronic disease. Nutrients. 15, 3129 (2023).
Hildreth, K., Kodani, S. D., Hammock, B. D. & Zhao, L. Cytochrome P450-derived linoleic acid metabolites EpOMEs and DiHOMEs: a review of recent studies. J. Nutr. Biochem 86, 108484 (2020).
Nava Lauson, C. B. et al. Linoleic acid potentiates CD8(+) T cell metabolic fitness and antitumor immunity. Cell Metab. 35, 633–650.e639 (2023).
Ma, K. et al. Hepatocellular carcinoma LINC01116 outcompetes T cells for linoleic acid and accelerates tumor progression. Adv. Sci., e2400676, (2024).
Liu, P. S. et al. CD40 signal rewires fatty acid and glutamine metabolism for stimulating macrophage anti-tumorigenic functions. Nat. Immunol. 24, 452–462 (2023).
An, L. et al. Qingfei oral liquid alleviates RSV-induced lung inflammation by promoting fatty-acid-dependent M1/M2 macrophage polarization via the Akt signaling pathway. J. Ethnopharmacol. 298, 115637 (2022).
Afonso, M. B. et al. RIPK3 acts as a lipid metabolism regulator contributing to inflammation and carcinogenesis in non-alcoholic fatty liver disease. Gut 70, 2359–2372 (2021).
Xu, M. et al. Activated TNF-α/RIPK3 signaling is involved in prolonged high fat diet-stimulated hepatic inflammation and lipid accumulation: inhibition by dietary fisetin intervention. Food Funct. 10, 1302–1316 (2019).
Schlaepfer, I. R. & Joshi, M. CPT1A-mediated fat oxidation, mechanisms, and therapeutic potential. Endocrinology 161, bqz046 (2020).
Bougarne, N. et al. Molecular actions of PPARα in lipid metabolism and inflammation. Endocr. Rev. 39, 760–802 (2018).
Wang, D. et al. Fibrates for secondary prevention of cardiovascular disease and stroke. Cochrane Database Syst. Rev. 2015, Cd009580 (2015).
Vonderheide, R. H. CD40 agonist antibodies in cancer immunotherapy. Annu. Rev. Med. 71, 47–58 (2020).
Fleischmann, R. et al. Placebo-controlled trial of tofacitinib monotherapy in rheumatoid arthritis. N. Engl. J. Med. 367, 495–507 (2012).
Sandborn, W. J. et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 376, 1723–1736 (2017).
Mease, P. et al. Tofacitinib or adalimumab versus placebo for psoriatic arthritis. N. Engl. J. Med. 377, 1537–1550 (2017).
Pérez-Baos, S. et al. Tofacitinib restores the inhibition of reverse cholesterol transport induced by inflammation: understanding the lipid paradox associated with rheumatoid arthritis. Br. J. Pharm. 174, 3018–3031 (2017).
Barrera, G., Pizzimenti, S. & Dianzani, M. U. Lipid peroxidation: control of cell proliferation, cell differentiation and cell death. Mol. Asp. Med. 29, 1–8 (2008).
Liu, J. et al. Chenodeoxycholic acid suppresses AML progression through promoting lipid peroxidation via ROS/p38 MAPK/DGAT1 pathway and inhibiting M2 macrophage polarization. Redox Biol. 56, 102452 (2022).
Lai, Z. W. et al. N-acetylcysteine reduces disease activity by blocking mammalian target of rapamycin in T cells from systemic lupus erythematosus patients: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 64, 2937–2946 (2012).
Li, M. et al. Early-stage lupus nephritis treated with N-acetylcysteine: a report of two cases. Exp. Ther. Med. 10, 689–692 (2015).
Altman, B. J., Stine, Z. E. & Dang, C. V. From Krebs to clinic: glutamine metabolism to cancer therapy. Nat. Rev. Cancer 16, 619–634 (2016).
Cluntun, A. A., Lukey, M. J., Cerione, R. A. & Locasale, J. W. Glutamine metabolism in cancer: understanding the heterogeneity. Trends Cancer 3, 169–180 (2017).
Hensley, C. T., Wasti, A. T. & DeBerardinis, R. J. Glutamine and cancer: cell biology, physiology, and clinical opportunities. J. Clin. Investig. 123, 3678–3684 (2013).
Martinez-Outschoorn, U. E. et al. Cancer metabolism: a therapeutic perspective. Nat. Rev. Clin. Oncol. 14, 11–31 (2017).
Sbirkov, Y. et al. Targeting glutaminolysis shows efficacy in both prednisolone-sensitive and in metabolically rewired prednisolone-resistant b-cell childhood acute lymphoblastic leukaemia cells. Int. J. Mol. Sci. 24, 3378 (2023).
Tang, Y. et al. Simultaneous glutamine metabolism and PD-L1 inhibition to enhance suppression of triple-negative breast cancer. J. Nanobiotechnol. 20, 216 (2022).
Huang, R. et al. Targeting glutamine metabolic reprogramming of SLC7A5 enhances the efficacy of anti-PD-1 in triple-negative breast cancer. Front. Immunol. 14, 1251643 (2023).
Pillai, R. et al. Glutamine antagonist DRP-104 suppresses tumor growth and enhances response to checkpoint blockade in KEAP1 mutant lung cancer. Sci. Adv. 10, eadm9859 (2024).
Sugimoto, K. et al. A clinically attainable dose of L-asparaginase targets glutamine addiction in lymphoid cell lines. Cancer Sci. 106, 1534–1543 (2015).
Liu, N. et al. Eubacterium rectale improves the efficacy of anti-PD1 immunotherapy in melanoma via l-serine-mediated NK cell activation. Research 6, 0127 (2023).
Liu, Y. et al. Lycorine eliminates B-cell acute lymphoblastic leukemia cells by targeting PSAT1 through the serine/glycine metabolic pathway. Eur. J. Pharm. 961, 176162 (2023).
Apostolova, P. & Pearce, E. L. Lactic acid and lactate: revisiting the physiological roles in the tumor microenvironment. Trends Immunol. 43, 969–977 (2022).
Ma, J. et al. Lithium carbonate revitalizes tumor-reactive CD8(+) T cells by shunting lactic acid into mitochondria. Nat. Immunol. 25, 552–561 (2024).
Plitzko, B. & Loesgen, S. Measurement of oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) in culture cells for assessment of the energy metabolism. Bio Protoc. 8, e2850 (2018).
Sakamuru, S., Attene-Ramos, M. S. & Xia, M. Mitochondrial membrane potential assay. Methods Mol. Biol. 1473, 17–22 (2016).
McGarry, T. et al. Rheumatoid arthritis CD14(+) monocytes display metabolic and inflammatory dysfunction, a phenotype that precedes clinical manifestation of disease. Clin. Transl. Immunol. 10, e1237 (2021).
Grayson, P. C. et al. Metabolic pathways and immunometabolism in rare kidney diseases. Ann. Rheum. Dis. 77, 1226–1233 (2018).
Shinohara, S., Hirohata, S., Inoue, T. & Ito, K. Phenotypic analysis of peripheral blood monocytes isolated from patients with rheumatoid arthritis. J. Rheumatol. 19, 211–215 (1992).
Chen, S. et al. Peripheral blood monocytes predict clinical prognosis and support tumor invasiveness through NF-κB-dependent upregulation of Snail in pancreatic cancer. Transl. Cancer Res. 10, 4773–4785 (2021).
Wu, Y. et al. Clinical significance of peripheral blood and tumor tissue lymphocyte subsets in cervical cancer patients. BMC Cancer 20, 173 (2020).
Tang, L., Ye, J., Shi, Y. & Zhu, X. Association between CD16(++) monocytes in peripheral blood and clinical features and short-term therapeutic effects of polycystic ovary syndrome. Int J. Gynaecol. Obstet. 145, 12–17 (2019).
Artyomov, M. N. & Van den Bossche, J. Immunometabolism in the Single-Cell Era. Cell Metab. 32, 710–725 (2020).
Vreijling, S. R. et al. Features of immunometabolic depression as predictors of antidepressant treatment outcomes: pooled analysis of four clinical trials. Br. J. Psychiatry 224, 89–97 (2024).
Sterner, R. C. & Sterner, R. M. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 11, 69 (2021).
Oo, Y. H. et al. Liver homing of clinical grade Tregs after therapeutic infusion in patients with autoimmune hepatitis. JHEP Rep. 1, 286–296 (2019).
Galli, F. et al. Relevance of immune cell and tumor microenvironment imaging in the new era of immunotherapy. J. Exp. Clin. Cancer Res. 39, 89 (2020).
Xiao, C. et al. Immunometabolism: a new dimension in immunotherapy resistance. Front. Med. 17, 585–616 (2023).
Clarke, A. J. et al. B1a B cells require autophagy for metabolic homeostasis and self-renewal. J. Exp. Med. 215, 399–413 (2018).
Zhou, Y. et al. Xanthones from Securidaca inappendiculata Hassk. attenuate collagen-induced arthritis in rats by inhibiting the nicotinamide phosphoribosyltransferase/glycolysis pathway and macrophage polarization. Int. Immunopharmacol. 111, 109137 (2022).
Jiang, W. et al. Peficitinib alleviated acute lung injury by blocking glycolysis through JAK3/STAT3 pathway. Int. Immunopharmacol. 132, 111931 (2024).
Acknowledgements
This work was supported by the National Natural Science Foundation (81970738), the Natural science foundation project of Sichuan (2024NSFSC0599), the Key Research and Development Program of Chengdu (2023-YF09-00052-SN), 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University (No.ZYGD23030), and the National Key Research and Development Program of China (No.2022YFC2304800).
Author information
Authors and Affiliations
Contributions
The author Tengyue Hu wrote the manuscript and created the figures and tables. The author Nannan Zhang provided the conceptual idea. The author Chang-Hai Liu, Min Lei, Qingmin Zeng, Li-Li and Hong Tang edited the manuscript. All authors have read and approved the article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hu, T., Liu, CH., Lei, M. et al. Metabolic regulation of the immune system in health and diseases: mechanisms and interventions. Sig Transduct Target Ther 9, 268 (2024). https://doi.org/10.1038/s41392-024-01954-6
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41392-024-01954-6
This article is cited by
-
Reprogramming the tumor immune microenvironment in cervical cancer: synergy between radiotherapy and immunotherapy
Molecular Cancer (2026)
-
Immuno-metabolic dysregulation in type 2 diabetes is associated with altered neutrophil functional plasticity, mitochondrial dysfunction, and compromised responses in sepsis
Journal of Translational Medicine (2026)
-
Lifelong cGAS deficiency leads to altered lipid storage and cholesterol homeostasis
Biological Research (2026)
-
Association between S100 calcium-binding protein A12 and sepsis associated-acute kidney injury: a prospective cohort study
BMC Nephrology (2026)
-
Lipid metabolism in homeostasis and disease
Signal Transduction and Targeted Therapy (2026)