Abstract
Accumulated evidence has implicated the diverse and substantial influence of lactate on cellular differentiation and fate regulation in physiological and pathological settings, particularly in intricate conditions such as cancer. Specifically, lactate has been demonstrated to be pivotal in molding the tumor microenvironment (TME) through its effects on different cell populations. Within tumor cells, lactate impacts cell signaling pathways, augments the lactate shuttle process, boosts resistance to oxidative stress, and contributes to lactylation. In various cellular populations, the interplay between lactate and immune cells governs processes such as cell differentiation, immune response, immune surveillance, and treatment effectiveness. Furthermore, communication between lactate and stromal/endothelial cells supports basal membrane (BM) remodeling, epithelial-mesenchymal transitions (EMT), metabolic reprogramming, angiogenesis, and drug resistance. Focusing on lactate production and transport, specifically through lactate dehydrogenase (LDH) and monocarboxylate transporters (MCT), has shown promise in the treatment of cancer. Inhibitors targeting LDH and MCT act as both tumor suppressors and enhancers of immunotherapy, leading to a synergistic therapeutic effect when combined with immunotherapy. The review underscores the importance of lactate in tumor progression and provides valuable perspectives on potential therapeutic approaches that target the vulnerability of lactate metabolism, highlighting the Heel of Achilles for cancer treatment.
Similar content being viewed by others
Introduction
Lactate secretion is widely recognized as a classic metabolic hallmark of cancer, often referred to as the Warburg effect. This phenomenon describes the preference of cancer cells to favor glycolysis for energy production, even in the presence of oxygen, leading to increased lactate production.1,2,3,4,5 Recent studies have not only confirmed this understanding but have also delved deeper into the role of lactate in cancer initiation and progression, highlighting its multifaceted contributions beyond merely being a byproduct of cellular metabolism.6,7,8,9,10
The traditional view of lactate as a waste product has been challenged by groundbreaking research employing advanced imaging techniques such as 18F-fluorodeoxyglucose-positron emission tomography (FDG-PET). A pivotal study by DeBerardinis et al. demonstrated that lactate serves as a vital nutrient for tumor regions, which fundamentally alters the perception of its role in cancer biology.11,12 In experiments involving non-small cell lung cancer (NSCLC) xenografts in mice, researchers injected both 13C-glucose and 13C-lactate. They discovered that metabolites derived from 13C-lactate in the tricarboxylic acid (TCA) cycle, such as citrate, glutamate, and malate, were found to be twice as abundant compared to those derived from glucose.13 This observation underscores the notion that lactate can serve as a more central and direct substrate in the TCA cycle, a role that has also been corroborated in healthy tissues as well as genetically engineered lung and pancreatic cancer models.14,15 Using isotope tracing techniques, scientists have gained insights into the transformation of metabolites within tumors, revealing that lactate is a direct carbon source for the TCA cycle. This innovative approach has allowed researchers to visualize how lactate contributes to the metabolic landscape of tumors, providing a clearer understanding of its role in cancer metabolism.
Abnormal lactate-related metabolisms instigate tumor progression. Lactate metabolism and aberrant glucose and fatty acid metabolism induced by lactate map the metabolic evolution in tumor ecosystem, which coordinates tumor progression.
In the context of tumor cells, the lactate shuttle—facilitating the exchange of lactate between anoxic and aerobic tumor regions—plays a crucial role in tumor surveillance and adaptation to changing metabolic conditions.16 Furthermore, lactate impacts both intracellular and extracellular signaling pathways within tumor cells, highlighting its influence on cancer cell behavior and response to therapy.17
What’s more, a significant milestone in lactate research is the discovery of lactylation, a post-translational modification that underscores the intersection of metabolism and epigenetics.18,19,20 Lactylation is not limited to histone proteins; it extends to non-histone proteins, thereby influencing a variety of cellular processes. This modification enhances the interaction between metabolic states and epigenetic regulation, accelerating tumor onset, proliferation, metastasis, and the development of drug resistance. Lactate-induced lactylation operates through multiple molecular mechanisms and signaling pathways, often intersecting with other epigenetic modifications to promote a malignant phenotype.20,21,22
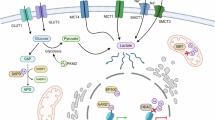
There is such a fountain of researches related to lactate, which is in vital need of further generalization and summarization.22,23,24,25,26,27,28 This review straightens out the circuit and physical role of lactate, sorts out the molecular mechanisms of lactate in tumor progression, and describes lactate-targeted strategies of tumor treatment, which strengthens and specifies the potent implementations of lactate in clinical applications and prognosis improvement (Fig. 1).
Lactate/Lactylation-targeted therapy stands for the Heel of Achilles for cancer treatment. Lactate/lactylation-targeted therapy mitigates the impact of lactate/lactylation on onco-metabolic reprogramming and tumor microenvironment (TME) remodeling, underscoring the Heel of Achilles for cancer treatment. Generated using Adobe Illustrator (Version 28.2). Abbreviations: BM basal membrane, CAFs cancer-associated fibroblasts; EMT epithelial-mesenchymal transitions; LDHA lactate dehydrogenase A
Research history of lactate metabolism and lactylation
In 1780, the Swedish chemist Carl Wilhelm Scheele was the first to isolate lactic acid from sour milk. Since then, its metabolic role and significance in tumors have gradually been clarified (Fig. 2). Soon, Jöns Jacob Berzelius discovered that lactic acid is also produced by muscles during exercise in 1808. Its structure was established by Johannes Wislicenus in 1873.29 Lactic acid is an organic acid with the chemical formula C3H6O3. Lactate is the ionized form of lactic acid. When lactic acid dissolves in water, it can lose a hydrogen ion (proton), resulting in lactate (C3H5O3−).30,31
Milestone events of research on lactate metabolism and lactylation. Since lactate was first discovered in 1780, its metabolic role and significance in tumors have gradually been clarified. With advances in isotope-tracing systems, single-cell sequencing, and probe-based metabolic imaging, the biological properties and functions of lactate and lactylation have been extensively explored. Generated using Adobe Illustrator (Version 28.2). Abbreviations: APOC2 apolipoprotein C-II, DCs dendritic cells, FDG-PET 18F-fluorodeoxyglucose-positron emission tomography, FFA free fatty acids, K80-lac lysine 80 lactylation, MCT1 monocarboxylate transporter 1, MCT4 monocarboxylate transporter 4
Lactate, the end product of glycolysis, was once mischaracterized as a waste since then.29,32,33 But recently, there are growing evidence that lactate is a metabolic fuel for skeletal muscle, heart, brain, and malignant cells, that contributes to cell fate decision-making processes.34,35,36,37 It is also regarded as a metabolic buffer that bridges oxidative phosphorylation (OXPHOS) and glycolysis.38
One classic metabolic reprogramming in tumors is the Warburg effect,36,39,40,41 which is associated with lactate production. In 1927, Warburg found that glycolysis remained the dominant mode of glucose metabolism under aerobic conditions in both homozygous mice and human tumor tissues. The Warburg effect was more dominant in malignant tumors compared to benign tumors.11,32,42,43,44 In 1956, He interpreted the mechanism of this phenomenon as a suppressed mitochondrial function and presented the theory that mitochondrial dysfunction underlies tumor aerobic glycolysis as the core of his metabolic theory regarding the origin of cancer.4,45 In 1972, researcher Efraim Racker was the first to introduce the term “Warburg effect” to describe the increased glycolytic capacity observed in cancer cells.46 However, some studies have challenged the validity of this idea, and the emerging view is that mitochondrial overload leads to an excessive release of lactate.42,47,48,49,50 In 1980, the adoption of the glucose analogue 18F-fluorodeoxyglucose (FDG) in positron emission tomography (PET) could indicate the activity of pyruvate kinase (PK) by tracing glucose metabolism, thus indirectly reflecting the intensity of lactate metabolism through the degree of glycolysis and enabling the quantification of lactate metabolism in vivo.51,52,53,54,55 Studies have shown that the conversion of 13C carbon from glucose to Krebs cycle intermediates (citrate and succinate) or related metabolites is increased in lung cancer samples compared to non-cancerous paraneoplastic tissues.56 Besides, at the single-cell level, it is likely that tumor cells, immune cells, and stromal cells within the TME, which encounter more severe oxygen deprivation, tend to upregulate both glycolysis and mitochondrial OXPHOS.57,58 Furthermore, OXPHOS showed a significant correlation with glycolysis in melanoma and head and neck squamous cell carcinoma (HNSCC) as well as with the response to hypoxia.58,59 This suggests that the traditional view of tumor cell metabolism under hypoxia, characterized by a switch between glycolysis and mitochondrial respiration, may be inaccurate.60,61 Lactate production is not a metabolic driver of cell proliferation and oxidation is the favored metabolic destiny of glucose.
While warburg effect stood for the hallmark of cancer metabolism for about 100 years, recent research as mentioned above have shown that his explanation of the mechanism of aerobic glycolysis is not fully correct.42,62,63,64,65 Lactate can be produced in significant amounts without impairing aerobic respiration and serves as a direct carbon source into the TCA cycle, which will be further described in chapter 3.2.13,14
Additional research has shown that lactate can build a bridge between epigenetics and metabolism through a novel epigenetic modification known as lactylation.66,67,68,69,70 Histone lactylation was first documented in 2019 by Zhang et al. the meaning of which was an addition of a lactyl (La) group to the lysine amino acid residues located in the tails of histone proteins.20 This study demonstrates the definition of histone lysine lactylation (Kla), a novel form of epigenetic modification, that is observed following the translation of proteins obtained from lactate.
As research on lactate and lactylation in cancer continues to flourish, strategies targeting lactate/lactylation have attracted significant interest as potential anticancer treatments.71,72,73,74,75 Consequently, AZD3965, an MCT1 (monocarboxylate transporter 1) inhibitor, has emerged as the first lactate metabolism-targeting drug currently undergoing a phase I/II clinical trial (NCT01791595) for the treatment of advanced solid tumors and non-Hodgkin lymphoma.76,77,78,79,80
Lactate production and transport
When the rate of demand for energy is high, glucose is catabolized and oxidized to pyruvate, which is primarily catalyzed by lactate dehydrogenase (LDH) to produce lactate81,82,83 (Fig. 2). Continuous generation of lactate facilitates the regeneration of NAD+ from NADH.84,85 During the reduction of pyruvate to lactate and the oxidation of NADH to NAD+, the NAD+ consumed by the oxidation of glyceraldehyde-3-phosphate in glycolysis is replenished, thus ensuring sustained glycolytic activity and energy production.86,87,88,89 To prevent lactate accumulation from leading to lactic acidosis, pyruvate dehydrogenase (PDH) catalyzes the production of acetyl coenzyme A from pyruvate, which joins the TCA cycle and irreversibly removes lactate.84,90,91 Lactate buildup has the potential to stimulate gluconeogenesis in skeletal muscle and liver cells, converting lactate into glucose and subsequently releasing it into the bloodstream.92,93,94
Lactate is transported in cells via four reversible monocarboxylate transporters (MCT; e.g., MCT1, MCT4).95,96,97,98 The MCT family achieves lactate exchange across the plasma membrane via H+/lactate cotransport, the direction of which is dependent on the concentration gradient of protons and monocarboxylates.99,100,101 The expulsion of lactate via MCTs removes protons, thereby preserving pH balance within the cytoplasm and inducing acidification in the extracellular environment.102,103 Among them, MCT1 is induced by c-Myc to be expressed in all cells and is responsible for the transport of lactate and pyruvate, whereas MCT4 (monocarboxylate transporter 4) is a highly efficient lactate transporter protein induced by hypoxia and is highly expressed in glycolytic tissues (e.g., white muscle fibers) and cancer cells.26,38,104,105,106 Most solid tumors rely on glycolysis for energy production, and upregulation of MCT in cancer contributes to the formulation of an acidic microenvironment, which has a critical impact on cancer cell viability by regulating pH allowing for sustained high rates of glycolysis.107,108,109 Herein, high expression of MCT1/4 in various tumors such as melanoma,110 glioblastoma111 and NSCLC,112 is associated with poor prognosis.
Lactate shuttle is a concept that was introduced in 1985 and has been continuously developed and refined.113,114,115,116 The lactate shuttle refers to the transport of lactate between cells, tissues, and organs as a product of glycolysis and a substrate for respiration, which summarizes the process of lactate transmembrane migration and serves as a bridge between anaerobic glycolysis and aerobic respiration.117,118 This connection persists under aerobic conditions29,38,119 (Fig. 3).
The overview of aberrant tumor lactate-related metabolism compared to physical conditions. In TME, cancer cells exhibit increased tumor glycolysis to meet their high energy demands and metabolic needs. This heightened glycolysis leads to elevated glucose consumption, resulting in excess lactate production and reduced ATP production in the cytoplasm. In normal cells, glycolysis involves ten steps, with the end product pyruvate entering the mitochondria for energy production via the TCA cycle. Besides participating in glucose metabolism, about 10% of the pyruvate is involved in other types of metabolism such as protein metabolism. Failure of pyruvate to enter the TCA cycle leads to decreased energy production compared to glucose molecules through altered glycolysis. Lactate in TME plays a crucial role in regenerating NAD+ molecules and directly join the TCA cycle under hypoxic conditions to sustain glycolysis and ATP production. In addition to glucose metabolism, increased glutaminolysis and lipogenic enzymes expression are also observed in TME. Generated using Adobe Illustrator (Version 28.2). Abbreviations: GLUT1/4 glucose transporter 1/4, TME tumor microenvironment
The lactate shuttle performs three physiological functions: 1. lactate is one of the major energy sources. 2. lactate serves as a glucose xenobiotic precursor. 3. lactate is a signaling molecule with autocrine, paracrine, and endocrine-like properties.29,118,120,121 The procedure in which muscle-generated lactate is re-transported to the muscle via hepatic glucose isomerization to glucose is known as the Cori cycle.122,123,124 The significance of this cycle is to 1. prevent muscle lactic acidosis under anaerobic conditions, 2. maintain muscle ATP supply, and 3. the Cori cycle stands for a more essential source of substrate for gluconeogenesis than food.125,126
Other than intracellular lactate metabolism, lactate may be transported into target cells via nonchannel pathways or MCTs through intercellular shuttling.127,128,129 To date, studies have uncovered that the lactate shuttle is involved in the TME during interactions between various cell populations and this aspect of lactate shuttling is defined as metabolic symbiosis, which is a vital phenomenon of tumor biology.130,131
Lactate shuttling between variable cell populations in the TME is a novel finding in oncology, thus revealing the tight association between lactate transport and the progression of tumors.130,132 Since lactate serves as a precursor for glycolysis and a substrate for the TCA cycle, the shuttling of this metabolite through TMEs containing both anoxic and aerobic cell populations is of paramount significance. There is an interrelationship and metabolic symbiosis across tumor cells in varied parts of a solid tumor. Because of the rapid growth of tumors, tumor region consist of anoxic and aerobic parts depending on whether they are located in the vicinity of blood vessels. In particular, anoxic tumor cells use lactate dehydrogenase A (LDHA) for lactate anabolism, which enters intercellular matrix through MCT4 and is taken up through MCT1 by aerobic tumor cells. Lactate dehydrogenase B (LDHB) may catalyzes this lactate to pyruvate to generate ATP.133 Solid tumor staining is consistent with this perspective. Immunofluorescence staining of pancreatic neuroendocrine tumors showed that MCT4 was predominantly expressed in hypoxic tumor compartments and MCT1 was mostly upregulated in MCT4-negative regions. Metabolic symbiosis invigorates the metabolic potential of tumor tissues for anaerobic environments and facilitates tumor proliferation and metastasis.130,134,135 It happens in hypoxic regions of tumor regression and increases invasion and metastasis in mouse models of pancreatic neuroendocrine carcinoma and glioblastoma (GBM).136 Studies unveiled that mTOR (mammalian target of rapamycin) mediated lactate shuttle induced by sunitinib/axitinib in PanNET, the inhibition of which significantly reduced tumor burden and viability.134
Multifaceted functions of lactate in cancer cells
Lactate has been found to play a crucial role in shaping the TME recently by influencing various cell populations.137,138,139 In tumor cells, lactate amplifies the lactate shuttle, affects cell signaling pathways, enhances oxidative stress resistance, and contributes to lactylation (Figs. 3, 4).
Lactate is an intracellular and extracellular signal transducer of great significance. As it fulfills a role intracellularly, lactate boosts tumor malignancy in hypoxic environments through both HIF-1-dependent and HIF-1-independent pathways. Lactate not only fulfills its function intracellularly, but also serves as an extracellular ligand to GPR81. Extracellularly, GPR81/GPR132 imports lactate and subsequent signaling boosts its utilization, resulting to anti-tumor immunity impairment. Meanwhile, Lactate intensifies the crosstalk between metabolism and epigenetics by editing lactylation modification. Generated using Adobe Illustrator (Version 28.2). Abbreviations: bFGF basic fibroblast growth factor, ERK1/2 extracellular signal-regulated kinase 1/2, GLUT1/4 glucose transporter 1/4, GPR81 G-protein-coupled receptor 81, Kla lysine lactyl; NDRG3, N-Myc downstream-regulated gene family member 3, PHD prolyl hydroxylases, PKA protein kinase A, TAZ transcriptional co-activator with PDZ-binding motif, VHL Von Hippel Lindau tumor suppressor
Circulating carbohydrate fuel of TCA cycle
Organisms rely primarily on OXPHOS and glycolysis to obtain energy from glucose.140,141,142 Traditionally, lactate was considered to be a minor byproduct of glucose utilization in anaerobic environments, but studies now indicate that lactate is an irreplaceable and primary fuel for energy and is crucial for the TCA cycle.143,144,145,146 In this study, a mouse 3T3-L1 fibroblast based in vitro models indicated that, apart from elevated glucose uptake and lactate release, proliferating fibroblasts showed improvements in mitochondrial respiration as well as coupling efficiency.147 Another study showed that pyruvate could not prevent glucose deprivation-induced cell death by facilitating glucose metabolism through gluconeogenesis, suggesting glucose is not one and only substrate for respiration in proliferating cells.148 Moreover, lactate is proven to be the main energy donor for the brain, which directly supports energy balance of preopiomelanocortin (POMC) neurons and excitatory neural activity in the brain.149,150,151
Mitochondrial OXPHOS fosters tumorigenesis, development, metastasis, and drug resistance.152,153,154,155,156 Pyruvate migrates into mitochondria during OXPHOS to engage in the TCA cycle.157,158 While research on the Warburg effect laid the foundation for the role of lactate in the TME through the glycolytic pathway, recent studies have revealed that lactate could be utilized as a carbon origin directly through the TCA cycle in tumor cells (Fig. 3). Isotope tracing showed that TCA metabolites labeled by 13C-lactate continued to outcompete that with 13C-glucose label in a mouse tumor model even in the presence of glucose.14 This reverses previous perceptions and establishes that lactate is a proximate fuel for the TCA cycle. Utilization of lactate by TCA cycle even correlates with the metastatic capacity of the tumor. Isotopic tracing of xenografts in mice showed that MCT1-mediated lactate utilization, reflected by TCA metabolites, was elevated in tumors with high metastatic potential compared with tumors with low metastatic potential.16 Other articles have also emphasized this emerging perspective that glucose serves as a specific fuel while lactate as a universal fuel.159,160 In a word, these studies illustrate that lactate is by no means just a useless byproduct of rapid tumor energy consumption in anaerobic stress. It is a direct and vital participant in the TCA cycle and OXPHOS.
Buffer of redox homeostasis
Lactate serves as a vital metabolic substrate and functions as an intercellular and intertissue redox signaling molecule.161,162 First, lactate production and removal uphold electron flux through a specific pathway, involving the conversion of NADH to NAD+ and H+ alongside LDH-mediated conversion of lactate to pyruvate.163 These reduced coenzymes (nicotinamide adenine dinucleotides (NAD+ or NADP+)) produce electrons as they undergo oxidation via either mitochondrial respiration or lactate fermentation, thereby sustaining redox balance.164 Besides, as a modulator of OXPHOS, lactate affects intracellular redox homeostasis.165 Last but not least, lactate benefits redox condition maintenance. Downregulation of MCT expression leads to loss-function of LDH, intracellular accumulation of lactate, cytoplasmic acidification, and cell death in cancer cells.166
Regulator of amino acid and fatty acid metabolism
As a specific feature of the process of lactate metabolism in cancer cells, glutamine provides a carbon source and promotes the utilization of lactate and TCA cycle intermediates. Regulated by cellular myelocytomatosis (c-Myc), glutamine is transported across cell membrane by amino acid transporter type 2 (ASCT2) and sodium-coupled neutral amino acid transporter 5 (SN2). Glutaminase (GLS/GLS2) catalyzes it and transforms it to glutamate. Next, glutamine enters the TCA cycle as α-ketoglutarate (α-KG). Via this above-mentioned pathway, glutamine becomes the second-largest carbon source of lactate in cancer cells.167 Additionally, lactate induces the expression of the proto-oncogene c-Myc. c-Myc transcriptionally binds to the promoter region of glutamine importers, ASCT2 and SN2, leading to increased glutamine uptake and tumor progression.168,169,170
Fatty acid metabolism fosters tumorigenesis, progression, and treatment resistance through enhanced lipid synthesis, storage, and catabolism.171,172 It is well known that lactate accumulation can promote intracellular fatty acid synthesis by promoting the activity of acetyl coenzyme A carboxylase (ACC), a key enzyme in fatty acid synthesis, and by supplementing the raw material for fatty acid synthesis, acetyl coenzyme A (acetyl-CoA).173,174 Accumulation of lipid droplets in the cytoplasm of cancer cells is correlated with cancer invasiveness and chemotherapy resistance.175,176 The expression of lipogenic enzymes is upregulated and their activity is on the rise in most tumors. For instance, citrate lyase is an indispensable modulator of histone acetylation in cancer cells.177 Lactate promotes intracellular fatty acid synthesis by supplementing acetyl-CoA, a raw material for fatty acid synthesis, and by increasing the activity of acetyl-CoA carboxylase, a key enzyme in fatty acid synthesis.178 Besides, as lactate acts as a favored energy source for muscle and heart cells through OXPHOS, it simultaneously inhibits lipolysis and blocks the import of free fatty acids (FFA) into mitochondria via carnitine palmitoyltransferase 1 (CPT1).179,180 Nonetheless, through the integration of multi-omics analysis and validation both in vitro and in vivo in NSCLC, a recent study disclosed that intracellular lactate drives extracellular lipolysis and FFA release via non-histone lactylation of apolipoprotein C-II (APOC2), bringing about immunotherapy resistance.181 These studies show that role of lactate in fatty acid metabolism differs between the TME and normal tissues, suggesting a complexity in its function. The particular signaling pathways by which lactate impacts fatty acid metabolism and their significance in tumor progression remain to be further explored.
Intracellular and extracellular signaling of tumor cells
Lactate is a predominant signal transducer of tumor cells (Fig. 4). Lactate inhibits 2-oxoglutarate-dependent prolyl hydroxylases (PHDs) (mainly PHD2), which in turn prevents Von Hippel Lindau tumor suppressor (VHL)-mediated ubiquitination of hypoxia-inducible factor 1 (HIF-1) and its proteasomal degradation, thus stabilizing HIF-1. Lactate-mediated PHD2 damage relies on the oxidation of lactate to pyruvate, which elicits suppression of PHD2 through pyruvate binding, alongside HIF-1-mediated elevation of VEGF.182
In addition, lactate induces angiogenesis and maintains tumor metabolism in hypoxic environments by inhibiting the PHD2/VHL system through an HIF-1-independent pathway that cooperates with the HIF-1 pathway. Lactate directly binds to N-Myc downstream-regulated gene family member 3 (NDRG3; NM_032013) protein and inhibits its binding to PHD2 and its deterioration, securing prolonged protein stability. Accumulation of NDRG3 leads to activation of Raf/ERK-mediated angiogenesis and proliferation, bringing about cellular adaptation to long-term hypoxia. Animal experiments showed that knockdown of NDRG3 inhibited the proliferation of hepatocellular carcinoma (HCC) subcutaneous tumors and angiogenesis of subcutaneous stromal plugs. Downregulation of lactate metabolism inhibited the proliferation of lymphoma cells at the cellular level and in subcutaneous tumors, and overexpression of NDRG3 reversed the growth inhibition caused by downregulation of lactate metabolism.17
Nevertheless, lactate, as a redox homeostasis regulator, can act as an antioxidant to resist excessive oxidative stress in tumor cells and reverse cellular DNA/RNA damage caused by massive reactive oxygen species (ROS) production, thus mediating treatment resistance and metastasis.162,183 Dou et al. unearthed that lactate enhanced the production of ROS through nicotinamide adenine dinucleotide phosphate oxidase 1 (NOX1), which induced the senescence-associated secretory phenotype (SASP). In contrast, inhibiting pyruvate dehydrogenase kinase 4 (PDK4) mitigates lactate-induced DNA damage and curbs the SASP.184,185,186,187 In addition, Hu et al. confirmed that in 4T1 and HeLa cells, LDHA mediated hydrogen peroxide production under oxidative stimuli in vivo and in vitro.188 Concerning cervical tumor, nuclear LDHA acquired a non-canonical enzymatic activity to produce α-hydroxybutyrate (α-HB), which facilitated interaction between disruptor of telomeric silencing 1-like (DOT1L) and LDHA, which mediated hypermethylation of histone H3K79. This process led to the activation of antioxidant genes and enhanced Wnt signaling pathway, thus promoting tumor growth.189 In patients with NSCLC, elevated LDHA expression is a negative prognostic factor linked to radiation resistance. Inhibition of LDHA by oxamate significantly boosted radiosensitivity and enhanced apoptosis, autophagy and cell cycle turbulence triggered by ionizing radiation (IR) in A549 and H1975 cancer cells.190 Response to fractionated irradiation correlates with lactate concentration of tumor regions in 10 xenografted human HNSCC tumor lines.191 Besides, disturbances in oxidative homeostasis due to lactate are also associated with metastasis. Studies elucidated that the heterogeneity of the metastatic process in melanoma depends on the differences in the expression levels of MCT1, and revealed that the molecular mechanism involved lies in the fact that melanomas with high expression of MCT1 can utilize lactate to resist oxidative stress, and thus obtain a stronger metastatic capability.13,16
Apart from its character as a signaling mediator intracellularly, meanwhile, lactate functions as an extracellular ligand.192,193,194,195,196 G-protein-coupled receptor 81 (GPR81), a G protein-coupled receptor for lactate, exists in colon, breast, lung, hepatocellular, salivary gland, cervical, and pancreatic cancer cell lines.197,198,199,200 Lactate supports energy metabolism in tumor cells through binding to GPR81. In pancreatic cancer samples, 94% (148/158) of patients expressed high levels of GPR81. Functionally, knockdown of GPR81 in lactate-containing low-glucose culture conditions resulted in decreased mitochondrial activity and massive death of pancreatic cancer cells. The addition of lactate to the culture medium induced the expression of genes involved in lactate uptake and metabolism, but not in GPR81-silenced cells. Under conditions that mimicked the TME (low glucose, glutamine, and pyruvate), the levels of MCT1, MCT4, cluster of differentiation 147 (CD147), peroxisome proliferator-activated receptor γ coactivator 1 α (PGC-1α) and other mRNAs were increased after 6 h of lactic acid treatment in parental pancreatic cancer cells expressing GPR81. In contrast, lactate treatment had no effect on the mRNA levels of these molecules mentioned above after GPR81 silencing. In addition to altering mitochondrial activity, mouse in situ pancreatic tumor models constructed by shGPR81 cell line had slower tumor growth, longer overall survival, and slower lung metastasis. In conclusion, GPR81-lactate transport is an important cancer cell transporter mechanism, which promotes energy consumption, proliferation and metastasis of pancreatic cancer.201 In TME, this pathway induces immunosuppression. In lung cancer cells, activation of GPR81 decreases intracellular cyclic adenosine monophosphate (cAMP) levels and inhibits protein kinase A (PKA) activity, leading to activation of Transcriptional co-activator with PDZ-binding motif (TAZ), which further activates the programmed cell death protein 1/programmed death-ligand 1 (PD-L1/PD-1) immune checkpoint pathway and impairs T-cell function.202,203 In addition to GPR81, G-protein coupled receptor G2A (GPR132) is an essential transmembrane lactate receptor which leads to immune suppression and metastasis as well.204,205 Lactate activated Gpr132 on macrophage, which facilitates M2 polarization and promoted adhesion, migration, and invasion of breast cancer and lung cancer.206,207 In vivo and in vitro experiment of colorectal cancer (CRC) clarified platelet reactive protein 2 (THBS2) induced HIF-1α/lactate/GPR132 pathway promoted M2 polarization of macrophages, resulting in inhibition of T-cell proliferation and cytotoxicity.208 In conclusion, lactate acts as an extracellular ligand and an intracellular signal transduction factor to facilitate the energy uptake, proliferation, migration, and immune escape processes of tumor cells.
Lactylation serves as the bridge between metabolism and epigenetics
Lactate intensifies the crosstalk between metabolism and epigenetics18,19,20,209,210 (Fig. 5). Recently, there have been histone and nonhistone aspects of Kla.157,211,212,213,214 Interestingly, numerous research have demonstrated the buildup of histone Kla on the genome in cytoplasm triggered by hypoxia, interferon-γ (IFNγ), lipopolysaccharide (LPS), or bacterial infection, bringing about lactate production.20,215
Histone/non-histone lactylation sites and their downstream genes following modification. Histone and non-histone lactylation sites and their downstream genes after modification are presented in the form of lactylation sites (downstream genes), with histone lactylation shown in brown and non-histone lactylation shown in green. Generated using Adobe Illustrator (Version 28.2). Abbreviations: AARS1, alanyl-tRNA synthetase 1; AK2 adenylate kinase 2, BCL2 B-cell lymphoma 2, CASP8 caspase 8, CBX3 chromobox 3, CD133 cluster of differentiation 133, CTGF connective tissue growth factor, CYR61 cysteine-rich protein 61, eEF1A2 elongation factor 1 alpha 2, FDX1 ferredoxin 1, GPI glucose-6-phosphate isomerase, HK1 hexokinase 1, HK2 hexokinase 2, IDH3G isocitrate dehydrogenase (NAD+) 3 gamma, LDHA lactate dehydrogenase A, METTL16 methyltransferase Like 16, MRE11 meiotic recombination 11, p21 p21^CIP1/WAF1, PDGFRβ platelet-derived growth factor receptor β, PKM pyruvate kinase M, PUMA p53 upregulated modulator of apoptosis, RUBCNL rubicon like autophagy enhancer, TEAD TEA domain transcription factor, XRCC1 X-ray repair cross-complementing 1, YTHDF2 YTH N (6)-methyladenosine RNA binding protein 2
Besides, Kla epigenetically regulates gene expression.216,217,218,219 Supplementation of exogenous sodium lactate (NaLa) to B-cell adapter for PI3K (BCAP)-deficient bone marrow-derived macrophages (BMDM) reverses the downregulated Arginase-1 (ARG1) and Krüppel-like factor 4 (KLF4) expression caused by BCAP deficiency.215 ChIP assay of lactate-treated BMDM reveals significant up-regulation of histone Kla in the promoter region of the ARG1, platelet-derived growth factor A (PDGFA), thrombospondin-1 (THBS1), and vascular endothelial growth factor A (VEGFA).220 It follows that Kla regulates gene expression of immune cells and plays an essential physiological role.
Histone Kla has been sequentially reported in a variety of cancers,221,222,223,224 especially histone H3 lysine 18 lactylation (H3K18la). H3K18la has been reported to play a part in multitudinous biological processes such as oncogenesis,221,225,226,227 progression,228,229 tumor immune escape28 and cancer cellular metabolism reprogramming21 (Figs. 4, 5). Yang et al. revealed that inactivated VHL upregulated H3K18la, which promoted the expression of platelet-derived growth factor receptor β (PDGFRβ) and formed a positive feedback loop, thereby promoting the proliferation and metastasis of clear cell renal cell carcinoma (ccRCC). Li et al. lately revealed that in CRC cells, high H3K18la level promoted the transcription of Rubicon-like autophagy enhancer (RUBCNL/Pacer), which enhanced autophagy through promoting autophagosome maturation, and contributed to CRC tumorigenesis and progression.230 Yu et al. elucidated histone Kla levels were greater in ocular melanoma than in normal tissue and were positively correlated with poor prognosis in patients. Additional exploration of potential mechanisms indicates a facilitated expression of YTH N (6)-methyladenosine RNA binding protein 2 (YTHDF2) in ocular melanoma cells, resulting from elevated Kla of its promoter. In addition, YTHDF2, as an N6-m6A reader, recognizes m6A-modified period circadian regulator 1 (PER1) and tumor protein p53 (TP53) mRNAs and promotes their degradation, thereby accelerating ocular melanoma tumorigenesis.221
In addition to promoting tumor proliferation, metastasis, and invasion by modulating the expression of epigenetically related genes in tumor cells, Kla is also capable of inducing the expression of TCA cycle-related enzymes.231,232 A global lactylome profiling of cancer and paracarcinoma tissues from patients with hepatitis B virus-related HCC (HCC) successfully identified lactylation modification sites located on both non-histone and histone proteins. It suggests that lactylation modification may be involved in a broader biological function besides transcriptional regulation. More importantly, Kla preferably affects enzymes involved in metabolic pathways, including glucose metabolism, TCA cycle, amino acid metabolism, fatty acid metabolism, and nucleotide metabolism, and that elevated Kla levels on metastasis-related substrate are strongly correlated with aggressive clinical features and driver mutations of HCC.21 Studies of NSCLC have shown that histone Kla leads to downregulated level of the glycolysis-related enzymes and concurrently elevated that of the TCA cycle-related enzymes.222 In summary, Kla improves glucose uptake by tumor cells by modifying the expression of metabolism-related genes and adds up to metabolic disorders of NSCLC.
Besides, Kla incorporates target therapy resistance.233,234 Scientists demonstrated downregulation of histone Kla enhanced the sensitivity of CRC cells to bevacizumab treatment in cell-based xenografts, patient-derived xenografts and patient-derived organoids models, which further broadens the role of Kla in antiangiogenic therapy.230
Regarding non-histone lactylation, other research also validated its effect on prompting tumor progression.235,236,237,238 Zong et al. uncovered that alanyl-tRNA synthetase 1 (AARS1) detected lactate and facilitated the site-specific lactylation of p53, which weakened its ability to bind DNA and underwent liquid-liquid phase separation (LLPS). As a result, tumor-suppressing functions of p53 were diminished in a CRC mouse model.239 Lysine acetyltransferase 8 (KAT8), a lysine acetyltransferase known for its pan-Kla writing capabilities, catalyzes the lactylation of elongation factor 1 alpha 2 (eEF1A2) at lysine (Κ)-408 in CRC, which promoted tumorigenesis.240 Besides, lactate leads to the Kla of methyltransferase-like 16 (METTL16)-K229, which further induces m6A modification of ferredoxin 1 (FDX1) mRNA, increases FDX1 mRNA expression, and ultimately leads to the death of gastric cancer cells via cuproptosis.241 Yang et al. discovered adenylate kinase 2 (AK2) K28 lactylation enhanced proliferation and metastasis of HCC cells.21 Furthermore, global lactylome profiling of gastrointestinal (GI) cancers, including liver, pancreatic, colorectal, and gastric cancers, further demonstrated that non-histone lactylation exhibits cross-talk with various forms of epigenetic regulation.21 Lactylation of chromobox 3 (CBX3) at K10 facilitates its binding to H3K9me3, which in turn drives the invasiveness of GI cancers.242
Moreover, similar to histone lactylation, non-histone lactylation modifications play a role in tumor treatment resistance. Chen et al. found that non-histone lactylation of meiotic recombination 11 (MRE11) boosted homologous recombination (HR) and chemoresistance in CRC.243 Li demonstrated that the interaction between aldehyde dehydrogenase (ALDH) 1 family member A3 (ALDHA3) and pyruvate kinase M2 (PKM2) increased lactate production, which in turn induced lactylation of XRCC1 at K247. This enhanced DNA damage repair and resulted in resistance to both radiotherapy and temozolomide (TMZ)-based chemotherapy.244 Nevertheless, recent reports have revealed the potential therapeutic effect of histone/non-histone lactylation in tumors, which will be further depicted in “Other lactate-targeted strategies”.245 In addition to lactylation, lactate is involved in regulating other epigenetic modifications that foster tumor progression. The Warburg effect results in a dramatic increase in intracellular levels of acetyl coenzyme A (Ac-CoA), which induces general control non-derepressible 5 protein/Spt-Ada Gcn5-acetyltransferase (Gcn5p/SAGA)-catalyzed acetylation of histone proteins, which induces downstream gene transcription and promotes cell growth.246,247 Apart from acetylation, lactate alters the anaphase-promoting complex (APC/C) by directly blocking the Small Ubiquitin-like Modifier protease (SUMO protease) sentrin/SUMO-specific protease 1 (SENP1) and stabilizing SUMOylation at two specific residues on APC4. The stabilization of SUMOylation induced by lactate accelerates the degradation of cell cycle proteins and ensures effective mitotic exit in actively dividing human cells.248
Previous studies have explored whether lactylation and acetylation have functional overlap.249 Their similarity lied in the fact that lactate and acetyl-CoA both were primarily derived from the glycolytic end product, pyruvate and had similar molecular structures.231,250 Besides, from the perspective of modification mechanisms, both lactylation and acetylation preferred targeting lysine (Lys) residues for epigenetic modulation, which utilized p300 as the “writer” for Lys catalysis and class I-III histone deacetylases (HDAC1-3) as “eraser”.251,252,253,254,255 However, recent studies have firmly established that lactylation functioned differently from acetylation. In 2019, Utilizing M1 macrophages exposed to bacteria as a model system, Zhang et al. reveal that histone lactylation follows a different temporal pattern compared to acetylation.20 Then, it was elucidated that lactylation exhibited slower kinetics at lysine compared to acetylation, and intracellular concentrations of lactyl-CoA were lower than those of acetyl-CoA.105 Recently, Zong et al. utilized a clustered regularly interspaced short palindromic repeat (CRISPR) screen, which identified AARS1 as an intracellular sensor for lactate and a transferase of lactyl to lysine residues. AARS1 binds directly to lactate, catalyzes the adenosine triphosphate (ATP)-dependent synthesis of lactate-AMP and mediates widespread lysine lactylation, including that of p53.239 AARS1 was also uncovered to lactylate and activate the Yes-associated protein-TEA domain transcription factor (YAP-TEAD) complex in gastric cancer. As a Hippo target gene that creates a positive-feedback loop with YAP-TEAD complex, AARS1 promotes proliferation of gastric cancer.256,257 Additionally, alanyl-tRNA synthetase 2 (AARS2) has been shown to act as a mitochondrial lactyltransferase as well, catalyzing the lactylation of K336 in pyruvate dehydrogenase complex 1 (PDHA1) and K457/8 in carnitine palmitoyltransferase 2 (CPT2) under hypoxic conditions. This lactylation process mediates mitochondrial proteins to regulate OXPHOS in muscle cells.258 Moreover, recent studies have provided deeper insights into the role of AARS1/2 in the transferase-catalyzed lactylation process, revealing that lactate is directly modified onto proteins via catalysis, eliminating the need for lactoyl-CoA formation.259 As AARS1/2 is not a pan-Kla writer, to summarize, these results unearth that lactylation and acetylation involve different enzymatic toolkits.
Lactate and lactylation in diverse cell populations
Deep-in-depth investigation of the TME complexity and collaborations between the abundant cell types within this niche stands for a stepping stone to precision cancer therapy.260,261,262 According to the fascinating and challenging features of TME, it consists of multiple populations of fibroblasts, an underdeveloped vascular system, and a varied and predominantly suppressive array of immune cells.263,264,265 The invasiveness, resistance to therapy, and heterogeneity of the tumors are therefore influenced to a significant extent by the non-malignant parts of the tumor, in addition to tumor cells themselves.266,267,268
In other cell populations, on the one hand, interaction between lactate and immune cells exerts an effect on impaired cell differentiation, reduced immune response, evasion of immune monitoring, and defective sensitivity upon treatment.269,270 On the other hand, lactate/lactylation crosstalk with stromal/endothelial cells reinforces basal membrane (BM) remodeling, epithelial-mesenchymal transitions (EMT), metabolism reprogramming, angiogenesis, and drug resistance (Fig. 6).
Lactic acid remodels variant cell populations in the TME. The TME consists of various cell types, including tumor, stromal, endothelial, and immune cells. Lactate impacts infiltrating immune cells by regulating their metabolism due to the Warburg effect, inhibiting the activation and proliferation of CD8 + T cells, natural killer (NK) cells, and dendritic cells, while promoting the immunosuppressive function of CD4 + CD25+ regulatory T (Treg) cells. Lactate also aids the polarization of macrophages towards an anti-inflammatory (M2-like) phenotype, supporting angiogenesis, tissue remodeling, and tumor progression. In cancer associated fibroblasts (CAFs), lactate production, driven by SIRT3/succinate-dependent HIF-1α activation, enhances BM remodeling, EMT, metastatic reprogramming, and treatment resistance. In endothelial cells, LDHB converts lactate to pyruvate, which enters the TCA cycle, influencing redox status, inducing reactive oxygen species (ROS), stabilizing HIF-1, and activating NF-κB signaling, which increases IL-8 and VEGF transcription. Thus, lactate significantly favors tumor progression, though detailed mechanisms remain unclear. Generated using Adobe Illustrator (Version 28.2). Abbreviations: bFGF, basic fibroblast growth factor; ERK1/2, extracellular signal eegulated kinase 1/2; GPR81, G-protein-coupled receptor 81; PHD, prolyl hydroxylases; PKA, protein kinase A; RUBCNL, Rubicon like autophagy enhancer; TAZ, transcriptional co-activator with PDZ-binding motif; VHL, Von Hippel Lindau tumor suppressor
Immune cells
Lactate/lactylation induces the generation of immunosuppressive TME in diverse types of tumors271,272,273,274,275 (Table 1). Xiong et al. elucidated that lactylation of methyltransferase like 3 (METTL3) was upregulated in tumor-infiltrating myeloid cells (TIMs), thus inducing immunosuppression CRC.28,276
Lactate represses differentiation and antigen presentation of dendritic cells (DCs).117,277 Previous study discovered that cocultures of melanoma and prostate carcinoma multicellular tumor spheroids (MCTSs) produced low levels of macrophage colony-stimulating factor (MF-CSF) and interleukin-6 (IL-6), while generating significant amounts of lactic acid. Furthermore, introducing lactic acid in the process of DCs differentiation in vitro led to a phenotype similar to that of tumor-associated dendritic cells (TADCs) formed within melanoma and prostate carcinoma MCTSs, marked by inhibited differentiation and reduced IL-12 secretion.278,279 Plebanek et al. discovered that lactate from melanoma stimulates sterol regulatory element-binding protein 2 (SREBP2) in tumor DCs, leading to the transformation of conventional DCs into cluster of differentiation 63 (CD63)+ mature regulatory DCs (mregDCs) through homeostatic or tolerogenic maturation. Targeted genetic silencing of SREBP2 in DCs, as well as its pharmacologic inhibition, enhanced antitumor CD8 + T cell activation and inhibited melanoma progression.280 Moreover, Caronni revealed that lactate inhibited DCs from presenting antigens and activating CD8 + T cells when co-cultured with Lewis lung carcinoma (LLC) cells, thus preventing them from initiating an immune response within LLC models in vivo.281 Furthermore, Nasi et al. uncovered that the lactate-induced changes in DCs might be density-dependent. In dense cultures, disrupting lactate production revealed its key role in reshaping DC functions, leading to increased production of interleukin-12 (IL-12) and decreased interleukin-10 (IL-10). However, in sparse cultures, the effects were reversed.282
Pertaining to microglia/macrophages, lactate and histone lactylation promote the conversion of macrophages to M2-type tumor-associated macrophages (TAM), while TAM enhances the transcription of M2-like genes hypoxia-inducible factor 2α (HIF-2α), ARG1, and VEGF.20,206,283,284,285 This polarization-inducing function may be mediated through extracellular signal-regulated kinase (ERK)/signal transducer and activator of transcription 3 (STAT3) pathway.286 Crosstalk with other epigenetic regulatory mechanisms may also occur during the lactate-induced M2 polarization. As a carbon source for the TCA cycle, lactate induces adenosine triphosphate–citrate lyase (ACLY)-dependent histone H3 lysine 9 acetylation (H3K9ac) in BMDM, thereby upregulating the expression of M2-like genes.287 Besides, lactate promotes tumor progression by reprogramming phenotype of microglia and monocyte/macrophages. Lactate is a pro-inflammatory mediator which encourages interleukin-23 (IL-23) transcription in Toll-like receptor (TLR)-stimulated monocytes and macrophages, thereby sustaining IL-23-dependent interleukin-17 (IL-17) secretion and polarizing the immune response against TH17 cells.288 Downregulation of LDHB skews TAMs to function as a lactate and sterol/oxysterol source for the proliferation of breast tumor cells.289 Additionally, as sentinel cells in the central nervous system, microglia upregulate the expression of insulin-like growth factor-binding protein 6 (IGFBP6) in response to lactate, thereby promoting M2 polarization and recruitment of microglia in the zebrafish GBM model.290,291,292 Then, H3K18la of TAM prohibited expression of retinoic acid receptor γ (RARγ), elevated IL-6 levels in the TME and activated STAT3 signaling in CRC cells, which in turn empowered macrophages to promote tumorigenesis.293,294
When it comes to Treg cells, the lactate-rich environment which is rich in lactate permits proliferation and immunosuppressive effect of Treg cells.281,295,296 Raychaudhuri et al. found that lactate weakened interferon-α (IFNα) induction and enhanced the recruitment of FoxP3 + CD4+ regulatory T (Treg) cells by plasmacytoid dendritic cells (pDCs) in a mouse breast cancer model. This impairment boosted the expansion of a specific group of Treg cells and promoted an immunosuppressive TME.297 Moreover, lactate fosters programmed death-ligand 1 (PD-1) level in Treg cells in rich-glycolysis TME, giving rise to treatment failure of immunotherapy.298,299 As the tumor-infiltrating Tregs require lactate uptake to sustain their immunosuppressive function, Treg-specific deletion of MCT1 demonstrates that while lactate blockage is not necessary for the functioning of peripheral Treg cells, it is essential within TME and leads to an impaired tumor growth and enhanced sensitivity to immunotherapy.300,301 To add up, Chen et al. manifested that lactate facilitates the lactylation of APOC2 at K-70, which stabilizes the protein and subsequently leads to the accumulation of Treg cells, immunotherapy resistance, and tumor metastasis of NSCLC.181
Furthermore, with respect to T cells and natural killer (NK) cells, Daneshmandi et al. observed elevated infiltration of NK cells and CD8+ cytotoxic T cells in melanoma cells deficient in LDHA.302 Additionally, Liu et al. found that lactate produced by KRAS-mutant colorectal cancer cells diminishes the sensitivity to anti-PD-1 therapy by inactivating nuclear factor-kappa B (NF-κB) and sensitizing CD8 + T cells to activation-induced cell death (AICD).303 Furthermore, during this process, circular RNA CircATXN7 may play a crucial role in inducing NF-κB inactivation and regulating T cell sensitivity to AICD. It has been identified as a potential target for enhancing anti-PD-1 therapy in mouse models of colorectal cancer, pancreatic cancer, and melanoma.304 Apart from that, Chang et al. demonstrated that prostate cancer released 1-Pyrroline-5-carboxylate (P5C) which inhibited T cell glycolysis through enhancing the activity of LDHB.305 With regard to mechanisms, lactate hinders IFN-γ production by downregulating T cell receptor (TCR)-triggered phosphorylation of JNK, c-Jun, and p38 in Cytotoxic T lymphocyte (CTL).306 Research also revealed that tumor-produced lactate inhibited focal adhesion kinase interacting protein (FIP) expression by downregulating nicotinamide adenine dinucleotide levels and simultaneously sensitizing the inhibitory effect of the adenylglycine uridylic acid-rich element in the untranslated region of the Fip200 mRNA, targeting naïve T-cells to evade the immune response.307 Besides, lactate and H+ ions exported to the TME impair immune surveillance of effector T cells and NK cells by respectively inhibiting glycolytic flux, granzyme B and IFN-γ secretion.23,302,308
More to the point, lactate-modulated immunosuppression hinders treatment sensitivity.309,310,311,312,313 Lin et al. uncovered that radiotherapy enhanced glycolysis and lactate secretion in pancreatic cancer, which bolstered myeloid-derived suppressor immune cells (MDSCs) and promoted a suppressive immune microenvironment, which in turn led to pancreatic cancer progression and recurrence.314 In brief, lactate modulates immune cells in TME, resulting in impaired differentiation, decreased immune response, evasion of immune surveillance, and treatment resistance.
Nonetheless, the latest research has revealed divergent viewpoints. Lactate stimulates the production of antitumor cytokines, such as IFNγ, IL-2, and TNFα, in T cells and boosts the proliferative and cytotoxic capabilities of CD8 + T cells.315 Furthermore, administering sodium lactate intraperitoneally (2 g/kg) results in reduced subcutaneous tumor growth of breast cancer, cutaneous melanoma, LLC and colon adenocarcinoma, with the effect being dependent on T cells.315 Besides, it is reported that lactate hinders the differentiation and promotes stemness of T cells, thus enhancing anti-tumor immunity. Feng et al. recently discovered that high sodium lactate concentrations boosted histone H3 lysine 27 acetylation (H3K27ac) levels at the T cell factor 7 (TCF7) super-enhancer locus by inhibiting histone deacetylase activity. This, in turn, led to higher T cell factor 1 (TCF1) expression and enhanced the stemness of CD8 + T cells.316,317,318 Lactate-induced extracellular acidosis blocks one-carbon metabolism that short-lived effector T cells are highly dependent on, subsequently bringing about decreased histone 3 lysine 27 trimethylation (H3K27me3) deposition of memory-related genes, thereby affecting differentiation, reserving sternness and facilitating anti-tumor cytotoxicity of T cells.318,319,320 It implies that lactate metabolism reprogramming towards T cells may be a double-edged sword which elicits a sophisticated effect on its antitumor immunity, which waits to be further investigated.
Stromal cells
Cancer-associated fibroblasts (CAFs) are essential components of the TME with multiple roles, encompassing stromal remodeling and deposition, extensive bidirectional signaling interactions with cancer cells, and communication with immune cells.321,322,323,324 Basal membrane (BM) remodeling is the process by which cells regulate cell-BM interactions by changing the structure and composition of BM.325,326,327 EMT describes the transformation from epithelial cells to mesenchymal cells.328,329,330,331 CAFs synthesize type I collagen and intensify tumor invasiveness by promoting BM remodeling and EMT.332,333 In CAFs coupled with prostate carcinoma cells models, contact between tumors and the stroma-activated CAFs through stabilization of HIF-1 that rely on sirtuin 3 or succinate, initiating mitochondrial oxidative stress, promoting mitophagy, upregulating expression of GLUT1 and glycolytic enzymes, and facilitating lactate biosynthesis. Meanwhile, CAFs metabolically reprogramed tumor cells so that prostate carcinoma cells tended to metabolize CAFs-sourced lactate rather than glucose through glycolysis.321 In parallel to promoting malignant phenotypes by facilitating BM remodeling, EMT, and metabolic reprogramming, CAFs have also been associated with treatment resistance. Apicella et al. revealed that the metabolic shift in tumor cells induced by tyrosine kinase inhibitors (TKIs) targeting mesenchymal-epithelial transition factor or epidermal growth factor receptor (EGFR), which results in increased lactate production, prompts CAFs to excessively produce HGF. This process ultimately reinforces drug resistance and promotes tumor progression.334
Endothelial cells
In addition to enhancing tumor invasion and metastasis via tumor cell lactate autocrine as mentioned above, paracrine secretion of lactate can directly modulate endothelial cell phenotype, thereby altering tumor vascular morphogenesis and perfusion.335,336,337,338 As for non-malignant endothelial cells at regular oxygen concentrations, lactate activates hypoxia-inducible factor 1α (HIF-1α) that enhances basic fibroblast growth factor (bFGF) and vascular endothelial growth factor receptor 2 (VEGFR2) expression, which synergizes with lactate-induced VEGF secretion.339 Lactate from tumor cells and stromal cells can enter endothelial cells via MCT1, promoting 2-oxoglutarate-dependent prolyl hydroxylase (PHD2) and ROS-dependent NF-κB activation in endothelial cells.340 Subsequently, endothelial cells produce IL-8, which mediates angiogenesis via autocrine.135 In addition to triggering the MCT1/NF-κB/IL-8 pathway, it was also found that lactate promotes angiogenesis by stimulating the phosphoinositide 3-kinase/protein kinase B signaling (PI3K/Akt) pathway. This activation developed through the engagement of three receptor tyrosine kinases—AXL receptor tyrosine kinase (Axl), TEK receptor tyrosine kinase 2 (Tie2), and VEGFR2 in endothelial cells.341 A-cyano-4-hydroxy-cinnamate (CHC) is an inhibitor of MCT1, which inhibits lactate metabolism.342,343,344 MCT1 inhibition downregulates VEGF expression, blocking lactate-induced endothelial cell migration, vascular outgrowth, and human umbilical vein endothelial cell (HUVEC) tube formation. The results of mouse experiments were consistent with cytological experiments that subcutaneous lactate matrix plugs promoted angiogenesis and that inhibitors of MCT1 inhibited angiogenesis in mouse HCC subcutaneous tumor model.339
Clinical application and interventions
Disruption of lactate homeostasis is one of the major mechanisms of tumor-targeted therapy.345,346,347 The major targets for lactate production and transport are LDH and MCTs348 (Table 2). LDH transforms pyruvate to lactate, and inhibition of its activity reduces lactate production. Besides, MCTs promote lactate transport. Targeting them disrupts lactate from release. On top of that, altered lactate levels may result in a therapeutic effect single-handedly or synergize with other conventional adjunctive anti-tumor treatments such as immunotherapy, chemotherapy, and thermotherapy for sensitizing effects.
Novel detection methods for lactate
Advanced molecular and imaging techniques are providing new insights into the mechanisms and functional importance of the fluctuation in tissue lactate/lactylation levels that occur during tumor progression.349,350,351
As for chemical probe for lactate detection, a fluorescently tagged analogue of L-lactate was employed as an L-lactate mimic to explore its transportation and metabolic processing within live cells.352 Aside from lactate-detecting probes, single-cell technique-based metabolomics analysis introduces a computational framework for profiling lactate metabolism and outlines key principles of the TME.353,354,355,356,357 For instance, metabolite set enrichment analysis revealed that metabolic pathways associated with the Warburg effect are linked to the metastatic potential of CRC cell lines.358 Subsequently, using a custom-built single-cell quantitative mass spectrometry platform, researchers monitored 14 identified metabolites in individual circulating tumor cells from CRC patients and developed a 4-metabolite fingerprint classifier, which includes lactate, to efficiently predict metastasis risk.359 Moreover, using isotope-tracing-based metabolic flux analysis, researchers can trace the path of each isotopic carbon atom, thereby assisting in gaining deeper insights into the particular intermediates and detailed metabolic processes caused by lactate.318 For example, with the assistance of isotope-tracing analysis, researchers proved that 13C-glucose labeled TCA intermediates were superior to that with 13C-lactate label in the brain, TCA labeling from lactate was significantly higher than infused 13C-glucose in other tissues.143 Besides, using 13C-labeled metabolic flux assays, researchers found that the preference for glycolysis and OXPHOS varies across variant stages of the cell cycle in breast cancer cell lines. Cells in the G1 phase primarily prefer OXPHOS, while cells in the S phase predominantly prefer glycolysis.360,361 To add up, by intravenously administering primed [U-13C]lactate (completely labeled with carbon-13 at three positions) and utilizing imaging mass spectrometry (IMS), Bartman et al. mapped TCA cycle flux across various tumor models.14,362,363 Their findings revealed that TCA flux in primary solid tumors, such as pancreatic cancer, NSCLC, and CRC, was lower than in corresponding normal tissues, while in hematological malignancies like NOTCH1-driven T cell acute lymphocytic leukemia, TCA flux in the spleen was elevated compared to normal spleen tissue.39,364,365 Additionally, in a breast cancer lung metastasis model, metastatic sites exhibited higher TCA flux compared to primary sites. These results suggest that despite an increase in glycolytic flux in tumors relative to normal tissues, the rate of ATP production is reduced.366,367 In addition, as for metabolic imaging, Li et al. reported a high-performance imaging technique for monitoring lactate, named FiLa, which achieves in situ, real-time, and quantitative dynamic tracking of lactate metabolism in live cells, subcellular structures, and in vivo.368 This method has made significant advances in understanding lactate spatial distribution, regulatory networks, drug screening, and clinical diagnostics. The FiLa probe, used for detecting lactate levels in subcellular organelles, showed that lactate concentrations in the nucleus are comparable to those in the cytoplasm, while mitochondrial lactate levels are markedly higher than those in the cytoplasm and nucleus.369
Since lactylation is a relatively new discovery, its presence on non-histone proteins and its subsequent functional impacts are not yet well unearthed, which proposes urgentexpectations for accurate detection methods of lactylation. Wan et al. presented a cyclic immonium (CycIm) ion of lactyllysine (Klac) that formed during tandem mass spectrometry, which allowed for precise assignment of protein lactylation. The sensitivity and specificity of this lactylation detecting method were confirmed through affinity-enriched lactylproteome analysis and extensive informatic evaluation of non-lactylated spectral libraries.370 Sun et al. also invented a chemical lactylation probe named sodium (S)-2-hydroxypent-4-ynoate (YnLac) which metabolically integrated into lactylated proteins, allowing them to be directly tagged with fluorescent or affinity markers for fluorescence visualization or proteomic analysis.371 Moreover, single-cell technologies play a crucial supportive role in studies related to tumor lactylation. It enables detailed correlation analyses based on cell type and the lactylation level, revealing genes associated with lactylation of specific cell population. It provides multi-dimensional indicators for evaluating tumor metabolic phenotypes and predicting tumor prognosis and has been applied in CRC.372,373 In summary, advancements in detection technologies are pressingly required to offer fresh insights into whether and how lactate/lactylation influences a plethora range of biological processes in different cell populations and plays a role in oncology.
Targeting LDHs
LDH is a tetrameric enzyme that mediates bidirectional transformation between pyruvate and lactate. LDHA is the prevailing isoform utilized by cancer cells to bypass OXPHOS. This diverts metabolic precursors of pyruvate into the pentose phosphate pathway, which supports cancer cell proliferation.74,189,374,375 A high level of LDHA indicates poor prognosis in several human malignancies.189,376,377 Meanwhile, overexpression of LDHB has been found in plenty of different cancers, including breast, thyroid, lung, and pancreatic cancer, which is significantly associated with unfavorable prognosis.378,379,380 Current efforts are focused on development of LDH inhibitors with better cellular potency, PK properties, and selective compounds and remain in preclinical state. AT-101 (gossypol), as an EGFR mutation targeted therapy, has been used in phase I/II randomized clinical trials of advanced non-small cell radiation-induced lung cancer, head and neck cancer and metastatic castration-resistant prostate cancer. Thus, standard chemotherapy with AT-101 has achieved potential benefits in high-risk patients or some patients with prolonged progression-free survival or overall survival (NCT01003769, NCT00988169, NCT00286780, NCT00540722).381 Nonetheless, AT-101 and its derivative FX-11, galloflavin, and N-hydroxyindole-based compounds are promising cell-active LDHA inhibitors, which pharmaceutic effect hasn’t been applied in clinical practice.26,382,383 Intravenous injection of LDHA/B inhibitor NCI-006 inhibits LDH activity and its growth in pancreatic cancer mouse models, so as oral administration of LDHA inhibitor GNE-140.384,385,386 Moreover, LDH PROteolysis TArgeting Chimeras (PROTAC) degrader, MS6105, time and ubiquitin-proteasome system-dependently degrades LDHA/B and inhibits the proliferation in multiple pancreatic cancer cell lines.387 The efficacy of LDHA inhibitors is limited due to LDH between different tumors and metabolic reprogramming-mediated LDH isoform transformation.343,388 Furthermore, a combination of LDHA inhibitor oxamate and respiratory complex I inhibitor metformin retards tumor progression in melanoma mice models.389
LDH-targeted therapy also achieves curative effect when combined with adjuvant treatments. Regardless of its effect as a tumor suppressor, emerging evidence validates the role of LDH inhibitors as an immunotherapy sensitizer.390 Using oxamate and PD-1 blockade pembrolizumab stimulated CD8 + T cell infiltration and hindered tumor proliferation in humanized mouse NSCLC model, thereby sensitizing immunotherapy.391 In preclinical melanoma mouse model, LDHA inhibitor GSK2837808A increased therapeutic effect of adoptive T cell therapy (ACT).392 Likewise, LDH inhibitors contribute to photothermal therapy (PTT). Zhao et al. Constructed a Zinc-enriched nanosystem which contained both glycolysis inhibitor LND and LDHA inhibitor Zinc for combined glycolysis modulation and photothermal therapy. In addition, LDHA inhibition induced by oxamate led to the accumulation of ROS and depletion of cellular ATP, leading to DNA damage, DNA repair activity impairment and boosted radiotherapy efficiency in NSCLC.190 Moreover, LDHA inhibitor restores sensitivity towards radioiodine (RAI) in papillary thyroid cancer (PTC). Shi unvealed that long noncoding RNAs (lncRNAs) glycine-rich long non-coding transcript (GLTC) hindered the succinylation of LDHA at K-155 by impeding the competitive inhibition of GLTC against the binding of sirtuin 5 (SIRT5) to LDHA. This restraint of LDHA enzymatic activity inhibited tumor progression and resistance to RAI in PTC.393
Results demonstrated that the presence of free zinc ions led to a concentration-dependent inhibition of LDHA activity and an elevation in LDH efflux, invigorating PTT treatment and synergistically suppressed primary melanoma and lung metastasis.394
Targeting MCTs
Targeting MCTs exerts significant effects on metabolic symbiosis.132 There are a variety of MCT inhibitors, including CHC,342,343,344,395 organomercurial compounds,396 photothialdehyde benzenesulfonate,396 as well as second-generation pharmaceuticals of more acceptable selectivity, such as AR-C155858 for MCT1/2397,398 and BAY8002, SR13800 for MCT1.399,400 In addition, AstraZenec’s compound AZ3965, which targets MCT1/2, has shown promising results in preclinical studies in small cell lung cancer (SCLC).78 Surely, AZ3965 is also therapeutically effective in models of MCT1-positive Burkit’s lymphoma, breast and gastric cancers.78,399 AZD3965 has already finished a Phase I/II clinical trial (NCT01791595) in patients with solid tumors diffuse large B-cell lymphoma,76,79 which reveals its pharmacokinetic characteristics and adverse effects and suggests that AZD3965 is tolerated at doses that produce target engagement. Dose-limiting toxicities were on-target and primarily dose-dependent, asymptomatic, reversible ocular changes. Preclinical evidence and retrospective analyses suggest MCT4 may serve as a compensatory option for MCT1 activity as long as MCT1 is downregulated. This study suggests the complexity of targeting MCT and potential resistance mechanisms.78 Moreover, targeting MCT1 boosts tumor reactivity of CD8 + T cells by exerting influence on lactate catabolism.
In addition to MCT1 inhibitors, MCT4 inhibitors have shown promising applications. In hypoxic TME, MCT4 expression is induced by HIF1α.38,104 Knocking down MCT4 reverses the changes in sensitivity of lung adenocarcinoma cell lines to glycolysis inhibitors and OXPHOS inhibitors under hypoxic conditions, indicating the vital role of MCT4 in lactate-targeted therapy.401 MCT4i is a promising therapeutic choice for gastric cancer,402 colorectal cancer,403 breast cancer,404,405 prostate cancer,406 lung adenocarcinoma (LUAD),407 and GBM.408,409 The combination of MCT1i (AZ3965) and MCT4i (AZ93) significantly inhibited proliferation in colorectal cancer cell lines.410 7-Aminocarboxycoumarins (7ACCs) compounds prevented MCT1 compensation resulting from MCT4 inhibition by simultaneously suppressing both MCT1 and MCT4, down-regulating mitochondrial pyruvate transport leading to intracellular pyruvate accumulation, and blocking lactate inward compensation.102 The assistance of the chaperone molecular chaperones CD147 or basigin (BSG) assures expression of MCT1 and MCT4 at the plasma membrane.411,412 Preclinical models of prostate cancer show that inhibition of CD147/BSG achieves modulation of lactate transport through MCT1/MCT4 activity, reducing lactate efflux and tumor growth.397 The CD147 dimerization inhibitor AC-73,413 the human/mouse chimeric IgG1 mAb of CD147 named metuzumab,414 the organomercurial reagent p-chloromercuribenzene sulfonate (pCMBS),415 which blocks MCT1/MCT4-CD147 binding 118 are available CD147-targeted anticancer drugs. Nonetheless, on a cautionary note, CD147 is ubiquitously expressed and interacts with other proteins at the cell surface. Thus, strategies selectively targeting CD147-MCT interactions ought to minimize drug toxicity and establish a therapeutic window.26
There is evidence that integration of MCT-targeted and other therapies fulfill a better therapeutic role. Li et al. revealed that in breast cancer mouse model, MCT inhibitor Syrosingopine downregulated the number of Treg cell and upregulated that of NK cells and M1 phenotype of TAM, suggesting reversal of the immunosuppressive TME.416 Meanwhile, Ma et al. found that in vivo and in vitro, Lithium carbonate (LC) assisted MCT1 localization to mitochondrial membranes and lactate influx into mitochondria. Revitalization of tumor-reactive CD8 + T cells induced by above-mentioned extra energy support sensitized immunotherapy towards CRC, melanoma and breast cancer.417 It sheds insight on the aspect that MCT targeted therapy may fulfill a role in synergy with immunotherapy.
Other lactate-targeted strategies
While development of LDH and MCT inhibitors is in full swing, there is emerging concern that they can disrupt the metabolism of healthy cells and cause severe non-specific toxicity. As for solution to overcoming these shortcomings, researchers put forward the opinion that lactate oxidase (LOx) was a therapeutic option which reduced lactate concentrations, released H2O2 and recruited immune cells, overcoming immunosuppression and sensitizing immunotherapy.418,419 Moreover, the drug delivery system of LOx evolves gradually from polymer nanocarriers into self‐assembled nanoparticles, the update refinement of which empowers its application towards chemotherapy and sonodynamic therapy (SDT) sensitization.420,421,422,423,424 The depletion of lactate catalyzed by LOx generates pyruvate, which in turn activates clustered regularly interspaced short palindromic repeat-associated protein 9 (CRISPR/Cas9)-mediated signal-regulatory protein alpha (SIRPα) genome-editing plasmids. When combined with a metal-organic framework (MOF), LOx and these plasmids are utilized to form nanoparticle named LPZ (LOx, Cas9/sgSIRPα plasmids, mannose-modified PEG loaded-ZIF-67) and facilitate the conversion of M2 macrophages to M1 macrophages, thereby inhibiting the growth of in situ breast cancer models.425 This approach offers a method for LOx-induced-CRISPR/Cas9-mediated macrophage gene editing directly within the tumor site and presents a potential strategy for enhancing immunotherapy.426,427 For instance, Luo et al. utilized nano-ZIF-8 as the carrier to construct the Hb-LOx-DOX-ZIF8@platelet membrane nanosystem (HLDZ@PM NPs) and effectively enhance the tumor sensitivity to DOX-induced chemotherapy.428 Anchor LOx onto the surface of lactobacillus (LA) also increased lesion targeting and delivery efficiency, enabled LOx to fully catalyze lactate oxidation and depletion of intra-tumor oxygen, thus activating the chemotoxicity drug to induce apoptosis.429,430 Zhang et al. developed a metal-phenolic network-based nanocomplex, incorporating LOx and the mitochondrial respiration inhibitor atovaquone (ATO) to reconstruct the immunosuppressive TME. This nanocomplex demonstrated superior pharmacological effects compared to single-agent therapy in breast cancer SDT.431
Besides, the newly-reported targeting strategy also aims to block AARS1, which serves as a bridge linking tumor cell metabolism with proteomic changes. Β-Alanine blocked the interaction between AARS1 and lactate, preventing subsequent lactyl transfer. As a result, the tumor suppressor gene p53 was not lactylated at K120 and K139, which inhibited tumor progression in a CRC mouse model.239 Downregulation of pan-Kla writer KAT8 by the histone deacetylase (HDAC) inhibitor MG149 blocks the KAT8-eEF1A2 Kla axis and suppresses CRC tumor growth, particularly in a high-lactic TME.240
Likewise, there are other elucidated strategies of dual regulation of metabolism and immunity. Li et al. invented an in-situ injection of a thermogel loaded with glucose transporter 1 (GLUT1) inhibitor-sensitized GBM immunotherapy of PD-1/PD-L1 blocker BMS-1 through alleviating lactate-driven Treg cells.432 Additionally, Niu et al. invented a novel single-atom nanozyme pyroptosis initiator: UK5099 and pyruvate oxidase (POx)-co-loaded Cu-NS single-atom nanozyme (Cu-NS@UK@POx), offering a dual-pronged approach that effectively enhanced the immunotherapeutic anti-tumor effects by inducing ROS storms and lactate/ATP depletion.433 Aside from the oxidative catabolism of lactate, neutralizing it with a basic salt is another potential strategy for targeted therapy. Inhibiting spontaneous metastases in mouse models of metastatic breast cancer was shown to be effective by neutralizing lactate in the tumor using a basic salt such as NaHCO3.434 Besides, acid-neutralizing CaCO3 nanoparticles were used to maintain the pH within the normal physiological range in breast cancer cells, which inhibited the proliferation and migration.435 To sum up, as other intervention in tumor lactate metabolism shows great potential to of chemotherapy and immunotherapy sensitization. The optimal scheme of organically combining lactate metabolic regulation with other therapies, which includes screening the most effective lactate regulation target, determining the best treatment time window, and identifying the most appropriate action site to maximize antitumor efficacy, remains to be further explored. Further exploration is needed to determine the optimal approach for effectively integrating lactate metabolic regulation with other therapies. This includes identifying efficient target, determining the optimal treatment time window, and pinpointing the most suitable action site to maximize the anti-tumor efficacy.
By inhibiting H3 histone lactylation (H3K9la and H3K56la), demethylzeylasteral decreased the tumorigenesis driven by liver cancer stem cells (LCSCs) both in vivo and in vitro. This indicated that lactylation inhibition served as a potential candidate for adjunctive tumor therapy.225 Besides, Xu et al. also found that by downregulating lactylation at H3K9la and H3K14la, lactate production was reduced by royal jelly acid (RJA), thereby inhibiting tumor invasion, migration, proliferation, and apoptosis of HCC.226
Inhibition of non-histone lactylation at the MRE11 K673 site through K673-peptide-3# (K673-pe) suppressed HR in CRC, thereby restoring its sensitivity to chemotherapy and poly ADP-ribose polymerase inhibitor (PARPi). Hearin, K673-pe exhibited a synergistic tumor-suppressive effect when combined with chemotherapy.243 D34-919 blocked the interaction between ALDH1A3 and PKM2 in GBM cells, thereby suppressing the downstream lactylation of XRCC1, which restored the sensitivity of GBM to TMZ-based chemotherapy and radiotherapy in GBM organoid models.244,436
Potential targeted therapeutic approaches may be possible by targeting lactate production and transport, particularly through LDH and MCTs. However, because these molecules are involved in complex interactions that control multiple signaling and metabolic events, it is challenging to determine the quantitative contribution of modulated lactate balance to therapeutic efficacy.437,438 In addition, metabolic targeting depends on specific metabolic requirements, by way of example, lactate metabolism-targeting drugs are ineffective against glutamine-dependent cells. The metabolic heterogeneity of diverse tumor types, varied clinical stages, distinct cell populations within the TME may be one of the factors contributing to the poor efficacy of metabolic-targeted drugs.439,440,441 To elaborate, in the study by Liu et al., it was shown that when the metabolic pathway of HCC, the most common type of primary liver cancer, shifted from glycolysis to OXPHOS, the proliferation of HCC cells and tumor growth were inhibited.442 However, for cholangiocarcinoma (CCA), the second most common type of primary liver cancer, mitochondrial OXPHOS metabolism helps maintain stem-like characteristics in CCA, thereby conferring tumorigenic potential and chemotherapy resistance in vivo.158,443 Apart from primary liver cancer, PD-1 resistant NSCLC cells were characterized by a markedly lower glycolytic reserve and exhibited a significantly higher reliance on OXPHOS compared to PD-1 sensitive NSCLC cells, which implies that tumor progression leads to complex changes in lactate metabolism flux.159,441 As well as primary liver cancer and NSCLC, CRC exhibits different lactate metabolism across various histological and molecular subtypes.444,445 Based on global genomic profiling, CRC can be classified into microsatellite stable (MSS) CRC and microsatellite unstable (MSI) CRC, with differences observed in mitochondrial DNA (mtDNA) copy numbers between these two types.446 Sun et al. found that MSS cancer cells with higher mtDNA copy numbers tended to rely on OXPHOS, which promoted malignant tumor development.447 Conversely, MSI cancer cells with lower mtDNA copy numbers depended on glycolysis, which contributed to tumorigenesis and cisplatin chemoresistance.160,446,448 In various cancers such as gastric cancer, breast cancer, HCC, NSCLC, endometrial cancers (EC) and renal cell carcinoma (RCC), mtDNA levels are decreased.449,450,451 Conversely, mtDNA copy numbers are elevated in other cancer types, including CRC, esophageal squamous cell carcinoma, acute lymphoblastic leukemia, head and HNSCC, and ovarian cancer.452,453,454,455,456,457 This suggests both complexity and potential commonalities in lactate metabolism characteristics across tumor types.
Meanwhile, when applied to tumors characterized with unlocking phenotype plasticity, lactate inhibitors are characterized with a high off-target rate and low specificity to lactate metabolism, which obscures their true pharmacological mechanisms and therapeutic effects.383,384,458,459 In this situation, targeting the metabolic symbiosis between cancer and stromal cells by inducing metabolic switching in the tumor TME leads to sensitization of targeted therapies. For instance, anti-angiogenic drugs may elicit a more hypoxic TME, thereby reducing the metabolic heterogeneity of the TME and enhancing the efficacy of targeted therapies. However, metabolic malleability in invasive tumors results in recurrence as well as drug-resistant clones with more complex metabolic profiles. Last but not least, changes in the non-cancerous cells during treatment are another being issue that metabolic targeting may confront. Lactate not only provides energy to neurons but also spreads beyond the active zone, influencing the function of neurons and astrocytes in nearby regions.179 Playing a role in processes ranging from neurovascular coupling to learning and memory, lactate serves a dual purpose as both a metabolic fuel and an intercellular signaling molecule.460,461 On the one hand, in relation to normal cells which lactate/lactylation exerts a pivotal effect on its physiological functions, lactate/lactylation targeted therapy may inflict a fatal blow to their structure and operation.462,463,464 For example, playing a role in processes ranging from neurovascular coupling to learning and memory, lactate serves a dual purpose as both a metabolic fuel and an intercellular signaling molecule.91,349 Also, current research demonstrated the critical role of lactate and lactate transport enzymes, such as LDHA, in biogenesis and function of phototransduction system.465,466,467,468 Lactate stimulated by insulin from the retinal pigment epithelium (RPE), supports the crucial visual functions of photoreceptors by enhancing glucose absorption in the retina.465 Last but not least, notably, the interplay between epigenetic mechanisms complicates the selective targeting of histone lactylation without influencing acetylation.21,469,470 On the other hand, immune system activation is accompanied by changes in immune cell metabolism.471 As an illustration, the activation of lymphocytes during the anti-tumor process of the immune system is dependent on the Warburg effect. Moreover, lactate, through arrestin β-2 (ARRB2) and GPR81, inhibits Toll-like receptor (TLR)-induced activation of the NOD-like receptor family pyrin domain containing 3 (NLRP3) inflammasome and the production of interleukin-1 beta (IL-1β).180 Thus, if an antimetabolic compound impairs tumor cell growth, it can also inhibit the anti-tumor immune response and anti-inflammasome-mediated inflammation immunomodulation.472,473
To overcome the heterogeneity and adaptability of tumor metabolism, enhance the specificity of targeted drugs, minimize adverse effects, and hinder the occrurence of treatment resistance, novel investigations in targeted therapy focus on devising novel small-molecule inhibitors, developing robust delivery systems that adhere to the 3 R delivery principle (right site, right timing and right dose) and exploring rational and effective drug combination strategies.474,475,476
Regarding novel lactate/lactylation inhibitors, PROTAC are novel small-molecule inhibitors that downregulate or even eliminate their target proteins via the ubiquitin-proteasome pathway.477 As PROTAC effectively degrade target proteins even under conditions of low affinity, they avoid several drawbacks associated with traditional small-molecule inhibitors, such as the need for high specificity to protein binding pockets, the development of resistance due to mutations at binding sites after prolonged use, and the cumulative toxicity from sustained high concentrations.478,479,480 Furthermore, PROTAC have successfully achieved dual targeting of LDHA/LDHB and proliferation inhibition in pancreatic cancer cell lines. Following intraperitoneal injection, the plasma of mice also indicated favorable drug bioavailability.387 This suggests that PROTAC could serve as a useful molecular tool for in-depth research to target lactate metabolism.
Considering delivery systems to effectively restore aberrant lactate and lactylation levels, catalytically active nanomaterials and gene-editing techniques have shown the therapeutic potential in previous studies.468,481 Catalytically active nanomaterials, often known as “nanozymes,” have emerged as promising alternatives. They present numerous benefits, such as improved catalytic efficiency, resistance to harsh environments, prolonged effectiveness, and precise targeting.421,482,483 These benefits grant nanozymes diverse and potent therapeutic potential in delivering small molecule lactate/lactylation inhibitors.479 Apart from nanozymes, gene editing techniques have also demonstrated possible functions in steamlinedly regulating lactate and lactylation. Adeno-associated virus (AAV) is viewed as among the leading viral vectors for gene delivery due to its capacity to infect diverse tissue types and its reputation as a comparatively low-risk option for gene transfer.484,485,486,487,488 AAV8-packaged vectors have been utilized for sclera-specific gene editing, which was employed to investigate how enhanced lactate/lactylation levels in the sclera promote myopia.481 This indicates the possible function of the AAV delivery system in targeted gene editing and its subsequent application in epigenetic therapy. Furthermore, LOx-catalyzed lactate depletion activates CRISPR/Cas9-mediated SIRPα genome-editing plasmids, which, when combined with a MOF, are used to promote the conversion of M2 macrophages to M1 macrophages in the breast cancer TME, thereby hinting the therapeutic effect of CRISPR/Cas9-mediated genome-editing.425,489,490,491 In a word, extensive breakthrough upon lactate targeted therapy requires further research into lactate/lactylation biology, related molecular pathways, and associated interactions between consonants of TME to provide foundational support.
Lactate assisted therapy
While lactate has long been considered a productive substrate for energy metabolism of tumors, recent studies have uncovered conflicting perspectives. Extracellular acidosis impedes one-carbon metabolism crucial for short-lived effector T cells, promoting their differentiation, resilience, and anti-tumor cytotoxicity.318,473 Concerning immune checkpoint blockade (ICB) therapy, it was demonstrated that inhibiting LDHA in melanoma mouse model significantly boosted the efficacy of anti-PD-1 therapy. This combined approach effectively disrupted the PD-1/PD-L1 pathway, leading to enhanced pro-inflammatory anti-tumor responses, including heightened infiltration and activity of NK cells and CD8+ cytotoxic T cells, and a decreased frequency of Treg cells.302 Additionally, extended study validated the immunotherapy sensitization effect of lactate in vivo. Subcutaneous administration of sodium lactate solution promotes antitumor immunity through CM8 + T cells and sensitizes immunotherapy in CRC, NSCLC and melanoma mouse models.316 This suggests that the reprogramming of lactate metabolism in T cells may have a nuanced impact on antitumor immunity, posing a potential dual effect that warrants further exploration. Besides, AlkB homolog 5 (ALKBH5) inhibitor ALK-04 enhances the sensitivity of anti–PD-1 immunotherapy and immune cell recruitment by downregulating MCT4 levels and lactate production in melanoma.492 Moreover, anti-APOC2K70-lac antibody interferes with K70 lactylaton of APOC2, thus thwarting FFA release, blocking the accumulation of Treg cells and sensitizing anti-tumor response of immunotherapy.181
Regarding lactate-targeted strategies for radiotherapy sensitization, Yao et al. developed a novel CoMnFe-layered double oxides (LDO) nanosheet with multienzyme activities, which not only enhanced ROS production during radiotherapy but also catalyzed lactate into pyruvate. This innovation offers a therapeutic strategy to eliminate lactate from the TME and improving radiotherapy efficacy for uveal melanoma (UM).493
In terms of chemotherapy sensitization, lactate has been found to confer chemoresistance by increasing the expression of multidrug resistance-associated protein 1 (MRP1, encoded by ATP-binding cassette sub-family C member 1 (ABCC1)), which induces drug efflux from cells. Nevertheless, the use of NaHCO3 to neutralize lactate may reverse this resistance, making NSCLC cells more susceptible to the chemotherapeutic drug etoposide.494 To add up, leveraging the acidity and high levels of lactate in the TME, the engineered LOx-immobilized Ce-benzenetricarboxylic acid (Ce-BTC) MOF facilitates the intratumoral production of hydroxyl radicals (·OH) through a cascade process. This strategy allows for pH-dependent, sensitized and targeted chemotherapy in HCC mouse model.495
In addition to immunotherapy, radiotherapy and chemotherapy, lactate-targeted therapy can also have a synergistic effect when combined with targeted therapies. It is demonstrated that lactate stabilized NF-κB within CAFs, prompting the secretion of tumor-promoting HGF, which induced TKIs resistance in tumor cells. When targeted therapies are used in combination with MCT1/2 inhibitor AZD3965 or LDHA inhibitor NHI-Glc-2, the sensitivity is restired in SLCLC and gastric cancer and they achieve more favorable efficacy.334 Lactate also induces resistance to pan-Akt inhibitor uprosertib in CRC. Notably, combining uprosertib with MCT1/2 inhibitor AZD3965 reversed this resistance through the inhibition of lactate uptake.496
Conclusion and future directions
Lactate is proven to be a key source of circulating carbohydrates that fuels the TCA cycle and promotes energy production in tumors. Changes in lactate metabolism impact tumor progression, as lactate can enter cells through various pathways, such as intercellular transport via MCT. This process, known as metabolic symbiosis, plays a crucial role in tumor biology by facilitating interactions between different cell populations in the TME. In tumor cells, lactate enhances the lactate shuttle as well as affecting cell signaling pathways, promoting resistance to oxidative stress, and leading to lactylation. In other cell populations, interaction between lactate and immune cells influences cell differentiation, immune response, immune surveillance, and sensitivity to treatment. Additionally, communication between lactate and stromal/endothelial cells reinforces invasiveness and progression of tumors.
Given lactate’s extensive role in cancer metabolism and its influence on both tumor and immune cells, targeting lactate metabolism has emerged as a promising strategy for cancer treatment. When it comes to detection of lactate/lactylation, advanced molecular and imaging techniques are shedding new light on the mechanisms and functional significance of fluctuations in tissue lactate and lactylation levels throughout tumor progression. In addition, researchers have explored various approaches to inhibit lactate production or block its transport within tumors, with the goal of disrupting the metabolic symbiosis that sustains tumor growth. Despite the potential of targeting lactate, most lactate-targeted therapies remain in the preclinical stage, with only a few advancing to clinical trials. One notable example is AZD3965, an inhibitor of MCT1/2, which has undergone a phase I clinical trial (NCT01791595) in patients with advanced cancer. This trial has provided insights into the pharmacokinetics and adverse effects of AZD3965, but further clinical trials are needed to assess its efficacy and therapeutic potential in a broader range of cancers.
However, significant challenges remain in the development of effective lactate/lactylation-targeted therapies. One major obstacle is that determining the quantitative contribution of modulating lactate balance to therapeutic efficacy remains challenging. Besides, according to the metabolic heterogeneity across different tumor types, clinical stages, and distinct cell populations within the TME, the heterogeneity and complexity of the TME make it difficult to achieve effective drug concentrations at the tumor site and complicate the effectiveness of metabolic-targeted drugs. Additionally, when lactate inhibitors are applied to tumors characterized by phenotypic plasticity, they often exhibit high off-target effects and low specificity to lactate metabolism, making it difficult to pinpoint their true pharmacological mechanisms and therapeutic benefits. Moreover, changes in non-cancerous cells during treatment introduce further complexities, as these indiscriminate alterations may undermine the effectiveness of immune response, confront normal physiological functions and lead to unintended side effects.
To tackle the challenges associated with lactate/lactylation-targeted cancer therapies, recent investigations have increasingly focused on several key areas of innovation that hold significant promise for improving treatment outcomes. One major area of focus is the design and optimization of novel small-molecule inhibitors that exhibit enhanced specificity and efficacy against tumor cells. These inhibitors aim to selectively disrupt lactate-related pathways, thereby reducing the cancer cells’ metabolic adaptability. In tandem with this, researchers are developing advanced drug delivery systems that are both robust and efficient, enhancing bioavailability and precision targeting of therapeutic agents. Such innovations are crucial for ensuring that drugs reach their intended sites of action in sufficient concentrations to exert their effects while minimizing side effects on healthy tissues. Furthermore, the exploration of rational, synergistic drug combination strategies is gaining traction. These combination therapies are designed to target multiple pathways simultaneously, thereby minimizing the potential for drug resistance and improving overall therapeutic efficacy. By employing a multi-faceted approach, these strategies aim to overcome the limitations of current therapies, ultimately leading to more favorable treatment outcomes and improved prognoses for cancer patients.497
In conclusion, although lactate-targeted therapies show considerable promise in the realm of cancer treatment, they are still in the early stages of development, and substantial efforts are required to unlock their full potential. A comprehensive understanding of how lactate interacts with various metabolic and epigenetic processes in cancer is crucial for creating more effective therapeutic strategies. By elucidating these complex relationships, researchers can develop novel cancer therapies that leverage the unique properties of lactate and lactylation. Such advancements in lactate/lactylation-targeted therapy are urgently needed to effectively suppress tumor growth, overcome drug resistance, and ultimately improve patient outcomes, paving the way for more personalized and successful treatment approaches in oncology.
Data availability
Not applicable.
References
Ge, T. et al. Crosstalk between metabolic reprogramming and epigenetics in cancer: updates on mechanisms and therapeutic opportunities. Cancer Commun. 42, 1049–1082 (2022).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Hanahan, D. Hallmarks of cancer: new dimensions. Cancer Discov. 12, 31–46 (2022).
Warburg, O. On the origin of cancer cells. Science 123, 309–314 (1956).
Liberti, M. V. & Locasale, J. W. The Warburg effect: how does it benefit cancer cells? Trends Biochem. Sci. 41, 211–218 (2016).
Thompson, C. B. et al. A century of the Warburg effect. Nat. Metab. 5, 1840–1843 (2023).
Zhong, X. et al. Warburg effect in colorectal cancer: the emerging roles in tumor microenvironment and therapeutic implications. J. Hematol. Oncol. 15, 160 (2022).
Lu, J. The Warburg metabolism fuels tumor metastasis. Cancer Metastasis Rev. 38, 157–164 (2019).
Liao, M. et al. Targeting the Warburg effect: a revisited perspective from molecular mechanisms to traditional and innovative therapeutic strategies in cancer. Acta Pharm. Sin. B 14, 953–1008 (2024).
Siska, P. J. et al. The immunological Warburg effect: can a metabolic-tumor-stroma score (MeTS) guide cancer immunotherapy? Immunol. Rev. 295, 187–202 (2020).
Hensley, C. T. et al. Metabolic heterogeneity in human lung tumors. Cell 164, 681–694 (2016).
Sushentsev, N. et al. Hyperpolarised 13C-MRI identifies the emergence of a glycolytic cell population within intermediate-risk human prostate cancer. Nat. Commun. 13, 466 (2022).
Faubert, B. et al. Lactate metabolism in human lung tumors. Cell 171, 358–371, (2017).
Hui, S. et al. Glucose feeds the TCA cycle via circulating lactate. Nature 551, 115–118 (2017).
Brooks, G. A. et al. Tracing the lactate shuttle to the mitochondrial reticulum. Exp. Mol. Med. 54, 1332–1347 (2022).
Tasdogan, A. et al. Metabolic heterogeneity confers differences in melanoma metastatic potential. Nature 577, 115–120 (2020).
Lee, D. C. et al. A lactate-induced response to hypoxia. Cell 161, 595–609 (2015).
Fan, H. et al. Lactylation: novel epigenetic regulatory and therapeutic opportunities. Am. J. Physiol. Endocrinol. Metab. 324, E330–E338 (2023).
Wang, T. et al. Lactate-induced protein lactylation: a bridge between epigenetics and metabolic reprogramming in cancer. Cell Prolif., e13478, (2023).
Zhang, D. et al. Metabolic regulation of gene expression by histone lactylation. Nature 574, 575–580 (2019).
Yang, Z. et al. Lactylome analysis suggests lactylation-dependent mechanisms of metabolic adaptation in hepatocellular carcinoma. Nat. Metab. 5, 61–79 (2023).
Rizwan, A. et al. Relationships between LDH-A, lactate, and metastases in 4T1 breast tumors. Clin. Cancer Res. 19, 5158–5169 (2013).
Brand, A. et al. LDHA-associated lactic acid production blunts tumor immunosurveillance by T and NK cells. Cell Metab. 24, 657–671 (2016).
Agathocleous, M. et al. Metabolic differentiation in the embryonic retina. Nat. Cell Biol. 14, 859–864 (2012).
Choi, S. Y. C. et al. Lessons from patient-derived xenografts for better in vitro modeling of human cancer. Adv. Drug Deliv. Rev. 79-80, 222–237 (2014).
Doherty, J. R. & Cleveland, J. L. Targeting lactate metabolism for cancer therapeutics. J. Clin. Investig. 123, 3685–3692 (2013).
Zhang, Y. & Yang, J.-M. Altered energy metabolism in cancer: a unique opportunity for therapeutic intervention. Cancer Biol. Ther. 14, 81–89 (2013).
Xiong, J. et al. Lactylation-driven METTL3-mediated RNA m6A modification promotes immunosuppression of tumor-infiltrating myeloid cells. Mol. Cell. 82, 1660–1677 (2022).
Brooks, G. A. The tortuous path of lactate shuttle discovery: From cinders and boards to the lab and ICU. J. Sport Health Sci. 9, 446–460 (2020).
Mäki-Arvela, P., Simakova, I. L., Salmi, T. & Murzin, D. Y. Production of lactic acid/lactates from biomass and their catalytic transformations to commodities. Chem. Rev. 114, 1909–1971 (2014).
Joan Dawson, M., Gadian, D. G. & Wilkie, D. R. Muscular fatigue investigated by phosphorus nuclear magnetic resonance. Nature 274, 861–866 (1978).
Koppenol, W. H., Bounds, P. L. & Dang, C. V. Otto Warburg’s contributions to current concepts of cancer metabolism. Nat. Rev. Cancer 11, 325–337 (2011).
Wang, Y. & Dang, C. V. The Warburg effect revisited through blood and electron flow. Cancer Res. 84, 2046–2048 (2024).
Lv, X., Lv, Y. & Dai, X. Lactate, histone lactylation and cancer hallmarks. Expert Rev. Mol. Med. 25, e7 (2023).
Dienel, G. A. Lactate shuttling and lactate use as fuel after traumatic brain injury: metabolic considerations. J. Cereb. Blood Flow. Metab. 34, 1736–1748 (2014).
Morandi, A. & Indraccolo, S. Linking metabolic reprogramming to therapy resistance in cancer. Biochim. Biophys. Acta Rev. Cancer 1868, 1–6 (2017).
Roumes, H. et al. Neuroprotective role of lactate in rat neonatal hypoxia-ischemia. J. Cereb. Blood Flow. Metab. 41, 342–358 (2021).
Halestrap, A. P. Monocarboxylic acid transport. Compr. Physiol. 3, 1611–1643 (2013).
Chang, C.-H. et al. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell 162, 1229–1241 (2015).
Finley, L. W. S. What is cancer metabolism? Cell 186, 1670–1688 (2023).
Urbano, A. M. Otto Warburg: the journey towards the seminal discovery of tumor cell bioenergetic reprogramming. Biochim. Biophys. Acta Mol. Basis Dis. 1867, 165965 (2021).
Wang, Y. & Patti, G. J. The Warburg effect: a signature of mitochondrial overload. Trends Cell Biol. 33, 1014–1020 (2023).
Vander Heiden, M. G., Cantley, L. C. & Thompson, C. B. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324, 1029–1033 (2009).
Warburg, O. & Minami, S. Versuche an Überlebendem Carcinom-gewebe. Klin. Wochenschr. 2, 776–777 (1923).
López-Ríos, F. et al. Loss of the mitochondrial bioenergetic capacity underlies the glucose avidity of carcinomas. Cancer Res. 67, 9013–9017 (2007).
Racker, E. Bioenergetics and the problem of tumor growth. Am. Sci. 60, 56–63 (1972).
Chance, B. & Castor, L. N. Some patterns of the respiratory pigments of ascites tumors of mice. Science 116, 200–202 (1952).
Chance, B. & Hess, B. Spectroscopic evidence of metabolic control. Science 129, 700–708, (1959).
Burk, D. & Schade, A. L. On respiratory impairment in cancer cells. Science 124, 270–272, (1956).
Weinhouse, S. Hepatomas. Science 158, 542–543 (1967).
Som, P. et al. A fluorinated glucose analog, 2-fluoro-2-deoxy-D-glucose (F-18): nontoxic tracer for rapid tumor detection. J. Nucl. Med. 21, 670–675 (1980).
Bordonne, M. et al. Brain (18)F-FDG PET for the diagnosis of autoimmune encephalitis: a systematic review and a meta-analysis. Eur. J. Nucl. Med. Mol. Imaging 48, 3847–3858 (2021).
Donnelly, R. et al. Meta-analysis of [(18)F]FDG-PET/CT in pulmonary sarcoidosis. Eur. Radiol. (2024).
Ahlman, M. A. & Grayson, P. C. Advanced molecular imaging in large-vessel vasculitis: adopting FDG-PET into a clinical workflow. Best. Pract. Res Clin. Rheumatol. 37, 101856 (2023).
Pijl, J. P. et al. FDG-PET/CT in intensive care patients with bloodstream infection. Crit. Care 25, 133 (2021).
Fan, T. W. M. et al. Altered regulation of metabolic pathways in human lung cancer discerned by (13)C stable isotope-resolved metabolomics (SIRM). Mol. Cancer 8, 41 (2009).
Lyssiotis, C. A. & Kimmelman, A. C. Metabolic interactions in the tumor microenvironment. Trends Cell Biol. 27, 863–875 (2017).
Xiao, Z., Dai, Z. & Locasale, J. W. Metabolic landscape of the tumor microenvironment at single cell resolution. Nat. Commun. 10, 3763 (2019).
Ghesquière, B., Wong, B. W., Kuchnio, A. & Carmeliet, P. Metabolism of stromal and immune cells in health and disease. Nature 511, 167–176 (2014).
Nakazawa, M. S., Keith, B. & Simon, M. C. Oxygen availability and metabolic adaptations. Nat. Rev. Cancer 16, 663–673 (2016).
Barretina, J. et al. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature 483, 603–607 (2012).
Vaupel, P. & Multhoff, G. Revisiting the Warburg effect: historical dogma versus current understanding. J. Physiol. 599, 1745–1757 (2021).
Kang, H. et al. The Warburg effect on radioresistance: survival beyond growth. Biochim. Biophys. Acta Rev. Cancer 1878, 188988 (2023).
Icard, P. et al. How the Warburg effect supports aggressiveness and drug resistance of cancer cells? Drug Resist. Updat. 38, 1–11 (2018).
Barba, I., Carrillo-Bosch, L. & Seoane, J. Targeting the Warburg effect in cancer: where do we stand? Int. J. Mol. Sci. 25, 3142 (2024).
Wang, N. et al. Histone lactylation boosts reparative gene activation post-myocardial infarction. Circ. Res. 131, 893–908 (2022).
Zhang, N. et al. α-myosin heavy chain lactylation maintains sarcomeric structure and function and alleviates the development of heart failure. Cell Res. 33, 679–698 (2023).
Rho, H., Terry, A. R., Chronis, C. & Hay, N. Hexokinase 2-mediated gene expression via histone lactylation is required for hepatic stellate cell activation and liver fibrosis. Cell Metab. 35, 1406–1423.e1408 (2023).
Pan, R. Y. et al. Positive feedback regulation of microglial glucose metabolism by histone H4 lysine 12 lactylation in Alzheimer’s disease. Cell Metab. 34, 634–648.e636 (2022).
Fan, M. et al. Lactate promotes endothelial-to-mesenchymal transition via Snail1 lactylation after myocardial infarction. Sci. Adv. 9, eadc9465 (2023).
Apostolova, P. & Pearce, E. L. Lactic acid and lactate: revisiting the physiological roles in the tumor microenvironment. Trends Immunol. 43, 969–977 (2022).
Colbert, L. E. et al. Tumor-resident Lactobacillus iners confer chemoradiation resistance through lactate-induced metabolic rewiring. Cancer Cell. 41, 1945–1962.e1911 (2023).
Lin, J. et al. Targeting lactate-related cell cycle activities for cancer therapy. Semin. Cancer Biol. 86, 1231–1243 (2022).
Sharma, D., Singh, M. & Rani, R. Role of LDH in tumor glycolysis: regulation of LDHA by small molecules for cancer therapeutics. Semin. Cancer Biol. 87, 184–195 (2022).
Ippolito, L. et al. Lactate rewires lipid metabolism and sustains a metabolic-epigenetic axis in prostate cancer. Cancer Res. 82, 1267–1282 (2022).
Halford, S. et al. A Phase I dose-escalation study of AZD3965, an oral monocarboxylate transporter 1 inhibitor, in patients with advanced cancer. Clin. Cancer Res. 29, 1429–1439 (2023).
Beloueche-Babari, M. et al. MCT1 inhibitor AZD3965 increases mitochondrial metabolism, facilitating combination therapy and noninvasive magnetic resonance spectroscopy. Cancer Res. 77, 5913–5924 (2017).
Polański, R. et al. Activity of the monocarboxylate transporter 1 inhibitor AZD3965 in small cell lung cancer. Clin. Cancer Res. 20, 926–937 (2014).
Noble, R. A. et al. Inhibition of monocarboxyate transporter 1 by AZD3965 as a novel therapeutic approach for diffuse large B-cell lymphoma and Burkitt lymphoma. Haematologica 102, 1247–1257 (2017).
Bola, B. M. et al. Inhibition of monocarboxylate transporter-1 (MCT1) by AZD3965 enhances radiosensitivity by reducing lactate transport. Mol. Cancer Ther. 13, 2805–2816 (2014).
DeBerardinis, R. J., Lum, J. J., Hatzivassiliou, G. & Thompson, C. B. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 7, 11–20 (2008).
Yao, S. et al. Astrocytic lactate dehydrogenase A regulates neuronal excitability and depressive-like behaviors through lactate homeostasis in mice. Nat. Commun. 14, 729 (2023).
Comandatore, A. et al. Lactate dehydrogenase and its clinical significance in pancreatic and thoracic cancers. Semin. Cancer Biol. 86, 93–100 (2022).
Arce-Molina, R. et al. A highly responsive pyruvate sensor reveals pathway-regulatory role of the mitochondrial pyruvate carrier MPC. Elife 9, e53917 (2020).
Cluntun, A. A. et al. The pyruvate-lactate axis modulates cardiac hypertrophy and heart failure. Cell Metab. 33, 629–648.e610 (2021).
Mitchell, P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature 191, 144–148 (1961).
Henderson, G. C., Horning, M. A., Wallis, G. A. & Brooks, G. A. Pyruvate metabolism in working human skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 292, E366 (2007).
Herzig, S. et al. Identification and functional expression of the mitochondrial pyruvate carrier. Science 337, 93–96 (2012).
Andreucci, E. et al. The acidic tumor microenvironment drives a stem-like phenotype in melanoma cells. J. Mol. Med. 98, 1431–1446 (2020).
Bennis, Y. et al. A study of associations between plasma metformin concentration, lactic acidosis, and mortality in an emergency hospitalization context. Crit. Care Med. 48, e1194–e1202 (2020).
Jha, M. K., Lee, I.-K. & Suk, K. Metabolic reprogramming by the pyruvate dehydrogenase kinase-lactic acid axis: linking metabolism and diverse neuropathophysiologies. Neurosci. Biobehav. Rev. 68, 1–19 (2016).
Pellerin, L. & Magistretti, P. J. Sweet sixteen for ANLS. J. Cereb. Blood Flow. Metab. 32, 1152–1166 (2012).
Miller, B. F. et al. Lactate and glucose interactions during rest and exercise in men: effect of exogenous lactate infusion. J. Physiol. 544, 963–975 (2002).
Emhoff, C.-A. W. et al. Gluconeogenesis and hepatic glycogenolysis during exercise at the lactate threshold. J. Appl Physiol. 114, 297–306 (2013).
Wang, N. et al. Structural basis of human monocarboxylate transporter 1 inhibition by anti-cancer drug candidates. Cell 184, 370–383.e313 (2021).
Kobayashi, M., Narumi, K., Furugen, A. & Iseki, K. Transport function, regulation, and biology of human monocarboxylate transporter 1 (hMCT1) and 4 (hMCT4). Pharm. Ther. 226, 107862 (2021).
Singh, M. et al. Targeting monocarboxylate transporters (MCTs) in cancer: How close are we to the clinics? Semin. Cancer Biol. 90, 1–14 (2023).
D’Aria, S. et al. Expression of the monocarboxylate transporter MCT1 is required for virus-specific mouse CD8(+) T cell memory development. Proc. Natl. Acad. Sci. USA 121, e2306763121 (2024).
Pereira-Nunes, A. et al. Targeting lactate production and efflux in prostate cancer. Biochim Biophys. Acta Mol. Basis Dis. 1866, 165894 (2020).
Noor, S. I. et al. A surface proton antenna in carbonic anhydrase II supports lactate transport in cancer cells. Elife 7, e35176 (2018).
Bonglack, E. N. et al. Monocarboxylate transporter antagonism reveals metabolic vulnerabilities of viral-driven lymphomas. Proc. Natl Acad. Sci. USA 118, e2022495118 (2021).
Corbet, C. et al. Interruption of lactate uptake by inhibiting mitochondrial pyruvate transport unravels direct antitumor and radiosensitizing effects. Nat. Commun. 9, 1208 (2018).
Droździk, M. et al. Monocarboxylate transporter 1 (MCT1) in liver pathology. Int. J. Mol. Sci. 21, 1606 (2020).
Ullah, M. S., Davies, A. J. & Halestrap, A. P. The plasma membrane lactate transporter MCT4, but not MCT1, is up-regulated by hypoxia through a HIF-1alpha-dependent mechanism. J. Biol. Chem. 281, 9030–9037 (2006).
Chen, A.-N. et al. Lactylation, a novel metabolic reprogramming code: current status and prospects. Front. Immunol. 12, 688910 (2021).
Monsorno, K. et al. Loss of microglial MCT4 leads to defective synaptic pruning and anxiety-like behavior in mice. Nat. Commun. 14, 5749 (2023).
Parks, S. K., Chiche, J. & Pouysségur, J. Disrupting proton dynamics and energy metabolism for cancer therapy. Nat. Rev. Cancer 13, 611–623 (2013).
Wang, K. et al. Di-methylation of CD147-K234 promotes the progression of NSCLC by enhancing lactate export. Cell Metab. 33, 160–173.e6 (2021).
De La Cruz-López, K. G. et al. Lactate in the regulation of tumor microenvironment and therapeutic approaches. Front. Oncol. 9, 1143 (2019).
Pinheiro, C. et al. The metabolic microenvironment of melanomas: prognostic value of MCT1 and MCT4. Cell Cycle 15, 1462–1470 (2016).
Miranda-Gonçalves, V. et al. MCT1 is a new prognostic biomarker and its therapeutic inhibition boosts response to temozolomide in human glioblastoma. Cancers 13, 3468 (2021).
Eilertsen, M. et al. Monocarboxylate transporters 1–4 in NSCLC: MCT1 is an independent prognostic marker for survival. PLoS One 9, e105038 (2014).
Brooks, G. A. Lactate shuttles in nature. Biochem. Soc. Trans. 30, 258–264 (2002).
She, X. et al. SETDB1 methylates MCT1 promoting tumor progression by enhancing the lactate shuttle. Adv. Sci. 10, e2301871 (2023).
Brooks, G. A. What the lactate shuttle means for sports nutrition. Nutrients 15, 2178 (2023).
Wu, A., Lee, D. & Xiong, W. C. Lactate metabolism, signaling, and function in brain development, synaptic plasticity, angiogenesis, and neurodegenerative diseases. Int. J. Mol. Sci. 24, 13398 (2023).
Certo, M. et al. Lactate modulation of immune responses in inflammatory versus tumour microenvironments. Nat. Rev. Immunol. 21, 151–161 (2021).
Brooks, G. A. The science and translation of lactate shuttle theory. Cell Metab. 27, 757–785 (2018).
Brooks, G. A. Cell-cell and intracellular lactate shuttles. J. Physiol. 587, 5591–5600 (2009).
D’Souza, P. et al. Commentary: Lactate, the astrocyte-neuron lactate shuttle, and neuroprotection in traumatic brain injury. Neurosurgery 90, e167–e169 (2022).
Brooks, G. A. et al. The blood lactate/pyruvate equilibrium affair. Am. J. Physiol. Endocrinol. Metab. 322, E34–e43 (2022).
Soeters, P. B. et al. The anabolic role of the Warburg, Cori-cycle and Crabtree effects in health and disease. Clin. Nutr. 40, 2988–2998 (2021).
Kulik, U., Moesta, C., Spanel, R. & Borlak, J. Dysfunctional Cori and Krebs cycle and inhibition of lactate transporters constitute a mechanism of primary nonfunction of fatty liver allografts. Transl. Res. 264, 33–65 (2024).
Manoj, K. M. et al. Murburn precepts for lactic-acidosis, Cori cycle, and Warburg effect: Interactive dynamics of dehydrogenases, protons, and oxygen. J. Cell Physiol. 237, 1902–1922 (2022).
Rogatzki, M. J., Ferguson, B. S., Goodwin, M. L. & Gladden, L. B. Lactate is always the end product of glycolysis. Front. Neurosci. 9, 22 (2015).
Katz, J. & Tayek, J. A. Gluconeogenesis and the Cori cycle in 12-, 20-, and 40-h-fasted humans. Am. J. Physiol. 275, E537–E542 (1998).
Zhao, P. et al. Targeting lactate metabolism and immune interaction in breast tumor via protease-triggered delivery. J. Control Release 358, 706–717 (2023).
Benjamin, D. An acid test for metformin(†). J. Pathol. 260, 365–367 (2023).
Zhu, J. et al. Blocking tumor-platelet crosstalk to prevent tumor metastasis via reprograming glycolysis using biomimetic membrane-hybridized liposomes. J. Control Release 366, 328–341 (2024).
Ippolito, L., Morandi, A., Giannoni, E. & Chiarugi, P. Lactate: a metabolic driver in the tumour landscape. Trends Biochem. Sci. 44, 153–166 (2019).
Gong, H. et al. Post-translational protein lactylation modification in health and diseases: a double-edged sword. J. Transl. Med. 22, 41 (2024).
Puri, S. & Juvale, K. Monocarboxylate transporter 1 and 4 inhibitors as potential therapeutics for treating solid tumours: a review with structure-activity relationship insights. Eur. J. Med. Chem. 199, 112393 (2020).
Dovmark, T. H. et al. Connexin-43 channels are a pathway for discharging lactate from glycolytic pancreatic ductal adenocarcinoma cells. Oncogene 36, 4538–4550 (2017).
Allen, E. et al. Metabolic symbiosis enables adaptive resistance to anti-angiogenic therapy that is dependent on mTOR signaling. Cell Rep. 15, 1144–1160 (2016).
Végran, F. et al. Lactate influx through the endothelial cell monocarboxylate transporter MCT1 supports an NF-κB/IL-8 pathway that drives tumor angiogenesis. Cancer Res. 71, 2550–2560 (2011).
Pàez-Ribes, M. et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell 15, 220–231 (2009).
Xia, L. et al. The cancer metabolic reprogramming and immune response. Mol. Cancer 20, 28 (2021).
Walenta, S. et al. High lactate levels predict likelihood of metastases, tumor recurrence, and restricted patient survival in human cervical cancers. Cancer Res. 60, 916–921 (2000).
Brooks, G. A. Lactate as a fulcrum of metabolism. Redox Biol. 35, 101454 (2020).
He, P. et al. Discovery of a potent and oral available complex I OXPHOS inhibitor that abrogates tumor growth and circumvents MEKi resistance. J. Med. Chem. 66, 6047–6069 (2023).
Jin, P. et al. Disrupting metformin adaptation of liver cancer cells by targeting the TOMM34/ATP5B axis. EMBO Mol. Med. 14, e16082 (2022).
Najjar, Y. G. et al. Tumor cell oxidative metabolism as a barrier to PD-1 blockade immunotherapy in melanoma. JCI Insight 4, e124989 (2019).
Rabinowitz, J. D. & Enerbäck, S. Lactate: the ugly duckling of energy metabolism. Nat. Metab. 2, 566–571 (2020).
Hui, S. et al. Quantitative fluxomics of circulating metabolites. Cell Metab. 32, 676–688, (2020).
Daw, C. C. et al. Lactate elicits ER-mitochondrial Mg2+ dynamics to integrate cellular metabolism. Cell 183, 474–489.e17 (2020).
Cai, X. et al. Lactate activates the mitochondrial electron transport chain independently of its metabolism. Mol. Cell. 83, 3904–3920.e3907 (2023).
Yao, C.-H. et al. Mitochondrial fusion supports increased oxidative phosphorylation during cell proliferation. Elife 8, e41351 (2019).
Sena, L. A. et al. Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity 38, 225–236 (2013).
Dienel, G. A. Brain glucose metabolism: integration of energetics with function. Physiol. Rev. 99, 949–1045 (2019).
Lhomme, T. et al. Tanycytic networks mediate energy balance by feeding lactate to glucose-insensitive POMC neurons. J. Clin. Investig. 131, e140521 (2021).
Gómez-Valadés, A. G. et al. Mitochondrial cristae-remodeling protein OPA1 in POMC neurons couples Ca2+ homeostasis with adipose tissue lipolysis. Cell Metab. 33, 1820–1835.e9 (2021).
Viale, A. et al. Oncogene ablation-resistant pancreatic cancer cells depend on mitochondrial function. Nature 514, 628–632 (2014).
Matassa, D. S. et al. Oxidative metabolism drives inflammation-induced platinum resistance in human ovarian cancer. Cell Death Differ. 23, 1542–1554 (2016).
LeBleu, V. S. et al. PGC-1α mediates mitochondrial biogenesis and oxidative phosphorylation in cancer cells to promote metastasis. Nat. Cell Biol. 16, 992–1003 (2014).
Weinberg, F. et al. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc. Natl Acad. Sci. USA 107, 8788–8793 (2010).
Viale, A., Corti, D. & Draetta, G. F. Tumors and mitochondrial respiration: a neglected connection. Cancer Res. 75, 3685–3686 (2015).
Li, X. et al. Lactate metabolism in human health and disease. Signal Transduct. Target Ther. 7, 305 (2022).
Raggi, C. et al. Mitochondrial oxidative metabolism contributes to a cancer stem cell phenotype in cholangiocarcinoma. J. Hepatol. 74, 1373–1385 (2021).
Chen, D. et al. Combination treatment with radiotherapy and a novel oxidative phosphorylation inhibitor overcomes PD-1 resistance and enhances antitumor immunity. J. Immunother. Cancer 8, e000289 (2020).
Deng, J. et al. Exosomal transfer leads to chemoresistance through oxidative phosphorylation-mediated stemness phenotype in colorectal cancer. Theranostics 13, 5057–5074 (2023).
Titov, D. V. et al. Complementation of mitochondrial electron transport chain by manipulation of the NAD+/NADH ratio. Science 352, 231–235 (2016).
Iatsenko, I., Boquete, J. P. & Lemaitre, B. Microbiota-derived lactate activates production of reactive oxygen species by the intestinal NADPH oxidase Nox and shortens drosophila lifespan. Immunity 49, 929–942.e925 (2018).
Tilton, W. M., Seaman, C., Carriero, D. & Piomelli, S. Regulation of glycolysis in the erythrocyte: role of the lactate/pyruvate and NAD/NADH ratios. J. Lab. Clin. Med. 118, 146–152 (1991).
Ying, W. NAD+/NADH and NADP+/NADPH in cellular functions and cell death: regulation and biological consequences. Antioxid. Redox Signal. 10, 179–206 (2008).
Luengo, A. et al. Increased demand for NAD+ relative to ATP drives aerobic glycolysis. Mol. Cell 81, 691–707.e6 (2021).
Benjamin, D. et al. Dual inhibition of the lactate transporters MCT1 and MCT4 is synthetic lethal with metformin due to NAD+ depletion in cancer cells. Cell Rep. 25, 3047–3058.e4 (2018).
DeBerardinis, R. J. et al. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc. Natl. Acad. Sci. USA 104, 19345–19350 (2007).
Jin, L., Alesi, G. N. & Kang, S. Glutaminolysis as a target for cancer therapy. Oncogene 35, 3619–3625 (2016).
Cluntun, A. A., Lukey, M. J., Cerione, R. A. & Locasale, J. W. Glutamine metabolism in cancer: understanding the heterogeneity. Trends Cancer 3, 169–180 (2017).
Yang, L., Venneti, S. & Nagrath, D. Glutaminolysis: a hallmark of cancer metabolism. Annu. Rev. Biomed. Eng. 19, 163–194 (2017).
Hoy, A. J., Nagarajan, S. R. & Butler, L. M. Tumour fatty acid metabolism in the context of therapy resistance and obesity. Nat. Rev. Cancer 21, 753–766 (2021).
Currie, E. et al. Cellular fatty acid metabolism and cancer. Cell Metab. 18, 153–161 (2013).
Pucino, V. et al. Lactate buildup at the site of chronic inflammation promotes disease by inducing CD4+ T cell metabolic rewiring. Cell Metab. 30, 1055–1074.e8 (2019).
Reddy, A. et al. Monocarboxylate transporters facilitate succinate uptake into brown adipocytes. Nat. Metab. 6, 567–577 (2024).
Yue, S. et al. Cholesteryl ester accumulation induced by PTEN loss and PI3K/AKT activation underlies human prostate cancer aggressiveness. Cell Metab. 19, 393–406 (2014).
Guillaumond, F. et al. Cholesterol uptake disruption, in association with chemotherapy, is a promising combined metabolic therapy for pancreatic adenocarcinoma. Proc. Natl. Acad. Sci. USA 112, 2473–2478 (2015).
Zaidi, N., Swinnen, J. V. & Smans, K. ATP-citrate lyase: a key player in cancer metabolism. Cancer Res. 72, 3709–3714, (2012).
Beloueche-Babari, M. et al. Monocarboxylate transporter 1 blockade with AZD3965 inhibits lipid biosynthesis and increases tumour immune cell infiltration. Br. J. Cancer 122, 895–903 (2020).
Bergersen, L. H. Lactate transport and signaling in the brain: potential therapeutic targets and roles in body-brain interaction. J. Cereb. Blood Flow. Metab. 35, 176–185 (2015).
Hoque, R. et al. Lactate reduces liver and pancreatic injury in Toll-like receptor- and inflammasome-mediated inflammation via GPR81-mediated suppression of innate immunity. Gastroenterology 146, 1763–1774 (2014).
Chen, J. et al. Lactylated apolipoprotein C‐II induces immunotherapy resistance by promoting extracellular lipolysis. Adv. Sci. 11, e2406333 (2024).
Lu, H. et al. Reversible inactivation of HIF-1 prolyl hydroxylases allows cell metabolism to control basal HIF-1. J. Biol. Chem. 280, 41928–41939 (2005).
Heneberg, P. Lactic acidosis in patients with solid cancer. Antioxid. Redox Signal. 37, 1130–1152 (2022).
Park, S., Kim, B. K. & Park, S. K. Effects of fisetin, a plant-derived flavonoid, on response to oxidative stress, aging, and age-related diseases in Caenorhabditis elegans. Pharmaceuticals 15, 1528 (2022).
Vermot, A., Petit-Härtlein, I., Smith, S. M. E. & Fieschi, F. NADPH oxidases (NOX): an overview from discovery, molecular mechanisms to physiology and pathology. Antioxidants 10, 890 (2021).
Dou, X. et al. PDK4-dependent hypercatabolism and lactate production of senescent cells promotes cancer malignancy. Nat. Metab. 5, 1887–1910 (2023).
Yoshimoto, S. et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature 499, 97–101 (2013).
Wu, H. et al. Lactate dehydrogenases amplify reactive oxygen species in cancer cells in response to oxidative stimuli. Signal Transduct. Target Ther. 6, 242 (2021).
Liu, Y. et al. Nuclear lactate dehydrogenase A senses ROS to produce α-hydroxybutyrate for HPV-induced cervical tumor growth. Nat. Commun. 9, 4429 (2018).
Yang, Y. et al. Targeting lactate dehydrogenase A improves radiotherapy efficacy in non-small cell lung cancer: from bedside to bench. J. Transl. Med. 19, 170 (2021).
Sattler, U. G. A. et al. Glycolytic metabolism and tumour response to fractionated irradiation. Radiother. Oncol. 94, 102–109 (2010).
Lee, Y. S. et al. Microbiota-derived lactate promotes hematopoiesis and erythropoiesis by inducing stem cell factor production from leptin receptor+ niche cells. Exp. Mol. Med. 53, 1319–1331 (2021).
Sun, J. et al. Lactate activates CCL18 expression via H3K18 lactylation in macrophages to promote tumorigenesis of ovarian cancer. Acta Biochim Biophys. Sin. 56, 1373–1386 (2024).
Lund, M. L. et al. Enterochromaffin 5-HT cells - A major target for GLP-1 and gut microbial metabolites. Mol. Metab. 11, 70–83 (2018).
Liu, X. et al. Myc-mediated inhibition of HIF1a degradation promotes M2 macrophage polarization and impairs CD8 T cell function through lactic acid secretion in ovarian cancer. Int. Immunopharmacol. 141, 112876 (2024).
Brown, T. P. & Ganapathy, V. Lactate/GPR81 signaling and proton motive force in cancer: role in angiogenesis, immune escape, nutrition, and Warburg phenomenon. Pharm. Ther. 206, 107451 (2020).
Zhao, B. et al. Effect of lactate export inhibition on anaplastic thyroid cancer growth and metabolism. J. Am. Coll. Surg. 234, 1044–1050 (2022).
Grochowalska, K., Pikul, P. & Piwkowska, A. Insights into the regulation of podocyte and glomerular function by lactate and its metabolic sensor G-protein-coupled receptor 81. J. Cell Physiol. 237, 4097–4111 (2022).
Shen, Z. et al. Inhibition of G protein-coupled receptor 81 (GPR81) protects against ischemic brain injury. CNS Neurosci. Ther. 21, 271–279 (2015).
Wang, M. et al. Dietary lactate supplementation can alleviate DSS-induced colitis in piglets. Biomed. Pharmacother. 158, 114148 (2023).
Roland, C. L. et al. Cell surface lactate receptor GPR81 is crucial for cancer cell survival. Cancer Res. 74, 5301–5310 (2014).
Feng, J. et al. Tumor cell-derived lactate induces TAZ-dependent upregulation of PD-L1 through GPR81 in human lung cancer cells. Oncogene 36, 5829–5839 (2017).
Holder, A. M. et al. Defining clinically useful biomarkers of immune checkpoint inhibitors in solid tumours. Nat. Rev. Cancer 24, 498–512 (2024).
Ngai, D., Schilperoort, M. & Tabas, I. Efferocytosis-induced lactate enables the proliferation of pro-resolving macrophages to mediate tissue repair. Nat. Metab. 5, 2206–2219 (2023).
Weiß, K. T. et al. Proton-sensing G protein-coupled receptors as regulators of cell proliferation and migration during tumor growth and wound healing. Exp. Dermatol. 26, 127–132 (2017).
Chen, P. et al. Gpr132 sensing of lactate mediates tumor-macrophage interplay to promote breast cancer metastasis. Proc. Natl. Acad. Sci. USA 114, 580–585 (2017).
Vadevoo, S. M. P. et al. The macrophage odorant receptor Olfr78 mediates the lactate-induced M2 phenotype of tumor-associated macrophages. Proc. Natl. Acad. Sci. USA 118, e2102434118 (2021).
Liu, Y., Jiang, C., Xu, C. & Gu, L. Systematic analysis of integrated bioinformatics to identify upregulated THBS2 expression in colorectal cancer cells inhibiting tumour immunity through the HIF1A/Lactic Acid/GPR132 pathway. Cancer Cell Int. 23, 253 (2023).
Yang, Z., Zheng, Y. & Gao, Q. Lysine lactylation in the regulation of tumor biology. Trends Endocrinol. Metab. 35, 720–731 (2024).
Wang, P. et al. H3K18 lactylation promotes the progression of arsenite-related idiopathic pulmonary fibrosis via YTHDF1/m6A/NREP. J. Hazard Mater. 461, 132582 (2024).
Gao, X. et al. Regulation of newly identified lysine lactylation in cancer. Cancer Lett. 587, 216680 (2024).
Su, J. et al. Functions and mechanisms of lactylation in carcinogenesis and immunosuppression. Front. Immunol. 14, 1253064 (2023).
He, C. et al. Lysine lactylation-based insight to understanding the characterization of cervical cancer. Biochim. Biophys. Acta Mol. Basis Dis. 1870, 167356 (2024).
Yang, H. et al. Microglia lactylation in relation to central nervous system diseases. Neural Regen. Res. 20, 29–40 (2025).
Irizarry-Caro, R. A. et al. TLR signaling adapter BCAP regulates inflammatory to reparatory macrophage transition by promoting histone lactylation. Proc. Natl. Acad. Sci. USA 117, 30628–30638 (2020).
Bian, X. et al. Regulation of gene expression by glycolytic and gluconeogenic enzymes. Trends Cell Biol. 32, 786–799 (2022).
Izzo, L. T. & Wellen, K. E. Histone lactylation links metabolism and gene regulation. Nature 574, 492–493 (2019).
Dichtl, S. et al. Lactate and IL6 define separable paths of inflammatory metabolic adaptation. Sci. Adv. 7, eabg3505 (2021).
Niu, Z. et al. HBO1 catalyzes lysine lactylation and mediates histone H3K9la to regulate gene transcription. Nat. Commun. 15, 3561 (2024).
Cui, H. et al. Lung myofibroblasts promote macrophage profibrotic activity through lactate-induced histone lactylation. Am. J. Respir. Cell Mol. Biol. 64, 115–125 (2021).
Yu, J. et al. Histone lactylation drives oncogenesis by facilitating m6A reader protein YTHDF2 expression in ocular melanoma. Genome Biol. 22, 85 (2021).
Jiang, J. et al. Lactate modulates cellular metabolism through histone lactylation-mediated gene expression in non-small cell lung cancer. Front. Oncol. 11, 647559 (2021).
Hua, G. et al. Targeting glucose metabolism in chondrosarcoma cells enhances the sensitivity to doxorubicin through the inhibition of lactate dehydrogenase-A. Oncol. Rep. 31, 2727–2734 (2014).
Peng, T. et al. Novel lactylation-related signature to predict prognosis for pancreatic adenocarcinoma. World J. Gastroenterol. 30, 2575–2602 (2024).
Pan, L. et al. Demethylzeylasteral targets lactate by inhibiting histone lactylation to suppress the tumorigenicity of liver cancer stem cells. Pharm. Res. 181, 106270 (2022).
Xu, H. et al. Royal jelly acid suppresses hepatocellular carcinoma tumorigenicity by inhibiting H3 histone lactylation at H3K9la and H3K14la sites. Phytomedicine 118, 154940 (2023).
Chai, P. et al. Lactate/lactylation in ocular development and diseases. Trends Mol. Med. (2024).
Yang, J. et al. A positive feedback loop between inactive VHL-triggered histone lactylation and pdgfrβ signaling drives clear cell renal cell carcinoma progression. Int J. Biol. Sci. 18, 3470–3483 (2022).
Wang, G. et al. The relationship and clinical significance of lactylation modification in digestive system tumors. Cancer Cell Int. 24, 246 (2024).
Li, W. et al. Tumor-derived lactate promotes resistance to bevacizumab treatment by facilitating autophagy enhancer protein RUBCNL expression through histone H3 lysine 18 lactylation (H3K18la) in colorectal cancer. Autophagy 20, 114–120 (2023).
Dai, X., Lv, X., Thompson, E. W. & Ostrikov, K. K. Histone lactylation: epigenetic mark of glycolytic switch. Trends Genet. 38, 124–127 (2022).
Wu, Q. et al. Integrated analysis of histone lysine lactylation (Kla)-specific genes suggests that NR6A1, OSBP2 and UNC119B are novel therapeutic targets for hepatocellular carcinoma. Sci. Rep. 13, 18642 (2023).
Chen, L. et al. Lactate-lactylation hands between metabolic reprogramming and immunosuppression. Int. J. Mol. Sci. 23, 11943 (2022).
Sun, X. et al. The diapause-like colorectal cancer cells induced by SMC4 attenuation are characterized by low proliferation and chemotherapy insensitivity. Cell Metab. 35, 1563–1579.e1568 (2023).
Jin, J. et al. SIRT3-dependent delactylation of cyclin E2 prevents hepatocellular carcinoma growth. EMBO Rep. 24, e56052 (2023).
Chen, M. et al. NUSAP1-LDHA-Glycolysis-Lactate feedforward loop promotes Warburg effect and metastasis in pancreatic ductal adenocarcinoma. Cancer Lett. 567, 216285 (2023).
Zhang, C. et al. H3K18 lactylation potentiates immune escape of non-small cell lung cancer. Cancer Res. 84, 3589–3601 (2024).
Zhang, X. W. et al. Thermal proteome profiling strategy identifies CNPY3 as a cellular target of gambogic acid for inducing prostate cancer pyroptosis. J. Med. Chem. 67, 10005–10011 (2024).
Zong, Z. et al. Alanyl-tRNA synthetase, AARS1, is a lactate sensor and lactyltransferase that lactylates p53 and contributes to tumorigenesis. Cell 187, 2375–2392.e2333 (2024).
Xie, B. et al. KAT8-catalyzed lactylation promotes eEF1A2-mediated protein synthesis and colorectal carcinogenesis. Proc. Natl. Acad. Sci. USA 121, e2314128121 (2024).
Sun, L. et al. Lactylation of METTL16 promotes cuproptosis via m6A-modification on FDX1 mRNA in gastric cancer. Nat. Commun. 14, 6523 (2023).
Duan, Y. et al. Integrated lactylome characterization reveals the molecular dynamics of protein regulation in gastrointestinal cancers. Adv. Sci. 11, e2400227 (2024).
Chen, Y. et al. Metabolic regulation of homologous recombination repair by MRE11 lactylation. Cell 187, 294-311.e21 (2023).
Sun, P., Ma, L. & Lu, Z. Lactylation: linking the Warburg effect to DNA damage repair. Cell Metab. 36, 1637–1639 (2024).
Yang, D. et al. Identification of lysine-lactylated substrates in gastric cancer cells. iScience 25, 104630 (2022).
Cai, L., Sutter, B. M., Li, B. & Tu, B. P. Acetyl-CoA induces cell growth and proliferation by promoting the acetylation of histones at growth genes. Mol. Cell. 42, 426–437 (2011).
Lu, C. & Thompson, C. B. Metabolic regulation of epigenetics. Cell Metab. 16, 9–17 (2012).
Liu, W. et al. Lactate regulates cell cycle by remodelling the anaphase promoting complex. Nature 616, 790–797 (2023).
Varner, E. L. et al. Quantification of lactoyl-CoA (lactyl-CoA) by liquid chromatography mass spectrometry in mammalian cells and tissues. Open Biol. 10, 200187 (2020).
Sabari, B. R., Zhang, D., Allis, C. D. & Zhao, Y. Metabolic regulation of gene expression through histone acylations. Nat. Rev. Mol. Cell Biol. 18, 90–101 (2017).
Wang, T. et al. Acetylation of lactate dehydrogenase B drives NAFLD progression by impairing lactate clearance. J. Hepatol. 74, 1038–1052 (2021).
Chisolm, D. A. & Weinmann, A. S. Connections between metabolism and epigenetics in programming cellular differentiation. Annu. Rev. Immunol. 36, 221–246 (2018).
Wang, Z. A. & Cole, P. A. The chemical biology of reversible lysine post-translational modifications. Cell Chem. Biol. 27, 953–969 (2020).
Moreno-Yruela, C. et al. Class I histone deacetylases (HDAC1–3) are histone lysine delactylases. Sci. Adv. 8, eabi6696 (2022).
Wagner, W., Ciszewski, W. M. & Kania, K. D. L- and D-lactate enhance DNA repair and modulate the resistance of cervical carcinoma cells to anticancer drugs via histone deacetylase inhibition and hydroxycarboxylic acid receptor 1 activation. Cell Commun. Signal. 13, 36 (2015).
Ju, J. et al. The alanyl-tRNA synthetase AARS1 moonlights as a lactyltransferase to promote YAP signaling in gastric cancer. J. Clin. Investig. 134, e174587 (2024).
Kuo, C.-C. et al. Metastatic colorectal cancer rewrites metabolic program through a glut3-YAP-dependent signaling circuit. Theranostics 9, 2526–2540 (2019).
Mao, Y. et al. Hypoxia induces mitochondrial protein lactylation to limit oxidative phosphorylation. Cell Res. 34, 13–30 (2024).
Li, H. et al. AARS1 and AARS2 sense L-lactate to regulate cGAS as global lysine lactyltransferases. Nature 634, 1229–1237 (2024).
Halbrook, C. J., Lyssiotis, C. A., Pasca di Magliano, M. & Maitra, A. Pancreatic cancer: advances and challenges. Cell 186, 1729–1754 (2023).
Peng, C. et al. TME-related biomimetic strategies against cancer. Int. J. Nanomed. 19, 109–135 (2024).
Chen, D., Zhang, X., Li, Z. & Zhu, B. Metabolic regulatory crosstalk between tumor microenvironment and tumor-associated macrophages. Theranostics 11, 1016–1030 (2021).
Bejarano, L., Jordāo, M. J. C. & Joyce, J. A. Therapeutic targeting of the tumor microenvironment. Cancer Discov. 11, 933–959 (2021).
Elhanani, O., Ben-Uri, R. & Keren, L. Spatial profiling technologies illuminate the tumor microenvironment. Cancer Cell. 41, 404–420 (2023).
Jin, M. Z. & Jin, W. L. The updated landscape of tumor microenvironment and drug repurposing. Signal Transduct. Target Ther. 5, 166 (2020).
Chhabra, Y. & Weeraratna, A. T. Fibroblasts in cancer: unity in heterogeneity. Cell 186, 1580–1609 (2023).
Zhou, Y., Cheng, L., Liu, L. & Li, X. NK cells are never alone: crosstalk and communication in tumour microenvironments. Mol. Cancer 22, 34 (2023).
D’Ambrosio, M. & Gil, J. Reshaping of the tumor microenvironment by cellular senescence: an opportunity for senotherapies. Dev. Cell. 58, 1007–1021 (2023).
Watson, M. J. & Delgoffe, G. M. Fighting in a wasteland: deleterious metabolites and antitumor immunity. J. Clin. Investig. 132, e148549 (2022).
Buck, M. D., Sowell, R. T., Kaech, S. M. & Pearce, E. L. Metabolic Instruction of Immunity. Cell 169, 570–586 (2017).
Hayes, C., Donohoe, C. L., Davern, M. & Donlon, N. E. The oncogenic and clinical implications of lactate induced immunosuppression in the tumour microenvironment. Cancer Lett. 500, 75–86 (2021).
Hirschhaeuser, F., Sattler, U. G. & Mueller-Klieser, W. Lactate: a metabolic key player in cancer. Cancer Res. 71, 6921–6925 (2011).
DeNardo, D. G. & Ruffell, B. Macrophages as regulators of tumour immunity and immunotherapy. Nat. Rev. Immunol. 19, 369–382 (2019).
DePeaux, K. & Delgoffe, G. M. Metabolic barriers to cancer immunotherapy. Nat. Rev. Immunol. 21, 785–797 (2021).
Diskin, C., Ryan, T. A. J. & O’Neill, L. A. J. Modification of proteins by metabolites in immunity. Immunity 54, 19–31 (2021).
Cheng, S. et al. A pan-cancer single-cell transcriptional atlas of tumor infiltrating myeloid cells. Cell 184, 792–809.e23 (2021).
Puig-Kröger, A. et al. Peritoneal dialysis solutions inhibit the differentiation and maturation of human monocyte-derived dendritic cells: effect of lactate and glucose-degradation products. J. Leukoc. Biol. 73, 482–492 (2003).
Gottfried, E. et al. Tumor-derived lactic acid modulates dendritic cell activation and antigen expression. Blood 107, 2013–2021 (2006).
Maier, B. et al. A conserved dendritic-cell regulatory program limits antitumour immunity. Nature 580, 257–262 (2020).
Plebanek, M. P. et al. A lactate-SREBP2 signaling axis drives tolerogenic dendritic cell maturation and promotes cancer progression. Sci. Immunol. 9, eadi4191 (2024).
Caronni, N. et al. Downregulation of membrane trafficking proteins and lactate conditioning determine loss of dendritic cell function in lung cancer. Cancer Res. 78, 1685–1699 (2018).
Nasi, A. & Rethi, B. Disarmed by density: a glycolytic break for immunostimulatory dendritic cells? OncoImmunology 2, e26744 (2013).
Colegio, O. R. et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature 513, 559–563 (2014).
Liu, N. et al. Lactate inhibits ATP6V0d2 expression in tumor-associated macrophages to promote HIF-2α-mediated tumor progression. J. Clin. Investig. 129, 631–646 (2019).
O’Neill, L. A. & Pearce, E. J. Immunometabolism governs dendritic cell and macrophage function. J. Exp. Med. 213, 15–23 (2016).
Mu, X. et al. Tumor-derived lactate induces M2 macrophage polarization via the activation of the ERK/STAT3 signaling pathway in breast cancer. Cell Cycle 17, 428–438 (2018).
Noe, J. T. et al. Lactate supports a metabolic-epigenetic link in macrophage polarization. Sci. Adv. 7, eabi8602 (2021).
Shime, H. et al. Tumor-secreted lactic acid promotes IL-23/IL-17 proinflammatory pathway. J. Immunol. 180, 7175–7183 (2008).
Frank, A.-C. et al. Lactate dehydrogenase B regulates macrophage metabolism in the tumor microenvironment. Theranostics 11, 7570–7588 (2021).
Longhitano, L. et al. Lactate modulates microglia polarization via IGFBP6 expression and remodels tumor microenvironment in glioblastoma. Cancer Immunol. Immunother. 72, 1–20 (2023).
Poon, C. C., Sarkar, S., Yong, V. W. & Kelly, J. J. P. Glioblastoma-associated microglia and macrophages: targets for therapies to improve prognosis. Brain 140, 1548–1560 (2017).
Brandenburg, S. et al. Resident microglia rather than peripheral macrophages promote vascularization in brain tumors and are source of alternative pro-angiogenic factors. Acta Neuropathol. 131, 365–378 (2016).
Xiao, Y., Zhou, L., Andl, T. & Zhang, Y. YAP1 controls the N-cadherin-mediated tumor-stroma interaction in melanoma progression. Oncogene 43, 884–898 (2024).
Li, X.-M. et al. Histone lactylation inhibits RARγ expression in macrophages to promote colorectal tumorigenesis through activation of TRAF6-IL-6-STAT3 signaling. Cell Rep. 43, 113688 (2024).
Angelin, A. et al. Foxp3 reprograms T cell metabolism to function in low-glucose, high-lactate environments. Cell Metab. 25, 1282–1293.e7 (2017).
Gerriets, V. A. et al. Foxp3 and toll-like receptor signaling balance Treg cell anabolic metabolism for suppression. Nat. Immunol. 17, 1459–1466 (2016).
Raychaudhuri, D. et al. Lactate induces pro-tumor reprogramming in intratumoral plasmacytoid dendritic cells. Front. Immunol. 10, 1878 (2019).
Kumagai, S. et al. Lactic acid promotes PD-1 expression in regulatory T cells in highly glycolytic tumor microenvironments. Cancer Cell. 40, 201–218.e9 (2022).
Zhang, Y. et al. Lactate acid promotes PD-1+ Tregs accumulation in the bone marrow with high tumor burden of Acute myeloid leukemia. Int. Immunopharmacol. 130, 111765 (2024).
Watson, M. J. et al. Metabolic support of tumour-infiltrating regulatory T cells by lactic acid. Nature 591, 645–651 (2021).
Kumagai, S. et al. An oncogenic alteration creates a microenvironment that promotes tumor progression by conferring a metabolic advantage to regulatory T cells. Immunity 53, 187–203.e188 (2020).
Daneshmandi, S., Wegiel, B. & Seth, P. Blockade of lactate dehydrogenase-A (LDH-A) improves efficacy of anti-programmed cell death-1 (PD-1) therapy in melanoma. Cancers 11, 450 (2019).
Liu, H. et al. Mutant KRAS drives immune evasion by sensitizing cytotoxic T-cells to activation-induced cell death in colorectal cancer. Adv. Sci. 10, e2203757 (2023).
Zhou, C. et al. Mutant KRAS-activated circATXN7 fosters tumor immunoescape by sensitizing tumor-specific T cells to activation-induced cell death. Nat. Commun. 15, 499 (2024).
Chang, L. et al. 1-Pyrroline-5-carboxylate inhibit T cell glycolysis in prostate cancer microenvironment by SHP1/PKM2/LDHB axis. Cell Commun. Signal. 22, 101 (2024).
Mendler, A. N. et al. Tumor lactic acidosis suppresses CTL function by inhibition of p38 and JNK/c-Jun activation. Int. J. Cancer 131, 633–640 (2012).
Xia, H. et al. Suppression of FIP200 and autophagy by tumor-derived lactate promotes naïve T cell apoptosis and affects tumor immunity. Sci. Immunol. 2, eaan4631 (2017).
Romero-Garcia, S., Moreno-Altamirano, M. M. B., Prado-Garcia, H. & Sánchez-García, F. J. Lactate contribution to the tumor microenvironment: mechanisms, effects on immune cells and therapeutic relevance. Front. Immunol. 7, 52 (2016).
Su, J. et al. Lactate/GPR81 recruits regulatory T cells by modulating CX3CL1 to promote immune resistance in a highly glycolytic gastric cancer. Oncoimmunology 13, 2320951 (2024).
Shi, W. et al. Lactic acid induces transcriptional repression of macrophage inflammatory response via histone acetylation. Cell Rep. 43, 113746 (2024).
Altinoz, M. A. & Ozpinar, A. Oxamate targeting aggressive cancers with special emphasis to brain tumors. Biomed. Pharmacother. 147, 112686 (2022).
Chen, S. et al. TAB182 regulates glycolytic metabolism by controlling LDHA transcription to impact tumor radiosensitivity. Cell Death Dis. 15, 209 (2024).
Magalhaes, I., Yogev, O., Mattsson, J. & Schurich, A. The metabolic profile of tumor and virally infected cells shapes their microenvironment counteracting T cell immunity. Front. Immunol. 10, 2309 (2019).
Yang, X. et al. Lactate-modulated immunosuppression of myeloid-derived suppressor cells contributes to the radioresistance of pancreatic cancer. Cancer Immunol. Res. 8, 1440–1451 (2020).
Wen, J. et al. Lactate anions participate in T cell cytokine production and function. Sci. China Life Sci. 64, 1895–1905 (2021).
Feng, Q. et al. Lactate increases stemness of CD8 + T cells to augment anti-tumor immunity. Nat. Commun. 13, 4981 (2022).
Gao, S. et al. Stem cell-like memory T cells: a perspective from the dark side. Cell Immunol. 361, 104273 (2021).
Cheng, H. et al. Extracellular acidosis restricts one-carbon metabolism and preserves T cell stemness. Nat. Metab. 5, 314–330 (2023).
Krishna, S. et al. Stem-like CD8 T cells mediate response of adoptive cell immunotherapy against human cancer. Science 370, 1328–1334 (2020).
Raynor, J. L., Chapman, N. M. & Chi, H. Metabolic control of memory T-cell generation and stemness. Cold Spring Harb. Perspect. Biol. 13, a037770 (2021).
Sahai, E. et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer 20, 174–186 (2020).
Pavlides, S. et al. The reverse Warburg effect: aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle 8, 3984–4001 (2009).
Zhang, D. et al. Metabolic reprogramming of cancer-associated fibroblasts by IDH3α downregulation. Cell Rep. 10, 1335–1348 (2015).
Kalluri, R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 16, 582–598 (2016).
Fiore, V. F. et al. Mechanics of a multilayer epithelium instruct tumour architecture and function. Nature 585, 433–439 (2020).
Li, L. et al. Human apical-out nasal organoids reveal an essential role of matrix metalloproteinases in airway epithelial differentiation. Nat. Commun. 15, 143 (2024).
Khalilgharibi, N. & Mao, Y. To form and function: on the role of basement membrane mechanics in tissue development, homeostasis and disease. Open Biol. 11, 200360 (2021).
Savagner, P. Epithelial-mesenchymal transitions: from cell plasticity to concept elasticity. Curr. Top. Dev. Biol. 112, 273–300 (2015).
Huang, Z. et al. Epithelial-mesenchymal transition: the history, regulatory mechanism, and cancer therapeutic opportunities. MedComm 3, e144 (2022).
Verstappe, J. & Berx, G. A role for partial epithelial-to-mesenchymal transition in enabling stemness in homeostasis and cancer. Semin. Cancer Biol. 90, 15–28 (2023).
Zhang, N. et al. Novel therapeutic strategies: targeting epithelial-mesenchymal transition in colorectal cancer. Lancet Oncol. 22, e358–e368 (2021).
Giannoni, E. et al. Targeting stromal-induced pyruvate kinase M2 nuclear translocation impairs OXPHOS and prostate cancer metastatic spread. Oncotarget 6, 24061–24074 (2015).
Pistore, C. et al. DNA methylation variations are required for epithelial-to-mesenchymal transition induced by cancer-associated fibroblasts in prostate cancer cells. Oncogene 36, 5551–5566 (2017).
Apicella, M. et al. Increased lactate secretion by cancer cells sustains non-cell-autonomous adaptive resistance to MET and EGFR-targeted therapies. Cell Metab. 28, 848–865.e6 (2018).
Li, F. & Simon, M. C. Cancer cells don’t live alone: metabolic communication within tumor microenvironments. Dev. Cell. 54, 183–195 (2020).
Zetter, B. R. Angiogenesis and tumor metastasis. Annu. Rev. Med. 49, 407–424 (1998).
Weidner, N., Semple, J. P., Welch, W. R. & Folkman, J. Tumor angiogenesis and metastasis-correlation in invasive breast carcinoma. N. Engl. J. Med. 324, 1–8 (1991).
Sherwood, L. M., Parris, E. E. & Folkman, J. Tumor angiogenesis: therapeutic implications. N. Engl. J. Med. 285, 1182–1186 (1971).
Sonveaux, P. et al. Targeting the lactate transporter MCT1 in endothelial cells inhibits lactate-induced HIF-1 activation and tumor angiogenesis. PLoS One 7, e33418 (2012).
De Bock, K. et al. Role of PFKFB3-driven glycolysis in vessel sprouting. Cell 154, 651–663 (2013).
Ruan, G.-X. & Kazlauskas, A. Lactate engages receptor tyrosine kinases Axl, Tie2, and vascular endothelial growth factor receptor 2 to activate phosphoinositide 3-kinase/Akt and promote angiogenesis. J. Biol. Chem. 288, 21161–21172 (2013).
Li, W. et al. Dual-inhibition of lactate metabolism and Prussian blue-mediated radical generation for enhanced chemodynamic therapy and antimetastatic effect. Nanoscale 15, 9214–9228 (2023).
McCleland, M. L. et al. Lactate dehydrogenase B is required for the growth of KRAS-dependent lung adenocarcinomas. Clin. Cancer Res. 19, 773–784 (2013).
Guan, X. & Morris, M. E. In vitro and in vivo efficacy of AZD3965 and alpha-cyano-4-hydroxycinnamic acid in the murine 4T1 breast tumor model. AAPS J. 22, 84 (2020).
Rey, S. et al. Targeting hypoxia-inducible factors for antiangiogenic cancer therapy. Trends Cancer 3, 529–541 (2017).
Whitfield, J. R., Beaulieu, M.-E. & Soucek, L. Strategies to inhibit myc and their clinical applicability. Front. Cell Dev. Biol. 5, 10 (2017).
Janku, F., Yap, T. A. & Meric-Bernstam, F. Targeting the PI3K pathway in cancer: are we making headway? Nat. Rev. Clin. Oncol. 15, 273–291 (2018).
Wang, Z. H. et al. Lactate in the tumour microenvironment: from immune modulation to therapy. EBioMedicine 73, 103627 (2021).
Barros, L. F. Metabolic signaling by lactate in the brain. Trends Neurosci. 36, 396–404 (2013).
Lopez Kolkovsky, A. L., Carlier, P. G., Marty, B. & Meyerspeer, M. Interleaved and simultaneous multi-nuclear magnetic resonance in vivo. Review of principles, applications and potential. NMR Biomed. 35, e4735 (2022).
Lai, F. L. & Gao, F. Auto-Kla: a novel web server to discriminate lysine lactylation sites using automated machine learning. Brief. Bioinform. 24, bbad070 (2023).
Rabuka, D. et al. A chemical reporter strategy to probe glycoprotein fucosylation. J. Am. Chem. Soc. 128, 12078–12079 (2006).
Levitin, H. M., Yuan, J. & Sims, P. A. Single-cell transcriptomic analysis of tumor heterogeneity. Trends Cancer 4, 264–268 (2018).
Patel, A. P. et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science 344, 1396–1401 (2014).
Tirosh, I. et al. Dissecting the multicellular ecosystem of metastatic melanoma by single-cell RNA-seq. Science 352, 189–196 (2016).
Chung, W. et al. Single-cell RNA-seq enables comprehensive tumour and immune cell profiling in primary breast cancer. Nat. Commun. 8, 15081 (2017).
Pan, N. et al. Quantification of drug molecules in live single cells using the single-probe mass spectrometry technique. Anal. Chem. 91, 9018–9024 (2019).
Khoo, B. L. et al. Expansion of patient-derived circulating tumor cells from liquid biopsies using a CTC microfluidic culture device. Nat. Protoc. 13, 34–58 (2018).
Zhang, W. et al. Single-cell metabolic fingerprints discover a cluster of circulating tumor cells with distinct metastatic potential. Nat. Commun. 14, 2485 (2023).
Liu, J. et al. Skp2 dictates cell cycle-dependent metabolic oscillation between glycolysis and TCA cycle. Cell Res. 31, 80–93 (2021).
Wang, H. et al. The metabolic function of cyclin D3-CDK6 kinase in cancer cell survival. Nature 546, 426–430 (2017).
Bartman, C. R. et al. Slow TCA flux and ATP production in primary solid tumours but not metastases. Nature 614, 349–357 (2023).
Befroy, D. E. et al. Direct assessment of hepatic mitochondrial oxidative and anaplerotic fluxes in humans using dynamic 13C magnetic resonance spectroscopy. Nat. Med. 20, 98–102 (2014).
Lapidot, T. et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature 367, 645–648 (1994).
Kamphorst, J. J. et al. Human pancreatic cancer tumors are nutrient poor and tumor cells actively scavenge extracellular protein. Cancer Res. 75, 544–553 (2015).
Yuan, J., Bennett, B. D. & Rabinowitz, J. D. Kinetic flux profiling for quantitation of cellular metabolic fluxes. Nat. Protoc. 3, 1328–1340 (2008).
Storz, P. Acinar cell plasticity and development of pancreatic ductal adenocarcinoma. Nat. Rev. Gastroenterol. Hepatol. 14, 296–304 (2017).
Wang, A. et al. Comprehensive multiscale analysis of lactate metabolic dynamics in vitro and in vivo using highly responsive biosensors. Nat. Protoc. 19, 1311–1347 (2024).
Li, X. et al. Ultrasensitive sensors reveal the spatiotemporal landscape of lactate metabolism in physiology and disease. Cell Metab. 35, 200–211.e209 (2023).
Wan, N. et al. Cyclic immonium ion of lactyllysine reveals widespread lactylation in the human proteome. Nat. Methods 19, 854–864 (2022).
Sun, Y., Chen, Y. & Peng, T. A bioorthogonal chemical reporter for the detection and identification of protein lactylation. Chem. Sci. 13, 6019–6027 (2022).
Huang, H. et al. A multi-dimensional approach to unravel the intricacies of lactylation-related signature for prognostic and therapeutic insight in colorectal cancer. J. Transl. Med. 22, 211 (2024).
Huang, A. et al. Novel hypoxia- and lactate metabolism-related molecular subtyping and prognostic signature for colorectal cancer. J. Transl. Med. 22, 587 (2024).
Liu, X. et al. LDHA and LDHB overexpression promoted the Warburg effect in malignantly transformed GES-1 cells induced by N-nitroso compounds. Food Chem. Toxicol. 180, 114007 (2023).
Qing, Y. et al. R-2-hydroxyglutarate attenuates aerobic glycolysis in leukemia by targeting the FTO/m6A/PFKP/LDHB axis. Mol. Cell. 81, 922–939.e9 (2021).
Dorneburg, C. et al. LDHA in neuroblastoma is associated with poor outcome and its depletion decreases neuroblastoma growth independent of aerobic glycolysis. Clin. Cancer Res. 24, 5772–5783 (2018).
Huo, N. et al. STAT3/LINC00671 axis regulates papillary thyroid tumor growth and metastasis via LDHA-mediated glycolysis. Cell Death Dis. 12, 799 (2021).
Pouysségur, J. et al. Warburg effect’ controls tumor growth, bacterial, viral infections and immunity—genetic deconstruction and therapeutic perspectives. Semin. Cancer Biol. 86, 334–346 (2022).
Zila, N. et al. Proteomic profiling of advanced melanoma patients to predict therapeutic response to Anti-PD-1 therapy. Clin. Cancer Res. 30, 159–175 (2024).
Cheng, A. et al. Aurora-A mediated phosphorylation of LDHB promotes glycolysis and tumor progression by relieving the substrate-inhibition effect. Nat. Commun. 10, 5566 (2019).
Renner, O. et al. Systematic review of Gossypol/AT-101 in cancer clinical trials. Pharmaceuticals 15, 144 (2022).
Rai, G. et al. Discovery and optimization of potent, cell-active pyrazole-based inhibitors of lactate dehydrogenase (LDH). J. Med. Chem. 60, 9184–9204 (2017).
Le, A. et al. Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. Proc. Natl. Acad. Sci. USA 107, 2037–2042 (2010).
Ward, R. A. et al. Design and synthesis of novel lactate dehydrogenase A inhibitors by fragment-based lead generation. J. Med. Chem. 55, 3285–3306 (2012).
Boudreau, A. et al. Metabolic plasticity underpins innate and acquired resistance to LDHA inhibition. Nat. Chem. Biol. 12, 779–786 (2016).
Oshima, N. et al. Dynamic imaging of LDH inhibition in tumors reveals rapid in vivo metabolic rewiring and vulnerability to combination therapy. Cell Rep. 30, 1798–1810.e4 (2020).
Sun, N. et al. Discovery of the first lactate dehydrogenase proteolysis targeting chimera degrader for the treatment of pancreatic cancer. J. Med. Chem. 66, 596–610 (2023).
Liu, J. et al. Aberrant FGFR tyrosine kinase signaling enhances the Warburg effect by reprogramming LDH isoform expression and activity in prostate cancer. Cancer Res. 78, 4459–4470 (2018).
Chaube, B. et al. Targeting metabolic flexibility by simultaneously inhibiting respiratory complex I and lactate generation retards melanoma progression. Oncotarget 6, 37281–37299 (2015).
Karlsson, M. J. et al. Inflammation and apolipoproteins are potential biomarkers for stratification of cutaneous melanoma patients for immunotherapy and targeted therapy. Cancer Res. 81, 2545–2555 (2021).
Qiao, T. et al. Inhibition of LDH-A by oxamate enhances the efficacy of Anti-PD-1 treatment in an NSCLC humanized mouse model. Front. Oncol. 11, 632364 (2021).
Cascone, T. et al. Increased tumor glycolysis characterizes immune resistance to adoptive T cell therapy. Cell Metab. 27, 977–987.e4 (2018).
Shi, L. et al. LncRNA GLTC targets LDHA for succinylation and enzymatic activity to promote progression and radioiodine resistance in papillary thyroid cancer. Cell Death Differ. 30, 1517–1532 (2023).
Meng, Z., Zhang, X., Tan, H. & Lian, H. Zinc-enriched nanosystem for dual glycolysis regulation and photothermal therapy to synergistically inhibit primary melanoma and lung metastasis. Chem. Eng. J. 435, 134781 (2022).
Halestrap, A. P. & Denton, R. M. The specificity and metabolic implications of the inhibition of pyruvate transport in isolated mitochondria and intact tissue preparations by alpha-Cyano-4-hydroxycinnamate and related compounds. Biochem. J. 148, 977–987.e4 (1975).
Poole, R. C. & Halestrap, A. P. Inhibition and labelling of the erythrocyte lactate transporter by stilbene disulphonates. Biochem. Soc. Trans. 18, 1245–1246 (1990).
Le Floch, R. et al. CD147 subunit of lactate/H+ symporters MCT1 and hypoxia-inducible MCT4 is critical for energetics and growth of glycolytic tumors. Proc. Natl. Acad. Sci. USA 108, 16663–16668 (2011).
Lopez, E. et al. Inhibition of lactate transport by MCT-1 blockade improves chimeric antigen receptor T-cell therapy against B-cell malignancies. J. Immunother. Cancer 11, e006287 (2023).
Doherty, J. R. et al. Blocking lactate export by inhibiting the Myc target MCT1 disables glycolysis and glutathione synthesis. Cancer Res. 74, 908–920 (2014).
Huang, C. et al. A screened GPR1 peptide exerts antitumor effects on triple-negative breast cancer. Mol. Ther. Oncol. 18, 602–612 (2020).
Yamaguchi, A. et al. Monocarboxylate transporter 4 involves in energy metabolism and drug sensitivity in hypoxia. Sci. Rep. 13, 1501 (2023).
Lee, J. Y. et al. MCT4 as a potential therapeutic target for metastatic gastric cancer with peritoneal carcinomatosis. Oncotarget 7, 43492–43503 (2016).
Kim, H. K. et al. MCT4 expression is a potential therapeutic target in colorectal cancer with peritoneal carcinomatosis. Mol. Cancer Ther. 17, 838–848 (2018).
Morais-Santos, F. et al. Targeting lactate transport suppresses in vivo breast tumour growth. Oncotarget 6, 19177–19189 (2015).
Baenke, F. et al. Functional screening identifies MCT4 as a key regulator of breast cancer cell metabolism and survival. J. Pathol. 237, 152–165 (2015).
Choi, S. Y. C. et al. The MCT4 gene: a novel, potential target for therapy of advanced prostate cancer. Clin. Cancer Res. 22, 2721–2733 (2016).
Qian, Y. et al. MCT4-dependent lactate secretion suppresses antitumor immunity in LKB1-deficient lung adenocarcinoma. Cancer Cell. 41, 1363–1380.e7 (2023).
Miranda-Gonçalves, V. et al. Monocarboxylate transporters (MCTs) in gliomas: expression and exploitation as therapeutic targets. Neuro Oncol. 15, 172–188 (2013).
Lim, K. S. et al. Inhibition of monocarboxylate transporter-4 depletes stem-like glioblastoma cells and inhibits HIF transcriptional response in a lactate-independent manner. Oncogene 33, 4433–4441 (2014).
Ždralević, M. et al. Disrupting the ‘Warburg effect’ re-routes cancer cells to OXPHOS offering a vulnerability point via ‘ferroptosis’-induced cell death. Adv. Biol. Regul. 68, 55–63 (2018).
Gallagher, S. M., Castorino, J. J., Wang, D. & Philp, N. J. Monocarboxylate transporter 4 regulates maturation and trafficking of CD147 to the plasma membrane in the metastatic breast cancer cell line MDA-MB-231. Cancer Res. 67, 4182–4189 (2007).
Marchiq, I. et al. Genetic disruption of lactate/H+ symporters (MCTs) and their subunit CD147/BASIGIN sensitizes glycolytic tumor cells to phenformin. Cancer Res. 75, 171–180 (2015).
Fu, Z.-G. et al. A novel small-molecule compound targeting CD147 inhibits the motility and invasion of hepatocellular carcinoma cells. Oncotarget 7, 9429–9447 (2016).
Zhang, Z. et al. Preclinical pharmacokinetics, tolerability, and pharmacodynamics of metuzumab, a novel CD147 human-mouse chimeric and glycoengineered antibody. Mol. Cancer Ther. 14, 162–173 (2015).
Wilson, M. C. et al. Basigin (CD147) is the target for organomercurial inhibition of monocarboxylate transporter isoforms 1 and 4: the ancillary protein for the insensitive MCT2 is EMBIGIN (gp70). J. Biol. Chem. 280, 27213–27221 (2005).
Tian, L.-R. et al. Nanodrug regulates lactic acid metabolism to reprogram the immunosuppressive tumor microenvironment for enhanced cancer immunotherapy. Biomater. Sci. 10, 3892–3900 (2022).
Ma, J. et al. Lithium carbonate revitalizes tumor-reactive CD8+ T cells by shunting lactic acid into mitochondria. Nat. Immunol. 25, 552–561 (2024).
Cao, Z. et al. Lactate oxidase nanocapsules boost T cell immunity and efficacy of cancer immunotherapy. Sci. Transl. Med. 15, eadd2712 (2023).
Zhou, J. et al. A lactate-depleting metal organic framework-based nanocatalyst reinforces intratumoral T cell response to boost anti-PD1 immunotherapy. J. Colloid Interface Sci. 660, 869–884 (2024).
Zheng, X. et al. Dual closed-loop of catalyzed lactate depletion and immune response to potentiate photothermal immunotherapy. ACS Appl. Mater. Interfaces 14, 23260–23276 (2022).
Zhao, S. et al. Nitrogen-centered lactate oxidase nanozyme for tumor lactate modulation and microenvironment remodeling. J. Am. Chem. Soc. 145, 10322–10332 (2023).
Desguin, B., Soumillion, P., Hausinger, R. P. & Hols, P. Unexpected complexity in the lactate racemization system of lactic acid bacteria. FEMS Microbiol. Rev. 41, S71–S83 (2017).
Lu, G. et al. Engineered biomimetic nanoparticles achieve targeted delivery and efficient metabolism-based synergistic therapy against glioblastoma. Nat. Commun. 13, 4214 (2022).
Zhao, J. et al. Insights into the effect of catalytic intratumoral lactate depletion on metabolic reprogramming and immune activation for antitumoral activity. Adv. Sci. 10, e2204808 (2023).
Li, Y. et al. Lactate-responsive gene editing to synergistically enhance macrophage-mediated cancer immunotherapy. Small 19, e2301519 (2023).
Yang, X. et al. Nanoscale ATP-responsive zeolitic imidazole framework-90 as a general platform for cytosolic protein delivery and genome editing. J. Am. Chem. Soc. 141, 3782–3786 (2019).
Alsaiari, S. K. et al. Endosomal escape and delivery of CRISPR/Cas9 genome editing machinery enabled by nanoscale zeolitic imidazolate framework. J. Am. Chem. Soc. 140, 143–146 (2018).
Luo, X. et al. Regulating acidosis and relieving hypoxia by platelet membrane-coated nanoparticle for enhancing tumor chemotherapy. Front. Bioeng. Biotechnol. 10, 885105 (2022).
Tang, J. et al. Openwork@dendritic mesoporous silica nanoparticles for lactate depletion and tumor microenvironment regulation. Angew. Chem. Int. Ed. Engl. 59, 22054–22062 (2020).
Wu, W. et al. Microbiotic nanomedicine for tumor-specific chemotherapy-synergized innate/adaptive antitumor immunity. Nano Today 42, 101377 (2022).
Zhang, Z. et al. Metal-phenolic network-enabled lactic acid consumption reverses immunosuppressive tumor microenvironment for sonodynamic therapy. ACS Nano 15, 16934–16945 (2021).
Li, T. et al. Metabolism/Immunity dual-regulation thermogels potentiating immunotherapy of glioblastoma through lactate-excretion inhibition and PD-1/PD-L1 blockade. Adv. Sci. 11, e2310163 (2024).
Niu, R. et al. Programmed targeting pyruvate metabolism therapy-amplified single-atom nanozyme-activated pyroptosis for immunotherapy. Adv. Mater. 36, e2312124 (2024).
Robey, I. F. et al. Bicarbonate increases tumor pH and inhibits spontaneous metastases. Cancer Res. 69, 2260–2268 (2009).
Lam, S. F. et al. Calcium carbonate nanoparticles stimulate cancer cell reprogramming to suppress tumor growth and invasion in an organ-on-a-chip system. Sci. Rep. 11, 9246 (2021).
Li, G. et al. Glycometabolic reprogramming-induced XRCC1 lactylation confers therapeutic resistance in ALDH1A3-overexpressing glioblastoma. Cell Metab. 36, 1696–1710.e1610 (2024).
Garber, K. First metabolic oncology inhibitor gets FDA green light, with record price tag. Nat. Biotechnol. 35, 895–895 (2017).
Huang, Y. Targeting glycolysis for cancer therapy using drug delivery systems. J. Control Release 353, 650–662 (2023).
Mullard, A. Cancer metabolism pipeline breaks new ground. Nat. Rev. Drug Discov. 15, 735–737 (2016).
Zheng, Y. et al. Shashen-Maidong Decoction inhibited cancer growth under intermittent hypoxia conditions by suppressing oxidative stress and inflammation. J. Ethnopharmacol. 299, 115654 (2022).
De Rosa, V. et al. Reversal of Warburg effect and reactivation of oxidative phosphorylation by differential inhibition of EGFR signaling pathways in non-small cell lung cancer. Clin. Cancer Res. 21, 5110–5120 (2015).
Liu, Y. et al. N-glycosylation stabilizes MerTK and promotes hepatocellular carcinoma tumor growth. Redox Biol. 54, 102366 (2022).
Banales, J. M. et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 17, 557–588 (2020).
Guinney, J. et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 21, 1350–1356 (2015).
Jass, J. R. Classification of colorectal cancer based on correlation of clinical, morphological and molecular features. Histopathology 50, 113–130 (2007).
Guo, J. et al. Frequent truncating mutation of TFAM induces mitochondrial DNA depletion and apoptotic resistance in microsatellite-unstable colorectal cancer. Cancer Res. 71, 2978–2987 (2011).
Sun, X. et al. Increased mtDNA copy number promotes cancer progression by enhancing mitochondrial oxidative phosphorylation in microsatellite-stable colorectal cancer. Signal Transduct. Target Ther. 3, 8 (2018).
Kather, J. N. et al. Deep learning can predict microsatellite instability directly from histology in gastrointestinal cancer. Nat. Med. 25, 1054–1056 (2019).
Maio, M. et al. Pembrolizumab in microsatellite instability high or mismatch repair deficient cancers: updated analysis from the phase II KEYNOTE-158 study. Ann. Oncol. 33, 929–938 (2022).
Vilar, E. & Gruber, S. B. Microsatellite instability in colorectal cancer-the stable evidence. Nat. Rev. Clin. Oncol. 7, 153–162 (2010).
Yurgelun, M. B. & Kastrinos, F. Tumor testing for microsatellite instability to identify lynch syndrome: new insights into an old diagnostic strategy. J. Clin. Oncol. 37, 263–265 (2019).
Hause, R. J., Pritchard, C. C., Shendure, J. & Salipante, S. J. Classification and characterization of microsatellite instability across 18 cancer types. Nat. Med. 22, 1342–1350 (2016).
Yamamoto, H. et al. Microsatellite instability: a 2024 update. Cancer Sci. 115, 1738–1748 (2024).
Amato, M. et al. Microsatellite instability: from the implementation of the detection to a prognostic and predictive role in cancers. Int. J. Mol. Sci. 23, 8726 (2022).
Lee, A. Serplulimab: first approval. Drugs 82, 1137–1141 (2022).
Ciombor, K. K. & Eng, C. Immunotherapy in localized microsatellite instability-high/mismatch repair deficient solid tumors: are we ready for a new standard of care? J. Clin. Oncol. 41, 2138–2140 (2023).
Chang, X. et al. Predicting colorectal cancer microsatellite instability with a self-attention-enabled convolutional neural network. Cell Rep. Med. 4, 100914 (2023).
Billiard, J. et al. Quinoline 3-sulfonamides inhibit lactate dehydrogenase A and reverse aerobic glycolysis in cancer cells. Cancer Metab. 1, 19 (2013).
Fantin, V. R., St-Pierre, J. & Leder, P. Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell. 9, 425–434 (2006).
Yang, C., Pan, R. Y., Guan, F. & Yuan, Z. Lactate metabolism in neurodegenerative diseases. Neural Regen. Res. 19, 69–74 (2024).
Hagihara, H. et al. Protein lactylation induced by neural excitation. Cell Rep. 37, 109820 (2021).
Jing, E. et al. Sirt3 regulates metabolic flexibility of skeletal muscle through reversible enzymatic deacetylation. Diabetes 62, 3404–3417 (2013).
Wang, J. et al. Ubiquitous protein lactylation in health and diseases. Cell Mol. Biol. Lett. 29, 23 (2024).
Millán-Zambrano, G., Burton, A., Bannister, A. J. & Schneider, R. Histone post-translational modifications - cause and consequence of genome function. Nat. Rev. Genet. 23, 563–580 (2022).
Iker Etchegaray, J. et al. Phagocytosis in the retina promotes local insulin production in the eye. Nat. Metab. 5, 207–218 (2023).
Vohra, R. & Kolko, M. Lactate: more than merely a metabolic waste product in the inner retina. Mol. Neurobiol. 57, 2021–2037 (2020).
Madaan, A. et al. Müller cell-localized G-protein-coupled receptor 81 (Hydroxycarboxylic Acid Receptor 1) regulates inner retinal vasculature via Norrin/Wnt pathways. Am. J. Pathol. 189, 1878–1896 (2019).
Takata, N. et al. Lactate-dependent transcriptional regulation controls mammalian eye morphogenesis. Nat. Commun. 14, 4129 (2023).
Fan, W. et al. A feedback loop driven by H3K9 lactylation and HDAC2 in endothelial cells regulates VEGF-induced angiogenesis. Genome Biol. 25, 165 (2024).
Park, J., Lee, K., Kim, K. & Yi, S. J. The role of histone modifications: from neurodevelopment to neurodiseases. Signal Transduct. Target Ther. 7, 217 (2022).
Obiedat, A. et al. The integrated stress response promotes B7H6 expression. J. Mol. Med. 98, 135–148 (2020).
Galluzzi, L. et al. Activating autophagy to potentiate immunogenic chemotherapy and radiation therapy. Nat. Rev. Clin. Oncol. 14, 247–258 (2017).
Li, G. et al. Intersection of immune and oncometabolic pathways drives cancer hyperprogression during immunotherapy. Cancer Cell. 41, 304–322.e307 (2023).
Zhang, R. X. et al. Nanomedicine of synergistic drug combinations for cancer therapy—strategies and perspectives. J. Control Release 240, 489–503 (2016).
Hu, Q., Sun, W., Wang, C. & Gu, Z. Recent advances of cocktail chemotherapy by combination drug delivery systems. Adv. Drug Deliv. Rev. 98, 19–34 (2016).
Slovin, S. Chemotherapy and immunotherapy combination in advanced prostate cancer. Clin. Adv. Hematol. Oncol. 10, 90–100 (2012).
Imaide, S. et al. Trivalent PROTACs enhance protein degradation via combined avidity and cooperativity. Nat. Chem. Biol. 17, 1157–1167 (2021).
Zheng, M. et al. Rational design and synthesis of novel dual PROTACs for simultaneous degradation of EGFR and PARP. J. Med. Chem. 64, 7839–7852 (2021).
Zhang, S. et al. PROTAC prodrug‐integrated nanosensitizer for potentiating radiation therapy of cancer. Adv. Mater. 36, e2314132 (2024).
Chirnomas, D., Hornberger, K. R. & Crews, C. M. Protein degraders enter the clinic - a new approach to cancer therapy. Nat. Rev. Clin. Oncol. 20, 265–278 (2023).
Lin, X. et al. Augmentation of scleral glycolysis promotes myopia through histone lactylation. Cell Metab. 36, 511–525.e517 (2024).
Lan, M. et al. Photosensitizers for photodynamic therapy. Adv. Health. Mater. 8, e1900132 (2019).
Pérez-Herrero, E. & Fernández-Medarde, A. Advanced targeted therapies in cancer: drug nanocarriers, the future of chemotherapy. Eur. J. Pharm. Biopharm. 93, 52–79 (2015).
Naso, M. F., Tomkowicz, B., Perry, W. L. 3rd & Strohl, W. R. Adeno-Associated Virus (AAV) as a vector for gene therapy. BioDrugs 31, 317–334 (2017).
He, X. et al. AAV for gene therapy in ocular diseases: progress and prospects. Research 6, 0291 (2023).
Aponte-Ubillus, J. J. et al. Molecular design for recombinant adeno-associated virus (rAAV) vector production. Appl. Microbiol. Biotechnol. 102, 1045–1054 (2018).
Kotterman, M. A., Chalberg, T. W. & Schaffer, D. V. Viral vectors for gene therapy: translational and clinical outlook. Annu. Rev. Biomed. Eng. 17, 63–89 (2015).
Dunbar, C. E. et al. Gene therapy comes of age. Science 359, e2314132 (2018).
He, X. Y. et al. Aptamer/peptide-functionalized genome-editing system for effective immune restoration through reversal of PD-L1-mediated cancer immunosuppression. Adv. Mater. 32, e2000208 (2020).
Alyami, M. Z. et al. Cell-type-specific CRISPR/Cas9 delivery by biomimetic metal organic frameworks. J. Am. Chem. Soc. 142, 1715–1720 (2020).
Li, T. et al. CRISPR/Cas9 therapeutics: progress and prospects. Signal Transduct. Target. Ther. 8, 36 (2023).
Li, N. et al. ALKBH5 regulates anti-PD-1 therapy response by modulating lactate and suppressive immune cell accumulation in tumor microenvironment. Proc. Natl. Acad. Sci. USA 117, 20159–20170 (2020).
Yao, Y. et al. A novel nanozyme to enhance radiotherapy effects by lactic acid scavenging, ROS generation, and hypoxia mitigation. Adv. Sci. 11, e2403107 (2024).
Dong, Q. et al. Lactate-induced MRP1 expression contributes to metabolism-based etoposide resistance in non-small cell lung cancer cells. Cell Commun. Signal. 18, 167 (2020).
Tian, Z. et al. Catalytically selective chemotherapy from tumor-metabolic generated lactic acid. Small 15, e1903746 (2019).
Barnes, E. M. E. et al. Lactic acidosis induces resistance to the pan-Akt inhibitor uprosertib in colon cancer cells. Br. J. Cancer 122, 1298–1308 (2020).
Wang, H. & Huang, Y. Combination therapy based on nano codelivery for overcoming cancer drug resistance. Med. Drug Discov. 6, 100024 (2020).
Wu, Y. W., Chik, C. L. & Knazek, R. A. An in vitro and in vivo study of antitumor effects of gossypol on human SW-13 adrenocortical carcinoma. Cancer Res. 49, 3754–3758 (1989).
Guan, X., Rodriguez-Cruz, V. & Morris, M. E. Cellular uptake of MCT1 inhibitors AR-C155858 and AZD3965 and their effects on MCT-mediated transport of L-lactate in murine 4T1 breast tumor cancer cells. AAPS J. 21, 13 (2019).
Khan, A. et al. Targeting metabolic activity in high-risk neuroblastoma through Monocarboxylate Transporter 1 (MCT1) inhibition. Oncogene 39, 3555–3570 (2020).
Dong, G. et al. Diisopropylamine dichloroacetate enhances radiosensitization in esophageal squamous cell carcinoma by increasing mitochondria-derived reactive oxygen species levels. Oncotarget 7, 68170–68178 (2016).
Bonnet, S. et al. A mitochondria-K+ channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer Cell. 11, 37–51 (2007).
Draoui, N. et al. Antitumor activity of 7-aminocarboxycoumarin derivatives, a new class of potent inhibitors of lactate influx but not efflux. Mol. Cancer Ther. 13, 1410–1418 (2014).
Spinello, I. et al. The small-molecule compound AC-73 targeting CD147 inhibits leukemic cell proliferation, induces autophagy and increases the chemotherapeutic sensitivity of acute myeloid leukemia cells. Haematologica 104, 973–985 (2019).
Luo, Y. et al. A nanounit strategy disrupts energy metabolism and alleviates immunosuppression for cancer therapy. Nano Lett. 22, 6418–6427 (2022).
Funding
Supported by grants from the Science and Technology Commission of Shanghai(22Y31900700); Program of Innovative Research Team of High‑Level Local Universities in Shanghai (SHSMU‑ZDCX20210902), Shanghai Municipal Health Commission(2022YQ01) and “New Star of Medical College” Young Medical Talents Training Program in Shanghai in 2020.
Author information
Authors and Affiliations
Contributions
Renbing Jia, Shiqiong Xu, and Shengfang Ge provided direction and guidance throughout the preparation of this manuscript. Jie Chen, Ziyue Huang, Hao Tian, and Ya Chen collected and interpreted studies and was a major contributor to the writing, editing and revising of the manuscript. Peiwei Chai, Yongning Shen, and Yiran Yao reviewed the manuscript and provided important guidance throughout its revision. All authors have read and approved the article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Not applicable.
Declaration of animal ethics approval
Studies cited in this review involving experiments with animals are all equipped with animal care in accordance with institution guidelines.
Additional information
Consent for publication Not applicable.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, J., Huang, Z., Chen, Y. et al. Lactate and lactylation in cancer. Sig Transduct Target Ther 10, 38 (2025). https://doi.org/10.1038/s41392-024-02082-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41392-024-02082-x
This article is cited by
-
Mechanisms of drug resistance in hepatocellular carcinoma
Biological Procedures Online (2025)
-
Unlocking the secret of glioblastoma multiforme: the role of lactylation in tumor progression, drug resistance and immune microenvironment
Cancer Cell International (2025)
-
Lactylation modifications in urological diseases: molecular mechanisms and biological implications
Clinical Epigenetics (2025)
-
Lactylation-regulated biomolecular condensates: metabolic control of phase separation in physiology and disease
Cell Communication and Signaling (2025)
-
Protein lipidation in the tumor microenvironment: enzymology, signaling pathways, and therapeutics
Molecular Cancer (2025)