Abstract
This prospective, nonrandomized, open-label phase 2 trial (Chinese Clinical Trial Registry, ChiCTR2200061906) aimed to evaluate the effectiveness of adding the PD-1 antibody tislelizumab to perioperative chemotherapy in patients with locally advanced gastroesophageal junction adenocarcinoma (GEJA). This study enrolled patients with GEJA clinically staged as cT3-4aNanyM0 or cT1-2N+M0 from October 2022 to June 2023. Eligible patients were administered three preoperative and five postoperative 3-week cycles of treatment with PD-1 antibody tislelizumab plus SOX (S-1 and oxaliplatin) regimen. The primary endpoint was major pathological response (MPR) rate. Thirty-two patients were enrolled. The median age was 60 years (range: 28–74 years), and 53.1% (17/32) patients were Siewert III type. All patients received at least one cycle of assigned preoperative treatment, and 93.8% (30/32) patients completed three cycles of assigned preoperative tislelizumab and SOX. The R0 resection rate was 96.9% (31/32). MPR, pathological complete response (pCR) of primary tumors and ypT0N0 rates were 50.0% (16/32, 95% CI: 31.9–68.1%), 28.1% (9/32, 95% CI: 13.7–46.7%) and 25.0% (8/32, 95% CI: 11.5–43.4%), respectively. The surgical morbidity rate was 15.6% (5/32), and no 30-day mortality was observed. In the preoperative and postoperative treatment periods, the rate of treatment-related grade 3–4 adverse events was 31.2% (10/32). At the date of 7th Jan 2025, 8 (25.0%) patients occurred recurrence. Therefore, perioperative tislelizumab plus chemotherapy demonstrated significantly improved pathological regression and might be a promising option for patients with locally advanced resectable GEJA.
Similar content being viewed by others
Introduction
Gastric and gastroesophageal junction adenocarcinoma ranks fifth among malignancies in terms of both incidence and mortality worldwide.1 While the overall prevalence of gastric adenocarcinoma has steadily decreased, an increase has been observed in the occurrence of gastroesophageal junction adenocarcinoma (GEJA) in the past 30 years.2,3 Moreover, about 61% of all GEJA case are diagnosed in China.3 GEJA cases are frequently diagnosed at a locally advanced stage,4 leading to a generally grim prognosis.5,6
Besides anatomical location, GEJA possesses unique molecular characteristics, such as a less common association with H. pylori infection, a more frequent association with chromosomal instability (CIN) subtype, and amplification of ERBB2 and EGFR.7 Patients diagnosed with GEJA should be categorized as having a distinct type of tumor and should receive specialized treatment. However, the scarcity of randomized controlled trials (RCTs) focusing on GEJA has resulted in a lack of globally accepted standard of care for these cases.8,9,10,11 Given that a substantial proportion of GEJA cases were included in RCTs assessing both esophageal carcinoma.12,13 and gastric carcinoma,14,15 the standard of care for patients with resectable GEJA corresponds to that of esophageal carcinoma or gastric carcinoma cases.8,9 Based on the successful outcomes of the FLOT4 study,14 perioperative fluorouracil, leucovorin, oxaliplatin and docetaxel (FLOT) is recommended for patients with locally advanced GEJA in non-Asian countries.8,11 In Japan and Korea,16,17 adjuvant chemotherapy following D2 gastrectomy is preferred in locally advanced GEJA due to the promising results of the CLASSIC and ACTS-GC studies.18,19 In 2021, the RESOLVE study reported a clinically meaningful improvement in disease-free survival after treatment with perioperative S-1 and oxaliplatin (SOX) versus adjuvant capecitabine and oxaliplatin (CapOx) in individuals with locally advanced gastric carcinoma or GEJA.15 These findings supported the adoption of perioperative SOX as the standard of care for localized GEJA cases in China.10 Although perioperative chemoradiotherapy is considered the standard care for patients with esophageal cancer,12 the recent TOPGEAR study indicated that incorporating radiotherapy into chemotherapy during the perioperative phase failed to improve survival outcomes for patients with locally advanced gastric cancer and GEJA.20 Therefore, there is an urgent need to develop effective treatment strategies for these patients.
Therapies including programmed cell death 1 (PD-1) or programmed death-ligand 1 (PD-L1) blockade, when combined with chemotherapy, improve patient survival in gastric carcinoma or GEJA and are thus endorsed for the first-line treatment of these cases.21,22,23,24,25,26,27 The RATIONALE-305 study demonstrated significantly improved overall survival (OS) after treatment with tislelizumab in combination with chemotherapy compared to placebo plus chemotherapy.24 In addition, tislelizumab also showed promising antitumor activity in the perioperative setting in individuals with localized tumors.28 Several RCTs have reported that addition of perioperative PD-1/PD-L1 inhibitors to chemotherapy substantially improves pathological response.29,30,31 However, little is known about the perioperative application of PD-1 inhibitors in individuals with locally advanced GEJA.
In this study, we report the results of the prospective, nonrandomized, open-label, phase 2 NEOSUMMIT-03 study that evaluated the efficacy of perioperative tislelizumab plus chemotherapy for locally advanced GEJA.
Results
Baseline patient characteristics
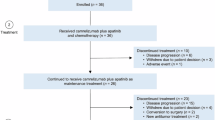
Between October 10, 2022 and June 15, 2023, 38 patients with GEJA were screened for eligibility (Fig. 1). Of these patients, 6 not meeting the inclusion criteria were excluded; therefore, 32 patients were included. The baseline patient characteristics are shown in Table 1. The median age was 60 years (range: 28–74 years), and 78.1% (25/32) patients were male. Of the 32 cases included, 17 (53.1%) were Siewert type III, 12 (37.5%) had the intestinal type, and 18 (56.3%) had a PD-L1 combined positive score (CPS) of 5 or more. All the 32 cases were mismatch repair-proficient (pMMR), and 2 (6.2%) were confirmed to be Epstein-Barr virus (EBV) positive. Diagnostic laparoscopic exploration was carried out in 22 (68.8%) of the 32 patients.
Neoadjuvant tislelizumab and chemotherapy
All the included 32 patients received at least one cycle of the assigned neoadjuvant tislelizumab and chemotherapy. Of these patients, 30 (93.8%) completed the planned 3 cycles of neoadjuvant therapy. One patient (3.1%) only completed one cycle of neoadjuvant tislelizumab and chemotherapy because of grade 3 diarrhea and per the patient’s request. One patient (3.1%) had grade 3 hypothyroidism after the third cycle of neoadjuvant tislelizumab and chemotherapy. Due to the high anesthetic risk at that time, the patient was deemed unsuitable for surgery, was subsequently administered two additional cycles of tislelizumab and chemotherapy, and started thyroid hormone replacement therapy. RECIST 1.1 evaluation was performed in 32 cases after completion of neoadjuvant treatment prior surgery: complete response, n = 1 (3.1%); partial response, n = 10 (31.3%); stable disease, n = 1 (3.1%); not evaluable, n = 20 (62.5%).
Surgical findings
After neoadjuvant treatment, all the 32 patients underwent surgical resection. The median time span from the end of the final round of neoadjuvant therapy to surgical intervention was 2.8 weeks. (range: 2.1–12.1 weeks). One patient had delayed surgery per the patient’s request. The surgery type and overall morbidity are shown in Supplementary Table 1. Total gastrectomy was carried out in 27 (84.4%) cases, whereas R0 resection was performed in 31 (96.9%) individuals. Five (15.6%) cases reported at least one serious adverse event associated with perioperative morbidity. There was no mortality reported within 30-day.
Pathological and tumor regression data are shown in Table 2 and Fig. 2a. The median tumor residual rate was 12.5% (range: 0–85%, Fig. 2a). The primary endpoint was major pathological response (MPR) of the included patients. Of the 32 patients, 16 (50.0%, 95% CI: 31.9–68.1) had MPR. The TRG results are summarized in Table 2. Nine (28.1%, 95% CI: 13.7–46.7) cases achieved pathological complete response (pCR) in the primary tumor, one of whom was T0 but with tumor cells in two nodes and was staged as ypT0N1. Therefore, eight (25.0% (8/32, 95% CI: 11.5–43.4%) cases achieved ypT0N0. Fourteen (43.8%, 95% CI: 26.4–62.3) cases achieved TRG 0/1.
Tumor response to neoadjuvant therapy and follow-up. a Waterfall plot of tumor regression rates after neoadjuvant therapy based on pathology. b Swimmer plot showing treatment events and follow-up in the population of all included patients. PD-L1, programmed cell death-ligand 1, CPS, combined positive score; EBV, Epstein-Barr virus; MPR, major pathological response
Adjuvant tislelizumab and chemotherapy
The adjuvant treatment is described in Fig. 1 and Fig. 2b. Of the 32 patients administered surgery, 31 (96.9%) cases were administered adjuvant tislelizumab and chemotherapy. One patient did not receive adjuvant treatment per the patient’s request. The median time span from surgery to the initiation of adjuvant therapy was 5.6 weeks (range: 3.0–11.1 weeks). Thirteen (40.6%) cases completed the planned 8 perioperative treatments with tislelizumab and chemotherapy.
Survival
At the data cutoff on January 7, 2025, the median follow-up time was 21.9 months (range: 11.2–27.3). Of the 32 cases, 22 (68.8%) were alive and free of recurrence. Six cases had peritoneal metastasis, two suffered from distant lymph node metastasis. Among 8 patients with recurrent disease, 6 patients were alive, 2 patients died of progressive disease. Two patient without recurrent disease died of non-tumor-related causes. Kaplan-Meier curves for overall survival (OS) and event-free survival (EFS) are shown in Fig. 3, respectively. The 18-month OS and EFS rates were 84.4% (95% CI: 72.7–97.9%) and 74.4% (95% CI: 60.5–91.4%), respectively.
Adverse events
Treatment-related adverse events (TRAEs) of any cause occurred in all patients during perioperative therapy (Supplementary Table 2). Grade 3 or 4 TRAEs were observed in 31.2% (10/32) of patients. Of these, thrombocytopenia (n = 3, 9.4%) was the most common grade 3 or 4 TRAE. Immune-relative AEs (irAEs) occurred in 8 (25.0%) patients, with most being grade 1 or 2. One patient experienced grade 3 hypothyroidism. There was no death considered to be treatment related.
Biomarker analysis
In post hoc analysis, subgroup analyses were carried out to assess tumor regression by clinical T stage, clinical N stage, Siewert type, Lauren classification, and PD-L1 CPS status (Fig. 4). Of note, a larger percentage of patients with intestinal type had MPR (9/12, 75.0% [95% CI: 42.8–94.5]) compared with cases with diffuse (3/9, 33.3% [95% CI: 7.5–70.1]; P = 0.087) or mixed (4/11, 36.4% [95% CI: 10.9–69.2]; P = 0.099) type. A higher ypT0N0 rate was observed in patients with intestinal type (7/12, 66.7% [95% CI: 30.4–86.2]) compared with cases with diffuse (0/9, 0% [95% CI: 0–33.6]; P = 0.007) or mixed (1/11, 9.1% [95% CI: 0.2–41.3]; P = 0.027) type. There were no significant differences in MPR or ypT0N0 rates between patients with a high CPS and those with a low CPS (Fig. 4b and Supplementary Fig. 1). We also performed subgroup analyses to assess the survival by PD-L1 CPS status (Supplementary Fig. 2). Interestingly, patients with a CPS of 5 or more appear to have shorter EFS and OS than those with a CPS of less than 5.
Subsequently, we conducted RNA sequencing on both paired baseline tumor biopsies and post-treatment samples from 28 patients to evaluate whether distinct immune cell components within the tumor immune microenvironment (TIME) control the treatment response (Fig. 5a, b). Of note, the baseline immune components could not predict the treatment response of neoadjuvant tislelizumab and chemotherapy. However, after neoadjuvant treatment, decreased levels of Treg, γδT and neutrophil cell infiltration were found in patients achieving MPR than in patients not achieving MPR (Fig. 5c–e). Besides, enhanced eosinophils infiltration was found in patients achieving MPR than in patients not achieving MPR (Fig. 5f).
Immune cell landscape of patients at baseline and after neoadjuvant treatment. a, b Heatmaps depicting 28 subpopulations of tumor-infiltrating lymphocytes at baseline and after neoadjuvant treatment in patients who achieved a MPR (a, top panel) and those who did not (b, bottom panel); (c–f) Paired analysis of Treg (c), γδT (d), neutrophil (e) and eosinophils (f) cell infiltration at baseline and after neoadjuvant treatment in patients who achieved a MPR (left panel) and those who did not (right panel). MPR major pathological response. Wilcoxon signed-rank test
Discussion
This NEOSUMMIT-03 phase 2 study evaluated the application of a PD-1 inhibitor perioperatively for locally advanced GEJA and reached its primary objective for MPR (50% vs the planned primary endpoint of 33%). In addition, perioperative tislelizumab plus chemotherapy resulted in a promising pCR in primary tumors of 28.1% and a rate of 25.0% for ypT0N0, without increasing TRAEs and perioperative morbidity.
In patients diagnosed with Siewert Type I GEJA, the staging and treatment strategy is aligned with that of esophageal carcinoma. For Siewert Type III cases, the staging and treatment approach refers to gastric carcinoma, while in Siewert Type II cases, the staging and treatment routine remains unspecified. In this study, we included patients with Siewert type II and III GEJA and adopted SOX as the perioperative chemotherapy regimen because of the high proportion of GEJA (36.5%) cases in the RESOLVE study.15
The effect of neoadjuvant immunotherapy on gastric cancer has been examined in several studies. The randomized phase 2 DANTE study of perioperative atezolizumab plus FLOT initially reported an improved pathological response (Becker TRG 1a/b: 49% vs 44%).27 At the 2023 American Society of Clinical Oncology (ASCO) annual meeting, we reported the results of the randomized phase 2 NEOSUMMIT-01 study, which revealed improved major (44.4% vs 20.4%) or complete pathological (22.2% vs 7.4%) response.29 Subsequently at the European Society for Medical Oncology (ESMO) 2023 congress, improved pathological response was also reported for the phase 3 KEYNOTE-585.30 (pCR: 12.9% vs 2.0%) and MATTERHORN (pCR: 19% vs 7%) studies.31 In our NEOSUMMIT-03 study, we specifically focused on GEJA, and all eligible cases were pMMR. Patients with locally advanced GEJA administered neoadjuvant tislelizumab plus chemotherapy had promising MPR (50.0%), pCR (28.1%) and ypT0N0 (25.0%), surpassing the pathological response rate previously observed in the NEOSUMMIT-01 study examining similar cases administered neoadjuvant chemotherapy.29 Intriguingly, corroborating previous studies,29,32 one case had pCR of the primary tumor with tumor cells in lymph nodes in this study, indicating heterogeneous immune responses between the primary tumor and metastatic lymph node metastasis.
In previous studies of first-line PD-1/PD-L1 inhibitors plus chemotherapy for advanced gastric carcinoma, enhanced survival benefits corresponding to the PD-L1 expression status were detected based on the CPS.21,22,23,24,25,27 However, in post hoc analysis of the NEOSUMMIT-03 study, no significant difference was found in pathological response rate between patients with a CPS ≥ 5 and cases with a CPS below 5, which was also reported by NEOSUMMIT-01.29 and KEYNOTE-585.30 In our study, the identification of PD-L1 status was reliant on pretreatment biopsies, and the potential reason for CPS’s inability to effectively predict patient outcomes may lie in the spatial heterogeneity of PD-L1 expression.33,34 Of note, a post hoc analysis revealed a more pronounced pathological response in cases with the intestinal type compared with those with the diffuse or mixed type. This suggests that Lauren histological classification could be more important in exhibiting the benefits of perioperative anti-PD-1 therapy compared with PD-L1 status based on CPS. However, this result should be interpreted with caution given the limited sample size and the phase 2 nature of this study.
Currently, there are limited reports concerning the alterations in the TIME following neoadjuvant treatment with PD-1 inhibitors and chemotherapy for locally advanced resectable gastric cancer. In this study, we did not observe apparent baseline differences in TIME composition between patients with MPR and those without, consistent with findings reported in the advanced gastric cancer.35 Eosinophils are granulocytes derived from the bone marrow that play crucial roles in maintaining tissue homeostasis and repair, eliminating parasites, and contributing to the pathophysiology of various conditions, such as allergic asthma and autoimmune disorders.36 A recent study reported that an increase in intratumoral eosinophils was observed in breast cancer patients responding to anti-PD-1 therapy.37 Similarly, we observed that following neoadjuvant treatment with tislelizumab in combination with chemotherapy, an intensified infiltration of eosinophils was noted in patients who achieved a MPR. Therefore, eosinophils, an innate immune cell type, deserve greater more focus regarding their role in anti-tumor immunity.
Perioperative safety is of great importance for operable tumors. In this NEOSUMMIT-03 study, the frequency of grade 3 or 4 TRAEs was 31.2%, corroborating previous findings.27,29,30 No new safety signal was detected in this study, with acceptable perioperative morbidity (15.6%) and no 30-day mortality. However, despite the high completion rate of preoperative tislelizumab plus chemotherapy (93.8%), only 40.6% of all included patients completed the 8 scheduled perioperative treatments with tislelizumab and chemotherapy, which was lower than reported for NEOSUMMIT-01.29 This finding also called for the possibility of long-course or total neoadjuvant anti-PD1 therapy plus chemotherapy for such patients (Chinese Clinical Trial Registry, identifier: ChiCTR2300069000).
The limitations of this study should be acknowledged. Given that this was a single-arm phase 2 trial with a small sample size, these results should be interpreted with caution. Therefore, a large-scale RCT has been conducted to validate the effectiveness of perioperative tislelizumab combined with chemotherapy in patients with GEJA (Chinese Clinical Trial Registry, identifier: ChiCTR2400081098). In addition, although pathological response (MPR or pCR) has been demonstrated to predict survival outcomes in operable patients administered perioperative anti-PD-1/PD-L1 therapy,38 pCR enhancement did not translate into a survival benefit based on EFS in KEYNOTE-585.30 Therefore, it is necessary to continue follow-up until maturation to evaluate EFS, recurrence-free survival (RFS) and OS in this study.
In conclusion, the present study demonstrated that perioperative tislelizumab plus chemotherapy significantly improves pathological regression and might be a promising option for patients with locally advanced resectable GEJA.
Materials and methods
Study population and trial design
NEOSUMMIT-03 is a single-arm, open-label, phase 2 trial performed in China. Patients were eligible if they had histological confirmed GEJA of Siewert type II or III and clinical stage cT3-4aNanyM0 or cT1-2N+M0 based on Siewert classification.39 and the 8th Edition of the International Union against Cancer tumor-node-metastasis classification. The clinical stage was assessed via physical examination, oesophagogastroduodenoscopy, and CT or MRI scan of the thoracic, abdominal, and pelvic regions. Diagnostic laparoscopy was recommended to exclude occult peritoneal seeding. Patients were also required to be aged between 18 and 75 years, with an Eastern Cooperative Oncology Group (ECOG) performance status of 0–1, life expectancy ≥ 3 months, no contraindications for surgery, and adequate organ function. Patients with a history of anti-tumor drug therapy, or surgical treatment for gastric cancer, those with a history of other malignancies within the past 5 years, or those allergic to the investigational drug were excluded. Eligibility criteria are available in the study protocol (Additional information).
The protocol and its amendments were approved by the institutional review board or independent ethics committee of Sun Yat-sen University Cancer Center, and the study was performed in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki. The results were reported in compliance with the CONSORT statement guidelines. All patients provided written informed consent.
Treatment
In the neoadjuvant phase, patients were administered intravenously tislelizumab at 200 mg on day 1 of each cycle every 3 weeks for three cycles. Oxaliplatin at 130 mg/m2 was administered intravenously on day 1 of each cycle plus S-1 at 40–60 mg orally twice daily for 2 weeks followed by a one-week rest. Surgery was performed within 2–4 weeks after the last cycle of the neoadjuvant phase. In the adjuvant phase, between 4 and 6 weeks after radical gastrectomy, patients were scheduled for tislelizumab treatment at 200 mg plus chemotherapy for five cycles. Dose reduction for tislelizumab was not permitted and the drug could only be interrupted or discontinued for adverse events. Patients could have a maximum of two chemotherapy dose reductions; the detailed modifications of chemotherapy are provided in the protocol (Additional information).
Assessments and outcomes
Imaging assessments by CT or MRI were carried out within 4 weeks before treatment initiation, after completion of cycle three of neoadjuvant treatment. Patients who had surgery and received adjuvant treatment were scheduled for repeat imaging every three months during the first three years, every six months until five years, and annually thereafter. The surgical procedures were performed by proficient abdominal surgeons with at least 100 gastric surgeries annually. In type II and III gastroesophageal junction adenocarcinoma cases, total or proximal gastrectomy with transhiatal distal esophagectomy was required. In this study, D2 lymphadenectomy was applied for total gastrectomy, and D1+lymphadenectomy for proximal gastrectomy. The quality of all surgeries was reviewed and evaluated by an experienced surgeon (YBC).
At baseline, Lauren histological classification, MMR status, PD-L1 status and EBV status were examined. The MMR status was determined by immunohistochemical (IHC) assessment of MLH1, PMS2, MSH2 and MSH6. PD-L1 expression was assessed using the 22C3 pharmDx assay (Agilent Technologies, Santa Clara, CA, USA) based on CPS., defined as the total number of PD-L1-positive tumor cells (partial or complete membrane staining), lymphocytes, and macrophages (membrane staining and/or intracellular staining) divided by the total number of viable tumor cells multiplied by 100. The EBV status was evaluated by EBER with an in-situ hybridization (ISH) kit (ISH-7001, Zhongshan Jinqiao Biotechnology Co., Ltd., Beijing, China/Leica Biosystems, Newcastle, UK).
The primary endpoint was MPR, defined as ≤ 10% residual viable tumor cells at the time of definitive surgery.40 Secondary endpoints included pCR of the primary tumor (ypT0), pathological regression response (TRG 0) or 1 as per the NCCN Guidelines for Gastric Cancer,8 margin-free resection (R0), RFS (duration from the initial treatment cycle to disease recurrence, metastasis, or death from any cause), EFS (time from the initial treatment cycle to disease progression, local or distant recurrence, or death from any cause), OS (time from initial treatment cycle to death from any cause), objective response rate (percentage of patients with partial or complete response as determined by investigators using RECIST version 1.1), surgical safety, treatment-related adverse events evaluated according to the CTCAE version 5.0, and quality of life.The pathological response was reviewed by a senior pathologist (MYC), whereas the radiological evaluation was carried out by a seasoned radiologist (ML).
Biomarker analysis
We conducted RNA sequencing on both paired baseline tumor biopsies and post-treatment samples from 28 patients to evaluate whether distinct immune cell components within the TIME control the treatment response. Using the Gene Set Enrichment Analysis (GSEA) strategy.41,42 alongside previously defined immune cell markers,43 we estimated a total of 28 subpopulations of tumor-infiltrating lymphocytes, including major types associated with adaptive immunity.
Statistical methods
The sample size was determined according to the assumption that addition of tislelizumab to chemotherapy would improve the MPR rate from the historical control rate of 13%.44 to 33%, with a one-sided α of 0.025 and a 80% power, accounting for a dropout rate of 10%. Therefore, it was expected that 32 patients would be included in the present study.
The population of all included patients was used to analyze the primary endpoint, surgical and pathological outcomes, non-surgical adverse events, serious adverse events, and survival outcomes. The primary efficacy endpoint (MPR rate) was reported as a proportion with 95% confidence interval (CI), determined by the Clopper-Pearson method. Other categorical variables were presented as frequency (percentage); continuous variables were reported as median (range). Survival data were estimated by the Kaplan-Meier method. Post hoc analyses were performed to evaluate tumor regression based on clinical T stage, clinical N stage, Siewert type, Lauren classification, and PD-L1 CPS status. We also performed subgroup analyses to assess the survival by PD-L1 CPS status. All P values were two-sided. SAS (version 9.4) was used for data analysis.
Data availability
The raw sequencing data reported in this paper have been deposited in the Genome Sequence Archive in National Genomics Data Center, China National Center for Bioinformation/Beijing Institute of Genomics, Chinese Academy of Sciences (GSAHuman: HRA008806) that are publicly accessible at https://ngdc.cncb.ac.cn/gsa-human. All requests for data will be reviewed by the leading clinical site, Sun Yat-Sen University Cancer Center, to verify whether the request is subject to any intellectual property or confidentiality obligations. Requests for access to the patient-level data from this study can be submitted via email to nierc@sysucc.org.cn with detailed proposals for approval. A signed data access agreement with the sponsor is required before accessing shared data. Source data are provided with this paper.
References
Bray, F. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 74, 229–263 (2024).
Liu, K. et al. Changes of esophagogastric junctional adenocarcinoma and gastroesophageal reflux disease among surgical patients during 1988-2012: a single-institution, high-volume experience in China. Ann. Surg. 263, 88–95 (2016).
Arnold, M., Ferlay, J., van Berge Henegouwen, M. I. & Soerjomataram, I. Global burden of oesophageal and gastric cancer by histology and subsite in 2018. Gut 69, 1564–1571 (2020).
Sano, T. et al. Proposal of a new stage grouping of gastric cancer for TNM classification: International Gastric Cancer Association staging project. Gastric Cancer 20, 217–225 (2017).
Nie, R. C. et al. Adjuvant chemotherapy for patients with adenocarcinoma of the esophagogastric junction: a retrospective, multicenter, observational study. Ann. Surg. Oncol. 30, 4014–4025 (2023).
Gavin, A. T. et al. Oesophageal cancer survival in Europe: a EUROCARE-4 study. Cancer Epidemiol. 36, 505–512 (2012).
Suh, Y. S. et al. Comprehensive molecular characterization of adenocarcinoma of the gastroesophageal junction between esophageal and gastric adenocarcinomas. Ann. Surg. 275, 706–717 (2022).
Ajani, J. A. et al. Gastric Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl Compr. Canc Netw. 20, 167–192 (2022).
Ajani, J. A. et al. Esophageal and esophagogastric junction cancers, Version 2.2023, NCCN clinical practice guidelines in oncology. J. Natl Compr. Canc Netw. 21, 393–422 (2023).
Wang, F. H. et al. The Chinese Society of Clinical Oncology (CSCO): Clinical guidelines for the diagnosis and treatment of gastric cancer, 2023. Cancer Commun. (Lond.) 44, 127–172 (2024).
Lordick, F. et al. Gastric cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 33, 1005–1020 (2022).
van Hagen, P. et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N. Engl. J. Med. 366, 2074–2084 (2012).
Faron, M. et al. Individual participant data network meta-analysis of neoadjuvant chemotherapy or chemoradiotherapy in esophageal or gastroesophageal junction carcinoma. J. Clin. Oncol. 41, 4535–4547 (2023).
Al-Batran, S. E. et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet 393, 1948–1957 (2019).
Zhang, X. et al. Perioperative or postoperative adjuvant oxaliplatin with S-1 versus adjuvant oxaliplatin with capecitabine in patients with locally advanced gastric or gastro-oesophageal junction adenocarcinoma undergoing D2 gastrectomy (RESOLVE): an open-label, superiority and non-inferiority, phase 3 randomised controlled trial. Lancet Oncol. 22, 1081–1092 (2021).
Japanese Gastric Cancer, A. Japanese Gastric Cancer Treatment Guidelines 2021 (6th edition). Gastric Cancer, 1-25 (2023).
Guideline Committee of the Korean Gastric Cancer Association, D. W. G. & Review, P. Korean Practice Guideline for Gastric Cancer 2018: an Evidence-based, Multi-disciplinary Approach. J Gastric Cancer 19, 1–48 (2019).
Sasako, M. et al. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J. Clin. Oncol. 29, 4387–4393 (2011).
Noh, S. H. et al. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol. 15, 1389–1396 (2014).
Leong, T. et al. Preoperative chemoradiotherapy for resectable gastric cancer. N. Engl. J. Med. 391, 1810–1821 (2024).
Janjigian, Y. Y. et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet 398, 27–40 (2021).
Kang, Y. K. et al. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): a randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 23, 234–247 (2022).
Rha, S. Y. et al. Pembrolizumab (pembro) plus chemotherapy (chemo) as first-line therapy for advanced HER2-negative gastric or gastroesophageal junction (G/GEJ) cancer: Phase III KEYNOTE-859 study. Ann. Oncol. 34, 319–320 (2023).
Moehler, M. H. et al. Phase 3 study of tislelizumab plus chemotherapy vs placebo plus chemotherapy as first-line treatment (1L) of advanced gastric or gastroesophageal junction adenocarcinoma (GC/GEJC). J. Clin. Oncol. 41, 286–286 (2023).
Rha, S. Y. et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for HER2-negative advanced gastric cancer (KEYNOTE-859): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 24, 1181–1195 (2023).
Xu, J. et al. Sintilimab plus chemotherapy for unresectable gastric or gastroesophageal junction cancer: the ORIENT-16 randomized clinical trial. JAMA 330, 2064–2074 (2023).
Lorenzen, S. et al. Perioperative atezolizumab plus fluorouracil, leucovorin, oxaliplatin, and docetaxel for resectable esophagogastric cancer: interim results from the randomized, multicenter, phase II/III DANTE/IKF-s633 Trial. J. Clin. Oncol. 42, 410–420 (2024).
Yin, Y. et al. Neoadjuvant tislelizumab and tegafur/gimeracil/octeracil (S-1) plus oxaliplatin in patients with locally advanced gastric or gastroesophageal junction cancer: Early results of a phase 2, single-arm trial. Front Oncol. 12, 959295 (2022).
Yuan, S. Q. et al. Perioperative toripalimab and chemotherapy in locally advanced gastric or gastro-esophageal junction cancer: a randomized phase 2 trial. Nat. Med 30, 552–559 (2024).
Shitara, K. et al. Neoadjuvant and adjuvant pembrolizumab plus chemotherapy in locally advanced gastric or gastro-oesophageal cancer (KEYNOTE-585): an interim analysis of the multicentre, double-blind, randomised phase 3 study. Lancet Oncol. 25, 212–224 (2024).
Janjigian, Y. Y. et al. Pathological complete response (pCR) to durvalumab plus 5-fluorouracil, leucovorin, oxaliplatin and docetaxel (FLOT) in resectable gastric and gastroesophageal junction cancer (GC/GEJC): Interim results of the global, phase III MATTERHORN study. Ann. Oncol. 34, S1315–S1316 (2023).
André, T. et al. Neoadjuvant nivolumab plus ipilimumab and adjuvant nivolumab in localized deficient mismatch repair/microsatellite instability-high gastric or esophagogastric junction adenocarcinoma: The GERCOR NEONIPIGA Phase II Study. J. Clin. Oncol. 41, 255–265 (2023).
Doroshow, D. B. et al. PD-L1 as a biomarker of response to immune-checkpoint inhibitors. Nat. Rev. Clin. Oncol. 18, 345–362 (2021).
Wang, Y. et al. Challenges coexist with opportunities: spatial heterogeneity expression of PD-L1 in cancer therapy. Adv. Sci. (Weinh.) 11, e2303175 (2024).
Kim, R. et al. Early tumor-immune microenvironmental remodeling and response to first-line fluoropyrimidine and platinum chemotherapy in advanced gastric cancer. Cancer Discov. 12, 984–1001 (2022).
Rosenberg, H. F., Dyer, K. D. & Foster, P. S. Eosinophils: changing perspectives in health and disease. Nat. Rev. Immunol. 13, 9–22 (2013).
Blomberg, O. S. et al. IL-5-producing CD4(+) T cells and eosinophils cooperate to enhance response to immune checkpoint blockade in breast cancer. Cancer Cell 41, 106–123.e10 (2023).
Nie, R. et al. Predictive value of radiological response, pathological response and relapse-free survival for overall survival in neoadjuvant immunotherapy trials: pooled analysis of 29 clinical trials. Eur. J. Cancer 186, 211–221 (2023).
Siewert, J. R. & Stein, H. J. Classification of adenocarcinoma of the oesophagogastric junction. Br. J. Surg. 85, 1457–1459 (1998).
Hellmann, M. D. et al. Pathological response after neoadjuvant chemotherapy in resectable non-small-cell lung cancers: proposal for the use of major pathological response as a surrogate endpoint. Lancet Oncol. 15, e42–e50 (2014).
Hänzelmann, S., Castelo, R. & Guinney, J. GSVA: gene set variation analysis for microarray and RNA-seq data. BMC Bioinforma. 14, 7 (2013).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 102, 15545–15550 (2005).
Charoentong, P. et al. Pan-cancer immunogenomic analyses reveal genotype-immunophenotype relationships and predictors of response to checkpoint blockade. Cell Rep. 18, 248–262 (2017).
Ge, X. et al. Comparison of preoperative concurrent chemoradiotherapy with chemotherapy alone in patients with locally advanced siewert II and III adenocarcinoma of the esophagogastric junction. Eur. J. Surg. Oncol. 44, 502–508 (2018).
Acknowledgements
We thank the patients who participated in this study and their families. We also thank the Prof. Ji-Bin Li for the statistical consultation and data analysis. This research was supported by the Noncommunicable Chronic Diseases-National Science and Technology Major Project (2023ZD0501500 to SQY), National Natural Science Foundation of China (82103586, to RCN; 82473358 to SQY), Beijing Xisike Clinical Oncology Research Foundation (Y-HR2022MS-0324, to SQY; Y-2023AZMETQN-0017to RCN; Y-2022METAZMS-0123to YFL), and the Guangdong Esophageal Cancer Institute Science and Technology Program (M202210). The funders played no role in the study’s design, conduct, or reporting.
Author information
Authors and Affiliations
Contributions
Y.-B.C., X.-S.Z., and R.-C.N. contributed to conception and design. S.-Q.Y., Y.D., Y.-M.C., Y.-F.L., C.-C.L., M.-Y.C., W.W., X.-W.S., D.-S.W., D.-D.L., J.-J.Z., X.-J.C., Y.-X.G., Z.-M.L., Y.L., Z.-W.Z., H.-B.Q., and collected. R.-C.N., M.L., J.C., and G.-M.C. performed statistical analysis and interpreted data. S.-Q.Y., Y.D., Y.-M.C., R.-C.N., and Y.-F.L. drafted the manuscript. All authors have read and approved the article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nie, RC., Yuan, SQ., Ding, Y. et al. Perioperative tislelizumab plus chemotherapy for locally advanced gastroesophageal junction adenocarcinoma (NEOSUMMIT-03): a prospective, nonrandomized, open-label, phase 2 trial. Sig Transduct Target Ther 10, 60 (2025). https://doi.org/10.1038/s41392-025-02160-8
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41392-025-02160-8