Abstract
Immunosenescence refers to the abnormal activation or dysfunction of the immune system as people age. Inflammaging is a typical pathological inflammatory state associated with immunosenescence and is characterized by excessive expression of proinflammatory cytokines in aged immune cells. Chronic inflammation contributes to a variety of age-related diseases, such as neurodegenerative disease, cancer, infectious disease, and autoimmune diseases. Although not fully understood, recent studies contribute greatly to uncovering the underlying mechanisms of immunosenescence at the molecular and cellular levels. Immunosenescence is associated with dysregulated signaling pathways (e.g., overactivation of the NF-κB signaling pathway and downregulation of the melatonin signaling pathway) and abnormal immune cell responses with functional alterations and phenotypic shifts. These advances remarkably promote the development of countermeasures against immunosenescence for the treatment of age-related diseases. Some anti-immunosenescence treatments have already shown promising results in clinical trials. In this review, we discuss the molecular and cellular mechanisms of immunosenescence and summarize the critical role of immunosenescence in the pathogenesis of age-related diseases. Potential interventions to mitigate immunosenescence, including reshaping immune organs, targeting different immune cells or signaling pathways, and nutritional and lifestyle interventions, are summarized. Some treatment strategies have already launched into clinical trials. This study aims to provide a systematic and comprehensive introduction to the basic and clinical research progress of immunosenescence, thus accelerating research on immunosenescence in related diseases and promoting the development of targeted therapy.
Similar content being viewed by others
Introduction
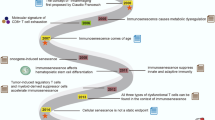
Immunosenescence, a concept proposed by Roy Walford in the 1960s, refers to the progressive remodeling and decline of immune function with aging. It is characterized by a diminished ability to respond to pathogens, reduced vaccine efficacy, and an increased risk of age-related diseases.1 This phenomenon encompasses both innate and adaptive immune dysregulation, which is characterized by thymic involution, chronic low-grade inflammation (“inflammaging”), and the accumulation of senescent cells (SnCs).1,2 Importantly, cellular senescence represents a stress response that induces irreversible cell cycle arrest, serving as a hallmark of aging.3,4 The pathological accumulation of SnCs not only amplifies age-related immunological alterations but also constitutes a key pathogenic driver of multiple age-associated comorbidities.5,6 Immune cells are pivotal modulators of cellular senescence.5,7 In this review, we focus on these immune populations, detailing how aging compromises their differentiation and functional capacity, while their dysregulation reciprocally accelerates the senescence process. Among these immune cell populations, T cells undoubtedly hold a predominant position. Thymic involution, impaired homeostatic proliferation of naïve T cells, and lifelong antigenic exposure collectively contribute to T cell senescence.8 This process drives the contraction of the naïve T cell compartment and expansion of the memory T cell pool, ultimately leading to a reduced diversity of the available T cell receptor (TCR) repertoire.9 Immunosenescence is also regulated by signaling pathways such as the nuclear factor-kappa B (NF-κB), mTOR, JAK-STAT, melatonin, and sirtuin pathways, whose dysregulation leads to aberrant immune responses and increased susceptibility to age-related diseases. Targeting these pathways may help mitigate immune decline in aging individuals.
Immunosenescence, characterized by dysregulation of signaling pathways and altered senescence/regulatory capacity across immune cell populations, may predispose individuals to diverse age-associated pathologies. These include neurodegenerative disorders, increased cancer incidence in geriatric populations, diminished efficacy of cancer immunotherapies, and heightened susceptibility to infectious and cardiovascular diseases. These diseases often present as multimorbidities, potentially leading to organ failure and death. Understanding the pathogenesis of these diseases and the driving factors behind their progression and deterioration can enhance our knowledge of immune system changes during immunosenescence. This insight can then guide the development of multifaceted strategies targeting immune organs, cells, and signaling pathways to restore immune competence, eliminate SnCs, and delay age-related dysfunction. Novel antiaging therapies targeting specific immune dysfunctions in elderly individuals are being proposed at an increasingly rapid pace, with some already entering clinical trials and demonstrating promising efficacy. This article systematically elaborates on various therapeutic interventions for immunosenescence.
In this review, we provide a systematic and comprehensive introduction to immunosenescence at three different levels: molecular, cellular and disease. More importantly, we provide a detailed summary of current strategies for targeting immunosenescence, ranging from targeted therapy to immunomodulation and lifestyle interventions. A concise summary of ongoing clinical trials targeting immune cells against immunosenescence is highlighted. By integrating these multifaceted strategies, this review not only addresses critical gaps in previous therapeutic frameworks but also highlights recent advancements and breakthroughs in fighting immunosenescence. The ultimate goal is to inspire future research to overcome existing research limitations and develop novel preventive and therapeutic approaches, thereby offering actionable solutions to mitigate aging-related diseases and extend the healthspan of the elderly population.
Signaling pathways in immunosenescence
Aberrant activation of various signaling pathways, including but not limited to the NF-κB, mTOR, JAK-STAT, cGAS (cyclic GMP-AMP synthase)-STING (stimulator of interferon genes), AMPK (AMP-activated protein kinase), melatonin, and sirtuin pathways, plays a crucial role in regulating immune function during aging. These pathways form a regulatory network to modulate immunosenescence (Fig. 1). Dysregulation of these pathways leads to impaired immune responses and increased susceptibility to age-related diseases. Understanding the intricate mechanisms by which these pathways influence immunosenescence is essential for developing targeted interventions to enhance immune function in elderly individuals.
Signaling pathways associated with immunosenescence. Immunosenescence is associated with aberrant activation of various signaling pathways, such as upregulation of the NF-κB, mTOR, JAK-STAT, and cGAS-STING signaling pathways and downregulation of the AMPK, melatonin, and sirtuin pathways. a Accumulation of endogenous DNA damage and oxidative stress cause overactivation of NF-κB signaling, which transcriptionally activates the mechanistic target of mTOR and upregulates antiapoptotic proteins, thus impairing the induction of autophagy and apoptotic clearance of SnCs. b mTOR functions through two distinct complexes: mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2). mTORC1 integrates signals from nutrients and growth factors to regulate various anabolic processes while inhibiting catabolic processes by phosphorylating ULK1/2 and sequestering lysosomal enzymes. mTORC2 regulates cytoskeletal organization and cell survival pathways through the activation of the AKT and SGK1-Foxo1 axes and the inhibition of GSK3β. c Overactivation of the JAK-STAT signaling pathway during aging contributes to immunosenescence by driving persistent inflammation and altering immune cell function and survival, including T cells and HSCs. d Accumulation of damaged DNA in aging cells activates the cGAS/STING pathway, which further induces NF-κB-dependent expression of inflammatory cytokines with impaired IFN-I production. e AMPK plays a crucial role in the regulation of cellular energy metabolism. It extends lifespan by promoting autophagy via mTOR inhibition and ULK1 activation. The activation of AMPK broadly suppresses proinflammatory signaling pathways, which inhibits the expansion and function of MDSCs and promotes the survival and memory formation of T cells. f Melatonin suppresses proinflammatory cytokines and enhances anti-inflammatory cytokines by inhibiting the NF-κB pathway. It directly scavenges ROS and upregulates the expression of antioxidant enzymes such as superoxide dismutase (SOD) and glutathione peroxidase (GPX), reducing oxidative damage and SASP accumulation in immune cells. Melatonin also mediates SIRT1 pathway activation, which optimizes mitochondrial function and autophagy. g Sirtuin family proteins play crucial roles in immune aging by regulating mitochondrial function, oxidative stress, and NF-κB signaling
Upregulated signaling pathways
NF-κB signaling pathway
NF-κB is a transcription factor that can be activated during cellular damage and stress. The activity of NF-κB increases with aging and aging-related diseases due to the accumulation of endogenous DNA damage and oxidative stress. In aged mice, NF-κB activation has been observed in a variety of cell types.10 Genetic depletion or pharmacological inhibition of NF-κB decreases oxidative DNA damage and stress in aged mice, leading to delayed cellular senescence and age-related symptoms and pathologies.10 Persistent NF-κB activation drives inflammaging, which impairs immune surveillance, reduces T cell diversity, and promotes tissue degeneration.11 Reactive oxygen species (ROS) accumulation during aging can activate NF-κB via IκBα phosphorylation or IKK modulation at critical cysteine residues, impairing its DNA-binding capacity and disrupting redox homeostasis.10,12,13 Furthermore, NF-κB suppresses autophagy by transcriptionally activating the mechanistic target of mTOR, a key inhibitor of autophagic flux.14 Impaired autophagy in aged immune cells leads to the accumulation of damaged mitochondria and protein aggregates, exacerbating oxidative stress and inflammasome activation.15 Additionally, NF-κB enhances the survival of dysfunctional immune cells by upregulating antiapoptotic proteins, preventing the clearance of SnCs.16 This apoptotic resistance contributes to reduced immune diversity and increased cancer risk during aging. These findings underscore NF-κB as a central driver of immune aging, linking oxidative stress, inflammaging, autophagy suppression, and immune cell survival dysregulation.
mTOR signaling pathway
The mTOR signaling pathway serves as a central regulator of cell survival and growth and cell cycle progression.17 mTOR functions through two distinct complexes, mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2), each of which play unique roles in cellular metabolism and immune function.18 mTORC1 integrates signals from nutrients and growth factors to regulate various anabolic processes, including protein synthesis, nucleotide production, lipid biosynthesis, and glycolysis, while inhibiting catabolic processes such as autophagy by phosphorylating ULK1/2 and sequestering lysosomal enzymes.19,20,21,22,23 The activation of mTORC1 has long been associated with cellular senescence24. However, in senescent CD8⁺ T cells, autophagy is not restored despite reduced mTORC1 activity, partly due to alternative inhibitory mechanisms such as chronic p38 MAPK activation, which impairs autophagy independently of mTORC1 and contributes to immune decline in senescent CD8⁺ T cells.23,25 Additionally, cytoplasmic p53 inhibits autophagy via mTOR activation under basal conditions, whereas nuclear p53 promotes autophagy through mTOR-independent mechanisms under stress.26,27 In addition to its role in autophagy regulation, mTORC1 also plays an important role in T cell function. In aged mice, in vitro inhibition of mTORC1 with metformin or everolimus increased IL-2 production and T cell proliferation and reduced oxidative stress in CD4⁺ T cells28. Moreover, in elderly humans, low-dose mTORC1 inhibition with RAD001 and BEZ235 reduced infection rates over 12 months, indicating an enhancement of immune function.29
mTORC2 has been increasingly recognized for its role in immune aging. In aged murine CD4⁺ T cells, increased mTORC2 signaling is associated with impaired TCR responsiveness and reduced proliferative capacity. These functional defects are related to mTORC2-mediated dysregulation of cytoskeletal organization (including actin polymerization) and cell survival pathways.30 In vivo studies in lymphocytic choriomeningitis virus-infected mice have shown that mTORC2 prevents ferroptosis in virus-specific memory CD4⁺ T cells by limiting lipid peroxidation and mitochondrial ROS accumulation, primarily through activation of AKT and inhibition of GSK3β, thereby supporting their long-term survival.31 Moreover, mTORC2 regulates CD8⁺ T cell differentiation via the SGK1-Foxo1 axis. The inhibition or genetic deletion of SGK1, a downstream effector of mTORC2, promotes the formation of memory precursors of CD8⁺ T cells and enhances their long-term survival.32
JAK-STAT signaling pathway
The JAK-STAT signaling pathway is fundamental to immune regulation and plays key roles in infection defense, immune tolerance, and tumor surveillance. During aging, the JAK-STAT signaling pathway is dysregulated, contributing to immunosenescence by driving persistent inflammation and altering immune cell function. JAK-STAT pathway alterations impair immune homeostasis by affecting immune cell development and function. Hyperactivation of STAT3 enhances the production of proinflammatory cytokines, including interleukin (IL)-6 and IL-23, promoting the senescence-associated secretory phenotype (SASP) and sustaining inflammatory signaling.33 JAK1/2 activation further amplifies the SASP, accelerating immune aging. Additionally, JAK3 and STAT5B mutations impair Foxp3 expression, disrupting regulatory T cell (Treg)-mediated immune tolerance.34 JAK3 mutations result in defective T and natural killer (NK) cell maturation, weakening immune responses against infections and tumors. Overactivation of STAT3 skews the immune balance by promoting T helper 17 (Th17) cell expansion while suppressing Treg function. Moreover, excessive JAK-STAT activation disrupts hematopoietic stem cell (HSC) differentiation, favoring myeloid rather than lymphoid lineage commitment, a hallmark of immune system aging.35
cGAS-STING
The cGAS-STING pathway senses cytosolic DNA under certain conditions, such as DNA damage, mitochondrial dysfunction, or nuclear envelope disruption. The accumulation of damaged DNA in the cytoplasm, which serves as a damage-associated molecular pattern (DAMP) to be recognized by DNA sensors, including the cGAS-STING pathway, is observed in aging cells.36 Through the cGAMP–STING–TBK/IKK axis, it induces NF-κB-dependent expression of inflammatory cytokines such as IL-6 and CXCL10, promoting the SASP.37 Notably, aging immune cells exhibit impaired secretion of type I interferons (IFN-I), which are downstream effector molecules of cGAS-STING signaling. For example, aging plasmacytoid dendritic cells (pDCs) exhibit limited production of IFN-I due to impaired IRF7 phosphorylation, resulting in decreased presentation of antigens to T lymphocytes.38
Downregulated signaling pathways
AMPK signaling pathway
AMPK is a pivotal serine/threonine protein kinase that plays an extensive role in the regulation of cellular energy metabolism39. It is critically involved in maintaining cellular homeostasis, mitigating oxidative stress, promoting cell survival and growth, and modulating cell death and autophagy40. As a central energy sensor, AMPK extends lifespan by promoting autophagy via mTOR inhibition and ULK1 activation, enhancing mitochondrial function through PGC-1α/SIRT1 signaling and improving the NAD+/NADH balance, suppressing inflammatory responses via NF-κB inhibition, and modulating stress resistance via the FOXO3/p53 pathways, thereby linking metabolic regulation to aging suppression41. The activation of AMPK broadly suppresses proinflammatory signaling pathways, including the JAK-STAT, NF-κB, C/EBPβ, CHOP, and HIF-1α pathways, which in turn inhibits the expansion and immunosuppressive function of myeloid-derived suppressor cells (MDSCs)42. Moreover, AMPKα1 is essential for CD8⁺ T cell memory formation because it senses glucose deprivation and suppresses mTORC1 activity, as AMPKα1-deficient CD8⁺ T cells fail to survive metabolic stress during immune contraction and exhibit impaired secondary responses43. In highly differentiated human CD4⁺ T cells (the CD27⁻CD28⁻ subset), AMPK activation under metabolic stress or DNA damage recruits TAB1 to induce p38 autophosphorylation, leading to telomerase suppression and proliferative arrest44. Given its regulatory effects on immune signaling and inflammation, AMPK may play a role in modulating immunosenescence, although further studies are needed to elucidate this potential connection.
Melatonin signaling pathway
Melatonin, a hormone produced by the pineal gland that plays a pivotal role in regulating circadian rhythms, significantly decreases in level as age progresses, manifesting as a deterioration of circadian rhythmicity.45 Melatonin counteracts immunosenescence via a multitarget regulatory network spanning cytokine balance, oxidative stress defense, immune cell functional restoration, signaling pathway crosstalk, disease-specific interventions, and circadian rhythm integration. Specifically, melatonin suppresses proinflammatory cytokines (e.g., IL-1β, IL-6, tumor necrosis factor-α (TNF-α), and interferon-γ (IFN-γ)) and enhances anti-inflammatory cytokines (e.g., IL-4 and IL-10) by inhibiting the NF-κB pathway, although its low-dose transient proinflammatory effects highlight dose- and pathology-dependent dynamics.46,47 It directly scavenges ROS and upregulates the expression of antioxidant enzymes such as superoxide dismutase (SOD) and glutathione peroxidase (GPX), reducing oxidative damage and SASP accumulation in immune cells.46,47,48 Through an “antioxidant cascade”, melatonin metabolites such as N1-acetyl-N2-formyl-5-methoxykynuramine (AFMK) and N1-acetyl-5-methoxykynuramine (AMK) further neutralize free radicals, protecting mitochondrial integrity, proteins, and DNA from oxidative destruction. At the immune cell level, melatonin enhances CD4+/CD8+ T cell proliferation and antigen responsiveness, modulates the Treg/Th1/Th2 balance, promotes macrophage polarization toward the anti-inflammatory M2 phenotype, and augments NK cell cytotoxicity.46,47 These effects are mediated by SIRT1 pathway activation, which optimizes mitochondrial function and autophagy, and by miRNA-dependent regulation (e.g., miR-146a targeting Nrf2/NF-κB pathways), although the mechanisms vary across cell types and microenvironments.46,47 Epigenetically, melatonin may inhibit histone deacetylases (HDACs) and modulate miRNA expression to reverse age-associated proinflammatory gene silencing in senescent immune cells, restoring functional competence.49 As a core circadian regulator, melatonin stabilizes clock genes (e.g., CLOCK protein), reduces cortisol-mediated immunosuppression, and coordinates rhythmic immune cell activities (e.g., diurnal fluctuations in macrophage phagocytosis), systemically delaying immunosenescence.46,49
Sirtuin signaling pathway
Sirtuin family proteins play crucial roles in immune aging by regulating mitochondrial function, oxidative stress, and NF-κB signaling, thereby maintaining immune homeostasis across various immune cell types. In HSCs, sirtuin 3 (SIRT3) preserves genomic stability and mitochondrial integrity, delaying cellular aging by increasing superoxide dismutase 2 (SOD2) activity and reducing oxidative stress.50 Restoring NAD+ levels can further reactivate SIRT3, improving stem cell reprogramming and lifespan extension.51 Within the innate immune system, SIRT1 and SIRT6 mitigate inflammatory responses in macrophages by inhibiting NF-κB signaling and suppressing excessive TNF-α and IL-1β expression, promoting endotoxin tolerance.52 In dendritic cells (DCs), SIRT1 modulates autophagy and cytokine secretion, enhancing antiviral responses while preventing excessive Th2/Th17-mediated inflammatory reactions.53 In NK cells, SIRT2 and SIRT6 contribute to exhaustion in colorectal cancer, suppressing NK cytotoxicity by downregulating glycolysis and mitochondrial respiration. Silencing these proteins restores the antitumor function of NK cells.54,55 Within adaptive immunity, SIRT1 is essential for T cell activation and peripheral tolerance, preventing autoimmune diseases by suppressing AP-1 transcription and IL-2 production in the absence of CD28 costimulation.56 Furthermore, both SIRT1 and SIRT7 regulate B cell class-switch recombination (CSR), influencing immunoglobulin (Ig) maturation.57 Overall, the sirtuin family acts as a critical regulator of immune aging by fine-tuning metabolic and inflammatory pathways in multiple immune cell types. Targeting these proteins may provide novel therapeutic strategies to mitigate immune decline and promote healthier aging.
Cellular mechanisms of immunosenescence
The aberrant signaling pathways during aging result in the dysfunction of immune cells, which interferes with almost all kinds of immune cells, ranging from HSCs to mature immune cells. Senescent immune cells under excessive loading contribute to age-related pathological changes, which can progress to age-related diseases. The proportion of HSCs, the progenitor cells of immune cells, is significantly increased in elderly individuals. Despite an increased tendency for self-renewal, their overall regenerative capacity declines due to impaired differentiation potential and functional deterioration. This is characterized by skewed hematopoietic output, accumulation of replication stress, and reduced adaptability to transplantation or hematopoietic challenges.58,59 Aged HSCs exhibit a myeloid-biased differentiation tendency, leading to a reduced generation of lymphoid lineage cells (T and B cells) and a decline in adaptive immune function (Fig. 2).60,61 Moreover, aging HSCs displayed impaired function, including reduced blood production and impaired engraftment after transplantation. Replication stress is a key driver of functional decline in aged HSCs, which is due to reduced expression of mini-chromosome maintenance genes and impaired DNA replication dynamics.59
Cellular mechanisms of immunosenescence. Aging-induced alterations in various immune cell populations are depicted with young cells in the top row and aged cells in the bottom row. (1) HSC: Aging increases the number of HSCs but weakens their function. SNS degeneration decreases ADRβ3 signaling, generating an inflammatory niche. Myeloid-biased differentiation reduces lymphoid output, weakening adaptive immunity. (2) Neutrophils: Aged neutrophils exhibit prolonged lifespan, hypersegmentation, and impaired chemotaxis but enhanced CXCL1-driven recruitment. Elevated NET formation, ROS, and TNF-α production promote chronic inflammation. (3) Macrophages and Monocytes: Aging increases the proportion of CD14⁺CD16⁺ monocytes, which exhibit a proinflammatory phenotype with increased TNF-α and IL-6 production. Reduced phagocytosis causes debris accumulation and chronic inflammation. Elevated ROS, NO, and β2M contribute to metabolic diseases and cognitive decline. (4) T cells: Aging reduces the levels of IL-7 and chemokines, impairing naïve T cell survival, proliferation, and lymph node entry and limiting renewal. Aging decreases CD8⁺ T cell diversity and number. CD160 and CD244 expression increases, resembling an exhausted phenotype. Aged CD8⁺ T cells show reduced cytotoxicity and produce less IFN-γ, granzyme B, and perforin. CD4⁺ T cell activation decreases in part due to elevated PD-1 expression. (5) Aged DCs have weaker antigen presentation (MHC/CD40 downregulation), resulting in weaker CD4⁺ T cell responses. (6) NK cells: Aging reduces the number of CD56bright NK cells and their activating receptors while increasing the number of inhibitory receptors (KIRs), impairing cytotoxicity. Degranulation and perforin secretion decline. NK cells shift toward a CD56dim subset, where they secrete more proinflammatory cytokines, contributing to chronic inflammation. (7) B cells: In elderly individuals, antibody production and class switching decline due to CD40 downregulation and weakened BCR signaling. ABC expansion disrupts immune balance, weakening humoral immunity
HSC aging can be induced by various mechanisms. Markus’s team reported that the myeloid-biased output of HSCs was mediated by interleukin-1 (IL-1) signaling in a mouse model.62 Knocking out IL-1 receptor 1 (IL-1R1) or pharmacologic inhibition of IL-1 signaling in older mice reversed the myeloid-biased hematopoietic output. Degeneration of the sympathetic nervous system (SNS) in the bone marrow microenvironment is another contributor to HSC aging. Interfering with the SNS nerves of adrenoreceptor β3 (ADRβ3) signaling resulted in premature HSC aging in young mice, whereas stimulating ADRβ3 in older mice rejuvenated the functions of HSCs.63 Zinc finger proteins in aged murine HSCs contribute to increased platelet bias and sustained myeloid HSC bias while suppressing lymphoid lineage output.64 Moreover, epigenetic changes in aged HSCs impair differentiation, including disrupted DNA methylation and histone modifications.58 The decrease in autophagy in aged HSCs leads to the accumulation of active and healthy mitochondria and increased metabolism, particularly increased oxidative phosphorylation, promoting accelerated myeloid differentiation of HSCs.65
Immunosenescence and aging-related dysregulation of myeloid lineage cells
Myeloid lineage cells are composed of various innate immune cells, such as neutrophils, macrophages, and monocytes. The myeloid-biased differentiation of aged HSCs leads to increased production of myeloid immune cells. In addition, mature myeloid immune cells display altered phenotypes and functions with elevated production of proinflammatory cytokines such as TNF-α and IL-6, which are closely related to age-related senescent inflammation, termed senoinflammation.66,67,68,69 Normally, neutrophils are short-lived innate immune cells. During aging, neutrophils display an extended lifespan and abnormal phenotypic features such as hypersegmentation in secondary lymphoid organs and bone marrow.70,71 Under inflammatory conditions, aging neutrophils can exhibit alterations in their functional state with increased integrin activation and the formation of neutrophil extracellular traps (NETs).72 Aged neutrophils remain active under lipopolysaccharide (LPS) stimulation, releasing many cytokines.73 Dysregulated activation of aging neutrophils may be associated with compromised calcium signaling pathways and increased metabolic byproducts (e.g., spontaneous ROS production and increased NAD+ levels).74,75 Notably, aging can also cause dysregulation of neutrophil recruitment in response to aberrant chemokine signaling and inflammatory responses. During influenza infection in mice, increased neutrophil recruitment to aging lungs or livers was observed upon C-X-C motif chemokine ligand 1 (CXCL1) stimulation, resulting in devastating inflammation and increased mortality.76,77 The upregulation of junctional adhesion molecule-C also promotes the accumulation of neutrophils in the lungs, leading to acute lung injury.78 In contrast, neutrophil depletion limits the secretion of neutrophil-activating cytokines and reduces mortality and long-term functional benefits in an ischemic stroke model in aged mice.79
Aging alters the phenotype and function of monocytes, increasing the proportions of nonclassical monocytes and intermediate monocytes.67,68,69 The proportion of the CD14+CD16+ subset of monocytes was elevated with downregulated expression of CX3CR1 and HLA-DRA during aging, whereas the CD14+CD16- subset was decreased.69,80 During aging, the level of β2-microglobulin (a component of major histocompatibility complex (MHC)-I) in plasma increases, leading to a proinflammatory phenotype in monocytes, cognitive decline, and regenerative impairments in the adult brain.81,82 Aging also promotes the proinflammatory polarization of monocytes by increasing the plasma saturated fatty acid concentration, thereby increasing the production of IL-6 and TNF-α but inhibiting the production of IL-10 and transforming growth factor-β (TGF-β1).83 Overproduction of IL-6 and TNF-α in the peripheral blood of aging monocytes may be related to human Toll-like receptor 2/6 (TLR2/6) signaling instead of TLR1/2 signaling.84 Epigenetic alterations such as histone modifications (e.g., H3K9me3 loss) contribute to the upregulation of inflammatory genes and an imbalance in macrophage polarization, further reinforcing systemic inflammation.85 Moreover, the phagocytic activity of macrophages and monocytes significantly decreases during aging, leading to the accumulation of unphagocytosed debris, chronic sterile inflammation, and the exacerbation of tissue aging and damage.86
Immunosenescence and aging-related dysregulation of lymphoid lineage cells
Lymphoid cells are composed of innate (such as NK cells) and adaptive immune cells (such as T cells and B cells). T and B cells can be further divided into a number of different subtypes. In general, aging is related to reduced production and impaired function of adaptive immune cells but elevated memory cell expansion, including both T and B cells.87,88,89,90,91 Thymic involution is a core characteristic of immunosenescence and is characterized by gradual shrinkage of the thymus and a significant reduction in thymic epithelial tissue, leading to a pronounced decline in the production of T cells, particularly naïve T cells.87,88,89 As the thymus shrinks, TCR diversity also decreases, impairing the ability of the immune system to respond effectively to novel pathogens92. In naïve T cells, aging disrupts their ability to enter and interact with survival factors, impairing their proliferation and function.93 Animal studies have indicated that age-related disruption of naïve T cell survival and homeostasis depends on alterations in the secondary lymphoid environment and IL-7 (a maintenance factor) signaling.94,95 Defects in the function of stromal cells in the secondary lymphoid organs of aged individuals play crucial roles in immune cell migration, activation, and survival of naïve T cells.96
A functional decline was observed in aging T and B cells. Unlike anergy and exhaustion, T cell senescence is an irreversible process.97,98 Senescent T cells are characterized by a phenotypic shift with the downregulation of the costimulatory molecules CD27 and CD28 and the upregulation of the killer cell lectin-like receptor subfamily G and CD57.99 Moreover, aging induces the expression of immune checkpoint-related molecules such as lymphocyte-activation gene 3 (LAG-3), programmed death protein 1 (PD-1), and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4).100,101 This is coupled with the upregulation of the cell cycle regulators P16, P21, and P53, leading to cell cycle arrest and diminished proliferative capacity.102 Compared with CD44lowCD8+ T cells, CD8+ T cells in aging individuals predominantly express high levels of CD44, which are of low quality.101 CD44highCD8+ T cells in aged mice presented similar transcriptional properties to exhausted CD8+ T cells during chronic viral infection and highly expressed inhibitory molecules, including CD160, CD244, LAG-3, and PD-1.101 Additionally, senescent T cells show reduced cytotoxicity with decreased production of functional immune molecules, including IFN-γ, granzyme B, and perforin.98,100,103 Compared with those of CD8+ T cells, the diversity and output of CD4+ T cells are more stable, although some studies have indicated the accumulation of CD4+ T cells expressing PD-1 and Tregs (CD4+CD25+Foxp3+) during aging.104,105,106,107 Notably, in Tregs, DNA methylation at the Foxp3 locus plays a crucial role in maintaining their anti-inflammatory function. Age-related alterations in this methylation pattern impair Treg activity, leading to dysregulated immune tolerance and contributing to the proinflammatory milieu associated with aging.108 With respect to B cells, aging B cells exhibit defects in antibody production and immunoglobulin (Ig) class switching.90,91 This is associated with reduced activation of the transcription factor 3/E47 transcription factor in aged B cells.91 Aging also decreases the AID enzyme (activation-induced cytidine deaminase), which is responsible for class-switch recombination and the production of high-affinity antibodies.90 Moreover, aging B cell activation was impaired by the downregulation of CD40 expression, which reduced responsiveness to B cell receptor (BCR) stimulation.109 During viral infection, aging is associated with a decline in B cell frequencies, reduced antibody responses, and weaker protection, ultimately reducing vaccine efficacy in elderly individuals.110
Some studies have indicated that elderly individuals possess a greater number and proportion of memory T cells (especially memory CD8+ T cells) and memory B cells.111,112 Chronic antigen stimulation and age-related inflammation contribute to this shift, accelerating naïve T cell decline while promoting memory T cell dominance.113 Vesna et al. discovered novel human memory CD8+ T cells with a naïve phenotype that accumulate with aging and can rapidly respond to persistent antigens by producing various cytokines.112 Age-related accumulation of memory-phenotype CD8+ T cells partially compensates for the loss of naïve T cells, enhancing responses to previously encountered pathogens.114,115 Age-associated B cells (ABCs), most of which are antigen-experienced memory B cells, are induced upon exposure to microbial infection and play a crucial role in pathogen clearance and control.116,117 TLR signaling is essential for B cell activation and differentiation, particularly for IgM⁺ memory B cells. In vitro studies have shown that TLR7 and TLR9 stimulation can expand IgM⁺ memory and plasma cells, promoting IgM secretion.118
NK cells are widely distributed cytotoxic innate lymphoid cells that can rapidly recognize and kill cancer cells or pathogen-infected cells.119 Aging impairs NK cell-mediated immune responses by reducing the number of mature NK cells and altering their activity. The number and proportion of NK cells gradually increased with age, but the CD56bright mature cell population decreased.120,121 Moreover, aging inhibited the expression of activating receptors but promoted the upregulation of killer cell inhibitory receptors (KIRs) on CD56bright NK cells.122,123 These changes weaken NK cell cytotoxicity to eliminate tumor cells and virus-infected cells.124 Aged NK cells exhibit reduced degranulation and impaired perforin secretion, which may be linked to alterations in Ca²⁺-dependent exocytosis regulated by Munc13-4, a key protein in NK cell cytotoxic granule release.125 At the molecular level, aging leads to the downregulation of key transcription factors such as EOMES and T-bet, hindering NK cell maturation.126,127 Epigenetic changes, such as reduced miR-181a-5p expression, contribute to an immature NK cell phenotype and functional defects.128 Additionally, nonhematopoietic cells, such as bone marrow stromal cells, may disrupt NK cell function through unknown signals.129 Aging also causes upregulation of the CD56dim immature NK subset with increased secretion of proinflammatory cytokines, exacerbating chronic inflammation.121 In conclusion, NK cell aging is characterized by hindered maturation, functional impairment, and a shift toward a proinflammatory phenotype.
DCs are a type of cell of special origin that can be derived from both lymphoid stem cells and myeloid stem cells. DCs play a vital role in activating adaptive immunity as professional antigen-presenting cells. In elderly individuals, the capacity of DCs to phagocytose antigens and migrate is significantly impaired.130 Moreover, DCs in aged mice presented downregulation of MHC and CD40 expression, weakening their ability to release proinflammatory cytokines in response to LPS stimulation and inducing CD4+ T cell immunity.131,132 Aged DCs exhibit dysfunctional mitochondria, with impaired energy production and increased oxidative stress133. Restoring mitochondrial health could increase the antigen-presenting ability of aged DCs.133 Aging-associated upregulation of WNT5A in the hematopoietic system activates the noncanonical WNT/CDC42 pathway, leading to impaired differentiation of plasmacytoid and conventional dendritic cells. Pharmacological inhibition of this pathway may restore DC development and function in aging.134 In aged mice, bone marrow-derived DCs exhibit increased IL-23 production and increased p19 mRNA expression upon TLR activation. This upregulation is associated with chromatin remodeling, which is characterized by di- and tri-methylation of histone H3K4 and preferential binding of c-Rel at the p19 promoter, contributing to age-related inflammatory responses.135 The increased basal activation levels of aged DCs disrupt respiratory epithelial function by altering cytokine and chemokine secretion, contributing to chronic airway inflammation and heightened susceptibility to respiratory infections in elderly individuals.136 In autoimmune diseases, DCs from aged mice exhibit reduced expression of TRIM28, a nuclear protein that silences gene expression. This resulted in increased T cell differentiation toward inflammatory effector cells.137 Therefore, aging has a significant effect on the activation, migration, and functions of DCs.
Effects of microbiome & sex differences on immunosenescence
During the aging process, the composition of the gut microbiota undergoes alterations and is closely associated with the progression of immunosenescence. In aged mice, the levels of anti-inflammatory bacteria such as Faecalibacterium prausnitzii and Bifidobacteria spp. are reduced. In aged individuals, the gut microbiota shifts toward a more Bacteroidetes-dominant structure, along with a decreased Firmicutes/Bacteroidetes (F/B) ratio.138,139 The F/B ratio is critical for the production of short-chain fatty acids (SCFAs), which play essential roles in maintaining intestinal and immune homeostasis. SCFAs, such as butyrate, inhibit HDAC activity and enhance Treg function.140,141 Age-related dysbiosis leads to increased intestinal permeability, allowing proinflammatory microbial products such as LPS to enter the circulation. This results in the upregulation of inflammatory molecules such as IL-6 and TNF-α, which promote chronic inflammation and further enhance the SASP.142 Notably, the gut microbiota of healthy centenarians is enriched with anti-inflammatory taxa such as Akkermansia and Christensenellaceae, which are potential producers of SCFAs.143 In addition, Lactobacillus is more abundant in healthy centenarians and produces the antioxidant L-ascorbic acid, which helps scavenge free radicals and reduce oxidative stress.144 Furthermore, Bifidobacterium longum subsp. longum has been shown to modulate the host immune transcriptome and suppress proinflammatory cytokine expression.145 Therefore, maintaining gut microbial homeostasis and enhancing both anti-inflammatory and antioxidant capacity may represent promising strategies for delaying immune aging and promoting healthy longevity.
Sex differences in immune aging are multifaceted, encompassing immune cell composition, functional responses, genetic background, and hormonal regulation. Females tend to have (1) higher CD4+ T cell levels and CD4/CD8 ratios, (2) a more activated phenotype in circulating monocytes, (3) higher frequencies of B cells, and (4) greater interferon production from pDCs. In contrast, males accumulate more senescent CD8+ T cells, reflecting a sex-biased trajectory of immunosenescence.146,147,148 These sex differences are concurrently influenced by sex hormones, with estrogens enhancing multiple immune parameters in a dose-dependent manner. Low concentrations of estrogens promote Th1 proinflammatory responses, whereas higher levels induce Th2 humoral immunity.149 Moreover, estrogens confer antioxidant advantages by inhibiting ROS-producing enzymes and upregulating antioxidant systems such as SOD and GPX in both rodents and humans.149 Sex chromosomes themselves also shape immune aging. At the molecular level, X-linked immune genes such as Tlr7 escape inactivation in females, promoting stronger antiviral responses, whereas age-related mosaic loss of the Y chromosome in males impairs leukocyte gene regulation.147 Functionally, females produce stronger vaccine-induced antibody responses than males do, underscoring the need for sex-specific immunization strategies for elderly individuals.150 As global aging accelerates, elucidating sex-based immune differences is critical for designing targeted interventions for age-related diseases.
Immunosenescence-related diseases
Neurodegenerative diseases
Aging leads to the establishment of an interdependent relationship between the nervous and immune systems, where changes in one system influence the other (Fig. 3).151 In elderly individuals, inflammaging, along with peripheral immunosenescence, modulates the activity and reactivity of neuronal immune cells. This results in chronic, low-grade inflammation within the central nervous system (CNS), referred to as neuro-inflammaging,151 with biomarkers including C-reactive protein (CRP), IL-6, and TNF-α.152 Preclinical studies have suggested that cytokine-driven glial activation may contribute to memory impairment and cognitive decline.152,153 These cytokines can enter the nervous system from the periphery, with the largest source being autoreactive T cells derived from the atrophied thymus, which strongly contribute to neurodegeneration.106 Immunosenescence and inflammaging together accelerate brain aging, cognitive decline, and memory loss. This interplay between the immune system and nervous system is evident in neurodegenerative diseases such as Alzheimer’s disease (AD) and Parkinson’s disease (PD), fuelling dementia progression.154
Immunosenescence in neurodegenerative diseases. In AD, immunosenescence and inflammaging drive chronic neuroinflammation, fostering neuronal damage and impairing Aβ clearance via dysfunctional microglia. Aβ deposition triggers the uncontrolled activation of microglia and astrocytes. Increased BBB permeability allows Th1/Th17 infiltration and the secretion of proinflammatory cytokines, exacerbating neurodegeneration, whereas Tregs help suppress inflammation and clear Aβ. In PD, misfolded α-synuclein aggregates into Lewy bodies, causing dopaminergic neuron loss in the substantia nigra. Peripheral CD4⁺ T cell infiltration and IL-17 signaling drive neuroinflammation and neuronal apoptosis. Activated microglia amplify this process by fostering a proinflammatory environment, whereas reduced Tregs fail to suppress excessive immune activation
AD is a progressive neurodegenerative disorder that is difficult to detect in its early stages, with cognitive impairment and memory problems manifesting in later stages and worsening over time.155 It is characterized by abnormal extracellular amyloid-beta (Aβ) aggregates, which form diffuse and neuritic plaques, as well as hyperphosphorylated tau aggregates, which form intraneuronal neurofibrillary tangles.156 With age, the failure of this immune barrier makes it more difficult for the innate immune system to respond. Senescent microglia (resident macrophages in the brain) exhibit increased proliferation and proinflammatory cytokine production but a reduced ability to clear Aβ, contributing to its accumulation in the brain.157 Conversely, the deposition of Aβ triggers the uncontrolled activation of microglia and astrocytes, which are ostensibly responsible for the clearance of damaged cells. However, this aberrant activation state precipitates excessive inflammation.158,159 Sustained neuroinflammation fosters mitochondrial dysfunction, neuronal injury, and cell death, which may contribute to the cognitive decline observed in neurodegenerative disorders, particularly memory impairment and, in some cases, language deficits.160,161 Furthermore, Aβ can function as a DAMP and activate the inflammasome via the TLR pathway, leading to the production of inflammatory cytokines such as IL-1β. This mechanism also significantly contributes to the pathogenesis of AD.162 Additionally, the meningeal lymphatic system is crucial for Aβ clearance, and its function is impaired with age, leading to cognitive decline. Enhancing meningeal lymphatic drainage can promote Aβ clearance and improve cognitive function in a mouse model.163 Changes in T cell senescence are also associated with cognitive decline.164 Compared with healthy young or elderly individuals, patients with AD exhibit an expansion of senescent T cells (within both the CD4+ and CD8+ populations) in the peripheral blood.165 CD4+ effector T cells in peripheral blood can differentiate into Th17 and Th1 subsets. Th1 and Th17 cells secrete cytokines that disrupt the tight junctions of the blood‒brain barrier (BBB), allowing inflammatory factors such as TNFα, IL-1β, and IL-6 to enter the brain, thereby accelerating the progression of AD.166,167 Tregs play crucial roles in suppressing neuroinflammation and facilitating the clearance of amyloid plaques. Their anti-inflammatory function not only helps regulate the immune response but also promotes cognitive function. Depletion of Tregs has been shown to improve cognitive performance and enhance the clearance of amyloid plaques in mouse models, highlighting their significant role in AD pathology.168,169 Aging affects B cell function, reducing antibody specificity and memory B cell formation, increasing the susceptibility of elderly individuals to infections and inflammation.170,171 In AD patients, B cells may produce autoantibodies that recognize misfolded Aβ peptides, potentially assisting microglia in clearing plaques.172 However, single-cell analyses revealed that microglia undergo dynamic transcriptional reprogramming into disease-associated microglia with altered phagocytic capacity.173 These observations suggest a possible interplay between B cell-mediated humoral immunity and microglial phagocytic function in AD pathogenesis.
PD, a neurodegenerative disorder closely linked to aging, involves a complex interplay between immunosenescence and neuroinflammation. Pathologically, PD is characterized by the misfolding of α-synuclein, leading to Lewy body formation and dopaminergic neuron loss in the substantia nigra.174,175 Oxidative stress, proteasome dysfunction, and protein aggregation, changes that frequently occur during aging, have been implicated in the pathogenesis of PD.176 While it remains debated whether neuroinflammation initiates or results from neurodegeneration, systemic inflammation is known to amplify CNS pathology. Activated microglia within the brain exacerbate neuronal damage by promoting a proinflammatory environment, further driving neuroinflammation and neurodegeneration in PD.177 Peripheral inflammation is associated with the activation of immune cells, including T cells, macrophages, and monocytes, which can breach the BBB due to their increased permeability in this disease state.178 This allows peripheral immune cells to infiltrate the central nervous system, contributing to neuroinflammation and accelerating neuronal damage.179 In PD, changes in immune cell function, particularly the accumulation of senescent T cells, significantly exacerbate the progression of the disease.180 Peripheral CD4+ T cells infiltrating the brain not only serve as primary mediators of dopamine toxicity but also respond to α-synuclein, promoting neuronal cell death. Additionally, they can influence oxidative stress and mitochondrial dysfunction, ultimately contributing to neurodegeneration.181 Like those in AD patients, the brain tissues of PD patients also contain Th17 cells. The IL-17 secreted by these cells binds to IL-17 receptors (IL-17Rs) expressed in midbrain neurons, inducing neuronal death through the upregulation of NF-κB and downstream signaling pathways, as demonstrated in human iPSC-derived neuronal models.182
Cancer
As individuals age, the risk of cancer increases, partly due to immune senescence, which involves a decline in immune system function and contributes to tumorigenesis (Fig. 4). In the tumor microenvironment, SnCs secrete SASP components.183 The SASP plays a crucial role in mediating the crosstalk between SnCs and their neighboring cells, often exacerbating pathways of cellular damage and leading to the disruption of immune balance.184,185,186 The role of the SASP in tumors can be summarized as follows: (1) It promotes the growth and proliferation of tumor cells. For example, fibroblast growth factor 10 (FGF10) secreted by senescent mesenchymal cells induces multifocal prostate cancer,187 and the expression of fibroblast growth factor 19 by skeletal muscle cells can lead to hepatocellular carcinoma.188 (2) It contributes to tumor invasion and metastasis. By remodeling the epithelial-mesenchymal transition (EMT), the SASP provides a conducive environment for tumor cell dissemination.183,189,190 Senescent fibroblasts secrete matrix metalloproteinase 3, which affects the morphological and functional differentiation of mammary epithelial cells, ultimately relaxing restrictions on cell migration/invasion.191 SnCs secrete a plethora of proangiogenic factors, thereby supporting tumor angiogenesis.192 Moreover, oxidative stress in the aging microenvironment contributes to tumor progression partly by promoting ROS-mediated platelet activation, which facilitates cancer cell protection and metastasis.193,194 Ferroptosis, which is induced by excessive ROS accumulation and lipid peroxidation, further amplifies oxidative stress and contributes to immune cell senescence in the tumor microenvironment.195 (3) It facilitates tumor evasion from immune surveillance, as the cytotoxicity of senescent NK cells and effector T cells is significantly reduced.196 It has been reported that senescence impedes tumor surveillance by DCs and the activation of OVA-specific T cells, ultimately resulting in suboptimal outcomes of OVA immunotherapy for melanoma.197 In another study, senescent DCs exhibited reduced secretion of IL-15, IL-18 and IFN-α, failing to activate NK cells and thereby exacerbating RMA-S lymphoma.198 However, some studies suggest that aging immune responses may have a suppressive effect on cancer.199 Compared with aged mice, young mice exhibit more rapid cancer growth and a deficiency in mature T and B lymphocytes.200 One of the antitumor mechanisms mediated by aging is the clearance of presenescent tumor cells through antigen-specific immune responses, thereby inhibiting tumor progression.201,202
Immunosenescence in cancer. In the left part of the figure, the SASP enhances tumor growth, invasion, and immune evasion, exacerbating immune suppression. Aging-related T cell exhaustion and an impaired TCR repertoire weaken immune surveillance. Additionally, reduced vaccine efficacy and diminished immune checkpoint blockade (ICB) responses are observed in the senescent TME. In the right part of the figure, therapy-induced senescence (TIS) reprograms tumor cells toward stem-like phenotypes and suppresses CD8⁺ T cell activation. This suppression of CD8⁺ T cell activity subsequently contributes to the development of an immunosuppressive environment. A similar effect is observed with the accumulation of senescent T cells, which also suppress CD8⁺ T cell activation and thereby promote immune suppression. Moreover, senescent T cells can recruit MDSCs and Tregs and further induce senescence in neighboring effector T cells, thereby reinforcing immune suppression and impeding effective antitumor responses within the immunosuppressive milieu. Furthermore, CAR-T cell immunotherapy itself can induce SASP-related cytokines. This adverse environment, together with the presence of senescent T cells, synergistically undermines the efficacy of CAR-T cell therapy
Moreover, immunosenescence may have significant implications for cancer immunotherapy.203 Conventional tumor therapies, typically chemotherapy, radiotherapy, and surgery, can induce both spontaneous and therapy-induced senescence (TIS), accompanied by the accumulation of SnCs. Upon entering TIS, tumor cells undergo intrinsic reprogramming to acquire stem-like properties, thereby promoting tumor progression and contributing to therapeutic failure.204 In this process, the prominent feature is the accumulation of senescent and failing T cells. It has been reported that TIS suppresses CD8+ T cell activation, fostering an immunosuppressive microenvironment that contributes to poor treatment outcomes.205 Cancer immunotherapy focuses primarily on T cell-mediated approaches, and T cell senescence is a critical component of immunosenescence. Senescent T cells can recruit MDSCs and Tregs205 and further induce senescence in neighboring effector T cells,206 thereby establishing an immunosuppressive microenvironment that impedes effective antitumor immune responses. In the context where immune checkpoint blockade (ICB) has demonstrated remarkable efficacy in cancer immunotherapy, several studies have revealed the reduced effectiveness of anti-PD-1/PD-L1 therapy in aged mice and elderly patients,207,208,209 leading to treatment resistance. This evidence suggests a connection between immunosenescence and the diminished efficacy of ICB. Furthermore, the ICB response to shared antigens is largely mediated by memory T cells.210 These memory responses remain functional even in older patients, highlighting that immunological memory is not necessarily strongly impaired by aging.8 While cancer vaccines have emerged as a groundbreaking therapeutic approach in recent years, their clinical application faces a significant challenge: markedly reduced immunogenic responses in elderly patients relative to younger populations. With advancing age, the magnitude of the germinal center response and its output are impaired, coupled with spatial dysregulation of follicular helper T (Tfh) cells.211,212 For example, age-related upregulation of CXCR4 leads to mislocalization of Tfh cells to the dark zone, along with a significant reduction in the follicular dendritic cell (FDC) network area and a marked decrease in the number of plasma cells.211 Consequently, older adults generate lower antibody titers than younger individuals do, and these titers wane more rapidly, resulting in diminished vaccine efficacy in elderly individuals.213,214 Recent research suggests that tumors often present new antigens arising from mutations within individual cancer cells, and these neoantigens are typically recognized by naïve T cells.215 With age, the reduced pool of naïve T cells may impair the ability of the immune system to detect these antigens, creating what is referred to as “holes in the repertoire.” This limitation could reduce the effectiveness of immunotherapies targeting neoantigens.203 Despite this, advances in cancer mutation analysis and TCR sequencing have made it possible to assess whether an individual patient lacks the necessary TCRs to recognize crucial neoantigens.216,217,218 JAK-STAT dysregulation has also been implicated in promoting tumor immune evasion. Persistent STAT3 activation, in particular, suppresses cytotoxic responses and fosters MDSC accumulation, further exacerbating cancer risk.33 Moreover, the dysregulation of microRNAs (miRNAs) in the tumor microenvironment plays a crucial role in modulating immune responses and promoting tumor progression.219 Exosome-mediated lipid metabolic communication also plays a vital role in modulating the tumor microenvironment and promoting digestive system neoplasms.220
Chimeric antigen receptor T cell (CAR-T) immunotherapy, which primarily targets hematologic malignancies, including B cell acute lymphoblastic leukemia (B-ALL), is currently being explored for solid tumors.221,222,223 However, only a minority of patients achieve long-term disease remission.224 The inefficacy of CAR-T cell therapy can be attributed to multiple factors, among which T cell senescence and exhaustion play pivotal inhibitory roles.225 One piece of supporting evidence demonstrated that patients exhibiting lower T cell differentiation (characterized by naïve or early memory T cell predominance) consistently exhibited superior clinical responses.226,227 Currently, the definitions of T cell senescence and exhaustion are not fully distinguished. However, it is clear that T cell functionality indeed impacts the outcomes of CAR-T cell therapy. Specifically, the antitumor activity of adoptively transferred T cells relies on their memory and stem-like properties, whereas T cells from patients with poor therapeutic responses exhibit increased exhaustion and apoptosis markers.228 Furthermore, CD8+ T cells expressing senescence-associated molecules such as LAG-3 and PD-1-related molecules are associated with an unfavorable prognosis in CLL patients receiving CAR-T cell therapy.228,229 Moreover, the tumor-suppressive microenvironment harbors MDSCs and Treg cells, which are associated with inflammatory senescence and further promote T cell senescence.184,230,231 Conversely, CAR-T cell therapy can induce SASP-related cytokines, ultimately contributing to the formation of an inflammatory milieu.232,233 These findings suggest that postinfusion, CAR-T cells may also exacerbate treatment efficacy through inflammation-driven senescence.
Other diseases
Age-related macular degeneration (AMD)
Immunosenescence plays a significant role in the onset and progression of AMD by altering the inflammatory response of the immune system (Fig. 5). AMD is an ocular disease that causes blurred central vision, primarily due to aging-related damage to the macula.234 Immunosenescence promotes the development of AMD by causing low-grade chronic inflammation in the retina and choroid.235,236 As the eyes age, significant changes in the retinal pigment epithelium (RPE) occur. The accumulation of ROS, lipofuscin, and other byproducts gradually disrupts the metabolism and function of RPE cells, leading to progressive deterioration of retinal health and accelerating the progression of AMD.236,237 Elevated levels of proinflammatory cytokines from various immune cells during aging further contribute to ongoing damage to the retina and choroid. Microglial cells are innate immune cells that have self-renewal ability and neuroprotectivity in the normal retina. In AMD, aging microglia stimulate chronic low-grade inflammatory responses and exacerbate immune-mediated damage to the retina and RPE.238,239 Senescent macrophages in the eyes secrete proinflammatory cytokines via STAT3 signaling, aggravating retinal damage and promoting neovascularization, a hallmark of AMD.240 Mast cells also play an important role in the pathogenesis of AMD. During AMD, increased mast cell numbers and degranulation are observed, with increased production of proinflammatory mediators such as CXCL1.241 Neutrophils participate in retinal immune responses by releasing NETs. These NETs help clear aged blood vessels and immune cells, but excessive NET formation exacerbates retinal damage, especially in the context of excessive inflammation.242 T cells and B cells also play critical roles in immune responses in AMD. With aging, T cell tolerance decreases, leading to increased secretion of proinflammatory cytokines and exacerbated retinal damage.243 Complement system activation recruits immune cells and disrupts the blood‒retinal barrier, further promoting retinal inflammation and the progression of AMD.244,245
Other immunosenescence-related diseases. (1) Infectious diseases: Immunosenescence increases susceptibility to infections (e.g., SARS-CoV-2, CMV) due to PD-1/Tim-3 overexpression in T cells. Chronic inflammation impairs lung function, contributing to COPD and IPF. Weakened vaccine responses reduce protection in older adults. (2) Autoimmune diseases: Senescent CD28⁻ T cells disrupt immune tolerance, exacerbating RA. Telomere attrition and epigenetic changes sustain chronic inflammation and systemic complications. (3) CVD: Aging-induced inflammation and oxidative stress drive CVD. DAMPs activate PRRs, triggering cytokine and ROS production. Senescent T cells worsen vascular dysfunction. (4) AMD: Dysregulated immune responses and chronic inflammation damage the retina. Dysregulated microglia and NK, T, and B cells drive inflammation, whereas mast cells and monocyte/macrophage activation exacerbate retinal damage through proinflammatory cytokine release. Neutrophil NET formation and complement activation further impair the blood‒retinal barrier, accelerating AMD progression. (5) Metabolic disorders: Immunosenescence promotes inflammation in T2D and obesity. Increased memory CD4⁺ T cells and senescent T cells enhance cytokine production, whereas double-negative B cells expand, leading to enhanced proinflammatory responses and autoantibody secretion. Reduced PBMC function weakens immune defense, exacerbating metabolic dysfunction
Metabolic disorders
Immunosenescence is closely linked to various metabolic disorders, particularly type 2 diabetes (T2D).246 The immune aging process results in functional alterations in immune cells, increasing the susceptibility of elderly individuals to metabolic dysfunction. Metabolic diseases such as T2D, obesity, and metabolic syndrome are associated with low-grade chronic inflammatory states (Fig. 5). This inflammation is called metabolic inflammation and is similar to the chronic inflammatory process that occurs during aging.247 Increased fat mass, metabolic dysfunction, and systemic inflammatory responses are key features of these metabolic diseases and can further exacerbate immune dysfunction associated with aging.164 In T2D, a hallmark of immune aging is a reduction in the proportion of naïve CD4+ T cells alongside increased memory CD4+ T cells and effector CD4+ and CD8+ T cells. These effector cells are the main producers of proinflammatory cytokines, including IFN-γ and TNF-α, leading to increased systemic inflammation.164,248 Additionally, an increase in the number of senescent T cells, including CD8+CD57+ and CD8+CD28− T cells, is considered a predictor of hyperglycemia development in humans.248 Apart from adaptive immunity, aging induces defects in the function and activation of the innate immune system in T2D. In T2D patients, the phagocytic capacity and TLR responsiveness of peripheral blood mononuclear cells (PBMCs), which are closely related to endoplasmic reticulum stress and poor blood sugar control, are significantly impaired.248,249 Obesity is another metabolic disorder associated with metabolic inflammation and has a similar phenotypic spectrum as aging, such as dysfunctional mitochondria, weakened immunity, and elevated systemic inflammation.250 Obesity significantly impacts B cell function. Studies have demonstrated that B cells from obese individuals exhibit a reduced capacity to generate pathogen-specific antibodies.251 Furthermore, both obesity and aging drive the expansion of double-negative (DN) B cells (CD27⁻IgD⁻), which display a proinflammatory phenotype marked by autoimmune antibody secretion and upregulated expression of activation markers (e.g., CD11c and T-bet).252 Exposure to plasma from obese individuals promoted the apoptosis, DNA damage, and mitochondrial dysfunction of PBMCs, resulting in increased production of IL-1β and IL-8.253 Moreover, CD8+ T cells present an immunosenescent phenotype with reduced expression of CD28, indicating that chronic systemic inflammation in individuals with obesity promotes immune system dysfunction and aging.253
Infectious diseases
Immunosenescence in the elderly population is closely related to increased susceptibility to infectious diseases such as severe acute respiratory syndrome (SARS-CoV) and cytomegalovirus (CMV) infection (Fig. 5).113 As people age, the immune system undergoes functional decline. During CMV infection, the oligoclonal expansion of CMV-specific CD8+ T cells is inhibited in elderly individuals, which limits the ability to combat viral infection.254 The coronavirus disease 2019 (COVID-19) pandemic has starkly illustrated the clinical consequences of immunosenescence. Elderly patients infected with SARS-CoV-2 frequently experience rapid disease progression due to high expression of PD-1 and Tim-3 on CD8+ T cells and dysregulated innate immunity, culminating in cytokine storms dominated by IL-6 and granulocyte‒macrophage colony‒stimulating factor (GM-CSF).255,256 Moreover, immunosenescence compromises vaccine efficacy. Although mRNA vaccines (e.g., BNT162b2) partially mitigate age-related immune deficits by enhancing GC reactions and memory B cell generation, their protective efficacy against COVID-19 in older adults remains suboptimal.257 Strategic booster doses, however, significantly improved cross-protection against variants such as Omicron, highlighting the necessity of age-tailored vaccination protocols.258 Similarly, elderly individuals exhibit weaker antibody responses upon immunization with influenza and pneumococcal vaccines, underscoring the need for enhanced immunization strategies.257,259 Moreover, JAK3 mutations have been shown to lead to severe immunodeficiencies, whereas STAT3 mutations impair IL-17 production, which increases susceptibility to bacterial and fungal infections. These findings suggest that specific genetic mutations may contribute to the diminished ability of the immune system to effectively fight infections in elderly individuals, potentially compounding the challenges posed by immunosenescence.34
Respiratory diseases
In chronic obstructive pulmonary disease (COPD), aging-associated immune dysfunction exacerbates lung damage through multiple pathways: epithelial barrier integrity deterioration, impaired mucus clearance, and alveolar macrophages with low phagocytic capacity.260,261 These changes amplify inflammation triggered by environmental insults such as cigarette smoke, while accelerated cellular aging (e.g., telomere shortening in alveolar cells) further drives oxidative stress and apoptosis.262 A parallel mechanism is observed in idiopathic pulmonary fibrosis (IPF), where telomere attrition in lung fibroblasts, combined with chronic inflammation and TGF-β pathway activation, promotes excessive collagen deposition and irreversible structural damage.263,264
Autoimmune diseases
Rheumatoid arthritis (RA) is a chronic inflammatory disorder characterized by symmetrical and destructive inflammation of joints and other organs/tissues. As individuals age, the immune system shifts toward a more proinflammatory state, marked by the accumulation of CD28- T cells. These senescent T cells disrupt immune tolerance, promote autoreactivity against self-antigens, and exacerbate disease severity, particularly in RA patients with extra-articular manifestations (Fig. 5).265 In addition, genetic factors, such as STAT3/STAT4 polymorphisms, contribute to the development of autoimmune diseases such as RA by impairing immune tolerance and increasing susceptibility to autoreactivity.33 Increased telomere attrition in these T cells exacerbates the inflammatory state and is associated with the development of cardiovascular disease in RA patients, indicating the systemic effects of immunosenescence.262,266 The complex interplay between immunosenescence and autoimmunity highlights the importance of further research and the development of novel therapeutic approaches to treat autoimmune diseases in the elderly population.
Cardiovascular diseases (CVDs)
Aging is the most significant risk factor for CVD, which remains the leading cause of death worldwide.267 CVD encompasses a range of heart and vascular diseases, which are associated with the biological process of aging, the loss of homeostasis, and increased morbidity and mortality rates (Fig. 5).268 Inflammaging is a key risk factor for CVD and involves elevated levels of proinflammatory cytokines, leading to endothelial damage, impaired vascular remodeling,269 and atherosclerosis.270 These inflammatory molecules are secreted primarily by senescent T cells and proinflammatory macrophages.271,272 This reflects the body’s inability to properly regulate immune responses during aging, driving tissue dysfunction and pathological alterations. During cardiac stress, ischemic injury, and metabolic syndrome in the cardiovascular system, necrotic cells release DAMPs, which are recognized by pattern recognition receptors on innate immune cells, triggering strong inflammatory responses.273 This leads to the secretion of proatherosclerotic cytokines, ROS, and reactive nitrogen species (RNS), amplifying oxidative stress. These cytokines also stimulate the proliferation of vascular smooth muscle cells (VSMCs) and the accumulation of oxidized low-density lipoprotein (LDL) particles, which are then captured by foam cells in vessel walls.270 Senescent T cells, particularly cytotoxic CD8+ T cells, also contribute to the pathophysiology of CVD. The expansion of CD8+CD28− T cells is a risk factor for vascular dysfunction.274 Studies have shown that the expansion of peripheral late-differentiated CD4+CD28− T cells that produce IFN-γ after persistent antigenic stimulation is observed in unstable angina.275 In older men, CMV infection-related atherosclerosis may be mediated by an increased proportion of memory CD4⁺ T cells.276
Therapeutic targets in immunosenescence
The study of aging has long been a primary objective for scientists. Over the past few decades, the mechanisms and pathways underlying this critical aspect of immune senescence have been explored via advanced biological techniques and genetic tools. Notably, these mechanisms and pathways represent essential targets for interventions aimed at mitigating immunosenescence. Consequently, in this chapter, we summarize the current status of interventions aimed at combating or delaying immunosenescence. The emerging strategies include targeted therapies, immune interventions, and lifestyle modifications, among which there are several promising results (Fig. 6).
Therapeutic strategies related to immunosenescence. The three types of therapeutic measures mentioned in the review are as follows: (1) Immune intervention, which is mainly divided into interventions targeting immune organs and immune cells. (2) Targeting signaling pathways related to aging; slowing the immune aging process by downregulating NF-κB, mTOR, and JAK-STAT; and upregulating AMPK, SIRT1 and other signaling pathways. (3) Nutritional and lifestyle intervention strategies
Targeting signaling pathways
NF-κB signaling pathway
Persistent NF-κB activation drives the SASP267. A study demonstrated that sustained inhibition of NF-κB for a period of two weeks can reverse tissue characteristics and the overall gene expression program.277 Direct inhibitors of the IKK/NF-κB pathway may confer clinical benefits for degenerative changes associated with both progeroid syndrome and normal aging.10,278 Genetic suppression of the IKK/NF-κB pathway, achieved through the deletion of one p65 allele, or pharmacological intervention via IKK inhibitors, such as 8K-NBD, has been shown to delay the onset and mitigate the severity of aging symptoms and age-related pathologies in the nervous system of murine models10,279. Bortezomib was demonstrated to inhibit the proteolysis of IκB, thereby preventing the activation of NF-κB;280 this represents a promising and innovative approach to delay aging (Table 1).281 Additionally, fisetin promotes the synthesis of the antioxidant glutathione and suppresses the activity of proinflammatory factors, including TNFα, IL-6, and the transcription factor NF-κB.282,283 Several natural phytochemicals, such as curcumin, have also been shown to inhibit NF-κB nuclear translocation while simultaneously activating Nrf2, an antioxidative pathway.284 The loss of PTEN activates the AKT/NF-κB pathway, thereby promoting alveolar epithelial cell senescence and the release of SASP285,286. EF24, a notable derivative of curcumin, has been demonstrated to increase PTEN expression and subsequently inhibit the NF-κB pathway (Table 1).287 Resveratrol activates SIRT1, leading to the deacetylation of the p65 subunit of NF-κB, thereby reducing its transcriptional activity.288 Notably, clustered regularly interspaced short palindromic repeats (CRISPR)-Cas is currently recognized as one of the most powerful gene-editing tools available. The hyperactivation of the gap junction protein connexin 43 (Cx43) increases the expression of p53, p16INK4a, and NF-κB, which are positively correlated with aging. CRISPR/Cas9-mediated downregulation of Cx43 inhibits the transition of chondrocytes into a senescent state.289
mTOR signaling pathway
There is substantial evidence demonstrating that the mTOR signaling pathway is a critical target for antiaging interventions. Inhibition of mTORC1 promotes autophagy, which facilitates the clearance of unwanted cytoplasmic proteins and reduces the accumulation of toxic metabolites, thereby mitigating cellular stress and extending lifespan.290,291 Another proposed mechanism is that mTOR regulates the crosstalk between mitochondria and the nucleus, stabilizing the key protein Clk-1, which is essential for mitochondrial signaling communication.292,293,294 The first-generation mTOR inhibitor rapamycin has been shown to extend lifespan across various organisms, making it the only known pharmacological agent that directly modulates aging (Table 1).295,296,297 However, Cloughesy et al. reported that while rapamycin reduces the proliferation of glioblastoma, its effects are not sustained.298 Consequently, the primary goal of second-generation mTOR inhibitors is to simultaneously target both the mTORC1 and mTORC2 signaling pathways, as well as their feedback loops, which were not addressed by first-generation inhibitors.299 By providing more comprehensive inhibition of the mTOR signaling pathway, second-generation mTOR inhibitors, including PP242, KU0063794, AZD3147, and eCF309, have shown promising results in preclinical and clinical trials.300,301 The most recent advancement, RapaLink-1, structurally resembles rapamycin and binds to mTOR, effectively inhibiting mTORC1.302 Compared with rapamycin, RapaLink-1 has been reported to exert a stronger inhibitory effect on T cell proliferation.302 Phase IIa and IIb clinical trials investigating the combination of the mTORC1 inhibitors RTB101 and RAD001 revealed that this regimen could reduce immunosenescence and enhance the response to influenza vaccination.29,303 However, the phase III trial of RTB101 alone failed to achieve the desired outcomes.303
JAK-STAT signaling pathway
During the aging process, sustained activation of the JAK-STAT signaling pathway induces the expression of the SASP, promoting cellular senescence and apoptosis, as well as impairing the function of T cells, B cells, and macrophages. Targeting the JAK-STAT signaling pathway can alleviate chronic inflammation, improve immune cell function, and delay the onset of aging-related phenotypes.34,304 Notably, the JAK pathway is more active in the adipose tissue of aged animals than in that of their younger counterparts. Compared with that in younger mice, the efficacy of the JAK inhibitor ruxolitinib in aged mice is superior, manifesting in the clearance of SnCs, enhancement of physical performance, and maintenance of adipose tissue homeostasis (Table 1). This evidence suggests that JAK inhibitors confer beneficial effects by modulating SnCs.304,305 The percentage of SnCs was significantly reduced in mice treated with the JAK inhibitor NVP-BSK805 in combination with docetaxel, thereby enhancing the antitumor response to docetaxel.306 Additionally, JAK inhibitors may promote hair growth by stimulating the activation and proliferation of stem cells.307 The underlying mechanism could be leveraged to directly target tissue stem cells and their respective niches.307 The administration of JAK inhibitors leads to a significant increase in satellite cell populations, facilitates robust muscle regeneration, and elevates functional capabilities, thereby presenting a viable and innovative therapeutic strategy for addressing muscle wasting conditions.308
AMPK signaling pathway
Metformin is a well-known activator of AMPK that functions by reducing the ADP/ATP and AMP/ATP ratios (Table 1).309 This reduction is achieved through partial inhibition of Complex I of the mitochondrial ETC. Consequently, this inhibition leads to direct or indirect activation of AMPK.310 In age-related diseases, metformin beneficially enhances mitochondrial function, increases Nrf2 activity, induces autophagy, or ameliorates accelerated aging defects in Hutchinson–Gilford progeria syndrome (HGPS) cells by altering gene splicing through its activation mechanisms.311 Metformin can also mitigate age-related hearing loss and neurodegeneration symptoms in D-galactose-induced aging rats by modulating the AMPK/extracellular signal-regulated kinase 1/2 (ERK1/2) signaling pathway.312 In addition, we cannot overlook the role that resveratrol plays in the AMPK pathway. It has been reported in the literature that resveratrol can prevent oxidative stress-induced aging and proliferation damage by activating the AMPK/FOXO3 signaling pathway.313 Oleanolic acid induces autophagy and apoptosis in colon cancer cells by modulating the AMPK-mTOR signaling pathway, thereby exerting therapeutic effects against colorectal cancer.314
Melatonin signaling pathway
The mechanisms by which melatonin-related signaling pathways exert their antiaging effects can be summarized as follows (Table 1): (1) Antioxidant function. Melatonin can directly scavenge ROS through its electron-rich indole ring, and it can also induce antioxidant enzymes such as GPX, SOD, and catalase to achieve antioxidative effects. Furthermore, melatonin enhances the efficacy of antioxidant vitamins. (2) Stabilization of mitochondrial function and enhancement of autophagy. As previously mentioned, mitophagy is a crucial step in protecting aging cells from damage caused by endogenous waste stress.315 Melatonin enhances mitophagy, which can alleviate the accumulation of dysfunctional mitochondria and restore mitochondrial quality.316,317 (3) Anti-inflammatory effects. Melatonin exerts its neuroprotective effects by inhibiting the release of cytochrome and the activation of caspases, as well as negatively regulating the production of proinflammatory cytokines.318 Early melatonin intervention disrupts the vicious cycle of Aβ-ROS-induced neuroinflammation in AD and ameliorates metabolic inflammation in T2D.47,319 The outcomes of clinical trials in these areas have been highly encouraging, underscoring its efficacy. However, while preclinical investigations of cardiovascular diseases have yielded promising results, melatonin has not met anticipated efficacy benchmarks in translational studies involving patients with established cardiovascular conditions.320,321 Nonetheless, it has shown notable protective effects against cardiovascular risk factors.321 Consequently, a deeper exploration of its mechanistic pathways and therapeutic potential is warranted, aiming to elucidate novel strategies and substantiate evidence for the prevention and management of these diseases.
Sirtuin signaling pathway
Sirtuins are a family of NAD+-dependent deacetylases that serve as crucial regulators in delaying cellular senescence and extending organismal lifespan.322 With advancing age, there is a notable decline in sirtuin activity, particularly in SIRT1 and SIRT3.323,324 The overexpression of SIRT1 has been demonstrated to inhibit the aging of nucleus pulposus cells, promote cell proliferation, and suppress apoptosis.325 Moreover, the activation of SIRT1 also inhibits the senescence of dermal fibroblasts induced by ultraviolet irradiation.326 On the basis of the aforementioned research, increasing interest has been directed toward various NAD+ precursors or small-molecule activators, which aim to increase the activity of sirtuins.323 Intriguingly, the first SIRT1 activator to capture widespread attention was resveratrol, which exerts its effects through several mechanisms (Table 1).327 For example, it can modulate the deacetylation state of the core autophagy protein ATG9A by activating SIRT1, thereby delaying age-related hearing loss.328 Additionally, resveratrol improves motor function in senescence-accelerated mice by attenuating the negative regulation of insulin and apoptotic signaling through the SIRT1/FOXO1 pathway.329 These findings underscore the capacity of resveratrol to exert its antiaging effects across diverse biological pathways.
Notably, SIRT1, which serves as a pivotal modulator in the promotion of healthspan, captures scientific intrigue largely because of its ability to orchestrate a multitude of signaling cascades. SIRT1 interacts with the RelA/p65 subunit of NF-κB, leading to the deacetylation of lysine residues on this subunit. This interaction ultimately diminishes the transcriptional activity of the NF-κB complex, and as a result, SIRT1-mediated deacetylation serves to inhibit NF-κB signaling.330 Concerning another significant pathway that promotes aging—the mTOR pathway—SIRT1 can obstruct mTOR signaling, thereby rectifying autophagy impairments induced by oxidative stress and consequently enhancing the survival rate of embryonic stem cells.331 In alignment with this, SIRT1 and AMPK can reciprocally enhance each other’s activity. SIRT1 deacetylates LKB1, thereby activating AMPK and inhibiting senescence332. Conversely, AMPK can increase intracellular NAD+ levels, which in turn boosts SIRT1 activity, ultimately delaying the aging process of chondrocytes in osteoarthritis.333,334
Other targets of signaling pathways
Dasatinib, a common drug for targeting SnCs, is a second-generation tyrosine kinase inhibitor that can downregulate the expression of senescence-associated biomarkers, including β-galactosidase, p16, and p21.335 Quercetin, which targets the Bcl-2 family member hypoxia-inducible factor-1α (HIF-1α) and the antiapoptotic PI3K/AKT and p21 signaling pathways, has demonstrated synergistic effects (Table 1).336 This combination has been shown to mitigate adipose tissue inflammation and cellular senescence in aged mice, concurrently enhancing systemic metabolic performance.337,338 It has been shown to prevent the progression of age-dependent intervertebral disc degeneration,339 reduce intestinal SnCs and excessive inflammation in aged mice,340 and effectively induce apoptosis, thereby eliminating SnCs and the SASP burden in adipose tissue.337 Additionally, navitoclax (also known as ABT-263) is a drug classified as a “Bcl-2 family inhibitor.” It specifically inhibits the antiapoptotic proteins Bcl-2, Bcl-xL, and Bcl-w within the Bcl family and induces apoptosis through the activation of caspase signaling pathways.341 For example, the oral administration of Navitoclax in mice subjected to sublethal irradiation or natural aging effectively depletes senescent hematopoietic stem cells and senescent muscle stem cells, thereby rejuvenating stem cell function.342 However, its side effects, such as transient thrombocytopenia and neutropenia, remain obstacles to clinical translation.342,343,344 It has been reported that p53 is a senescence marker and that the p53 signaling pathway regulates cellular senescence.181 In another study, FOXO4 was found to be elevated in SnCs and contributed to the senescence process.345,346 FOXO4-DRI, a cell-permeable peptide, disrupted the FOXO4-p53 interaction, thereby reducing senescence-induced FOXO4 activity and selectively targeting SnCs for p53-dependent apoptosis (Table 1).346 FOXO4-DRI inhibits renal tubular senescence and restores renal function in vivo347. Additionally, it effectively eliminates senescent chondrocytes in vitro and ameliorates pathological changes and collagen deposition in the lungs of mice with pulmonary fibrosis.348,349
Targeting immune cells
Hematopoietic stem cells
Dysfunction of HSCs may serve as a fundamental basis for the aging of the myeloid and lymphoid lineage systems.350 For example, aged HSCs exhibit a diminished capacity to adhere to stromal cells, which may perturb the interactions between stem cells and the hematopoietic niche, ultimately leading to alterations in the functionality of aging HSCs.351,352 Therefore, the underlying causes of stem cell aging are likely to represent potential therapeutic targets. One contributing factor to the changes observed in senescent stem cells may be the age-dependent acquisition of defects in telomeres, genomic DNA, or mitochondrial DNA.353,354,355,356 During the process of replicative aging of HSCs in both humans and mice, telomeres progressively shorten.357 The function of telomerase in HSCs is to counteract the rate of telomere shortening during cell division, thereby preventing premature telomere attrition and extending the replicative capacity of HSCs.357 The in vitro culture of telomere-binding protein protection of telomeres 1 (Pot1) in HSCs has been demonstrated to maintain self-renewal capacity and regulate the activity of HSCs in elderly individuals, which has significant implications for the ex vivo cultivation of human HSCs.358 Similarly, gene therapy employing adeno-associated virus (AAV) 9 vectors to overexpress telomerase reverse transcriptase (TERT) has been demonstrated to alleviate age-related telomere damage and delay senescence in mouse models of cancer resistance.359
The diminished efficacy of HSCs can be attributed not only to telomere shortening but also to an increased risk of DNA damage360 and elevated levels of ROS production.361 In the context of ataxia-telangiectasia mutated (ATM) deficiency, treatment with the permeable thiol antioxidant N-acetyl-l-cysteine (NAC) was shown to restore the reconstitution capacity of HSCs, thereby preventing bone marrow failure.361 Furthermore, inhibition of the upregulated tumor suppressors p16INK4a and p19ARF in the context of ATM deficiency has been demonstrated to restore the replicative function of HSCs.361 However, p16INK4a does not universally induce HSC senescence in all contexts, and its role in maintaining HSC homeostasis has not been reported in other studies.362 A new study revealed that depleting aged murine HSCs with myeloid-biased outputs (my-HSCs) via antibodies such as anti-CD150 can restore features of a younger immune system in mice. This intervention increased naïve T cells and mature B cells in aged mice while reducing the levels of inflammatory markers.363 Interestingly, in aging tissues, the inhibition of key molecules with high activity in oxidative stress and strong ROS inducers, such as p38, can restore the function of HSCs.350,364 SIRT3, a prominent member of the sirtuin family, enhances antioxidant activity and exerts an inhibitory effect on ROS. Upregulation of SIRT3 rescues functional impairments in aged HSCs.365 Notably, mTOR and cell division control protein 42 (CDC42) are closely associated with the aging process and have been identified as promising targets for rejuvenating HSCs.295,366,367,368 The small-molecule CDC42-targeting drug CASIN has significant potential in mobilizing murine HSCs, thereby contributing to overall lifespan extension.369,370 Furthermore, targeted therapies against these proteins may not only lead to the rejuvenation of HSCs but also potentially induce systemic rejuvenation of the entire organism.371,372 Inflammation associated with aging induces the expression of interleukin-27 receptor α (IL27Ra) on the surface of HSCs through the TNF-α-ERK-ETS1 signaling pathway, leading to HSC senescence.373 The pharmacological targeting of key molecules within this signaling pathway may provide new therapeutic avenues for combating immune aging and related diseases caused by HSC senescence.373 In summary, the concept of revitalizing tissue-specific stem cells to restore tissue function and, ultimately, rejuvenating the whole organism represents a promising avenue worthy of further exploration. Hematopoietic stem cell transplantation is a treatment method with promising therapeutic outcomes. An intervention involving the shared circulation of blood between two individuals of different ages has been reported to rejuvenate the state and functionality of stem cells in the brain, liver, and muscle tissues of older individuals.374,375,376 However, subsequent studies demonstrated that this rejuvenation effect is attributed to noncellular factors present in the blood rather than the cells themselves.375,377,378
T cell
In recent decades, it has become increasingly recognized that T cell senescence is a significant aspect of the aging process. The development of targeted therapies specifically aimed at modifying senescent T cells remains an ongoing area of research for the treatment of diseases associated with immune aging. Like HSCs, T cells are also affected by telomere shortening. On this basis, a specific subset of T cells with elevated expression of CD28 (a costimulatory molecule required for T cell activation) is capable of maintaining robust telomerase activity upon stimulation379. Therefore, the methods previously described for telomere extension and telomerase-mediated approaches can still reactivate T cells (Fig. 7). Furthermore, an intriguing discovery has been made that naïve and central memory T cells can elongate their telomeres by acquiring telomeric components from antigen-presenting cells (APCs) through immunological synapses.380 This mechanism may also represent a potential breakthrough for extending T cell telomeres. By employing the safe and feasible gene-editing tool CRISPR/Cas9 to engineer T cells, this method can mitigate exhaustion-inducing signaling pathways, preserve antitumor activity, and increase the in vivo persistence of CAR-T cells, thereby further overcoming limitations in cancer therapy (Fig. 6).225,381,382 The incorporation of additional costimulatory domains (such as 4-1BB or CD28) into engineered T cells enhances the persistence of CAR-T cells.383,384
Targeting T cell senescence. a At the genetic level, modifying T cell functionality or engineering chimeric antigen receptor (CAR)-T cells with CD28 may prolong the duration of adoptive cell therapy by mitigating T cell senescence. b Thymic rejuvenation through thymic remodeling supports T cell development and maturation. c By directly targeting senescence-associated molecules in aged T cells, this approach can reverse T cell senescence. d Regulating signaling pathways associated with T cell senescence
Direct targeting of pathogenic T cells constitutes a widely investigated therapeutic strategy (Fig. 7). For example, the proliferation and functional restoration of T cells can be stimulated through the use of cytokines. IL-7 modulates the size of the peripheral T cell pool and plays a pivotal role in regulating T cell homeostasis. In the context of hematopoietic cell transplantation, the administration of exogenous IL-7 can facilitate T cell reconstitution, thereby promoting the homeostatic proliferation of T cells and conferring antiapoptotic effects on peripheral T cells.385,386 In addition to IL-7, several other cytokines that play pivotal regulatory roles in T cell regeneration have been identified in preclinical studies. IL-12 acts as an enhancer cytokine, potentiating the IL-2 and IL-7 signaling pathways, thereby sustaining thymic T cell function and supporting their development during aging.387 IL-15 is critical for enhancing the development and functional efficacy of CD8+ T cells.388,389 IL-21 has demonstrated efficacy in promoting the reconstitution of the peripheral naïve T cell compartment across diverse models of immune injury.389 Additionally, IL-22 facilitates the development of thymic-derived peripheral T cells.390 Together, these cytokines collectively contribute to the maintenance and restoration of T cell homeostasis, underscoring their therapeutic potential in immune regeneration and repair processes.
On the basis of the previously discussed evidence, keratinocyte growth factor (KGF) has been demonstrated to significantly enhance thymic regeneration. The therapeutic administration of recombinant KGF expedites the recovery of the peripheral T cell compartment and replenishes T cell populations subsequent to immune insults such as irradiation, cyclophosphamide administration, and dexamethasone treatment. In clinical practice, the utilization of KGF holds promise for the efficient reconstitution of the T cell compartment, thereby reinstating the ability to elicit a potent adaptive immune response.391,392 In an experimental study evaluating the effects of KGF therapy on thymic architecture and T cell immune reconstitution following myeloablative total body irradiation and autologous peripheral blood progenitor cell (PBPC) transplantation in healthy rhesus macaques, KGF treatment significantly increased de novo T cell production and the recovery of naïve T cells. These findings underscore the potential of KGF to promote robust thymic regeneration and immune restoration in the context of intensive conditioning regimens.393 Furthermore, the combined administration of KGF and androgens resulted in the accelerated reconstitution of naïve CD4+ and CD8+ T cells with a diverse Vβ repertoire, significantly contributing to the restoration of peripheral T cell populations.394
The capacity of sex hormones to influence lymphocyte development has been recognized for decades. In particular, the ability of estrogen to suppress postnatal thymocyte development and T cell production has been well-documented.395 Sex steroid ablation has been widely demonstrated to be beneficial for T cell reconstitution. A study showed that castration in 9-month-old mice rapidly reversed thymic involution, restoring the numbers of CD4+ and CD8+ cells to those of 2-month-old mice.396 Therapeutically, strategies that target sex steroid-related pathways, such as luteinizing hormone-releasing hormone (LHRH), or directly block sex steroid receptors can also achieve similar effects. A nonrandomized trial indicated that administration of the LHRH agonist goserelin (Zoladex) increased the numbers of various T cell subsets, particularly naïve CD4+ T cells, and improved the restoration of TCR repertoire diversity.397 LHRH agonists represent an effective and rational strategy for enhancing thymic function, not only in immunocompromised patients but also during normal aging.398 AR inhibitors and LHRH antagonists, which have the advantage of bypassing the sex steroid surge observed with LHRH agonists, may offer a greater opportunity for T cell reconstitution.399 However, it is worth considering that the regenerative effects of sex steroid inhibition on T cell development may be sustained only if sex steroid levels remain suppressed and that sex may also influence the efficacy of these interventions.400
The CD153 vaccine has been demonstrated to attenuate the presence of CD153+ senescent T cells within the adipose tissue of diet-induced obese mice through the induction of mouse IgG2 antibodies following administration.401 Additionally, the adoptive transfer of in vitro expanded or genetically engineered CD4+ T cells has emerged as a promising therapeutic approach to rejuvenating immune function in the context of immunosenescence. Notably, specific pharmacological interventions have shown potential in mitigating immunosenescence via T cell modulation. For example, dasatinib, a senolytic agent that inhibits tyrosine kinase (TK) activity and regulates the SASP, has been shown to promote the differentiation of CD4+ T cells toward a more juvenile phenotype.402 Furthermore, the mitochondrion-targeted antioxidant plastoquinonyl-decyltriphenylphosphonium has been shown to increase the intrathymic CD4+ T-to-CD8+ T cell ratio and prolong lifespan in diverse species.403 In elderly populations, metformin administration may ameliorate age-related impairments in autophagy and its downstream effects on CD4+ T cells.404 Recent studies have reported that micro/nanomaterial-based artificial antigen-presenting cell platforms can induce T memory stem cells in vitro and sustain long-term T cell immunity, thereby providing durable immune competence in elderly individuals.405
Other immune cells
Experimental evidence indicates that mice lacking MyD88, a critical adapter molecule for TLR signaling, lack ABCs and that pharmacological inhibition of this signaling axis effectively impedes the commitment of B cells to the ABC lineage.116,406,407,408 The downstream mediators of TLR7 and TLR9 signaling include IFN-γ and IL-21. In the context of IFN-γ deficiency, IL-4 exerts a suppressive effect on ABC differentiation, and targeted inhibition of IFN-γ or IL-21 represents a potential therapeutic avenue.406 Several studies have shown that follicular B (FO B) cells can serve as progenitors for ABCs, and their findings further revealed that neither MHC-II-deficient nor CD40-deficient FO B cells are capable of generating ABCs.408,409,410 Moreover, CD154-deficient mice exhibit an age-dependent failure to develop natural ABCs. Another recently characterized age-associated B cell population is aged adipose B cells (AABs), which localize to fat-associated lymphoid clusters and display a phenotypic profile distinct from that of ABCs.411 The expansion of AABs is mediated by Nlrp3 inflammasome activation.411 Therapeutic strategies involving the blockade of IL-1 signaling to inhibit Nlrp3-dependent B cell accumulation or the targeted depletion of B cells within adipose tissue via anti-CD20 antibodies have been shown to mitigate metabolic dysfunction in aged AT.411 CASIN, which is utilized for the treatment of aged HSCs, has also been shown to restore B cell populations and extend the posttransplant lifespan.366 Exogenous supplementation with spermidine can effectively promote the translation of TFEB, a transcription factor involved in autophagosome and lysosome biogenesis, through hypusinated EIF5A. This process reverses the age-dependent decline in the EIF5A-TFEB-autophagy axis and restores B cell function.412
Therapeutic blockade of NKG2A, an inhibitory receptor that modulates NK cell cytotoxic activity, enhances NK cell-mediated immunosurveillance and significantly diminishes senescent cell accumulation in aged murine models.413,414 The development and functionality of NK cells are regulated by a variety of miRNAs. Among these, the expression of miR-181a-5p notably decreases with age.128 This reduction is partially mediated through the upregulation of NLK and BCL2, which may contribute to the functional impairment observed in NK cells during aging.128,415 Consequently, the targeted delivery of miR-181a-5p to NK cells represents a clinically significant avenue of research aimed at enhancing the maturation and functional efficacy of NK cells in elderly individuals. Furthermore, it is well established that immunosenescence and inflammation are intricately interconnected. Immune cells that mediate the initiation and progression of inflammatory responses consequently represent promising therapeutic targets for intervention in age-related immune dysfunction. In monocyte/macrophage lineages, pharmacological modulation via the metabolic agent metformin achieves dual therapeutic effects by impairing monocyte-to-macrophage differentiation, consequently attenuating age-related macrophage senescence and systemic immune dysfunction.416 Pharmacological intervention through p38-MAPK signaling pathway inhibition or COX-2 suppression has demonstrated efficacy in restoring monocyte functional competence.417 Notably, cytokine-based intervention with IL-4 administration confers cytoprotective effects on macrophages while enhancing their survival capacity, ultimately ameliorating age-associated physiological decline and increasing health span parameters in geriatric mouse cohorts.418 Interestingly, recent studies have revealed that immunoglobulin G (IgG) induces both senescence and a proinflammatory state in macrophages from mice and humans. An antisense oligonucleotide targeting Fcgrt (encoded by FcRn depletion, which reduces tissue IgG levels) decreases IgG levels and reduces senescence-associated marker levels.419 Moreover, transplantation of eosinophils from young donors into aged recipient mice transiently reversed age-associated alterations in the HSC pool, leading to measurable improvements in physiological and immune health parameters that were partially mediated by eosinophil-derived IL-4.420 Brahmakshatriya and colleagues demonstrated that adoptive transfer of ex vivo activated bone marrow-derived DCs into aged mice enhances postimmunization GC formation and Tfh cell responses.421 Importantly, a separate study revealed that the reduction in the number of type 2 conventional dendritic cells (cDC2s) within the DC compartment may account for the attenuated vaccine efficacy observed in aged populations.422 Topical administration of the TLR7 agonist imiquimod was shown to increase antigen-bearing cDC2 populations, resulting in partial restoration of vaccination efficacy through targeted phenotypic restoration.422
Reshaping immune organs
Thymus
Thymic involution is indeed one of the key characteristics of immunosenescence. As people age, the thymus gradually atrophies, leading to a decrease in T cell production, which in turn affects the function of the immune system. Therefore, thymic regeneration is considered a potential strategy to combat immunosenescence. Thymic epithelial cells (TECs) primarily maintain thymic development to ensure their integrity and support T cell development (Figs. 6, 7). Therefore, it is feasible to develop a developmental niche within the thymus by amplifying TECs and to concentrate on the interplay between TECs and thymocytes to increase the flux of thymopoiesis.423,424 Importantly, Forkhead box N1 (FOXN1) regulates the formation and expansion of TECs, making it a key regulatory factor in thymic development and even regeneration.425 Therefore, for the rejuvenation of the aging thymus, our strategies focused on the FOXN1-TEC axis, such as (1) the enhancement of thymic rejuvenation by the exogenous expression of FoxN1 in TECs via FoxN1 cDNA plasmids and FoxN1 transgenic models;426 (2) the implantation of FOXN1-reprogrammed embryonic fibroblasts (FREF) into the senescent native thymus to restore thymic function and potentially counteract age-related inflammation;427,428 (3) extracellular vesicles and exosomes derived from young, healthy serum might contribute to the recovery of thymic aging by enhancing FoxN1 expression, characterized by partial reversal of thymic involution and enhanced negative selection signals;429 and (4) furthermore, key cytokines and growth factors essential for maintaining TEC function, including mesenchymal-derived KGF, macrophages and T lymphocyte-derived IGF-1, and thymic stromal cell-derived bone morphogenetic protein-4 (BMP4), as well as critical TEC functions such as the production of cytokines (e.g., IL-7 and Kit ligands) and chemokines (e.g., SDF-1 and TECK), can improve thymic function in mice.424,430,431,432,433,434 Deficiency of the epithelium-specific microRNA-205 (miR-205) can lead to significant thymic involution.435,436
Bone marrow
Bone marrow (BM) can provide niches for plasma cells and memory T cells and is influenced by aging. Immunosenescence is widely acknowledged to play a role in the pathogenesis of AD.437 A previous study revealed that young bone marrow transplantation (BMT) can rejuvenate peripheral cells, alleviate aging-associated signaling pathways, restore altered intercellular communication in senescent PBMCs, and reduce the levels of SASP factors in the blood (Fig. 6).438 Pangrazzi et al. revealed that the expression of molecules responsible for sustaining immune memory in the human BM undergoes age-related alterations. On the basis of this insight, the application of antioxidants could mitigate inflammatory levels within the BM and promote the generation of adequate “appropriate” survival factors for memory cells. Consequently, such interventions may enhance the preservation of immune memory during aging.439 Most importantly, clarifying the concept of the hematopoietic niche, which refers to the spatial localization and unique microenvironment of HSCs and immune cells within the bone marrow, is essential. The critical role of HSCs in immunosenescence is clear. As the bone marrow serves as the site of the hematopoietic niche, targeting senescent niches within the bone marrow holds broad application prospects. For example, targeting HIF transcription factors (involved in ROS production pathways) has been shown to improve outcomes in patients with hematopoietic disorders by reconstructing the niche composition. Additionally, supplementation with β3-adrenergic receptor agonists can enhance sympathetic innervation in the bone marrow and promote hematopoietic function.
Peripheral immune organs
Although thymic regeneration represents a promising therapeutic strategy for addressing immunosenescence, emerging evidence suggests that age-associated fibrosis in lymph nodes may counteract the benefits conferred by thymic rejuvenation (Fig. 6).440 Consequently, lymph node fibrosis has emerged as a potential therapeutic target in the context of rejuvenating the aged immune system. Alternatively, the restoration of lymphatic and immune cell functions through the utilization of functional synthetic lymphoid organoids composed of stromal progenitor cells and decellularized extracellular matrix scaffolds represents a novel and innovative approach.441 In addition, as the largest secondary immune organ in the body, the spleen is closely associated with immune aging.442 Reports indicate that transplanting splenic cells from young mice can reduce aging and tissue damage in aged mice.443 Furthermore, systemic AAV vector-mediated LAV-BPIFB4 gene transfer has been shown to redirect circulating SnCs to the spleen and increase the activity of immune cells within the spleen, thereby effectively eliminating senescent immune cells.444 However, immune organs do not function in isolation, and targeting a single organ still has numerous limitations. Therefore, combining this approach with other strategies is essential to achieve long-lasting restoration of immune function.
Nutritional and lifestyle interventions
A nutritionally optimized diet coupled with a balanced lifestyle exerts indispensable immunomodulatory effects through the potentiation of innate and adaptive immune responses, as well as the attenuation of chronic low-grade inflammation (inflammaging). These synergistic interventions constitute critical determinants in preventing age-related immunosenescence and mitigating the pathogenesis of various inflammation-associated disorders, including metabolic syndrome, cardiovascular diseases, and neurodegenerative conditions (Fig. 6).
Omega-3 polyunsaturated fatty acids (PUFAs), which are enriched in fatty fish and exhibit potent anti-inflammatory activities through multiple molecular mechanisms, are of particular interest.445,446 Extensive research has shown that omega-3 PUFAs exert pleiotropic regulatory effects on virtually all immune cell populations, including but not limited to T cells, B lymphocytes, macrophages, and neutrophils, thereby increasing their functional competence.447,448,449,450 Furthermore, clinical evidence has substantiated the adjunctive therapeutic efficacy of omega-3 PUFAs in the management of various pathological conditions.445 Of particular importance are the antioxidant compounds that play pivotal roles in cellular defense mechanisms, which are derived primarily from vegetable and fruit sources.451,452 These bioactive molecules play crucial roles in protecting cellular integrity against oxidative stress and modulating immune homeostasis. A notable example is vitamin C (ascorbic acid) and its stable derivative, L-ascorbic acid 2-phosphate, which significantly enhance the proliferative capacity and effector functions of γδ T cells, a unique subset of T lymphocytes that bridge innate and adaptive immunity.453 Emerging evidence indicates that exogenous stimuli, including fungal components, may drive and perpetuate immunosenescence.454 Strategic modulation of the gut mycobiota through dietary interventions, fecal microbiota transplantation, probiotic supplementation, and prebiotic administration has therapeutic potential in enhancing immunotherapy efficacy while mitigating immune-related adverse events.455,456
In addition to a healthy diet, regular moderate-intensity exercise has been demonstrated to enhance various aspects of immune function. A substantial body of literature confirms that consistent physical activity can reduce the levels of proinflammatory cytokines, including IL-6 and TNF-α, while increasing lymphocyte proliferation and NK cell activity. Furthermore, regular exercise helps maintain the mass and function of the thymus gland.457,458,459,460 Importantly, muscles act as modulators of the immune system. IL-15, produced by muscle, enhances the cytotoxicity of NK cells and increases cytokine secretion. Additionally, myokines, which are secreted by skeletal muscle, possess anti-inflammatory properties and contribute to the enhancement of immune responses.196,461 This evidence underscores the critical role of exercise in modulating immune responses, and the mechanisms underlying these benefits are thought to involve both systemic and cellular adaptations, including improved circulation of immune cells, reduced chronic low-grade inflammation, and enhanced stress resistance.462 Thus, integrating regular moderate exercise into lifestyle practices is a scientifically supported strategy for optimizing immune health. Chronic stress has been scientifically validated to accelerate immunosenescence, characterized by heightened systemic inflammation and a decline in immune efficacy.463,464 Interventions such as meditation, yoga, and controlled breathing techniques have demonstrated efficacy in attenuating stress responses and bolstering immune resilience.463,465 Concurrently, adherence to robust sleep hygiene protocols and the management of sleep disorders are imperative for sustaining immune homeostasis and overall physiological well-being.466,467 Empirical evidence indicates that smoking exacerbates immune aging, thereby increasing susceptibility to a spectrum of age-related pathologies.468 Smoking cessation has been associated with a reduction in the expression of proinflammatory markers and the restoration of immune cell functionality, including the normalization of T cells and NK cell dynamics.469,470 Furthermore, the timely administration of age-appropriate vaccines is crucial for counteracting the waning immune responsiveness observed in the elderly population.471,472 These multifaceted, evidence-based interventions underscore the pivotal role of lifestyle modifications in preserving immune integrity and ameliorating the deleterious effects of immunosenescence.
Clinical applications
Various clinical trials have been conducted to investigate the aforementioned therapeutic strategies. For example, in the strategy of targeting immune organ rejuvenation, a phase II clinical trial demonstrated the potential of miR-205 mimics to restore thymopoiesis in settings of diminished or impaired T cell output. The underlying mechanism involves the upregulation of FOXN1 and FOXN1-regulated chemokines.436 Additionally, in the context of immune cell-targeted therapies, allogeneic hematopoietic stem cell transplantation (allo-HSCT) has emerged as a primary clinical modality for counteracting immunosenescence and treating associated conditions by targeting the restoration of HSC vitality and function (NCT06484049) (Table 2). Of particular interest is the continuous emergence of clinical trials utilizing de novo T cell generation to treat diseases. A phase I/IIa randomized, placebo-controlled, multicenter study reported that repeated administration of glycosylated recombinant human IL-7 (rhIL-7) in patients infected with human immunodeficiency virus-1 (HIV-1) may facilitate the achievement and maintenance of normal circulating CD4+ T cell counts. More importantly, this treatment promotes the expansion of rejuvenated T cell populations, such as naïve T cells (NCT0047732).473 Furthermore, rhIL-7 significantly expands the diversity of the circulating TCR repertoire. These findings suggest that rhIL-7 therapy can potentiate and broaden immune responses, particularly in individuals with limited naïve T cell populations and reduced TCR repertoire diversity, such as those experiencing advanced age, HIV infection, or iatrogenic (chemotherapy-induced) lymphodepletion.474,475 Consequently, rhIL-7 holds promise for maintaining T cell homeostasis, increasing T cell numbers, and enhancing their cytotoxic functions, not only in idiopathic CD4+ lymphopenia but also in the context of various cancer therapies (Table 2).476 Several clinical trials, including NCT00376935, NCT02356159, and NCT00593554, have aimed primarily to investigate the impact of recombinant human KGF (palifermin) on peripheral T cell reconstitution. However, palifermin may exacerbate thymic dysfunction following alemtuzumab treatment and should not be used to promote T cell recovery392. Importantly, palifermin is generally not utilized as a standalone therapeutic agent but rather in conjunction with other immune-enhancing therapies. Consequently, its potential to enhance immune recovery following T cell-depleting, total body irradiation-based hematopoietic stem cell transplantation is being explored when it is coadministered with leuprolide acetate (NCT01746849) (Table 2) or in combination with busulfan, melphalan, and fludarabine (NCT00629798). Allogeneic CAR-T cell therapy targeting B cell maturation antigens has demonstrated clinical feasibility in relapsed/refractory multiple myeloma.477 Similarly, other types of lymphocyte-B cells have been investigated to determine whether restoring B cell production in the bone marrow or reconstructing the peripheral B cell repertoire could enhance the immune responsiveness of elderly individuals to neoantigen challenges (NCT00863187) (Table 2). Furthermore, as highlighted in the previous section regarding therapeutic strategies, the critical relationship between immunosenescence and inflammation has prompted clinical trials to investigate targeting macrophages (NCT01045512), endothelial cells (NCT04537884), and neutrophils (NCT02441205) (Table 2) in an effort to mitigate immunosenescence and combat conditions such as congestive heart failure, neovascular age-related macular degeneration, and diabetes. However, owing to interindividual variability, specific genetic or epigenetic characteristics may influence therapeutic outcomes. Larger-scale clinical studies are needed to identify optimal treatment strategies that achieve the best health outcomes with minimal side effects.
Even more intriguing is that advancements in artificial intelligence (AI) have the potential to significantly propel the field of personalized medicine forward. By leveraging vast databases, AI can analyze large-scale genomic and epigenomic data to identify patients who are most likely to benefit from specific rejuvenation therapies.478 This capability is based on age-related changes in the immune system and alterations in signaling pathways to predict treatment outcomes, design clinical trials, and tailor personalized therapeutic strategies.478,479 For example, a recently reported integrated bioinformatics analysis utilized machine learning to extract a predefined immunosenescence index from aggregated gene expression data, which can predict treatment outcomes and drug sensitivity in melanoma patients.480 Furthermore, the predictive modeling capabilities of AI can help biologists gain deeper insights into the mechanisms and biological implications of immune aging. Machine learning algorithms can analyze high-dimensional datasets (genomic, transcriptomic, and proteomic) to identify the most relevant biomarkers and potential therapeutic targets, thereby reducing time costs and enhancing treatment efficiency.481,482 Similarly, using AI to track the patient’s physical changes in real time throughout the whole process is conducive to medical staff’s understanding of the patient’s treatment effect and recovery ability, thus providing convenience for medical staff to update the treatment plan.483
Conclusions and future directions
In summary, this review delineates the multifactorial determinants underlying the pathogenesis of immunosenescence and its contributory role in diverse age-related pathologies. The dysregulation of immunosenescence-associated signaling pathways and the unique phenotypic alterations observed in senescent immune cells within these regulatory networks were summarized. Emphasis is placed on how to use targeted therapies, immunomodulatory strategies at the cellular and organ levels, or nutritional and lifestyle interventions to fight immunosenescence. Clinical advances in the development of immunosenescence-targeted treatment methods are also discussed.
Given the inextricable link between immune system dysregulation and organismal aging, future research on preventing or mitigating immunosenescence may prioritize the integrated application of the therapeutic strategies discussed herein. The therapeutic strategy mentioned in the text involves targeting immune organs to counteract immunosenescence by restoring thymic vitality, rejuvenating bone marrow niches, and improving the function of peripheral immune organs, ultimately enhancing the generation and functionality of immune cells. Targeting various immune cells aims to restore their cellular functions, with T cells being the most critical. The senescence of T cells can be reversed through IL-7473 and KGF391,392 therapies, thereby ameliorating associated pathological conditions. Inhibiting NF-κB reverses SASP and delays aging-related pathologies via IKK/NF-κB inhibitors (e.g., 8K-NBD) or phytochemicals such as curcumin and resveratrol.10,278,279,288 MTORC1 inhibition (e.g., rapamycin, RapaLink-1) extends lifespan by enhancing autophagy and metabolic regulation, with clinical trials targeting immunosenescence.295,296,297,302 Mitigating immunosenescence can be achieved through dietary optimization, regular moderate exercise, stress management, sufficient sleep, smoking cessation, and timely vaccination. Crucially, synergistic interplay between these modalities, such as combining senolytics with epigenetic reprogramming or mTOR inhibition, should be rigorously explored to increase efficacy while minimizing unintended adverse effects. A central conundrum in immunosenescence research lies in navigating the dual risks of therapeutic interventions: suppressing age-associated inflammation (“inflammaging”) may inadvertently exacerbate infection susceptibility or compromise basal immune homeostasis, whereas overactivating inflammatory pathways to restore immune function risks precipitates autoimmune pathologies or acute inflammatory sequelae. Achieving this delicate equilibrium demands precision in intervention dosing. Notably, the integration of personalized medicine frameworks and AI into immune aging management is a strategic but challenging frontier. As this field evolves, interdisciplinary collaborations-spanning immunology and computational biology—will be integral to translating these insights into therapies that improve immune resilience and healthy longevity.
In the past few decades, our understanding of immunosenescence has increased, and various treatment methods, such as immunological intervention measures and pathway-targeted drugs, have emerged. However, despite the existence of many treatment methods, the biological changes and underlying mechanisms behind immunosenescence remain unclear. The effects of a single treatment method are not ideal, and various side effects faced in clinical translation hinder the treatment of immunosenescence. In addition, aging has always been regarded as a natural process, and the use of various means to impede this biological process may present many doubts. For example, the genetic changes introduced by gene editing can be passed on to future generations through germline modification, which may lead to unforeseeable genetic complications or ecological impacts on the human gene pool. This poses considerable ethical challenges in both the health and social fields. Moreover, although personalized medicine provides one-on-one treatment for patients, which further improves the treatment plan for patients and is conducive to their further recovery and improvement in their quality of life, the associated cost may be high for ordinary people. As a result, these personalized medical treatments may become exclusive to wealthy individuals or countries, thus deepening the global health gap. Artificial intelligence and machine learning have broad prospects in the field of aging treatment and the importance of further research and development. However, the data security issues they cause are the greatest concern for people. How to balance the relationship between the security of patients’ private data and the transparent management of artificial intelligence is the key to achieving a win‒win situation through the cooperation of various disciplines.
References
Franceschi, C. et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N. Y Acad. Sci. 908, 244–254 (2000).
Palmer, S., Albergante, L., Blackburn, C. C. & Newman, T. J. Thymic involution and rising disease incidence with age. Proc. Natl Acad. Sci. USA 115, 1883–1888 (2018).
McHugh, D., Durán, I. & Gil, J. Senescence as a therapeutic target in cancer and age-related diseases. Nat. Rev. Drug Discov. 24, 57–71 (2025).
Baker, D. J. et al. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature 530, 184–189 (2016).
Liu, Z. et al. Immunosenescence: molecular mechanisms and diseases. Signal Transduct. Target Ther. 8, 200 (2023).
Admasu, T. D. & Yu, J. S. Harnessing immune rejuvenation: advances in overcoming T cell senescence and exhaustion in cancer immunotherapy. Aging Cell 24, e70055 (2025).
Li, X. et al. Inflammation and aging: signaling pathways and intervention therapies. Sig. Transduct. Target Ther. 8, 239 (2023).
Goronzy, J. J. & Weyand, C. M. Mechanisms underlying T cell ageing. Nat. Rev. Immunol. 19, 573–583 (2019).
Yu, P. J., Zhou, M., Liu, Y. & Du, J. Senescent T cells in age-related diseases. Aging Dis. 16, 321–344 (2024).
Tilstra, J. S. et al. NF-κB inhibition delays DNA damage-induced senescence and aging in mice. J. Clin. Invest. 122, 2601–2612 (2012).
Salminen, A. & Kaarniranta, K. NF-kappaB signaling in the aging process. J. Clin. Immunol. 29, 397–405 (2009).
Morgan, M. J. & Liu, Z. G. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 21, 103–115 (2011).
Kabe, Y. et al. Redox regulation of NF-kappaB activation: distinct redox regulation between the cytoplasm and the nucleus. Antioxid. Redox Sig.7, 395–403 (2005).
Djavaheri-Mergny, M. et al. NF-kappaB activation represses tumor necrosis factor-alpha-induced autophagy. J. Biol. Chem. 281, 30373–30382 (2006).
Aman, Y. et al. Autophagy in healthy aging and disease. Nat. Aging 1, 634–650 (2021).
Karin, M. & Lin, A. NF-kappaB at the crossroads of life and death. Nat. Immunol. 3, 221–227 (2002).
Glaviano, A. et al. PI3K/AKT/mTOR signaling transduction pathway and targeted therapies in cancer. Mol. Cancer 22, 138 (2023).
Werlen, G., Jain, R. & Jacinto, E. MTOR signaling and metabolism in early T cell development. Genes 12, 728 (2021).
Zhang, Y. et al. Coordinated regulation of protein synthesis and degradation by mTORC1. Nature 513, 440–443 (2014).
Ali, E. S. et al. The mTORC1-SLC4A7 axis stimulates bicarbonate import to enhance de novo nucleotide synthesis. Mol. Cell 82, 3284–3298.e3287 (2022).
Han, J. & Wang, Y. mTORC1 signaling in hepatic lipid metabolism. Protein Cell 9, 145–151 (2018).
Fan, H. et al. Critical role of mTOR in regulating aerobic glycolysis in carcinogenesis (Review). Int J. Oncol. 58, 9–19 (2021).
Kim, J., Kundu, M., Viollet, B. & Guan, K. L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 13, 132–141 (2011).
Carroll, B. et al. Persistent mTORC1 signaling in cell senescence results from defects in amino acid and growth factor sensing. J. Cell Biol. 216, 1949–1957 (2017).
García-Prat, L. et al. Autophagy maintains stemness by preventing senescence. Nature 529, 37–42 (2016).
Tasdemir, E. et al. Regulation of autophagy by cytoplasmic p53. Nat. Cell Biol. 10, 676–687 (2008).
Kenzelmann Broz, D. et al. Global genomic profiling reveals an extensive p53-regulated autophagy program contributing to key p53 responses. Genes Dev. 27, 1016–1031 (2013).
Asghari, F., Karimi, M. H. & Pourfathollah, A. A. mTORC1 inhibition may improve T lymphocytes affected by aging. Immunopharmacol. Immunotoxicol. 45, 719–729 (2023).
Mannick, J. B. et al. TORC1 inhibition enhances immune function and reduces infections in the elderly. Sci. Transl. Med. 10, eaaq1564 (2018).
Perkey, E., Fingar, D., Miller, R. A. & Garcia, G. G. Increased mammalian target of rapamycin complex 2 signaling promotes age-related decline in CD4 T cell signaling and function. J. Immunol. 191, 4648–4655 (2013).
Wang, Y. et al. The kinase complex mTORC2 promotes the longevity of virus-specific memory CD4(+) T cells by preventing ferroptosis. Nat. Immunol. 23, 303–317 (2022).
Patel, C. H. et al. Cutting edge: mTORC2 Regulates CD8+ effector and memory T cell differentiation through serum and glucocorticoid kinase 1. J. Immunol. 209, 2287–2291 (2022).
Villarino, A. V., Kanno, Y., Ferdinand, J. R. & O’Shea, J. J. Mechanisms of Jak/STAT signaling in immunity and disease. J. Immunol. 194, 21–27 (2015).
Xu, M., Tchkonia, T. & Kirkland, J. L. Perspective: targeting the JAK/STAT pathway to fight age-related dysfunction. Pharm. Res. 111, 152–154 (2016).
Flanagan, S. E. et al. Activating germline mutations in STAT3 cause early-onset multi-organ autoimmune disease. Nat. Genet 46, 812–814 (2014).
Glück, S. & Ablasser, A. Innate immunosensing of DNA in cellular senescence. Curr. Opin. Immunol. 56, 31–36 (2019).
Zhou, J., Zhuang, Z., Li, J. & Feng, Z. Significance of the cGAS-STING Pathway In Health And Disease. Int. J. Mol. Sci. 24, 13316 (2023).
Schmitz, C. R. R. et al. cGAS-STING pathway as a potential trigger of immunosenescence and inflammaging. Front. Immunol. 14, 1132653 (2023).
Han, X. et al. AMPK activation protects cells from oxidative stress-induced senescence via autophagic flux restoration and intracellular NAD(+) elevation. Aging Cell 15, 416–427 (2016).
Wang, S., Kandadi, M. R. & Ren, J. Double knockout of Akt2 and AMPK predisposes cardiac aging without affecting lifespan: role of autophagy and mitophagy. Biochim. Biophys. Acta Mol. Basis Dis. 1865, 1865–1875 (2019).
Ge, Y. et al. Role of AMPK mediated pathways in autophagy and aging. Biochimie 195, 100–113 (2022).
Salminen, A., Kauppinen, A. & Kaarniranta, K. AMPK activation inhibits the functions of myeloid-derived suppressor cells (MDSC): impact on cancer and aging. J. Mol. Med. 97, 1049–1064 (2019).
Rolf, J. et al. AMPKα1: a glucose sensor that controls CD8 T-cell memory. Eur. J. Immunol. 43, 889–896 (2013).
Lanna, A., Henson, S. M., Escors, D. & Akbar, A. N. The kinase p38 activated by the metabolic regulator AMPK and scaffold TAB1 drives the senescence of human T cells. Nat. Immunol. 15, 965–972 (2014).
Atayik, M. C. & Çakatay, U. Melatonin-related signaling pathways and their regulatory effects in aging organisms. Biogerontology 23, 529–539 (2022).
Acuña-Castroviejo, D. et al. Extrapineal melatonin: sources, regulation, and potential functions. Cell Mol. Life Sci. 71, 2997–3025 (2014).
Hardeland, R. Aging, melatonin, and the pro- and anti-inflammatory networks. Int. J. Mol. Sci. 20, 1223 (2019).
El, Mouatassim, S., Guérin, P. & Ménézo, Y. Expression of genes encoding antioxidant enzymes in human and mouse oocytes during the final stages of maturation. Mol. Hum. Reprod. 5, 720–725 (1999).
Manchester, L. C. et al. Melatonin: an ancient molecule that makes oxygen metabolically tolerable. J. Pineal Res. 59, 403–419 (2015).
Tang, Y. L. et al. Ginsenoside Rg1 protects against Sca-1(+) HSC/HPC cell aging by regulating the SIRT1-FOXO3 and SIRT3-SOD2 signaling pathways in a γ-ray irradiation-induced aging mice model. Exp. Ther. Med. 20, 1245–1252 (2020).
Son, M. J., Kwon, Y., Son, T. & Cho, Y. S. Restoration of mitochondrial NAD(+) levels delays stem cell senescence and facilitates reprogramming of aged somatic cells. Stem Cells 34, 2840–2851 (2016).
Liu, T. F. et al. NAD+-dependent SIRT1 deacetylase participates in epigenetic reprogramming during endotoxin tolerance. J. Biol. Chem. 286, 9856–9864 (2011).
Owczarczyk, A. B. et al. Sirtuin 1 regulates dendritic cell activation and autophagy during respiratory syncytial virus-induced immune responses. J. Immunol. 195, 1637–1646 (2015).
Jiang, B. et al. Sirtuin 2 up-regulation suppresses the anti-tumour activity of exhausted natural killer cells in mesenteric lymph nodes in murine colorectal carcinoma. Scand. J. Immunol. 98, e13317 (2023).
Xiao, F. et al. Sirtuin 6 is a negative regulator of the anti-tumor function of natural killer cells in murine inflammatory colorectal cancer. Mol. Immunol. 158, 68–78 (2023).
Zhang, J. et al. The type III histone deacetylase Sirt1 is essential for maintenance of T cell tolerance in mice. J. Clin. Invest 119, 3048–3058 (2009).
Gámez-García, A. & Vazquez, B. N. Nuclear sirtuins and the aging of the immune system. Genes 12, 2021 (1856).
Sun, D. et al. Epigenomic profiling of young and aged HSCs reveals concerted changes during aging that reinforce self-renewal. Cell Stem Cell 14, 673–688 (2014).
Flach, J. et al. Replication stress is a potent driver of functional decline in ageing haematopoietic stem cells. Nature 512, 198–202 (2014).
Pang, W. W. et al. Human bone marrow hematopoietic stem cells are increased in frequency and myeloid-biased with age. Proc. Natl Acad. Sci. USA 108, 20012–20017 (2011).
Beerman, I. et al. Functionally distinct hematopoietic stem cells modulate hematopoietic lineage potential during aging by a mechanism of clonal expansion. Proc. Natl Acad. Sci. USA 107, 5465–5470 (2010).
Kovtonyuk, L. V. et al. IL-1 mediates microbiome-induced inflammaging of hematopoietic stem cells in mice. Blood 139, 44–58 (2022).
Maryanovich, M. et al. Adrenergic nerve degeneration in bone marrow drives aging of the hematopoietic stem cell niche. Nat. Med. 24, 782–791 (2018).
Grover, A. et al. Single-cell RNA sequencing reveals molecular and functional platelet bias of aged haematopoietic stem cells. Nat. Commun. 7, 11075 (2016).
Ho, T. T. et al. Autophagy maintains the metabolism and function of young and old stem cells. Nature 543, 205–210 (2017).
Chung, H. Y. et al. Redefining Chronic Inflammation in Aging and Age-Related Diseases: Proposal of the Senoinflammation Concept. Aging Dis. 10, 367–382 (2019).
Seidler, S. et al. Age-dependent alterations of monocyte subsets and monocyte-related chemokine pathways in healthy adults. BMC Immunol. 11, 30 (2010).
Verschoor, C. P. et al. Blood CD33(+)HLA-DR(-) myeloid-derived suppressor cells are increased with age and a history of cancer. J. Leukoc. Biol. 93, 633–637 (2013).
Hearps, A. C. et al. Aging is associated with chronic innate immune activation and dysregulation of monocyte phenotype and function. Aging Cell 11, 867–875 (2012).
Van Avondt, K. et al. Neutrophils in aging and aging-related pathologies. Immunol. Rev. 314, 357–375 (2023).
Gullotta, G. S. et al. Age-induced alterations of granulopoiesis generate atypical neutrophils that aggravate stroke pathology. Nat. Immunol. 24, 925–940 (2023).
Maier-Begandt, D. et al. Neutrophils-biology and diversity. Nephrol. Dial. Transpl. 39, 1551–1564 (2024).
Sapey, E. et al. Phosphoinositide 3-kinase inhibition restores neutrophil accuracy in the elderly: toward targeted treatments for immunosenescence. Blood 123, 239–248 (2014).
Vázquez-Prieto, M. et al. Sex-dependent effect of aging on calcium signaling and expression of TRPM2 and CRAC channels in human neutrophils. Hum. Immunol. 83, 645–655 (2022).
Richer, B. C., Salei, N., Laskay, T. & Seeger, K. Changes in Neutrophil Metabolism upon Activation and Aging. Inflammation 41, 710–721 (2018).
Lagnado, A. et al. Neutrophils induce paracrine telomere dysfunction and senescence in ROS-dependent manner. EMBO J. 40, e106048 (2021).
Kulkarni, U. et al. Excessive neutrophil levels in the lung underlie the age-associated increase in influenza mortality. Mucosal Immunol. 12, 545–554 (2019).
Aurrand-Lions, M. et al. Junctional adhesion molecule-C regulates the early influx of leukocytes into tissues during inflammation. J. Immunol. 174, 6406–6415 (2005).
Roy-O’Reilly, M. A. et al. Aging exacerbates neutrophil pathogenicity in ischemic stroke. Aging12, 436–461 (2020).
Nyugen, J., Agrawal, S., Gollapudi, S. & Gupta, S. Impaired functions of peripheral blood monocyte subpopulations in aged humans. J. Clin. Immunol. 30, 806–813 (2010).
Hilt, Z. T. et al. Platelet-derived β2m regulates age related monocyte/macrophage functions. Aging 11, 11955–11974 (2019).
Smith, L. K. et al. β2-microglobulin is a systemic pro-aging factor that impairs cognitive function and neurogenesis. Nat. Med. 21, 932–937 (2015).
Pararasa, C. et al. Age-associated changes in long-chain fatty acid profile during healthy aging promote pro-inflammatory monocyte polarization via PPARγ. Aging Cell 15, 128–139 (2016).
van Duin, D. et al. Age-associated defect in human TLR-1/2 function. J. Immunol. 178, 970–975 (2007).
Ivashkiv, L. B. Epigenetic regulation of macrophage polarization and function. Trends Immunol. 34, 216–223 (2013).
Li, W. Phagocyte dysfunction, tissue aging and degeneration. Ageing Res. Rev. 12, 1005–1012 (2013).
George, A. J. & Ritter, M. A. Thymic involution with ageing: obsolescence or good housekeeping?. Immunol. Today 17, 267–272 (1996).
Aspinall, R. & Andrew, D. Thymic involution in aging. J. Clin. Immunol. 20, 250–256 (2000).
Haynes, B. F. et al. The role of the thymus in immune reconstitution in aging, bone marrow transplantation, and HIV-1 infection. Annu. Rev. Immunol. 18, 529–560 (2000).
Frasca, D. et al. Aging down-regulates the transcription factor E2A, activation-induced cytidine deaminase, and Ig class switch in human B cells. J. Immunol. 180, 5283–5290 (2008).
Frasca, D., Van der Put, E., Riley, R. L. & Blomberg, B. B. Reduced Ig class switch in aged mice correlates with decreased E47 and activation-induced cytidine deaminase. J. Immunol. 172, 2155–2162 (2004).
Yager, E. J. et al. Age-associated decline in T cell repertoire diversity leads to holes in the repertoire and impaired immunity to influenza virus. J. Exp. Med. 205, 711–723 (2008).
Goronzy, J. J., Lee, W. W. & Weyand, C. M. Aging and T-cell diversity. Exp. Gerontol. 42, 400–406 (2007).
Pido-Lopez, J., Imami, N., Andrew, D. & Aspinall, R. Molecular quantitation of thymic output in mice and the effect of IL-7. Eur. J. Immunol. 32, 2827–2836 (2002).
Velardi, E., Tsai, J. J. & van den Brink, M. R. M. T cell regeneration after immunological injury. Nat. Rev. Immunol. 21, 277–291 (2021).
Mueller, S. N. & Germain, R. N. Stromal cell contributions to the homeostasis and functionality of the immune system. Nat. Rev. Immunol. 9, 618–629 (2009).
Zhao, Y., Shao, Q. & Peng, G. Exhaustion and senescence: two crucial dysfunctional states of T cells in the tumor microenvironment. Cell Mol. Immunol. 17, 27–35 (2020).
Crespo, J. et al. T cell anergy, exhaustion, senescence, and stemness in the tumor microenvironment. Curr. Opin. Immunol. 25, 214–221 (2013).
Weng, N. P., Akbar, A. N. & Goronzy, J. CD28(-) T cells: their role in the age-associated decline of immune function. Trends Immunol. 30, 306–312 (2009).
Lian, J., Yue, Y., Yu, W. & Zhang, Y. Immunosenescence: a key player in cancer development. J. Hematol. Oncol. 13, 151 (2020).
Decman, V. et al. Defective CD8 T cell responses in aged mice are due to quantitative and qualitative changes in virus-specific precursors. J. Immunol. 188, 1933–1941 (2012).
Krishnamurthy, J. et al. p16INK4a induces an age-dependent decline in islet regenerative potential. Nature 443, 453–457 (2006).
Yang, O. O. et al. Decreased perforin and granzyme B expression in senescent HIV-1-specific cytotoxic T lymphocytes. Virology 332, 16–19 (2005).
Shimatani, K. et al. PD-1+ memory phenotype CD4+ T cells expressing C/EBPalpha underlie T cell immunodepression in senescence and leukemia. Proc. Natl Acad. Sci. USA 106, 15807–15812 (2009).
Zhao, L. et al. Changes of CD4+CD25+Foxp3+ regulatory T cells in aged Balb/c mice. J. Leukoc. Biol. 81, 1386–1394 (2007).
Thomas, R., Wang, W. & Su, D. M. Contributions of age-related thymic involution to immunosenescence and inflammaging. Immun. Ageing 17, 2 (2020).
Czesnikiewicz-Guzik, M. et al. T cell subset-specific susceptibility to aging. Clin. Immunol. 127, 107–118 (2008).
Floess, S. et al. Epigenetic control of the foxp3 locus in regulatory T cells. PLoS Biol. 5, e38 (2007).
Kogut, I., Scholz, J. L., Cancro, M. P. & Cambier, J. C. B cell maintenance and function in aging. Semin. Immunol. 24, 342–349 (2012).
Dhakal, S. et al. Estradiol mediates greater germinal center responses to influenza vaccination in female than male mice. mBio 15, e0032624 (2024).
Fukushima, Y., Minato, N. & Hattori, M. The impact of senescence-associated T cells on immunosenescence and age-related disorders. Inflamm. Regen. 38, 24 (2018).
Pulko, V. et al. Human memory T cells with a naive phenotype accumulate with aging and respond to persistent viruses. Nat. Immunol. 17, 966–975 (2016).
Nikolich-Žugich, J. The twilight of immunity: emerging concepts in aging of the immune system. Nat. Immunol. 19, 10–19 (2018).
Mold, J. E. et al. Divergent clonal differentiation trajectories establish CD8(+) memory T cell heterogeneity during acute viral infections in humans. Cell Rep. 35, 109174 (2021).
Le Saout, C., Mennechet, S., Taylor, N. & Hernandez, J. Memory-like CD8+ and CD4+ T cells cooperate to break peripheral tolerance under lymphopenic conditions. Proc. Natl Acad. Sci. USA 105, 19414–19419 (2008).
Cancro, M. P. Age-associated B cells. Annu Rev. Immunol. 38, 315–340 (2020).
Phalke, S. et al. Molecular mechanisms controlling age-associated B cells in autoimmunity. Immunol. Rev. 307, 79–100 (2022).
Simchoni, N. & Cunningham-Rundles, C. TLR7- and TLR9-responsive human B cells share phenotypic and genetic characteristics. J. Immunol. 194, 3035–3044 (2015).
Crinier, A., Narni-Mancinelli, E., Ugolini, S. & Vivier, E. SnapShot: natural killer cells. Cell 180, 1280–1280.e1281 (2020).
Solana, R., Campos, C., Pera, A. & Tarazona, R. Shaping of NK cell subsets by aging. Curr. Opin. Immunol. 29, 56–61 (2014).
Chidrawar, S. M. et al. Ageing is associated with a decline in peripheral blood CD56bright NK cells. Immun. Ageing 3, 10 (2006).
Campos, C. et al. Expression of NKp30, NKp46 and DNAM-1 activating receptors on resting and IL-2 activated NK cells from healthy donors according to CMV-serostatus and age. Biogerontology 16, 671–683 (2015).
Manser, A. R. & Uhrberg, M. Age-related changes in natural killer cell repertoires: impact on NK cell function and immune surveillance. Cancer Immunol. Immunother. 65, 417–426 (2016).
Brauning, A. et al. Aging of the immune system: focus on natural killer cells phenotype and functions. Cells 11, 1017 (2022).
Bin, N. R. et al. C2 domains of Munc13-4 are crucial for Ca(2+)-dependent degranulation and cytotoxicity in NK cells. J. Immunol. 201, 700–713 (2018).
Wong, P. et al. T-BET and EOMES sustain mature human NK cell identity and antitumor function. J. Clin. Invest. 133, e162530 (2023).
Shehata, H. M., Hoebe, K. & Chougnet, C. A. The aged nonhematopoietic environment impairs natural killer cell maturation and function. Aging Cell 14, 191–199 (2015).
Lu, J. et al. Declined miR-181a-5p expression is associated with impaired natural killer cell development and function with aging. Aging Cell 20, e13353 (2021).
Najar, M. et al. Mesenchymal stromal cells of the bone marrow and natural killer cells: cell interactions and cross modulation. J. Cell Commun. Sig.12, 673–688 (2018).
Agrawal, A. et al. Altered innate immune functioning of dendritic cells in elderly humans: a role of phosphoinositide 3-kinase-signaling pathway. J. Immunol. 178, 6912–6922 (2007).
Pereira, L. F., de Souza, A. P., Borges, T. J. & Bonorino, C. Impaired in vivo CD4+ T cell expansion and differentiation in aged mice is not solely due to T cell defects: decreased stimulation by aged dendritic cells. Mech. Ageing Dev. 132, 187–194 (2011).
Sridharan, A. et al. Age-associated impaired plasmacytoid dendritic cell functions lead to decreased CD4 and CD8 T cell immunity. Age33, 363–376 (2011).
Chougnet, C. A. et al. Loss of phagocytic and antigen cross-presenting capacity in aging dendritic cells is associated with mitochondrial dysfunction. J. Immunol. 195, 2624–2632 (2015).
Zhou, H. & Wu, L. The non-canonical Wnt pathway leads to aged dendritic cell differentiation. Cell Mol. Immunol. 15, 871–872 (2018).
El Mezayen, R., El Gazzar, M., Myer, R. & High, K. P. Aging-dependent upregulation of IL-23p19 gene expression in dendritic cells is associated with differential transcription factor binding and histone modifications. Aging Cell 8, 553–565 (2009).
Wu, Y., Goplen, N. P. & Sun, J. Aging and respiratory viral infection: from acute morbidity to chronic sequelae. Cell Biosci. 11, 112 (2021).
Chikuma, S. et al. TRIM28 expression on dendritic cells prevents excessive T cell priming by silencing endogenous retrovirus. J. Immunol. 206, 1528–1539 (2021).
Thevaranjan, N. et al. Age-associated microbial dysbiosis promotes intestinal permeability, systemic inflammation, and macrophage dysfunction. Cell Host Microbe 21, 455–466.e454 (2017).
Johansson, A., Ho, N. P. & Takizawa, H. Microbiome and Hemato-immune Aging. Exp. Hematol. 141, 104685 (2025).
Arpaia, N. & Rudensky, A. Y. Microbial metabolites control gut inflammatory responses. Proc. Natl Acad. Sci. USA 111, 2058–2059 (2014).
Corrêa-Oliveira, R. et al. Regulation of immune cell function by short-chain fatty acids. Clin. Transl. Immunol. 5, e73 (2016).
Boyajian, J. L. et al. Microbiome and human aging: probiotic and prebiotic potentials in longevity, skin health and cellular senescence. Nutrients. 13, 4550 (2021).
Zhang, S. et al. Gut microbiota composition and metabolic potential of long-living people in China. Front. Aging Neurosci. 14, 820108 (2022).
Byrd, A. L., Belkaid, Y. & Segre, J. A. The human skin microbiome. Nat. Rev. Microbiol 16, 143–155 (2018).
Longhi, G. et al. Highly conserved bifidobacteria in the human gut: Bifidobacterium longum subsp. longum as a potential modulator of elderly innate immunity. Benef. Microbes 15, 241–258 (2024).
Uppal, S. S., Verma, S. & Dhot, P. S. Normal values of CD4 and CD8 lymphocyte subsets in healthy indian adults and the effects of sex, age, ethnicity, and smoking. Cytom. B Clin. Cytom. 52, 32–36 (2003).
Arakelyan, N. A., Kupriyanova, D. A., Vasilevska, J. & Rogaev, E. I. Sexual dimorphism in immunity and longevity among the oldest old. Front. Immunol. 16, 1525948 (2025).
Zeng, Y. et al. Sex differences in genetic associations with longevity. JAMA Netw. Open 1, e181670 (2018).
Suarez, L. M. et al. Sex differences in neuroimmunoendocrine communication. Involvement on longevity. Mech. Ageing Dev. 211, 111798 (2023).
Yanicke, S. & Ucar, D. Sex differences in immune system aging and responsiveness to vaccination. Public Policy Aging Rep. 33, 125–127 (2023).
Costantini, E., D’Angelo, C. & Reale, M. The role of immunosenescence in neurodegenerative diseases. Mediators Inflamm. 2018, 6039171 (2018).
Murdaca, G. et al. Neuro-inflammaging and psychopathological distress. Biomedicines. 10, 2133 (2022).
Griffin, W. S. Inflammation and neurodegenerative diseases. Am. J. Clin. Nutr. 83, 470s–474s (2006).
Heppner, F. L., Ransohoff, R. M. & Becher, B. Immune attack: the role of inflammation in Alzheimer disease. Nat. Rev. Neurosci. 16, 358–372 (2015).
Burns, A. & Iliffe, S. Alzheimer’s disease. BMJ 338, b158 (2009).
Duyckaerts, C., Delatour, B. & Potier, M. C. Classification and basic pathology of Alzheimer disease. Acta Neuropathol. 118, 5–36 (2009).
Floden, A. M. & Combs, C. K. Microglia demonstrate age-dependent interaction with amyloid-β fibrils. J. Alzheimers Dis. 25, 279–293 (2011).
Li, Q. et al. Microglia and immunotherapy in Alzheimer’s disease. Acta Neurol. Scand. 145, 273–278 (2022).
Njie, E. G. et al. Ex vivo cultures of microglia from young and aged rodent brain reveal age-related changes in microglial function. Neurobiol. Aging 33, 195.e191–112 (2012).
Dhapola, R. et al. Recent advances in molecular pathways and therapeutic implications targeting neuroinflammation for Alzheimer’s disease. Inflammopharmacology 29, 1669–1681 (2021).
Luo, X. G., Ding, J. Q. & Chen, S. D. Microglia in the aging brain: relevance to neurodegeneration. Mol. Neurodegener. 5, 12 (2010).
Heneka, M. T., Kummer, M. P. & Latz, E. Innate immune activation in neurodegenerative disease. Nat. Rev. Immunol. 14, 463–477 (2014).
Louveau, A. et al. Structural and functional features of central nervous system lymphatic vessels. Nature 523, 337–341 (2015).
Barbé-Tuana, F. et al. The interplay between immunosenescence and age-related diseases. Semin. Immunopathol. 42, 545–557 (2020).
Larbi, A. et al. Dramatic shifts in circulating CD4 but not CD8 T cell subsets in mild Alzheimer’s disease. J. Alzheimers Dis. 17, 91–103 (2009).
Machhi, J. et al. CD4+ effector T cells accelerate Alzheimer’s disease in mice. J. Neuroinflamm.18, 272 (2021).
Xia, T. et al. Benefit delayed immunosenescence by regulating CD4(+)T cells: a promising therapeutic target for aging-related diseases. Aging Cell 23, e14317 (2024).
Dansokho, C. et al. Regulatory T cells delay disease progression in Alzheimer-like pathology. Brain 139, 1237–1251 (2016).
Dombrowski, Y. et al. Regulatory T cells promote myelin regeneration in the central nervous system. Nat. Neurosci. 20, 674–680 (2017).
Frasca, D. & Blomberg, B. B. B cell function and influenza vaccine responses in healthy aging and disease. Curr. Opin. Immunol. 29, 112–118 (2014).
Dorshkind, K. & Swain, S. Age-associated declines in immune system development and function: causes, consequences, and reversal. Curr. Opin. Immunol. 21, 404–407 (2009).
Britschgi, M. et al. Neuroprotective natural antibodies to assemblies of amyloidogenic peptides decrease with normal aging and advancing Alzheimer’s disease. Proc. Natl Acad. Sci. USA 106, 12145–12150 (2009).
Keren-Shaul, H. et al. A unique microglia type associated with restricting development of Alzheimer’s disease. Cell 169, 1276–1290.e1217 (2017).
Spillantini, M. G. et al. Alpha-synuclein in Lewy bodies. Nature 388, 839–840 (1997).
Dexter, D. T. & Jenner, P. Parkinson disease: from pathology to molecular disease mechanisms. Free Radic. Biol. Med. 62, 132–144 (2013).
Singleton, A. B. et al. alpha-Synuclein locus triplication causes Parkinson’s disease. Science 302, 841 (2003).
Block, M. L., Zecca, L. & Hong, J. S. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat. Rev. Neurosci. 8, 57–69 (2007).
Kortekaas, R. et al. Blood-brain barrier dysfunction in parkinsonian midbrain in vivo. Ann. Neurol. 57, 176–179 (2005).
Perry, V. H. Contribution of systemic inflammation to chronic neurodegeneration. Acta Neuropathol. 120, 277–286 (2010).
Gao, Y. et al. CD4(+) T-cell senescence in neurodegenerative disease: pathogenesis and potential therapeutic targets. Cells. 13, 749 (2024).
Gao, H. et al. Role of hypoxia in cellular senescence. Pharm. Res. 194, 106841 (2023).
Sommer, A. et al. Th17 lymphocytes induce neuronal cell death in a human iPSC-based model of Parkinson’s disease. Cell Stem Cell 24, 1006 (2019).
Coppé, J. P., Desprez, P. Y., Krtolica, A. & Campisi, J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu. Rev. Pathol. 5, 99–118 (2010).
Yasuda, T. et al. Inflammation-driven senescence-associated secretory phenotype in cancer-associated fibroblasts enhances peritoneal dissemination. Cell Rep. 34, 108779 (2021).
Coppé, J. P. et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 6, 2853–2868 (2008).
Haston, S. et al. Clearance of senescent macrophages ameliorates tumorigenesis in KRAS-driven lung cancer. Cancer Cell 41, 1242–1260.e1246 (2023).
Memarzadeh, S. et al. Enhanced paracrine FGF10 expression promotes formation of multifocal prostate adenocarcinoma and an increase in epithelial androgen receptor. Cancer Cell 12, 572–585 (2007).
Nicholes, K. et al. A mouse model of hepatocellular carcinoma: ectopic expression of fibroblast growth factor 19 in skeletal muscle of transgenic mice. Am. J. Pathol. 160, 2295–2307 (2002).
Kim, Y. H. et al. Senescent tumor cells lead the collective invasion in thyroid cancer. Nat. Commun. 8, 15208 (2017).
Demaria, M. et al. Cellular senescence promotes adverse effects of chemotherapy and cancer relapse. Cancer Discov. 7, 165–176 (2017).
Parrinello, S., Coppe, J. P., Krtolica, A. & Campisi, J. Stromal-epithelial interactions in aging and cancer: senescent fibroblasts alter epithelial cell differentiation. J. Cell Sci. 118, 485–496 (2005).
Oubaha, M. et al. Senescence-associated secretory phenotype contributes to pathological angiogenesis in retinopathy. Sci. Transl. Med. 8, 362ra144 (2016).
Zhang, X. et al. Research progress on the interaction between oxidative stress and platelets: another avenue for cancer?. Pharm. Res. 191, 106777 (2023).
Cao, Z. et al. Reshaping the immune microenvironment and reversing immunosenescence by natural products: Prospects for immunotherapy in gastric cancer. Semin. Cancer Biol. 110, 1–16 (2025).
Wang, Y. et al. Ferroptosis and immunosenescence in colorectal cancer. Semin Cancer Biol. 106-107, 156–165 (2024).
Liu, Z. et al. Targeting immunosenescence for improved tumor immunotherapy. MedComm5, e777 (2024).
Grolleau-Julius, A., Harning, E. K., Abernathy, L. M. & Yung, R. L. Impaired dendritic cell function in aging leads to defective antitumor immunity. Cancer Res 68, 6341–6349 (2008).
Guo, Z., Tilburgs, T., Wong, B. & Strominger, J. L. Dysfunction of dendritic cells in aged C57BL/6 mice leads to failure of natural killer cell activation and of tumor eradication. Proc. Natl Acad. Sci. USA 111, 14199–14204 (2014).
Takasugi, M., Yoshida, Y. & Ohtani, N. Cellular senescence and the tumour microenvironment. Mol. Oncol. 16, 3333–3351 (2022).
Oh, J. et al. Age-related tumor growth in mice is related to integrin α 4 in CD8+ T cells. JCI Insight. 3, e122961 (2018).
Eggert, T. et al. Distinct functions of senescence-associated immune responses in liver tumor surveillance and tumor progression. Cancer Cell 30, 533–547 (2016).
Kang, T. W. et al. Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature 479, 547–551 (2011).
Pawelec, G. Immunosenescence and cancer. Biogerontology 18, 717–721 (2017).
Milanovic, M. et al. Senescence-associated reprogramming promotes cancer stemness. Nature 553, 96–100 (2018).
Ruhland, M. K. et al. Stromal senescence establishes an immunosuppressive microenvironment that drives tumorigenesis. Nat. Commun. 7, 11762 (2016).
Levard, D. et al. Central nervous system-associated macrophages modulate the immune response following stroke in aged mice. Nat. Neurosci. 27, 1721–1733 (2024).
Zhivaki, D. et al. Correction of age-associated defects in dendritic cells enables CD4(+) T cells to eradicate tumors. Cell 187, 3888–3903.e3818 (2024).
Maggiorani, D. et al. Senescence drives immunotherapy resistance by inducing an immunosuppressive tumor microenvironment. Nat. Commun. 15, 2435 (2024).
Nie, R. C. et al. Efficacy of anti-PD-1/PD-L1 monotherapy or combinational therapy in patients aged 75 years or older: a study-level meta-analysis. Front Oncol. 11, 538174 (2021).
Wherry, E. J. & Kurachi, M. Molecular and cellular insights into T cell exhaustion. Nat. Rev. Immunol. 15, 486–499 (2015).
Silva-Cayetano, A. et al. Spatial dysregulation of T follicular helper cells impairs vaccine responses in aging. Nat. Immunol. 24, 1124–1137 (2023).
Blaeser, A., McGlauchlen, K. & Vogel, L. A. Aged B lymphocytes retain their ability to express surface markers but are dysfunctional in their proliferative capability during early activation events. Immun. Ageing 5, 15 (2008).
Levin, E. G. et al. Waning Immune Humoral Response to BNT162b2 Covid-19 Vaccine over 6 Months. N. Engl. J. Med. 385, e84 (2021).
Zhu, F. C. et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 396, 479–488 (2020).
Schumacher, T. N. & Schreiber, R. D. Neoantigens in cancer immunotherapy. Science 348, 69–74 (2015).
Kemeny, H. R. et al. Targeting PD-L1 initiates effective antitumor immunity in a murine model of cushing disease. Clin. Cancer Res. 26, 1141–1151 (2020).
Genolet, R. et al. TCR sequencing and cloning methods for repertoire analysis and isolation of tumor-reactive TCRs. Cell Rep. Methods 3, 100459 (2023).
Stadtmauer, E. A. et al. CRISPR-engineered T cells in patients with refractory cancer. Science. 367, eaba7365 (2020).
Wu, X. et al. MicroRNA: another pharmacological avenue for colorectal cancer?. Front. Cell Dev. Biol. 8, 812 (2020).
Li, S. et al. Exosomes: Another intercellular lipometabolic communication mediators in digestive system neoplasms?. Cytokine Growth Factor Rev. 73, 93–100 (2023).
O’Leary, M. C. et al. FDA approval summary: tisagenlecleucel for treatment of patients with relapsed or refractory B-cell precursor acute lymphoblastic leukemia. Clin. Cancer Res 25, 1142–1146 (2019).
Berdeja, J. G. et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): a phase 1b/2 open-label study. Lancet 398, 314–324 (2021).
Locke, F. L. et al. Axicabtagene ciloleucel as second-line therapy for large B-cell lymphoma. N. Engl. J. Med. 386, 640–654 (2022).
Rejeski, K. et al. The CAR-HEMATOTOX risk-stratifies patients for severe infections and disease progression after CD19 CAR-T in R/R LBCL. J. Immunother. Cancer 10, e004475 (2022).
Rejeski, K., Jain, M. D. & Smith, E. L. Mechanisms of resistance and treatment of relapse after CAR T-cell therapy for large B-cell lymphoma and multiple myeloma. Transpl. Cell Ther. 29, 418–428 (2023).
Singh, N., Perazzelli, J., Grupp, S. A. & Barrett, D. M. Early memory phenotypes drive T cell proliferation in patients with pediatric malignancies. Sci. Transl. Med. 8, 320ra323 (2016).
Hinrichs, C. S. et al. Adoptively transferred effector cells derived from naive rather than central memory CD8+ T cells mediate superior antitumor immunity. Proc. Natl Acad. Sci. USA 106, 17469–17474 (2009).
Fraietta, J. A. et al. Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat. Med. 24, 563–571 (2018).
Grosso, J. F. et al. LAG-3 regulates CD8+ T cell accumulation and effector function in murine self- and tumor-tolerance systems. J. Clin. Invest. 117, 3383–3392 (2007).
Yan, Z. X. et al. Cholesterol efflux from C1QB-expressing macrophages is associated with resistance to chimeric antigen receptor T cell therapy in primary refractory diffuse large B cell lymphoma. Nat. Commun. 15, 5183 (2024).
Hernandez-Segura, A., Nehme, J. & Demaria, M. Hallmarks of cellular senescence. Trends Cell Biol. 28, 436–453 (2018).
Kochenderfer, J. N. et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood 119, 2709–2720 (2012).
Lee, D. W. et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet 385, 517–528 (2015).
Thomas, C. J., Mirza, R. G. & Gill, M. K. Age-related macular degeneration. Med Clin. North Am. 105, 473–491 (2021).
Nussenblatt, R. B. & Ferris, F. 3rd Age-related macular degeneration and the immune response: implications for therapy. Am. J. Ophthalmol. 144, 618–626 (2007).
Khan, A. H., Chowers, I. & Lotery, A. J. Beyond the Complement Cascade: Insights into Systemic Immunosenescence and Inflammaging in Age-Related Macular Degeneration and Current Barriers to Treatment. Cells. 12, 1708 (2023).
Doyle, S. L. et al. NLRP3 has a protective role in age-related macular degeneration through the induction of IL-18 by drusen components. Nat. Med. 18, 791–798 (2012).
Karlstetter, M. & Langmann, T. Microglia in the aging retina. Adv. Exp. Med. Biol. 801, 207–212 (2014).
Alves, C. H., Fernandes, R., Santiago, A. R. & Ambrósio, A. F. Microglia contribution to the regulation of the retinal and choroidal vasculature in age-related macular degeneration. Cells. 9, 1217 (2020).
Nakamura, R. et al. IL10-driven STAT3 signalling in senescent macrophages promotes pathological eye angiogenesis. Nat. Commun. 6, 7847 (2015).
Bhutto, I. A. et al. Increased choroidal mast cells and their degranulation in age-related macular degeneration. Br. J. Ophthalmol. 100, 720–726 (2016).
Zeng, J. et al. Neutrophil extracellular traps boost laser-induced mouse choroidal neovascularization through the activation of the choroidal endothelial cell TLR4/HIF-1α pathway. FEBS J. 290, 5395–5410 (2023).
Choi, Y. J. et al. Chemokine receptor profiles of T cells in patients with age-related macular degeneration. Yonsei Med. J. 63, 357–364 (2022).
Kubicka-Trząska, A. et al. Genetic variants of complement factor H Y402H (rs1061170), C2 R102G (rs2230199), and C3 E318D (rs9332739) and response to intravitreal anti-VEGF treatment in patients with exudative age-related macular degeneration. Medicina. 58, 658 (2022).
Maugeri, A. et al. Complement system and age-related macular degeneration: implications of gene-environment interaction for preventive and personalized medicine. Biomed. Res. Int. 2018, 7532507 (2018).
Franceschi, C. et al. Inflammaging and ‘Garb-aging. Trends Endocrinol. Metab. 28, 199–212 (2017).
Esser, N. et al. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res. Clin. Pr. 105, 141–150 (2014).
Lee, Y. H. et al. Senescent T cells predict the development of hyperglycemia in humans. Diabetes 68, 156–162 (2019).
Thimmappa, P. Y. et al. Neutrophil (dys)function due to altered immuno-metabolic axis in type 2 diabetes: implications in combating infections. Hum. Cell 36, 1265–1282 (2023).
Tam, B. T., Morais, J. A. & Santosa, S. Obesity and ageing: two sides of the same coin. Obes. Rev. 21, e12991 (2020).
Green, W. D. & Beck, M. A. Obesity impairs the adaptive immune response to influenza virus. Ann. Am. Thorac. Soc. 14, S406–s409 (2017).
Frasca, D., Romero, M. & Blomberg, B. B. Similarities in B cell defects between aging and obesity. J. Immunol. 213, 1407–1413 (2024).
Parisi, M. M. et al. Immunosenescence induced by plasma from individuals with obesity caused cell signaling dysfunction and inflammation. Obesity25, 1523–1531 (2017).
Tu, W. & Rao, S. Mechanisms underlying T cell immunosenescence: aging and cytomegalovirus infection. Front. Microbiol. 7, 2111 (2016).
Zhou, Y. et al. Pathogenic T-cells and inflammatory monocytes incite inflammatory storms in severe COVID-19 patients. Natl Sci. Rev. 7, 998–1002 (2020).
Del Valle, D. M. et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat. Med. 26, 1636–1643 (2020).
Collier, D. A. et al. Age-related immune response heterogeneity to SARS-CoV-2 vaccine BNT162b2. Nature 596, 417–422 (2021).
Muik, A. et al. Neutralization of SARS-CoV-2 Omicron by BNT162b2 mRNA vaccine-elicited human sera. Science 375, 678–680 (2022).
Goronzy, J. J. & Weyand, C. M. Understanding immunosenescence to improve responses to vaccines. Nat. Immunol. 14, 428–436 (2013).
Christenson, S. A., Smith, B. M., Bafadhel, M. & Putcha, N. Chronic obstructive pulmonary disease. Lancet 399, 2227–2242 (2022).
Cho, W. K., Lee, C. G. & Kim, L. K. COPD as a disease of immunosenescence. Yonsei Med. J. 60, 407–413 (2019).
Alder, J. K. et al. Short telomeres are a risk factor for idiopathic pulmonary fibrosis. Proc. Natl Acad. Sci. USA 105, 13051–13056 (2008).
Armanios, M. Y. et al. Telomerase mutations in families with idiopathic pulmonary fibrosis. N. Engl. J. Med. 356, 1317–1326 (2007).
Fernandez, I. E. & Eickelberg, O. The impact of TGF-β on lung fibrosis: from targeting to biomarkers. Proc. Am. Thorac. Soc. 9, 111–116 (2012).
Fessler, J. et al. Senescent T-cells promote bone loss in rheumatoid arthritis. Front. Immunol. 9, 95 (2018).
Chen, B., Yan, Y., Wang, H. & Xu, J. Association between genetically determined telomere length and health-related outcomes: a systematic review and meta-analysis of Mendelian randomization studies. Aging Cell 22, e13874 (2023).
López-Otín, C. et al. Hallmarks of aging: an expanding universe. Cell 186, 243–278 (2023).
Franceschi, C. et al. Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 14, 576–590 (2018).
Donato, A. J., Morgan, R. G., Walker, A. E. & Lesniewski, L. A. Cellular and molecular biology of aging endothelial cells. J. Mol. Cell Cardiol. 89, 122–135 (2015).
Libby, P., Ridker, P. M. & Hansson, G. K. Inflammation in atherosclerosis: from pathophysiology to practice. J. Am. Coll. Cardiol. 54, 2129–2138 (2009).
Leon, M. L. & Zuckerman, S. H. Gamma interferon: a central mediator in atherosclerosis. Inflamm. Res. 54, 395–411 (2005).
Franceschi, C. & Campisi, J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J. Gerontol. A Biol. Sci. Med. Sci. 69, S4–9 (2014).
Mauro, A. G. et al. NLRP3 inflammasome in acute myocardial infarction. J. Cardiovasc. Pharm. 74, 175–187 (2019).
Youn, J. C. et al. Immunosenescent CD8+ T cells and C-X-C chemokine receptor type 3 chemokines are increased in human hypertension. Hypertension 62, 126–133 (2013).
Liuzzo, G. et al. Perturbation of the T-cell repertoire in patients with unstable angina. Circulation 100, 2135–2139 (1999).
Kirkham, F. et al. Cytomegalovirus infection is associated with an increase in aortic stiffness in older men which may be mediated in part by CD4 memory T-cells. Theranostics 11, 5728–5741 (2021).
Adler, A. S. et al. Motif module map reveals enforcement of aging by continual NF-kappaB activity. Genes Dev. 21, 3244–3257 (2007).
Kim, H. J. et al. Antioxidant alpha-lipoic acid inhibits osteoclast differentiation by reducing nuclear factor-kappaB DNA binding and prevents in vivo bone resorption induced by receptor activator of nuclear factor-kappaB ligand and tumor necrosis factor-alpha. Free Radic. Biol. Med. 40, 1483–1493 (2006).
Sivandzade, F., Prasad, S., Bhalerao, A. & Cucullo, L. NRF2 and NF-қB interplay in cerebrovascular and neurodegenerative disorders: Molecular mechanisms and possible therapeutic approaches. Redox Biol. 21, 101059 (2019).
Sogbein, O. et al. Bortezomib in cancer therapy: mechanisms, side effects, and future proteasome inhibitors. Life Sci. 358, 123125 (2024).
Chondrogianni, N. et al. Proteasome activation: an innovative promising approach for delaying aging and retarding age-related diseases. Ageing Res. Rev. 23, 37–55 (2015).
Yousefzadeh, M. J. et al. Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine 36, 18–28 (2018).
Ijima, S. et al. Fisetin reduces the senescent tubular epithelial cell burden and also inhibits proliferative fibroblasts in murine lupus nephritis. Front. Immunol. 13, 960601 (2022).
Izadi, M. et al. Longevity and anti-aging effects of curcumin supplementation. Geroscience 46, 2933–2950 (2024).
Justice, J. N. et al. Senolytics in idiopathic pulmonary fibrosis: results from a first-in-human, open-label, pilot study. EBioMedicine 40, 554–563 (2019).
Nambiar, A. et al. Senolytics dasatinib and quercetin in idiopathic pulmonary fibrosis: results of a phase I, single-blind, single-center, randomized, placebo-controlled pilot trial on feasibility and tolerability. EBioMedicine 90, 104481 (2023).
Zhang, Y. et al. Curcumin analogue EF24 prevents alveolar epithelial cell senescence to ameliorate idiopathic pulmonary fibrosis via activation of PTEN. Phytomedicine 133, 155882 (2024).
Zhu, H. et al. Resveratrol alleviates inflammation and ER stress through SIRT1/NRF2 to delay ovarian aging in a short-lived fish. J. Gerontol. A Biol. Sci. Med. Sci. 78, 596–602 (2023).
Varela-Eirín, M. et al. Targeting of chondrocyte plasticity via connexin43 modulation attenuates cellular senescence and fosters a pro-regenerative environment in osteoarthritis. Cell Death Dis. 9, 1166 (2018).
Salminen, A. & Kaarniranta, K. Regulation of the aging process by autophagy. Trends Mol. Med. 15, 217–224 (2009).
Carosi, J. M., Fourrier, C., Bensalem, J. & Sargeant, T. J. The mTOR-lysosome axis at the centre of ageing. FEBS Open Bio 12, 739–757 (2022).
Sena, L. A. & Chandel, N. S. Physiological roles of mitochondrial reactive oxygen species. Mol. Cell 48, 158–167 (2012).
Dillin, A. et al. Rates of behavior and aging specified by mitochondrial function during development. Science 298, 2398–2401 (2002).
Monaghan, R. M. et al. A nuclear role for the respiratory enzyme CLK-1 in regulating mitochondrial stress responses and longevity. Nat. Cell Biol. 17, 782–792 (2015).
Harrison, D. E. et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460, 392–395 (2009).
Bjedov, I. et al. Mechanisms of life span extension by rapamycin in the fruit fly Drosophila melanogaster. Cell Metab. 11, 35–46 (2010).
Robida-Stubbs, S. et al. TOR signaling and rapamycin influence longevity by regulating SKN-1/Nrf and DAF-16/FoxO. Cell Metab. 15, 713–724 (2012).
Cloughesy, T. F. et al. Antitumor activity of rapamycin in a Phase I trial for patients with recurrent PTEN-deficient glioblastoma. PLoS Med. 5, e8 (2008).
Feldman, M. E. et al. Active-site inhibitors of mTOR target rapamycin-resistant outputs of mTORC1 and mTORC2. PLoS Biol. 7, e38 (2009).
Marafie, S. K., Al-Mulla, F. & Abubaker, J. mTOR: its critical role in metabolic diseases, cancer, and the aging process. Int. J. Mol. Sci. 25, 6141 (2024).
Jhanwar-Uniyal, M. et al. Distinct signaling mechanisms of mTORC1 and mTORC2 in glioblastoma multiforme: a tale of two complexes. Adv. Biol. Regul. 57, 64–74 (2015).
Wang, N. et al. RapaLink-1 outperforms rapamycin in alleviating allogeneic graft rejection by inhibiting the mTORC1-4E-BP1 pathway in mice. Int. Immunopharmacol. 125, 111172 (2023).
Mannick, J. B. et al. Targeting the biology of ageing with mTOR inhibitors to improve immune function in older adults: phase 2b and phase 3 randomised trials. Lancet Healthy Longev. 2, e250–e262 (2021).
Xu, M. et al. JAK inhibition alleviates the cellular senescence-associated secretory phenotype and frailty in old age. Proc. Natl Acad. Sci. USA 112, E6301–6310 (2015).
Meyer, S. C. & Levine, R. L. Molecular pathways: molecular basis for sensitivity and resistance to JAK kinase inhibitors. Clin. Cancer Res. 20, 2051–2059 (2014).
Toso, A. et al. Enhancing chemotherapy efficacy in Pten-deficient prostate tumors by activating the senescence-associated antitumor immunity. Cell Rep. 9, 75–89 (2014).
Harel, S. et al. Pharmacologic inhibition of JAK-STAT signaling promotes hair growth. Sci. Adv. 1, e1500973 (2015).
Price, F. D. et al. Inhibition of JAK-STAT signaling stimulates adult satellite cell function. Nat. Med. 20, 1174–1181 (2014).
Kaisar, M. A. et al. Offsetting the impact of smoking and e-cigarette vaping on the cerebrovascular system and stroke injury: is Metformin a viable countermeasure?. Redox Biol. 13, 353–362 (2017).
Hardie, D. G. AMP-activated protein kinase: maintaining energy homeostasis at the cellular and whole-body levels. Annu. Rev. Nutr. 34, 31–55 (2014).
Finley, J. Cellular stress and AMPK activation as a common mechanism of action linking the effects of metformin and diverse compounds that alleviate accelerated aging defects in Hutchinson-Gilford progeria syndrome. Med. Hypotheses 118, 151–162 (2018).
Cai, H. et al. Metformin attenuates the D‑galactose‑induced aging process via the UPR through the AMPK/ERK1/2 signaling pathways. Int. J. Mol. Med. 45, 715–730 (2020).
Liu, J. et al. Mitochondrial dysfunction launches dexamethasone-induced skeletal muscle atrophy via AMPK/FOXO3 signaling. Mol. Pharm. 13, 73–84 (2016).
Hu, C. et al. Oleanolic acid induces autophagy and apoptosis via the AMPK-mTOR signaling pathway in colon cancer. J. Oncol. 2021, 8281718 (2021).
Chen, G., Kroemer, G. & Kepp, O. Mitophagy: an emerging role in aging and age-associated diseases. Front. Cell Dev. Biol. 8, 200 (2020).
Wang, J. et al. Antioxidant and pro-oxidant activities of melatonin in the presence of copper and polyphenols in vitro and in vivo. Cells. 8, 903 (2019).
Reiter, R. J., Ma, Q. & Sharma, R. Melatonin in mitochondria: mitigating clear and present dangers. Physiology 35, 86–95 (2020).
Zhang, H. M. & Zhang, Y. Melatonin: a well-documented antioxidant with conditional pro-oxidant actions. J. Pineal Res. 57, 131–146 (2014).
Hardeland, R., Cardinali, D. P., Brown, G. M. & Pandi-Perumal, S. R. Melatonin and brain inflammaging. Prog. Neurobiol. 127-128, 46–63 (2015).
Cardinali, D. P. & Hardeland, R. Inflammaging, metabolic syndrome and melatonin: a call for treatment studies. Neuroendocrinology 104, 382–397 (2017).
Dominguez-Rodriguez, A. & Abreu-Gonzalez, P. Future strategies for acute cardioprotection: ‘melatonin as promising therapy’. Cardiovasc. Res. 113, 1418 (2017).
Lee, S. H., Lee, J. H., Lee, H. Y. & Min, K. J. Sirtuin signaling in cellular senescence and aging. BMB Rep. 52, 24–34 (2019).
Bonkowski, M. S. & Sinclair, D. A. Slowing ageing by design: the rise of NAD(+) and sirtuin-activating compounds. Nat. Rev. Mol. Cell Biol. 17, 679–690 (2016).
Xu, C. et al. SIRT1 is downregulated by autophagy in senescence and ageing. Nat. Cell Biol. 22, 1170–1179 (2020).
Guo, J. et al. Role of Sirt1 plays in nucleus pulposus cells and intervertebral disc degeneration. Spine 42, E757–e766 (2017).
Lim, C. J. et al. Aquatide activation of SIRT1 reduces cellular senescence through a SIRT1-FOXO1-autophagy axis. Biomol. Ther. 25, 511–518 (2017).
Howitz, K. T. et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 425, 191–196 (2003).
Pang, J. et al. SIRT1 protects cochlear hair cell and delays age-related hearing loss via autophagy. Neurobiol. Aging 80, 127–137 (2019).
Sin, T. K. et al. Effects of long-term resveratrol-induced SIRT1 activation on insulin and apoptotic signalling in aged skeletal muscle. Acta Diabetol. 52, 1063–1075 (2015).
Yeung, F. et al. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 23, 2369–2380 (2004).
Ou, X. et al. SIRT1 positively regulates autophagy and mitochondria function in embryonic stem cells under oxidative stress. Stem Cells 32, 1183–1194 (2014).
Kauppinen, A. et al. Antagonistic crosstalk between NF-κB and SIRT1 in the regulation of inflammation and metabolic disorders. Cell Signal 25, 1939–1948 (2013).
Zhang, H. et al. Genistein protects against ox-LDL-induced senescence through enhancing SIRT1/LKB1/AMPK-mediated autophagy flux in HUVECs. Mol. Cell Biochem. 455, 127–134 (2019).
Wang, Y. et al. Mitochondrial biogenesis is impaired in osteoarthritis chondrocytes but reversible via peroxisome proliferator-activated receptor γ coactivator 1α. Arthritis Rheumatol. 67, 2141–2153 (2015).
Gu, Y. et al. The tyrosine kinase inhibitor Dasatinib reduces cardiac steatosis and fibrosis in obese, type 2 diabetic mice. Cardiovasc. Diabetol. 22, 214 (2023).
Ulusoy, H. G. & Sanlier, N. A minireview of quercetin: from its metabolism to possible mechanisms of its biological activities. Crit. Rev. Food Sci. Nutr. 60, 3290–3303 (2020).
Islam, M. T. et al. Senolytic drugs, dasatinib and quercetin, attenuate adipose tissue inflammation, and ameliorate metabolic function in old age. Aging Cell 22, e13767 (2023).
Murakami, T., Inagaki, N. & Kondoh, H. Cellular senescence in diabetes mellitus: distinct senotherapeutic strategies for adipose tissue and pancreatic β cells. Front Endocrinol. (Lausanne) 13, 869414 (2022).
Novais, E. J. et al. Long-term treatment with senolytic drugs Dasatinib and Quercetin ameliorates age-dependent intervertebral disc degeneration in mice. Nat. Commun. 12, 5213 (2021).
Saccon, T. D. et al. Senolytic combination of dasatinib and quercetin alleviates intestinal senescence and inflammation and modulates the gut microbiome in aged mice. J. Gerontol. A Biol. Sci. Med. Sci. 76, 1895–1905 (2021).
Zhu, Y. et al. Identification of a novel senolytic agent, navitoclax, targeting the Bcl-2 family of anti-apoptotic factors. Aging Cell 15, 428–435 (2016).
Chang, J. et al. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat. Med. 22, 78–83 (2016).
Le Couteur, D. G. et al. Aging biology and novel targets for drug discovery. J. Gerontol. A Biol. Sci. Med. Sci. 67, 168–174 (2012).
Croker, B. A. et al. Fas-mediated neutrophil apoptosis is accelerated by Bid, Bak, and Bax and inhibited by Bcl-2 and Mcl-1. Proc. Natl Acad. Sci. USA 108, 13135–13140 (2011).
Eijkelenboom, A. & Burgering, B. M. FOXOs: signalling integrators for homeostasis maintenance. Nat. Rev. Mol. Cell Biol. 14, 83–97 (2013).
Baar, M. P. et al. Targeted apoptosis of senescent cells restores tissue homeostasis in response to chemotoxicity and aging. Cell 169, 132–147.e116 (2017).
Barnes, T. C. et al. Endothelial activation and apoptosis mediated by neutrophil-dependent interleukin 6 trans-signalling: a novel target for systemic sclerosis?. Ann. Rheum. Dis. 70, 366–372 (2011).
Huang, Y., He, Y., Makarcyzk, M. J. & Lin, H. Senolytic peptide FOXO4-DRI selectively removes senescent cells from in vitro expanded human chondrocytes. Front. Bioeng. Biotechnol. 9, 677576 (2021).
Liu, Y. et al. FOXO4-D-Retro-Inverso targets extracellular matrix production in fibroblasts and ameliorates bleomycin-induced pulmonary fibrosis in mice. Naunyn Schmiedebergs Arch. Pharm. 396, 2393–2403 (2023).
Geiger, H., de Haan, G. & Florian, M. C. The ageing haematopoietic stem cell compartment. Nat. Rev. Immunol. 13, 376–389 (2013).
Geiger, H. & Rudolph, K. L. Aging in the lympho-hematopoietic stem cell compartment. Trends Immunol. 30, 360–365 (2009).
Xing, Z. et al. Increased hematopoietic stem cell mobilization in aged mice. Blood 108, 2190–2197 (2006).
Vijg, J. & Dollé, M. E. Large genome rearrangements as a primary cause of aging. Mech. Ageing Dev. 123, 907–915 (2002).
Rossi, D. J. et al. Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature 447, 725–729 (2007).
Niedernhofer, L. J. et al. A new progeroid syndrome reveals that genotoxic stress suppresses the somatotroph axis. Nature 444, 1038–1043 (2006).
Rudolph, K. L. et al. Longevity, stress response, and cancer in aging telomerase-deficient mice. Cell 96, 701–712 (1999).
Allsopp, R. C. et al. Telomerase is required to slow telomere shortening and extend replicative lifespan of HSCs during serial transplantation. Blood 102, 517–520 (2003).
Hosokawa, K. et al. The telomere binding protein Pot1 maintains haematopoietic stem cell activity with age. Nat. Commun. 8, 804 (2017).
Bernardes de Jesus, B. et al. Telomerase gene therapy in adult and old mice delays aging and increases longevity without increasing cancer. EMBO Mol. Med. 4, 691–704 (2012).
Park, Y. & Gerson, S. L. DNA repair defects in stem cell function and aging. Annu. Rev. Med. 56, 495–508 (2005).
Ito, K. et al. Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature 431, 997–1002 (2004).
Attema, J. L. et al. Hematopoietic stem cell ageing is uncoupled from p16 INK4A-mediated senescence. Oncogene 28, 2238–2243 (2009).
Ross, J. B. et al. Depleting myeloid-biased haematopoietic stem cells rejuvenates aged immunity. Nature 628, 162–170 (2024).
Jang, Y. Y. & Sharkis, S. J. A low level of reactive oxygen species selects for primitive hematopoietic stem cells that may reside in the low-oxygenic niche. Blood 110, 3056–3063 (2007).
Brown, K. et al. SIRT3 reverses aging-associated degeneration. Cell Rep. 3, 319–327 (2013).
Florian, M. C. et al. Cdc42 activity regulates hematopoietic stem cell aging and rejuvenation. Cell Stem Cell 10, 520–530 (2012).
Carrillo-García, C. & Janzen, V. Restoring cell polarity: an HSC fountain of youth. Cell Stem Cell 10, 481–482 (2012).
Hua, H. et al. Targeting mTOR for cancer therapy. J. Hematol. Oncol. 12, 71 (2019).
Liu, W. et al. Rational identification of a Cdc42 inhibitor presents a new regimen for long-term hematopoietic stem cell mobilization. Leukemia 33, 749–761 (2019).
Florian, M. C. et al. Inhibition of Cdc42 activity extends lifespan and decreases circulating inflammatory cytokines in aged female C57BL/6 mice. Aging Cell 19, e13208 (2020).
Liu, L. & Rando, T. A. Manifestations and mechanisms of stem cell aging. J. Cell Biol. 193, 257–266 (2011).
Conboy, I. M. & Rando, T. A. Heterochronic parabiosis for the study of the effects of aging on stem cells and their niches. Cell Cycle 11, 2260–2267 (2012).
He, H. et al. Aging-induced IL27Ra signaling impairs hematopoietic stem cells. Blood 136, 183–198 (2020).
Brack, A. S. et al. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science 317, 807–810 (2007).
Conboy, I. M. et al. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 433, 760–764 (2005).
Villeda, S. A. et al. Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nat. Med. 20, 659–663 (2014).
Villeda, S. A. et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature 477, 90–94 (2011).
Baht, G. S. et al. Exposure to a youthful circulaton rejuvenates bone repair through modulation of β-catenin. Nat. Commun. 6, 7131 (2015).
Pangrazzi, L. & Weinberger, B. T cells, aging and senescence. Exp. Gerontol. 134, 110887 (2020).
Lanna, A. et al. An intercellular transfer of telomeres rescues T cells from senescence and promotes long-term immunological memory. Nat. Cell Biol. 24, 1461–1474 (2022).
Lamarche, C. et al. Tonic-signaling chimeric antigen receptors drive human regulatory T cell exhaustion. Proc. Natl Acad. Sci. USA 120, e2219086120 (2023).
Qasim, W. Genome-edited allogeneic donor “universal” chimeric antigen receptor T cells. Blood 141, 835–845 (2023).
Gomes-Silva, D. et al. Tonic 4-1BB costimulation in chimeric antigen receptors impedes T cell survival and is vector-dependent. Cell Rep. 21, 17–26 (2017).
Poorebrahim, M. et al. Genetically modified immune cells targeting tumor antigens. Pharm. Ther. 214, 107603 (2020).
Alpdogan, O. et al. IL-7 enhances peripheral T cell reconstitution after allogeneic hematopoietic stem cell transplantation. J. Clin. Invest. 112, 1095–1107 (2003).
Perales, M. A. et al. Recombinant human interleukin-7 (CYT107) promotes T-cell recovery after allogeneic stem cell transplantation. Blood 120, 4882–4891 (2012).
Li, L. et al. IL-12 inhibits thymic involution by enhancing IL-7- and IL-2-induced thymocyte proliferation. J. Immunol. 172, 2909–2916 (2004).
Tieu, R. et al. Tissue-resident memory T cell maintenance during antigen persistence requires both cognate antigen and interleukin-15. Sci. Immunol. 8, eadd8454 (2023).
Nguyen, R. et al. Cooperative armoring of CAR and TCR T cells by T cell-restricted IL15 and IL21 universally enhances solid tumor efficacy. Clin. Cancer Res 30, 1555–1566 (2024).
Dudakov, J. A. et al. Loss of thymic innate lymphoid cells leads to impaired thymopoiesis in experimental graft-versus-host disease. Blood 130, 933–942 (2017).
Rossi, S. W. et al. Keratinocyte growth factor (KGF) enhances postnatal T-cell development via enhancements in proliferation and function of thymic epithelial cells. Blood 109, 3803–3811 (2007).
Coles, A. J. et al. Keratinocyte growth factor impairs human thymic recovery from lymphopenia. JCI Insight. 5, e125377 (2019).
Seggewiss, R. et al. Keratinocyte growth factor augments immune reconstitution after autologous hematopoietic progenitor cell transplantation in rhesus macaques. Blood 110, 441–449 (2007).
Kelly, R. M. et al. Keratinocyte growth factor and androgen blockade work in concert to protect against conditioning regimen-induced thymic epithelial damage and enhance T-cell reconstitution after murine bone marrow transplantation. Blood 111, 5734–5744 (2008).
Barr, I. G. et al. Dihydrotestosterone and estradiol deplete corticosensitive thymocytes lacking in receptors for these hormones. J. Immunol. 128, 2825–2828 (1982).
Heng, T. S. et al. Effects of castration on thymocyte development in two different models of thymic involution. J. Immunol. 175, 2982–2993 (2005).
Sutherland, J. S. et al. Enhanced immune system regeneration in humans following allogeneic or autologous hemopoietic stem cell transplantation by temporary sex steroid blockade. Clin. Cancer Res. 14, 1138–1149 (2008).
Roden, A. C. et al. Augmentation of T cell levels and responses induced by androgen deprivation. J. Immunol. 173, 6098–6108 (2004).
van Poppel, H. & Nilsson, S. Testosterone surge: rationale for gonadotropin-releasing hormone blockers?. Urology 71, 1001–1006 (2008).
Hun, M. L. et al. Gender disparity impacts on thymus aging and LHRH receptor antagonist-induced thymic reconstitution following chemotherapeutic damage. Front. Immunol. 11, 302 (2020).
Yoshida, S. et al. The CD153 vaccine is a senotherapeutic option for preventing the accumulation of senescent T cells in mice. Nat. Commun. 11, 2482 (2020).
Ghamar Talepoor, A., Khosropanah, S. & Doroudchi, M. Partial recovery of senescence in circulating follicular helper T cells after Dasatinib treatment. Int. Immunopharmacol. 94, 107465 (2021).
Obukhova, L. A., Skulachev, V. P. & Kolosova, N. G. Mitochondria-targeted antioxidant SkQ1 inhibits age-dependent involution of the thymus in normal and senescence-prone rats. Aging 1, 389–401 (2009).
Bharath, L. P. et al. Metformin enhances autophagy and normalizes mitochondrial function to alleviate aging-associated inflammation. Cell Metab. 32, 44–55.e46 (2020).
Hasani-Sadrabadi, M. M. et al. Harnessing biomaterials to amplify immunity in aged mice through T memory stem cells. ACS Nano 18, 6908–6926 (2024).
Naradikian, M. S. et al. Cutting edge: IL-4, IL-21, and IFN-γ interact to govern T-bet and CD11c expression in TLR-activated B cells. J. Immunol. 197, 1023–1028 (2016).
Treml, L. S. et al. TLR stimulation modifies BLyS receptor expression in follicular and marginal zone B cells. J. Immunol. 178, 7531–7539 (2007).
Hao, Y. et al. A B-cell subset uniquely responsive to innate stimuli accumulates in aged mice. Blood 118, 1294–1304 (2011).
Messaoudi, I., Warner, J. & Nikolich-Zugich, J. Age-related CD8+ T cell clonal expansions express elevated levels of CD122 and CD127 and display defects in perceiving homeostatic signals. J. Immunol. 177, 2784–2792 (2006).
Russell Knode, L. M. et al. Age-associated B cells express a diverse repertoire of V(H) and Vκ genes with somatic hypermutation. J. Immunol. 198, 1921–1927 (2017).
Camell, C. D. et al. Aging induces an Nlrp3 inflammasome-dependent expansion of adipose B cells that impairs metabolic homeostasis. Cell Metab. 30, 1024–1039.e1026 (2019).
Metur, S. P. & Klionsky, D. J. The curious case of polyamines: spermidine drives reversal of B cell senescence. Autophagy 16, 389–390 (2020).
André, P. et al. Anti-NKG2A mAb is a checkpoint inhibitor that promotes anti-tumor immunity by unleashing both T and NK cells. Cell 175, 1731–1743.e1713 (2018).
Pereira, B. I. et al. Senescent cells evade immune clearance via HLA-E-mediated NK and CD8(+) T cell inhibition. Nat. Commun. 10, 2387 (2019).
Ouyang, Y. B., Lu, Y., Yue, S. & Giffard, R. G. miR-181 targets multiple Bcl-2 family members and influences apoptosis and mitochondrial function in astrocytes. Mitochondrion 12, 213–219 (2012).
Vasamsetti, S. B. et al. Metformin inhibits monocyte-to-macrophage differentiation via AMPK-mediated inhibition of STAT3 activation: potential role in atherosclerosis. Diabetes 64, 2028–2041 (2015).
De Maeyer, R. P. H. et al. Blocking elevated p38 MAPK restores efferocytosis and inflammatory resolution in the elderly. Nat. Immunol. 21, 615–625 (2020).
Zhou, Z. et al. Type 2 cytokine signaling in macrophages protects from cellular senescence and organismal aging. Immunity 57, 513–527.e516 (2024).
Ma, S. et al. Spatial transcriptomic landscape unveils immunoglobin-associated senescence as a hallmark of aging. Cell 187, 7025–7044.e7034 (2024).
Brigger, D. et al. Eosinophils regulate adipose tissue inflammation and sustain physical and immunological fitness in old age. Nat. Metab. 2, 688–702 (2020).
Brahmakshatriya, V. et al. IL-6 production by TLR-activated APC broadly enhances aged cognate CD4 helper and B cell antibody responses in vivo. J. Immunol. 198, 2819–2833 (2017).
Stebegg, M. et al. Rejuvenating conventional dendritic cells and T follicular helper cell formation after vaccination. Elife. 9, e52473 (2020).
Berzins, S. P., Godfrey, D. I., Miller, J. F. & Boyd, R. L. A central role for thymic emigrants in peripheral T cell homeostasis. Proc. Natl Acad. Sci. USA 96, 9787–9791 (1999).
Chu, Y. W. et al. Exogenous insulin-like growth factor 1 enhances thymopoiesis predominantly through thymic epithelial cell expansion. Blood 112, 2836–2846 (2008).
Moses, A. et al. Comprehensive phenotypic analysis of diverse FOXN1 variants. J. Allergy Clin. Immunol. 152, 1273–1291.e1215 (2023).
Sun, L. et al. Declining expression of a single epithelial cell-autonomous gene accelerates age-related thymic involution. Aging Cell 9, 347–357 (2010).
Bredenkamp, N. et al. An organized and functional thymus generated from FOXN1-reprogrammed fibroblasts. Nat. Cell Biol. 16, 902–908 (2014).
Oh, J., Wang, W., Thomas, R. & Su, D. M. Thymic rejuvenation via FOXN1-reprogrammed embryonic fibroblasts (FREFs) to counteract age-related inflammation. JCI Insight. 5, e140313 (2020).
Wang, W. et al. Extracellular vesicles extracted from young donor serum attenuate inflammaging via partially rejuvenating aged T-cell immunotolerance. FASEB J. 32, fj201800059R (2018).
Min, D. et al. Sustained thymopoiesis and improvement in functional immunity induced by exogenous KGF administration in murine models of aging. Blood 109, 2529–2537 (2007).
Tsai, P. T., Lee, R. A. & Wu, H. BMP4 acts upstream of FGF in modulating thymic stroma and regulating thymopoiesis. Blood 102, 3947–3953 (2003).
Henson, S. M. et al. An IL-7 fusion protein that shows increased thymopoietic ability. J. Immunol. 175, 4112–4118 (2005).
Suzuki, G. et al. Loss of SDF-1 receptor expression during positive selection in the thymus. Int. Immunol. 10, 1049–1056 (1998).
Wurbel, M. A. et al. The chemokine TECK is expressed by thymic and intestinal epithelial cells and attracts double- and single-positive thymocytes expressing the TECK receptor CCR9. Eur. J. Immunol. 30, 262–271 (2000).
Park, C. Y. et al. A resource for the conditional ablation of microRNAs in the mouse. Cell Rep. 1, 385–391 (2012).
Hoover, A. R. et al. MicroRNA-205 maintains T cell development following stress by regulating forkhead Box N1 and selected chemokines. J. Biol. Chem. 291, 23237–23247 (2016).
Sims, R., Hill, M. & Williams, J. The multiplex model of the genetics of Alzheimer’s disease. Nat. Neurosci. 23, 311–322 (2020).
Sun, P. Y. et al. Rejuvenation of peripheral immune cells attenuates Alzheimer’s disease-like pathologies and behavioral deficits in a mouse model. Sci. Adv. 10, eadl1123 (2024).
Pangrazzi, L. et al. Inflamm-aging” influences immune cell survival factors in human bone marrow. Eur. J. Immunol. 47, 481–492 (2017).
Thompson, H. L. et al. Lymph nodes as barriers to T-cell rejuvenation in aging mice and nonhuman primates. Aging Cell 18, e12865 (2019).
Lenti, E. et al. Therapeutic regeneration of lymphatic and immune cell functions upon lympho-organoid transplantation. Stem Cell Rep. 12, 1260–1268 (2019).
El-Naseery, N. I. et al. Aging-associated immunosenescence via alterations in splenic immune cell populations in rat. Life Sci. 241, 117168 (2020).
Yousefzadeh, M. J. et al. An aged immune system drives senescence and ageing of solid organs. Nature 594, 100–105 (2021).
Ciaglia, E. et al. Transfer of the longevity-associated variant of BPIFB4 gene rejuvenates immune system and vasculature by a reduction of CD38(+) macrophages and NAD(+) decline. Cell Death Dis. 13, 86 (2022).
Pangrazzi, L. & Meryk, A. Molecular and cellular mechanisms of immunosenescence: modulation through interventions and lifestyle changes. Biology 14, 17 (2024).
Gutiérrez, S., Svahn, S. L. & Johansson, M. E. Effects of omega-3 fatty acids on immune cells. Int. J. Mol. Sci. 20, 5028 (2019).
Roessler, C. et al. Impact of polyunsaturated fatty acids on miRNA profiles of monocytes/macrophages and endothelial cells-a pilot study. Int. J. Mol. Sci. 18, 284 (2017).
Summers, C. et al. Neutrophil kinetics in health and disease. Trends Immunol. 31, 318–324 (2010).
Venet, F. & Monneret, G. Advances in the understanding and treatment of sepsis-induced immunosuppression. Nat. Rev. Nephrol. 14, 121–137 (2018).
Baumgarth, N. The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat. Rev. Immunol. 11, 34–46 (2011).
Pawelec, G. Hallmarks of human “immunosenescence”: adaptation or dysregulation?. Immun. Ageing 9, 15 (2012).
Aiello, A. et al. Immunosenescence and its hallmarks: how to oppose aging strategically? A review of potential options for therapeutic intervention. Front. Immunol. 10, 2247 (2019).
Kouakanou, L. et al. Vitamin C promotes the proliferation and effector functions of human γδ T cells. Cell Mol. Immunol. 17, 462–473 (2020).
Xu, B. et al. Fungi, immunosenescence and cancer. Semin. Cancer Biol. 109, 67–82 (2025).
Lee, K. A. et al. Role of the gut microbiome for cancer patients receiving immunotherapy: dietary and treatment implications. Eur. J. Cancer 138, 149–155 (2020).
Lam, K. C. et al. Microbiota triggers STING-type I IFN-dependent monocyte reprogramming of the tumor microenvironment. Cell 184, 5338–5356.e5321 (2021).
Chastin, S. F. M. et al. Effects of regular physical activity on the immune system, vaccination and risk of community-acquired infectious disease in the general population: systematic review and meta-analysis. Sports Med. 51, 1673–1686 (2021).
Sellami, M. et al. Effects of acute and chronic exercise on immunological parameters in the elderly aged: can physical activity counteract the effects of aging?. Front Immunol. 9, 2187 (2018).
Duggal, N. A. et al. Can physical activity ameliorate immunosenescence and thereby reduce age-related multi-morbidity?. Nat. Rev. Immunol. 19, 563–572 (2019).
Nieman, D. C. & Wentz, L. M. The compelling link between physical activity and the body’s defense system. J. Sport Health Sci. 8, 201–217 (2019).
Shen, B. et al. A mechanosensitive peri-arteriolar niche for osteogenesis and lymphopoiesis. Nature 591, 438–444 (2021).
Tylutka, A., Morawin, B., Gramacki, A. & Zembron-Lacny, A. Lifestyle exercise attenuates immunosenescence; flow cytometry analysis. BMC Geriatr. 21, 200 (2021).
Black, D. S. & Slavich, G. M. Mindfulness meditation and the immune system: a systematic review of randomized controlled trials. Ann. N. Y Acad. Sci. 1373, 13–24 (2016).
Glaser, R. & Kiecolt-Glaser, J. K. Stress-induced immune dysfunction: implications for health. Nat. Rev. Immunol. 5, 243–251 (2005).
Weyh, C., Krüger, K. & Strasser, B. Physical activity and diet shape the immune system during aging. Nutrients 12, 622 (2020).
Ganz, F. D. Sleep and immune function. Crit. Care Nurse 32, e19–25 (2012).
Mander, B. A., Winer, J. R. & Walker, M. P. Sleep and Human Aging. Neuron 94, 19–36 (2017).
Sheng, C. et al. Early-life smoking, cardiovascular disease risk, and the mediating role of DNA methylation biomarkers of aging. J. Transl. Med. 23, 484 (2025).
Sopori, M. Effects of cigarette smoke on the immune system. Nat. Rev. Immunol. 2, 372–377 (2002).
Stämpfli, M. R. & Anderson, G. P. How cigarette smoke skews immune responses to promote infection, lung disease and cancer. Nat. Rev. Immunol. 9, 377–384 (2009).
Cadar, A. N., Martin, D. E. & Bartley, J. M. Targeting the hallmarks of aging to improve influenza vaccine responses in older adults. Immun. Ageing 20, 23 (2023).
Allen, J. C., Toapanta, F. R., Chen, W. & Tennant, S. M. Understanding immunosenescence and its impact on vaccination of older adults. Vaccine 38, 8264–8272 (2020).
Lévy, Y. et al. Effects of recombinant human interleukin 7 on T-cell recovery and thymic output in HIV-infected patients receiving antiretroviral therapy: results of a phase I/IIa randomized, placebo-controlled, multicenter study. Clin. Infect. Dis. 55, 291–300 (2012).
Sportès, C. et al. Administration of rhIL-7 in humans increases in vivo TCR repertoire diversity by preferential expansion of naive T cell subsets. J. Exp. Med. 205, 1701–1714 (2008).
Rosenberg, S. A. et al. IL-7 administration to humans leads to expansion of CD8+ and CD4+ cells but a relative decrease of CD4+ T-regulatory cells. J. Immunother. 29, 313–319 (2006).
Sheikh, V. et al. Administration of interleukin-7 increases CD4 T cells in idiopathic CD4 lymphocytopenia. Blood 127, 977–988 (2016).
Mailankody, S. et al. Allogeneic BCMA-targeting CAR T cells in relapsed/refractory multiple myeloma: phase 1 UNIVERSAL trial interim results. Nat. Med. 29, 422–429 (2023).
Gomes, B. & Ashley, E. A. Artificial intelligence in molecular medicine. N. Engl. J. Med. 388, 2456–2465 (2023).
Cunningham, J. W. et al. Artificial intelligence in cardiovascular clinical trials. J. Am. Coll. Cardiol. 84, 2051–2062 (2024).
Zhu, L. et al. Machine learning-derived immunosenescence index for predicting outcome and drug sensitivity in patients with skin cutaneous melanoma. Genes Immun. 25, 219–231 (2024).
Mann, M., Kumar, C., Zeng, W. F. & Strauss, M. T. Artificial intelligence for proteomics and biomarker discovery. Cell Syst. 12, 759–770 (2021).
Goodyear, K. et al. Estimating apparent age using artificial intelligence: Quantifying the effect of blepharoplasty. J. Plast. Reconstr. Aesthet. Surg. 85, 336–343 (2023).
Saliev, T. & Singh, P. B. From bench to bedside: translating cellular rejuvenation therapies into clinical applications. Cells. 13, 2052 (2024).
Negi, G., Kumar, A. & Sharma, S. S. Nrf2 and NF-κB modulation by sulforaphane counteracts multiple manifestations of diabetic neuropathy in rats and high glucose-induced changes. Curr. Neurovasc. Res. 8, 294–304 (2011).
Wang, X. et al. Nrf2 upregulates ATP binding cassette transporter expression and activity at the blood-brain and blood-spinal cord barriers. J. Neurosci. 34, 8585–8593 (2014).
Mao, L. et al. Disruption of Nrf2 exacerbated the damage after spinal cord injury in mice. J. Trauma Acute Care Surg. 72, 189–198 (2012).
Nakshatri, H. et al. Constitutive activation of NF-kappaB during progression of breast cancer to hormone-independent growth. Mol. Cell Biol. 17, 3629–3639 (1997).
Paule, B. et al. The NF-kappaB/IL-6 pathway in metastatic androgen-independent prostate cancer: new therapeutic approaches?. World J. Urol. 25, 477–489 (2007).
Jentsch, S. The ubiquitin-conjugation system. Annu. Rev. Genet. 26, 179–207 (1992).
Hou, S. J., Zhang, S. X., Li, Y. & Xu, S. Y. Rapamycin responds to Alzheimer’s disease: a potential translational therapy. Clin. Inter. Aging 18, 1629–1639 (2023).
Senkevich, K. & Gan-Or, Z. Autophagy lysosomal pathway dysfunction in Parkinson’s disease; evidence from human genetics. Parkinsonism Relat. Disord. 73, 60–71 (2020).
Lu, Z. et al. mTOR inhibitor PP242 increases antitumor activity of sulforaphane by blocking Akt/mTOR pathway in esophageal squamous cell carcinoma. Mol. Biol. Rep. 49, 451–461 (2022).
Qin, Y., Zhao, X. & Fang, Y. PP242 synergizes with suberoylanilide hydroxamic acid to inhibit growth of ovarian cancer cells. Int. J. Gynecol. Cancer 24, 1373–1380 (2014).
Verstovsek, S. et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N. Engl. J. Med. 366, 799–807 (2012).
Zeiser, R. et al. Ruxolitinib for glucocorticoid-refractory chronic graft-versus-host disease. N. Engl. J. Med. 385, 228–238 (2021).
Chitimus, D. M. et al. Melatoninas impact on antioxidative and anti-inflammatory reprogramming in homeostasis and disease. Biomolecules 10, 1211 (2020).
Pyo, I. S. et al. Mechanisms of aging and the preventive effects of resveratrol on age-related diseases. Molecules 25, 4649 (2020).
Kim, D. et al. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer’s disease and amyotrophic lateral sclerosis. EMBO J. 26, 3169–3179 (2007).
Acknowledgements
This work was supported by the National Key R&D Program of China (2020YFA0710700, X.L.Y.), the National Science and Technology Major Projects of New Drugs (2018ZX09201018-013, P.C.), the National Key Research and Development Program of China (No. 2023YFC3403303, P.C.) and the National Natural Science Foundation of China Young Student Basic Research Program (823B2065, A.A.). We acknowledge BioRender (https://biorender.com) for the production of the figures in the article.
Author information
Authors and Affiliations
Contributions
X.L.Y., P.C., and H.S.S. conceived the idea, edited, and revised the manuscript. Y.C.F. and B.H.W., and A.A. were the major contributors to the writing of the manuscript. Y.C.F., B.H.W., and A.A. created all the figures. B.H.W. created all the tables. W.Q.H., H.L., and X.M.H. revised the manuscript. All the authors have read and approved the article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent for publication
All the authors have read and approved the final manuscript.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fu, Y., Wang, B., Alu, A. et al. Immunosenescence: signaling pathways, diseases and therapeutic targets. Sig Transduct Target Ther 10, 250 (2025). https://doi.org/10.1038/s41392-025-02371-z
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41392-025-02371-z
This article is cited by
-
Oestrogen changes at menopause: insights into obesity-associated breast risk and outcomes
Nature Reviews Endocrinology (2026)
-
Autophagy-NLRP3 Inflammasome Crosstalk in Microglia: A Therapeutic Target for Multiple Sclerosis
Inflammation (2026)