Abstract
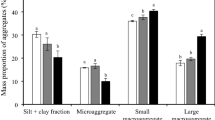
Stable soils provide valuable ecosystem services and mechanical soil stability is enhanced by the presence of arbuscular mycorrhizal fungi (AMF). Soil aggregation, which is the major driver of mechanical soil stability, is often treated as a static phenomenon, even though aggregate turnover is continually ongoing. In fact, some breakdown of macroaggregates is necessary to allow new aggregate formation and inclusion of new organic matter into microaggregates. We determined how aggregate turnover times were affected by AMF by tracking movement of rare earth elements (REE), applied as their immobile oxides, between aggregate size classes, and using X-ray fluorescence microscopy to spatially localize REEs in a sample of aggregates. Here we show that AMF increased large macroaggregate formation and slowed down disintegration of large and small macroaggregates. Microaggregate turnover was increased in the presence of AMF. Internal aggregate organization suggested that although formation of microaggregates by accretion of soil to particulate organic matter is common, it is not the only mechanism in operation.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Data availability
Raw data for X-ray fluorescence microscopy were generated at the Advanced Photon Source at Argonne National Laboratory. Derived data supporting the findings of this study are included in the paper and its supplementary information files. Other data, REE concentrations, and aggregate size class distributions are available from the corresponding author upon reasonable request.
References
Hartge KH, Stewart BA. Soil structure: its development and function. Boca Raton, FL: CRC, Lewis Publishers; 1995.
Jastrow JD, Miller RM. Soil aggregate stabilization and carbon sequestration: feedbacks through organomineral associations. In: Lal R, Kimble JM, Follett RF, Stewart BA, editors. Soil processes and the carbon cycle. Boca Raton, FL: CRC Press; 1997. p. 207–23.
Miller RM, Jastrow JD. The role of mycorrhizal fungi in soil conservation. In: Bethlenfalvay GJ, Linderman RG, editors. Mycorrhizae in sustainable agriculture. Madison, WI: American Society of Agronomy; 1992. p. 29–44.
Oades JM. Soil organic matter and structural stability: mechanisms and implications for management. Plant Soil. 1984;76:319–37.
Rillig MC, Mummey DL. Mycorrhizas and soil structure. New Phytol. 2006;171:41–53.
Six J, Conant RT, Paul EA, Paustian K. Stabilization mechanisms of soil organic matter: implications for C-saturation of soils. Plant Soil. 2002a;241:155–76.
Jiménez JJ, Lal R. Mechanisms of C sequestration in soils of Latin America. CRC Crit Rev Plant Sci. 2006;25:337–65.
Blanco-Canqui H, Lal R. Mechanisms of carbon sequestration in soil aggregates. CRC Crit Rev Plant Sci. 2004;23:481–504.
Six J, Elliott ET, Paustian K, Doran JW. Aggregation and soil organic matter accumulation in cultivated and native grassland soils. Soil Sci Soc Am J. 1998;62:1367–77.
Wilcke W, Bol R, Amelung W. Fate of dung-applied copper in a British grassland soil. Geoderma. 2002;106:273–88.
von Lutzow M, Kogel-Knabner I, Ekschmitt K, Matzner E, Guggenberger G, Marschner B, et al. Stabilization of organic matter in temperate soils: mechanisms and their relevance under different soil conditions—a review. Eur J Soil Sci. 2006;57:426–45.
Six J, Bossuyt H, Degryze S, Denef K. A history of research on the link between (micro)aggregates, soil biota, and soil organic matter dynamics. Soil & Tillage Res. 2004;79:7–31.
Jastrow JD, Amonette JE, Bailey VL. Mechanisms controlling soil carbon turnover and their potential application for enhancing carbon sequestration. Clim Change. 2007;80:5–23.
Parwada C, Van Tol J. Effects of litter source on the dynamics of particulate organic matter fractions and rates of macroaggregate turnover in different soil horizons. Eur J Soil Sci. 2018;69:1126–36.
Barto EK, Alt F, Oelmann Y, Wilcke W, Rillig MC. Contributions of biotic and abiotic factors to soil aggregation across a land use gradient. Soil Biol Biochem. 2010;42:2316–24.
Lehmann A, Zheng W, Rillig MC. Soil biota contributions to soil aggregation. Nat Ecol & Evol. 2017;1:1828–35.
Lynch JM, Bragg E. Microorganisms and soil aggregate stability. In: Stewart BA, editor. Advances in soil science. New York: Springer Verlag; 1985. p. 133–71. .
Bast A, Wilcke W, Graf F, Lüscher P, Gärtner H. Does mycorrhizal inoculation improve plant survival, aggregate stability, and fine root development on a coarse-grained soil in an alpine eco-engineering field experiment? J Geophys Res: Biogeosciences. 2016;121:2158–71.
Miller RM, Jastrow JD. Mycorrhizal fungi influence soil structure. In: Kapulnik Y, Douds DD, editors. Arbuscular Mycorrhizae: molecular biology and physiology. Kluwer Academic Press, Dordrecht, MA; 2000.
Tisdall JM, Oades JM. Organic matter and water-stable aggregates in soils. J Soil Sci. 1982;33:141–63.
Degens BP. Macro-aggregation of soils by biological bonding and binding mechanisms and the factors affecting these: a review. Aust J Soil Res. 1997;35:431–59.
Harris RF, Chesters G, Allen ON, Attoe OLJ. Mechanisms involved in soil aggregate stabilization by fungi and bacteria. Soil Sci Soc Am Proc. 1964;28:529–32.
Rillig M, Aguilar-Trigueros C, Bergmann J, Verbruggen E, Veresoglou S, Lehmann A. Plant root and mycorrhizal fungal traits for understanding soil aggregation. New Phytol. 2015;205:1385–8.
Peng X, Zhu Q, Zhang Z, Hallett P. Combined turnover of carbon and soil aggregates using rare earth oxides and isotopically labelled carbon as tracers. Soil Biol Biochem. 2017;109:81–94.
Zhang XC, Friedrich JM, Nearing MA, Norton LD. Potential use of rare earth oxides as tracers for soil erosion and aggregation studies. Soil Sci Soc Am J. 2001;65:1508–15.
De Gryze S, Six J, Merckx R. Quantifying water-stable soil aggregate turnover and its implication for soil organic matter dynamics in a model study. Eur J Soil Sci. 2006;57:693–707.
Majumdar S, Peralta-Videa JR, Castillo-Michel H, Hong J, Rico CM, Gardea-Torresdey JL. Applications of synchrotron mu-XRF to study the distribution of biologically important elements in different environmental matrices: a review. Anal Chim Acta. 2012;755:1–16.
Paunesku T, Vogt S, Maser J, Lai B, Woloschak G. X-ray fluorescence microprobe imaging in biology and medicine. J Cell Biochem. 2006;99:1489–502.
Friese CF, Allen MF. The spread of VA mycorrhizal fungal hyphae in the soil: inoculum types and external hyphal architecture. Mycologia. 1991;83:409–18.
Tisdall JM. Possible role of soil microorganisms in aggregation of soils. Plant Soil. 1994;159:115–21.
Plante A, McGill W. Soil aggregate dynamics and the retention of organic matter in laboratory-incubated soil with differing simulated tillage frequencies. Soil & Tillage Res. 2002;66:79–92.
Hallett PD, Feeney DS, Bengough AG, Rillig MC, Scrimgeour CM, Young IM. Disentangling the impact of AM fungi versus roots on soil structure and water transport. Plant Soil. 2009;314:183–96.
Six J, Elliott E, Paustian K. Aggregate and soil organic matter dynamics under conventional and no-tillage systems. Soil Sci Soc Am J. 1999;63:1350–8.
Six J, Elliott E, Paustian K. Soil macroaggregate turnover and microaggregate formation: a mechanism for C sequestration under no-tillage agriculture. Soil Biol Biochem. 2000;32:2099–103.
Vogel C, Mueller C, Höschen C, Buegger F, Heister K, Schulz S, et al. Submicron structures provide preferential spots for carbon and nitrogen sequestration in soils. Nat Commun. 2014;5:2947.
Six J, Feller C, Denef K, Ogle SM, Sa JCD, Albrecht A. Soil organic matter, biota and aggregation in temperate and tropical soils—effects of no-tillage. Agronomie. 2002b;22:755–75.
Bronick CJ, Lal R. Soil structure and management: a review. Geoderma. 2005;124:3–22.
IUSS Working Group WRB (2014). World Reference Base for Soil Resources 2014. International soil classification system for naming soils and creating legends for soil maps. World Soil Resources Reports No. 106. Rome: FAO.
Vierheilig H, Coughlan A, Wyss U, Piche Y. Ink and vinegar, a simple staining technique for arbuscular-mycorrhizal fungi. Appl Environ Microbiol. 1998;64:5004–7.
McGonigle TP, Miller MH, Evans DG, Fairchild GL, Swan JA. A new method which gives an objective measure of colonization of roots by vesicular-arbuscular mycorrhizal fungi. New Phytol. 1990;115:495–501.
Sainju U. Carbon and nitrogen pools in soil aggregates separated by dry and wet sieving methods. Soil Sci. 2006;171:937–49.
R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2017.
Vogt S. MAPS: a set of software tools for analysis and visualization of 3D X-ray fluorescence data sets. J De Phys Iv. 2003;104:635–8.
Vogt S, Maser J, Jacobsen C. Data analysis for x-ray fluorescence imaging. J De Phys IV: JP. 2003;104:617–22.
Acknowledgements
We would like to thank Sabine Artelt and Sabine Buchert for help in the lab at FU Berlin, and the Bundesanstalt für Materialforschung und –prüfung for help preparing thin sections of soil aggregates. This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357.
Author information
Authors and Affiliations
Contributions
EKM, DJPM, and MCR designed the experiments. EKM and DJPM completed REE labeling and incubation. EKM, DJPM, and S-CG completed X-ray fluorescence microscopy. MB and WW completed ICP-MS work. EKM, DJPM, SV, and S-CG completed data analysis. EKM and DJPM wrote the manuscript, and all authors edited the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Morris, E.K., Morris, D.J.P., Vogt, S. et al. Visualizing the dynamics of soil aggregation as affected by arbuscular mycorrhizal fungi. ISME J 13, 1639–1646 (2019). https://doi.org/10.1038/s41396-019-0369-0
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41396-019-0369-0
This article is cited by
-
Effects of litter inputs on soil aggregate C turnover and flow differ among three natural forest ecosystems along a climate gradient in China
Ecological Processes (2026)
-
The multifunctional roles of arbuscular mycorrhizal fungi in soil health and nutrient dynamics
Biology and Fertility of Soils (2026)
-
Arbuscular mycorrhizal fungi communities and glomalin mediate particulate and mineral-associated organic carbon formation in grassland patches
Communications Earth & Environment (2025)
-
Hairy Vetch Intercropping Attenuates Mycorrhizal Benefits to Walnut Growth and Soil Organic Carbon Sequestration via Glomalin
Microbial Ecology (2025)
-
The role of vegetation restoration in shaping the structure and stability of soil bacterial community of alpine mining regions
Plant and Soil (2025)