Abstract
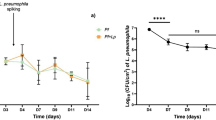
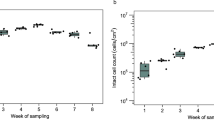
The water-borne bacterium Legionella pneumophila is the causative agent of Legionnaires’ disease. In the environment, the opportunistic pathogen colonizes different niches, including free-living protozoa and biofilms. The physiological state(s) of sessile Legionella in biofilms and their functional consequences are not well understood. Using single-cell techniques and fluorescent growth rate probes as well as promoter reporters, we show here that sessile L. pneumophila exhibits phenotypic heterogeneity and adopts growing and nongrowing (“dormant”) states in biofilms and microcolonies. Phenotypic heterogeneity is controlled by the Legionella quorum sensing (Lqs) system, the transcription factor LvbR, and the temperature. The Lqs system and LvbR determine the ratio between growing and nongrowing sessile subpopulations, as well as the frequency of growth resumption (“resuscitation”) and microcolony formation of individual bacteria. Nongrowing L. pneumophila cells are metabolically active, express virulence genes and show tolerance toward antibiotics. Therefore, these sessile nongrowers are persisters. Taken together, the Lqs system, LvbR and the temperature control the phenotypic heterogeneity of sessile L. pneumophila, and these factors regulate the formation of a distinct subpopulation of nongrowing, antibiotic tolerant, virulent persisters. Hence, the biofilm niche of L. pneumophila has a profound impact on the ecology and virulence of this opportunistic pathogen.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Data availability
All data is available in the main text or the Supplementary Material and provided as source data files.
References
Newton HJ, Ang DK, van Driel IR, Hartland EL. Molecular pathogenesis of infections caused by Legionella pneumophila. Clin Microbiol Rev. 2010;23:274–98.
Hilbi H, Hoffmann C, Harrison CF. Legionella spp. outdoors: colonization, communication and persistence. Environ Microbiol Rep. 2011;3:286–96.
Fields BS. The molecular ecology of Legionella. Trends Microbiol. 1996;4:286–90.
Greub G, Raoult D. Microorganisms resistant to free-living amoebae. Clin Microbiol Rev. 2004;17:413–33.
Hoffmann C, Harrison CF, Hilbi H. The natural alternative: protozoa as cellular models for Legionella infection. Cell Microbiol. 2014;16:15–26.
Boamah DK, Zhou G, Ensminger AW, O’Connor TJ. From many hosts, one accidental pathogen: The diverse protozoan hosts of Legionella. Front Cell Infect Microbiol. 2017;7:477.
Swart AL, Harrison CF, Eichinger L, Steinert M, Hilbi H. Acanthamoeba and Dictyostelium as cellular models for Legionella infection. Front Cell Infect Microbiol. 2018;8:61.
Sherwood RK, Roy CR. A Rab-centric perspective of bacterial pathogen-occupied vacuoles. Cell Host Microbe. 2013;14:256–68.
Asrat S, de Jesus DA, Hempstead AD, Ramabhadran V, Isberg RR. Bacterial pathogen manipulation of host membrane trafficking. Annu Rev Cell Dev Biol. 2014;30:79–109.
Finsel I, Hilbi H. Formation of a pathogen vacuole according to Legionella pneumophila: how to kill one bird with many stones. Cell Microbiol. 2015;17:935–50.
Qiu J, Luo ZQ. Legionella and Coxiella effectors: strength in diversity and activity. Nat Rev Microbiol. 2017;15:591–605.
Personnic N, Bärlocher K, Finsel I, Hilbi H. Subversion of retrograde trafficking by translocated pathogen effectors. Trends Microbiol. 2016;24:450–62.
Steiner B, Weber S, Hilbi H. Formation of the Legionella-containing vacuole: phosphoinositide conversion, GTPase modulation and ER dynamics. Int J Med Microbiol. 2018;308:49–57.
Declerck P. Biofilms: the environmental playground of Legionella pneumophila. Environ Microbiol. 2010;12:557–66.
Abdel-Nour M, Duncan C, Low DE, Guyard C. Biofilms: the stronghold of Legionella pneumophila. Int J Mol Sci. 2013;14:21660–75.
Pécastaings S, Allombert J, Lajoie B, Doublet P, Roques C, Vianney A. New insights into Legionella pneumophila biofilm regulation by c-di-GMP signaling. Biofouling. 2016;32:935–48.
Hochstrasser R, Hilbi H. Intra-species and inter-kingdom signaling of Legionella pneumophila. Front Microbiol. 2017;8:79.
Mampel J, Spirig T, Weber SS, Haagensen JAJ, Molin S, Hilbi H. Planktonic replication is essential for biofilm formation by Legionella pneumophila in a complex medium under static and dynamic flow conditions. Appl Environ Microbiol. 2006;72:2885–95.
Hindré T, Brüggemann H, Buchrieser C, Héchard Y. Transcriptional profiling of Legionella pneumophila biofilm cells and the influence of iron on biofilm formation. Microbiology. 2008;154:30–41.
Pécastaings S, Berge M, Dubourg KM, Roques C. Sessile Legionella pneumophila is able to grow on surfaces and generate structured monospecies biofilms. Biofouling. 2010;26:809–19.
Wai SN, Mizunoe Y, Yoshida S. How Vibrio cholerae survive during starvation. FEMS Microbiol Lett. 1999;180:123–31.
Balaban NQ, Merrin J, Chait R, Kowalik L, Leibler S. Bacterial persistence as a phenotypic switch. Science. 2004;305:1622–5.
Harms A, Maisonneuve E, Gerdes K. Mechanisms of bacterial persistence during stress and antibiotic exposure. Science. 2016;354:aaf4268.
Claudi B, Sprote P, Chirkova A, Personnic N, Zankl J, Schurmann N, et al. Phenotypic variation of Salmonella in host tissues delays eradication by antimicrobial chemotherapy. Cell. 2014;158:722–33.
Hélaine S, Cheverton AM, Watson KG, Faure LM, Matthews SA, Holden DW. Internalization of Salmonella by macrophages induces formation of nonreplicating persisters. Science. 2014;343:204–8.
Conlon BP, Rowe SE, Gandt AB, Nuxoll AS, Donegan NP, Zalis EA, et al. Persister formation in Staphylococcus aureus is associated with ATP depletion. Nat Microbiol. 2016;1:16051.
Personnic N, Striednig B, Lezan E, Manske C, Welin A, Schmidt A, et al. Quorum sensing modulates the formation of virulent Legionella persisters within infected cells. Nat Commun. 2019;10:5216.
Molofsky AB, Swanson MS. Differentiate to thrive: lessons from the Legionella pneumophila life cycle. Mol Microbiol. 2004;53:29–40.
Hammer BK, Swanson MS. Co-ordination of Legionella pneumophila virulence with entry into stationary phase by ppGpp. Mol Microbiol. 1999;33:721–31.
Dalebroux ZD, Yagi BF, Sahr T, Buchrieser C, Swanson MS. Distinct roles of ppGpp and DksA in Legionella pneumophila differentiation. Mol Microbiol. 2010;76:200–19.
Tiaden A, Spirig T, Hilbi H. Bacterial gene regulation by α-hydroxyketone signaling. Trends Microbiol. 2010;18:288–97.
Personnic N, Striednig B, Hilbi H. Legionella quorum sensing and its role in pathogen-host interactions. Curr Opin Microbiol. 2018;41:29–35.
Spirig T, Tiaden A, Kiefer P, Buchrieser C, Vorholt JA, Hilbi H. The Legionella autoinducer synthase LqsA produces an α-hydroxyketone signaling molecule. J Biol Chem. 2008;283:18113–23.
Tiaden A, Spirig T, Sahr T, Wälti MA, Boucke K, Buchrieser C, et al. The autoinducer synthase LqsA and putative sensor kinase LqsS regulate phagocyte interactions, extracellular filaments and a genomic island of Legionella pneumophila. Environ Microbiol. 2010;12:1243–59.
Kessler A, Schell U, Sahr T, Tiaden A, Harrison C, Buchrieser C, et al. The Legionella pneumophila orphan sensor kinase LqsT regulates competence and pathogen-host interactions as a component of the LAI-1 circuit. Environ Microbiol. 2013;15:646–62.
Tiaden A, Spirig T, Weber SS, Brüggemann H, Bosshard R, Buchrieser C, et al. The Legionella pneumophila response regulator LqsR promotes host cell interactions as an element of the virulence regulatory network controlled by RpoS and LetA. Cell Microbiol. 2007;9:2903–20.
Tiaden A, Spirig T, Carranza P, Brüggemann H, Riedel K, Eberl L, et al. Synergistic contribution of the Legionella pneumophila lqs genes to pathogen-host interactions. J Bacteriol. 2008;190:7532–47.
Schell U, Simon S, Sahr T, Hager D, Albers MF, Kessler A, et al. The α-hydroxyketone LAI-1 regulates motility, Lqs-dependent phosphorylation signalling and gene expression of Legionella pneumophila. Mol Microbiol. 2016;99:778–93.
Hochstrasser R, Hutter CAJ, Arnold FM, Bärlocher K, Seeger MA, Hilbi H. The structure of the Legionella response regulator LqsR reveals amino acids critical for phosphorylation and dimerization. Mol Microbiol. 2020;113:1070–84.
Hochstrasser R, Kessler A, Sahr T, Simon S, Schell U, Gomez-Valero L, et al. The pleiotropic Legionella transcription factor LvbR links the Lqs and c-di-GMP regulatory networks to control biofilm architecture and virulence. Environ Microbiol. 2019;21:1035–53.
Hochstrasser R, Hilbi H. Legionella quorum sensing meets cyclic-di-GMP signaling. Curr Opin Microbiol. 2020;55:9–16.
Simon S, Schell U, Heuer N, Hager D, Albers MF, Matthias J, et al. Inter-kingdom signaling by the Legionella quorum sensing molecule LAI-1 modulates cell migration through an IQGAP1-Cdc42-ARHGEF9-dependent pathway. PLoS Pathog. 2015;11:e1005307.
Faucher SP, Friedlander G, Livny J, Margalit H, Shuman HA. Legionella pneumophila 6S RNA optimizes intracellular multiplication. Proc Natl Acad Sci USA. 2010;107:7533–8.
Faucher SP, Shuman HA. Small regulatory RNA and Legionella pneumophila. Front Microbiol. 2011;2:98.
Balaban NQ, Hélaine S, Lewis K, Ackermann M, Aldridge B, Andersson DI, et al. Definitions and guidelines for research on antibiotic persistence. Nat Rev Microbiol. 2019;17:441–8.
Brauner A, Fridman O, Gefen O, Balaban NQ. Distinguishing between resistance, tolerance and persistence to antibiotic treatment. Nat Rev Microbiol. 2016;14:320–30.
Personnic N, Striednig B, Hilbi H. Single cell analysis of Legionella and Legionella-infected Acanthamoeba by agarose embedment. Methods Mol Biol. 2019;1921:191–204.
Byrne B, Swanson MS. Expression of Legionella pneumophila virulence traits in response to growth conditions. Infect Immun. 1998;66:3029–34.
Lewis K. Persister cells, dormancy and infectious disease. Nat Rev Microbiol. 2007;5:48–56.
Lewis K. Multidrug tolerance of biofilms and persister cells. Curr Top Microbiol Immunol. 2008;322:107–31.
Hall CW, Mah TF. Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiol Rev. 2017;41:276–301.
Brenzinger S, van der Aart LT, van Wezel GP, Lacroix JM, Glatter T, Briegel A. Structural and proteomic changes in viable but non-culturable Vibrio cholerae. Front Microbiol. 2019;10:793.
Defraine V, Fauvart M, Michiels J. Fighting bacterial persistence: current and emerging anti-persister strategies and therapeutics. Drug Resist Updates. 2018;38:12–26.
Wu B, Liang W, Kan B. Growth phase, oxygen, temperature, and starvation affect the development of viable but non-culturable state of Vibrio cholerae. Front Microbiol. 2016;7:404.
Ackermann M. A functional perspective on phenotypic heterogeneity in microorganisms. Nat Rev Microbiol. 2015;13:497–508.
Epstein SS. Microbial awakenings. Nature 2009;457:1083.
Sturm A, Dworkin J. Phenotypic diversity as a mechanism to exit cellular dormancy. Curr Biol. 2015;25:2272–7.
Carlson HK, Vance RE, Marletta MA. H-NOX regulation of c-di-GMP metabolism and biofilm formation in Legionella pneumophila. Mol Microbiol. 2010;77:930–42.
Loh E, Righetti F, Eichner H, Twittenhoff C, Narberhaus F. RNA thermometers in bacterial pathogens. Microbiol Spectr. 2018;6.
Terskikh A, Fradkov A, Ermakova G, Zaraisky A, Tan P, Kajava AV, et al. “Fluorescent timer”: protein that changes color with time. Science. 2000;290:1585–8.
Acknowledgements
We thank Selina Niggli for initial cloning and Ramon Hochstrasser for comments and discussions. We would also like to thank Sébastien Faucher (McGill University, Canada) for providing the Δ6S RNA mutant and parental strains. Research of NP in the laboratory of HH was supported by the Swiss National Science Foundation (SNF) Ambizione program (PZ00P3_161492 & PZ00P3_185529) awarded to NP. Research in the laboratory of HH was supported by the SNF (31003A_153200, 31003A_175557), the Novartis Foundation for Medical-Biological Research, the OPO Foundation, and the German Research Foundation (DFG; SPP 1617). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
NP conceived the study with input from HH. NP designed the experiments. NP and BS performed the experiments. NP and HH wrote the paper with input from BS.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Personnic, N., Striednig, B. & Hilbi, H. Quorum sensing controls persistence, resuscitation, and virulence of Legionella subpopulations in biofilms. ISME J 15, 196–210 (2021). https://doi.org/10.1038/s41396-020-00774-0
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41396-020-00774-0
This article is cited by
-
A bacterial toxin-antitoxin system involved in an unusual response to genotoxic stress
EMBO Reports (2025)
-
The sleeping bacterium: shedding light on the resuscitation mechanism
European Biophysics Journal (2025)
-
Phagotrophic protists preserve antibiotic-resistant opportunistic human pathogens in the vegetable phyllosphere
ISME Communications (2023)
-
A clash of quorum sensing vs quorum sensing inhibitors: an overview and risk of resistance
Archives of Microbiology (2023)
-
Regulatory and innovative mechanisms of bacterial quorum sensing–mediated pathogenicity: a review
Environmental Monitoring and Assessment (2023)