Abstract
Pharmacogenetics (PGx) is increasingly implemented in the adult population, but its potential in children remains uncertain. The aim of this study was to investigate PGx drug utilization in children in Switzerland, using Helsana claims data between 2017 and 2021. We identified 82 drugs with paediatric guideline annotations associated with variants in 24 genes from the Pharmacogenomics Knowledgebase. Of 159 172 children continuously insured, 66.1% claimed at least one PGx drug during the study period. The three PGx drugs with the highest user numbers were systemically administered ibuprofen (59.1%), ondansetron (8.3%), and locally administered fluorouracil (7.5%). Over 96% of all potential drug-gene interactions were caused by seven genes (CYP2C9, CYP2D6, DPYD, CYP2C19, MT-RNR1, CACNA1S, and RYR1). The high number of children claiming PGx drugs in Switzerland implies that a significant number of children could benefit from PGx testing.
Similar content being viewed by others
Introduction
Drug-gene-interactions (DGIs) refer to situations where a genetic variant and a drug interact, resulting in an altered drug response [1,2,3]. Individual genetic factors affecting pharmacodynamics or pharmacokinetics in DGIs can lead to variability in drug exposure and /or response resulting in treatment failure or toxicity [1, 2]. Pharmacogenomics aims to enhance an individuals’ drug response through a personalised, and therefore safer and more effective therapy [4].
Children are a vulnerable patient group and frequently affected by adverse drug reactions [5]. In adults, pharmacogenetic (PGx) testing has been demonstrated to reduce the number of adverse drug reactions, which is associated with fewer hospital admissions due to adverse drug reactions and with improved treatment responses [6,7,8,9,10], but these results cannot easily be transferred to children. Children represent a heterogeneous group ranging from preterm new-borns to adolescents. In the postnatal phase, both age and genotype affect enzyme expression and activity [11]. Moreover, ontogenesis influences the activity of drug-metabolizing enzymes and transporters [12]. Of particular significance in this context are individuals below the age of two years, as ontogenetic variability is most pronounced in this age group [12].
The implementation of PGx for children has proceeded slower than anticipated and is still limited [13, 14]. PGx dosing guidelines applicable for children by the Clinical Pharmacogenetics Implementation Consortium (CPIC) [15, 16], the Dutch Pharmacogenetics Working Group (DPWG) [17, 18], and the Canadian Pharmacogenomics Network for Drug Safety (CPNDS) [19, 20] are available on the Pharmacogenomics Knowledgebase (PharmGKB, www.pharmgkb.org) [21]. These guidelines shall facilitate the implementation of PGx in clinical practice by helping paediatricians and pharmacists to interpret PGx findings.
The majority of existing PGx studies in children focus on the hospital setting and /or specific medical conditions such as mental illnesses, sickle cell anaemia, or children with burns and surgery [22,23,24,25,26,27]. To assess the benefit of PGx testing in children, it is crucial to understand the utilisation of PGx drugs in paediatrics. To date, most studies examining the prevalence of PGx drugs in children have been conducted in the United States of America (USA) and Canada [23, 28,29,30,31,32]. Currently, there is insufficient research on the frequency of PGx prescriptions in paediatrics in Europe [33].
Therefore, the aim of this study was to analyse the utilisation of PGx drugs in children and adolescents in Switzerland. The objectives were to assess the prevalence of PGx drug prescriptions, to identify the most frequently used PGx drugs, and thereby to determine the most relevant PGx genes in different age groups.
Materials and methods
Data source
We used claims data from one of the largest Swiss health insurance providers (Helsana Group), which covers approximately 1.2 million people (15% of the Swiss population) annually across all 26 cantons with basic health insurance. The Helsana database is representative of the Swiss population. This database has previously been used for several drug safety and drug utilisation studies [34,35,36,37]. The dataset included demographic information of insured individuals, such as canton of residence, year of birth, and sex. Additionally, it contained records of both therapeutic treatments and diagnostic evaluations carried out in the outpatient setting. Drug claims were recorded using the Anatomical Therapeutical Chemical (ATC) classification. Information on lifestyle factors or over the counter (OTC) drug sales was not available. In our analyses we included claims from children under 18 years of age who were continuously insured from the 1st of January 2017 to the 31st of December 2021. For supplementary analyses of individual 1-year periods, we included claims from children under 18 years of age who were at least insured during the respective year of the study period.
PGx drugs
PGx drugs were defined as drugs with a dosing or testing recommendation applicable to children on the PharmGKB in April 2023 [21]. We identified 82 drugs as PGx drugs associated with variants in 24 genes (Table 1). We differentiated local vs. systemic applications using the ATC code for the following 12 drugs: amikacin, dapsone, doxepin, fluorouracil, flurbiprofen, gentamicin, ibuprofen, kanamycin, meloxicam, piroxicam, tacrolimus, and tobramycin. If an ATC code included both systemic and local application, we included it in the systemically administered category. We excluded 41 drugs due to lack of guideline recommendations (n = 40) or lack of ATC code (toluidine blue). Additionally, the PGx drug claims were categorised into different anatomical groups, using the first level of the ATC code.
Age groups
Children’s ages were calculated at the end of the calendar year. Children were stratified into five age groups adapted from the Eunice Kennedy Shriver National Institute of Child Health and Human Development: infants (<1 year old), toddlers (1 year old), early childhood (2–4 years old), late childhood (5–10 years old), and adolescence (11–17 years old) [38]. Children can change the age group during the study period.
Statistical analysis
We performed a retrospective and descriptive analysis and assessed PGx drug claims in all registered children, stratified by age groups. Counts and percentages for drug claims are based on the number of distinct children, rather than the number of claims. Furthermore, we computed the average quantity of both any drug and PGx drugs that were claimed by each child. Additionally, PGx drugs and associated genes were ranked based on the number of children with claims.
All analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC, USA).
Ethics approval
Ethics approval and informed consent was not necessary according to article 22 of the Swiss Federal law on data protection, as the study was retrospective and used anonymized data [39].
Results
Study population
A total of 309 123 children were insured by Helsana between 2017 and 2021, with 159 172 (51.5%) registered throughout the entire five-year period. Of the children present during the entire five-year period, 48.6% were female, 5.4% < 1 year old, 11.1% 1 year old, 28.3% 2–4 years old, 57.9% 5–10 years old, and 60.1% 11–17 years old at least once during the five-year period. Throughout the five-year period, 95.3% of children claimed at least one drug, 95.8% in females and 94.8% in males. Drug prevalence ranged from 88.1% in adolescents to 98.3% in toddlers and was similar in boys and girls. On average, children claimed 10.7 ± 8.0 drugs during the 5-year study period, 10.7 ± 8.1 in girls and 10.6 ± 8.0 in boys (Table 2). The characteristics of the study population in each individual year (2017, 2018, 2019, 2020, and 2021) are shown in the appendix (Appendix Table 1).
PGx drugs
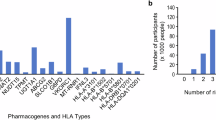
105 192 of the 159 172 children, who were registered for the full five-year study period, claimed at least one PGx drug (66.1%) during the study period. More than two thirds (69.4%) of the children having at least one drug claim for any drug claimed one of the 82 PGx drugs included in our study. Only 6% of all children claimed ≥ 3 PGx drugs. Boys more frequently claimed PGx drugs in children up to 10 years old, while girls older than 10 years more frequently claimed PGx drugs. When excluding ibuprofen and locally administered PGx drugs 18.2% of children claimed PGx drugs during the five-year period. The total number of children claiming PGx drugs ranged from 94 092 for systemically administered ibuprofen to one each in atazanavir, fluvastatin, methylene blue, paromomycin, phenytoin, primaquine, and tenoxicam (Table 3 and Appendix Table 2). The top three claimed PGx drugs regardless of sex were systemically administered ibuprofen, ondansetron, and locally administered fluorouracil with 94 092, 13 269, and 11 982 users, respectively (Table 3). 25 out of the 82 drugs were not claimed during the five-year study period, because 20 PGx drugs were either not approved for use in children in Switzerland or not approved at all. Furthermore, amikacin, doxepin, flurbiprofen, gentamicin, and ibuprofen were not administered locally at any point during the five-year-period. However, only flurbiprofen and ibuprofen were approved for local administration in adolescents in Switzerland during the study period. Grouping the drugs by anatomical group revealed that the majority of children claimed PGx drugs from the musculo-skeletal system, followed by those targeting the alimentary tract and metabolism, and antineoplastic and immunomodulating agents (Fig. 1). After excluding locally administered drugs, we found that most children regardless of sex claimed PGx drugs from the musculo-skeletal system, the alimentary tract and metabolism, and the nervous system (Appendix Figure 1). The distribution after additionally excluding ibuprofen is provided in the appendix (Appendix Figure 2).
The anatomical groups are ranked by the proportion of children who claimed a PGx drug in the total population. Anatomical groups excluded, because < 1.0%: dermatologicals, antiinfectives for systemic use, cardiovascular system, blood and blood forming organs, antiparasitic products, insecticides and repellents, various, genito urinary system and sex hormones, systemic hormonal preparations, excl. sex hormones and insulins. PGx pharmacogenetic, % percentage of the total number of children per age group.
Comparisons across the different age groups revealed that the PGx drug prevalence ranged from 12.4% in infants to 60.6% in children 2–4 years old. Based on the number of subjects claiming PGx drugs in the respective age groups, systemic ibuprofen ranked first across all age groups in boys and girls, while second and third rank varied in the different age groups. Locally administered tobramycin was more frequently used by younger children, while locally administered fluorouracil was primarily used by children aged 5 and over. Ondansetron was frequently used across all age groups. Gender differences in PGx drug utilization were observed for certain drugs. For instance, girls were found to use sertraline (0.7 vs. 0.3%), escitalopram (0.4 vs. 0.2%), and nitrofurantoin (0.4 vs. <0.1%) more frequently, while boys were found to use risperidone (0.2 vs. 0.6%) and atomoxetine (0.1 vs. 0.3%) more frequently during the five-year period. The number of users during the whole five-year period, as well as annual PGx drug prevalences stratified by age groups, are available in the appendix (Appendix Table 2 to Appendix Table 8).
PGx genes
The ranking of genes associated with PGx drugs showed that CYP2C9, CYP2D6, and DPYD were the genes most frequently associated with the drugs used by the whole study population (Table 4), but the frequency of exposure and the rank within the different age groups varied across childhood and adolescence. During the study period, no children claimed drugs associated with CFTR and CYP4F2.
96.1% of all potential DGIs found in the total population were attributed to seven genes: CYP2C9, CYP2D6, DPYD, CYP2C19, MT-RNR1, CACNA1S, and RYR1 (Fig. 2). When PGx drugs administered locally were excluded, five genes were found to cause 98.7% of all potential DGIs in the total population. These genes were: CYP2C9, CYP2D6, CYP2C19, RYR1, and CACNA1S (Appendix Figure 3). After additionally excluding ibuprofen the following five genes CYP2D6, CYP2C19, RYR1, CACNA1S, and CYP2B6 were associated with 97.0% of all potential DGIs in the total population (Appendix Figure 4). The proportion of CYP2D6 increased with the age of the children and had a greater impact on older children. In contrast, MT-RNR1 held the largest proportion of all potential DGIs in infants. CYP2C19 was responsible for a significant proportion of all potential DGIs in infants.
Discussion
This study determined prescription patterns of PGx drugs and assessed the potential of PGx testing in paediatrics for the first time in Switzerland. During the five-year study period, 66.1% of children claimed at least one PGx drug, which is high compared to PGx drug prevalences reported in previous studies [30, 31, 33, 40]. Comparisons across different studies are complicated by various factors. For example, PGx dosing guidelines, such as CPIC or DPWG guidelines, are regularly updated. Additionally, different countries have different drugs on the market and use different criteria to define paediatric age groups. Furthermore, studies covered different study periods. The CPIC guideline for ibuprofen, which was a highly prevalent drug in our study, has only been published in 2020; for that reason, it was not included in some of the previous studies [23, 28,29,30, 40]. Ramsey et al. reported an annual PGx drug exposure prevalence of 8.0–10.6% in children in the USA [30]. Their investigation was, however, limited to systemically applied CPIC level A drugs in persons < 21 years. Ibuprofen was not classified as a CPIC level A drug at the time of their study, which explains at least in part the large difference in PGx drug prevalence, as ibuprofen was the drug with the highest prevalence in our study [30]. Samwald et al. estimated that between 11.2 and 14.0% of children aged 0–13 years received at least one PGx drug during a four-year span. They did also not include ibuprofen as a PGx drug and excluded locally administered PGx drugs [40]. Liu et al. found a 29% prevalence of PGx drug exposure in children living in the USA aged 0–17 years within a three-year period [31]. Only Liu et al. included ibuprofen as a PGx drug [31]. To increase the validity of comparisons with previous studies, we did additional analyses in which we excluded ibuprofen and locally administered drugs. We then observed a five-year PGx drug prevalence of 18.2% in our study population.
When we focus on claims of at least two or three systemic PGx drugs (excluding ibuprofen), the prevalence was 4.0 and 1.0%, respectively, amongst the paediatric population, which is also higher than in previous studies from other countries. Samwald et al. reported that exposure to at least two PGx drugs was observed in 1.1–1.8% of children, while exposure to at least three PGx drugs was seen in 0.2–0.5% of children [40]. Ramsey et al. observed an increase in exposure to at least two PGx drugs from 1.5% in 2011 to 2.2% in 2017 [30].
In our study we observed a high ibuprofen prevalence of 59.1%. Ibuprofen is used in children to treat pain and fever [41]. The only other study to include ibuprofen as a PGx drug was conducted by Liu et al., using claims data from 2017–2019 in the USA. In children aged 0–17 years, the three-year prevalence of ibuprofen was 2.9% [31]. The prevalence for ibuprofen in our study was much higher. Non-PGx studies from the USA, Finland, and the Netherlands also found lower prevalences for ibuprofen and NSAIDs [42,43,44]. Differences in prevalences can have many causes: they are influenced by how the health system is organised, which reimbursement is offered by insurance companies, who is insured, and what drugs in which pharmaceutical forms are available for children OTC. In Switzerland, ibuprofen can be bought OTC and is therefore likely to even be underestimated in our study [45].
The antiemetic drug ondansetron (8.3%) was also quite prevalent in our study population, an observation which corresponds to results from other studies [28,29,30,31,32]. Besides its approved indications, ondansetron is also used off-label for the treatment of acute gastroenteritis [46, 47]. Additionally, it is the sole antiemetic approved for treating postoperative and radiation-induced vomiting in Switzerland from the first month of life [45].
Compared to the high prevalence of depression in adolescents (33%) [48], the number of claims of PGx antidepressants is low (escitalopram = 0.5%, sertraline = 0.8%). One explanation for this result is that, according to current guidelines, sertraline is the second-line treatment for unipolar depression in children and adolescents. The first choice is fluoxetine, which is not a PGx antidepressant and was therefore not investigated further in our study [49]. Tricyclic antidepressants, which are also PGx drugs, have shown no benefit in treating depression in children and are therefore unlikely to be used in our population [50].
In contrast to other studies, we included locally applied drugs as PGx drugs [30, 31, 40], because there are some reports on toxicity with locally applied PGx drugs [51,52,53,54]. In our study, three locally administered PGx drugs (fluorouracil, tacrolimus, and tobramycin) were among the top PGx drugs. The prevalence of local and systemic PGx drugs varied by age groups. Locally administered fluorouracil was mainly used in children aged 5–10 years. As locally administered fluorouracil can be used to treat warts, which are common in this age group [55], we assume that this explains why it is more prevalent in this than in other age groups in our study. There is only one locally applied product containing fluorouracil approved for use in children in Switzerland. It is a solution containing 5% fluorouracil [45]. According to the medicinal product information, the solution is applied 2–3 times a day for an average of 6 weeks. The solution should be applied to a maximum of 25 cm2 of body surface area, resulting in the application of 1 mg of fluorouracil [45]. Cohen et al. described a case of an adult male using 5% fluorouracil cream who developed severe neutropenia after 14 applications of the cream over 11 days [51]. The doses used in this case and in children in Switzerland overlap. We therefore think that toxic reactions to locally applied fluorouracil may indeed be relevant in children. Topical tobramycin was more prevalent in children under 4 years of age than older children. This was also observed in a French study, where children under 6 years were more likely to receive ophthalmic anti-infectives than older children [56]. In children aged 0–10 years, omeprazole was used more often than pantoprazole. However, pantoprazole was more commonly used in adolescents. In Switzerland, omeprazole is approved for infants, while pantoprazole is approved from the age of 12 years on [45]. Both substances can be used off-label from the age of one month [57]. Omeprazole is available in a multiple-unit-pellet-system (MUPS) formulation, making it easy to suspend for use in children [45]. Pantoprazole also has a granule formulation, but unlike MUPS-omeprazole, it is not reimbursed by health insurance [45, 58]. These differences could explain why omeprazole was used more often in younger children.
Out of 24 genes associated with the 82 studied drugs, CYP2C9 ranked first when assessing the number of children who claimed a drug associated with this gene, followed by CYP2D6 and DPYD. This is not surprising as ibuprofen, the most prevalent drug in our study population, is associated with CYP2C9 [59]. In contrast to other studies, we observed no drugs claims for drugs associated with CFTR and CYP4F2. Ivacaftor, which is associated with CFTR, was not claimed in the paediatric population, although it is available in Switzerland. Warfarin, which is associated with CYP4F2, is not approved in Switzerland and has therefore never been claimed [45]. For a better comparison with previous studies, we again excluded locally administered drugs and ibuprofen from the analysis. CYP2D6, CYP2C19, and CACNA1S/ RYR1 ranked first, second, and third, which corresponds to the results by Ramsey et al. [30]. The genes CYP2C9, CYP2D6, DPYD, MT-RNR1, CYP2C19, CACNA1S, and RYR1 were responsible for over 96% of all potential DGIs. Studies from the Netherlands, the UK, and Switzerland came to similar conclusions, although they did not focus on the paediatric population [33, 60, 61]. These three studies found that a higher percentage of DGIs were caused by SLCO1B1 [33, 60, 61]. SLCO1B1 is most commonly associated with statins, which are rarely used in children [62].
Our study has a number of strengths and limitations. A notable strength of this study lies in its use of multiple sources of recommendations for the definition of PGx drugs. Furthermore, solely PGx guidelines with recommendations were included, to ensure that the study is relevant to the current clinical decision-making process. We included medications applied locally and systemically, and we present results for both, which - to our knowledge - no other study has done yet. The classification of PGx drugs as either locally or systemically administered was challenging, due to the absence of differentiation in some ATC codes. Another challenge was the fact that age groups are defined differently in various countries, and they do not have the same duration. Such disparities in definitions make it difficult to compare our results with those of others. Our study is based on claims data and is therefore subject to the limitations of those data. We did not have information regarding the utilisation of OTC medication. Our research may have underestimated the prevalence of three PGx drugs (codeine and flurbiprofen both approved for use from the age of 12 years, and ibuprofen approved for use from the age of 6 months) that are available OTC for children in Switzerland. Additionally, we lacked information on drugs used in hospitals, as these are billed at a case rate except for highly expensive drugs [63]. Hence, our study may have underestimated medications frequently used in hospitals. Furthermore, the used claims database does not provide diagnoses; thus, it is impossible to determine the indication of the administered drugs. Importantly, the database lacks any genetic data of individuals. Consequently, it is impossible to determine the frequency of actionable DGIs and the impact of PGx testing on children. Only about 50% of the children registered during the five-year period were continuously insured. As Swiss insurances are private companies, the fees for standard insurance fluctuate annually. In Switzerland it is mandatory to have basic health insurance, but Swiss people are free to choose and to switch between insurances on an annual basis [64]. As a result, some individuals do indeed change their insurance every year, resulting in this fluctuation. Everybody is required to pay a (small) yearly amount before they can be reimbursed. This can lead to some drugs that have not been billed to the health insurance and may have been missed. As out-of-pocket payment has a greater impact on acute than on chronic medication, it is possible that some drug claims have been omitted. This could have resulted in a minor underestimation of acute medication in our findings. Furthermore, it should be noted that health care claims data cannot determine if the medication was actually taken by the individuals. Therefore, it is probable that the actual exposure to PGx drugs may be lower if the medication was not taken.
To our knowledge, this study is the first to evaluate PGx drug claims among children residing in Switzerland. Our study indicates a high utilisation of PGx drugs among children in Switzerland, with 66.1% claiming PGx drugs during a five-year period. Among PGx drugs, the NSAID ibuprofen, the antiemetic ondansetron and locally administered fluorouracil had the highest number of users. We identified CYP2C9, CYP2D6, DPYD, and CYP2C19 as relevant pharmacogenes in paediatrics. As the utilisation of PGx drugs is frequent and few genes are responsible for a high amount of potential DGIs, we propose to rather use a preemptive testing panel in paediatrics than single gene testing. However, further studies are required to evaluate the clinical impact of PGx in the paediatric population.
Data availability
The datasets generated and/or analysed during the current study are not publicly available due to confidentiality requirements issued by Helsana. Analysis codes and datasets can be made available by the corresponding author (s.allemann@unibas.ch) upon reasonable request and with permission of Helsana.
Code availability
Analysis codes can be made available by the corresponding author (s.allemann@unibas.ch) upon reasonable request and with permission of Helsana.
References
Westervelt P, Cho K, Bright DR, Kisor DF. Drug-gene interactions: inherent variability in drug maintenance dose requirements. P T. 2014;39:630–7.
Hahn M, Roll SC. The influence of pharmacogenetics on the clinical relevance of pharmacokinetic drug–drug interactions: drug–gene, drug–gene–gene and drug–drug–gene interactions. Pharmaceuticals. 2021;14:487. https://doi.org/10.3390/ph14050487.
Tannenbaum C, Sheehan NL. Understanding and preventing drug–drug and drug–gene interactions. Expert Rev Clin Pharmacol. 2014;7:533–44. https://doi.org/10.1586/17512433.2014.910111.
Lee JW, Aminkeng F, Bhavsar AP, Shaw K, Carleton BC, Hayden MR, et al. The emerging era of pharmacogenomics: current successes, future potential, and challenges. Clin Genet. 2014;86:21–8. https://doi.org/10.1111/cge.12392.
Smyth RMD, Gargon E, Kirkham J, Cresswell L, Golder S, Smyth R, et al. Adverse drug reactions in children—a systematic review. PLoS ONE. 2012;7:e24061. https://doi.org/10.1371/JOURNAL.PONE.0024061.
Swen JJ, van der Wouden CH, Manson LE, Abdullah-Koolmees H, Blagec K, Blagus T, et al. A 12-gene pharmacogenetic panel to prevent adverse drug reactions: an open-label, multicentre, controlled, cluster-randomised crossover implementation study. Lancet. 2023;401:347–56. https://doi.org/10.1016/S0140-6736(22)01841-4.
Vassy JL, Chun S, Advani S, Ludin SA, Smith JG, Alligood EC. Impact of SLCO1B1 pharmacogenetic testing on patient and healthcare outcomes: a systematic review. Clin Pharmacol Ther. 2019;106:360–73. https://doi.org/10.1002/cpt.1223.
Alfirevic A, Pirmohamed M, Marinovic B, Harcourt-Smith L, Jorgensen AL, Cooper TE. Genetic testing for prevention of severe drug-induced skin rash. Cochrane Database Syst Rev. 2019;7:CD010891. https://doi.org/10.1002/14651858.CD010891.pub2.
Ontario Health. Multi-gene pharmacogenomic testing that includes decision-support tools to guide medication selection for major depression: a health technology assessment. Ont Health Technol Assess Ser. 2021;21:1–214.
David V, Fylan B, Bryant E, Smith H, Sagoo GS, Rattray M. An analysis of pharmacogenomic-guided pathways and their effect on medication changes and hospital admissions: a systematic review and meta-analysis. Front Genet. 2021;12:698148. https://doi.org/10.3389/fgene.2021.698148.
Stevens JC, Marsh SA, Zaya MJ, Regina KJ, Divakaran K, Le M, et al. Developmental changes in human liver CYP2D6 expression. Drug Metab Dispos. 2008;36:1587–93. https://doi.org/10.1124/DMD.108.021873.
Barker CIS, Groeneweg G, Maitland-van der Zee AH, Rieder MJ, Hawcutt DB, Hubbard TJ, et al. Pharmacogenomic testing in paediatrics: clinical implementation strategies. Br J Clin Pharmacol. 2022;88:4297–310. https://doi.org/10.1111/BCP.15181.
Sing CW, Cheung CL, Wong ICK. Pharmacogenomics – how close/far are we to practising individualized medicine for children? Br J Clin Pharmacol. 2015;79:419–28. https://doi.org/10.1111/BCP.12338.
Scodellaro S, Cohn RD, Cohn I. Urgent call for guidance supporting gene-based drug dosing in children and adolescents. Paediatr Child Health. 2023;28:205–7. https://doi.org/10.1093/PCH/PXAD003.
Bousman CA, Stevenson JM, Ramsey LB, Sangkuhl K, Hicks JK, Strawn JR, et al. Clinical pharmacogenetics implementation consortium (CPIC) guideline for CYP2D6, CYP2C19, CYP2B6, SLC6A4, and HTR2A genotypes and serotonin reuptake inhibitor antidepressants. Clin Pharmacol Ther. 2023;114:51–68. https://doi.org/10.1002/cpt.2903.
Amstutz U, Henricks LM, Offer SM, Barbarino J, Schellens JHM, Swen JJ, et al. Clinical pharmacogenetics implementation consortium (CPIC) guideline for dihydropyrimidine dehydrogenase genotype and fluoropyrimidine dosing: 2017 update. Clin Pharmacol Ther. 2018;103:210–6. https://doi.org/10.1002/cpt.911.
Beunk L, Nijenhuis M, Soree B, de Boer-Veger NJ, Buunk A-M, Guchelaar HJ, et al. Dutch pharmacogenetics working group (DPWG) guideline for the gene-drug interaction between CYP2D6, CYP3A4 and CYP1A2 and antipsychotics. Eur J Hum Genet. 2024;32:278–85. https://doi.org/10.1038/s41431-023-01347-3.
Matic M, Nijenhuis M, Soree B, de Boer-Veger NJ, Buunk AM, Houwink EJF, et al. Dutch pharmacogenetics working group (DPWG) guideline for the gene–drug interaction between CYP2D6 and opioids (codeine, tramadol and oxycodone). Eur J Hum Genet. 2021;30:1105–13. https://doi.org/10.1038/s41431-021-00920-y.
Aminkeng F, Ross CJD, Rassekh SR, Hwang S, Rieder MJ, Bhavsar AP, et al. Recommendations for genetic testing to reduce the incidence of anthracycline-induced cardiotoxicity. Br J Clin Pharmacol. 2016;82:683–95. https://doi.org/10.1111/BCP.13008.
Lee JW, Pussegoda K, Rassekh RS, Monzon JG, Liu G, Hwang S, et al. Clinical practice recommendations for the management and prevention of cisplatin-induced hearing loss using pharmacogenetic markers. Ther Drug Monit. 2016;38:423–31. https://doi.org/10.1097/FTD.0000000000000298.
Whirl-Carrillo M, Huddart R, Gong L, Sangkuhl K, Thorn CF, Whaley R, et al. An evidence-based framework for evaluating pharmacogenomics knowledge for personalized medicine. Clin Pharmacol Ther. 2021;110:563–72. https://doi.org/10.1002/CPT.2350.
Lunenburg CATC, Ishtiak-Ahmed K, Werge T, Gasse C. Life-time actionable pharmacogenetic drug use: a population-based cohort study in 86 040 young people with and without mental disorders in Denmark. Pharmacopsychiatry. 2022;55:95–107. https://doi.org/10.1055/a-1655-9500.
Roberts TA, Wagner JA, Sandritter T, Black BT, Gaedigk A, Stancil SL. Retrospective review of pharmacogenetic testing at an academic Children’s hospital. Clin Transl Sci. 2021;14:412–21. https://doi.org/10.1111/cts.12895.
Gammal RS, Crews KR, Haidar CE, Hoffman JM, Baker DK, Barker PJ, et al. Pharmacogenetics for safe codeine use in sickle cell disease. Pediatrics. 2016;138:e20153479. https://doi.org/10.1542/PEDS.2015-3479/52565.
Grimsrud KN, Davis RR, Tepper CG, Palmieri TL. Pharmacogenetic gene–drug associations in pediatric burn and surgery patients. J Burn Care Res. 2022;43:987–96. https://doi.org/10.1093/jbcr/irac062.
Ramsey LB, Bishop JR, Strawn JR. Pharmacogenetics of treating pediatric anxiety and depression. Pharmacogenomics. 2019;20:867–70. https://doi.org/10.2217/pgs-2019-0088.
Ariefdjohan M, Lee YM, Stutzman DL, LeNoue S, Wamboldt MZ. The utility of pharmacogenetic-guided psychotropic medication selection for pediatric patients: a retrospective study. Pediatr Rep. 2021;13:421–33. https://doi.org/10.3390/PEDIATRIC13030049.
Aka I, Bernal CJ, Carroll R, Maxwell-Horn A, Oshikoya KA, Van Driest SL. Clinical pharmacogenetics of cytochrome P450-associated drugs in children. J Pers Med. 2017;7:14. https://doi.org/10.3390/jpm7040014.
Fan M, Yarema MC, Box A, Hume S, Aitchison KJ, Bousman CA. Identification of high-impact gene–drug pairs for pharmacogenetic testing in Alberta, Canada. Pharmacogenet Genomics. 2021;31:29–39. https://doi.org/10.1097/FPC.0000000000000418.
Ramsey LB, Ong HH, Schildcrout JS, Shi Y, Tang LA, Hicks JK, et al. Prescribing prevalence of medications with potential genotype-guided dosing in pediatric patients. JAMA Netw Open. 2020;3:e2029411. https://doi.org/10.1001/jamanetworkopen.2020.29411.
Liu D, Olson KL, Manzi SF, Mandl KD. Patients dispensed medications with actionable pharmacogenomic biomarkers: rates and characteristics. Genet Med. 2021;23:782–6. https://doi.org/10.1038/S41436-020-01044-2.
Gallaway KA, Cann K, Oetting K, Rothenberger M, Raibulet A, Slaven JE, et al. The potential impact of preemptive pharmacogenetic genotyping in the neonatal intensive care unit. J Pediatr. 2023;259:113489. https://doi.org/10.1016/j.jpeds.2023.113489.
Alshabeeb MA, Deneer VHM, Khan A, Asselbergs FW. Use of pharmacogenetic drugs by the dutch population. Front Genet. 2019;10:567. https://doi.org/10.3389/fgene.2019.00567.
Twerenbold S, Schur N, Wittwer N, Schneider C, Marxer C, Spöndlin J, et al. Helsana-arzneimittelreport für die Schweiz 2022. 2022. https://reports.helsana.ch/en/drugs2022/. (accessed January 7, 2023).
Schneider R, Reinau D, Schur N, Blozik E, Früh M, Signorell A, et al. Coverage rates and timeliness of nationally recommended vaccinations in swiss preschool children: a descriptive analysis using claims data. Vaccine. 2020;38:1551–8. https://doi.org/10.1016/j.vaccine.2019.11.057.
Spoendlin J, Blozik E, Graber S, Rauch M, Marxer C, Regg S, et al. Use of valproate in pregnancy and in women of childbearing age between 2014 and 2018 in Switzerland: a retrospective analysis of Swiss healthcare claims data. Swiss Med Wkly. 2021;151:w20386. https://doi.org/10.4414/smw.2021.20386.
Reinau D, Schur N, Twerenbold S, Blozik E, Früh M, Signorell A, et al. Utilisation patterns and costs of lipid-lowering drugs in Switzerland 2013–2019. Swiss Med Wkly. 2021;151:w30018. https://doi.org/10.4414/smw.2021.w30018.
National Institute of Child Health and Human Development (NICHD). NICHD pediatric terminology files. NICHD Pediatr Terminol Files. 2023. https://evs.nci.nih.gov/ftp1/Pediatric_Terminologies/NICHD/About.html. (accessed January 17, 2023).
Fedlex. Federal act on data protection (FADP). 2019. https://www.fedlex.admin.ch/eli/cc/1993/1945_1945_1945/en. (accessed December 24, 2021).
Samwald M, Xu H, Blagec K, Empey PE, Malone DC, Ahmed SM, et al. Incidence of exposure of patients in the United States to multiple drugs for which pharmacogenomic guidelines are available. PLoS ONE. 2016;11:e0164972. https://doi.org/10.1371/journal.pone.0164972.
Dao K, Laubscher B, Buclin T. Paracetamol und ibuprofen in der pädiatrie. Paediatrica. 2016;27:28–32.
Chai G, Governale L, McMahon AW, Trinidad JP, Staffa J, Murphy D. Trends of outpatient prescription drug utilization in US children, 2002–2010. Pediatrics. 2012;130:23–31. https://doi.org/10.1542/PEDS.2011-2879.
Helin-Salmivaara A, Klaukka T, Huupponen R. Heavy users of non-steroidal anti-inflammatory drugs: a nationwide prescription database study in Finland. Eur J Clin Pharmacol. 2003;59:477–82. https://doi.org/10.1007/S00228-003-0635-X/FIGURES/2.
Kosse RC, Koster ES, de Vries TW, Bouvy ML. Drug utilisation among dutch adolescents: a pharmacy prescription records study. Arch Dis Child. 2019;104:745–8. https://doi.org/10.1136/archdischild-2017-314692.
refdata. Medicinal product information n.d. https://www.swissmedicinfo.ch/. (accessed August 12, 2023).
Bonvanie IJ, Weghorst AAH, Holtman GA, Russchen HA, Fickweiler F, Verkade HJ, et al. Oral ondansetron for paediatric gastroenteritis in primary care: a randomised controlled trial. Br J Gen Pract. 2021;71:e728–35. https://doi.org/10.3399/BJGP.2021.0211.
Niño-Serna LF, Acosta-Reyes J, Veroniki AA, Florez ID. Antiemetics in children with acute gastroenteritis: a meta-analysis. Pediatrics. 2020;145:20193260. https://doi.org/10.1542/PEDS.2019-3260/36986.
Barrense-Dias Y, Chok L, Surís J A picture of the mental health of adolescents in Switzerland and Liechtenstein | Unisanté. Lausanne: Unisanté – Cent Univ Médecine Générale Santé Publique; 2021. https://doi.org/10.16908/issn.1660-7104/323.
Brent DA, Gibbons RD, Wilkinson P, Dubicka B. Antidepressants in paediatric depression: do not look back in anger but around in awareness. BJPsych Bull. 2018;42:1–4. https://doi.org/10.1192/bjb.2017.2.
Hazell P, Mirzaie M. Tricyclic drugs for depression in children and adolescents. Cochrane Database Syst Rev. 2013;2013:CD002317. https://doi.org/10.1002/14651858.CD002317.pub2.
Cohen PR. Topical application of 5-fluorouracil 5 percent cream associated with severe neutropenia: discussion of a case and review of systemic reactions after topical treatment with 5-fluorouracil. Dermatol Online J. 2018;24:13030/qt974797j7. https://doi.org/10.5070/D3244039360.
Sargen M, Wanat KA, Jambusaria A, Rosenbach M, Sobanko J, Miller CJ. Systemic toxicity from occlusive therapy with topical 5-fluorouracil: a case report and review of the literature. Dermatol Surg. 2012;38:1756–9. https://doi.org/10.1111/J.1524-4725.2012.02511.X.
Membrive Jiménez C, Pérez Ramírez C, Sánchez Martín A, Vieira Maroun S, Arias Santiago S, Ramírez Tortosa MC, et al. Clinical application of pharmacogenetic markers in the treatment of dermatologic pathologies. Pharmaceuticals. 2021;14:905. https://doi.org/10.3390/ph14090905.
Bollinger A, Jeiziner C, Meyer zu Schwabedissen HE, Hersberger KE, Allemann SS, Stäuble CK. Severe systemic adverse reactions to ophthalmic timolol in a CYP2D6 homozygous *4 allele carrier: a case report. Pharmacogenomics. 2023;24:739–46. https://doi.org/10.2217/pgs-2023-0122.
Van Haalen FM, Bruggink SC, Gussekloo J, Assendelft WJJ, Eekhof JAH. Warts in primary schoolchildren: prevalence and relation with environmental factors. Br J Dermatol. 2009;161:148–52. https://doi.org/10.1111/J.1365-2133.2009.09160.X.
Taine M, Offredo L, Dray-Spira R, Weill A, Chalumeau M, Zureik M. Paediatric outpatient prescriptions in France between 2010 and 2019: a nationwide population-based study. Lancet Reg Health Eur. 2021;7:100129. https://doi.org/10.1016/j.lanepe.2021.100129.
Tilen R, Panis D, Aeschbacher S, Sabine T, Meyer zu Schwabedissen HE, Berger C. Development of the swiss database for dosing medicinal products in pediatrics. Eur J Pediatr. 2021;181:1221–31. https://doi.org/10.1007/s00431-021-04304-8.
Federal Office of Public Health (FOPH). Specialties list (SL) 2023. http://www.spezialitätenliste.ch/. (accessed April 7, 2022).
Theken KN, Lee CR, Gong L, Caudle KE, Formea CM, Gaedigk A, et al. Clinical pharmacogenetics implementation consortium guideline (CPIC) for CYP2C9 and nonsteroidal anti‐inflammatory drugs. Clin Pharmacol Ther. 2020;108:191–200. https://doi.org/10.1002/cpt.1830.
Wittwer NL, Meier CR, Huber CA, Meyer zu Schwabedissen HE, Allemann S, Schneider C. Utilization of drugs with pharmacogenetic dosing recommendations in Switzerland: a descriptive study using the helsana database. Pharmgenomics Pers Med. 2022;15:967–76. https://doi.org/10.2147/PGPM.S382214.
Youssef E, Kirkdale CL, Wright DJ, Guchelaar H-J, Thornley T. Estimating the potential impact of implementing pre-emptive pharmacogenetic testing in primary care across the UK. Br J Clin Pharmacol. 2021;87:2907–25. https://doi.org/10.1111/BCP.14704.
Liberman JN, Berger JE, Lewis M. Prevalence of antihypertensive, antidiabetic, and dyslipidemic prescription medication use among children and adolescents. Arch Pediatr Adolesc Med. 2009;163:357–64. https://doi.org/10.1001/ARCHPEDIATRICS.2009.5.
Federal Office of Public Health (FOPH). Inpatient tariff structures 2021. https://www.bag.admin.ch/bag/en/home/versicherungen/krankenversicherung/krankenversicherung-leistungen-tarife/Spitalbehandlung/Tarifsystem-SwissDRG.html. (accessed November 24, 2023).
Biller-Andorno N, Zeltner T. Individual responsibility and community solidarity — the swiss health care system. N Engl J Med. 2015;373:2193–7. https://doi.org/10.1056/NEJMp1508256.
Acknowledgements
We used Deepl Write (Deepl SE, Germany) for final language editing.
Funding
Open access funding provided by University of Basel.
Author information
Authors and Affiliations
Contributions
NLW contributed to the study conception and design, analysed the data, interpreted the data, wrote the first draft of the manuscript, revised and approved the manuscript. CRM contributed to the study conception and design, interpreted the data, revised and approved the manuscript. CAH contributed to the study conception and design, acquired the data, interpreted the data, revised and approved the manuscript. RT contributed to the study conception and design, interpreted the data, revised and approved the manuscript. CY contributed to the study conception and design, analysed the data, interpreted the data, revised and approved the manuscript. HEMzS contributed to the study conception and design, interpreted the data, revised and approved the manuscript. SA contributed to the study conception and design, interpreted the data, revised and approved the manuscript. CS contributed to the study conception and design, analysed the data, interpreted the data, revised and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
Ethics approval was not necessary according to article 22 of the Swiss Federal law on data protection, as the study was retrospective and used anonymized data.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wittwer, N.L., Meier, C.R., Huber, C.A. et al. Utilisation of drugs with pharmacogenetic recommendations in children in Switzerland. Pharmacogenomics J 25, 19 (2025). https://doi.org/10.1038/s41397-025-00378-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41397-025-00378-x