Abstract
Electronic cigarettes (e-cigs) use, especially among youngsters, has been on the rise in recent years. However, little is known about the long-term effects of the use of e-cigs on brain functional activity. We acquired the resting-state functional magnetic resonance imaging (rs-fMRI) data from 93 e-cigs users with nicotine dependence and 103 health controls (HC). The local synchronization was analyzed via the regional homogeneity (ReHo) method at voxel-wise level. The functional connectivity (FC) between the nucleus accumbens (NAcc), the ventral tegmental area (VTA), and the insula was calculated at ROI-wise level. The support vector machining classification model based on rs-fMRI measures was used to identify e-cigs users from HC. Compared with HC, nicotine-dependent e-cigs users showed increased ReHo in the right rolandic operculum and the right insula (p < 0.05, FDR corrected). At the ROI-wise level, abnormal FCs between the NAcc, the VTA, and the insula were found in e-cigs users compared to HC (p < 0.05, FDR corrected). Correlation analysis found a significant negative correlation between ReHo in the left NAcc and duration of e-cigs use (r = −0.273, p = 0.008, FDR corrected). The following support vector machine model based on significant results of rs-fMRI successfully differentiates chronic e-cigs users from HC with an accuracy of 73.47%, an AUC of 0.781, a sensitivity of 67.74%, and a specificity of 78.64%. Dysregulated spontaneous activity and FC of addiction-related regions were found in e-cigs users with nicotine dependence, which provides crucial insights into the prevention of its initial use and intervention for quitting e-cigs.
Similar content being viewed by others
Introduction
Electronic cigarettes (e-cigs) are novel vaporizing devices that deliver nicotine with battery power. Due to its potential as an alternative to smoking cessation and its appeal to teenagers, the number of e-cigs users is rapidly increasing in many countries [1]. In European countries, about 30% of current smokers ever used e-cigs [2]. In the United States, the rate of e-cigs use among high school students was 19.6%, while it was 4.7% among middle school students [3, 4]. A survey conducted in China revealed that approximately a quarter of young Chinese adults have ever used e-cigs [5]. Chronic use of e-cigs may increase the risk of developing nicotine dependence and have negative effects on psychological well-being such as increased depressive symptoms, decreased sleep quality and so on [6,7,8]. Given the dramatic increase in e-cigs use in recent years, there is growing concern about the associated adverse effects. However, the impact of repeated use of e-cigs on brain structure and function remains largely unknown. Such information may provide valuable insights for developing programs aimed at optimizing the prevention and treatment of e-cigs dependence.
Some studies support e-cigs as a valid alternative for quitting combustible cigarettes, while others suggest that repeated use of e-cigs may have negative health effects [6, 9]. The effects of e-cigs on lung and microvascular function are similar to or even greater than those of conventional cigarettes [8, 10]. For instance, a study on healthy participants revealed that lung perfusion increased after exposure to e-cigs [11]. Another study showed that healthy nonsmokers exposed to e-cigs experienced increased resistivity index and aortic pulse wave velocity [12]. Additionally, exposure to e-cigs has been also found to exhibit neurotoxic effects on brain [13, 14]. Animal experiments have revealed neurotoxic effects associated with e-cigarette aerosol exposure, including increased expression of α-7 nicotinic acetylcholine receptors (nAChRs) in the prefrontal cortex and striatum, reduced expression of glutamate transporter-1 in the striatum [15], increased oxidative stress in the prefrontal cortex [16], elevated metal levels in the prefrontal cortex and striatum [16], disruption of the blood-brain barrier integrity, and promotion of neurovascular inflammation [17], reduced brain glucose uptake and glucose transporters [18]. Moreover, exposure to e-cigs exhibited potentially harmful neurodevelopmental effects during early pregnancy or early-life exposure. For example, maternal exposure to e-cigs containing nicotine had been shown to increase offspring bodyweight and impair motor skill learning [19].
However, the impact of e-cigs, especially in the long-term effect, on the brain functional activity of individuals with nicotine dependence remains unknown. E-cigs likely promote the same addictive behaviors as traditional combustible cigarettes, resulting in increasing chronic and repeated use [20]. Studies found adolescents who have ever used e-cigs were more likely to use combustible tobacco compared with nonusers [21]. E-cigs users exhibited higher levels of nicotine dependence than traditional tobacco smokers [20, 22]. Existing evidence suggested e-cigs use, especially long-term use, may lead to human brain functional activity alterations.
Resting-state functional magnetic resonance imaging (rs-fMRI) is a useful and noninvasive tool to measure human brain functional activity [23]. So far, studies using fMRI were focused on measuring the acute effects of e-cigs intake in the brain with a small sample. A study with 9 participants found abnormal functional connectivity (FC) between the insula and prefrontal cortex following e-cigs use [24]. Another study found youth who had tried e-cigs showed higher activation in the nucleus accumbens during viewing advertisements about sweet or fruit flavor e-cigs [25].
The current study aimed to measure brain functional activity alternations among e-cigs users who have nicotine dependence/addiction. Studies on substance dependence have begun to suggest more precise links between functional properties of some brain regions (including the insula, the nucleus accumbens (NAcc), and the ventral tegmental area (VTA)) with initiation and maintain of addiction [26, 27]. Some of these findings may be relevant to understanding the potential effects of repeated use of e-cigs. In this study, we measured regional homogeneity (ReHo) map at voxel-wise level, and FC between the NAcc, the VTA, and the insula at ROI-wise level. The relationship between significant functional measures and clinical behavior scales was explored. The support vector machine was used to test if these altered functional activities can classify chronic e-cigs users from health control at the individual level. We hypothesized that (1) chronic e-cigs users showed abnormal brain functional activity in the NAcc, the VTA, and the insula; (2) these abnormal brain regions can be used to classify chronic e-cigs users from HC.
Methods
Subjects
We recruited 93 e-cigs users with nicotine dependence and 103 healthy controls via social media advertisement. All subjects were Han Chinese and aged between 18–45. E-cigs users with nicotine dependence need to meet the following criteria: (1) they had used e-cigs containing nicotine for more than one year, and (2) they had a diagnosis of nicotine dependence based on the Diagnostic and Statistical Manual of Mental Disorders (DSM-V) criteria, assessed using the Structured Clinical Interview for DSM Disorders (SCID). Both chronic e-cigs users and healthy control subjects were excluded from the study if they: (1) had learning disabilities or central nervous system dysfunctions; (2) had a history of head injury, psychiatric disorders, or a loss of consciousness lasting more than 10 min; (3) were left-handed or mixed-handed; (4) had undergone electroconvulsive therapy (ECT), brain stimulation therapies or received the anti-psychotic drugs within the last 3 months; (5) had a family history of psychotic disorders; (6) met the criteria for substance dependence (excluding nicotine for subjects in the e-cigs group); (7) were pregnant or had contraindications for MRI.
Subjects underwent cotinine urine dipsticks test to further identify their smoking status with a 200 ng/ml cut-off for urine cotinine. All participants meet the criteria for cotinine levels, with the control group having cotinine levels below the specified cut-off and the e-cigs users having cotinine levels above the specified cut-off. Among all chronic e-cigs users, the study identified 47 individuals who exclusively use e-cigs and 46 individuals who are dual users, using both combustible cigarettes and e-cigs. This classification was based on a criterion of self-reported smoking more than 100 tobacco cigarettes within the last 3 months. All participants completed questionnaires to provide demographic and clinical information, and they also underwent MRI scans.
The study protocol was approved by the Institutional Review Board (IRB) of Sir Run Run Shaw Hospital, Zhejiang University School of Medicine (No. 2022–401–01) and was conducted in accordance with the Helsinki Declaration. All subjects signed and gave written informed consent before the formal experiment.
Clinical measurements
The demographic characteristics from every subject were acquired, including age, sex, educational level, ethnicity and handedness. Depressive symptoms were estimated by the 21-item Beck Depression Intervention (BDI). For chronic e-cigs users, the characteristics of addictive substance use were also estimated, including tobacco smoking, and e-cigs smoking. Carving for e-cigs was measure by the 10-score Visual Analogue Scale (VAS). Dependence for e-cigs was accessed by the 10-score VAS and the Fagerström Test for Cigarette Dependence (FTCD), respectively.
MRI data acquisition
All images were acquired using a 3.0 T Siemens Magentom Trio scanner (Allegra; Siemens, Erlangen, Germany) at the Hunan Provincial People’s Hospital, China. The 3D T1-weighted images were obtained through a magnetization-prepared rapid acquisition with gradient echo (MPRAGE) with the following parameters: 176 sagittal slices of 1 mm thickness without gap, TR = 2000 ms, TE = 2.26 ms, FOV = 256 × 256 mm2, flip angle = 8°, matrix size = 256 × 256. The rs-fMRI images were obtained using an echo planar imaging (EPI) scan sequence with the following parameters: (TR = 2000 ms, TE = 30 ms, FOV = 220 × 220 mm2, flip angle = 90°, 36 axial, 200 volumes). During the acquisition of fMRI data, especially in R-fMRI scans, subjects were asked to relax with closed eyes, think of nothing and not fall asleep.
Image data processing
All neuroimage data were processed using DPABI (http://www.rfmri.org/), SPM (http://www.fil.ion.ucl.ac.uk/spm/) and written scripts. Firstly, we excluded the first 10 volumes of functional images at the individual level to account for magnetization equilibration effects and participants’ adaptation to the circumstances. Subsequently, the remaining images were temporally corrected for time delay between slices, and aligned to the first volume for head-motion correction. To realign the images, the movement of the participant’s head was calculated by estimating the displacement along each axis and the rotation around each axis for every consecutive volume. Each participant displayed a peak displacement of under 2 mm along all axes and an angular movement of fewer than 2 degrees for each axis. Linear regression method was performed to regress out confounding factors including 24 motion parameters and the average time series of signals from the cerebrospinal fluid and white matter. The images were subsequently normalized to a stereotactic space that conforms to the Montreal Neurological Institute’s standard with a resampling voxel size of 3 mm × 3 mm × 3 mm. Finally, a temporal bandpass filter between 0.01 and 0.08 Hz was applied to the images.
ReHo calculations
Regional homogeneity (ReHo) is a widely-applied measure to delineate the local synchronization of BOLD signals at the voxel level recorded by rs-fMRI [28]. The ReHo is defined by estimating the relationship between the time series of a given voxel and its spatially adjacent voxels [29]. We calculated the Kendall concordance coefficient of a given voxel with the time courses of 27 neighboring voxels as the ReHo value of a given voxel [28, 30]. For further analysis, the mean ReHo (mReHo) value was estimated by divided by the whole-brain averaged ReHo. The mReHo was spatial smoothed (FWHM = 6 mm). It is noted that the preprocessed steps did not include spatial smooth before the ReHo calculation, to reduce the regional homogeneity among voxels. The ReHo values were extracted specifically from the NAcc, the VTA, and the insular subregions, as described below.
Functional connectivity analysis
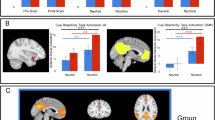
We used the Brainnetome atlas to define the bilateral NAcc [31]. For the VTA, we used a spherical seed with a 3-mm radius centered on the ROI at Talairach coordinates (x = 0, y = −16, z = −7) [32]. Due to the functional heterogeneity of the insular cortex [33, 34], we defined six subregions within the insula. These subregions included the following: the left ventral anterior insula (MNI coordinates: x = −33, y = 13, z = −7), the right ventral anterior insula (MNI coordinates: x = 32, y = 10, z = −6), the left dorsal anterior insula (MNI coordinates: x = −38, y = 6, z = 2), the right dorsal anterior insula (MNI coordinates: x = 35, y = 7, z = 3), the left posterior insula (MNI coordinates: x = −38, y = −6, z = 5), and the right posterior insula (MNI coordinates: x = 35, y = −11, z = 6). These subregions were defined by a spherical mask with a 6-mm radius (Fig. 1A).
A Eight regions of interest. B Aberrant functional connectivity map in chronic e-cigarette users compared to control groups. This map involved the insular subregions (represented by blue circles), the nucleus accumbens (represented by yellow circles), and the ventral tegmental area (represented by red circles). Each abnormal functional connectivity is represented by a blue line, with its thickness indicating the t-value magnitude. L, left; R, right; L.NAcc, left nucleus accumbens; VTA, the ventral tegmental area; L.PI, left ventral anterior insula; L.DAI, left dorsal anterior insula; L.PI, left posterior insula.
Functional connectivity (FC) is a method used to examine the functional interactions between different brain areas by measuring the temporal coherence of their activity. In this study, we extracted the mean time series from the identified brain regions. To quantify the FC between these regions, we employed the Pearson correlation method, which measures the linear relationship between two-time series. This resulted in the calculation of 36 functional connections between the selected brain regions. To facilitate further analysis and statistical comparisons, we transformed the correlation coefficients to z values using Fisher’s r-to-z transformation. This transformation helps to normalize the correlation values and allows for more appropriate statistical analyses.
Statistical analyses
The two-sample t-test and χ2 test was used to compare demographic data between the two groups (e-cigs users and HC). To investigate group differences in rs-fMRI measures, we conducted a general linear model (GLM) analysis. Age, sex, and years of education were included as covariates. The rs-fMRI measures included voxel-wise ReHo maps, ReHo of the NAcc, the VTA, and the insular subregions at the ROI level, and functional connectivity between the NAcc and the VTA, as well as the insular subregions. The Pearson correlation method was used to estimate the relationship between the significant findings from ReHo or FC analysis and the characteristic of e-cigs use within chronic e-cigs users. The false discovery rate (FDR) method with a threshold p < 0.05 was performed for multiple comparison corrections. To further identify the effect of long-term e-cigs on the brain, we also compare exclusive e-cigs users and dual users in the rs-fMRI measures using GLM with age, sex, and years of education as covariates.
Support vector machine (SVM) is a widely used classifier in neuroimaging research, providing a framework to study brain images at the individual level [35]. In this study, we employed SVM based on rs-fMRI measures that exhibited significant group differences to classify chronic e-cigs users from HC using a leave-one-out cross-validation (LOOCV) approach. To assess the performance of the classifier, the permutation test was performed with 10,000 iterations through randomly relabeling the class labels and recalculating the classification accuracy each time. We considered the classification performance to be reliable if the generalization rate obtained by the classifier trained on the actual class labels exceeded the 95% confidence interval of the classifier trained on randomly relabeled class labels. Furthermore, we calculated the area under the receiver-operating characteristic curve (AUC), sensitivity and specificity to evaluate the performance of the classifier model. Classification measures the area under the receiver operating characteristic (ROC) curve. Sensitivity measures the proportion of true positive samples (e-cigs users) correctly identified by the classifier, while specificity measures the proportion of true negative samples (healthy controls) correctly identified by the classifier.
Results
Sample characteristics
Table 1 presents demographics, and clinical characteristics for the total of 93 chronic e-cigs users and 103 healthy controls. We did not find significant group differences between chronic e-cigs users and HC in age and educational level variates. However, there was a significant difference in sex between the two groups (χ2 = 19.355, p < 0.001). We also found that chronic e-cigs users exhibited higher BDI scores than HC. Age and educational level variates did not differ from exclusive e-cigs users to dual users, while sex showed a significant difference (χ2 = 7.355, p < 0.001, see Table 1 in supplementary materials).
Between-group differences in ReHo
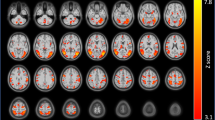
We found that chronic e-cigs users showed significantly increased ReHo in the right rolandic operculum (p < 0.05, FDR corrected) and the right insula (p < 0.05, FDR corrected) relative to HCs (Fig. 2). There was no compared difference in two significant clusters between exclusive e-cigs users and dual users (p > 0.05, FDR corrected).
Local synchronization of insular subregions, VTA and NAc
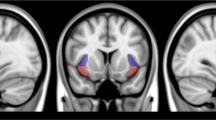
As shown in Fig. 3, we found chronic e-cigs users showed significantly lower ReHo value in the bilateral NAcc (p < 0.05, FDR corrected) than HC, while there were no significant changes in the VTA (p < 0.05, FDR corrected). With regard to the insular subregions, chronic e-cigs users significantly increased ReHo in the bilateral dorsal anterior insula and the right posterior insula compared to HC, while significantly decreased in the left ventral anterior insula (p < 0.05, FDR corrected). However, we did not find any significant group difference between exclusive e-cigs users and dual users (p > 0.05).
The group difference between chronic e-cigs users and HC A, exclusive e-cigs users and dual users in the NAcc B. The group difference between chronic e-cigs users and HC C, and exclusive e-cigs users and dual users in insular subregions D. The significance threshold was p < 0.05, FDR corrected. L.NAcc, left nucleus accumbens; R.NAcc, right nucleus accumbens; L.PI, left ventral anterior insula; R.PI, right posterior insula; L.DAI, left dorsal anterior insula; R.DAI, right dorsal anterior insula.
Connectivity of the insular subregions, NAcc and VTA
At the ROI-wise level, as described in Fig. 1, the FC between the bilateral NAcc and the VTA in chronic e-cigs users significantly decreased compared to HC (p < 0.05, FDR corrected). The functional connectivity of the VTA and all insular subregions showed significant group differences between chronic e-cigs users and HC (p < 0.05, FDR corrected). We also found that chronic e-cigs users showed a significantly decreased in the connectivity of the right NAcc with the bilateral dorsal anterior insula, the right ventral anterior insula and the posterior insula when compared to HC (p < 0.05, FDR corrected). And significantly decreased functional connectivity between the left NAcc bilateral dorsal anterior insula, the right ventral anterior insula and the posterior insula were found (p < 0.05, FDR corrected). Decreased functional connectivity within the subregions of the insular cortex (between the bilateral dorsal anterior insula and the bilateral posterior insula, the right ventral anterior insula and the bilateral posterior insula, the left dorsal anterior insula and the right posterior insula, the left posterior insula and the right posterior insula) was found in e-cigs users compared to HC. However, we did not find any significant group difference between exclusive e-cigs users and dual users (p > 0.05).
The relationship between the brain and e-cigs use characteristics
We found a significant negative correlation between ReHo in the left NAcc and years of e-cigs use within chronic e-cigs users (r = −2.73, p = 0.008, FDR corrected, Fig. 4). However, we found no significant associations between rs-fMRI measures and other characteristics of e-cigs use.
Machine learning analysis
The SVM based on all significant results can differentiate chronic e-cigs users from HC with an accuracy of 73.47%, an AUC of 0.781, a sensitivity of 67.74%, and a specificity of 78.64%. Permutation tests found that the classification accuracy based on actual labels was significantly higher when compared to the random label (p < 0.001).
Discussion
Using a rs-fMRI technique, we identified brain regions with brain functional activity alterations among chronic e-cigs users with nicotine dependence. In the voxel-wise analysis, we observed significant increases in ReHo of the right rolandic operculum and the right insula in e-cigs users with nicotine dependence compared to the HC group. At the ROI-wise level, abnormalities in ReHo were detected in the NAcc and insular subregions. Furthermore, FC analysis revealed significant alterations in connectivity patterns between the NAcc, the VTA, and the insular subregions among nicotine-dependent e-cigs users when compared to HC. The classification model based on these results successfully distinguished chronic e-cigs users from HC. Correlation analysis revealed a negative relationship between ReHo in the left NAcc and years of e-cigs use. Subgroup analysis did not reveal any significant differences between exclusive e-cigs users and dual users. Together, these findings suggested that chronic cigs use alternates brain functional activity.
The finding of altered local synchronization of BOLD signals in the NAcc and the insular subregions associated with chronic e-cigs use was lined with our hypothesis, which showed the impact of e-cigs on brain regions related to addictive behaviors. The NAcc, a key structure within the basal ganglia, plays a role in goal-directed behaviors by integrating information from cortical and limbic regions [36]. Chronic drug exposure disrupts plasticity in the NAcc, leading to an abnormal drive to seek drugs when exposed to drug-associated cues. For example, a positron emission tomography study found altered cue-induced activity in the NAcc among tobacco smokers compared to nonsmokers [37]. The insular cortex, a crucial hub within the salience network involved in processing interoceptive signals and emotions, is associated with nicotine addiction [38]. Altered ReHo in the insula may contribute to the maintenance of e-cigs use. Additionally, we observed altered local synchronized activity in the right rolandic operculum among chronic e-cigs users. These findings align with a VBM meta-analysis demonstrating reduced gray matter volume in the bilateral rolandic operculum, the bilateral prefrontal cortex, and the bilateral insula in addiction-related disorders compared to healthy controls [39].
Altered FC between the insular cortex, the NAcc, and the VTA were found in chronic e-cigs users relative to HC. The insula was able to be segmented into three different subregions with distinct function [40]. The posterior insular cortex was involved in pain perception, sensorimotor processing and language processing [40]. The dorsal anterior insular cortex was responsible for an executive control function, while the ventral anterior insular cortex was associated with social-emotional processing and autonomic function [40, 41]. The unbalance of interoceptive signals originating from physiological states (the posterior insular cortex) and positive hedonic emotions (the anterior insular cortex) was believed to play a key role in the affective learning of the effects of drugs and their associations with specific contexts [42], which was lined with our findings of abnormal internal connectivity within the insula. Abnormal NAcc-VTA connectivity found among e-cigs users are consistent with findings of decreased FC between the VTA and the NAcc in chronic cocaine users [43]. The NAcc and the VTA were crucial components of the mesolimbic reward system, which is widely recognized as playing a key role in the neurobiology of addiction [41]. Dopamine neurons within the VTA projected to the NAcc through the mesolimbic pathway and associations between these regions were essential for drug reward and implicated in incentive motivation, such as substance-related craving [44, 45]. We speculated that reduced functional connectivity between the VTA and the NAcc may represent a vulnerability for the development of e-cigs dependence. The altered connections of the insular subregions and the mesolimbic reward system (the NAcc and VTA) were observed in the current study. Evidence from diffusion tensor imaging (DTI) analysis suggested that the connectivity of the right anterior insula and the NAcc was associated with subsequent relapse to stimulant use [46]. Another study has shown that there is a correlation between the connectivity of the insula and VTA and the severity of nicotine dependence [47]. These findings suggest that the repetitive and sustained use of e-cigs may be linked to the altered FC between the insula, NAcc, and VTA.
We found these altered results were able to successfully differentiate e-cigs users with nicotine dependence from non-users using an SVM classification model. By employing machine learning algorithms, we provided a novel framework to assess disorder-related biomarkers at the individual level [35, 48]. Our findings suggest that the insula, the NAcc, and the VTA may have diagnostic potential for e-cigs dependence. These brain regions appear to play an important role in identifying individuals with a higher susceptibility to develop impulsive drug-seeking behaviors.
Brain and behaviors analysis found ReHo in the left NAcc was negatively related to more long years of e-cigs use. This inverse relationship was consistent with previous studies which revealed brain functional activity in the NAcc was years of addictive substance [43]. Moreover, previous studies have demonstrated that the NAcc plays a crucial role in processing goal-directed actions and mediates the positive reinforcing effects of drugs [49, 50]. Deficiencies in goal-directed behavior have been associated with drug-seeking tendencies and compulsive behaviors, representing notable risk factors for relapse. With prolonged use of electronic cigarettes, it is plausible that this could lead to a decrease in NAcc functional activity, thereby potentially exacerbating drug-seeking behavior [51]. Furthermore, the insular cortex has been recognized as a pivotal predictive factor for anticipating relapse to substance use [46, 52]. We speculated that the left NAcc may potentially serve as a target for neuromodulation, such as through Transcranial Magnetic Stimulation (TMS) [53]. Although some studies using resting-state FC found an inverse relationship between mesolimbic functional activity and subjective craving [32, 54], we did not find similar findings in the current study. One possible explanation is that the subjects in our study had been using e-cigs for over a year, which may have caused a shift from reward-directed behavior to habitual and compulsive behavior [49].
Subgroup analysis found no difference in fMRI indices between exclusive e-cigs users and dual users. Previous studies found that traditional cigarette users, e-cigs users, and dual users all exhibited perceived high stress and depressive mood compared to HC [55, 56]. These results suggest that the impact of exclusive e-cigs use on brain functional activity is comparable to that of traditional cigarettes and e-cigs. Therefore, it is important for smokers, regardless of their specific smoking habits, to quit smoking in order to maximize potential health benefits. Nevertheless, the profiles of brain functional activity observed in this study may serve as markers indicative of an increased propensity for the development of drug use behaviors. These findings contribute to our understanding of the neural mechanisms underlying e-cigs addiction and highlight the potential of utilizing brain imaging techniques for diagnostic purposes.
There are some limitations in this study. First, this study did not include traditional cigarette users. Given that e-cigs users often have a history of combustible cigarette use or engage in dual use [56], future research should compare the effects of e-cigs and traditional cigarettes to better understand their respective impacts on the brain. Second, the current study focused on ROI-wise FC analysis to investigate the effects of chronic e-cigs use on the brain. It would be beneficial to include more brain regions and construct a larger functional network in future studies, as this could provide a more comprehensive understanding of the effects of e-cigs use. Third, while all e-cig users in our study consumed nicotine, it’s important to note that different e-cig brands may contain different substances beyond nicotine. We anticipated that future studies will delve into the effects of these specific substances in e-cigs. Fourth, some studies have reported that white signals may contain functional information, suggesting that signals were not regressed in the processing. Furthers studies should take account into this factor and investigate the temporal properties of white signals. Additionally, while we have employed general linear models to account for the influence of age and sex, we acknowledge that there may still be residual effects of these variables on the outcomes. Given the sample size imbalance (e-cigarette group: 26 females and 67 males), future research should further investigate whether age or sex has an impact on the neural activity associated with e-cigarette use. Finally, while the classification model based on the significant group differences achieved relatively high accuracy, it is important to note that the generalizability of this model is unknown due to the absence of independent test sets. Future studies with larger sample sizes should aim to build a reliable diagnostic model.
In conclusion, this study has identified abnormalities in nicotine-dependent e-cigs users in the functional activity of the NAcc, the VTA, and the insula, which were all linked to the initiation and maintenance of addiction. Although e-cigs can potentially aid smokers in quitting combustible cigarettes, it is important to consider its addiction and changes in brain function associated with their use. Given the insufficiency of existing regulations in China to safeguard youth from e-cigarette usage and nicotine addiction, it is imperative for policy-makers to place a strong emphasis on early prevention of youth e-cigs use.
Data availability
The data that was used in this study would be available if the readers have reasonable aims to use these data and acquire an agreement from the corresponding author.
References
Callahan-Lyon P. Electronic cigarettes: human health effects. Tob Control. 2014;23:ii36–40.
Farsalinos KE, Poulas K, Voudris V, Le Houezec J. Electronic cigarette use in the European Union: analysis of a representative sample of 27 460 Europeans from 28 countries. Addiction. 2016;111:2032–40.
Wang TW, Gentzke AS, Neff LJ, Glidden EV, Jamal A, Park-Lee E, et al. Characteristics of e-cigarette use behaviors among US Youth, 2020. JAMA Netw Open. 2021;4:e2111336.
Lee SJ, Rees VW, Yossefy N, Emmons KM, Tan ASL. Youth and young adult use of pod-based electronic cigarettes from 2015 to 2019: a systematic review. JAMA Pediatr. 2020;174:714–20.
Wang X, Zhang X, Xu X, Gao Y. Perceptions and use of electronic cigarettes among young adults in China. Tob Induc Dis. 2019;17:17.
Rom O, Pecorelli A, Valacchi G, Reznick AZ. Are E-cigarettes a safe and good alternative to cigarette smoking? Ann N Y Acad Sci. 2015;1340:65–74.
Chadi N, Li G, Cerda N, Weitzman ER. Depressive symptoms and suicidality in adolescents using e-cigarettes and Marijuana: a secondary data analysis from the youth risk behavior survey. J Addict Med. 2019;13:362–5.
Zvolensky MJ, D’Souza J, Garey L, Alfano CA, Mayorga NA, Peraza N, et al. Subjective sleep quality and electronic cigarette dependence, perceived risks of use, and perceptions about quitting electronic cigarettes. Addict Behav. 2020;102:106199.
Bullen C, Howe C, Laugesen M, McRobbie H, Parag V, Williman J, et al. Electronic cigarettes for smoking cessation: a randomised controlled trial. Lancet. 2013;382:1629–37.
Eltorai AE, Choi AR, Eltorai AS. Impact of electronic cigarettes on various organ systems. Respir Care. 2019;64:328–36.
Nyilas S, Bauman G, Korten I, Pusterla O, Singer F, Ith M, et al. MRI shows lung perfusion changes after vaping and smoking. Radiology. 2022;304:195–204.
Caporale A, Langham MC, Guo W, Johncola A, Chatterjee S, Wehrli FW. Acute effects of electronic cigarette aerosol inhalation on vascular function detected at Quantitative MRI. Radiology. 2019;293:97–106.
Orzabal M, Ramadoss J. Impact of electronic cigarette aerosols on pregnancy and early development. Curr Opin Toxicol. 2019;14:14–20.
Archie SR, Sifat AE, Zhang Y, Villalba H, Sharma S, Nozohouri S, et al. Maternal e-cigarette use can disrupt postnatal blood-brain barrier (BBB) integrity and deteriorates motor, learning and memory function: influence of sex and age. Fluids Barriers CNS. 2023;20:17.
Alasmari F, Crotty Alexander LE, Nelson JA, Schiefer IT, Breen E, Drummond CA, et al. Effects of chronic inhalation of electronic cigarettes containing nicotine on glial glutamate transporters and α-7 nicotinic acetylcholine receptor in female CD-1 mice. Prog Neuropsychopharmacol Biol Psychiatry. 2017;77:1–8.
Re DB, Hilpert M, Saglimbeni B, Strait M, Ilievski V, Coady M, et al. Exposure to e-cigarette aerosol over two months induces accumulation of neurotoxic metals and alteration of essential metals in mouse brain. Environ Res. 2021;202:111557.
Kaisar MA, Villalba H, Prasad S, Liles T, Sifat AE, Sajja RK, et al. Offsetting the impact of smoking and e-cigarette vaping on the cerebrovascular system and stroke injury: is metformin a viable countermeasure? Redox Biol. 2017;13:353–62.
Sifat AE, Vaidya B, Kaisar MA, Cucullo L, Abbruscato TJ. Nicotine and electronic cigarette (E-Cig) exposure decreases brain glucose utilization in ischemic stroke. J Neurochem. 2018;147:204–21.
Bakker C, Chivers E, Chia XW, Quintrell E, Wyrwoll C, Larcombe A. Switching from tobacco cigarettes in very early pregnancy: the effects of in utero e-cigarette exposure on mouse offspring neurodevelopment and behaviour. Physiol Behav. 2023;263:114118.
Jankowski M, Krzystanek M, Zejda JE, Majek P, Lubanski J, Lawson JA, et al. E-cigarettes are more addictive than traditional cigarettes-a study in highly educated young people. Int J Environ Res Public Health. 2019;16:2279.
Leventhal AM, Strong DR, Kirkpatrick MG, Unger JB, Sussman S, Riggs NR, et al. Association of electronic cigarette use with initiation of combustible tobacco product smoking in early adolescence. Jama. 2015;314:700–7.
Hammond D, Reid JL, Cole AG, Leatherdale ST. Electronic cigarette use and smoking initiation among youth: a longitudinal cohort study. Cmaj. 2017;189:E1328–e1336.
Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–11.
Hobkirk AL, Nichols TT, Foulds J, Yingst JM, Veldheer S, Hrabovsky S, et al. Changes in resting state functional brain connectivity and withdrawal symptoms are associated with acute electronic cigarette use. Brain Res Bull. 2018;138:56–63.
Garrison KA, O’Malley SS, Gueorguieva R, Krishnan-Sarin S. A fMRI study on the impact of advertising for flavored e-cigarettes on susceptible young adults. Drug Alcohol Depend. 2018;186:233–41.
Lindsey KP, Gatley SJ, Volkow ND. Neuroimaging in drug abuse. Curr Psychiatry Rep. 2003;5:355–61.
Wise RA, Koob GF. The development and maintenance of drug addiction. Neuropsychopharmacology. 2014;39:254–62.
Zang Y, Jiang T, Lu Y, He Y, Tian L. Regional homogeneity approach to fMRI data analysis. Neuroimage. 2004;22:394–400.
Zuo XN, Xu T, Jiang L, Yang Z, Cao XY, He Y, et al. Toward reliable characterization of functional homogeneity in the human brain: preprocessing, scan duration, imaging resolution and computational space. Neuroimage. 2013;65:374–86.
Feng Z-J, Deng X-P, Zhao N, Jin J, Yue J, Hu Y-S, et al. Resting-state fMRI functional connectivity strength predicts local activity change in the dorsal cingulate cortex: a multi-target focused rTMS study. Cerebral Cortex. 2021;32:2773–84.
Fan L, Li H, Zhuo J, Zhang Y, Wang J, Chen L, et al. The human brainnetome Atlas: a new brain atlas based on connectional architecture. Cereb Cortex. 2016;26:3508–26.
Zhang JT, Ma SS, Yip SW, Wang LJ, Chen C, Yan CG, et al. Decreased functional connectivity between ventral tegmental area and nucleus accumbens in Internet gaming disorder: evidence from resting state functional magnetic resonance imaging. Behav Brain Funct. 2015;11:37.
Bi Y, Yuan K, Guan Y, Cheng J, Zhang Y, Li Y, et al. Altered resting state functional connectivity of anterior insula in young smokers. Brain Imaging Behav. 2017;11:155–65.
Uddin LQ, Nomi JS, Hébert-Seropian B, Ghaziri J, Boucher O. Structure and function of the human insula. J Clin Neurophysiol. 2017;34:300–6.
Sun Y, Zhao L, Lan Z, Jia XZ, Xue SW. Differentiating boys with ADHD from those with typical development based on whole-brain functional connections using a machine learning approach. Neuropsychiatr Dis Treat. 2020;16:691–702.
Scofield MD, Heinsbroek JA, Gipson CD, Kupchik YM, Spencer S, Smith AC, et al. The nucleus accumbens: mechanisms of addiction across drug classes reflect the importance of glutamate homeostasis. Pharmacol Rev. 2016;68:816–71.
Yasuno F, Ota M, Ando K, Ando T, Maeda J, Ichimiya T, et al. Role of ventral striatal dopamine D1 receptor in cigarette craving. Biol Psychiatry. 2007;61:1252–9.
Paulus MP, Stewart JL. Interoception and drug addiction. Neuropharmacology. 2014;76:342–50.
Zhang M, Gao X, Yang Z, Wen M, Huang H, Zheng R, et al. Shared gray matter alterations in subtypes of addiction: a voxel-wise meta-analysis. Psychopharmacology (Berl). 2021;238:2365–79.
Droutman V, Read SJ, Bechara A. Revisiting the role of the insula in addiction. Trends Cogn Sci. 2015;19:414–20.
Chang LJ, Yarkoni T, Khaw MW, Sanfey AG. Decoding the role of the insula in human cognition: functional parcellation and large-scale reverse inference. Cereb Cortex. 2013;23:739–49.
Naqvi NH, Gaznick N, Tranel D, Bechara A. The insula: a critical neural substrate for craving and drug seeking under conflict and risk. Ann N Y Acad Sci. 2014;1316:53–70.
Gu H, Salmeron BJ, Ross TJ, Geng X, Zhan W, Stein EA, et al. Mesocorticolimbic circuits are impaired in chronic cocaine users as demonstrated by resting-state functional connectivity. Neuroimage. 2010;53:593–601.
Cooper S, Robison AJ, Mazei-Robison MS. Reward circuitry in addiction. Neurotherapeutics. 2017;14:687–97.
Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry. 2016;3:760–73.
Tisdall L, MacNiven KH, Padula CB, Leong JK, Knutson B. Brain tract structure predicts relapse to stimulant drug use. Proc Natl Acad Sci USA. 2022;119:e2116703119.
Claus ED, Blaine SK, Filbey FM, Mayer AR, Hutchison KE. Association between nicotine dependence severity, BOLD response to smoking cues, and functional connectivity. Neuropsychopharmacology. 2013;38:2363–72.
Zhao L, Xue SW, Sun YK, Lan Z, Zhang Z, Xue Y, et al. Altered dynamic functional connectivity of insular subregions could predict symptom severity of male patients with autism spectrum disorder. J Affect Disord. 2022;299:504–12.
Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–9.
Voon V, Joutsa J, Majuri J, Baek K, Nord CL, Arponen E, et al. The neurochemical substrates of habitual and goal-directed control. Transl Psychiatry. 2020;10:84.
MacNiven KH, Jensen ELS, Borg N, Padula CB, Humphreys K, Knutson B. Association of neural responses to drug cues with subsequent relapse to stimulant use. JAMA Netw Open. 2018;1:e186466.
Lichenstein SD, Scheinost D, Potenza MN, Carroll KM, Yip SW. Dissociable neural substrates of opioid and cocaine use identified via connectome-based modelling. Mol Psychiatry. 2021;26:4383–93.
Joutsa J, Moussawi K, Siddiqi SH, Abdolahi A, Drew W, Cohen AL, et al. Brain lesions disrupting addiction map to a common human brain circuit. Nat Med. 2022;28:1249–55.
Bracht T, Soravia L, Moggi F, Stein M, Grieder M, Federspiel A, et al. The role of the orbitofrontal cortex and the nucleus accumbens for craving in alcohol use disorder. Transl Psychiatry. 2021;11:267.
Kim CY, Paek YJ, Seo HG, Cheong YS, Lee CM, Park SM, et al. Dual use of electronic and conventional cigarettes is associated with higher cardiovascular risk factors in Korean men. Sci Rep. 2020;10:5612.
Pisinger C, Rasmussen SKB. The health effects of real-world dual use of electronic and conventional cigarettes versus the health effects of exclusive smoking of conventional cigarettes: a systematic review. Int J Environ Res Public Health. 2022;19:13687.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (Grant no. U22A20302), by the STI 2030—Major projects (Grant no. 2022ZD0211200) and by the Provincial Natural Science Foundation of Hunan (Grant nos. 2020JJ5306 and 21B0083). The funders had no role in study design, data collection and analysis, the decision to write the report or to submit the paper for publication. We thank all subjects who participated in this study.
Author information
Authors and Affiliations
Contributions
LYH conceived the study. SYK, XA., FYH, LL, and CHB were responsible for clinical data collection. SYK analyzed the data and drafted the manuscript, with TJS and LYH involved in review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sun, Y., Xie, A., Fang, Y. et al. Altered insular functional activity among electronic cigarettes users with nicotine dependence. Transl Psychiatry 14, 293 (2024). https://doi.org/10.1038/s41398-024-03007-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41398-024-03007-6